Abstract
Rabbit is a unique species to study human embryology; however, there are limited reports on the key transcription factors and epigenetic events of rabbit embryos. This study examined the Oct-4 and acetylated H4K5 (H4K5ac) patterns in rabbit embryos using immunochemistry staining. The average intensity of the Oct-4 signal in the nuclei of the whole embryo spiked upon fertilization, then decreased until the 8-cell stage and increased afterwards until the compact morula (CM) stage. It decreased thereafter from the CM stage to the early blastocyst (EB) stage, with a minimum at the expanded blastocyst (EXPB) stage and came back to a level similar to that of the CM-stage embryos in the hatching blastocysts (HB). The Oct-4 signal was observed in both the inner cell mass (ICM) and the trophectoderm (TE) cells of blastocysts. The average H4K5ac signal intensity of the whole embryo increased upon fertilization, started to decrease at the 4-cell stage, reached a minimum at the 8-cell stage, increased again at the EXPB stage and peaked at the HB stage. While TE cells maintained similar levels of H4K5ac throughout the blastocyst stages, ICM cells of HB showed higher levels of H4K5ac than those of EB and EXPB.
Keywords: H4K5 acetylation, inner cell mass, Oct-4, preimplantation, rabbit, trophectoderm
Introduction
A series of genetic and epigenetic events define early embryo development. Disturbances in these highly co-ordinated processes are believed to contribute to developmental failures and defects in mammals (Jaenisch and Bird, 2003, Corry et al., 2009, Palini et al., 2011). For example, cell lineage formation at the blastocyst stage is regulated by the POU-domain transcription factor Oct-4 (also known as Pou5f1) (Pesce and Scholer, 2001, Ovitt and Scholer, 1998). When Oct-4 was mutated, mouse embryos failed to establish an inner cell mass (ICM) and eventually died prematurely (Nichols et al., 1998, Boiani and Scholer, 2005). Therefore understanding key genetic and epigenetic events during early embryo development will help to identify the factors contributing to embryo losses and consequently improve embryo survival rates in human and other mammalian species (Mamo et al., 2008, Maher et al., 2003). It is reported that about 15–50% of mammalian embryos die during the preimplantation period (Warner et al., 1998, Mamo et al., 2008).
The majority of studies on early embryo development use mouse models; however, mouse embryos are not always representative of the earliest stages of mammalian development (Berg et al., 2011, Winston and Johnson, 1992). For example, the restrictive expression of Oct-4 in the ICM, but not in the trophectoderm (TE), appears to be unique in the mouse (Dietrich and Hiiragi, 2007, Palmieri et al., 1994, Yeom et al., 1996, Ovitt and Scholer, 1998, Pesce et al., 1998). In human, cattle, pig and rabbit embryos, Oct-4 expression was present in both ICM and TE cells even until the expanded blastocyst stage (Berg et al., 2011, Hansis et al., 2000, Kirchhof et al., 2000). It was suggested that the regulatory circuitry determining ICM/TE identity has been rewired in the mouse, to allow rapid TE differentiation and early blastocyst implantation (Berg et al., 2011). Alternative animal models are needed for better understanding of human embryology and stem cell biology.
The rabbit is a classic agricultural species and a useful model animal for biomedical research (Fan and Watanabe, 2003). Rabbits are genetically and physiologically closer to humans than mice. In comparison with larger animals, such as pigs and monkeys, rabbits can be housed indoor, have a short gestation and produce multiple-offspring litter. These advantages make rabbit a unique species for the study of human physiology. As a preferred laboratory species for many human disease studies such as atherosclerosis, it is also a pioneer species in the development of several embryo biotechnologies, such as IVF, transgenesis, animal cloning, embryo cryopreservation and embryonic stem cells (ESC) (Fan and Watanabe, 2003, Lin et al., 2011).
There are limited studies on key transcription factors and epigenetic programming events in preimplantation-stage rabbit embryos. Using quantitative real-time PCR, the gene for Oct-4, one of the few transcription factors studied in rabbit embryos, was found abundantly expressed in oocytes and zygotes, then gradually reduced until the activation of the embryonic genome and thereafter continuously increased until the blastocyst stage (Mamo et al., 2008, Kobolak et al., 2009). Oct-4 mRNA was found in both the ICM and the TE, a pattern similar to that of the human embryos. Studies on epigenetic events during early rabbit embryo development are mostly based on nuclear transfer experiments. Immunostaining results showed that the acetylation patterns of histones H3K14, H4K12 and H4K5 were different between fertilized and cloned embryos. When cloned embryos were treated with trichostatin A, a histone deacetylation inhibitor, they displayed an acetylation pattern of H3K14, H4K12 and H4K5 more similar to that of normally fertilized embryos than those not treated with trichostatin A, suggesting that increased levels of global acetylation in cloned embryos may improve genetic reprogramming and consequently embryo development competence in rabbits (Shi et al., 2008).
As far as is known, the distribution pattern of Oct-4 has not been thoroughly examined in preimplantation-stage rabbit embryos at the protein level. Previous studies have only examined at the mRNA expression level (Mamo et al., 2008, Kobolak et al., 2009). The acetylated H4K5 (H4K5ac) pattern in different cell lineages (i.e. ICM and TE cells) in rabbit blastocysts is not available yet. H4K5ac is a good indicator for the global activation of genes (Grunstein, 1997, Rundlett et al., 1998, Shi et al., 2008), as the acetylation of histone H4 occurs initially at lysine 16 (H4K16), then at K8 or K12, and ultimately at K5 (Corry et al., 2009).
The present study used the immunochemistry approach to examine the spatial and temporal profiles of Oct-4 and H4K5ac in rabbit embryos at different developmental stages from zygotes to hatching blastocysts. This study also compared the patterns of these two important biomarkers in ICM and TE cells in blastocyst-stage embryos.
Materials and methods
All chemicals were purchased from Sigma Chemical Co (St. Louis, MO, USA), unless otherwise indicated.
Animal maintenance and hormone administration
All animal maintenance, care and use procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the National Taiwan University and the University Committee on the Use and Care of Animals of the University of Michigan. Sexually mature (6–18 months old) New Zealand White female rabbits were maintained under a 12/12 light/dark cycle and superovulated with hormones using a routine regime (Du et al., 2009), consisting of two 0.3-mg, two 0.4-mg and two 0.6-mg injections of FSH (folltropin-V; Bioniche Animal Health Canada, Belleville, Ontario, Canada) at intervals of 12 h, followed by 200 IU of human chorionic gonadotrophin (HCG; Chorulon; Intervet, Millsboro, DE, USA). Superovulated rabbits were either mated with fertile males and served as embryo donors or not mated and served as oocyte donors.
Embryo collection and culture
Embryo collection and culture were performed as described previously (Lin et al., 2011). Briefly, Dulbecco's phosphate-buffered saline (DPBS; 15240-013; Gibco, Grand Island, NY, USA) containing 0.1% polyvinyl alcohol (P-8136) was used for flushing embryos from oviducts (PBS-PVP). Medium 199 with Earle's Salts, L-glutamine, 2.2 g/l sodium bicarbonate and 25 mmol/l HEPES (12340-014, Gibco) supplemented with 10% fetal bovine serum (FBS; SH0070.03; Hyclone, Logan, UT, USA) was used as the standard manipulation medium. Metaphase II (MII) oocytes were flushed at 12–14 h post HCG from oocyte donors. Zygotes were collected at 18– 20 post HCG from embryo donors and cultured in B2 medium (Laboratories CCD, Paris, France) supplemented with 2.5% FBS at 38.5°C in 5% CO2 and humidified air.
Immunostaining of rabbit embryos
Embryos at different stages – i.e. 2-cell, 4-cell, 8-cell, 16-cell, compact morulae (CM), early blastocyst (EB, diameter ≤135 μm), expanded blastocyst (EXPB, diameter >135 μm with intact zona pellucida) and hatching blastocyst (HB) – were fixed with fresh 4% paraformaldehyde in DPBS for 10 min and stored in DPBS at 4°C until ready for processing. After washing in DPBS for 10 min, permeabilization was achieved by treatment of 0.5% Triton-X 100 for 15–30 min and washing in 0.25% DPBS/Tween 20 (PBST) for 30 min at room temperature (20–25°C). Treatment of DPBS supplemented with 2% bovine serum albumin for 1 h at room temperature was used to block nonspecific binding sites. Immunostaining was performed by incubation with the first primary monoclonal antibody versus Oct-4 (1:150; MAB4401; Millipore, Billerica, MA, USA) overnight at 4°C and then washed in PBST three times for 15 min at room temperature. Incubation with the first secondary antibody Alexa Fluor 488 goat anti-mouse IgG (1:500; A11029; Invitrogen, Carlsbad, CA, USA) was performed for 1 h at 37°C. Then embryos were washed in PBST twice for 30 min at room temperature. Incubation of the second primary monoclonal antibody versus acetylated H4K5 (1:250; ab51997; Abcam, Cambridge, UK) was performed for 1.5 h at room temperature and then washed in PBST three times for 15 min at room temperature. Embryos were then incubated with the second secondary antibody Alexa Fluor 594 donkey anti-rabbit IgG (1:500; A21207; Invitrogen) for 1 h at 37°C. Embryos were washed by PBST for 30 min at room temperature. Finally, the embryos were stained with 4(,6-diamidino-2-phenylindole) (DAPI; 100 ng/ml; D9564) for 10 min and mounted on slides with 50% glycerol in DPBS.
Image processing and quantification
Image sections of individual embryos stained with DAPI, Oct-4 or H4K5ac were observed and captured by laser scanning confocal microscopy (Olympus IX71 with UltraVIEW confocal software; PerkinElmer, Covina, California, USA). Volocity (version 5.3.1; Improvision) and ImageJ (version 1.45b; National Institutes of Health) were applied for intensity analysis of these images.
For Oct-4 intensity of the whole embryo, image planes of each individual embryo were merged by Volocity without any modification of fluorescent intensity. Images were first converted to 8-bit greyscale and the background value was eliminated by the background subtract function. The nuclear intensities of integrated fluorescent images were measured by manually outlining all nuclei of embryos at the zygote stage to the 16-cell stage, randomly selected 20 nuclei at the CM stage and 30 nuclei at the blastocyst stages, as previously described (Shi et al., 2008).
For comparison of Oct-4 intensity between the ICM and TE regions in blastocyst-stage embryos (EB, EXPB and HB), a representative single plane across both the ICM and the TE regions of each embryo was selected. The test areas (i.e. the ICM and TE regions) were visually identified and all nuclei of the selected region were measured using ImageJ.
The same measurement procedures were applied for H4K5ac intensity analysis.
Statistical analysis
Statistical analyses were performed with Prism (version 5.0C; GraphPad Software, La Jolla, CA, USA). Differences of Oct-4 or H4K5ac intensity among different stages of in-vitro cultured embryos were subjected to one-way ANOVA. Paired t-test was used to compare the intensity difference between the ICM and TE regions in blastocyst-stage embryos. A P-value less than 0.05 was considered statistically significant.
Results
Oct-4 protein signals in in-vitro cultured rabbit embryos
After testing different Oct-4 antibodies, the purified monoclonal antibody MAB4401 was used. This human Oct-4 antibody cross-reacted with rabbit Oct-4 satisfactorily, as previously described (Zhang and Wang, 2010).
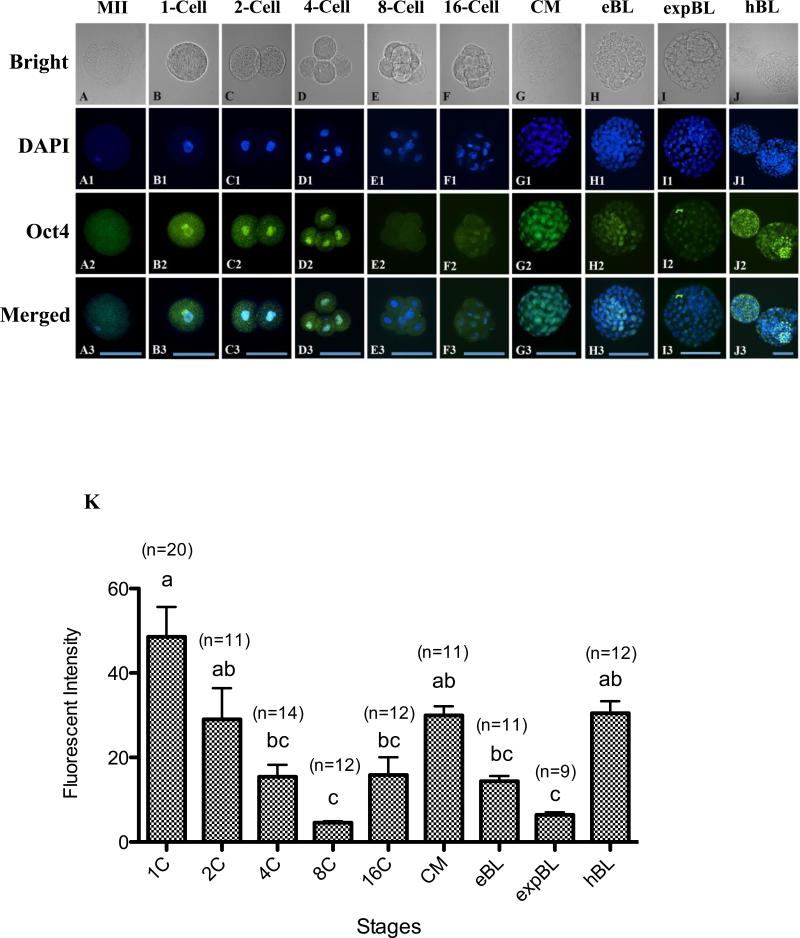
A diffuse signal of Oct-4 was observed in the cytoplasm but not on the chromosomes of MII oocytes (Figure 1A). A strong Oct-4 signal in the nuclei was observed at the 1-cell stage (Figure 1B,K), gradually decreased at the 2- and 4-cell stages and reached its lowest level at the 8-cell stage (P < 0.05; Figure 1C–E,K). Embryos at the 8-cell stage showed a very weak Oct-4 signal in the cytoplasm and diminished signal in the nuclei (Figure 1E). The signal started to increase at the 16-cell stage (Figure 1F) and became very intense in all nuclei at the CM stage (Figure 1G). In all blastocysts examined, at the EB, EXPB and HB stages, the Oct-4 signal was present in the nuclei of both ICM and TE cells (Figure 1H–J). Interestingly, the average nucleus intensity of the Oct-4 signal decreased again at the EB stage and reached another minimum at the EXPB stage (P < 0.05; Figure 1H,I,K). The embryos regained the Oct-4 signal in the nuclei at the HB stage to a level similar to the CM-stage embryos (Figure 1J,K).
Figure 1.
(A–J3) Oocytes and embryos at different stages doubly labelled for DNA (4(,6-diamidino-2-phenylindole); DAPI; blue) and Oct-4 (green). Oct-4 staining of metaphase-II oocytes (MII) showed visible but diffuse signals distributed throughout the cytoplasm (A–A3). Oct-4 signals were detected both in the cytoplasm and the nuclei of zygotes (B–B3) and 2-cell (C–C3) and 4-cell (D–D3) embryos. A very weak Oct-4 signal was found in the cytoplasm and diminished in the nuclei of 8-cell embryos (E-E3). Nuclear Oct-4 staining was evident again at the 16-cell stage (F–F3) and became more intense at the compact morula stage (CM; G–G3). A wave of Oct-4 signal intensity change was observed in blastocyst embryos (H-J3): the average intensity of Oct-4 signal decreased at the early blastocyst stage (EB; H–H3), reached a minimum at the expanded blastocyst stage (EXPB; I–I3) and spiked at the hatching blastocyst stage (HB; J–J3). Bars = 100 μm (A–J3). (K) Quantification of Oct-4 intensity at different stages of in-vitro cultured rabbit embryos. Values are mean ± SEM. Numbers of embryos used for each stage are indicated above each bar. Different letters (a–c) indicate a statistically significant difference (P < 0.05).
Oct-4 protein signals in the ICM and TE regions of blastocysts
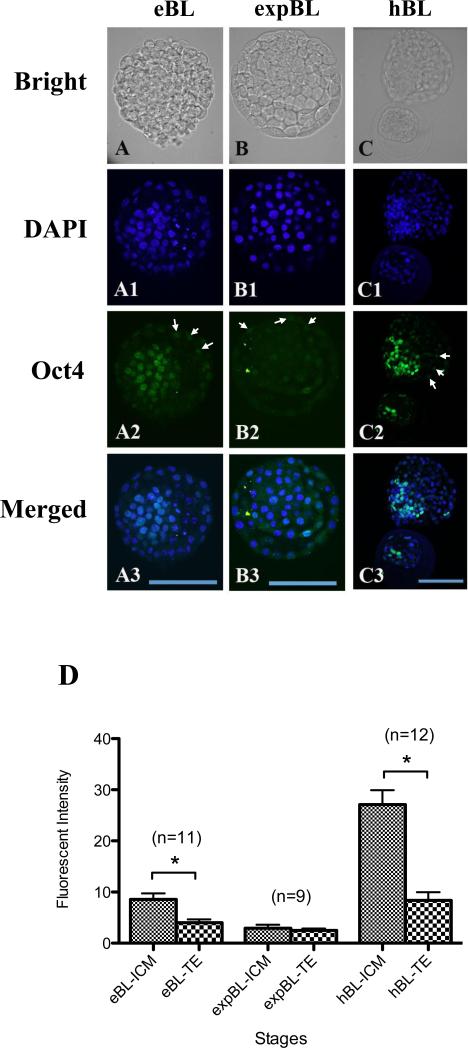
The Oct-4 intensity in the nuclei between two cell lineage types, ICM and TE cells, was compared at the EB, EXPB and HB stages. At the EB stage, clear and strong Oct-4 staining in the nuclei of ICM cells was observed, in contrast to diffuse but visible signals in the nuclei of TE cells (Figure 2A,D). At the EXPB stage, both ICM and TE nuclei displayed a weak Oct-4 signal. No intensity difference was found between these two cell types (Figure 2B,D). At the HB stage, the Oct-4 signal in the nuclei of ICM cells was much higher than those in TE cells of the same embryo and ICM cells of EB and EXPB embryos (Figure 2C,D; P < 0.05). A wave of Oct-4 signal intensity in the nuclei of ICM cells was observed during the EB (moderate), EXPB (lowest) to HB (highest) stages, while such signal intensity remained at similar levels in TE cells throughout these stages.
Figure 2.
(A–C3) Inner cell mass (ICM) and trophectoderm (TE) at the early blastocyst (EB), expanded blastocyst (EXPB) and hatching blastocyst (HB) stages doubly labelled for DNA (4(,6-diamidino-2-phenylindole); DAPI; blue) and Oct-4 (green). Oct-4 fluorescence associated with the ICM was strong in EB (A–A3), dramatically declined after expansion (B–B3), but then elevated to a higher level in HB (C–C3). The Oct-4 signal was detectable in TE cells throughout the blastocyst stages (arrows, A2–C2). Bars = 100 μm (A–C3). (D) Quantification of Oct-4 intensity in the nuclei of ICM and TE cells at different blastocyst stages. The Oct-4 signal in ICM cells at the HB stage was much higher than that in the EB and EXPB stages, whereas the signal intensity was similar in TE cells of all three stages. Oct-4 intensity of the ICM versus the TE regions was also analysed. Significantly higher intensity of the Oct-4 signal was found in ICM cells than in TE cells in both EB- and the HB-stage embryos. The Oct-4 signal was similarly low between these two groups of cells in EXPB-stage embryos. Values are mean ± SEM. Numbers of embryos used for each stage are indicated above each bar. Asterisks indicate statistically significant differences (P < 0.05).
Acetylated H4K5 distribution in rabbit in-vitro cultured embryos
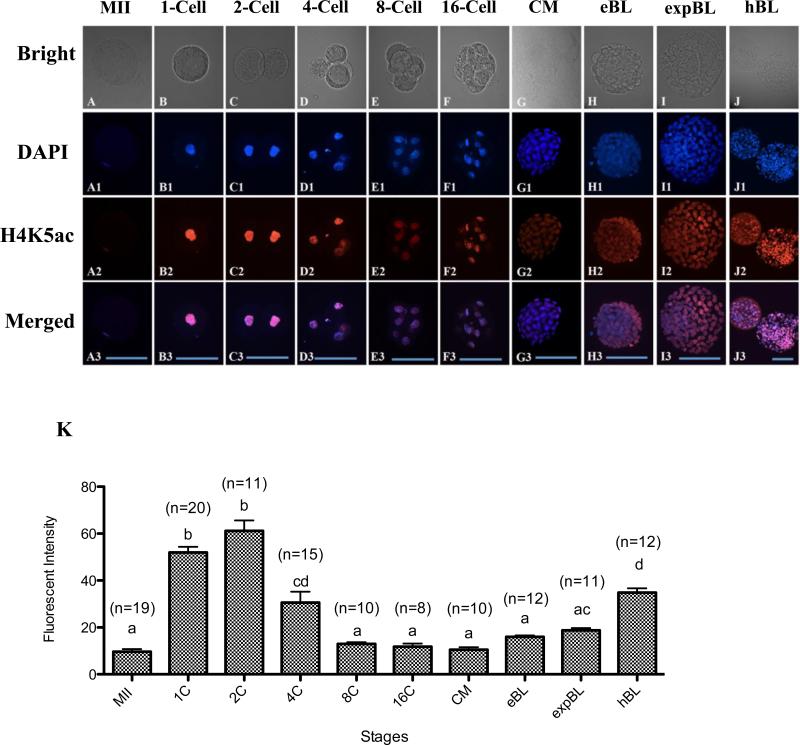
The terminal histone acetylation marker of histone 4, acetylated H4K5, was examined in rabbit oocytes and preimplantation-stage embryos. Immunocytochemistry with specific antibodies against acetylated H4K5 was negative in spermatozoa (data not shown) and weak in MII chromosomes (Figure 3A,K). Hyper-acetylated H4K5 was found in both parental pronuclei at the zygote stage (Figure 3B,K). The hyper-acetylated H4K5 status peaked at the 2-cell stage (Figure 3C,K), decreased at the 4-cell stage, reached a minimum at the 8-cell stage and remained low until the EB stage (Figure 3D–H,K). The average signal intensity of H4K5ac of the whole embryo increased again at the EXPB stage and reached the highest level at the HB stage (P < 0.05; Figure 3I–K).
Figure 3.
(A–J3) Oocytes and embryos at different stages doubly labelled for DNA (4(,6-diamidino-2-phenylindole); DAPI; blue) and acetylated H4K5 (H4K5ac; red). A visible but weak H4K5ac signal was detected on MII chromosomes (A–A3). Strong signals associated with nuclei were observed in zygotes (B–B3) and 2-cell embryos (C–C3). Declined intensity of H4K5ac was shown in 4-cell embryos (D–D3). Low fluorescence with various staining intensity on nuclei was detected at the 8-cell (E–E3), 16-cell (F–F3), compact morula (CM; G–G3) and early blastocyst (EB; H–H3) stages. The average H4K5ac signal of the whole embryo started to increase at the expanded blastocyst stage (EXPB; G–G3) and peaked at the hatching blastocyst stage (HB; J–J3). Bars = 100 μm (A–J3). (K) Quantification of H4K5ac intensity at different in-vitro cultured stages. Values are mean ± SEM. Numbers of embryos used for each stage are indicated above each bar. Different letters (a–d) indicate a statistically significant difference (P < 0.05).
Acetylated H4K5 distribution in the ICM and TE regions of blastocysts
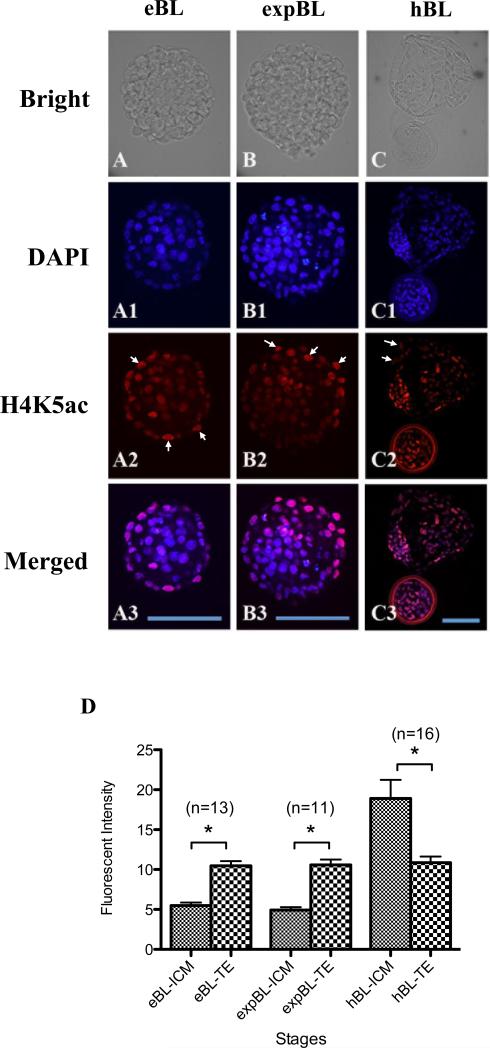
The H4K5ac signals of ICM and TE cells were compared at the EB, EXPB and HB stages. In EB- and EXPB-stage embryos, the H4K5ac signal was significantly higher in the nuclei of TE cells than in ICM cells (P < 0.05; Figure 4A,B,D). In contrast, the nuclear H4K5ac signal was stronger in the ICM than in the TE in embryos at the HB stage (P < 0.05; Figure 4C,D). The H4K5ac signal in the nuclei of ICM cells at the HB stage was higher than in ICM cells of EB- and EXPB-stage embryos, whereas the signal in TE cells was almost unchanged throughout these three blastocyst stages.
Figure 4.
(A–C3) Inner cell mass (ICM) and trophectoderm (TE) at the early blastocyst (EB), expanded blastocyst (EXPB) and hatching blastocyst (HB) stages doubly labelled for DNA (4(,6-diamidino-2-phenylindole); DAPI; blue) and acetylated H4K5 (H4K5ac; red). Similar levels of H4K5ac signals were observed in ICM cells of EB (A–A3) and EXPB (B–B3). The H4K5ac signal in ICM cells increased at the HB stage (C–C3). On the other hand, the H4K5ac signal in TE cells remained similar throughout these three stages (arrows, A2–C2). Bars = 100 μm (A– C3). (D) Quantification of H4K5ac intensity in the nuclei of ICM and TE cells at different blastocyst stages. The H4K5ac signal was higher in TE cells than in ICM cells at the EB and EXPB stages. The trend reversed at the HB stage, where the H4K5ac signal was higher in ICM cells than in TE cells. Values are mean ± SEM. The number of embryos used for each stage is indicated above each bar. Asterisks indicate statistically significant differences (P < 0.05).
Discussion
The present work studied the spatial and temporal distribution of the Oct-4 protein at different stages throughout early embryo development in rabbits. Previously, the expression pattern of Oct-4 in rabbit embryos was examined using reverse-transcription PCR (Mamo et al., 2008). It was found that the mRNA levels of Oct-4 gradually decreased from the zygote stage until zygotic genomic activation (8- to 16-cell stage for rabbits), then increased and reached the highest level at the blastocyst stage. The present results using the immunostaining approach revealed a similar pattern where the Oct-4 signal was present in the zygote stage, decreased gradually and reached its lowest level at the 8-cell stage and increased again at the 16-cell stage.
However, several of the present main findings, such as the second wave of Oct-4 signal change from the EB to the HB, were not observed by Mamo et al. (2008). Notably, while this study reports low Oct-4 protein signals in the ICM cells of EXPB-stage embryos, Mamo et al. (2008) reported high Oct-4 mRNA levels in pooled blastocysts. This is most likely because the present study collected blastocysts at different stages (EB, EXPB and HB) and performed comparisons between ICM and TE cells, whereas Mamo et al. (2008) collected all blastocysts at one time point (103 h post HCG) and did not make the comparison between ICM and TE cells. As a result, this study is able to report the Oct-4 profiles at a higher spatial and temporal resolution (i.e. in a specific cell lineage at a specific blastocyst stage), while Mamo et al. (2008) could only report at the whole embryo level for pooled blastocysts. However, the present study cannot exclude the possibility that Oct-4 expression in rabbit blastocysts is regulated at the post-transcriptional level (e.g. translation of mRNA is influenced by small RNA) (Ambros, 2001). If this is the case, rabbit embryos at the EXPB stage would show high mRNA expression (as suggested by Mamo et al., 2008) and low protein (as reported in the present study) at the same time. Further experiments are necessary to elucidate if such regulation exists or not.
The use of different rabbit strains and culture media may also contribute to the different observations in the present study and Mamo et al. (2008). It has been shown that gene expression patterns in preimplantation-stage embryos differ in different mouse strains (Rambhatla and Latham, 1995), and that embryo culture conditions could affect gene expression patterns in mammalian embryos (Rinaudo and Schultz, 2004).
It is important to point out that this study confirmed that the Oct-4 signal was present in both ICM and TE cells in blastocyst-stage rabbit embryos. This is different from the Oct-4 expression pattern in mouse embryos, predominately in ICM cells, but not in TE cells (Dietrich and Hiiragi, 2007, Palmieri et al., 1994, Yeom et al., 1996, Ovitt and Scholer, 1998, Pesce et al., 1998). Human embryos as well as cattle and pig embryos also express Oct-4 in both ICM and TE cells (Berg et al., 2011, Hansis et al., 2000, Kirchhof et al., 2000). The fact that Oct-4 is considered one of the most important pluripotent genes and that mouse embryos and human embryos differ in their patterns of Oct-4 expression indicates that the mouse is not always a good model for the human, particularly in the context of embryo development, cell lineage formation and ESC biology. In fact, it is speculated that the regulatory mechanisms determining ICM/TE identity in the mouse is different from most if not all other species, to allow rapid TE differentiation and early blastocyst implantation (Berg et al., 2011). Such differences may have contributed to the relatively high success rates in authentic ESC derivation in mice and the general lack of success with other species, such as cattle, pigs and rabbits (Wang et al., 2005, Fang et al., 2006, Keefer et al., 2007). The present findings on Oct-4 patterns, along with the findings by several other groups (Berg et al., 2011, Kobolak et al., 2009, Mamo et al., 2008) support the argument that the rabbit could serve as a better model than the mouse for human embryology and stem cell studies.
Interestingly, regarding EB-stage embryos, the ratio of the Oct-4 signal between ICM and TE cells of different species seems to be associated with the evolutionary distance from human. In mouse EB-stage embryos, Oct-4 expression is restricted to the ICM and is very low in the TE (Ovitt and Scholer, 1998). In rabbit EB-stage embryos, the Oct-4 signal is high in the ICM but low in the TE (present study). In bovine EB-stage embryos, Oct-4 expression is high in the ICM and moderate in the TE (Berg et al., 2011). In monkey and human EB-stage embryos, Oct-4 signal is high in both ICM and TE cells (Cauffman et al., 2005, Mitalipov et al., 2003). Such correlations have not observed for later stages (e.g. EXPB and HB).
From the immunostaining results, two waves of Oct-4 signal change during early embryo development in rabbits were identified. The first wave reached a minimum at the 8-cell stage. Nuclear Oct-4 staining in rabbit embryos became evident again at the 16-cell stage and a strong signal associated with each nucleus was observed at the CM stage. This coincides with the timing of zygotic genomic activation in rabbits, suggesting that the embryonic expression of Oct-4 is following the overall pattern of genomic activation. Different from rabbits, zygotic genomic activation in mouse embryos is seen at the first cell cycle (2-cell stage), whereas the zygotic Oct-4 expression is detected at the 8-cell stage (Palmieri et al., 1994, Kurosaka et al., 2004).
The second wave of Oct-4 signal change occurred in the ICM cells, where it bottomed at the EXPB stage and spiked at the HB stage. This finding was unexpected. In mouse studies, Oct-4 signal intensity in ICM cells was strong from EB to HB stages (Nicholset al., 1998, Chang et al., 2005, Ovitt and Scholer, 1998). In early studies examining the Oct-4 mRNA levels in rabbit embryos, the total mRNA of the whole embryos was found at similar levels in unhatched and hatched blastocysts (Kobolak et al., 2009); however, comparison of the Oct-4 mRNA levels between ICM and TE cells was not conducted at different blastocyst stages. As far as is known, the present work is the first report on Oct-4 protein levels between ICM and TE cells at different blastocyst stages. A quick loss of the Oct-4 signal in the ICM at the EXPB stage and the quick re-emergence of the signal at the HB stage may reflect critical regulating events on pluripotent cell lineage formation. It is speculated that this may be associated with the switch of the distal enhancer (DE) of Oct-4 to proximal enhancer (PE). The regulatory region of Oct-4 is highly conserved among species and usually contains four conserved regions (CR1–4) within the promoter (Yeom et al., 1996, Ovitt and Scholer, 1998, Nordhoff et al., 2001, Chew et al., 2005). Reporter gene expression experiments in mouse (Yeom et al., 1996, Bao et al., 2009) identified two elements in the promoter that regulate Oct-4 expression at distinct developmental stages, including the PE within CR2 and CR3 and the DE within CR4. DE is responsible for driving Oct-4 expression in ICM and primordial germ cells as well as in in-vitro derivatives (e.g. ESC and embryonic germ cells). By contrast, PE is active and directs Oct-4 expression in epiblast cells and their in-vitro counterparts, epiblast stem cells. Thus, the regulatory machinery to express Oct-4 is a useful tool to distinguish the pluripotent cell property and its origins (Bao et al., 2009). In rabbits, the gene structure and the sequence of Oct-4 were demonstrated to be highly conserved compared with different species (Yeom et al., 1996, Kobolak et al., 2009). Expression analysis of rabbit Oct-4 promoter regions in mouse ESC demonstrated that the function of the rabbit CR4 regulator domain (containing DE) was indistinguishable to that of the mouse counterpart (Kobolak et al., 2009). On the basis of the present and previous observations, it is speculated that the unique Oct-4 reactivation at the HB stage may reflect the enhancer switching from DE to PE and emergence of epiblast precursor cells. Further, it is postulated that the EB-stage embryos may serve better for authentic ESC derivation in rabbits, as the DE driving Oct-4 expression is associated with the ICM and the ESC (Bao et al., 2009).
Histone acetylation is one of the major epigenetic regulatory mechanisms during preimplantation embryo development (Corry et al., 2009). A single report on H4K5ac in rabbit embryos (Shi et al., 2008) reported different acetylation profiles of H3K14, H4K12 and H4K5 between fertilized and cloned embryos in rabbits. Relevant to the present study, Shi et al. (2008) documented that in fertilized rabbit embryos the H4K5ac signal was high at the pronuclear stage, dramatically decreased at the 2-cell stage, resurged at the 4-cell stage and then gradually decreased at the 8-cell, CM and blastocyst stage. In comparison, the present work included five additional stages (MII oocyte, 16-cell, EB, EXPB and HB) and included comparisons between ICM and TE cells in blastocysts. Similar to the findings of Shi et al. (2008), the present study observed that the signal intensity of H4K5ac was low in MII oocytes and became higher after fertilization. This suggests that the fertilized embryos have a more open chromatin structure, consistent with the need for epigenetic reprogramming of male and female pronuclei. Additionally, both the present study and Shi et al. (2008) report a decrease of the H4K5ac signal at the 4-cell stage. However, the present study did not observe a sudden decrease of H4K5ac signal between the 1- and 2-cell stages; instead it was maintained at a similar level. Consequently, instead of a resurgence at the 4-cell stage, as reported by Shi et al. (2008), there was a decrease of H4K5ac signal from the 2-cell to the 4-cell stage, which is similar to a previous report on H4K5ac in cleaved mouse embryos (Ma and Schultz, 2008). Several factors, including biological materials, external environment and experiment protocols, may have contributed to the discrepancy between the present study and Shi et al. (2008). For example, the present study used a monoclonal antibodies to detect H4K5ac, B2 medium for embryo culture and New Zealand White rabbits whereas Shi et al. (2008) used polyclonal antibodies, medium 199 and Japanese big-eared white rabbits.
This study examined, as far as is known for the first time, the H4K5ac in different regions of the embryos (i.e. the ICM versus the TE) at the EB, EXPB and HB stages.
The H4K5ac signal was present in both types of cells in all stages yet with different relative levels. While the H4K5ac signal in TE cells was at a similar level among all three blastocyst stages examined, the signal in ICM cells was higher at the HB stage than at the EB and EXPB stages. As a result, the H4K5ac signal of TE cells was higher than that of ICM cells at the EB and EXPB stages; however at the HB stage, it was lower in TE cells than in ICM cells.
In cloned rabbit and bovine embryos (Shiratsuki et al., 2011, Shi et al., 2008), as well as in mouse ESC (Hattori et al., 2007), improved histone acetylation by trichostatin A treatment is correlated with increased Oct-4 expression, implying an interplay between the histone acetylation and Oct-4 expression. Examining the dynamics of histone acetylation including H4K5ac, particularly in the context of Oct-4, will improve the understanding of these highly co-ordinated genetic and epigenetic events during early embryo development. Using chromatin immunoprecipitation on mouse embryos, it was revealed that the acetylation level of H4K16 at the promoter region of Oct-4 was highly correlated with the Oct-4 concentration in ICM cells (O'Neill et al., 2006). The present study did not observe such association of the acetylation level of H4K5ac and concentration of Oct-4. This is likely because the global H4K5ac patterns were examined, but not that at the promoter region of Oct-4. It may also be the result of a difference between rabbits and mice. Further work is needed to examine the interplay of these two important processes.
It is noted that different cell lineages displayed different signature profiles of Oct-4 and H4K5ac (Table 1). Unlike mouse embryos where the Oct-4 signal is consistently present in the ICM but not in the TE, rabbit embryos showed the Oct-4 signal in both cell types. At the EXPB stage, both ICM and TE showed a low Oct-4 signal, indicating that Oct-4 may not be used as a unique biomarker for cell lineage at this stage. On the other hand, the relative ratio of the H4K5ac levels between the ICM and TE cell types reversed the trend at the HB stage, indicating that H4K5ac alone is not a good biomarker for cell lineage identification either. This study also compared the Oct-4 and H4K5ac signal intensities between the outside and inside cells of embryos at the CM stage and found no difference (Table 1). These findings suggest that a combination of Oct-4, H4K5ac and possibly other biomarkers such as Cdx-2 is needed to accurately identify different lineages of cells in morula- and blastocyst-stage rabbit embryos.
Table 1.
Oct-4 and acetylated H4K5 (H4K5ac) profiles in different cell lineages at different embryo stages.
| CM | EB | EXPB | HB | |||||
|---|---|---|---|---|---|---|---|---|
| Inside | Outside | ICM | TE | ICM | TE | ICM | TE | |
| Oct-4 | Similar | Similar | High | Low | Low | Low | High | Low |
| H4K5ac | Similar | Similar | Low | High | Low | High | High | Low |
CM = compact morulae; EB = early blastocyst; EXPB = expanded blastocyst; HB = hatching blastocyst; ICM = inner cell mass; TE = trophectoderm.
As far as is known, the present work represents a first effort in understanding the genetic and epigenetic events during early embryo development in rabbits. Embryos were flushed at the zygote stage and cultured in vitro until used for analysis. Therefore, some of observations may be artefacts of the in-vitro embryo culture system. Studies have revealed that in-vitro cultured embryos may differ from in-vivo-derived embryos in many ways, such as gene expression profiles (Mamo et al., 2007, Niemann and Wrenzycki, 2000), embryo morphology, cell number, embryo size and development speed (Hyttel and Niemann, 1990, Machaty et al., 1998). A separate project is examining Oct-4 and H4K5ac, as well as several other transcription factors and epigenetic mechanisms using in-vivo-derived embryos. Also, the current work utilized only a single method (immunostaining) and further work, such as Western blotting and reverse-transcription PCR, is needed to confirm and better quantify the present findings.
In conclusion, this study reports the temporal and spatial distribution of Oct-4 and acetylated H4K5 during rabbit embryo development. Future work proposes to use a combination of biomarkers (Oct-4, H4K5ac and others) to accurately identify different cell lineages in rabbit embryos. This study revealed a novel wave of Oct-4 intensity change in ICM cells from the early blastocyst to the hatching blastocyst. It is postulated that such wave may have reflected the regulation of Oct-4 through enhancer switching.
SUMMARY.
Understanding key genetic and epigenetic events during early embryo development will help to identify factors contributing to embryo losses and consequently improve embryo survival rates. As a preferred laboratory species for many human disease studies such as atherosclerosis, rabbit is also a pioneer species in the development of several embryo biotechnologies, such as IVF, transgenesis, animal cloning, embryo cryopreservation and embryonic stem cells. However, there are limited reports on key transcription factors and epigenetic events of rabbit embryos. In the present study, we documented the temporal and spatial distribution of Oct-4 protein and H4K5 acetylation during early embryo development using the immunostaining approach. We also compared the patterns of these two important biomarkers between the inner cell mass (ICM) and the trophectoderm (TE) cells in blastocyst-stage embryos. Our findings suggest that a combination of Oct-4, H4K5ac and possibly other biomarkers such as Cdx-2 is needed to accurately identify different lineages of cells in morula and blastocyst stage rabbit embryos. Importantly, we revealed a novel wave of Oct-4 intensity change in the ICM cells of rabbit blastocysts. The signal was high at the early blastocyst stage, reached a minimum at the expanded blastocyst stage and returned to a high level at the hatching blastocyst stage. We hypothesize that the signal may have reflected the regulation of Oct-4 through enhancer switching and therefore may be related to cell lineage formation in rabbit embryos. These findings enrich our understanding on key genetic and epigenetic programming events during early embryo development in rabbits.
Acknowledgements
This study was supported by the National Institutes of Health, USA (grant number 5R44RR023774 to JX) and the National Science Council, Taiwan, ROC (grant number 97–2313-B-002–006-MY3 and 100–2313-B-002–045-MY3) to L-YS.
Biography
 Chien-Hong Chen was awarded a BA in Animal Science in 2000 and a MSc in embryology in 2003, both from the National Chung–Hsing University, Taichung, Taiwan. From 2004 to 2008, he worked as an assistant research fellow at the Animal Technology Institute in Taiwan. Since 2010, he has been is a graduate student at the National Taiwan University, Taipei, Taiwan. He is a member of the Taiwan Society for Stem Cell Research.
Chien-Hong Chen was awarded a BA in Animal Science in 2000 and a MSc in embryology in 2003, both from the National Chung–Hsing University, Taichung, Taiwan. From 2004 to 2008, he worked as an assistant research fellow at the Animal Technology Institute in Taiwan. Since 2010, he has been is a graduate student at the National Taiwan University, Taipei, Taiwan. He is a member of the Taiwan Society for Stem Cell Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration: The authors report no financial or commercial conflicts of interest.
References
- Ambros V. microRNAs: Tiny regulators with great potential. Cell. 2001;107:823–26. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- Bao S, Tang F, Li X, Hayashi K, Gillich A, Lao K, Surani MA. Epigeneticf reversion of post-implantation epiblast to pluripotent embryonic stem cells. Nature. 2009;461:1292–5. doi: 10.1038/nature08534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg DK, Smith CS, Pearton DJ, Wells DN, Broadhurst R, Donnison M, Pfeffer PL. Trophectoderm lineage determination in cattle. Dev Cell. 2011;20:244–55. doi: 10.1016/j.devcel.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Boiani M, Scholer HR. Regulatory networks in embryo-derived pluripotent stem cells. Nature reviews. Molecular cell biology. 2005;6:872–84. doi: 10.1038/nrm1744. [DOI] [PubMed] [Google Scholar]
- Cauffman G, van de Velde H, Liebaers I, van Steirteghem A. Oct-4 mRNA and protein expression during human preimplantation development. Molecular human reproduction. 2005;11:173–81. doi: 10.1093/molehr/gah155. [DOI] [PubMed] [Google Scholar]
- Chang HC, Liu H, Zhang J, Grifo J, Krey LC. Developmental incompetency of denuded mouse oocytes undergoing maturation in vitro is ooplasmic in nature and is associated with aberrant Oct-4 expression. Human reproduction. 2005;20:1958–68. doi: 10.1093/humrep/dei003. [DOI] [PubMed] [Google Scholar]
- Chew JL, Loh YH, Zhang W, Chen X, Tam WL, Yeap LS, Li P, Ang YS, Lim B, Robson P, Ng HH. Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Molecular and cellular biology. 2005;25:6031–46. doi: 10.1128/MCB.25.14.6031-6046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corry GN, Tanasijevic B, Barry ER, Krueger W, Rasmussen TP. Epigenetic regulatory mechanisms during preimplantation development. Birth defects research. Part C, Embryo today: reviews. 2009;87:297–313. doi: 10.1002/bdrc.20165. [DOI] [PubMed] [Google Scholar]
- Dietrich JE, Hiiragi T. Stochastic patterning in the mouse pre-implantation embryo. Development. 2007;134:4219–31. doi: 10.1242/dev.003798. [DOI] [PubMed] [Google Scholar]
- Du F, Xu J, Zhang J, Gao S, Carter MG, He C, Sung LY, Chaubal S, Fissore RA, Tian XC, Yang X, Chen YE. Beneficial effect of young oocytes for rabbit somatic cell nuclear transfer. Cloning Stem Cells. 2009;11:131–40. doi: 10.1089/clo.2008.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Watanabe T. Transgenic rabbits as therapeutic protein bioreactors and human disease models. Pharmacol Ther. 2003;99:261–82. doi: 10.1016/s0163-7258(03)00069-x. [DOI] [PubMed] [Google Scholar]
- Fang ZF, Gai H, Huang YZ, Li SG, Chen XJ, Shi JJ, Wu L, Liu A, Xu P, Sheng HZ. Rabbit embryonic stem cell lines derived from fertilized, parthenogenetic or somatic cell nuclear transfer embryos. Experimental cell research. 2006;312:3669–82. doi: 10.1016/j.yexcr.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–52. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- Hansis C, Grifo JA, Krey LC. Oct-4 expression in inner cell mass and trophectoderm of human blastocysts. Molecular human reproduction. 2000;6:999–1004. doi: 10.1093/molehr/6.11.999. [DOI] [PubMed] [Google Scholar]
- Hattori N, Imao Y, Nishino K, Ohgane J, Yagi S, Tanaka S, Shiota K. Epigenetic regulation of Nanog gene in embryonic stem and trophoblast stem cells. Genes to cells: devoted to molecular and cellular mechanisms. 2007;12:387–96. doi: 10.1111/j.1365-2443.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- Hyttel P, Niemann H. Ultrastructure of porcine embryos following development in vitro versus in vivo. Mol Reprod Dev. 1990;27:136–44. doi: 10.1002/mrd.1080270208. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–54. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Keefer CL, Pant D, Blomberg L, Talbot NC. Challenges and prospects for the establishment of embryonic stem cell lines of domesticated ungulates. Anim Reprod Sci. 2007;98:147–68. doi: 10.1016/j.anireprosci.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Kirchhof N, Carnwath JW, Lemme E, Anastassiadis K, Scholer H, Niemann H. Expression pattern of Oct-4 in preimplantation embryos of different species. Biol Reprod. 2000;63:1698–705. doi: 10.1095/biolreprod63.6.1698. [DOI] [PubMed] [Google Scholar]
- Kobolak J, Kiss K, Polgar Z, Mamo S, Rogel-Gaillard C, Tancos Z, Bock I, Baji AG, Tar K, Pirity MK, Dinnyes A. Promoter analysis of the rabbit POU5F1 gene and its expression in preimplantation stage embryos. BMC molecular biology. 2009;10:88. doi: 10.1186/1471-2199-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaka S, Eckardt S, Mclaughlin KJ. Pluripotent lineage definition in bovine embryos by Oct4 transcript localization. Biol Reprod. 2004;71:1578–82. doi: 10.1095/biolreprod.104.029322. [DOI] [PubMed] [Google Scholar]
- Lin TA, Chen CH, Sung LY, Carter MG, Chen YE, Du F, Ju JC, Xu J. Open-pulled straw vitrification differentiates cryotolerance of in vitro cultured rabbit embryos at the eight-cell stage. Theriogenology. 2011;75:760–8. doi: 10.1016/j.theriogenology.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Ma P, Schultz RM. Histone deacetylase 1 (HDAC1) regulates histone acetylation, development, and gene expression in preimplantation mouse embryos. Dev Biol. 2008;319:110–20. doi: 10.1016/j.ydbio.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machaty Z, Day BN, Prather RS. Development of early porcine embryos in vitro and in vivo. Biol Reprod. 1998;59:451–5. doi: 10.1095/biolreprod59.2.451. [DOI] [PubMed] [Google Scholar]
- Maher ER, Afnan M, Barratt CL. Epigenetic risks related to assisted reproductive technologies: epigenetics, imprinting, ART and icebergs? Human reproduction. 2003;18:2508–11. doi: 10.1093/humrep/deg486. [DOI] [PubMed] [Google Scholar]
- Mamo S, Gal AB, Bodo S, Dinnyes A. Quantitative evaluation and selection of reference genes in mouse oocytes and embryos cultured in vivo and in vitro. BMC developmental biology. 2007;7:14. doi: 10.1186/1471-213X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamo S, Gal AB, Polgar Z, Dinnyes A. Expression profiles of the pluripotency marker gene POU5F1 and validation of reference genes in rabbit oocytes and preimplantation stage embryos. BMC molecular biology. 2008;9:67. doi: 10.1186/1471-2199-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitalipov SM, Kuo HC, Hennebold JD, Wolf DP. Oct-4 expression in pluripotent cells of the rhesus monkey. Biol Reprod. 2003;69:1785–92. doi: 10.1095/biolreprod.103.019455. [DOI] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–91. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Niemann H, Wrenzycki C. Alterations of expression of developmentally important genes in preimplantation bovine embryos by in vitro culture conditions: implications for subsequent development. Theriogenology. 2000;53:21–34. doi: 10.1016/s0093-691x(99)00237-x. [DOI] [PubMed] [Google Scholar]
- Nordhoff V, Hubner K, Bauer A, Orlova I, Malapetsa A, Scholer HR. Comparative analysis of human, bovine, and murine Oct-4 upstream promoter sequences. Mammalian genome: official journal of the International Mammalian Genome Society. 2001;12:309–17. doi: 10.1007/s003350010279. [DOI] [PubMed] [Google Scholar]
- O'neill LP, Vermilyea MD, Turner BM. Epigenetic characterization of the early embryo with a chromatin immunoprecipitation protocol applicable to small cell populations. Nat Genet. 2006;38:835–41. doi: 10.1038/ng1820. [DOI] [PubMed] [Google Scholar]
- Ovitt CE, Scholer HR. The molecular biology of Oct-4 in the early mouse embryo. Molecular human reproduction. 1998;4:1021–31. doi: 10.1093/molehr/4.11.1021. [DOI] [PubMed] [Google Scholar]
- Palini S, de Stefani S, Scala V, Dusi L, Bulletti C. Epigenetic regulatory mechanisms during preimplantation embryo development. Annals of the New York Academy of Sciences. 2011;1221:54–60. doi: 10.1111/j.1749-6632.2010.05937.x. [DOI] [PubMed] [Google Scholar]
- Palmieri SL, Peter W, Hess H, Scholer HR. Oct-4 transcription factor is differentially expressed in the mouse embryo during establishment of the first two extraembryonic cell lineages involved in implantation. Dev Biol. 1994;166:259–67. doi: 10.1006/dbio.1994.1312. [DOI] [PubMed] [Google Scholar]
- Pesce M, Scholer HR. Oct-4: gatekeeper in the beginnings of mammalian development. Stem Cells. 2001;19:271–8. doi: 10.1634/stemcells.19-4-271. [DOI] [PubMed] [Google Scholar]
- Pesce M, Wang X, Wolgemuth DJ, Scholer H. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mechanisms of development. 1998;71:89–98. doi: 10.1016/s0925-4773(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Rambhatla L, Latham KE. Strain-specific progression of alpha-amanitin-treated mouse embryos beyond the two-cell stage. Mol Reprod Dev. 1995;41:16–9. doi: 10.1002/mrd.1080410104. [DOI] [PubMed] [Google Scholar]
- Rinaudo P, Schultz RM. Effects of embryo culture on global pattern of gene expression in preimplantation mouse embryos. Reproduction. 2004;128:301–11. doi: 10.1530/rep.1.00297. [DOI] [PubMed] [Google Scholar]
- Rundlett SE, Carmen AA, Suka N, Turner BM, Grunstein M. Transcriptional repression by UME6 involves deacetylation of lysine 5 of histone H4 by RPD3. Nature. 1998;392:831–5. doi: 10.1038/33952. [DOI] [PubMed] [Google Scholar]
- Shi LH, Ai JS, Ouyang YC, Huang JC, Lei ZL, Wang Q, Yin S, Han ZM, Sun QY, Chen DY. Trichostatin A and nuclear reprogramming of cloned rabbit embryos. Journal of animal science. 2008;86:1106–13. doi: 10.2527/jas.2007-0718. [DOI] [PubMed] [Google Scholar]
- Shiratsuki S, Iwata H, Kimura K, Kuge T, Monji Y, Kuwayama T. Trichostatin A-treated eight-cell bovine embryos had increased histone acetylation and gene expression, with increased cell numbers at the blastocyst stage. Theriogenology. 2011;75:841–8. doi: 10.1016/j.theriogenology.2010.10.026. [DOI] [PubMed] [Google Scholar]
- Wang L, Duan E, Sung LY, Jeong BS, Yang X, Tian XC. Generation and characterization of pluripotent stem cells from cloned bovine embryos. Biol Reprod. 2005;73:149–55. doi: 10.1095/biolreprod.104.037150. [DOI] [PubMed] [Google Scholar]
- Warner CM, Exley GE, Mcelhinny AS, Tang C. Genetic regulation of preimplantation mouse embryo survival. The Journal of experimental zoology. 1998;282:272–9. [PubMed] [Google Scholar]
- Winston NJ, Johnson MH. Can the mouse embryo provide a good model for the study of abnormal cellular development seen in human embryos? Human reproduction. 1992;7:1291–6. doi: 10.1093/oxfordjournals.humrep.a137844. [DOI] [PubMed] [Google Scholar]
- Yeom YI, Fuhrmann G, Ovitt CE, Brehm A, Ohbo K, Gross M, Hubner K, Scholer HR. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development. 1996;122:881–94. doi: 10.1242/dev.122.3.881. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang JH. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC musculoskeletal disorders. 2010;11:10. doi: 10.1186/1471-2474-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]