Abstract
Heparin, a sulfated glycosaminoglycan, is a widely used injectable anticoagulant. This polysaccharide is a natural product extracted from porcine intestinal tissue. A specific pentasaccharide sequence is responsible for heparin’s high affinity towards anti-thrombin III, which undergoes a conformational change and, as a result, inhibits the blood coagulation Factor Xa, a critical serine protease at the convergence on the intrinsic and extrinsic activation pathway of the coagulation cascade. Due to its structural complexity and heterogeneity, the synthesis of the anti-thrombin III-binding sequence of heparin has been limited to a few approaches. The heparin contamination crisis in 2007 has motivated the development of alternative methods for the efficient preparation of safe heparin products. In this article, we discuss the current methods and recent advances in heparin and low MW heparin syntheses and the recent successful chemoenzymatic preparation of ultralow MW heparins.
Heparin has been used as an anticoagulant for over 75 years since it entered clinical trials in 1935 [1]. It was first discovered in 1916 by McLean and Howell at Johns Hopkins University and is one of the oldest drugs still in clinical use. Heparin is a highly sulfated polysaccharide belonging to the family of heparin/heparan sulfate (HS) glycosaminoglycans (GAGs), with an average MW of 12–15 kDa and a MW range of 5–40 kDa [2].
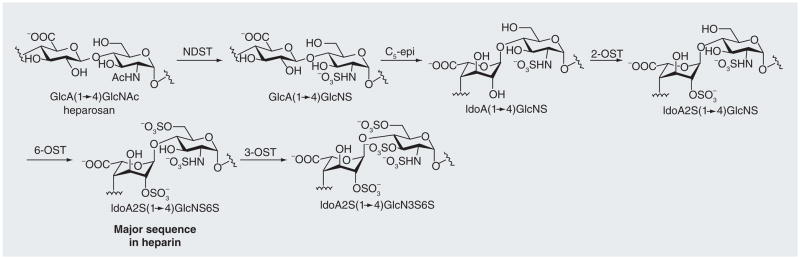
In the biosynthetic pathway for heparin [3,4], glycosyltransferases, called polysaccharide synthases, sequentially transfer activated monosaccharides, called uridinediphosphate-sugars, to an acceptor, polymerizing them into heparosan. Heparosan has a repeating, 1→4-linked, β-D-glucuronic acid (GlcA) and D-N-acetylglucosamine (GlcNAc) disaccharide unit, and is the starting polysaccharide in heparin biosynthesis. Subsequent modifications through the action of N-deacetylase/N-sulfotransferase, C5-epimerase (C5-epi), and 2-, 6- and 3-O-sulfotransferases, result in heparin (Figure 1). The number and position of sulfo groups (S) varies throughout the chain and the most common sequence is the iduronic acid (IdoA)2S (1→4) GlcNS6S, trisulfated disaccharide. A less commonly occurring sequence can contain an IdoA or GlcA (or very rarely a GlcA2S) 1→4 linked to a GlcNAc or GlcNAc6S (or rarely a GlcNS3S6S) residue. HS is structurally similar to heparin but contains more GlcA and GlcNAc residues than heparin [5]. These variable patterns of sulfo-group substitution throughout its chain give heparin and HS their structural diversity and heterogeneity, providing its anionic character, allowing it to interact with a variety of basic proteins [6,7]. While well studied, the structure–activity relationship of heparin/HS with heparin-binding proteins is not yet fully understood, due to the difficulties of preparing structurally defined GAGs [8].
Figure 1. Modification of heparosan in the biosynthetic pathway of heparin[3].
The heparosan polysaccharide is sequentially modified through the action of N-deacetylase/N-sulfotransferase, C5-epimerase, 2-O-sulfotransferase (OST) and 6-OST to make the major sequence found in heparin, IdoA2SGlcNS6S. A third of the chain is modified by 3-OST to add another sulfate in the glucosamine residue.
Anticoagulant mechanism of heparin
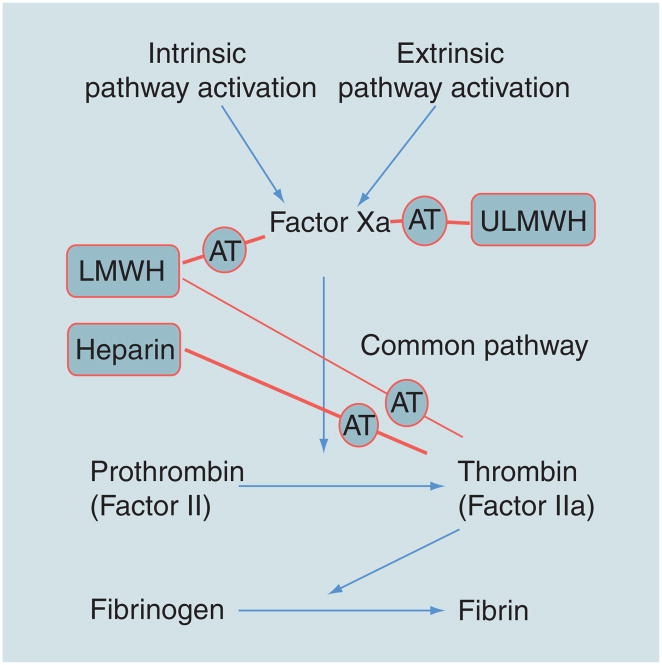
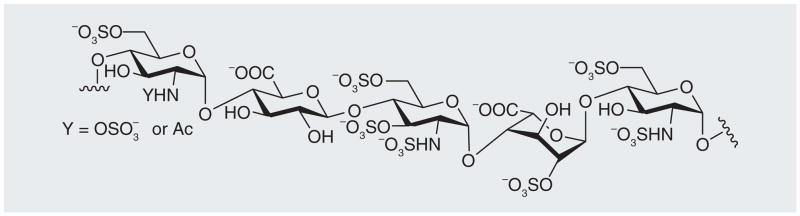
The most well-studied heparin-binding protein is anti-thrombin III (AT), a serine proteinase inhibitor that, in its activated form, inhibits the blood-clotting cascade (Figure 2) [9]. Heparin’s application as an anticoagulant arises primarily from a specific pentasaccharide sequence, shown in Figure 3 [10,11]. This rare sequence, containing an unusual 3-O-sulfo group in a GlcNS6S residue, is found in only approximately a third of heparin’s chains [12]. The negative charges on the sulfo and carboxyl groups are essential for the tight binding of heparin and the plasma proteinase inhibitor AT [13]. Although AT in its native form is a weak protease inhibitor, on binding to the pentasaccharide in heparin AT undergoes a conformational change altering its reactive site loop, resulting in a 300-fold increase in the inhibition of the critical serine protease Factor Xa, located at the convergence on the intrinsic and extrinsic activation pathways of the coagulation cascade (Figure 2) [14]. Heparin bound to AT can also bind to, and inhibit, thrombin (Factor IIa). While thrombin does not require a specific sequence within heparin to bind, if the AT-binding site on heparin is flanked by a contiguous stretch of additional five to six trisulfated disaccharides, heparin can also effectively bind thrombin [15,16]. This ternary complex, consisting of heparin–AT–thrombin, blocks thrombin’s ability to convert soluble fibrinogen into an insoluble fibrin clot (Figure 2).
Figure 2. Simplified coagulation pathway showing the different points at which heparin, low and ultralow MW heparins inhibit coagulation through anti-thrombin III.
The arrows indicate the direction of the coagulation cascade (from activation to fibrin clot formation). The lines passing through AT show the points in the pathway inhibited by heparin, low and ultralow MW heparins through their interactions with AT. The thinner line passing through AT indicates the low level of thrombin inhibition by low MW heparins. AT: Anti-thrombin III; LMWH: Low MW heparin; ULMWH: Ultralow MW heparin.
Figure 3. The anti-thrombin III-binding pentasaccharide found in heparin.
The 3-O-sulfo group in GlcNS3S6S is crucial for its binding to anti-thrombin III.
The heparin contamination crisis
Commercial heparin is currently prepared by animal tissue extraction, usually porcine intestine. After processing, porcine intestinal mucosa contains other heparin-like GAGs that have a different charge state from heparin. To isolate heparin, the mixture of GAGs is passed through an ion-exchange resin, which fractionates GAGs based on their charge density. Heparin, having 2.6 sulfo groups per disaccharide on average, has the highest negative charge among them. The yield for heparin is ~300 mg/animal, which corresponds to 30,000–50,000 U.
From late 2007 to early 2008, many patients suffered from severe allergic-type reactions after being injected with heparin and some of these reactions were quite severe, being associated with the death of nearly 100 patients in the USA alone. The cause was linked to an adulterant in heparin [17], which was later identified as oversulfated chondroitin sulfate [18]. Oversulfated chondroitin sulfate, a semi-synthetic polysaccharide prepared through the chemical persulfonation of chondroitin sulfate [19], is structurally similar to, and mimics, heparin in the anticoagulant pathway. However, unlike heparin, it causes anaphylactoid side effects [20]. After the heparin crisis, the US FDA enhanced its inspection of heparin-manufacturing facilities and the US Pharmacopeia has revised its monograph on heparin. Additional testing of heparin-active pharmaceutical ingredients, required by the FDA and US Pharmacopeia, has markedly decreased the availability and increased the cost of this critical drug.
Low MW heparins
Heparin is the anticoagulant of choice for cardiovascular surgery, the prevention of thromboembolism and hemodialysis. It is administered intravenously and exhibits some undesirable side effects including hemorrhagic complications [21] and heparin-induced thrombocytopenia (HIT) [22], which can result in the generation of antibodies to platelet factor 4–heparin complex that lead to platelet loss. In an effort to reduce undesirable side effects of heparin, many heparin-based mimetics have been developed[23].
Low MW heparins (LMWHs), or fractionated heparin with a MW of approximately 3–8 kDa, were first introduced in the late 1980s as substitutes for heparin in certain applications. LMWHs exhibit anticoagulant activity similar to heparin and may slightly lower the risk of bleeding complications and HIT compared with unfractionated heparin, but most critically, LMWHs are subcutaneously bioavailable [24,25]. Due to their more predictable pharmacodynamics and anticoagulant effects, LMWHs are used for patients with pulmonary embolism. They are given once or twice daily, as their half-lives are longer than heparin, which is usually administered continuously or immediately prior to procedures of brief duration [26]. Due to their shorter chain lengths, LMWHs show reduced activity towards thrombin (Factor IIa) and have antifactor Xa/antifactor IIa ratios of approximately four [27].
There are a number of different LMWHs currently in use that are prepared by the controlled chemical or enzymatic degradation of heparin [28,29]. Since the LMWHs are derivatives of heparin, they are polydisperse and structurally heterogeneous and, as a result of their preparation by a chemical or enzymatic process, they often carry an unnatural anhydromannitol, 1,6-anhydro sugar or unsaturated uronic acid residues at the ends of their chains. Each LMWH is prepared differently and, therefore, have slightly differing properties from one another [30]. Moreover, LMWHs are all derived from porcine intestinal heparin and some varieties were found to be severely contaminated during the heparin crisis between 2007 and 2008.
Ultralow MW heparins
Ultralow MW heparins (ULMWHs) have MWs of less than 3 kDa, corresponding to chain lengths that are insufficient to exhibit measurable antifactor IIa activity and, thus, are generally regarded as specific antifactor Xa agents. Fondaparinux is the first synthetic ULMWH and a pentasaccharide based on the AT-binding site of heparin. First marketed in 2002 by Sanofi, it is now sold by GlaxoSmithKline, under the trade name Arixtra®. The FDA recently approved a generic version of fondaparinux, synthesized by Reddy’s Laboratories.
Choay et al. first accomplished the total synthesis of fondaparinux in the 1980s, shortly after the AT-binding domain of heparin was elucidated [11,31,32]. The reducing end of fondaparinux is protected with an O-methyl group, as it prevented side reactions during its synthesis (Figure 4). Due to its small molecular size, fondaparinux indirectly inhibits thrombin, by specifically inhibiting Factor Xa, but not act directly on thrombin (Factor IIa) [33]. It also binds less tightly to platelet factor 4, significantly reducing the risk for HIT. Finally, it can be administered subcutaneously resulting in an increased half-life [34]. Since it is structurally defined, the heparin pentasaccharide has more predictable pharmacokinetics and pharmacodynamics and it is easier to chemically characterize. Unfortunately, fondaparinux is prepared by a long and complex chemical synthesis, making it an expensive anticoagulant.
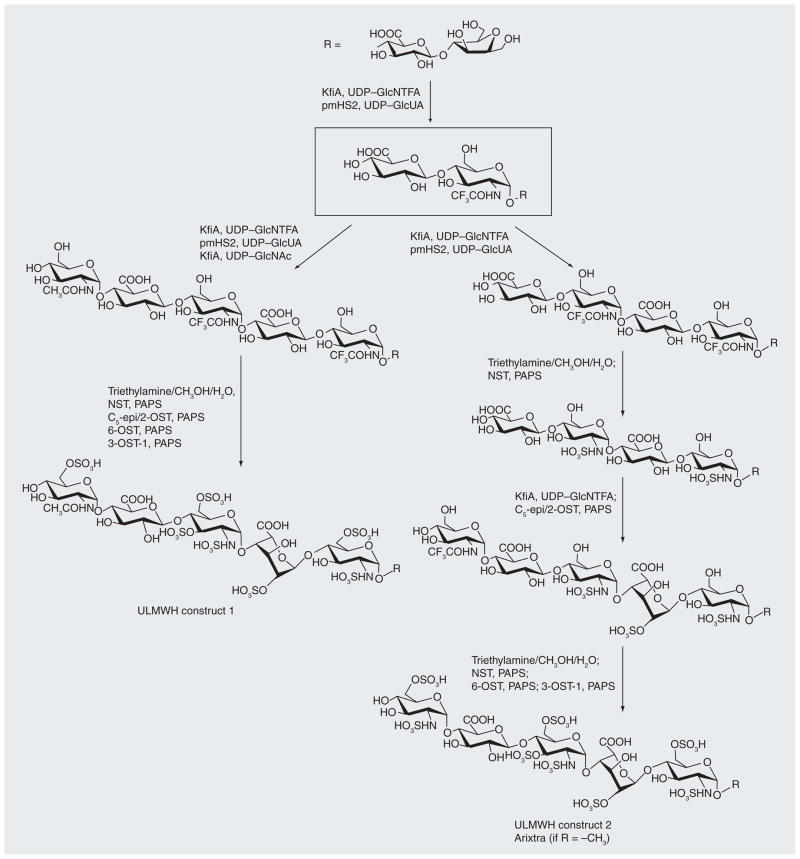
Figure 4. Chemoenzymatic synthesis of ultralow MW heparin constructs 1 and 2.
NST: N-sulfotransferase; NTFA: N-trifluoracetate; PAPS: 3′-phosphoadenosine 5′-phoshphosulfate; ULMWH: Ultralow MW heparin. Modified with permission from [47].
Sanofi is currently developing a novel ULMWH called semuloparin (AVE5026) for the prevention of venous thromboembolism. It is a hemisynthetic ULMWH that is prepared by a selective and controlled depolymerization of heparin using a phosphazene base [35]. Due to its bulky structure, the base cleaves the heparin chain through a β-elimination reaction only at the less hindered regions, leaving the crowded anti-thrombin-binding site intact. The generated AT-binding hexasaccharide is isolated by strong anion-exchange chromatography. Studies in patients showed the antifactor Xa/antifactor IIa ratio of semuloparin to be above 30, indicating nearly pure antifactor Xa activity [36]. Although the preparation cost is significantly lower than that of fondaparinux, it is neither homogeneous nor structurally defined and, since it is still derived from porcine intestinal heparin semuloparin, could be subject to contamination or adulteration.
Although there have been many successful chemical syntheses of complex molecules of pharmaceutical significance [37], these typically involve numerous steps and result in low overall yields. Chemists are starting to turn towards enzymatic or chemoenzymatic synthesis to circumvent these problems [38,39]. Enzymes are proteins that are used under mild (20–60°C) and generally aqueous conditions, which catalyze the chemical reaction of a substrate or substrates. Unlike most chemical reactions, these enzymatic reactions are highly chemospecific, regiospecific and stereospecific. For example, glycosyltransferases can be used to glycosylate a saccharide unit without protecting or directing groups affording only either α- or β-glycosylation at a specific site within the acceptor.
Using recombinant technology, glycosyltransferases and heparin biosynthetic enzymes have been cloned and expressed, and are under study for the synthesis of heparin [40,41]. Initial efforts towards a chemoenzymatic preparation of heparin used C5-epi to convert the GlcA of the heparosan polysaccharide to IdoA, but relied primarily on chemical modifications for N- and O-sulfation, creating unwanted sulfation sites [42]. An enzymatic synthesis of an oligosaccharide based on the structure of HS has been accomplished using the heparin/HS modification enzymes [43] and glycosyltransferases [44]. A concerted effort is currently underway to chemoenzymatically synthesize a bioengineered heparin [45]. Such a bioengineered heparin might one day be approved as a generic heparin and also used in the preparation of LMWHs, increasing the supply and safety of these heparin products [46].
Recently, fondaparinux-like oligosaccharides have been chemoenzymatically synthesized using heparin biosynthetic enzymes [47]. Two such ULMWs, constructs 1 and 2 (Figure 4), are heptasaccharides that showed excellent in vivo and in vitro anticoagulant activity. More notably, by using a chemoenzymatic approach, these homogeneous heptasaccharides were synthesized in ten or 12 steps at a multimilligram scale and in approximately 40% overall yield.
The synthetic strategy for ULMWH constructs 1 and 2 was biomimetic to heparin biosynthesis. The backbones of these oligosaccharides were first constructed using glycosyltransferases and, thereafter, the sulfotransferases were used to position the sulfo groups (Figure 4). Both heparin constructs were synthesized on a heparosan-derived disaccharide acceptor containing a ring-contracted anhydromannitol residue. Using KfiA (an N-acetyl glucosaminyl-transferase) and pmHS2 (a heparosan synthase) [48], the acceptor was elongated stepwise from a disaccharide to a heptasaccharide. Instead of the natural GlcNAc, an unnatural N-trifluoroacetyl glucosamine (GlcNTFA) was installed, which is distinguishable from GlcNAc at the chemical de-N-trifluoroacetylation step. The NTFA group is susceptible to deprotection under mildly basic conditions (Et3N:MeOH:H2O = 1:1:0.5), while leaving the NAc group intact, as this requires strongly basic conditions with heating to cleave. KfiA transferred GlcNTFA smoothly, demonstrating that uridinediphosphate–GlcNTFA is a compatible unnatural substrate for KfiA. In contrast with chemical glycosylation, enzymatic glycosylation proceeded with >80% yield and in a stereospecific manner, giving the correct stereochemistry at each anomeric center as confirmed by 2D NMR analyses.
Subsequent step-by-step modifications on the backbone involved epimerization of GlcA to IdoA, chemical de-N-trifluoroacetylation, and enzymatic N- and O-sulfations. Chemical de-N-trifluoroacetylation cleaved only the NTFA group, leaving the oligosaccharide backbone intact as it required mildly basic conditions. The order of modifications that took place was carefully chosen as these enzymes are substrate specific. For instance, C5-epi/2-OST is specific to the GlcA residue flanked by two GlcNS residues. Therefore, in synthesizing heparin construct 2, which carries two possible sites for C5-epimerization/2-O-sulfation, it was crucial to add another GlcNTFA residue at the nonreducing end, allowing the modification to take place at the GlcA residue on the reducing end. A small-scale reaction, using radioisotope-labeled 3′-phosphoadenosine 5′-phoshphosulfate was carried out in parallel to ensure that each reaction was complete. The reaction containing the radioisotope reagent was monitored by HPLC, whose chromatogram showed one product upon completion of the reaction. With subsequent 6-O-sulfation and 3-O-sulfation, multimilligrams of ULMW heparins 1 and 2 were synthesized in a ten- and 12-step scheme, respectively, with an overall yield of approximately 40%; a remarkably high yield compared with a total chemical synthesis of an oligosaccharide. In addition, the chemoenzymatic approach proved to be scalable as the ULMW heparin 1 synthesis was scaled up by 15-fold, resulting in 50 mg of the final product.
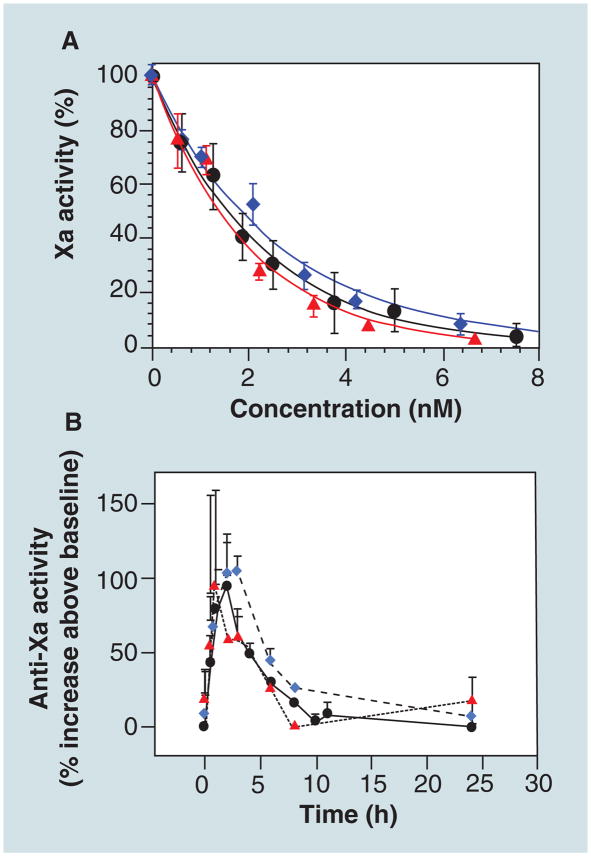
The in vivo and in vitro anticoagulant activities of ULMWH construct 1 and 2 were determined and compared with fondaparinux (Figure 5) [47]. The binding affinities of ULMWH construct 1 and 2 to AT were 5.2 ± 0.2 and 9.1 ± 0.2 nM, respectively, similar to that of fondaparinux at 5.9 ± 1.5 nM. The in vitro anti-Xa acitivity assay (Figure 5A) showed that the IC50 values of ULMW heparin 1 and 2 were 2.8 and 3.6 nM, respectively, close to that of fondaparinux at 3.0 nM. In vivo anti-Xa assays were carried out in a rabbit model, which showed that the heparin constructs also have similar pharmacokinetic properties to fondaparinux (Figure 5B). Moreover, unpublished results showed that neither heparin construct showed HIT, making them promising new anticoagulant agents.
Figure 5. In vitro and in vivo assays of ultralow MW heparins.
(A) In vitro anti-Xa activity determination using a chromogenic substrate and (B) in vivo pharmacokinetics profiles in rabbits. Arixtra® (circle), ultralow MW heparins construct 1 (triangle) and ultralow MW heparins construct 2 (diamond).
Reproduced with permisssion from [47].
Future perspective
Heparin and LMWHs are widely used anticoagulants and with the recent developments in synthesis, biotechnology and analytical techniques, methods for preparing new heparin-based anti-thrombotic drugs are emerging at a rapid pace. The chemoenzymatic synthesis of ULMWHs has demonstrated a novel efficient approach to structurally defined heparin oligosaccharides. Using a similar approach, it is now possible to custom design novel heparin oligosaccharides with varying sulfate patterns, a LMWH or even heparin itself. Defined heparin/HS oligosaccharides will also be extremely useful in understanding a specific structure–activity relationship between heparin and its interacting proteins, leading to the design of novel anticoagulants.
Executive summary.
Background
Heparin, a highly sulfated glycosaminoglycan, is a widely used anticoagulant that is structurally complex and heterogeneous.
Anticoagulant mechanism of heparin
Heparin inhibits blood coagulation by binding to anti-thrombin III through a specific pentasaccharide sequence.
The heparin contamination crisis
Commercial heparin is currently extracted from porcine intestines, but the heparin contamination crisis of 2007 has motivated a search for non-animal sourced heparin.
Low MW heparins
Heparin is administered intravenously and has undesirable side effects such as heparin-induced thrombocytopenia and hemorrhagic complication, whereas low MW heparins can be administered subcutaneously and have a lower incidence of heparin-induced thrombocytopenia.
Ultralow MW heparins
Fondaparinux is a chemically synthesized pentasaccharide of heparin, but is the most expensive of the heparin anticoagulants.
Semuloparin, developed by Sanofi, is a novel hemi-synthetic anticoagulant derived from heparin and is currently in clinical trials.
Two fondaparinux analogs were synthesized on a multi-milligram scale by Liu et al. using heparin biosynthetic enzymes. In vitro and in vivo they showed promising results as anticoagulants.
Key Terms
- Heparin
A highly sulfated glycosaminoglycan with high levels of a disaccharide-repeating unit of IdoA2S (1→4) GlcNS6S and an anticoagulant drug
- Glycosaminoglycans
A class of unbranched linear polysaccharides with a repeating disaccharide unit of hexuronic acid and hexosamine
- Anti-thrombin III
A serine proteinase inhibitor whose effect is significantly enhanced upon binding with heparin and, as a result, inhibits blood clotting
- Factor Xa
A serine protease in the thrombin pathway that is inactivated when heparin binds to anti-thrombin III
- Oversulfated chondroitin sulfate
An adulterant that was identified in commercial heparin during the heparin contamination crisis
- Low MW heparins
Depolymerized chains of heparin with a MW of 3–8 kDa
- Ultralow MW heparins
Even shorter chains of heparin having a MW of <3 kDa
Footnotes
For reprint orders, please contact reprints@future-science.com
Financial & competing interests disclosure
This work was supported by NIH grants HL62244, HL096972, GM090257 (to RJL). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Linhardt RJ. Heparin: an important drug enters its seventh decade. Chem Indust. 1991;2:45–50. [Google Scholar]
- 2.Laurent TC, Tengblad A, Thunberg L, Hook M, Lindahl U. The molecular-weight-dependence of the anti-coagulant activity of heparin. Biochem J. 1978;175(2):691–701. doi: 10.1042/bj1750691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3▪.Lindahl U, Feingold DS, Roden L. Biosynthesis of heparin. Trends Biochem Sci. 1986;11(5):221–225. Explains how heparin is biosynthesized. [Google Scholar]
- 4.DeAngelis PL. Microbial glycosaminoglycan glycosyltransferases. Glycobiology. 2002;12(1):9R–16R. doi: 10.1093/glycob/12.1.9r. [DOI] [PubMed] [Google Scholar]
- 5.Lindahl U, Kjellen L. Heparin or heparan sulfate – what is the difference? Thromb Haemost. 1991;66(1):44–48. [PubMed] [Google Scholar]
- 6.Capila I, Linhardt RJ. Heparin–protein interactions. Angew Chem Int Ed Engl. 2002;41(3):391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi NS, Mancera RL. The structure of glycosaminoglycans and their interactions with proteins. Chem Biol Drug Des. 2008;72(6):455–482. doi: 10.1111/j.1747-0285.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 8.Codée JDC, Overkleeft HS, van der Marel G, van Boeckel CAA. The synthesis of well-defined heparin and heparan sulfate fragments. Drug Discov Today Technol. 2004;1(3):317–326. doi: 10.1016/j.ddtec.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg RD, Damus PS. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973;248(18):6490–6505. [PubMed] [Google Scholar]
- 10.Thunberg L, Backstrom G, Lindahl U. Further characterization of the antithrombin-binding sequence in heparin. Carbohydr Res. 1982;100:393–410. doi: 10.1016/s0008-6215(00)81050-2. [DOI] [PubMed] [Google Scholar]
- 11.Choay J, Petitou M, Lormeau JC, Sinay P, Casu B, Gatti G. Structure–activity relationship in heparin: a synthetic pentasaccharide with high affinity for antithrombin III and eliciting high antifactor Xa activity. Biochem Biophys Res Commun. 1983;116(2):492–499. doi: 10.1016/0006-291x(83)90550-8. [DOI] [PubMed] [Google Scholar]
- 12.Lindahl U, Backstrom G, Thunberg L, Leder IG. Evidence for a 3-O-sulfated D-glucosamine residue in the antithrombin-binding sequence of heparin. Proc Natl Acad Sci USA. 1980;77(11):6551–6555. doi: 10.1073/pnas.77.11.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin L, Abrahams JP, Skinner R, Petitou M, Pike RN, Carrell RW. The anticoagulant activation of antithrombin by heparin. Proc Natl Acad Sci USA. 1997;94(26):14683–14688. doi: 10.1073/pnas.94.26.14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olson ST, Bjork I, Sheffer R, Craig PA, Shore JD, Choay J. Role of the antithrombin-binding pentasaccharide in heparin acceleration of antithrombin–proteinase reactions. Resolution of the antithrombin conformational change contribution to heparin rate enhancement. J Biol Chem. 1992;267(18):12528–12538. [PubMed] [Google Scholar]
- 15▪.VanBoeckel CAA, Petitou M. The unique antithrombin III binding domain of heparin: a lead to new synthetic antithrombotics. Angew Chemie Int Ed. 1993;32(12):1671–1690. Describes approaches for designing oligosaccharide-based anti-thrombotics. [Google Scholar]
- 16.Olson ST, Halvorson HR, Bjork I. Quantitative characterization of the thrombin-heparin interaction. Discrimination between specific and nonspecific binding models. J Biol Chem. 1991;266(10):6342–6352. [PubMed] [Google Scholar]
- 17.Blossom DB, Kallen AJ, Patel PR, et al. Outbreak of adverse reactions associated with contaminated heparin. N Engl J Med. 2008;359(25):2674–2684. doi: 10.1056/NEJMoa0806450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18▪▪.Guerrini M, Beccati D, Shriver Z, et al. Oversulfated chondroitin sulfate is a major contaminant in heparin associated with adverse clinical events. Nat Biotechnol. 2008;26(6):669–675. doi: 10.1038/nbt1407. Explains the heparin contamination crisis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maruyama T, Toida T, Imanari T, Yu GY, Linhardt RJ. Conformational changes and anticoagulant activity of chondroitin sulfate following its O-sulfonation. Carbohydr Res. 1998;306(1–2):35–43. doi: 10.1016/s0008-6215(97)10060-x. [DOI] [PubMed] [Google Scholar]
- 20.Li B, Suwan J, Martin JG, et al. Oversulfated chondroitin sulfate interaction with heparin-binding proteins: new insights into adverse reactions from contaminated heparins. Biochem Pharmacol. 2009;78(3):292–300. doi: 10.1016/j.bcp.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gervin AS. Complications of heparin therapy. Surg Gynecol Obst. 1975;140(5):789–796. [PubMed] [Google Scholar]
- 22.Kelton JG, Warkentin TE. Heparin-induced thrombocytopenia: a historical perspective. Blood. 2008;112(7):2607–2616. doi: 10.1182/blood-2008-02-078014. [DOI] [PubMed] [Google Scholar]
- 23.Gandhi NS, Mancera RL. Heparin/heparan sulphate-based drugs. Drug Discov Today. 2010;15(23–24):1058–1069. doi: 10.1016/j.drudis.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Quinlan DJ, McQuillan A, Eikelboom JW. Low-molecular-weight heparin compared with intravenous unfractionated heparin for treatment of pulmonary embolism: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2004;140(3):175–183. doi: 10.7326/0003-4819-140-3-200402030-00008. [DOI] [PubMed] [Google Scholar]
- 25.Kakkar AK. Low- and ultra-low-molecular-weight heparins. Best Prac Res Clin Haematol. 2004;17(1):77–87. doi: 10.1016/j.beha.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Koch A, Bouges S, Ziegler S, Dinkel H, Daures JP, Victor N. Low molecular weight heparin and unfractionated heparin in thrombosis prophylaxis after major surgical intervention: update of previous meta-analyses. Br J Surg. 1997;84(6):750–759. [PubMed] [Google Scholar]
- 27.Verstraete M. Pharmacotherapeutic aspects of unfractionated and low molecular weight heparins. Drugs. 1990;40(4):498–530. doi: 10.2165/00003495-199040040-00003. [DOI] [PubMed] [Google Scholar]
- 28.Hoppensteadt D, Walenga JM, Fareed J, Bick RL. Heparin, low-molecular-weight heparins and heparin pentasaccharide: basic and clinical differentiation. Hematol Oncol Clin N Am. 2003;17(1):313–341. doi: 10.1016/s0889-8588(02)00091-6. [DOI] [PubMed] [Google Scholar]
- 29.Gomez-Outes A, Suarez-Gea ML, Lecumberri R, Rocha E, Pozo-Hernandez C, Vargas-Castrillon E. New parenteral anticoagulants in development. Ther Adv Cardiovasc Dis. 2011;5(1):33–59. doi: 10.1177/1753944710387808. [DOI] [PubMed] [Google Scholar]
- 30.Linhardt RJ. Production and chemical processing of low molecular weight heparins. Semin Thromb Hemost. 1999;25:5–16. [PubMed] [Google Scholar]
- 31▪▪.Petitou M, Duchaussoy P, Lederman I, et al. Synthesis of heparin fragments: a methyl alpha-pentaoside with high affinity for antithrombin III. Carbohydr Res. 1987;167:67–75. doi: 10.1016/0008-6215(87)80268-9. Describes the first synthesis of fondaparinux. [DOI] [PubMed] [Google Scholar]
- 32▪▪.Petitou M, van Boeckel CAA. A synthetic antithrombin III binding pentasaccharide is now a drug! What comes next? Angew Chem Int Ed. 2004;43(24):3118–3133. doi: 10.1002/anie.200300640. Explains the prospects in developing new heparin-based drugs. [DOI] [PubMed] [Google Scholar]
- 33.Bauer KA, Hawkins DW, Peters PC, et al. Fondaparinux, a synthetic pentasaccharide: the first in a new class of antithrombotic agents – the selective factor Xa inhibitors. Cardiovasc Drug Rev. 2002;20(1):37–52. doi: 10.1111/j.1527-3466.2002.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 34.Toschi V, Lettino M. Fondaparinux: pharmacology and clinical experience in cardiovascular medicine. Mini-Rev Med Chem. 2007;7(4):383–387. doi: 10.2174/138955707780363819. [DOI] [PubMed] [Google Scholar]
- 35.Viskov C, Just M, Laux V, Mourier P, Lorenz M. Description of the chemical and pharmacological characteristics of a new hemisynthetic ultra-low-molecular-weight heparin, AVE5026. J Thromb Haem. 2009;7(7):1143–1151. doi: 10.1111/j.1538-7836.2009.03447.x. [DOI] [PubMed] [Google Scholar]
- 36.Lassen MR, Dahl OE, Mismetti P, Destree D, Turpie AGG. AVE5026, a new hemisynthetic ultra-low-molecular-weight heparin for the prevention of venous thromboembolism in patients after total knee replacement surgery – TREK: a dose-ranging study. J Thromb Haem. 2009;7(4):566–572. doi: 10.1111/j.1538-7836.2009.03301.x. [DOI] [PubMed] [Google Scholar]
- 37.Nicolaou KC, Snyder SA. The essence of total synthesis. Proc Natl Acad Sci USA. 2004;101(33):11929–11936. doi: 10.1073/pnas.0403799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gijsen HJ, Qiao L, Fitz W, Wong CH. Recent advances in the chemoenzymatic synthesis of carbohydrates and carbohydrate mimetics. Chem Rev. 1996;96(1):443–473. doi: 10.1021/cr950031q. [DOI] [PubMed] [Google Scholar]
- 39.Karst NA, Linhardt RJ. Recent chemical and enzymatic approaches to the synthesis of glycosaminoglycan oligosaccharides. Curr Med Chem. 2003;10(19):1993–2031. doi: 10.2174/0929867033456891. [DOI] [PubMed] [Google Scholar]
- 40.Orellana A, Hirschberg CB, Wei Z, et al. Molecular cloning and expression of a glycosaminoglyan N-acetylglucosaminyl N-deacetyltase/N-sulfotransferase from a heparin-producing cell-line. J Biol Chem. 1994;269(3):2270–2276. [PubMed] [Google Scholar]
- 41.DeAngelis PL, White CL. Identification and molecular cloning of a heparosan synthase from Pasteurella multocida type D. J Biol Chem. 2002;277(9):7209–7213. doi: 10.1074/jbc.M112130200. [DOI] [PubMed] [Google Scholar]
- 42.Naggi A, Torri G, Casu B, et al. Toward a biotechnological heparin through combined chemical and enzymatic modification of the Escherichia coli K5 polysaccharide. Semin Thromb Haem. 2001;27(5):437–443. doi: 10.1055/s-2001-17954. [DOI] [PubMed] [Google Scholar]
- 43▪.Kuberan B, Lech MZ, Beeler DL, Wu ZLL. Rosenberg RD. Enzymatic synthesis of antithrombin III-binding heparan sulfate pentasaccharide. Nat Biotechnol. 2003;21(11):1343–1346. doi: 10.1038/nbt885. The first enzymatic synthesis of an anti-thrombin-binding heparan sulfate oligosaccharide. [DOI] [PubMed] [Google Scholar]
- 44.Liu R, Xu Y, Chen M, et al. Chemoenzymatic design of heparan sulfate oligosaccharides. J Biol Chem. 2010;285(44):34240–34249. doi: 10.1074/jbc.M110.159152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z, McCallum SA, Xie J, et al. Solution structures of chemoenzymatically synthesized heparin and its precursors. J Am Chem Soc. 2008;130(39):12998–13007. doi: 10.1021/ja8026345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46▪.Bhaskar U, Sterner E, Hickey AM, et al. Engineering of routes to heparin and related polysaccharides. Appl Microbiol Biotechnol. 2011;93(1):1–16. doi: 10.1007/s00253-011-3641-4. The approach to the chemoenzymatic synthesis of a bioengineered heparin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47▪▪.Xu Y, Masuko S, Takieddin M, et al. Chemoenzymatic synthesis of structurally homogeneous ultra-low molecular weight heparins. Science. 2011;334:498–501. doi: 10.1126/science.1207478. The chemoenzymatic synthesis of homogenous ultralow MW heparins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sismey-Ragatz AE, Green DE, Otto NJ, Rejzek M, Field RA, DeAngelis PL. Chemoenzymatic synthesis with distinct Pasteurella heparosan synthases: monodisperse polymers and unnatural structures. J Biol Chem. 2007;282(39):28321–28327. doi: 10.1074/jbc.M701599200. [DOI] [PubMed] [Google Scholar]