Abstract
The outer membrane (OM) of Gram negative bacteria is an essential organelle that serves as a selective permeability barrier by keeping toxic compounds out of the cell while allowing vital nutrients in. How the OM and its constituent lipid and protein components are assembled remains an area of active research. In this review, we describe our current understanding of how outer membrane proteins (OMPs) are delivered to and then assembled in the OM of the model Gram-negative organism Escherichia coli.
Bacteria are more than just bags of enzymes
With each passing year, the structural complexities of bacteria are becoming increasingly apparent. Gram negative bacteria can be divided into several subcellular compartments [1]. There are two aqueous compartments called the cytoplasm and the periplasm. The cytoplasm is enclosed by a phospholipid bilayer called the Inner Membrane (IM), which is itself surrounded by an asymmetric bilayer called the Outer Membrane (OM). The periplasm lies in the space between the IM and OM, and is home to the peptidoglycan cell wall (CW). Present throughout these compartments are proteins with diverse and important biological functions. Some of these proteins are membrane-embedded and allow transfer of molecules between compartments. Others are soluble enzymes involved in metabolic reactions. Much work has been devoted toward understanding how each of these compartments is formed and maintained. In this review, we focus on a particular aspect of OM biogenesis, namely the assembly of integral outer membrane proteins (OMPs).
An OMP is born!
Like all cellular proteins, OMPs are synthesized in the cytoplasm. Since OMPs are destined for the cell envelope, they are synthesized as pre-proteins with an N-terminal signal sequence [2]. The signal sequence is a targeting element that routes proteins to specific protein translocases embedded in the IM. In the case of OMPs, the Sec translocase provides a path across the IM. The Sec pathway is responsible for transporting the bulk of all exported proteins in E. coli [3]. Only unfolded proteins can be accommodated by the Sec pathway. To keep precursor OMPs transport-competent, the cytoplasmic chaperone SecB maintains them in an unfolded conformation [4]. Translocation of unfolded proteins through SecYEG is powered by the essential ATPase SecA [5]. Many elegant genetic and biochemical experiments have elucidated the mechanism of Sec-mediated protein export in considerable detail and have been reviewed elsewhere [3,6].
Chaperones: always watching, judging
Signal sequence cleavage releases translocated OMPs into the periplasm. Some OMPs possess a C-terminal signature sequence that facilitates their insertion into the OM. This signature sequence consists of several hydrophobic residues, as well as an aromatic residue (usually phenylalanine) at the final position [7]. Mutation of the final phenylalanine residue can prevent OMP assembly in vivo. There is also evidence that sorting is aided by the C-terminal most beta-strand of the unfolded beta-barrel [8]. Since beta-barrel proteins will ultimately reside in the OM, it is not surprising that these proteins are prone to aggregation in aqueous environments. To prevent aggregation and misfolding these hydrophobic residues must be shielded from the aqueous periplasm; failure to do so can trigger cellular stress responses.
In E. coli, there are parallel pathways of chaperone activity [9]. The chaperone SurA functions in one pathway and the chaperone Skp and the chaperone/protease DegP in the other. SurA can function to assemble all OMPs efficiently, and some OMPs, including the essential LPS assembly factor LptD prefer this pathway [10,11]. No OMPs that prefer the Skp/DegP pathway have been identified. However, in the absence of SurA, Skp/DegP can assemble most OMPs efficiently. The importance of SurA is evidenced by the increased permeability, OMP assembly defects, and envelope stress factor induction observed in surA mutants [12]. In contrast, in Neisseria meningitidis, where the LptD homolog is dispensable for growth, the OMP assembly defects observed in a surA mutant are mild [13]. Such defects are much more pronounced in a skp mutant, suggesting that Skp might be the major periplasmic chaperone in this organism.
The Bam Complex puts OMPs in their place
After traversing the periplasm, OMPs are delivered to a multi-subunit machine in the OM called the Bam complex [2]. The multiprotein Bam complex was originally identified in E. coli, but Bam (for beta-barrel assembly machine) has also been studied in N. meningitidis and Caulobacter crescentus [14–16]. At the center of the complex is BamA, which is itself an essential OMP. In addition to the beta-barrel domain, BamA possess an N-terminal extension that stretches into the periplasm. This extension is composed of 5 discrete POTRA (polypeptide translocation associated) domains P1–P5, the structures of which have been determined experimentally [17–19]. The POTRAs are important contact points for the accessory Bam lipoproteins [17]. P1 is dispensable for these interactions, but the other domains are needed to interact with BamB. Only P5 is needed to preserve interactions between BamA and BamCDE. The interactions with BamA are stable; they can be detected by protein pull-down experiments in the absence of chemical crosslinking. This is in contrast to the interaction between BamA and SurA which is only evident when chemical crosslinkers are employed, suggesting this interaction occurs transiently [12].
The other four members BamBCDE are OM lipoproteins, all of which are directed to the OM by the well-characterized Lol system [20]. The connectivity of the Bam complex has been probed biochemically [14,21,22]. BamA and BamB interact directly, as do BamA and BamD. Both of these interactions are independent of one another. BamC and BamE form contacts with BamD and the BamCDE subcomplex can be stably isolated in vitro [23]. The entire complex has been reconstituted in proteoliposomes that contain purified Bam proteins, and it is now possible to monitor assembly of the model substrate OmpT in vitro [23,24]. The in vitro assembly reaction requires SurA and all 5 Bam proteins to proceed efficiently, and occurs in the absence of any added energy source. Recently, the efficiency of the in vitro system has been increased which allowed analysis of a mutant Bam complex with lower activity [24]. This remarkable achievement will be an invaluable tool for dissecting the mechanism of how other OMPs are assembled, and what role each Bam protein plays in this process.
Aside from BamA, BamD is the next most highly conserved member of the Bam complex and this protein is essential for growth in E. coli [22,25]. Depletion of BamA or BamD causes identical OMP assembly defects, indicating these two proteins play a central role during OMP assembly [22]. The structure of BamD reveals a series of tetratricopeptide repeat (TPR) motifs which are often important for mediating protein-protein interactions [26,27]. Indeed, deletion of the final C-terminal helix of BamD weakens interactions with BamC and BamE, as well as BamA [22]. Beyond this structural information, not much else is know about how BamD functions. The Neisseria BamD homolog, designated ComL, is also essential for growth and has been shown to associate with peptidoglycan [15,28]. Whether this interaction is relevant to OMP assembly is unclear, but it has been suggested that BamD/ComL could alter peptidoglycan structure in some way to accommodate OMPs as they penetrate the CW. However, E. coli BamD does not bind peptidoglycan (unpublished).
The remaining lipoproteins BamBCE are all dispensable for growth in E. coli. Of the three, loss of BamB causes the most severe defect in OMP assembly [29]. Several groups have now published the structure of BamB, all of which revealed an 8-bladed beta-propeller [26,30,31]. Mutagenesis of BamB identified two clusters of residues important for interaction with BamA, which map near each other on the crystal structures [32]. Beyond these interactions, little is known about the function of BamB. A study of LamB assembly showed that both bamB and surA mutants exhibit the same kinetic defect in folding LamB monomers prior to trimer formation [33]. This suggests that BamB is involved in early steps of OMP assembly, possibly including the delivery of OMPs to the OM. Interestingly, it was recently reported that the beta-barrel domain of the autotransporter (AT) protein EspP interacts with BamA, BamB, and BamD, and that the interactions with BamB and BamD persist longer than those with BamA [34]. The implication here is that BamB and BamD function at a later step of AT assembly; whether BamB and BamD normally function late, or if this is a property restricted to assembly of ATs remains to be established.
Loss of either BamC or BamE causes only a slight defect in OM biogenesis [14,21]. However, coupling null mutations of bamC or bamE with mutations in other OMP assembly factors leads to dramatic synthetic phenotypes. For example, both bamB bamC and bamC bamE double mutants display growth defects under standard laboratory conditions. Strikingly, a bamB bamE double mutant is lethal as it cannot be constructed under any condition [21]. This synthetic phenotype suggests that BamB and BamE share a partially overlapping essential function. However, these proteins clearly have distinct functions as the phenotypes of bamB and bamE single mutants are quite different. At least in vitro, BamC and BamE are needed to assemble OmpT [23]. Whether all the accessory Bam lipoproteins are needed to efficiently assemble all OMPs remains an open question.
Don’t envy your neighbor’s Bam Complex
For the sake of comparison, it is worth briefly describing the composition of the Bam complex in the two other organisms where it has been studied. N. meningitidis has homologs of BamA (Omp85), BamD (ComL), BamC, and BamE, but no detectable homolog of BamB [15,25,35]. Another protein, RmpM, was identified as forming a high molecular weight complex that contains BamA [15]. Although RmpM can bind to peptidoglycan and to OMPs, it does not appear to have any role in OMP biogenesis.
In C. crescentus, there are homologs of BamA, BamB, BamD, and BamE [25]. While there is no obvious BamC homolog, the sequence of BamE is slightly longer as compared to E. coli. It is tempting to speculate that this region might encode an enhanced activity for this BamE homolog. Alternatively, other as yet uncharacterized proteins might fulfill the role normally played by the missing Bam component. Interestingly, the lipoprotein Pal was found to co-immunoprecipitate with BamA [16]. The Tol-Pal multi-protein complex extends from the IM to the OM [36]. Pal is an OM lipoprotein and as such is anchored in the OM via the N-terminal lipid anchor, while able to interact with peptidoglycan or the periplasmic protein TolB at the C-terminus [37]. Both TolB and Pal are capable of binding to TolA, which is integrated in the IM along with TolQ and TolR. The functional significance of the interaction between Bam and Pal for OMP assembly is not clear. One suggestion was that the Pal-Bam complex interaction helps position the Bam complex to better enable receipt of emergent OMPs. In any case, it is clear that in different organisms, membership in the Bam complex can vary. This undoubtedly is a reflection of differences in the OM and the subset of OMPs found in different Gram negative organisms.
When misfolded OMPs attack…
The journey an unfolded OMP takes from the IM to the OM is filled with peril. Any diversion from or delay in the normal assembly pathway, whether caused by aberrant interactions with chaperone or defects in the Bam machinery, can cause OMPs to fold improperly and aggregate in the periplasm. It is well established that accumulation of misfolded protein aggregates serves as an inducing cue for cellular stress responses including the σE and Cpx systems [9]. The σE stress response is mediated by a regulated proteolytic cascade triggered by misfolded OMPs [38]. (Regulation of the σE response is discussed in more detail by Ho and Ellermeier elsewhere in this issue.) RseA is an IM-spanning anti-sigma factor that sequesters cytoplasmic σE. Under normal conditions, RseA interacts with the periplasmic protein RseB which protects it from the protease DegS [39]. To activate the system, two signals are required. First, DegS is activated by binding to specific C-terminal residues of misfolded OMPs [40]. The second signal, recently identified as a beta-strand motif (BSM) also in the C-terminal portion of misfolded OMPs, binds RseB [41]. Cleavage of RseA by DegS allows additional cleavage by RseP and cytoplasmic proteases such as ClpXP, which allows release of σE [42,43].
The σE stress response increases the odds of properly assembling OMPs while cleaning up the mess of misfolded OMP aggregates. This is accomplished by increasing the expression of genes encoding chaperones like SurA and Skp, the Bam machinery, and the periplasmic protease DegP. In addition, the expression of several small regulatory RNAs is also increased. These small RNAs down-regulate OMP levels by binding to and targeting OMP mRNA for degradation [44]. Reducing the load of OMPs that need to be assembled also helps the cell cope with envelope stress.
What’s next?
The last decade has seen an incredible increase in our understanding of OMP biogenesis. It seems that all of the key players have been identified and crystal structures of all the lipoprotein components and the periplasmic domain of BamA have been determined. In addition, the assembly reaction has been reconstituted from purified components. However, despite these advances, the mechanism by which beta-barrels are folded and inserted into the OM bilayer still lacks detail. Is the barrel domain folded prior to membrane insertion? Do the essential proteins BamA and BamD each interact with substrate? What roles are played by the accessory Bam lipoproteins in recognizing and folding precursor OMPs? Are different classes of OMPs (for example, monomers vs. multimers) assembled in the same way? An understanding of this complex reaction will require productive interplay between genetics and biochemistry. Our hope is that mutations that alter the Bam machinery in specific ways will provide tools for understanding the mechanism(s) of this amazing reaction.
Highlights.
Outer membrane protein (OMP) assembly requires the Bam complex.
Several structures of the Bam complex members are now available.
The OMP assembly reaction has been reconstituted in vitro using purified Bam proteins and requires chaperone for full activity.
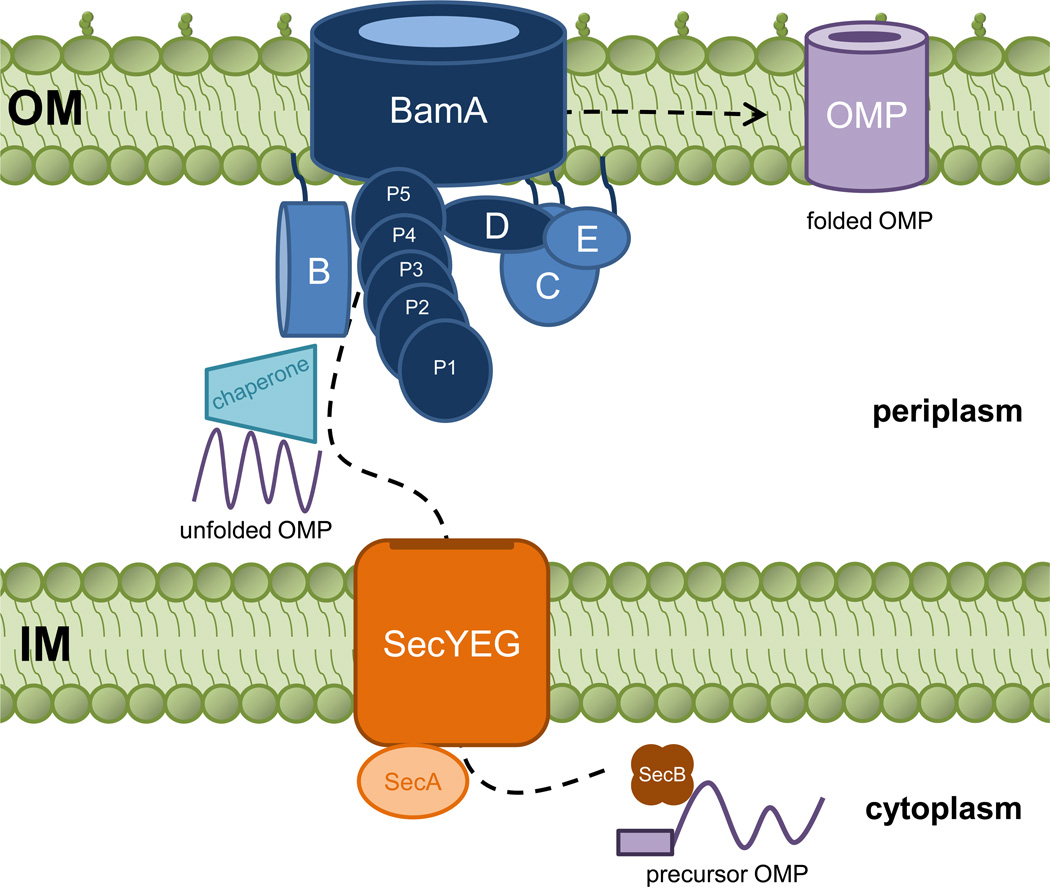
Figure 1. The OMP assembly pathway in E. coli.
Outer membrane proteins (OMPs) are synthesized as unfolded precursors in the cytoplasm and translocated across the inner membrane (IM) via the Sec translocase. Once in the periplasm, chaperones recognize unfolded OMPs and prevent them from forming misfolded aggregates. The multi-subunit Bam complex folds and inserts OMPs into the outer membrane (OM).
Acknowledgments
This work was supported by NIH postdoctoral fellowship GM093768 (N.W.R) and National Institute of General Medical Sciences grant GM34821 (T.J.S). We thank Holly Cardoso, Penny Mahoney, and Dante Ricci for reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricci DP, Silhavy TJ. The Bam machine: A molecular cooper. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbamem.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Driessen AJ, Nouwen N. Protein translocation across the bacterial cytoplasmic membrane. Annu Rev Biochem. 2008;77:643–667. doi: 10.1146/annurev.biochem.77.061606.160747. [DOI] [PubMed] [Google Scholar]
- 4.Bechtluft P, Nouwen N, Tans SJ, Driessen AJ. SecB--a chaperone dedicated to protein translocation. Mol Biosyst. 2010;6:620–627. doi: 10.1039/b915435c. [DOI] [PubMed] [Google Scholar]
- 5.Kusters I, Driessen AJ. SecA, a remarkable nanomachine. Cell Mol Life Sci. 2011;68:2053–2066. doi: 10.1007/s00018-011-0681-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.du Plessis DJ, Nouwen N, Driessen AJ. The Sec translocase. Biochim Biophys Acta. 2011;1808:851–865. doi: 10.1016/j.bbamem.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 7.de Cock H, Struyve M, Kleerebezem M, van der Krift T, Tommassen J. Role of the carboxy-terminal phenylalanine in the biogenesis of outer membrane protein PhoE of Escherichia coli K-12. J Mol Biol. 1997;269:473–478. doi: 10.1006/jmbi.1997.1069. [DOI] [PubMed] [Google Scholar]
- 8.Kutik S, Stojanovski D, Becker L, Becker T, Meinecke M, Kruger V, Prinz C, Meisinger C, Guiard B, Wagner R, et al. Dissecting membrane insertion of mitochondrial beta-barrel proteins. Cell. 2008;132:1011–1024. doi: 10.1016/j.cell.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 9.Merdanovic M, Clausen T, Kaiser M, Huber R, Ehrmann M. Protein Quality Control in the Bacterial Periplasm. Annu Rev Microbiol. 2011 doi: 10.1146/annurev-micro-090110-102925. [DOI] [PubMed] [Google Scholar]
- 10.Vertommen D, Ruiz N, Leverrier P, Silhavy TJ, Collet JF. Characterization of the role of the Escherichia coli periplasmic chaperone SurA using differential proteomics. Proteomics. 2009;9:2432–2443. doi: 10.1002/pmic.200800794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ruiz N, Chng SS, Hiniker A, Kahne D, Silhavy TJ. Nonconsecutive disulfide bond formation in an essential integral outer membrane protein. Proc Natl Acad Sci U S A. 2010;107:12245–12250. doi: 10.1073/pnas.1007319107. LptD has two nonconsecutive disulfide bonds and requires its lipoprotein partner for proper assembly
- 12.Sklar JG, Wu T, Kahne D, Silhavy TJ. Defining the roles of the periplasmic chaperones SurA, Skp, DegP in Escherichia coli. Genes Dev. 2007;21:2473–2484. doi: 10.1101/gad.1581007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Volokhina EB, Grijpstra J, Stork M, Schilders I, Tommassen J, Bos MP. Role of the periplasmic chaperones, Skp, SurA, and DegQ in outer membrane protein biogenesis in Neisseria meningitidis. J Bacteriol. 2011;193:1612–1621. doi: 10.1128/JB.00532-10. This study demonstrates how the relative importance of periplasmic chaperones differs by organism. Deletion of skp has a major impact on OMP assembly in N. meningitidis while deletion of surA causes little change. The opposite is true in E. coli
- 14.Wu T, Malinverni J, Ruiz N, Kim S, Silhavy TJ, Kahne D. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell. 2005;121:235–245. doi: 10.1016/j.cell.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Volokhina EB, Beckers F, Tommassen J, Bos MP. The beta-barrel outer membrane protein assembly complex of Neisseria meningitidis. J Bacteriol. 2009;191:7074–7085. doi: 10.1128/JB.00737-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anwari K, Poggio S, Perry A, Gatsos X, Ramarathinam SH, Williamson NA, Noinaj N, Buchanan S, Gabriel K, Purcell AW, et al. A modular BAM complex in the outer membrane of the alpha-proteobacterium Caulobacter crescentus. PLoS One. 2010;5:e8619. doi: 10.1371/journal.pone.0008619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S, Malinverni JC, Sliz P, Silhavy TJ, Harrison SC, Kahne D. Structure and function of an essential component of the outer membrane protein assembly machine. Science. 2007;317:961–964. doi: 10.1126/science.1143993. [DOI] [PubMed] [Google Scholar]
- 18.Gatzeva-Topalova PZ, Warner LR, Pardi A, Sousa MC. Structure and flexibility of the complete periplasmic domain of BamA: the protein insertion machine of the outer membrane. Structure. 2010;18:1492–1501. doi: 10.1016/j.str.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gatzeva-Topalova PZ, Walton TA, Sousa MC. Crystal structure of YaeT: conformational flexibility and substrate recognition. Structure. 2008;16:1873–1881. doi: 10.1016/j.str.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okuda S, Tokuda H. Lipoprotein Sorting in Bacteria. Annu Rev Microbiol. 2011 doi: 10.1146/annurev-micro-090110-102859. [DOI] [PubMed] [Google Scholar]
- 21.Sklar JG, Wu T, Gronenberg LS, Malinverni JC, Kahne D, Silhavy TJ. Lipoprotein SmpA is a component of the YaeT complex that assembles outer membrane proteins in Escherichia coli. Proc Natl Acad Sci U S A. 2007;104:6400–6405. doi: 10.1073/pnas.0701579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malinverni JC, Werner J, Kim S, Sklar JG, Kahne D, Misra R, Silhavy TJ. YfiO stabilizes the YaeT complex and is essential for outer membrane protein assembly in Escherichia coli. Mol Microbiol. 2006;61:151–164. doi: 10.1111/j.1365-2958.2006.05211.x. [DOI] [PubMed] [Google Scholar]
- 23. Hagan CL, Kim S, Kahne D. Reconstitution of outer membrane protein assembly from purified components. Science. 2010;328:890–892. doi: 10.1126/science.1188919. This is the first study demonstrating reconstitution of the Bam machinery in vitro. The unfolded protease OmpT is used as a model substrate, and the folding reaction proceeds devoid of an energy source. Notably, the chaperone SurA is required for full activity
- 24.Hagan CL, Silhavy TJ, Kahne D. beta-Barrel membrane protein assembly by the Bam Complex. Annu Rev Biochem. 2011;80:189–210. doi: 10.1146/annurev-biochem-061408-144611. [DOI] [PubMed] [Google Scholar]
- 25.Gatsos X, Perry AJ, Anwari K, Dolezal P, Wolynec PP, Likic VA, Purcell AW, Buchanan SK, Lithgow T. Protein secretion and outer membrane assembly in Alphaproteobacteria. FEMS Microbiol Rev. 2008;32:995–1009. doi: 10.1111/j.1574-6976.2008.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Albrecht R, Zeth K. Structural basis of outer membrane protein biogenesis in bacteria. J Biol Chem. 2011;286:27792–27803. doi: 10.1074/jbc.M111.238931. This is one of several recent reports describing the crystal structure of Bam complex proteins. Included here are structures of all four accessory Bam lipoproteins
- 27.Sandoval CM, Baker SL, Jansen K, Metzner SI, Sousa MC. Crystal structure of BamD: an essential component of the beta-Barrel assembly machinery of gram-negative bacteria. J Mol Biol. 2011;409:348–357. doi: 10.1016/j.jmb.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fussenegger M, Facius D, Meier J, Meyer TF. A novel peptidoglycan-linked lipoprotein (ComL) that functions in natural transformation competence of Neisseria gonorrhoeae. Mol Microbiol. 1996;19:1095–1105. doi: 10.1046/j.1365-2958.1996.457984.x. [DOI] [PubMed] [Google Scholar]
- 29.Charlson ES, Werner JN, Misra R. Differential effects of yfgL mutation on Escherichia coli outer membrane proteins and lipopolysaccharide. J Bacteriol. 2006;188:7186–7194. doi: 10.1128/JB.00571-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noinaj N, Fairman JW, Buchanan SK. The crystal structure of BamB suggests interactions with BamA and its role within the BAM complex. J Mol Biol. 2011;407:248–260. doi: 10.1016/j.jmb.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim KH, Paetzel M. Crystal structure of Escherichia coli BamB, a lipoprotein component of the beta-barrel assembly machinery complex. J Mol Biol. 2011;406:667–678. doi: 10.1016/j.jmb.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 32.Vuong P, Bennion D, Mantei J, Frost D, Misra R. Analysis of YfgL and YaeT interactions through bioinformatics, mutagenesis, and biochemistry. J Bacteriol. 2008;190:1507–1517. doi: 10.1128/JB.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ureta AR, Endres RG, Wingreen NS, Silhavy TJ. Kinetic analysis of the assembly of the outer membrane protein LamB in Escherichia coli mutants each lacking a secretion or targeting factor in a different cellular compartment. J Bacteriol. 2007;189:446–454. doi: 10.1128/JB.01103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ieva R, Tian P, Peterson JH, Bernstein HD. Sequential and spatially restricted interactions of assembly factors with an autotransporter beta domain. Proc Natl Acad Sci U S A. 2011;108:E383–E391. doi: 10.1073/pnas.1103827108. Photocrosslinking probes the sequential interaction of an autotransporter with the Bam complex
- 35.Voulhoux R, Bos MP, Geurtsen J, Mols M, Tommassen J. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science. 2003;299:262–265. doi: 10.1126/science.1078973. [DOI] [PubMed] [Google Scholar]
- 36.Lloubes R, Cascales E, Walburger A, Bouveret E, Lazdunski C, Bernadac A, Journet L. The Tol-Pal proteins of the Escherichia coli cell envelope: an energized system required for outer membrane integrity? Res Microbiol. 2001;152:523–529. doi: 10.1016/s0923-2508(01)01226-8. [DOI] [PubMed] [Google Scholar]
- 37.Godlewska R, Wisniewska K, Pietras Z, Jagusztyn-Krynicka EK. Peptidoglycan-associated lipoprotein (Pal) of Gram-negative bacteria: function, structure, role in pathogenesis and potential application in immunoprophylaxis. FEMS Microbiol Lett. 2009;298:1–11. doi: 10.1111/j.1574-6968.2009.01659.x. [DOI] [PubMed] [Google Scholar]
- 38.Ades SE. Regulation by destruction: design of the sigmaE envelope stress response. Curr Opin Microbiol. 2008;11:535–540. doi: 10.1016/j.mib.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Kim DY, Kwon E, Choi J, Hwang HY, Kim KK. Structural basis for the negative regulation of bacterial stress response by RseB. Protein Sci. 2010;19:1258–1263. doi: 10.1002/pro.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walsh NP, Alba BM, Bose B, Gross CA, Sauer RT. OMP peptide signals initiate the envelope-stress response by activating DegS protease via relief of inhibition mediated by its PDZ domain. Cell. 2003;113:61–71. doi: 10.1016/s0092-8674(03)00203-4. [DOI] [PubMed] [Google Scholar]
- 41. Kulp A, Kuehn MJ. The recognition of {beta}-strand motifs by RseB is required for {sigma}E activity in Escherichia coli. J Bacteriol. 2011 doi: 10.1128/JB.05657-11. By fusing the C-terminus of various OMPs to the periplasmic carrier protein alkaline phosphatase, the authors identified beta-strand structure as the second signal required to initiate the σE envelope stress response
- 42.Li X, Wang B, Feng L, Kang H, Qi Y, Wang J, Shi Y. Cleavage of RseA by RseP requires a carboxyl-terminal hydrophobic amino acid following DegS cleavage. Proc Natl Acad Sci U S A. 2009;106:14837–14842. doi: 10.1073/pnas.0903289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flynn JM, Levchenko I, Sauer RT, Baker TA. Modulating substrate choice: the SspB adaptor delivers a regulator of the extracytoplasmic-stress response to the AAA+ protease ClpXP for degradation. Genes Dev. 2004;18:2292–2301. doi: 10.1101/gad.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valentin-Hansen P, Johansen J, Rasmussen AA. Small RNAs controlling outer membrane porins. Curr Opin Microbiol. 2007;10:152–155. doi: 10.1016/j.mib.2007.03.001. [DOI] [PubMed] [Google Scholar]