Abstract
Background
So far, many studies have investigated the distribution of CCR5 genotype between HIV-1 infected patients and uninfected people. However, no definite results have been put forward about whether heterozygosity for a 32-basepair deletion in CCR5 gene (CCR5-Δ32) can affect HIV-1 susceptibility.
Methods
We performed a meta-analysis of 18 studies including more than 12000 subjects for whom the CCR5-Δ32 polymorphism was genotyped. Odds ratio (OR) with 95% confidence interval (CI) were employed to assess the association of CCR5-Δ32 polymorphism with HIV-1 susceptibility.
Results
Compared with the wild-type CCR5 homozygotes, the pooled OR for CCR5-Δ32 heterozygotes was 1.02 (95%CI, 0.88–1.19) for healthy controls (HC) and 0.95 (95%CI, 0.71–1.26) for exposed uninfected (EU) controls. Similar results were found in stratified analysis by ethnicity, sample size and method of CCR5-Δ32 genotyping.
Conclusions
The meta-analysis indicated that HIV-1 susceptibility is not significantly affected by heterozygosity for CCR5-Δ32.
Introduction
Inter-individual variability in susceptibility to HIV-1 infection, transmission, disease progression, and response to antiviral therapy has been attributed to host variability in multiple genes [1]. CC chemokine receptor 5 (CCR5) and CXC chemokine receptor 4 (CXCR4) are co-receptors for the entry of human immunodeficiency virus type 1 (HIV-1) into target cells [2]. A natural knockout deletion of 32 bases in CCR5 gene introduces a premature stop codon resulting in truncated protein product [3]. People homozygous for CCR5-Δ32 are naturally resistant to R5 HIV infection and the heterozygous state is associated with up to 2–4 years delay in disease progression [4] ,. Recently, Allers et al reported that they have successfully cured a HIV infected patient through CCR5-Δ32/Δ32 stem cell transplantation [5], [6]. On the other hand, the evidence for protection from HIV-1 infection among CCR5-Δ32 heterozygotes is mixed. A meta-analysis of Despina et al suggested that perinatal infection rates are not strongly determined by the number of functional CCR5 receptors in the children [7]. For adults, some studies have reported that CCR5-Δ32 heterozygotes could be protective against HIV transmission [8]–[15], whereas others have not confirmed that [16]–[28]. Therefore, we performed a meta-analysis of the accumulated data to address this question definitively.
Materials and Methods
Search Strategy and Study Selection
English database of Google Scholar (GS), PubMed and Chinese database of CNKI were searched till June 2011 using key words: CCR5-Δ32 and HIV-1. Studies satisfying the following criteria were included: case-control studies reporting the association of CCR5-Δ32 genotype with HIV-1 susceptibility, distribution of CCR5-Δ32 genotype between the cohorts was shown, not a prenatal HIV-1 infection study.
Data Extraction and Statistical Analysis
Two reviewers( SiJie Liu, Jie Wu) independently performed data extraction and then checked the results together. The following information was extracted from included studies: authors, year of publication, ethnicity, country, sample size, method of CCR5-Δ32 genotyping and CCR5-Δ32 genotype of cohorts.
Odds ratio (OR) and its 95% confidence intervals (CI) were used to evaluate the association of CCR5-Δ32 heterozygotes with HIV-1 susceptibility. Subgroups were identified by ethnicity, sample size and method of CCR5-Δ32 genotyping. A chi-square-based Q-test was carried out to assess heterogeneity across studies [29]. A P value less than 0.10 was used to denote statistical significance. Fixed effects (Mantel and Haenszel) model was employed to pool the effects of studies without heterogeneity, otherwise the random effects (Dersirmonian and Laird) model was used [30], [31]. Publication bias was evaluated by Egger’s and Begg’s test with funnel plots [32], [33]. Asymmetry of the funnel plot suggests publication bias. A P value less than 0.05 was used to denote statistical significance. One-way sensitivity analyses were performed to examine the influence of individual studies on meta-analysis’s results. Data were analyzed using Stata version 10.0 (StataCorp, College Station, Tex).
Results
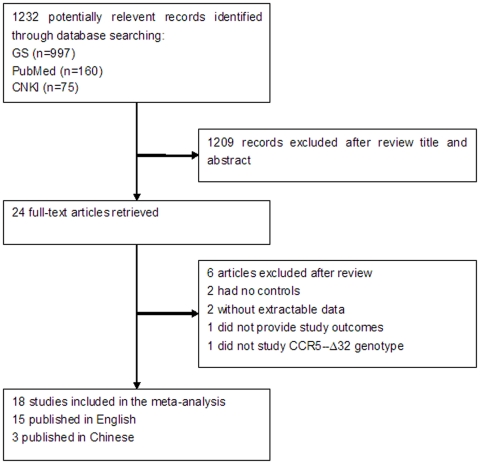
Figure 1 summarized the selection process of literatures. The electronic search yielded 1232 records, after screening over titles and/or abstracts, 24 articles were selected for further review. Finally, 18 studies involving 6427 cases and 5809 controls were included in the meta-analysis. Study sample size ranged from 140 to 2605 subjects. Study characteristics of the 18 eligible studies were summarized in Table 1. Distribution of CCR5 genotype among subjects was shown in Table 2. Briefly, 9 studies involved Caucasian subjects [8], [10]–[12], [17], [22]–[24], 4 studies involved Mongoloid subjects [16], [21], [25], [28], 3 studies involved African subjects [8], [12], [24], 3 studies involved Latina subjects [12], [18], [27]. In addition to CCR5-Δ32 genotype, 8 studies provided the subjects’ CCR2-64I genotype [10], [16], [19]–[21], [26]–[28], 5 studies provided the subjects’ SDF-1 genotype [16], [20], [21], [26], [27]. All studies were done in subjects of mixed genders except that by Downer et al [24], which only included women.
Figure 1. Selection process of studies included in the meta-analysis.
Table 1. Characteristics of selected studies in the meta-analysis.
| Study (year) | Country | Genotyping method | Ethnicity | Sample size (case/control) |
| Battiloro (2000) [17] | Italy | PCR | Caucasian | 256/806 |
| Deng (2004) [28] | China | PCR | Mongoloid | 88/119 |
| Diaz (2000) [18] | Colombia | PCR | Latina | 29/188 |
| Downer (2002) [24] | USA | PCR-RFLP | Mixed | 929/445 |
| Grimaldi(2002)[13] | Brazil | PCR | Mixed | 113/549 |
| Li (2003) [26] | China | PCR | Mongoloid | 94/46 |
| Liu (2004) [20] | USA | PCR | Mixed | 316/513 |
| Lockett (1999) [19] | Britain | PCR | Mixed | 86/105 |
| Oh(2008)[8] | German | PCR | Caucasian | 610/427 |
| African | 35/26 | |||
| Papa (2000) [10] | Greece | PCR | Caucasian | 138/239 |
| Paz-y-Mino (2005) [27] | Ecuador | PCR | Latina | 295/50 |
| Philpott (2003) [12] | USA | PCR-RFLP | Mixed | 2047/558 |
| Takacova (2008) [22] | Slovakia | PCR | Caucasian | 162/198 |
| Tan (2010) [16] | China | PCR | Mongoloid | 250/237 |
| Tang (2010) [25] | China | PCR-LDR | Mongoloid | 245/223 |
| Trecarichi(2006)[11] | Italy | PCR | Caucasian | 120/120 |
| Veloso(2010)[23] | Span | PCR | Caucasian | 184/236 |
| Wang (2003) [21] | China | PCR | Mongoloid | 330/474 |
Table 2. Distribution of CCR5 genotype of included studies.
| Study | Ethnicity | HIV-infected | Healthy Controls | Exposed but uninfected | |||
| +/+1 | +/△2 | +/+ | +/△ | +/+ | +/△ | ||
| Battiloro | Caucasian | 232 | 24 | 744 | 62 | ||
| Deng | Mongoloid | 88 | 0 | 117 | 2 | ||
| Diaz | Latina | 28 | 1 | 142 | 8 | 37 | 1 |
| Downer | Mixed | 879 | 50 | 422 | 23 | ||
| Grimaldi | Mixed | 103 | 10 | 520 | 29 | ||
| Li | Mongoloid | 90 | 4 | 45 | 1 | ||
| Liu | Mixed | 261 | 55 | 354 | 68 | 69 | 22 |
| Lockett | Mixed | 63 | 23 | 38 | 10 | 40 | 17 |
| Oh | Caucasian | 595 | 115 | 352 | 75 | ||
| African | 35 | 0 | 25 | 1 | |||
| Papa | Caucasian | 132 | 6 | 216 | 23 | ||
| Paz-y-Mino | Latina | 292 | 3 | 50 | 0 | ||
| Philpott | Mixed | 1940 | 107 | 513 | 45 | ||
| Takacova | Caucasian | 137 | 25 | 164 | 34 | ||
| Tan | Mongoloid | 226 | 24 | 222 | 15 | ||
| Tang | Mongoloid | 221 | 24 | 209 | 14 | ||
| Trecarichi | Caucasian | 111 | 9 | 24 | 6 | ||
| Veloso | Caucasian | 144 | 40 | 174 | 26 | 31 | 5 |
| Wang | Mongoloid | 329 | 1 | 473 | 1 | ||
CCR5 homozygotes.
CCR5-Δ32 heterozygotes.
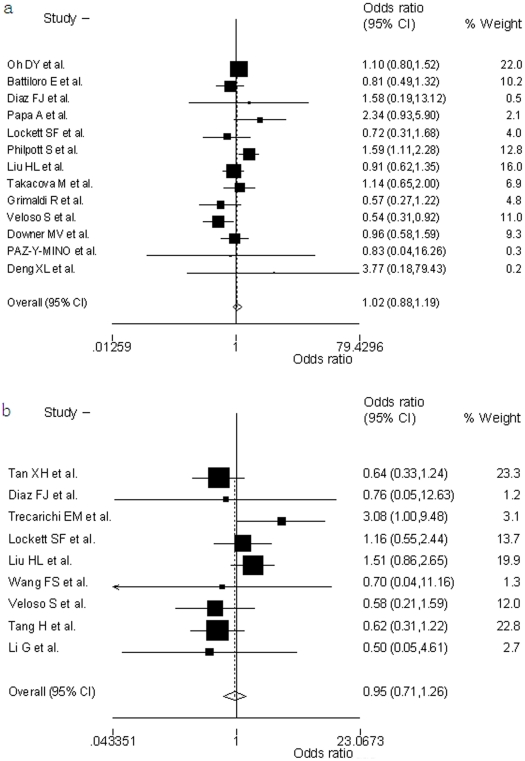
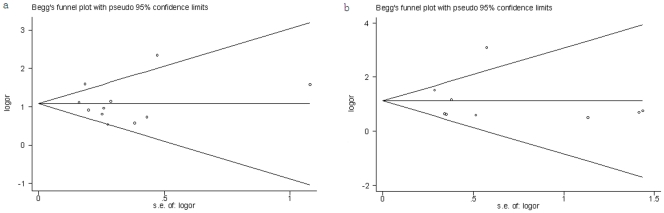
Compared with the wild-type CCR5 homozygotes, the pooled OR for CCR5-Δ32 heterozygotes was 1.02 (95%CI, 0.88–1.19, p = 0.073) for healthy controls (HC) (figure 2a) and 0.95 (95%CI, 0.71–1.26, p = 0.182) for exposed uninfected (EU) controls (figure 2b). There was no significant between-study heterogeneity. No asymmetry is observed in the funnel plots (figure 3a, 3b).
Figure 2. Odds ratio of HIV-1 infection of CCR5-Δ32 heterozygotes versus wild type CCR5 homozygotes.
The area of the black square reflects the weight of each study. The diamonds represent the combined odds ratio and 95% confidence interval using the fixed effects model for (a) healthy controls (HC) and (b) exposed uninfected controls (EU).
Figure 3. Funnel plots to detect publication bias in the meta-analysis.
(a) Healthy controls considered; (b) exposed uninfected controls considered. The horizontal line indicates the pooled log odds ratio (OR) and guidelines to assist in visualizing the funnel are pooled at 95% pseudo confidence limits for this estimate.
We also performed stratified analysis by ethnicity, sample size and method of CCR5-Δ32 genotyping. The results were summarized in Table 3. All the results were consisted with overall analysis and no publication bias were observed.
Table 3. Stratified analysis of CCR5-Δ32 heterozygotes and susceptibility to HIV-1.
| Variable | Healthy Controls | Exposed but uninfected | ||
| OR (95%CI) | P 1 | OR (95%CI) | P | |
| Ethnicity | ||||
| African | 1.17 (0.71–1.93) | 0.604 | ||
| Caucasian | 1.06 (0.73–1.53) | 0.005 | 1.31 (0.25–6.85) | 0.029 |
| Latina | 1.28 (0.62–2.61) | 0.942 | ||
| Mongoloid | 0.63 (0.39–1.01) | 0.995 | ||
| Genotyping Method | ||||
| PCR | 0.94 (0.79–1.12) | 0.239 | 1.04 (0.76–1.44) | 0.233 |
| PCR-RFLP | 1.32 (0.99–1.78) | 0.110 | ||
| Sample Size | ||||
| >800 | 1.09 (0.91–1.30) | 0.152 | ||
| <800 | 0.87 (0.65–1.16) | 0.144 | 0.81 (0.57–1.14) | 0.233 |
P value of Q-test for heterogeneity test.
Sensitive analysis was conducted by deleting one study at a time to examine the influence of individual data-set to the pooled ORs. All of the corresponding pooled ORs were not materially altered (Data not shown).
Discussion
Meta-analysis offers a powerful method to synthesize information of independent studies with similar intention. It has been proved that CCR5-Δ32 homozygotes are associated with near complete protection to HIV-1 infection. Moreover, published data have demonstrated that a disease-retarding effect of CCR5-Δ32 heterozygosity in HIV-1 infected individuals [34]. Whereas it remained unclear if a heterozygosity for CCR5-Δ32 could affect HIV-1 susceptibility. Thus we performed this meta-analysis involving 18 eligible studies with 6427 patients and 5809 controls. The study demonstrated that CCR5-Δ32 heterozygosity has little or no protective effect against HIV-1 infection among adults. This result is similar to a previous study of perinatal HIV-1 infection [7]. Several factors might underlie the lack of observed association between CCR5-Δ32 heterozygosity and HIV-1 susceptibility. First, the expression of CCR5 is influenced by factors other than CCR5 genotype. Even an individual with CCR5-Δ32 heterozygosity could still express high level of CCR5 [35], [36]. Second, susceptibility to HIV-1 infection is affected by a combination of genes besides CCR5. The CCR5-Δ32 heterozygotes couldn’t provide a full resistant to HIV-1 infection as the homozygotes. It is possible that a single Δ32 allele exerts a protective effect against HIV-1 infection only if it occurs combined with other protective factors [9].
There are a number of limitations to our study. First, although test of publication bias have generated negative results, studies solely in conference or in local journals may have been overlooked. Second, HIV-1 of X4 strain take advantage of CXCR4 as co-receptor. It has been reported that new infections in individuals are primarily established by strains that use R5 [37]–[39]. Currently, it is remains controversial about if HIV could use CXCR4 as co-receptor in primary HIV infection [40]. Primary infection with CXCR4-using HIV-1 strains is believed to be a rare event [41]. Thus, we might believe that in most of the cases HIV-1 R5 strain cause the initial infection, rather than the X4 strain. Although we couldn’t exclude the interference of X4 viruses, it is unlikely that virus of X4 strain would significantly affect the results. Third, controls of some studies were solely derived from healthy individuals. For studies concerning disease susceptibility, it’ll be more proper to take samples from exposed uninfected people as controls. Fourth, susceptibility to HIV-1 is influenced by multiple factors other than CCR5, they might interfere the precision of analysis.
In conclusion, our study involving more than 12000 subjects suggested that CCR5-Δ32 heterozygosity has little effect on protecting from HIV-1 infection. Therefore, other chemokine receptors and transmission mechanisms may play a more important role.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work is supported by National Grand Program on Key Infectious Disease (2012ZX10001-003), National Natural Science Funding of China (No.31171247). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kaur G, Mehra N. Genetic determinants of HIV-1 infection and progression to AIDS: susceptibility to HIV infection. Tissue Antigens; 2009;73:289–301. doi: 10.1111/j.1399-0039.2009.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Brien SJ, Moore JP. The effect of genetic variation in chemokines and their receptors on HIV transmission and progression to AIDS. Immunol Rev; 2000;177:99–111. doi: 10.1034/j.1600-065x.2000.17710.x. [DOI] [PubMed] [Google Scholar]
- 3.Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, et al. Homozygous defects in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell: 1996;86:367–77. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 4.Dean M, Carrington M, Winkler C, Huttley GA, Smith MW, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science: 1996;273:1856–62. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 5.Hütter G, Nowak D, Mossner M, Ganepola S, Müssiq A, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med; 2009;360:692–8. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 6.Allers K, Hütter G, Hofmann J, Loddenkemper C, Rieger K, et al. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood; 2011;117:2791–9. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 7.Contopoulos-Ioannidis DG, O’Brien TR, Goedert JJ, Rosenberg PS, Ioannidis JP. Effect of CCR5-delta32 heterozygosity on the risk of perinatal HIV-1 infection: a meta-analysis. J Acquir Immune Defic Syndr; 2003;32:70–6. doi: 10.1097/00126334-200301010-00010. [DOI] [PubMed] [Google Scholar]
- 8.Oh DY, Jessen H, Kücherer C, Neumann K, Oh N, et al. CCR5D32 Genotypes in a German HIV-1 Seroconverter Cohort and Report of HIV-1 Infection in a CCR5D32 Homozygous Individual. PLoS One; 2008;3:e2747. doi: 10.1371/journal.pone.0002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hladik F, Liu H, Speelmon E, Livingston-Rosanoff D, Wilson S, et al. Combined effect of CCR5-Delta32 heterozygosity and the CCR5 promoter polymorphism -2459 A/G on CCR5 expression and resistance to human immunodeficiency virus type 1 transmission. J Virol; 2005;79:11677–84. doi: 10.1128/JVI.79.18.11677-11684.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papa A, Papadimitriou E, Adwan G, Clewley JP, Malissiovas N, et al. HIV-1 co-receptor CCR5 and CCR2 mutations among Greeks. FEMS Immunol Med Microbiol; 2000;28:87–9. doi: 10.1111/j.1574-695X.2000.tb01461.x. [DOI] [PubMed] [Google Scholar]
- 11.Trecarichi EM, Tumbarello M, de Gaetano Donati K, Tamburrini E, Cauda R, et al. Partial protective effect of CCR5-Delta 32 heterozygosity in a cohort of heterosexual Italian HIV-1 exposed uninfected individuals. AIDS Res Ther; 2006;3:22. doi: 10.1186/1742-6405-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Philpott S, Weiser B, Tarwater P, Vermund SH, Kleeberger CA, et al. CC chemokine receptor 5 genotype and susceptibility to transmission of human immunodeficiency virus type 1 in women. J Infect Dis; 2003;187:569–75. doi: 10.1086/367995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimaldi R, Shindo N, Acosta AX, Dourado I, Brites C, et al. Prevalence of the CCR5Delta32 mutation in Brazilian populations and cell susceptibility to HIV-1 infection. Hum Genet; 2002;111:102–4. doi: 10.1007/s00439-002-0747-x. [DOI] [PubMed] [Google Scholar]
- 14.Pasi KJ, Sabin CA, Jenkins PV, Devereux HL, Ononye C, et al. The effects of the 32-bp CCR-5 deletion on HIV transmission and HIV disease progression in individuals with haemophilia. Br J Haematol; 2000;111:136–142. doi: 10.1046/j.1365-2141.2000.02325.x. [DOI] [PubMed] [Google Scholar]
- 15.Marmor M, Sheppard HW, Donnell D, Bozeman S, Celum C, et al. Homozygous and heterozygous CCR5-Delta32 genotypes are associated with resistance to HIV infection. J Acquir Immune Defic Synd; 2001;27:472–81. doi: 10.1097/00126334-200108150-00009. [DOI] [PubMed] [Google Scholar]
- 16.Tan XH, Zhang JY, Di CH, Hu AR, Yang L, et al. Distribution of CCR5-Delta32, CCR5m303A, CCR2-64I and SDF1-3'A in HIV-1 infected and uninfected high-risk Uighurs in Xinjiang, China. Infect Genet Evol; 2010;10:268–72. doi: 10.1016/j.meegid.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Battiloro E, Andreoni M, Parisi SG, Mura MS, Sotgiu G, et al. Distribution of the CCR5 delta32 allele in Italian HIV type 1-infected and normal individuals. AIDS Res Hum Retroviruses; 2000;16:181–2. doi: 10.1089/088922200309520. [DOI] [PubMed] [Google Scholar]
- 18.Díaz FJ, Vega JA, Patiño PJ, Bedoya G, Nagles J, et al. Frequency of CCR5 delta-32 mutation in human immunodeficiency virus (HIV)-seropositive and HIV-exposed seronegative individuals and in general population of Medellin, Colombia. Mem Inst Oswaldo Cruz; 2000;95:237–42. doi: 10.1590/s0074-02762000000200018. [DOI] [PubMed] [Google Scholar]
- 19.Lockett SF, Alonso A, Wyld R, Martin MP, Robertson JR, et al. Effect of chemokine receptor mutations on heterosexual human immunodeficiency virus transmission. J Infect Dis; 1999;180:14–21. doi: 10.1086/314918. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Hwangbo Y, Holte S, Lee J, Wang C, et al. Analysis of genetic polymorphisms in CCR5, CCR2, stromal cell-derived factor-1, RANTES, and dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin in seronegative individuals repeatedly exposed to HIV-1. J Infect Dis; 2004;190:055–8. doi: 10.1086/423209. [DOI] [PubMed] [Google Scholar]
- 21.Wang FS, Hong WG, Cao Y, Liu MX, Jin L, et al. Population survey of CCR5 delta32, CCR5 m303, CCR2b 64I, and SDF1 3'A allele frequencies in indigenous Chinese healthy individuals, and in HIV-1-infected and HIV-1-uninfected individuals in HIV-1 risk groups. J Acquir Immune Defic Syndr; 2003;32:24–30. doi: 10.1097/00126334-200302010-00002. [DOI] [PubMed] [Google Scholar]
- 22.Takacova M, Nogova P, Habekova M, Staneková D. Prevalence of a 32 bp deletion in the gene for human immunodeficiency virus 1 co-receptor ccr5 in slovak population. Acta Virol; 2008;52:61–4. [PubMed] [Google Scholar]
- 23.Veloso S, Olona M, García F, Domingo P, Alonso-Villaverde C, et al. Effect of TNF-alpha genetic variants and CCR5 Delta 32 on the vulnerability to HIV-1 infection and disease progression in Caucasian Spaniards. BMC Med Genet; 2010;11:3. doi: 10.1186/1471-2350-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Downer MV, Hodge T, Smith DK, Qari SH, Schuman P, et al. Regional variation in CCR5-Delta32 gene distribution among women from the US HIV Epidemiology Research Study (HERS). Genes Immun; 2002;3:95–8. doi: 10.1038/sj.gene.6363884. [DOI] [PubMed] [Google Scholar]
- 25.Tang H, Guo SX, Zhao RL, Zhang JY, Tan XH. Research on the Polymorphism of HIV Infection-Related Gene CCR5 -Delta32 in HIV Highly-Infected Uygur Population in Yili Prefecture of Xinjiang Uygur Autonomous Region. Journal of Shihezi University{Natural Science); 2010;28:98–201. [Google Scholar]
- 26.Li GH, Wei FL, He Y, Sun F, Zhou ZQ, et al. Impact of the polymorphisms of CCR5, CCR2 and SDF1 on HIV??1 heterosexual transmission. Chin J Microbiol Immunol, 2003;23:65–9. [Google Scholar]
- 27.Paz-y-Mino C, Morillo SA, Celi AP, Witte T, et al. CCR5delta32, CCR2-64I, and SDF1-3'A polymorphisms related to resistance to HIV-1 infection and disease in the Ecuadorian population. Hum Biol; 2005;77:21–6. doi: 10.1353/hub.2005.0068. [DOI] [PubMed] [Google Scholar]
- 28.Deng XL, Hong KX, Chen JP, Ruan YH, Xu MY, et al. Genetic polymorphism of human immunodeficiency virus coreceptor CCR5-Delta32 and CCR2-64I alleles in Chinese Yi Ethnic group in Sichuan. Chin J Epidemiol; 2004;25:050–3. [PubMed] [Google Scholar]
- 29.Cochran WG. The combination of estimates from different experiments. Biometrics; 1954;10:9. [Google Scholar]
- 30.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Nat Cancer Inst; 1959;22:19–48. [PubMed] [Google Scholar]
- 31.Dersimonian R, Larid N. Mata-analysis in clinical trials. Control Clin Trials; 1986;7:77–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 32.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ; 1997;315:29–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics; 1994;50:088–100. [PubMed] [Google Scholar]
- 34.Ioannidis JP, Rosenberg PS, Goedert JJ, Ashton LJ, Benfield TL, et al. Effects of CCR5-Delta32, CCR2-64I, and SDF-1 3'A alleles on HIV-1 disease progression: An international meta-analysis of individual-patient data. Ann Intern Med; 2001;135:82–95. doi: 10.7326/0003-4819-135-9-200111060-00008. [DOI] [PubMed] [Google Scholar]
- 35.Wu L, Paxton WA, Kassam N, Ruffing N, Rottman JB, et al. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med; 1997;85:681–91. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Roda Husman AM, Blaak H, Brouwer M, Schuitemaker H. CC chemokine receptor 5 cell-surface expression in relation to CC chemokine receptor 5 genotype and the clinical course of HIV-1 infection. J Immunol; 1999;163:597–603. [PubMed] [Google Scholar]
- 37.Schuitemaker H, Kootstra NA, de Goede RE, de Wolf F, Miedema F, et al. Monocytotropic human immunodeficiency virus type 1 (HIV-1) variants detectable in all stages of HIV-1 infection lack T-cell line tropism and syncytium-inducing ability in primary T-cell culture[J]. J Virol, 1991;65:356–63. doi: 10.1128/jvi.65.1.356-363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Brien SJ, Nelson GW. Human genes that limit AIDS. Nat Genet; 2004;36:565–74. doi: 10.1038/ng1369. [DOI] [PubMed] [Google Scholar]
- 39.Ghaffari G, Tuttle DL, Briggs D, Burkhardt BR, Bhatt D, et al. Complex determinants in human immunodeficiency virus type 1 envelope gp120 mediate CXCR4-dependent infection of macrophages. J. Virol; 2005;79:13250–61. doi: 10.1128/JVI.79.21.13250-13261.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Isaacman-Beck J, Hermann EA, Yi Y, Ratcliffe SJ, Mulenga J, et al. Heterosexual transmission of human immunodeficiency virus type 1 subtype C: Macrophage tropism, alternative coreceptor use, and the molecular anatomy of CCR5 utilization. J Virol; 2009;83:8208–20. doi: 10.1128/JVI.00296-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuitemaker H, van ‘t Wout AB, Lusso P. Clinical significance of HIV-1 coreceptor usage. J Transl Med ;Suppl. 2009;1:S5. doi: 10.1186/1479-5876-9-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]