Abstract
Metastasis requires tumor cell dissemination to different organs from the primary tumor. Dissemination is a complex cell motility phenomenon that requires the molecular coordination of the protrusion, chemotaxis, invasion and contractility activities of tumor cells to achieve directed cell migration. Recent studies of the spatial and temporal activities of the small GTPases have begun to elucidate how this coordination is achieved. The direct visualization of the pathways involved in actin polymerization, invasion and directed migration in dissemination competent tumor cells will help identify the molecular basis of dissemination and allow the design and testing of more specific and selective drugs to block metastasis.
Keywords: metastasis, motility, invadopodia, actin-binding proteins, Rho GTPases
Introduction to cancer metastasis
Metastasis is a multistep process where tumor cells disseminate from the primary tumor and colonize distant organs [1]. Metastasis accounts for more than 90% of all cancer related deaths [2, 3]. The steps include tumor cell invasion of basement membranes and the surrounding tissue, intravasation into blood vessels, survival there, extravasation and/or growth at different organ sites. To achieve these steps, precise coordination of cell movement and matrix remodeling are essential [4]. At any given time, only a small proportion of tumor cells are invading and disseminating [5, 6]. Understanding the mechanisms that drive motility and invasion of these tumor cells is crucial to better understand metastasis.
Escaping the tumor: Protrusions with matrix degradation activity
EMT (Epithelial-meshenchymal transition) is believed to be an important early step in the conversion of tumor cells into a migratory population capable of systemic metastasis [7, 8]. To become migratory, the first barrier that tumor cells must overcome is their epithelial basement membrane (Figure 1). In order to cross basement membranes, tumor cells are postulated to form invadopodia. There is growing evidence that the formation of invadopodia is part of the EMT process [9•, 10]. It is unknown the degree to which carcinoma cells must proteolytically digest the epithelial basement membrane as they initially invade and whether stromal cells are also involved in this process. However, there is good evidence for the involvement of invadopodium-mediated proteolysis of vascular basement membranes during intravasation and metastasis [11].
Figure 1. Invasion, migration and intravasation in tumors.
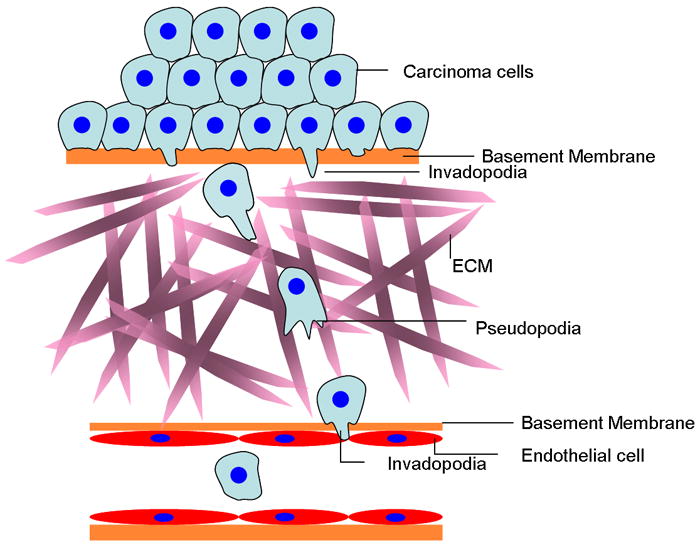
Tumor cells undergoing EMT form invadopodia and acquire a migratory phenotype. Degradation of basement membranes and extracellular matrix during migration is achieved by invadopodia. Cells migrate on the extracellular matrix (ECM) forming pseudopodia. There is evidence that invadopodia are involved in directional migration and chemotaxis at this step. Once tumor cells reach a blood vessel they are believed to again use invadopodia to degrade the basement membrane and enter the blood stream.
Invadopodia are defined as invasive actin polymerization dependent protrusions found specifically on cancer cells that degrade matrix. They were first described in invasive tumor cells such as the human malignant breast tumor cell MDA-MB-231[12] and human malignant melanoma cells [13]. In this review we are going to focus on describing invadopodia which, by definition, are only formed by invasive tumor cells and since they have unique characteristics that differentiate them from other matrix degrading organelles described in other cell types [14]. These other degrading organelles are called podosomes (macrophages), rosettes (fibroblasts) and more generally invadosomes (reviewed in [15••]).
When plated on 2D surfaces, tumor cells form invadopodia on their ventral surfaces which degrade extracellular matrix through the delivery of metalloproteases like MT1-MMP [16, 17]. Recent studies have shown that the formation of invadopodia is a multistep process involving actin-binding proteins and complex signaling cascades [18]. Initiation of invadopodium assembly involves “invadopodium precursor” formation prior to maturation to the degradation competent invadopodium. Cortactin phosphorylation by Arg-kinase regulates cortactin binding to other proteins including cofilin and NCK1 and this phosphorylation plays a critical role in regulating actin polymerization and precursor assembly [18-20] (reviewed in [21••]). It has been proposed that at the membrane level, caveolin regulates invadopodium formation through the regulation of membrane cholesterol levels [22], and also that extracellular matrix rigidity can influence the formation of invadopodia [23] suggesting that extra-cytoskeletal factors also regulate their formation.
Invadopodia in chemotaxis
Recent results have demonstrated that invadopodia are involved in directional polarization, signal sensing and directional protrusion during chemotaxis in 2D [24] as observed for podosomes [25]. These results raise an interesting question: are invadopodia only necessary for degradation, or do they have other functions such as orienting tumor cells towards chemotactic signals such as those associated with blood vessels in vivo? The importance of chemotaxis in tumor and stromal cell migration in vivo and in tumor cell dissemination and metastasis has been reviewed recently in detail [21••]. An important insight to come from these considerations is that invadopodia are involved in chemotaxis during migration in both 2 and 3D (Figure 2). Previous reports of human tumor cells migrating in three dimensional matrices indicate that matrix-degrading invadopodia localize to the leading edge of the invading cell where the secretory machinery and metalloproteases are located [26]. Recently, Magalhaes et al., [27] were able to identify invadopodia during cell invasion in 3D matrix. MDA-MB-231 cells in a 3D matrix contain cortactin, Tks5, MT1-MMP and matrix degradation (which together in tumor cells are unique markers of invadopodia) localized in protrusive structures at the leading front of the cell in dense native matrix consistent with studies of 3D cell migration by breast carcinoma cells [28]. Similar results implicating invadopodium-mediated proteolysis at the cell front during invasion and migration in vivo in mammary tumors have been observed [11]. Wolf and coworkers [29] have reported that tumor cells invading 3D pepsin digested collagen also exhibit pericellular degradation to widen the hole available for pseudopodium-mediated migration but did not observe proteolysis at the cell front in this pepsin treated collagen. Taken together, these 3D and in vivo studies indicate that proteolytic activity at the cell front is necessary for tumor cell invasion and migration in 3D and that this requirement is relaxed in pepsin treated collagens lacking telopeptides and crosslinking. Furthermore, common signaling and cytoskeletal pathways used by both locomotory protrusions, such as pseudopodia, and invasive protrusions, ie. invadopodia, indicate a high degree of molecular integration and cross talk between pseudopodia and invadopodia allowing efficient invasion coupled migration in both 2 and 3D (Figures 1 and 2) [21••].
Figure 2. Invadopodia and locomotory protrusions are functionally coupled during both 2 and 3-D migration.
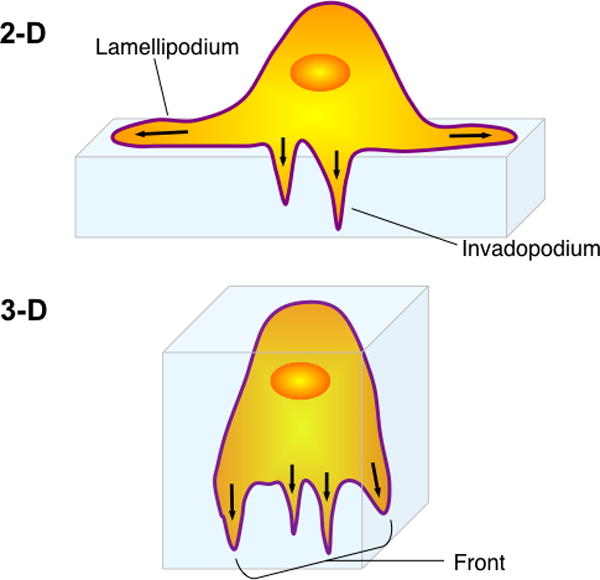
In 2-D invadopodia and pseudopodia (lamellipodia) are separated in space. In 3-D both types of protrusions are found together at the cell front. Common signaling and cytoskeletal pathways used by both motility supporting protrusions such as pseudopodia and invasion specific protrusions such as invadopodia indicate a high degree of molecular integration and cross talk between pseudopodia and invadopodia allowing efficient invasion coupled migration in 2 and 3D.
Types of tumor cell motility
For some time it has been generally agreed that tumor cell motility is necessary for tumor cells to metastasize [30]. Once tumor cells have passed through their basement membrane they have to migrate through the extracellular matrix for long distances to disseminate efficiently via blood vessels and lymphatics (Figure 1). But, how do tumor cells move long distances in the tumor? The development of novel imaging techniques has illuminated these processes [31-33]. High resolution multiphoton imaging of tumors in vivo has shown that tumor cells use both collective and single cell motility (reviewed in references [21••, 34]). Breast carcinoma cell motility is characterized by solitary amoeboid movement. Cells undergoing amoeboid movement in vivo move at high speeds (~ 4 μm/min) compared to other types of cell motility described in vivo [31].
An important question is: why do tumor cells display these different types of motility? A common feature of cell motility in vivo is the formation of F-actin rich protrusions which cells use to extend forward to adhere to their surroundings, followed by contraction of the trailing end. These coordinated events, called the motility cycle, propel the cell forward. Coupled to these events is the formation of invasive protrusions used to penetrate through the extracellular matrix and to overcome matrix barriers to migration. Historically, protrusions at the leading edge of motile cells were named based on their shape: filopodia (needle shape), pseudopodia (round), lobopodia (cylindrical) and lamellipodia (flat veils) [35]. Lamellipodia are observed when cells are plated on 2D substrates. All are F-actin rich and actin polymerization is required for their protrusion [36, 37]. Invasive tumor cells form pseudopodia in vivo (functionally equivalent to lamellipodia but three dimensional [38]) in response to the EGF secreted by the tumor associated macrophages, as part of the tumor cell/macrophage paracrine loop described in breast tumors [21••, 39]. F-actin rich pseudopodia are the defining morphological feature of fast moving amoeboid cells and are involved during chemotaxis to direct tumor cells toward blood vessels before intravasation [11, 31] (Figure 1). Different reports have described the importance of amoeboid motility for cell migration in vivo [40-42]. This work identifies ROCK , and its regulator PDK1, as important mediators of cortical actomyosin contractility part of the motility cycle [41, 42], and Rac, as a central GTPase involved in the interchangeability of different modes of tumor cell motility [40, 43].
The formation of these distinct protrusive structures during the cell motility cycle of tumor cells during migration and dissemination in vivo highlights the fact that tumor cells adapt to different types of matrix composition, as proposed for other cell types [44]. We hypothesize that the tumor microenvironment is responsible for these different motility behaviors. The interactions with different stromal cell types, different matrix compositions and different chemoattractants further determine how tumor cells behave within, and escape from, the primary tumor. For example, the formation of invasive protrusions (invadopodia) and locomotory protrusions (pseudopodia) is regulated by stromal cells in the tumor microenvironment including fibroblasts (Squamos Cell Carcinoma) [45] and macrophages (breast carcinoma) [21••].
Regulation of actin polymerization and Metastasis
The formation of long, force-supporting membrane protrusions requires actin polymerization. Signaling pathways are altered in invasive tumor cells to increase their actin polymerization activity and motility [5, 6]. The Rho-family GTPases have been directly linked to motility and protrusion formation through their ability to activate the signaling targets that direct upstream actin cytoskeleton modifying proteins. Among the 20 members of the family, the most studied have been RhoA, Rac1 and Cdc42. The close isoforms of Rho including RhoA, RhoC and RhoB, play different roles in cancer [46-49]. RhoC expression is upregulated in metastatic tumor cells isolated from lung metastases [50•] and invasive human breast tumor cells [48••]. RhoC plays a critical role in metastasis regulating the spatial formation of invadopodia to tightly focus the matrix degradation [48••]. However, RhoA seems to play a different role in invasion, regulating MMP trafficking and Rac1 activation [47-49]. Effectors of GTPases, like the formins; and the Arp2/3 complex, have also been shown to be involved in metastasis through their roles in motility and protrusion formation (reviewed in [51]).
The cofilin activity cycle is regulated by GTPases through ROCK [52]. Cofilin plays a critical role in promoting protrusions through the formation of free actin barbed ends needed for new actin polymerization. The cofilin pathway is upregulated both at the level of gene expression and protein activity [5, 53] in metastatic tumor cells where the cofilin activity cycle is involved in actin polymerization, protrusion formation, chemotaxis, motility and invasion [48••, 54]. To explain the activation of cofilin locally, a Local Excitation Global Inhibition (LEGI) model has been proposed. Global inhibition of cofilin activity through cofilin phosphorylation mediated by RhoC, restricts cofilin activity just at the very tip of a motility supporting protrusion or within the core of an invadopodium [48••, 53] suggesting how these two types of protrusions might be coordinated at the cell front.
Rac1 GTPase, a member of the Rac GTPase subfamily, is necessary for the formation of lamellipodia. It has been proposed that the anti-capping protein Mena is involved in acting as a negative regulator of Rac1 activity [55]. In addition, it has been shown that the splicing patterns of Mena isoforms change in invasive versus non invasive tumor cells in vivo. Invasive tumor cells upregulate MENAINV and downregulate another Mena isoform, MENA 11a [56]. Overexpression of MENAINV promotes invadopodium formation and matrix degradation and also lamellipodium formation contributing to tumor cell invasion, dissemination and metastasis [57-60].
RhoGTPases and invadopodia: Spatiotemporal RhoC GTPase signaling
Different GTPases have been implicated in the formation and function of invadopodia including Cdc42, RhoA and Rac1 [17, 61, 62]. However, recent reports show that GTPases display spatiotemporal activation dynamics important for the precise control of different signaling pathways [63•]. Different regulators including GEFs, GAPs and GDIs regulate the GTPase activation cycle. To decipher such complex and dynamic activation kinetics, traditional approaches including biochemical strategies or simply tagging these molecules with fluorescent proteins, fall short in detecting these phenomena in living cells. Fluorescent biosensors for the detection of Rho GTPase activation have emerged as critical and powerful tools allowing direct interrogation of GTPase activation at subcellular resolution and in the time scale of seconds [64-66] (Figure 3). These fluorescent biosensors based on the fluorescence resonance energy transfer (FRET) rely on the conformational reorientation within the engineered molecule upon “activation” of the built-in GTPase through the guanine nucleotide exchange. This event causes the shift in the dipole-dipole coupling angle between the two fluorescent proteins within the biosensor to affect FRET; thus, through monitoring the ratio of the FRET to donor fluorescent emissions, one can determine the relative protein activation states within a single living cell.
Figure 3. Examples of Rho-family GTPase biosensors based on FRET.
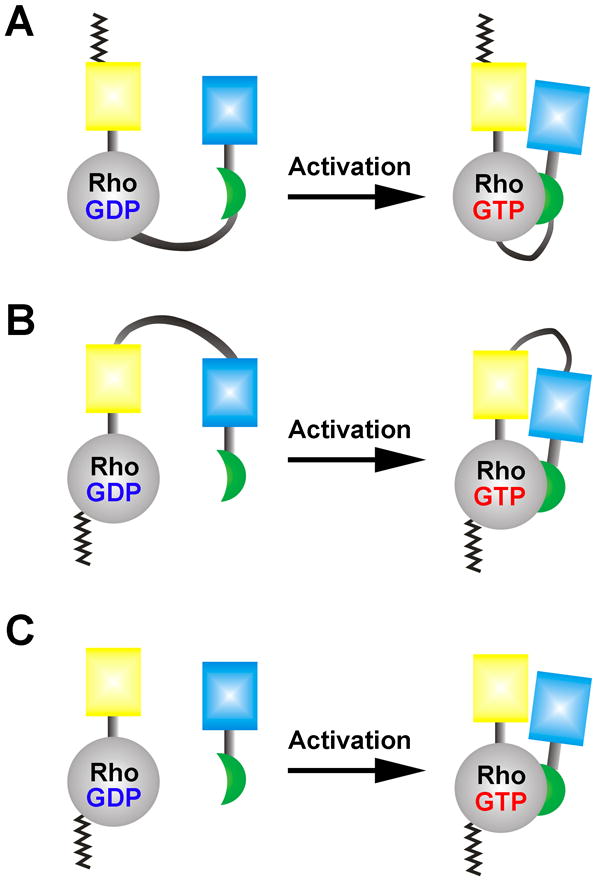
In all examples, the guanine nucleotide loading state (GDP versus GTP) confers either a conformational change within the molecule or to bring the binding pairs together to affect FRET between the cyan and yellow fluorescent proteins. A: The fluorescent protein pair for FRET is placed at the terminal ends of the molecule, necessitating an attachment of a CAAX-box at the C-terminus for the plasma membrane insertion [67•]. This design compromises the GDI-GTPase interaction that requires the C-terminal lipid-moiety in addition to the Switch I/II accessibility by the GDI. B: The Rho GTPase biosensor based on a design that maintains the GDI-GTPase interaction by placing a full-length RhoA at the C-terminus of the molecule [65•]. The RhoC GTPase biosensor is based on a similar design as B [48] . C: Bimolecular FRET biosensor for Rho GTPases [63]. This approach maximizes the total dynamic range of FRET while making the data processing and interpretation more challenging due to the non-equimolar distribution of the biosensor components in living cells.
Using a FRET-based RhoC biosensor, a novel pathway controlling spatial restriction of invadopodium activity has been described (Figure 4). RhoC is activated surrounding the invadopodium center generating a ring pattern achieved by the spatial placement of p190RhoGAP inside the center, and p190RhoGEF outside the center. The combination of a specific GEF and GAP in these locations tightly regulates and focuses the region of RhoC activation (Figure 4). As a consequence, the activation of the RhoC/ROCK/LIMK pathway results in cofilin phosphorylation in the outer ring thereby focusing the cofilin activity to generate barbed ends and actin polymerization within the center, focusing the matrix degradation activity for efficient invasion [48••]. These results show that controlling the RhoGTPase signaling is not only achieved by the quantity of GEFs and GAPs acting on a specific GTPase, but also by the spatial and temporal placement of these components that will specifically target the activity of the GTPase and its signaling pathway.
Figure 4. A model for the spatial regulation of RhoC activity during invadopodium protrusion.
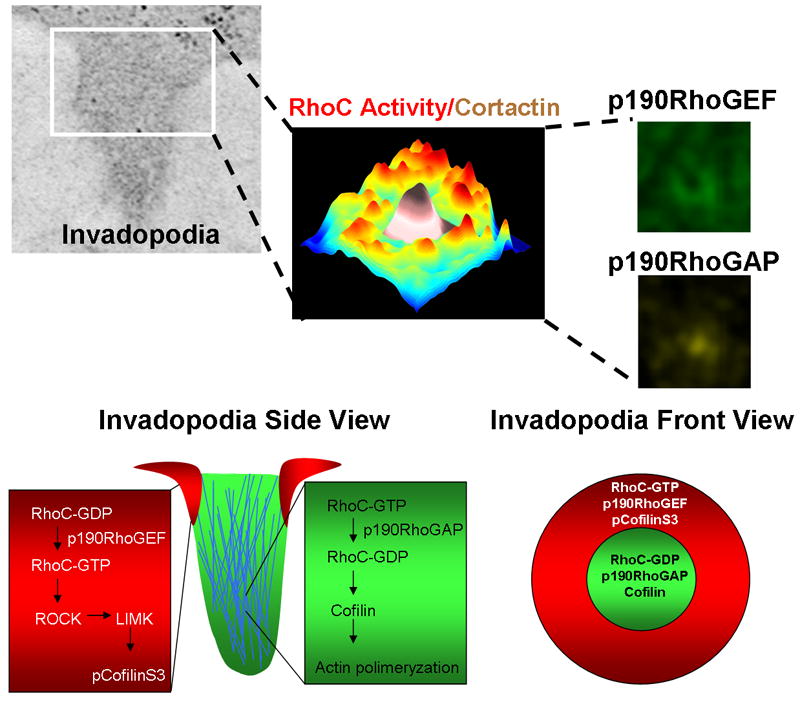
Formation of a focused invadopodium is mediated by the spatiotemporal localization of RhoC activity outside invadopodia. RhoC activity increases around the invadopodial core structure, as shown by this plot of the maximum projection over time of RhoC activity. Pseudo-color shows low RhoC activity levels (blue) to high RhoC activity levels (red) in relation to low (white) and high (brown) cortactin intensity where cortactin marks the central core of the invadopodium. This activation pattern is achieved by activation of p190RhoGEF (green) outside and p190RhoGAP inside (yellow), restricting RhoC activity just outside the invadopodium core. This spatial restriction localizes active cofilin to the core of the structure and focuses actin polymerization so as to achieve optimum protrusion elongation and invasion (bottom part adapted from [68]).
Concluding Remarks and future directions
To date, drugs capable of blocking metastasis are not available. At least two reasons for this are 1) that such a complex process is still not well understood and 2) there are no reliable end point markers for evaluating drug performance in blocking metastasis. Invadopodia are unique protrusions with matrix degrading activity formed specifically by invasive tumor cells that have an essential role in invasion, migration and chemotaxis during metastasis, making them an attractive functional target to interfere with metastasis and as a potential marker for assessing drug performance. Defining the molecular features of invadopodia under physiological conditions will help in the design of specific drugs that could block metastasis in the future. Proteins involved in the formation of invadopodia are upregulated in the invasive and migrating population of tumor cells. The signaling pathways involved in invadopodium formation may not be present in normal cells. Understanding these signaling pathways will clarify the molecular basis of metastasis and allow the design and testing of more specific and selective drugs to block metastasis.
Highlights.
Invadopodium degrading protrusion facilitate degradation of basement membranes by tumor cells
Motility is an important feature of invasive tumor cells
FRET-based GTPases biosensors allow direct visualization of GTPases activation in living cells during invasion.
RhoC mechanism of invadopodium protrusion confinement.
Acknowledgments
The authors would like to thank members of the Condeelis and Hodgson laboratories for helpful discussions. We thank Current Biology Journal for granting copyright permission to adapt Figure 4 bottom part (from Figure 1 of MacGrath, S.M., and Koleske, A.J (2011)) We thank especially Dr. Minna Roh for her critical comments and suggestions for this manuscript and Dr. Robert Eddy for generously provide models for figure 2. The authors apologize to those whose work is not cited owing to space limitations. The authors’ research is funded by grants: GM093121 (LH, JJB-C), “Sinsheimer Foundation Young Investigator Award” (LH) and CA150344 (JC and JJB-C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
-
•
of special interest
-
••
of outstanding interest
- 1.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70:5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Sporn MB. The war on cancer. Lancet. 1996;347:1377–1381. doi: 10.1016/s0140-6736(96)91015-6. [DOI] [PubMed] [Google Scholar]
- 4.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Goswami S, Lapidus K, Wells AL, Wyckoff JB, Sahai E, Singer RH, Segall JE, Condeelis JS. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer research. 2004;64:8585–8594. doi: 10.1158/0008-5472.CAN-04-1136. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Wyckoff JB, Goswami S, Wang Y, Sidani M, Segall JE, Condeelis JS. Coordinated regulation of pathways for enhanced cell motility and chemotaxis is conserved in rat and mouse mammary tumors. Cancer research. 2007;67:3505–3511. doi: 10.1158/0008-5472.CAN-06-3714. [DOI] [PubMed] [Google Scholar]
- 7.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 8.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. The Journal of clinical investigation. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •9.Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, Yang J. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer cell. 2011;19:372–386. doi: 10.1016/j.ccr.2011.01.036. This paper shows that TWIST1, a central regulator of EMT, promotes invadopodia formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 11.Gligorijevic B, Wyckoff J, Yamaguchi H, Wang Y, Roussos ET, C J. N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors. Journal of Cell Science. doi: 10.1242/jcs.092726. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen WT, Lee CC, Goldstein L, Bernier S, Liu CH, Lin CY, Yeh Y, Monsky WL, Kelly T, Dai M, et al. Membrane proteases as potential diagnostic and therapeutic targets for breast malignancy. Breast cancer research and treatment. 1994;31:217–226. doi: 10.1007/BF00666155. [DOI] [PubMed] [Google Scholar]
- 13.Aoyama A, Chen WT. A 170-kDa membrane-bound protease is associated with the expression of invasiveness by human malignant melanoma cells. Proc Natl Acad Sci U S A. 1990;87:8296–8300. doi: 10.1073/pnas.87.21.8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oser M, Dovas A, Cox D, Condeelis J. Nck1 and Grb2 localization patterns can distinguish invadopodia from podosomes. Eur J Cell Biol. 2011;90:181–188. doi: 10.1016/j.ejcb.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••15.Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Annual review of cell and developmental biology. 2011;27:185–211. doi: 10.1146/annurev-cellbio-092910-154216. A comprehensive review on the different degrading structures found in a variety of cell types. [DOI] [PubMed] [Google Scholar]
- 16.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–3043. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- 17.Sakurai-Yageta M, Recchi C, Le Dez G, Sibarita JB, Daviet L, Camonis J, D’Souza-Schorey C, Chavrier P. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol. 2008;181:985–998. doi: 10.1083/jcb.200709076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, Desmarais V, van Rheenen J, Koleske AJ, Condeelis J. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009;186:571–587. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oser M, Mader CC, Gil-Henn H, Magalhaes M, Bravo-Cordero JJ, Koleske AJ, Condeelis J. Specific tyrosine phosphorylation sites on cortactin regulate Nck1-dependent actin polymerization in invadopodia. J Cell Sci. 2010;123:3662–3673. doi: 10.1242/jcs.068163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mader CC, Oser M, Magalhaes MA, Bravo-Cordero JJ, Condeelis J, Koleske AJ, Gil-Henn H. An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Cancer Res. 2011;71:1730–1741. doi: 10.1158/0008-5472.CAN-10-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••21.Roussos ET, Condeelis JS, Patsialou A. Chemotaxis in cancer. Nat Rev Cancer. 2011;11:573–587. doi: 10.1038/nrc3078. A comprehensible review about how chemotaxis is essential to cancer progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caldieri G, Giacchetti G, Beznoussenko G, Attanasio F, Ayala I, Buccione R. Invadopodia biogenesis is regulated by caveolin-mediated modulation of membrane cholesterol levels. Journal of cellular and molecular medicine. 2009;13:1728–1740. doi: 10.1111/j.1582-4934.2008.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parekh A, Ruppender NS, Branch KM, Sewell-Loftin MK, Lin J, Boyer PD, Candiello JE, Merryman WD, Guelcher SA, Weaver AM. Sensing and modulation of invadopodia across a wide range of rigidities. Biophysical journal. 2011;100:573–582. doi: 10.1016/j.bpj.2010.12.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desmarais V, Yamaguchi H, Oser M, Soon L, Mouneimne G, Sarmiento C, Eddy R, Condeelis J. N-WASP and cortactin are involved in invadopodium-dependent chemotaxis to EGF in breast tumor cells. Cell Motil Cytoskeleton. 2009;66:303–316. doi: 10.1002/cm.20361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dovas A, Gevrey JC, Grossi A, Park H, Abou-Kheir W, Cox D. Regulation of podosome dynamics by WASp phosphorylation: implication in matrix degradation and chemotaxis in macrophages. J Cell Sci. 2009;122:3873–3882. doi: 10.1242/jcs.051755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bravo-Cordero JJ, Marrero-Diaz R, Megias D, Genis L, Garcia-Grande A, Garcia MA, Arroyo AG, Montoya MC. MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. Embo J. 2007;26:1499–1510. doi: 10.1038/sj.emboj.7601606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magalhaes M, L DR, Mader C, Bravo-Cordero JJ, Gil-Henn H, Oser M, Chen X, Koleske AJ, Condeelis J. Cortactin phosphorylation regulates cell invasion through a pH dependent pathway. Journal of Cell Biology. 2011 doi: 10.1083/jcb.201103045. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Packard BZ, Artym VV, Komoriya A, Yamada KM. Direct visualization of protease activity on cells migrating in three-dimensions. Matrix biology : journal of the International Society for Matrix Biology. 2009;28:3–10. doi: 10.1016/j.matbio.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 30.Liotta LA. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986;46:1–7. [PubMed] [Google Scholar]
- 31.Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nat Rev Cancer. 2003;3:921–930. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 32.Kedrin D, Gligorijevic B, Wyckoff J, Verkhusha VV, Condeelis J, Segall JE, van Rheenen J. Intravital imaging of metastatic behavior through a mammary imaging window. Nat Methods. 2008;5:1019–1021. doi: 10.1038/nmeth.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexander S, Koehl GE, Hirschberg M, Geissler EK, Friedl P. Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model. Histochem Cell Biol. 2008;130:1147–1154. doi: 10.1007/s00418-008-0529-1. [DOI] [PubMed] [Google Scholar]
- 34.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 35.Taylor DL, Condeelis JS. Cytoplasmic structure and contractility in amoeboid cells. Int Rev Cytol. 1979;56:57–144. doi: 10.1016/s0074-7696(08)61821-5. [DOI] [PubMed] [Google Scholar]
- 36.Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. II. “RRuffling”. Exp Cell Res. 1970;60:437–444. doi: 10.1016/0014-4827(70)90537-9. [DOI] [PubMed] [Google Scholar]
- 37.Condeelis JS, Wyckoff JB, Bailly M, Pestell R, Lawrence D, Backer J, Segall JE. Lamellipodia in invasion. Semin Cancer Biol. 2001;11:119–128. doi: 10.1006/scbi.2000.0363. [DOI] [PubMed] [Google Scholar]
- 38.Wang W, Wyckoff JB, Frohlich VC, Oleynikov Y, Huttelmaier S, Zavadil J, Cermak L, Bottinger EP, Singer RH, White JG, et al. Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res. 2002;62:6278–6288. [PubMed] [Google Scholar]
- 39.Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005;17:559–564. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 41.Pinner S, Sahai E. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nat Cell Biol. 2008;10:127–137. doi: 10.1038/ncb1675. [DOI] [PubMed] [Google Scholar]
- 42.Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol. 2006;16:1515–1523. doi: 10.1016/j.cub.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 43.Sanz-Moreno V, Marshall CJ. The plasticity of cytoskeletal dynamics underlying neoplastic cell migration. Curr Opin Cell Biol. 2010;22:690–696. doi: 10.1016/j.ceb.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 44.Van Goethem E, Poincloux R, Gauffre F, Maridonneau-Parini I, Le Cabec V. Matrix architecture dictates three-dimensional migration modes of human macrophages: differential involvement of proteases and podosome-like structures. J Immunol. 2010;184:1049–1061. doi: 10.4049/jimmunol.0902223. [DOI] [PubMed] [Google Scholar]
- 45.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 46.Liu AX, Rane N, Liu JP, Prendergast GC. RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Molecular and cellular biology. 2001;21:6906–6912. doi: 10.1128/MCB.21.20.6906-6912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simpson KJ, Dugan AS, Mercurio AM. Functional analysis of the contribution of RhoA and RhoC GTPases to invasive breast carcinoma. Cancer Res. 2004;64:8694–8701. doi: 10.1158/0008-5472.CAN-04-2247. [DOI] [PubMed] [Google Scholar]
- ••48.Bravo-Cordero JJ, Oser M, Chen X, Eddy R, Hodgson L, Condeelis J. A novel spatiotemporal RhoC activation pathway locally regulates cofilin activity at invadopodia. Curr Biol. 2011;21:635–644. doi: 10.1016/j.cub.2011.03.039. This paper shows, for the first time, RhoC activation at invadopodia. It describes how RhoC activity is spatiotemporally regulated by p190RhoGEF/p190RhoGAP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vega FM, Fruhwirth G, Ng T, Ridley AJ. RhoA and RhoC have distinct roles in migration and invasion by acting through different targets. J Cell Biol. 2011;193:655–665. doi: 10.1083/jcb.201011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •50.Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–535. doi: 10.1038/35020106. First paper identifying RhoC as a gene upregulated in metastatic tumor cells. [DOI] [PubMed] [Google Scholar]
- 51.Nurnberg A, Kitzing T, Grosse R. Nucleating actin for invasion. Nat Rev Cancer. 2011;11:177–187. doi: 10.1038/nrc3003. [DOI] [PubMed] [Google Scholar]
- 52.Oser M, Condeelis J. The cofilin activity cycle in lamellipodia and invadopodia. J Cell Biochem. 2009;108:1252–1262. doi: 10.1002/jcb.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang W, Eddy R, Condeelis J. The cofilin pathway in breast cancer invasion and metastasis. Nat Rev Cancer. 2007;7:429–440. doi: 10.1038/nrc2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Rheenen J, Condeelis J, Glogauer M. A common cofilin activity cycle in invasive tumor cells and inflammatory cells. J Cell Sci. 2009;122:305–311. doi: 10.1242/jcs.031146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Higashi M, Ishikawa C, Yu J, Toyoda A, Kawana H, Kurokawa K, Matsuda M, Kitagawa M, Harigaya K. Human Mena associates with Rac1 small GTPase in glioblastoma cell lines. PloS one. 2009;4:e4765. doi: 10.1371/journal.pone.0004765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goswami S, Philippar U, Sun D, Patsialou A, Avraham J, Wang W, Di Modugno F, Nistico P, Gertler FB, Condeelis JS. Identification of invasion specific splice variants of the cytoskeletal protein Mena present in mammary tumor cells during invasion in vivo. Clin Exp Metastasis. 2009;26:153–159. doi: 10.1007/s10585-008-9225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, Giampieri S, Wang Y, Goswami S, Wyckoff JB, Lauffenburger DA, et al. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Developmental cell. 2008;15:813–828. doi: 10.1016/j.devcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roussos ET, Balsamo M, Alford SK, Wyckoff JB, Gligorijevic B, Wang Y, Pozzuto M, Stobezki R, Goswami S, Segall JE, et al. Mena invasive (MenaINV) promotes multicellular streaming motility and transendothelial migration in a mouse model of breast cancer. J Cell Sci. 2011;124:2120–2131. doi: 10.1242/jcs.086231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roussos ET, Goswami S, Balsamo M, Wang Y, Stobezki R, Adler E, Robinson BD, Jones JG, Gertler FB, Condeelis JS, et al. Mena invasive (Mena(INV)) and Mena11a isoforms play distinct roles in breast cancer cell cohesion and association with TMEM. Clin Exp Metastasis. 2011;28:515–527. doi: 10.1007/s10585-011-9388-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roussos ET, Wang Y, Wyckoff JB, Sellers RS, Wang W, Li J, Pollard JW, Gertler FB, Condeelis JS. Mena deficiency delays tumor progression and decreases metastasis in polyoma middle-T transgenic mouse mammary tumors. Breast Cancer Res. 2010;12:R101. doi: 10.1186/bcr2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, et al. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakahara H, Otani T, Sasaki T, Miura Y, Takai Y, Kogo M. Involvement of Cdc42 and Rac small G proteins in invadopodia formation of RPMI7951 cells. Genes to cells : devoted to molecular & cellular mechanisms. 2003;8:1019–1027. doi: 10.1111/j.1365-2443.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- •63.Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. A seminal paper describing the spatiotemporal RhoGTPases activation during cell protrusion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hodgson L, Shen F, Hahn K. Biosensors for characterizing the dynamics of rho family GTPases in living cells. In: Bonifacino Juan S, et al., editors. Current protocols in cell biology. Chapter 14. 2010. pp. Unit 14 11 11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •65.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. This paper describes the first design of the RhoA single chain biosensor that retains it original C-terminus permitting the interaction with endogenous GDI. [DOI] [PubMed] [Google Scholar]
- 66.Nalbant P, Hodgson L, Kraynov V, Toutchkine A, Hahn KM. Activation of endogenous Cdc42 visualized in living cells. Science. 2004;305:1615–1619. doi: 10.1126/science.1100367. [DOI] [PubMed] [Google Scholar]
- •67.Kurokawa K, Matsuda M. Localized RhoA activation as a requirement for the induction of membrane ruffling. Molecular biology of the cell. 2005;16:4294–4303. doi: 10.1091/mbc.E04-12-1076. This paper is the first description of a single chain biosensor for RhoA. In this design the Rho GTPase and the small binding domain are placed within the internal portion of the single-chain biosensor construct, not permitting the interaction with endogenous GDI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacGrath SM, Koleske AJ. Invadopodia: RhoC runs rings around cofilin. Curr Biol. 2011;21:R280–282. doi: 10.1016/j.cub.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]


