Abstract
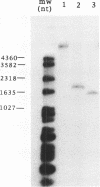
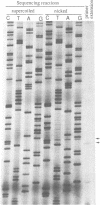
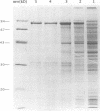
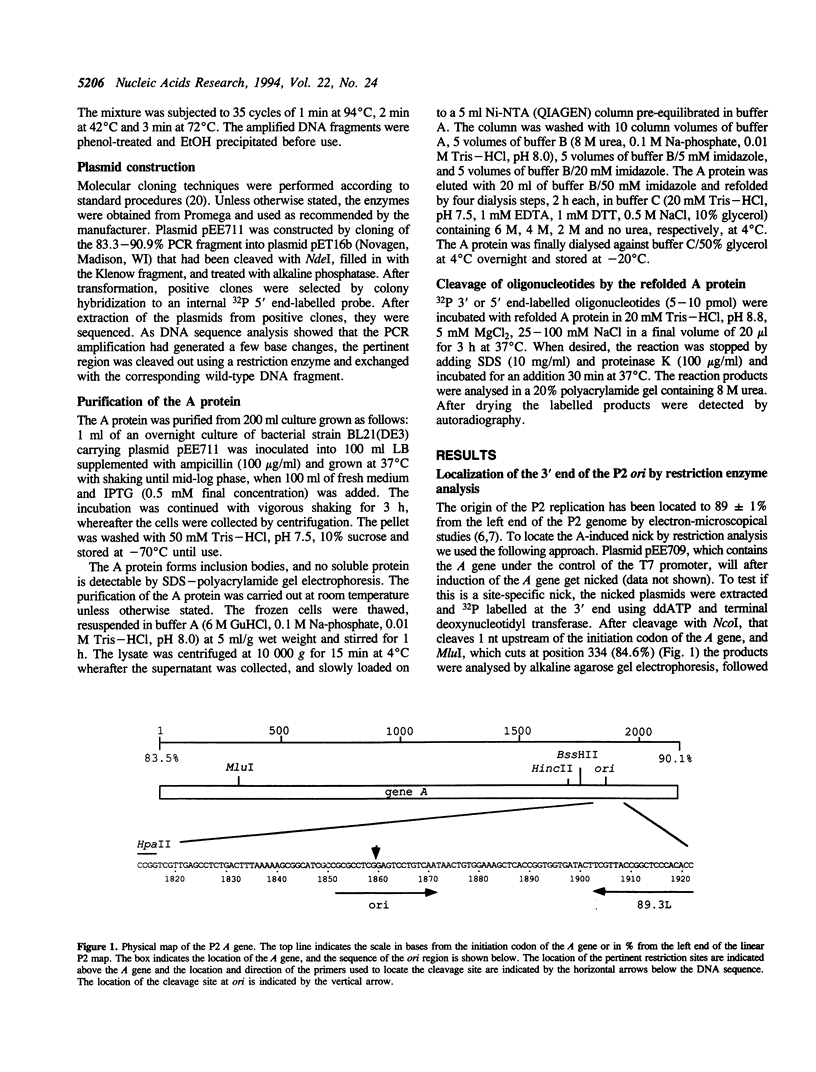
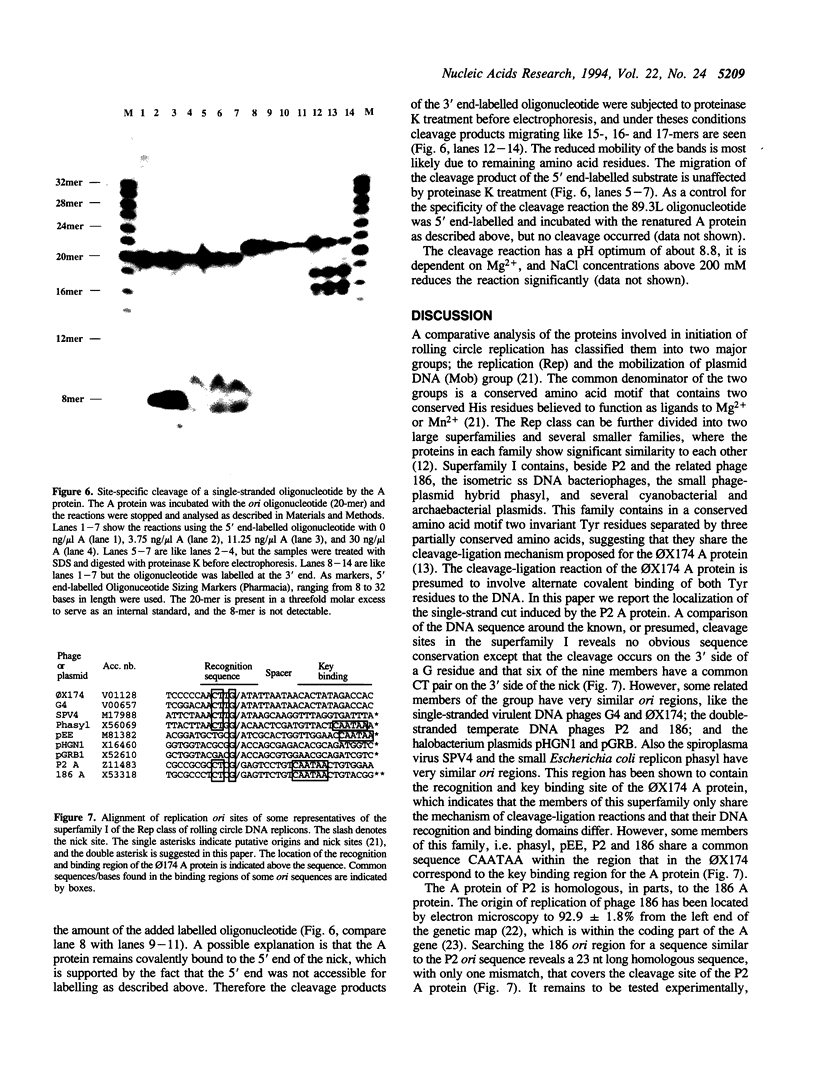
Bacteriophage P2 replicates via a modified rolling circle-type of mechanism, where the P2 A protein acts as an initiator of the replication by inducing a single-stranded cut at the origin of replication (ori). The exact location of the cut induced by the A protein in vivo is determined in this report by: (i) restriction analysis; (ii) DNA sequence analysis; and (iii) primer extensions. It is located 89.2% from the left end of the P2 genome, which is within the coding part of the A gene, in a region devoid of secondary structures. The A gene has been cloned into an expression vector, and the A protein has been purified. The purified A protein does not bind to double-stranded ori containing DNA, but it cleaves single-stranded ori containing DNA, which indicates that a special DNA structure and/or protein is required to make the ori accessible for the A protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertani G., Ljungquist E., Jagusztyn-Krynicka K., Jupp S. Defective particle assembly in wild type P2 bacteriophage and its correction by the lg mutation. J Gen Virol. 1978 Feb;38(2):251–261. doi: 10.1099/0022-1317-38-2-251. [DOI] [PubMed] [Google Scholar]
- Bowden D. W., Twersky R. S., Calendar R. Escherichia coli deoxyribonucleic acid synthesis mutants: their effect upon bacteriophage P2 and satellite bacteriophage P4 deoxyribonucleic acid synthesis. J Bacteriol. 1975 Oct;124(1):167–175. doi: 10.1128/jb.124.1.167-175.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calendar R., Lindqvist B., Sironi G., Clark A. J. Characterization of REP- mutants and their interaction with P2 phage. Virology. 1970 Jan;40(1):72–83. doi: 10.1016/0042-6822(70)90380-6. [DOI] [PubMed] [Google Scholar]
- Chattoraj D. K., Inman R. B. Origin and direction of replication of bacteriophage 186 DNA. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1768–1771. doi: 10.1073/pnas.70.6.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattoraj D. K. Strand-specific break near the origin of bacteriophage P2 DNA replication. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1685–1689. doi: 10.1073/pnas.75.4.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta S., Mukhopadhyay G., Papp P. P., Lewis M. S., Chattoraj D. K. Activation of DNA binding by the monomeric form of the P1 replication initiator RepA by heat shock proteins DnaJ and DnaK. J Mol Biol. 1993 Jul 5;232(1):23–34. doi: 10.1006/jmbi.1993.1367. [DOI] [PubMed] [Google Scholar]
- Funnell B. E., Inman R. B. Bacteriophage P2 DNA replication. Characterization of the requirement of the gene B protein in vivo. J Mol Biol. 1983 Jun 25;167(2):311–334. doi: 10.1016/s0022-2836(83)80338-6. [DOI] [PubMed] [Google Scholar]
- Geisselsoder J. Strand-specific discontinuity in republicating P2 DNA. J Mol Biol. 1976 Jan 5;100(1):13–22. doi: 10.1016/s0022-2836(76)80030-7. [DOI] [PubMed] [Google Scholar]
- Haggård-Ljungquist E., Kockum K., Bertani L. E. DNA sequences of bacteriophage P2 early genes cox and B and their regulatory sites. Mol Gen Genet. 1987 Jun;208(1-2):52–56. doi: 10.1007/BF00330421. [DOI] [PubMed] [Google Scholar]
- Hooper I., Egan J. B. Coliphage 186 infection requires host initiation functions dnaA and dnaC. J Virol. 1981 Nov;40(2):599–601. doi: 10.1128/jvi.40.2.599-601.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D. S., Crooke E., Kornberg A. Aggregated dnaA protein is dissociated and activated for DNA replication by phospholipase or dnaK protein. J Biol Chem. 1990 Nov 5;265(31):19244–19248. [PubMed] [Google Scholar]
- Ilyina T. V., Koonin E. V. Conserved sequence motifs in the initiator proteins for rolling circle DNA replication encoded by diverse replicons from eubacteria, eucaryotes and archaebacteria. Nucleic Acids Res. 1992 Jul 11;20(13):3279–3285. doi: 10.1093/nar/20.13.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y., Wada C., Yura T. Roles of Escherichia coli heat shock proteins DnaK, DnaJ and GrpE in mini-F plasmid replication. Mol Gen Genet. 1990 Jan;220(2):277–282. doi: 10.1007/BF00260494. [DOI] [PubMed] [Google Scholar]
- Koonin E. V., Ilyina T. V. Computer-assisted dissection of rolling circle DNA replication. Biosystems. 1993;30(1-3):241–268. doi: 10.1016/0303-2647(93)90074-m. [DOI] [PubMed] [Google Scholar]
- Lengyel J. A., Calendar R. Control of bacteriophage P2 protein and DNA synthesis. Virology. 1974 Feb;57(2):305–313. doi: 10.1016/0042-6822(74)90170-6. [DOI] [PubMed] [Google Scholar]
- Lindahl G. Bacteriophage P2: replication of the chromosome requires a protein which acts only on the genome that coded for it. Virology. 1970 Oct;42(2):522–533. doi: 10.1016/0042-6822(70)90295-3. [DOI] [PubMed] [Google Scholar]
- Lindahl G. Characterization of conditional lethal mutants of bacteriophage P2. Mol Gen Genet. 1974 Feb 6;128(3):249–260. doi: 10.1007/BF00267114. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H. Vegetative DNA of temperate coliphage P2. Mol Gen Genet. 1971;110(2):178–196. doi: 10.1007/BF00332647. [DOI] [PubMed] [Google Scholar]
- Liu Y., Saha S., Haggård-Ljungquist E. Studies of bacteriophage P2 DNA replication. The DNA sequence of the cis-acting gene A and ori region and construction of a P2 mini-chromosome. J Mol Biol. 1993 May 20;231(2):361–374. doi: 10.1006/jmbi.1993.1288. [DOI] [PubMed] [Google Scholar]
- Ljungquist E. Association of nonreplicating P2 DNA to fast-sedimenting cell material following infection with satellite phage P4. Virology. 1976 Sep;73(2):402–412. doi: 10.1016/0042-6822(76)90401-3. [DOI] [PubMed] [Google Scholar]
- Osipiuk J., Georgopoulos C., Zylicz M. Initiation of lambda DNA replication. The Escherichia coli small heat shock proteins, DnaJ and GrpE, increase DnaK's affinity for the lambda P protein. J Biol Chem. 1993 Mar 5;268(7):4821–4827. [PubMed] [Google Scholar]
- Pansegrau W., Lanka E., Barth P. T., Figurski D. H., Guiney D. G., Haas D., Helinski D. R., Schwab H., Stanisich V. A., Thomas C. M. Complete nucleotide sequence of Birmingham IncP alpha plasmids. Compilation and comparative analysis. J Mol Biol. 1994 Jun 24;239(5):623–663. doi: 10.1006/jmbi.1994.1404. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y. The dnaK gene of Escherichia coli functions in initiation of chromosome replication. J Bacteriol. 1988 Feb;170(2):972–979. doi: 10.1128/jb.170.2.972-979.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki I., Bertani G. Growth abnormalities in Hfr derivatives of Escherichia coli strain C. J Gen Microbiol. 1965 Sep;40(3):365–376. doi: 10.1099/00221287-40-3-365. [DOI] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Starting point and direction of replication in P2 DNA. J Mol Biol. 1971 Jan 14;55(1):31–38. doi: 10.1016/0022-2836(71)90278-6. [DOI] [PubMed] [Google Scholar]
- Sivaprasad A. V., Jarvinen R., Puspurs A., Egan J. B. DNA replication studies with coliphage 186. III. A single phage gene is required for phage 186 replication. J Mol Biol. 1990 Jun 5;213(3):449–463. doi: 10.1016/S0022-2836(05)80207-4. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Waters V. L., Guiney D. G. Processes at the nick region link conjugation, T-DNA transfer and rolling circle replication. Mol Microbiol. 1993 Sep;9(6):1123–1130. doi: 10.1111/j.1365-2958.1993.tb01242.x. [DOI] [PubMed] [Google Scholar]
- Wickner S., Skowyra D., Hoskins J., McKenney K. DnaJ, DnaK, and GrpE heat shock proteins are required in oriP1 DNA replication solely at the RepA monomerization step. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10345–10349. doi: 10.1073/pnas.89.21.10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mansfeld A. D., van Teeffelen H. A., Baas P. D., Jansz H. S. Two juxtaposed tyrosyl-OH groups participate in phi X174 gene A protein catalysed cleavage and ligation of DNA. Nucleic Acids Res. 1986 May 27;14(10):4229–4238. doi: 10.1093/nar/14.10.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]