Abstract
Disease can be an important driver of host population dynamics and epizootics can cause severe host population declines. Batrachochytrium dendrobatidis (Bd), the pathogen causing amphibian chytridiomycosis, may occur epizootically or enzootically and can harm amphibian populations in many ways. While effects of Bd epizootics are well documented, the effects of enzootic Bd have rarely been described. We used a state-space model that accounts for observation error to test whether population trends of a species highly susceptible to Bd, the midwife toad Alytes obstetricans, are negatively affected by the enzootic presence of the pathogen. Unexpectedly, Bd had no negative effect on population growth rates from 2002–2008. This suggests that negative effects of disease on individuals do not necessarily translate into negative effects at the population level. Populations of amphibian species that are susceptible to the emerging disease chytridiomycosis can persist despite the enzootic presence of the pathogen under current environmental conditions.
Introduction
Parasites and pathogens can be important drivers of host population dynamics by altering host behaviour, demography or genetics [1]–[4]. The most extreme effect of a pathogen on the host is host extinction [5]. However, host extinction is rare for two main reasons. First, parasites and their hosts generally share a common evolutionary history and extinction of either antagonist is hence unlikely [6]. Second, when host density declines, the pathogen's transmission rate is also expected to drop, unless there is frequency-dependent transmission or a reservoir host [5]. Emerging diseases, however, are different from established pathogens because pathogen interactions with novel hosts are unpredictable. In the most extreme –albeit rare– case they can lead to host population declines and extinctions [7], [8].
While the effects of disease on vital rates of captive populations are well documented, the effects of disease on dynamics of wild populations are still poorly understood [2], [4], [9], [10]. For example, a reduction in population size is only expected if pathogen-induced mortality is additive rather than compensatory [4], [11]. If pathogen-induced mortality is additive, then overall mortality is the sum of pathogen-induced mortality plus mortality inflicted by all other causes. In contrast, if mortality is compensatory, then increased mortality due to the presence of a pathogen is countered by a reduction in mortality due to other causes, often in a density-dependent manner [12]. Here, our goal is to contribute to a better understanding of host population dynamics influenced by an emerging infectious disease.
An emerging pathogen of amphibians, the chytrid fungus Batrachochytrium dendrobatidis (hereafter Bd), has contributed to amphibian declines and extinctions on most continents [13]–[15]. Many amphibian populations have collapsed after emergence of the pathogen, which is still appearing in new areas [16]–[18]. However, host extinction is not the only outcome of Bd emergence in new localities. The amphibian host-chytrid pathogen models by Briggs et al. [19], [20] suggest that enzootic Bd-infection may initially cause a reduction in abundance, but thereafter, populations remain stationary (i.e., mean abundance does not change anymore). Yet, due to a lack of time series data on the abundance of amphibian populations coexisting with enzootic Bd, we cannot state with certainty that amphibian populations with enzootic Bd-infection are stationary. In contrast to the model predictions, some mark-recapture studies have shown that enzootic Bd depresses both individual survival and population growth rates [21]–[23].
Our goal was to quantify the effects of enzootic Bd on populations of an amphibian species that is known to be susceptible to Bd [24]–[26]. Ideally, one would compare population sizes before and after the emergence of Bd in a population. Unfortunately, such data are rarely available [16], [17], [27]. We compare population monitoring data from sites where Bd is present with population monitoring data from sites where Bd was not detected. We analyse short time series of counts of calling males from 26 populations of the common midwife toad, Alytes obstetricans, in the Swiss canton Lucerne. Assuming that Bd leads to chytridiomycosis-induced mortality of individuals [24]–[26] and that mortality is additive rather than compensatory [4], [11], we expect to find declining populations in the presence of the pathogen while populations free of the pathogen should be either stationary or growing. The pathogen has been present in Switzerland since at least the early 1980 s [28], is widespread, and prevalence often high ([29], U. Tobler & B. R. Schmidt, unpublished data). Although no chytridiomycosis-induced mass mortality has been observed in Switzerland, including our study area, we know that Bd-associated mortality occurs in the field because we have detected dead metamorphs at our study sites that tested positive for Bd (U. Tobler, C. C. Geiger & B. R. Schmidt, unpublished data). Additionally, high (up to 90%) Bd-related mortality has been reported in a laboratory experiment on postmetamorphic Alytes obstetricans [25]. Although mortality in the field may be lower than in the laboratory, these results led us to expect Bd-induced population declines.
Alytes obstetricans is listed as “endangered” (IUCN category EN) on the Swiss national red list of threatened amphibians [30]. The species is therefore the target of conservation action [31]. Knowing whether and to what degree Bd poses a threat to Alytes population survival in this area is vital in order to develop suitable conservation strategies. Currently, no habitat mitigation methods are available barring ex-situ captive breeding although mitigation methods using antifungal chemicals or bacterial treatments are currently being tested [32]. With this study, we aim to test whether or not chytridiomycosis represents an additional threat to host populations of an endangered species.
Materials and Methods
Study sites
Based on the availability of population count data, 26 sites in canton Lucerne, Switzerland, were included in the analysis. All sites are situated between 46.871° and 47.258° N and 7.882° and 8.382° E (Figure 1) and along an altitudinal gradient ranging from 402 to 1330 m.a.s.l. Mean summer temperatures range from 12.3 to 17.5°C [33]. Habitat types include quarries, ponds in open meadows, garden ponds, fire water reservoirs, and alpine and pre-alpine streams.
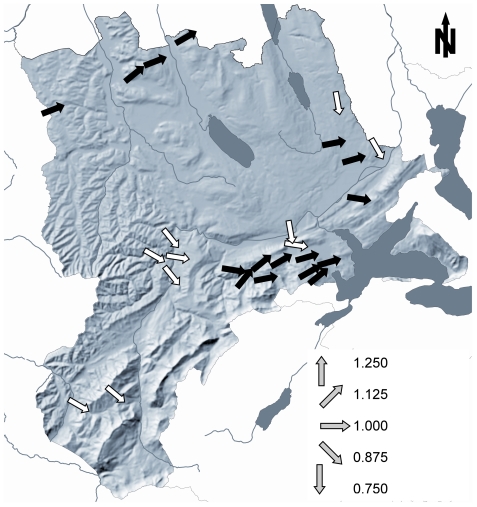
Figure 1. Topographic map of the canton Lucerne and study sites.
Lakes are shaded in grey. White arrows mark sites where we did not detect Bd; black arrows mark Bd-positive sites. The slope of the arrows reflect population growth rates (Table 2).
Population monitoring
Populations of Alytes obstetricans were regularly monitored as part of the midwife toad action plan of the Swiss canton Lucerne [34], [35]. Counts of the number of calling Alytes obstetricans males were obtained every year from 2002 to 2008 except for four sites where Alytes obstetricans was reintroduced and calling males only occurred after 2002. Every site was visited at least twice a year by experienced volunteers. Volunteers were free to choose the nights for the monitoring, but advised to do so during optimal warm and humid nights when detectability of Alytes obstetricans is high because many males are calling [36]. The number of calling males, an index to population size [37], was recorded during every visit. We used the highest number of calling males within a year in the analysis. In our analysis of population trends, detectability may vary among years (the statistical model accounts for observation error; see below) but we assume that detectability shows no temporal trend such that population trends can be reliably estimated from counts of calling males [38], [39]. Trend estimates are unlikely to depend on Bd infection status because observers were unaware of Bd infection status of a site. Hence, we can exclude an interaction between Bd presence and detectability that would have biased our conclusion on the impact of Bd on population trends. In addition to the data on the numbers of calling males, presence or absence of tadpoles (based on visual encounter surveys [40]) was noted; this provided us with the explanatory variable “number of years tadpoles were observed”, which indicates the number of years in which tadpoles were observed in the pond.
Sampling for Bd
During summer 2007 and spring 2008 or 2009, all sites were sampled for the presence of Bd in the amphibian population. To test the populations for Bd, 16 to 47 (mean ± SD: 26.0±6.6) amphibians were caught and swabbed with a sterile rayon swab (Copan Italia S.p.A., Brescia, Italy). Exceptions are sites “Sagerhüsli”, where only two dead Alytes metamorphs were sampled, and “Hombrig”, where sample size was six. Because both these sites tested positive for Bd we do not expect the small sample sizes to affect our results because the goal was to determine presence or absence of Bd. Sample size at sites where we did not detect Bd was 26.5±7.6 (mean ± SD). Hence, we cannot exclude that we may have missed Bd at some sites where prevalence was low (with a sample size of n = 26 a prevalence of 10% may not be detected; see [41]). Sampling was done opportunistically, i.e. all available amphibian species that could be captured were tested for Bd. Apart from midwife toads (where we swabbed tadpoles), the other amphibians sampled (always adults) were the fire-bellied toad Bombina variegata, the waterfrogs Pelophylax lessonae and Pelophylax esculentus (the two taxa were pooled because they form a hybridogenetic complex [42]), the alpine newt Mesotriton alpestris and the palmate newt Lissotriton helveticus (scientific names are based on [43]). To swab tadpoles, we made five swipes across the mouthparts. To sample adult amphibians, we swiped the underside of each foot five times and the ventral abdominal skin five times for a total of 25 swipes per amphibian. In 6 out of 11 sites where we did not detect Bd, samples were exclusively obtained from Alytes tadpoles. Due to the prolonged larval period Alytes tadpoles are more likely to be infected than any other species or life stage in this system (see results). Standard hygiene recommendations were followed during field work [44], [45].
We followed the rt-PCR protocol of Boyle et al [46] for the extraction and analysis of Bd-DNA from swabs. We used Bd-specific primers and standards to quantify the amount of Bd-DNA (infection load). To prevent inhibition by the extraction reagent, the extractions were diluted 1∶10 with water prior to PCR analysis. Hence, we calculated the original zoospore equivalent by multiplying the PCR output by 10. We ran each sample twice and the PCR was repeated if the two wells returned unequal results. Reactions below 0.1 genomic equivalents were scored Bd-negative [25].
Statistical analysis
We tested for differences in prevalence of Bd among the sampled species using a generalised linear mixed model (GLMM) with a binomial error distribution. We tested for differences in infection intensity among species using a linear mixed model (LMM) with a normal error distribution. In both analyses, we used site as grouping (random) variable. Both analyses were done in R 2.8.1 [47].
We used WinBUGS 1.4 [48] to fit a a state-space model to the 26 time series [49], [50] and to assess whether the presence of Bd and the number of years tadpoles were observed affected population trends. State-space models disentangle the effects of the biological process and the observation process and thereby account for observation error. Modeling followed closely the approach described in Kéry & Schaub [50]. We built a model that estimates the observation and biological process at two hierarchical levels. The time series counts ci,t are described by
| (1) |
where Ni,t is the unobserved true population size of site i at time t and 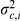 the observation variance at site i. This part of the model describes the observation process and removes observation error from the time series. The biological process is described by
the observation variance at site i. This part of the model describes the observation process and removes observation error from the time series. The biological process is described by
| (2) |
whereλi,t is the population growth rate of site i at time t which is assumed to be have a normal distribution
| (3) |
where 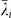 is the mean population growth rate at site i and
is the mean population growth rate at site i and 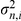 is the process variance at site i.
is the process variance at site i. 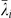 was further modeled as a function of site-specific presence of Bd (Bdi) and of the site-specific number of years tadpoles were observed (tadi) using a linear relationship:
was further modeled as a function of site-specific presence of Bd (Bdi) and of the site-specific number of years tadpoles were observed (tadi) using a linear relationship:
| (4) |
Uniform priors were used for the observation (U(0, 30) for each site) and for the process standard deviations (U(0, 10) for each site). A normal prior with a wide variance N(0, 100) was used for α, while for both β we used a uniform prior U(−5,5). Further, we used Normal priors for the population size at the first occasion for each site with variance 1 and the mean equal to the site-specific counts in that year. We ran three independent MCMC chains for 25,000 iterations, each with a burn-in of 10,000 iterations. The chains were thinned by a thinning factor of three. Convergence was assumed if the Gelman-Rubin statistic Rhat<1.1 [51].
Ethics statement
The experiment was conducted under permit number 110/2007 by the veterinary office of the canton Zurich; collecting permits were provided by the office for Landwirtschaft und Wald (lawa) of the canton Lucerne.
Results
We detected Bd in 16 out of the 26 (61.5%) sites and in 16.5% of all sampled amphibians, including the sites where we did not detect Bd (Table 1). In sites where Bd was detected, 28% of all sampled amphibians tested positive for Bd. Bd was not found in any site in the Entlebuch valley, which encompassed the south-western cluster of populations (Figure 1). The two dead metamorphs collected at Sagerhüsli in 2007 tested positive for Bd with infection intensities of 226.1 and 161.9 genomic equivalents. Four of five dead metamorphs collected at Schauensee in 2010 tested positive for Bd with an average infection intensity of 2.9±1.8 genomic equivalents (U. Tobler, C. C. Geiger & B. R. Schmidt, unpublished data). Bd data will be deposited at http://www.bd-maps.eu/.
Table 1. Estimates of observed infection prevalence and intensity in Alytes tadpoles and in all other species (pooled) for all study ponds.
| Alytes | other species | |||||
| Population | N | observed prevalence | infection intensity | N | observed prevalence | infection intensity |
| Ämmenmatt | 7 | 0 | 23 | 0 | ||
| Ballwil | 0 | - | - | 34 | 0.147 | 0.35 (±0.25) |
| Chalchloch | 24 | 0 | 0 | - | - | |
| Chräuel | 14 | 0.857 | 6.86 (±7.24) | 17 | 0.294 | 2.39 (±3.16) |
| Egghütten | 22 | 0 | - | 0 | - | - |
| Ehrendingen | 24 | 0 | - | 0 | - | - |
| Einsamkeit | 0 | - | - | 27 | 0.407 | 8.68 (±10.22) |
| Fontanne | 21 | 0 | - | 0 | - | - |
| Grisigen | 20 | 1.000 | 2446.76 (±2764.18) | 6 | 0.167 | 122.60 |
| Hergiswald | 17 | 0.706 | 21.58 (±26.01) | 13 | 0.462 | 1.43 (±1.41) |
| Hilferenmättili | 33 | 0 | - | 14 | 0 | - |
| Hiltbrunnen | 4 | 0.750 | 175.53 (±37.89) | 23 | 0.652 | 16.99 (±26.07) |
| Hinter Rohren | 6 | 0.333 | 215.96 (±58.52) | 19 | 0 | - |
| Hochrüti | 0 | - | - | 16 | 0 | - |
| Hohenrain | 0 | - | - | 30 | 0 | - |
| Hombrig | 5 | 0.200 | 29.20 | 1 | 0 | - |
| Lätten | 1 | 0 | - | 33 | 0.121 | 1.86 (±1.95) |
| Linden | 0 | - | - | 25 | 0.080 | 4.16 (±0.32) |
| Ottigenbüel | 0 | - | - | 26 | 0.731 | 18.23 (±27.39) |
| Pfaffwil | 0 | - | - | 24 | 0 | - |
| Räschenhus | 6 | 0.833 | 66.59 (±80.18) | 19 | 0.211 | 1.11 (±0.60) |
| Rossei | 25 | 0 | - | 0 | - | - |
| Sagerhüsli | 2 | 1.000 | 194.05 (±45.39) | 0 | - | - |
| Schauensee | 0 | - | - | 30 | 0.233 | 3.44 (±4.21) |
| Schlagweiher | 5 | 0.800 | 6032.2 (±3609.54) | 20 | 0.500 | 235.36 ±(645.43) |
| Unter Utigen | 0 | 1.000 | - | 27 | 0.296 | 42.97 (±85.57) |
Prevalence is the proportion of infected individuals, infection intensity are mean zoospore equivalents (genomic equivalents) among infected individuals ±1 SD.
Within positive sites, Alytes tadpoles had higher observed infection prevalence than the other species (mean proportion of individuals carrying Bd per pond (mean, [range]): A. obstetricans tadpoles: 0.57 [0–0.95]; Pelophylax spp. adults: 0.27 [0.18–0.68]; M. alpestris adults: 0.24 [0–0.67]; L. helveticus adults: 0.14 [0–0.60]). Among infected individuals, infection intensity was higher in Alytes tadpoles than in the other species (A. obstetricans: 1266.5±322.2 (SE) genomic equivalents, Pelophylax spp. adults.: 29.1±11.4, M. alpestris adults: 46.1±36.2, L. helveticus adults: 17.9±9.3). The species differed significantly in observed infection prevalence (GLMM, z = −4.250, p = 0.0175) and intensity ( = Bd genomic equivalents; LMM, t = −3.551, p<0.001), respectively (Table 1).
Alytes populations had an average of 10.8±9.1 (SD) calling males (range within single years: 0–75 calling males; range of mean number of calling males per population: 2–43; Figure 2). Between 2002 and 2009, tadpoles were observed in 22 out of the 26 ponds during at least one visit; in those ponds where tadpoles were observed, they were seen in 4.8±1.7 years on average out of the 8 study years.
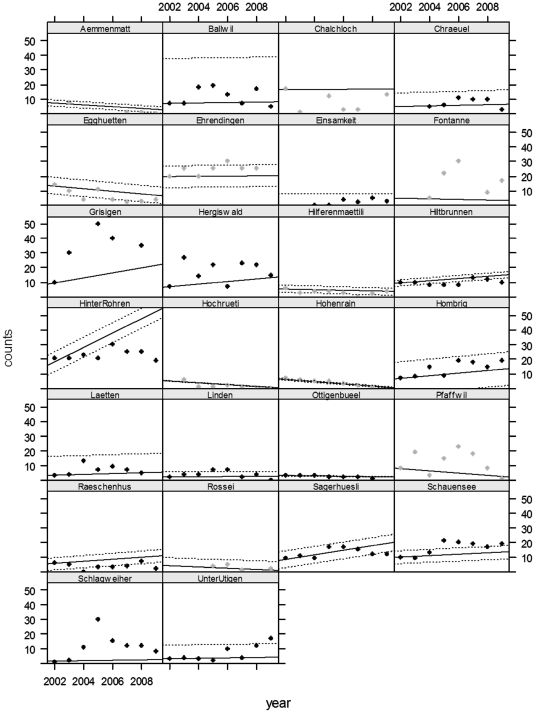
Figure 2. Observed counts and model-estimated trends per population and year.
The circles show the observed counts of calling males, with sites where we did not detect Bd in grey and Bd-positive sites in black. The solid line shows the predicted population trend (with 95% CRI (dotted lines)).
Among populations, the mean population growth rates (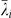 ; see equation 3) varied from 0.773 to 1.135; the average population growth rate of all populations during the 8-year study period was (mean ± SD) 0.987±0.105 (Table 2). This means that on average the number of calling males in the region was stationary. For 14 out of the 26 study sites, the 95% credible interval (CRI) for the estimated population trend included 1, i.e., the populations were neither growing nor declining (Figure 2). Populations that tested Bd-positive had on average a higher
; see equation 3) varied from 0.773 to 1.135; the average population growth rate of all populations during the 8-year study period was (mean ± SD) 0.987±0.105 (Table 2). This means that on average the number of calling males in the region was stationary. For 14 out of the 26 study sites, the 95% credible interval (CRI) for the estimated population trend included 1, i.e., the populations were neither growing nor declining (Figure 2). Populations that tested Bd-positive had on average a higher 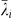 value than populations where we did not detect Bd. The difference in
value than populations where we did not detect Bd. The difference in 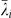 values was 0.191 (95% CRI 0.046–0.353; this is the regression coefficient for the effect of Bd on population growth rate (see equation 4 and Table 3)). Populations where tadpoles were observed in a higher number of study years had an increased growth rate compared to populations where tadpoles were not or only rarely observed; every year in which tadpoles were observed increased the growth rate by 0.024. The 95% CRI marginally included zero (95% CRI −0.007–0.051; Table 3). Repeating the analyses excluding the reintroduced populations did not alter the results qualitatively because parameter estimates were almost equal (results not shown).
values was 0.191 (95% CRI 0.046–0.353; this is the regression coefficient for the effect of Bd on population growth rate (see equation 4 and Table 3)). Populations where tadpoles were observed in a higher number of study years had an increased growth rate compared to populations where tadpoles were not or only rarely observed; every year in which tadpoles were observed increased the growth rate by 0.024. The 95% CRI marginally included zero (95% CRI −0.007–0.051; Table 3). Repeating the analyses excluding the reintroduced populations did not alter the results qualitatively because parameter estimates were almost equal (results not shown).
Table 2. Population growth rates as predicted by the state-space model.
| Population | Bd status |
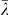
|
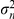
|
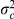
|
| Aemmenmatt | 0 | 0.871±0.063 (0.740–0.989) | 5.271±12.056 (0.122–43.832) | 3.140±21.863 (0.001–18.802) |
| Ballwil | 1 | 1.013±0.052 (0.921–1.123) | 0.536±1.391 (0.002–2.828) | 43.395±57.075 (0.153–190.000) |
| Chalchloch | 0 | 0.968±0.094 (0.757–1.128) | 4.654±11.069 (0.008–38.782) | 68.574±91.777 (1.212–327.005) |
| Chräuel | 1 | 1.037±0.045 (0.953–1.132) | 0.592±1.802 (0.002–3.098) | 13.555±33.003 (0.007–83.283) |
| Egghütten | 0 | 0.895±0.067 (0.752–1.017) | 0.462±1.054 (0.002–2.515) | 8.220±10.648 (0.014–35.720) |
| Ehrendingen | 0 | 0.968±0.094 (0.757–1.128) | 0.092±0.246 (0.001–0.446) | 10.466±33.411 (0.003–58.931) |
| Einsamkeit | 1 | 0.964±0.074 (0.843–1.121) | 7.355±13.905 (0.005–52.242) | 11.565±18.070 (0.491–52.482) |
| Fontanne | 0 | 0.920±0.074 (0.756–1.049) | 7.130±12.229 (0.008–45.040) | 190.983±214.982 (0.056–761.725) |
| Grisigen | 1 | 1.110±0.052 (1.015–1.212) | 1.657±2.398 (0.107–6.936) | 146.905±225.389 (0.032–791.812) |
| Hergiswald | 1 | 1.086±0.045 (1.001–1.174) | 0.518±1.668 (0.000–3.637) | 140.024±120.709 (13.609–493.800) |
| Hilferenmättili | 0 | 0.920±0.074 (0.756–1.049) | 0.321±1.214 (0.000–1.946) | 3.417±3.903 (0.437–12.890) |
| Hiltbrunnen | 1 | 1.061±0.043 (0.980–1.144) | 0.072±0.107 (0.000–0.308) | 3.015±6.509 (0.002–13.750) |
| Hinter Rohren | 1 | 1.135±0.062 (1.021–1.256) | 0.098±0.121 (0.005–0.371) | 9.704±23.339 (0.003–58.094) |
| Hochrüti | 0 | 0.773±0.079 (0.610–0.936) | 1.137±1.770 (0.209–4.371) | 0.030±0.417 (0.000–0.248) |
| Hohenrain | 0 | 0.773±0.079 (0.610–0.936) | 0.061±0.114 (0.000–0.288) | 0.501±1.150 (0.000–2.653) |
| Hombrig | 1 | 1.086±0.045 (1.001–1.174) | 0.166±0.323 (0.000–0.901) | 16.112±22.979 (0.084–70.225) |
| Lätten | 1 | 1.061±0.043 (0.980–1.144) | 1.006±2.917 (0.001–5.459) | 18.746±31.239 (0.035–90.531) |
| Linden | 1 | 1.013±0.052 (0.921–1.123) | 0.745±1.570 (0.004–3.461) | 4.973±8.869 (0.006–25.180) |
| Ottigenbüel | 1 | 0.964±0.074 (0.843–1.121) | 0.098±0.356 (0.000–0.464) | 0.297±0.716 (0.001–1.614) |
| Pfaffwil | 0 | 0.846±0.062 (0.724–0.966) | 1.044±2.237 (0.005–5.817) | 91.589±107.273 (1.827–405.707) |
| Räschenhus | 1 | 1.086±0.045 (1.001–1.174) | 0.766±2.305 (0.019–4.071) | 6.120±9.027 (0.456–26.250) |
| Rossei | 0 | 0.846±0.062 (0.724–0.966) | 3.107±8.811 (0.001–26.770) | 8.242±31.220 (0.006–54.762) |
| Sagerhüsli | 1 | 1.110±0.052 (1.015–1.212) | 0.114±0.197 (0.001–0.488) | 8.125±14.127 (0.023–38.550) |
| Schauensee | 1 | 1.037±0.045 (0.953–1.132) | 0.114±0.198 (0.004–0.464) | 6.438±14.275 (0.002–37.001) |
| Schlagweiher | 1 | 1.086±0.045 (1.001–1.174) | 4.180±6.715 (0.030–21.820) | 76.575±130.830 (0.024–457.207) |
| Unter Utigen | 1 | 1.037±0.045 (0.953–1.132) | 1.731±3.615 (0.010–10.011) | 13.619±24.959 (0.003–71.705) |
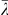 is the average population growth rate.
is the average population growth rate. 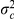 is the observation variance and
is the observation variance and 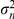 is the process variance. All estimates are given as means ± standard deviation with the 95% CRI in brackets. Bd status 0 means that Bd was not detected while Bd status 1 means that Bd was detected.
is the process variance. All estimates are given as means ± standard deviation with the 95% CRI in brackets. Bd status 0 means that Bd was not detected while Bd status 1 means that Bd was detected.
Table 3. Parameter estimates (equation 4), standard deviations and 95% CRI for the effects of the presence of Bd and the number of years in which tadpoles were observed on population growth rates.
| parameter | mean | SD | 95% CRI |
| α | 0.773 | 0.079 | 0.610–0.936 |
| βBd | 0.191 | 0.076 | 0.046–0.353 |
| βT | 0.024 | 0.015 | −0.007–0.051 |
Discussion
We found that Alytes populations in the Swiss canton Lucerne remained stable despite the presence of the pathogen, but that they were small with an average of only 10 calling males. Bd-positive populations did not have lower population growth rates than populations where we did not detect Bd. The result is robust even if we failed to detect Bd infection when it was present in some populations. If all populations where we did not detect Bd were Bd-positive, then the presence of Bd would still not lead to negative population trends since the average population growth rate is close to one.
The result was unexpected. The absence of negative effects of Bd on population trends contrasts strongly with many studies on other species that report dramatic negative effects of Bd on amphibian populations, including global extinction of species ([14]–[18], [22], but see [52]). It is even more surprising since Alytes obstetricans is known to be highly susceptible to Bd [23]–[25]. In a laboratory experiment, we showed that Bd-associated mortality of Alytes obstetricans shortly after metamorphosis was up to 90% [25]. Thus, there can be strong individual-level effects of Bd.
Population models suggest that high juvenile mortality lowers population growth rates in species with complex life cycles [53]–[56]. Hence, we expected that high chytridiomycosis-associated juvenile mortality in Alytes obstetricans in the laboratory [25] would lead to population declines. Even though we observed Bd-infected dead metamorphs in the field, there was apparently no effect of Bd-associated juvenile mortality on population trends. There are several possible explanations why there were no population-level effects of Bd in the field despite the strong individual-level effects in the laboratory [25]. The explanations are not mutually exclusive.
The first explanation is based on the fact that environmental conditions, especially those related to altitude and temperature, may mediate the effects of Bd on amphibian populations [26], [57], [58]. Under the prevailing environmental conditions, juvenile mortality in the field may be lower than in the laboratory [25]. It may be that environmental conditions, especially climate, in our study area may be such that there is some Bd–induced mortality of individuals but no population declines. However, we do not think that the populations that we studied experienced environmental conditions hostile to Bd. First, some dead and Bd-positive metamorphs were observed at two of our study sites. Admittedly, metamorphs found dead in the field had very low Bd loads perhaps indicating a cause of death other than chytridiomycosis (we did not do post mortem examinations to determine the cause of death). Second, one fifth of our populations were within the summer temperature range within which fatal chytridiomycosis is observed in Spain [26]. Further, because Bd occurred at all elevations and thus all climate regimes within our study region, we can exclude altitude as being confounded with Bd presence.
A second explanation may be that Bd-induced mortality could be compensatory rather than additive when Bd is enzootic. A decline in population size is only expected if mortality due to disease is additive, i.e. individuals die that would not die for other reasons in the absence of the disease [11]. While disease-induced mortality during Bd epizootics is obviously additive (e.g. when Bd epidemics caused Alytes obstetricans population declines in Spain [24]), this may not be the case for enzootic Bd. Compensatory mortality can result in a lack of disease effects on host abundance [4], [11]. If mortality is compensatory, increased mortality due to the presence of Bd would have to be countered by a reduction in mortality due to other causes, often in a density-dependent manner [12]. Density-dependence in the terrestrial stages of amphibian populations may indeed occur [59]–[61].
The third explanation may be that effects of Bd on abundance occurred in the past and are no longer measurable. The Briggs et al. host-pathogen models [19], [20] suggests that amphibian populations may decline to lower abundance after the emergence of Bd but remain stationary at a smaller size after Bd became enzootic. Indeed, strong Alytes obstetricans population declines were observed in our study area in the 1980 s and 1990 s [31]. Since Bd has occurred in Switzerland since at least the early 1980 s [28], it may be that Bd contributed to these declines in the past. Today, the Alytes obstetricans populations may have reached the stationary state predicted by the models such that an effect of Bd on abundance is no longer detectable. This is also supported by the low infection intensities observed in the field today. If populations have stabilized at lower abundance – which is highly plausible given the small population sizes observed in this study – they may now be less resilient [62] or more prone to environmental and/or demographic stochasticity because of reduced abundance. It is also possible that unusual environmental conditions could interact with Bd to affect populations in the future.
Although there are several possible explanations for why we did not observe the expected negative effect of Bd on abundance, the question remains open as to why Bd-positive populations had higher growth rates than Bd-negative populations. The more years tadpoles were observed at a given site, the more likely the population was to grow (Table 2). This may suggest that recruitment may determine population growth [55], [63]. Hence, the compensatory mechanism that we alluded to above may simply be increased recruitment. Increased recruitment of Bd-positive populations of Anaxyrus (Bufo) boreas may allow these populations to persist despite the presence of Bd [22].
In conclusion, we demonstrated that populations of a species that is susceptible to an emerging pathogen can grow despite a high prevalence of the pathogen. Evidently, individual-level effects of disease (mortality of individuals) did not translate into population-level effects (negative population growth rates). Our results are phenomenological and we do not know the mechanisms that allow the populations to persist. Understanding why an amphibian species that is known to be susceptible to Bd can have growing populations despite high prevalence of the pathogen would be a key to successful mitigation of the effects of chytridiomycosis.
Acknowledgments
We thank all volunteers who contributed data on counts of calling males and all field assistants during the Bd survey, Uli Reyer for supporting our research, and Matthew Fisher, Michael Schaub, Jasmin Winkler, Florian Altermatt, Doug Woodhams and Erin Muths for comments on the manuscript. Michael Schaub and Marc Kéry helped with the WinBUGS analysis.
Footnotes
Competing Interests: The authors have the following competing interest: This study was partly funded by Zoo Zürich. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: Funding was provided by the Zoological Institute and the Forschungskredit of the University of Zurich, Vontobel Stiftung, Janggen-Pöhn Stiftung, Basler Stiftung für biologische Forschung, Stiftung Dr. Joachim De Giacomi, Zoo Zürich, Grün Stadt Zürich, European Union of Aquarium Curators, and Zürcher Tierschutz. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Anderson RM, May RM. Population biology of infectious diseases: Part I. Nature. 1979;280:361–367. doi: 10.1038/280361a0. [DOI] [PubMed] [Google Scholar]
- 2.Tompkins DM, Begon M. Parasites can regulate wildlife populations. Parasitol Today. 1999;15:311–313. doi: 10.1016/s0169-4758(99)01484-2. [DOI] [PubMed] [Google Scholar]
- 3.Ibelings BW, De Bruin A, Kagami M, Rijkeboer M, Brehm M, et al. Host parasite interactions between freshwater phytoplankton and chytrid fungi (Chytridiomycota). J Phycol. 2004;40:437–453. [Google Scholar]
- 4.Jolles AE, Etienne RS, Olff H. Independent and competing disease risks: Implications for host populations in variable environments. Amer Nat. 2006;167:745–757. doi: 10.1086/503055. [DOI] [PubMed] [Google Scholar]
- 5.de Castro F, Bolker B. Mechanisms of disease-induced extinction. Ecol Lett. 2005;8:117–126. [Google Scholar]
- 6.Ebert D, Hamilton WD. Sex against virulence: the coevolution of parasitic diseases. Trends Ecol Evol. 1996;11:A79–A82. doi: 10.1016/0169-5347(96)81047-0. [DOI] [PubMed] [Google Scholar]
- 7.Ebert D. Virulence and local adaptation of a horizontally transmitted parasite. Science. 1994;265:1084–1086. doi: 10.1126/science.265.5175.1084. [DOI] [PubMed] [Google Scholar]
- 8.Read AF. The evolution of virulence. Trends Microbiol. 1994;72:73–76. doi: 10.1016/0966-842x(94)90537-1. [DOI] [PubMed] [Google Scholar]
- 9.Tompkins DM, Dobson AP, Arneberg P, Begon ME, Cattadori IM, et al. Parasites and host population dynamics. In: Hudson PJ, Rizzoli A, Grenfell BT, Heesterbeek H, Dobson AP, editors. The ecology of wildlife diseases. Oxford: Oxford University Press; 2001. pp. 45–62. [Google Scholar]
- 10.Deem SL, Ezenwa VO, Ward JR, Wilcox BA. Research frontiers in ecological systems: evaluating the impacts of disease on ecosystems. In: Ostfeld RS, Keesing F, Eviner VT, editors. Infectious disease ecology: effects of ecosystems on disease and of disease on ecosystems. Princeton: Princeton University Press; 2008. pp. 304–318. [Google Scholar]
- 11.Burnham KP, Anderson DR. Tests of compensatory vs. additive hypotheses of mortality in mallards. Ecology. 1984;65:105–112. [Google Scholar]
- 12.Lebreton JD. Dynamical and statistical models for exploited populations. Austral New Zealand J Stat. 2005;47:49–63. [Google Scholar]
- 13.Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- 14.Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, et al. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth. 2007;4:125–134. [Google Scholar]
- 15.Fisher MC, Garner TWJ, Walker SF. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu Rev Microbiol. 2009;63:291–310. doi: 10.1146/annurev.micro.091208.073435. [DOI] [PubMed] [Google Scholar]
- 16.Laurance WF, McDonald KR, Speare R. Epidemic disease and the catastrophic decline of Australian rain forest frogs. Conserv Biol. 1996;10:406–413. [Google Scholar]
- 17.Lips KR, Brem F, Brenes R, Reeve JD, Alford RA, et al. Emerging infectious disease and the loss of biodiversity in a neotropical amphibian community. Proc Nat Acad Sci USA. 2006;103:3165–3170. doi: 10.1073/pnas.0506889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc Nat Acad Sci USA. 2010;107:9689–9694. doi: 10.1073/pnas.0914111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briggs CJ, Vredenburg VT, Knapp RA, Rachowicz LJ. Investigating the population-level effects of chytridiomycosis: An emerging infectious disease of amphibians. Ecology. 2005;86:3149–3159. [Google Scholar]
- 20.Briggs CJ, Knapp RA, Vredenburg VT. Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proc Nat Acad Sci USA. 2010;107:9695–9700. doi: 10.1073/pnas.0912886107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilliod DS, Muths E, Scherer RD, Partelt PE, Corn PS, et al. Effects of amphibian chytrid fungus on individual survival probability in wild boreal toads. Conserv Biol. 2010;24:1259–1267. doi: 10.1111/j.1523-1739.2010.01506.x. [DOI] [PubMed] [Google Scholar]
- 22.Muths E, Scherer RD, Pilliod DS. Compensatory effects of recruitment and survival when amphibian populations are perturbed by disease. J Appl Ecol. 2011;48:873–879. [Google Scholar]
- 23.Longo AV, Burrowes PA. Persistence with chytridiomycosis does not assure survival of direct-developing frogs. EcoHealth. 2010;7:185–195. doi: 10.1007/s10393-010-0327-9. [DOI] [PubMed] [Google Scholar]
- 24.Bosch J, Martínez-Solano I, García-París M. Evidence of a chytrid fungus infection involved in the decline of the common midwife toad (Alytes obstetricans) in protected areas of central Spain. Biol Conserv. 2001;97:331–337. [Google Scholar]
- 25.Tobler U, Schmidt BR. Within- and among-population variation in chytridiomycosis-induced mortality in the toad Alytes obstetricans. PLoS ONE. 2010;5:e10927. doi: 10.1371/journal.pone.0010927. doi: 10.1371/journal.pone.0010927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker SF, Bosch J, Gomez V, Garner TWJ, Cunningham AA, et al. Factors driving pathogenicity vs. prevalence of amphibian panzootic chytridiomycosis in Iberia. Ecol Lett. 2010;13:372–382. doi: 10.1111/j.1461-0248.2009.01434.x. [DOI] [PubMed] [Google Scholar]
- 27.Teacher AGF, Cunningham AA, Garner TWJ. Assessing the long-term impact of Ranavirus infection in wild common frog populations. Anim Conserv. 2010;13:514–522. [Google Scholar]
- 28.Peyer NF. Historical evidence for the presence of the emerging amphibian pathogen Batrachochytrium dendrobatidis (Longcore et al. 1999) in Switzerland. M.Sc. thesis. Zurich: University of Zurich; 2010. [Google Scholar]
- 29.Garner TWJ, Walker S, Bosch J, Hyatt AD, Cunningham AA, et al. Chytrid fungus in Europe. Emerg Infect Dis. 2005;11:1639–1641. doi: 10.3201/eid1110.050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt BR, Zumbach S. Rote Liste der gefährdeten Amphibien der Schweiz. Bern: Bundesamt für Umwelt, Wald und Landschaft; 2005. [Google Scholar]
- 31.Borgula A, Zumbach S. Verbreitung und Gefährdung der Geburtshelferkröte (Alytes obstetricans) in der Schweiz. Z Feldherpetol. 2003;10:11–26. [Google Scholar]
- 32.Woodhams DC, Bosch J, Briggs CJ, Cashins S, Davis LR, et al. Mitigating amphibian disease: strategies to maintain wild populations and control chytridiomycosis. Front Zool. 2011;8:8. doi: 10.1186/1742-9994-8-8. doi: 10.1186/1742-9994-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. [Google Scholar]
- 34.Amt für Natur- und Landschaftsschutz. Artenhilfsprogramm Geburtshelferkröte 2000–2009. Luzern: Kanton Luzern; 2000. [Google Scholar]
- 35.Borgula A, Zuberbühler N. Artenhilfsprogramm Geburtshelferkröte 2010–2019. Luzern: Kanton Luzern; 2010. [Google Scholar]
- 36.Schmidt BR. Monitoring the distribution of pond-breeding amphibians when species are detected imperfectly. Aquat Conserv. 2005;15:681–692. [Google Scholar]
- 37.Schmidt BR. Declining amphibian populations: The pitfalls of count data in the study of diversity, distributions, dynamics, and demography. Herpetol J. 2004;14:167–174. [Google Scholar]
- 38.Bart J, Droege S, Geissler P, Peterjohn B, Ralph CJ. Density estimation in wildlife surveys. Wildl Soc Bull. 2004;32:1242–1247. [Google Scholar]
- 39.Kéry M, Schmidt BR. Imperfect detection and its consequences for monitoring for conservation. Comm Ecol. 2008;9:207–216. [Google Scholar]
- 40.Skelly DK, Richardson JL. Larval sampling. In: Dodd CK, editor. In Amphibian ecology and conservation: a handbook of techniques. Oxford: Oxford University Press; 2009. pp. 55–70. [Google Scholar]
- 41.DiGiacomo RF, Koepsell TD. Sampling for detection of infection or disease in animal populations. J Amer Vet Med Assoc. 1986;189:22–23. [PubMed] [Google Scholar]
- 42.Schmidt BR. Are hybridogenetic frogs cyclical parthenogens? Trends Ecol Evol. 1993;8:271–273. doi: 10.1016/0169-5347(93)90252-K. [DOI] [PubMed] [Google Scholar]
- 43.Vences M. The Amphibian Tree of Life: Ideologie, Chaos oder biologische Realität? Z Feldherpet. 2007;14:153–162. [Google Scholar]
- 44.Schmidt BR, Furrer S, Kwet A, Lötters S, Rödder D, et al. Desinfektion als Maßnahme gegen die Verbreitung der Chytridiomykose bei Amphibien. In: Hachtel M, Schlüpmann M, Thiesmeier B, Weddeling K, editors. In Methoden der Feldherpetologie. Bielefeld: Laurenti Verlag; 2009a. pp. 229–241. [Google Scholar]
- 45.Schmidt BR, Geiser C, Peyer N, Keller N, von Rütte M. Assessing whether disinfectants against the fungus Batrachochytrium dendrobatidis have negative effects on tadpoles and zooplankton. Amphibia-Reptilia. 2009b;30:313–319. [Google Scholar]
- 46.Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Org. 2004;60:141–148. doi: 10.3354/dao060141. [DOI] [PubMed] [Google Scholar]
- 47.R Development Core Team. R: A language and environment for statistical computing. 2008. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. Available: http://www.R-project.org.
- 48.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comp. 2000;10:325–337. [Google Scholar]
- 49.de Valpine P, Hastings A. Fitting population models incorporating process noise and observation error. Ecol Monogr. 2002;72:57–76. [Google Scholar]
- 50.Kéry M, Schaub M. Bayesian Population Analysis using WinBUGS: A hierarchical perspective. London: Elsevier; 2012. [Google Scholar]
- 51.Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- 52.Retallick RWR, McCallum H, Speare R. Endemic infection of the amphibian chytrid fungus in a frog community post-decline. PLoS Biol. 2004;2:e351. doi: 10.1371/journal.pbio.0020351. DOI: 10.1371/journal.pbio.0020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conroy SDS, Brook BW. Demographic sensitivity and persistence of the threatened white- and orange-bellied frogs of Western Australia. Pop Ecol. 2003;45:105–114. [Google Scholar]
- 54.Hels T, Nachman G. Simulating viability of a spadefoot toad Pelobates fuscus metapopulation in a landscape fragmented by a road. Ecography. 2002;25:730–744. [Google Scholar]
- 55.Lampo M, De Leo GA. The invasion ecology of the toad Bufo marinus: from South America to Australia. Ecol Appl. 1998;8:388–396. [Google Scholar]
- 56.Di Minin E, Griffiths RA. Viability analysis of a threatened amphibian population: modelling the past, present and future. Ecography. 2011;34:162–169. [Google Scholar]
- 57.Bosch J, Carrascal LM, Duran L, Walker S, Fisher MC. Climate change and outbreaks of amphibian chytridiomycosis in a montane area of Central Spain; is there a link? Proc Roy Soc B. 2007;274:253–260. doi: 10.1098/rspb.2006.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Puschendorf R, Hoskin CJ, Cashins SD, McDonald KR, Skerratt LF, et al. Environmental refuge from disease-driven amphibian extinction. Conserv Biol. 2011;25:956–964. doi: 10.1111/j.1523-1739.2011.01728.x. [DOI] [PubMed] [Google Scholar]
- 59.Altwegg R. Multistage density dependence in an amphibian. Oecologia. 2003;136:46–50. doi: 10.1007/s00442-003-1248-x. [DOI] [PubMed] [Google Scholar]
- 60.Berven KA. Density dependence in the terrestrial stage of wood frogs: evidence from a 21-year population study. Copeia. 2009;2009:328–338. [Google Scholar]
- 61.Patrick DA, Harper EB, Hunter ML, Calhoun AJK. Terrestrial habitat selection and strong density-dependent mortality in recently metamorphosed amphibians. Ecology. 2008;89:2563–2574. doi: 10.1890/07-0906.1. [DOI] [PubMed] [Google Scholar]
- 62.Jolles AE, Cooper DV, Levin SA. Hidden effects of chronic tuberculosis in African buffalo. Ecology. 2005;86:2358–2364. [Google Scholar]
- 63.Grafe TU, Kaminsky SK, Bitz JH, Lussow H, Linsenmair KE. Demographic dynamics of the afro-tropical pig-nosed frog, Hemisus marmoratus: effects of climate and predation on survival and recruitment. Oecologia. 2004;141:40–46. doi: 10.1007/s00442-004-1639-7. [DOI] [PubMed] [Google Scholar]