Abstract
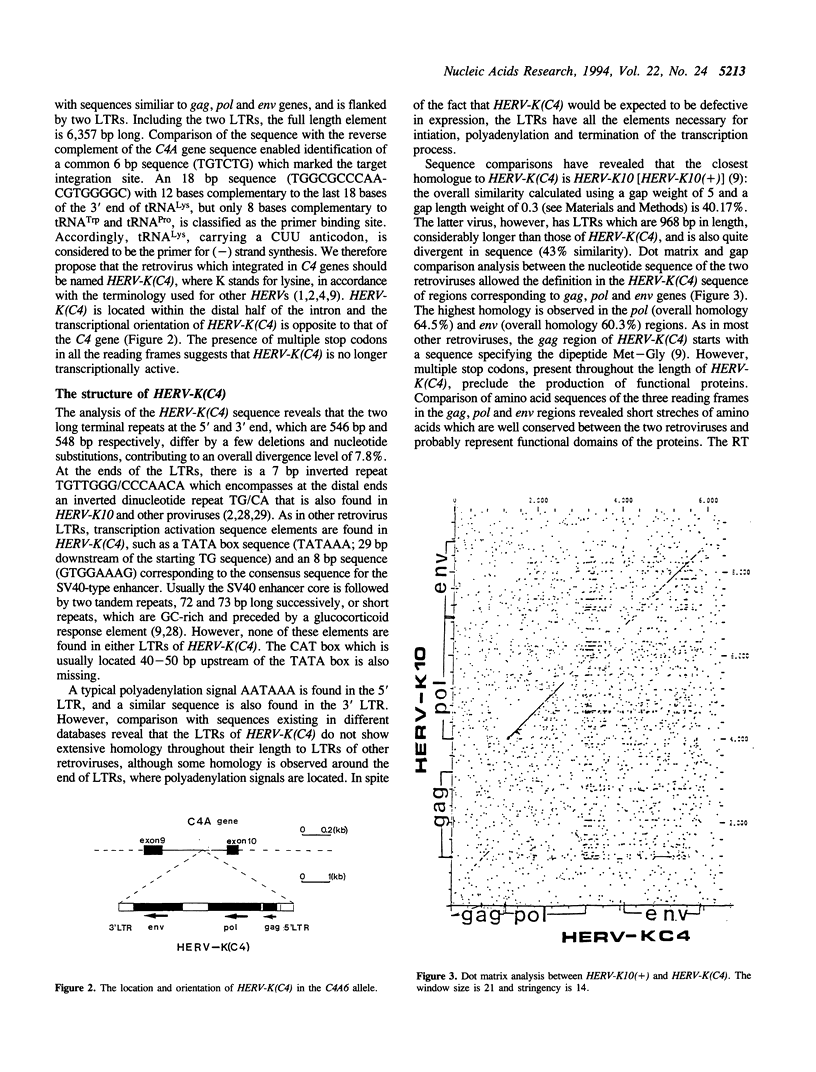
We have identified a novel family of about 10-50 human endogenous retrovirus elements (HERVs) and have characterized one family member (HERV-KC4). This retrovirus element is integrated within intron 9 of and complement C4A genes and also in some C4B genes, and is a principal contribution to interlocus and interallelic length heterogeneity of C4 genes. The HERV-K(C4) sequence has a typical retrovirus structure with elements of gag, pol and env domains, flanked by two long terminal repeats (LTRs) and is similar to type A, B and D retroviruses. Multiple termination codons preclude the existence of long open reading frames, suggesting that the HERV-K(C4) sequence is no longer functional. Zoo blot hybridization reveals that New World monkeys appear to lack sequences similar to HERV-K(C4), suggesting that integration has occurred after the divergence of Old and New World monkeys. Retrotransposition of prototype viruses is presumed to have led to the amplification and integration of the members of the family in different loci, which in humans, appear to be dispersed over several chromosomes. The absence of the HERV-K(C4) element in some C4B genes in both humans and orangutangs indicate that the retrovirus inserted into the C4A gene after the duplication of the cluster. Subsequent spread of the HERV-K(C4) sequence to C4B genes presumably occurred by interlocus sequence exchange mechanisms, such as unequal crossover and gene conversion-like mechanisms.
Full text
PDF

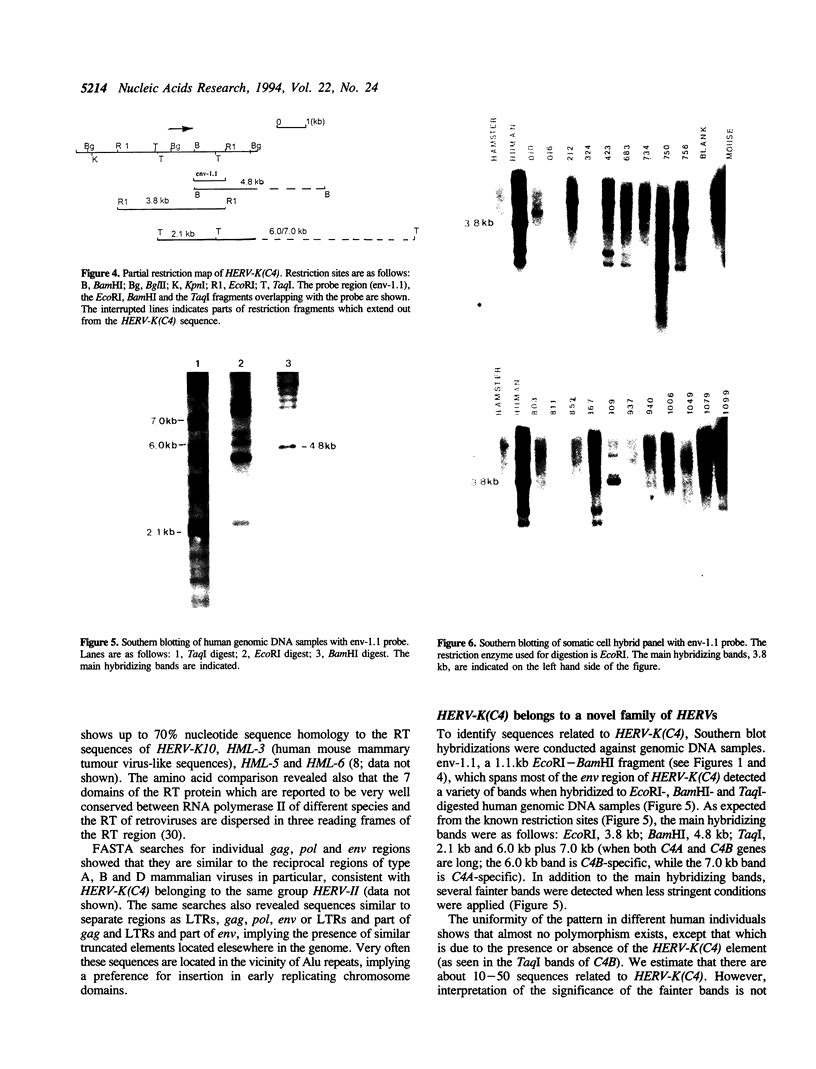



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Anderson M. J., Milner C. M., Cotton R. G., Campbell R. D. The coding sequence of the hemolytically inactive C4A6 allotype of human complement component C4 reveals that a single arginine to tryptophan substitution at beta-chain residue 458 is the likely cause of the defect. J Immunol. 1992 May 1;148(9):2795–2802. [PubMed] [Google Scholar]
- Bontrop R. E., Broos L. A., Otting N., Jonker M. J. Polymorphism of C4 and CYP21 genes in various primate species. Tissue Antigens. 1991 Apr;37(4):145–151. doi: 10.1111/j.1399-0039.1991.tb01862.x. [DOI] [PubMed] [Google Scholar]
- Carroll M. C., Belt T., Palsdottir A., Porter R. R. Structure and organization of the C4 genes. Philos Trans R Soc Lond B Biol Sci. 1984 Sep 6;306(1129):379–388. doi: 10.1098/rstb.1984.0098. [DOI] [PubMed] [Google Scholar]
- Carroll M. C., Campbell R. D., Bentley D. R., Porter R. R. A molecular map of the human major histocompatibility complex class III region linking complement genes C4, C2 and factor B. Nature. 1984 Jan 19;307(5948):237–241. doi: 10.1038/307237a0. [DOI] [PubMed] [Google Scholar]
- Gitelman S. E., Bristow J., Miller W. L. Mechanism and consequences of the duplication of the human C4/P450c21/gene X locus. Mol Cell Biol. 1992 May;12(5):2124–2134. doi: 10.1128/mcb.12.5.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild N. L., Wilkinson D. A., Mager D. L. Recent evolutionary expansion of a subfamily of RTVL-H human endogenous retrovirus-like elements. Virology. 1993 Oct;196(2):778–788. doi: 10.1006/viro.1993.1535. [DOI] [PubMed] [Google Scholar]
- Kambhu S., Falldorf P., Lee J. S. Endogenous retroviral long terminal repeats within the HLA-DQ locus. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4927–4931. doi: 10.1073/pnas.87.13.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Gruss P. Enhancer elements. Cell. 1983 Jun;33(2):313–314. doi: 10.1016/0092-8674(83)90410-5. [DOI] [PubMed] [Google Scholar]
- Kröger B., Horak I. Isolation of novel human retrovirus-related sequences by hybridization to synthetic oligonucleotides complementary to the tRNA(Pro) primer-binding site. J Virol. 1987 Jul;61(7):2071–2075. doi: 10.1128/jvi.61.7.2071-2075.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N., Kim H. S. Three independent insertions of retrovirus-like sequences in the haptoglobin gene cluster of primates. Genomics. 1990 Dec;8(4):671–683. doi: 10.1016/0888-7543(90)90254-r. [DOI] [PubMed] [Google Scholar]
- Mager D. L., Henthorn P. S. Identification of a retrovirus-like repetitive element in human DNA. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7510–7514. doi: 10.1073/pnas.81.23.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani-Costantini R., Horn T. M., Callahan R. Ancestry of a human endogenous retrovirus family. J Virol. 1989 Nov;63(11):4982–4985. doi: 10.1128/jvi.63.11.4982-4985.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. A., Bryan T., Rasheed S., Khan A. S. Identification and cloning of endogenous retroviral sequences present in human DNA. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4892–4896. doi: 10.1073/pnas.78.8.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medstrand P., Blomberg J. Characterization of novel reverse transcriptase encoding human endogenous retroviral sequences similar to type A and type B retroviruses: differential transcription in normal human tissues. J Virol. 1993 Nov;67(11):6778–6787. doi: 10.1128/jvi.67.11.6778-6787.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M. Molecular cloning and long terminal repeat sequences of human endogenous retrovirus genes related to types A and B retrovirus genes. J Virol. 1986 Jun;58(3):937–944. doi: 10.1128/jvi.58.3.937-944.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Yasunaga T., Miyata T., Ushikubo H. Nucleotide sequence of human endogenous retrovirus genome related to the mouse mammary tumor virus genome. J Virol. 1986 Nov;60(2):589–598. doi: 10.1128/jvi.60.2.589-598.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
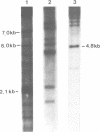
- Palsdottir A., Fossdal R., Arnason A., Edwards J. H., Jensson O. Heterogeneity of human C4 gene size. A large intron (6.5 kb) is present in all C4A genes and some C4B genes. Immunogenetics. 1987;25(5):299–304. doi: 10.1007/BF00404422. [DOI] [PubMed] [Google Scholar]
- Partanen J., Campbell R. D. Restriction fragment analysis of non-deleted complement C4 null genes suggests point mutations in C4A null alleles, but gene conversions in C4B null alleles. Immunogenetics. 1989;30(6):520–523. doi: 10.1007/BF02421186. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske R., Steele P. E., O'Neill R. R., Rabson A. B., Martin M. A. Nucleotide sequence of a full-length human endogenous retroviral segment. J Virol. 1985 Jun;54(3):764–772. doi: 10.1128/jvi.54.3.764-772.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent C. A., Anderson M. J., Hsieh S. L., Kendall E., Gomez-Escobar N., Campbell R. D. Characterisation of the novel gene G11 lying adjacent to the complement C4A gene in the human major histocompatibility complex. Hum Mol Genet. 1994 Mar;3(3):481–488. doi: 10.1093/hmg/3.3.481. [DOI] [PubMed] [Google Scholar]
- Schendel D. J., O'Neill G. J., Wank R. MHC-linked class III genes. Analysis of C4 gene frequencies, complotypes and associations with distinct HLA haplotypes in German Caucasians. Immunogenetics. 1984;20(1):23–31. doi: 10.1007/BF00373444. [DOI] [PubMed] [Google Scholar]
- Shih C. C., Stoye J. P., Coffin J. M. Highly preferred targets for retrovirus integration. Cell. 1988 May 20;53(4):531–537. doi: 10.1016/0092-8674(88)90569-7. [DOI] [PubMed] [Google Scholar]
- Stavenhagen J. B., Robins D. M. An ancient provirus has imposed androgen regulation on the adjacent mouse sex-limited protein gene. Cell. 1988 Oct 21;55(2):247–254. doi: 10.1016/0092-8674(88)90047-5. [DOI] [PubMed] [Google Scholar]
- Steele P. E., Martin M. A., Rabson A. B., Bryan T., O'Brien S. J. Amplification and chromosomal dispersion of human endogenous retroviral sequences. J Virol. 1986 Sep;59(3):545–550. doi: 10.1128/jvi.59.3.545-550.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taruscio D., Manuelidis L. Integration site preferences of endogenous retroviruses. Chromosoma. 1991 Dec;101(3):141–156. doi: 10.1007/BF00355364. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Structure, variation and synthesis of retrovirus long terminal repeat. Cell. 1981 Nov;27(1 Pt 2):1–3. doi: 10.1016/0092-8674(81)90353-6. [DOI] [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. A., Goodchild N. L., Saxton T. M., Wood S., Mager D. L. Evidence for a functional subclass of the RTVL-H family of human endogenous retrovirus-like sequences. J Virol. 1993 Jun;67(6):2981–2989. doi: 10.1128/jvi.67.6.2981-2989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Eickbush T. H. Origin and evolution of retroelements based upon their reverse transcriptase sequences. EMBO J. 1990 Oct;9(10):3353–3362. doi: 10.1002/j.1460-2075.1990.tb07536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C. Y. The complete exon-intron structure of a human complement component C4A gene. DNA sequences, polymorphism, and linkage to the 21-hydroxylase gene. J Immunol. 1991 Feb 1;146(3):1057–1066. [PubMed] [Google Scholar]
- Zhu Z. B., Jian B., Volanakis J. E. Ancestry of SINE-R.C2 a human-specific retroposon. Hum Genet. 1994 May;93(5):545–551. doi: 10.1007/BF00202821. [DOI] [PubMed] [Google Scholar]