Abstract
The cell surface of Gram-positive pathogens represents a complex association of glycopolymers that control cell division, homeostasis, immune evasion, tissue invasion, and resistance to antimicrobials. These glycopolymers include the peptidoglycan cell wall, wall-teichoic acids, lipoteichoic acids, and capsular polysaccharide. Disruption of individual factors often results in pleiotropic effects, making it difficult to discern regulation and function. In this review we collate recent work describing these pleiotropic phenotypes, and propose that this is due to coordinated regulation of biosynthesis or modification of these cell surface components. A better understanding of the regulatory networks that control the relative prevalence of each factor on the cell surface or their modulated functions may help facilitate the identification of new targets for antimicrobial therapy.
Introduction
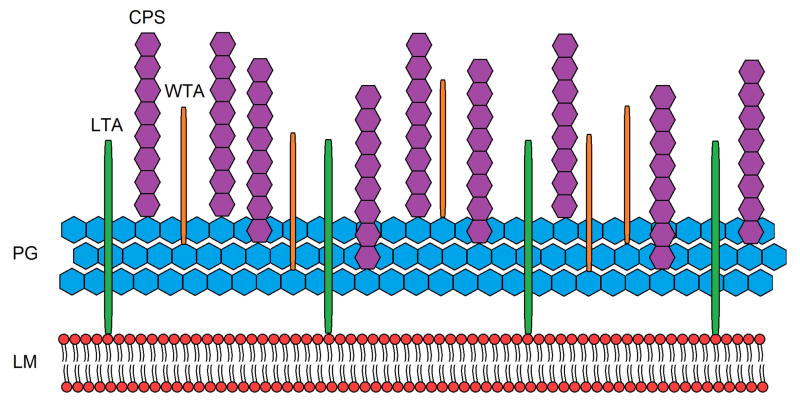
Gram-positive pathogens utilize complex defense mechanisms in response to extracellular stress, be it environmental or host-derived. One such defense mechanism involves modification of individual molecules of the cell surface. Unlike Gram-negative bacteria, Gram-positive species have only a single cell membrane composed of the well-characterized phospholipid bi-layer (Fig. 1). Protection for the membrane is provided by the adjacent thick peptidoglycan layer (PG), which also acts as a scaffold for other components of the cell surface. Interspersed throughout the multilayered peptidoglycan are the anionic teichoic acid molecules that provide cell wall integrity as one of their many functions, as well as supplying the major portion of the overall negative charge of the cell surface. Cell surface-exposed proteins are attached to the cell wall and function in multiple interactions with the extracellular environment. Lastly, a polysaccharide capsule (CPS) serves as the outer most layer of the cell surface, providing additional protection from extracellular assaults. This highly structured consortium functions cooperatively to provide stability and protection from the external environment. Recent work on individual structural components of the cell surface has demonstrated that disturbance of a single factor can result in pleiotropic effects, underscoring the interdependence of each component to the overall function of the cell surface. Consequently, loss or disturbance of any one of these closely associated factors may affect the regulation or ultimate function of another factor, resulting in a change in the equilibrium in which the cell surface typically exists.
Figure 1.
Simplified overview of the cell surface of Gram-positive bacteria, excluding proteins, with the lipid membrane (LM), peptidoglycan (PG), lipoteichoic acid (LTA), capsular polysaccharide (CPS), and wall teichoic acid (WTA).
Although a complete understanding of the cell surface network is currently beyond our grasp, this review will highlight recent work that has provided important new insights into the complex associations occurring between multiple elements that contribute to overall pathogen survival. Consideration of these distinctions is important to our understanding of bacterial pathogen virulence, as the cell surface remains an extremely important target for future antimicrobial therapy [1–3].
Peptidoglycan
The cell surface of Gram-positive pathogens is a highly complex assembly consisting of a lipid bilayer, cell wall peptidoglycan (PG) [4], cell wall associated teichoic acids (WTA) [5], membrane associated lipoteichoic acids (LTA) [6], capsular polysaccharide (CPS) [7], and a variety of proteins associated with the cell membrane [8–10] or covalently attached to the PG of the cell wall [11–13]. Peptidoglycan of the cell surface exists as alternating repeats of N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM) with peptide side chains attached to the NAM residue. Although made up of simple repeating subunits, a complex association network of PG with multiple cell surface components occurs through cross-linking and covalent and non-covalent interactions. Although a number of methods have been employed to arrive at current models of peptidoglycan macromolecular structure, including atomic force microscopy (AFM), nuclear magnetic resonance (NMR), and cryo-transmission electron microscopy (TEM) [14], major obstacles still exist, including the structural diversity of PG between different organisms. Future strategies will likely focus on not just PG structures, but the full complement of interactions between PG, WTAs, LTAs, and CPS, with subsequent comparisons to surface structures in which one or more components are missing or altered by targeted mutations. For an excellent overview on the coordination of peptidoglycan biosynthesis and cell division in streptococcal species, see review by Sham et al., in this issue.
The importance of elucidating the actual structure of PG is highlighted by recent studies involving the deletion of genes with homology to lytR, a member of the lytR_cpsA_psr family, which is associated with transcriptional attenuation of PG hydrolases, their processing and transport. The interruption of LytR function results in pleiotropic effects, such as defective cell division, asymmetric septation, and altered antimicrobial sensitivity [15–17]. Furthermore, the production of multiple phenotypes can be difficult to reconcile, as shown in Streptococcus mutans, where deletion of lytR resulted in longer chain length despite an increase in autolysis [15], two traits typically considered to be the product of opposing processes. A similar phenomenon has been observed with insertional inactivation of cpsA, the putative regulator of the capsule locus in Streptococcus iniae, resulting in much longer chains of cocci [18] and increased autolysis when treated with non-ionic detergents or when grown in culture (B.H. and M.N., unpublished data). These seemingly contradictory findings emphasize the complexity of the PG cell wall network and its regulation by a number of factors, and indicate that unidentified targets of regulation likely exist for LytR family members beyond the PG hydrolase system that has been described thus far. For example, in another Gram-positive pathogen, Staphylococcus aureus, the LytSR two component system (no homology to the streptococcal LytR proteins) controls PG hydrolase activity as well as proteins involved in bacterial programmed cell death, as described in the review by Sadykov and Bayles in this issue. Therefore, a better understanding of the structural form of PG under conditions in which regulatory elements such as LytR are disrupted may provide insight on mechanisms responsible for controlling cell wall integrity.
In addition to regulation of synthesis and recycling of PG, a number of modifications can be made to PG, resulting in altered function. These include O-acetylation of NAM residues catalyzed by the protein OatA, and N-deacetylation of NAG through the functions of the PgdA protein. Both of these modifications confer resistance to lysozyme cleavage of the glycosidic bond between NAM and NAG residues [19]. Regulation of OatA appears to be enacted through two-component systems that sense cell wall stress resulting in upregulation of oatA expression [20]. Importantly, WTA is covalently attached to the same C-6 atom of NAM that is O-acetylated, suggesting there may be some cross-regulation of these processes [5]. Expression of pgdA has been shown to be induced by oxidative stress in the Gram-negative pathogen Helicobacter pylori [21], and similar regulation may exist for PgdA homologues recently identified in Gram-positive pathogens [22]. Taken together, a number of regulatory schemes involved in PG synthesis, turnover through autolysin activity, and modification of PG residues converge to provide bacteria with a stable and functional cell wall.
Lipoteichoic acids and wall-teichoic acids
Teichoic acids of Gram-positive species represent an interesting subset of the cell surface, demonstrating a wide variety of functions including invasion of host tissue [23,24], regulation of autolysis [25–27], and regulation of cell division [5,26,28]. Cell wall-associated teichoic acid (WTA) and lipoteichoic acid (LTA) differ in overall structure, with WTA covalently attached to NAM [5] and LTA anchored to the membrane via a glycolipid [29]. However, similar modifications are made to both WTA and LTA, such as D-alanylation [30], which has been shown to facilitate resistance to cationic antimicrobial peptides, glycopeptides, lytic enzymes produced by neutrophils [5] and reduced autolytic activity [27]. The similar processing of WTA and LTA appears to provide some functional redundancy as disruption of both pathways simultaneously is lethal [26], however, the phenotypes exhibited by individual disruption of LTA or WTA vary considerably. Disruption of LTA has been associated with a decrease in autolysis in Staphylococcus aureus, and this seems to be associated with reduced levels of cell wall-associated hydrolases [26]. This is consistent with the prediction that LTA actively recruits autolysins to septal regions during cell division to facilitate daughter cell separation [31]. The association between LTA and autolysins is not currently known, but could include direct binding of autolysins to LTA, or enhanced substrate accessibility for autolysins in the presence of LTA.
The observations for LTA are in striking contrast to those found for WTA in which disruption results in increased autolysis [32] and a concomitant decrease in lysozyme resistance [25]. In S. aureus, WTA is hypothesized to indirectly mediate targeting of autolysins to newly synthesized PG by excluding its access to older PG where WTA is present, thus promoting its access to septal PG where WTA is absent and assisting with daughter cell separation [32]. These hypotheses are supported by the observation that loss of WTA results in indiscriminate binding of autolysins to the cell surface instead of preferential localization to the septum [32]. In addition to spatial regulation of autolysin activity, WTA has been shown to regulate peptidoglycan crosslinking in a spatial and temporal manner [25]. Localized synthesis of intermediate forms of WTA at the septum appears to indicate the presence of a mature cell wall, and temporally triggers penicillin-binding proteins (PBP) to initiate crosslinking. Temporal regulation of this process may be important in permitting the introduction of proteins and glycopolymers that may not be able to penetrate a highly crosslinked cell wall [25].
Taken together, LTA and WTA appear to exert opposing and complementary functions during daughter cell separation with LTA promoting autolysis at the septum while WTA selectively blocks autolysis elsewhere on the cell. WTA intermediates subsequently accumulate at the septum, recruiting PBPs which crosslink the PG, forming a mature cell wall. These observations suggest a tight regulatory scheme over the localization of both elements during cell division, the mechanism of which is still not fully described. This principle may explain observed abnormalities in Bacillus subtilis morphology [28] and S. aureus cell division [26] when LTA is disrupted. Similarly, CPS and WTA have been demonstrated to have direct effects on each other. Phase variation in Streptococcus pneumoniae to a form that results in increased virulence relies on a switch from relative low levels of CPS and high levels of WTA to relative high levels of CPS and low levels of WTA [33]. Whether this results from direct competition for covalent attachment to PG, or if it is due to a regulatory pathway that co-regulates levels of both CPS and WTA is not understood. Clearly, WTA and LTA exert a fine tuned control over a number of important processes, including cell division and resistance to cell wall reactive antimicrobial agents. The effect that differing levels of CPS has on these traits has not been explored in depth, and it may be that the absence or presence of CPS contributes to the dynamic equilibrium experienced by components of the cell surface.
Capsule
The CPS of Gram-positive organisms can be covalently linked to a variety of surface structures, with attachment to the peptide moiety of PG for Bacillus anthracis [34], attachment to N-acetylglucosamine of PG for Streptococcus agalactiae [35], and covalent attachment to the PG or membrane for S. pneumonia [7]. The enzyme that catalyzes the covalent addition of CPS to these locations has not been determined for many Gram-positive species, including S. agalactiae and S. pneumoniae [7]. The location of CPS linkage is important to consider in the context of the cell surface as WTA may compete for these ligation sites, or experience steric hindrance in the presence of CPS [7]. CPS appears to be generally linked to NAG instead of NAM in S. agalactiae and S. pneumoniae [7], therefore steric limitation may explain the relative balance between WTA and CPS described above for S. pneumoniae. An understanding of how CPS ligation is controlled may shed light on regulation of the other modifications that occur at or near this location.
Recent reports indicate that the presence or absence of CPS has a significant effect on minimum bactericidal activity of a number of cell wall reactive agents for Streptococcus suis [36] as well as vancomycin resistance in S. pneumoniae [37]. Insertional inactivation of cpsA, which encodes the putative regulator of capsule synthesis in S. iniae, results in various changes to antimicrobial sensitivity from cell wall-targeted compounds, with the cpsA mutant demonstrating decreased capsule levels in conjunction with increased resistance to lysozyme and bacitracin, and decreased resistance to ampicillin and methicillin (B.H. and M.N., unpublished data). These results indicate a clear association between expression of CPS and cell wall integrity, which may be mediated by the CpsA regulator protein. The exact mechanism mediating these events is unclear, though reasonable suggestions have been proposed based on simple occlusion of antibiotics via capsular stereochemistry or its contribution to structural stability [36,37]. These effects are most likely the result of more specific actions associated with perturbation of the cell surface, and these phenotypes may be explained by considering the relative changes in PG, WTA, and LTA and the pleiotropic effects that may occur with loss of CPS. Whether these regulatory events happen in response to the host environment is currently not known; however, evidence exists for the regulation of CPS expression in-vivo, with the observation that levels of CPS are decreased when S. pneumoniae cocci come in contact with the surface of epithelial cells [38]. This scenario suggests that bacteria may dynamically regulate levels or processing of CPS, WTA, and LTA in response to host signals, essentially altering the cell surface from prevalent CPS and immune evasion function to prevalent D-alanylated WTA and LTA with attachment and colonization function, as WTA and LTA have been shown to mediate adhesion to host cells [39]. Supporting this model is the previously mentioned observation that CPS and WTA levels are coordinately altered during phase variation in S. pneumoniae [33].
CPS in S. pneumoniae also has a direct effect on the number of bacterial cells present in a single chain [40], with the presence of capsule generally leading to longer chains in S. pneumoniae. The observation that this trait varies when secondary mutations are made to genes responsible for regulation of cell division further underscores the complexity of the cell surface and its regulation [40,41]. The deletion of cpsA in S. agalactiae and S. iniae results in decreased production of CPS [18,42] which actually coincides with longer chains (18), (B.H. and M.N., unpublished). Whether this phenotype is a consequence of reduced capsule is unclear, or alternatively, CpsA may actively contribute to regulation of cell division. The discrepancy between these observations suggests that fundamental differences exist between S. pneumoniae and S. agalactiae concerning regulation of the cell surface, and may relate to the presence of multiple cell wall processing enzymes in S. pneumoniae that are absent in S. agalactiae, such as the PG hydrolases LytA and LytC. As with the other components of the cell surface, this indicates that the role of CPS and its effects on regulation of the cell surface in Gram-positive pathogens may indeed be species-specific.
Conclusions
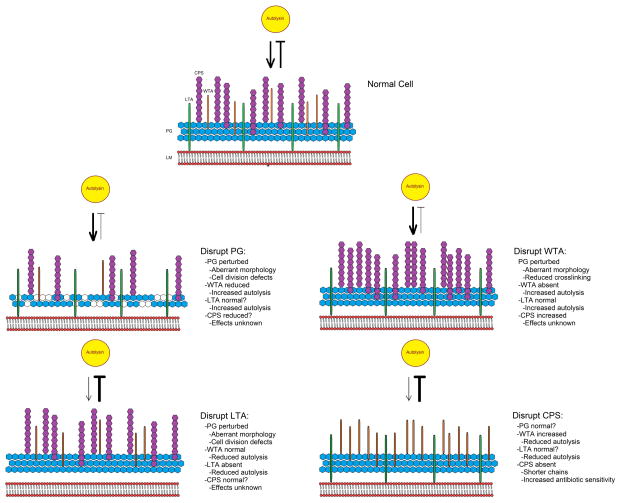
Clearly, analysis of the bacterial surfome should include the contributions made by PG, WTAs, LTAs, and CPS as each plays an individual role in pathogenicity and can have profound effects on the associations occurring in the overall architectural network. “Fingerprinting” or “protein profiling” is routinely performed to determine specific protein components of various strains and species using two-dimensional electrophoresis along with Mass spectrometry. Such “fingerprinting” methods would be useful for characterization of the composition and relative abundance of surface associated glycosaminoglycan structures, allowing differentiation between species and strains under specific conditions, particularly as the intricate associations between cell surface components and the corresponding implications for virulence and antimicrobial treatment are unraveled. Accomplishing this goal necessitates technological enhancements in the methods currently used to probe the bacterial cell surface. The determination that PG, LTA, WTA, and CPS have a shared pool of precursors and, with the exception of LTA, also share the undecaprenyl-phosphate acceptor (Und-P) for repeat unit synthesis [7,30] raises interesting questions about how precursor fate and prioritization of Und-P for different substrates is controlled. Ostensibly, this series of interconnected pathways has important points of regulation (Fig. 2), and work describing regulation of branch points in this network or the point at which precursor fate is decided is currently incomplete. A better understanding of how each of these components relates functionally to one another in Gram-positive bacteria and the coordinated control of the enzymes that facilitate their construction and eventual fate remains an exceedingly important task in a future beset by the onset of antimicrobial resistance and vaccine escape through serotype diversity.
Figure 2.
Proposed model detailing the pleiotropic effects caused by disruption of individual components of the cell surface. LM: Lipid membrane; PG: peptidoglycan; LTA: lipoteichoic acid; CPS: capsular polysaccharide; WTA: wall-teichoic acid. Yellow circle represents an autolysin. Bold lines indicate an increase in function, while narrow lines indicate a decrease in function.
Table 1.
Effects on the cell surface by individual component disruption
| Altered componenta | ||||
|---|---|---|---|---|
| PG | CPS | WTA | LTA | |
| PG | Abnormal morphology [16] Assymetric septum [16] Increased autolysis [15] Increased chain length [15] |
Increased sensitivity to antimicrobials [36,37] Altered chain length [39,40] |
Increased autolysis [32] Decreased lysozyme resistance [25] Decreased PG crosslinking [23,25] Perturbation of PG [23] Aberrant morphology [23] |
Decreased autolysis [26] Abnormal morphology [28] Aberrant cell division [26] Increased chain length [28] |
| CPS | Covalent attachment of CPS to PG [7,34,35] | Not considered [7] | Increased levels of CPS [33] | Unknown |
| WTA | Covalent attachment of WTA to PG [5] | Increased levels of WTA [33] | Not considered [30,3] | Can compensate for essential function of LTA [5,26] |
| LTA | β-lactam treatment causes release of LTA [42] | Unknown | Can compensate for essential function of WTA [5,26] | Not considered [3,30] |
A collection of phenotypes exhibited when components at the top are disrupted in some way in relation to the other components of the cell surface. Categories “Not considered” were judged to be outside the scope of this summary and have been described at length in the associated citation. Categories that are “Unknown” did not have significant findings to present in this context.
Highlights.
Peptidoglycan, teichoic acids and capsule are a major part of the cell surface network
Cell surface components function cooperatively to provide stability and protection
Recent work highlights the importance of understanding surface component associations
Greater understanding of cell surface network will provide new antimicrobial targets
Acknowledgments
Work in the Neely lab was funded by Public Health Service Grants AI52141 and AI078147 from the NIAID of the National Institutes of Health.
Due to the abbreviated length of this review, it was not possible to discuss all of the recent work done on regulation of Gram-positive cell surface components and we apologize for those which we were not able to include.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Brett R. Hanson, Email: brhanson@med.wayne.edu.
Melody N. Neely, Email: mneely@med.wayne.edu.
References
* of special interest
** of outstanding interest
- 1.Bugg TDH, Braddick D, Dowson CG, Roper DI. Bacterial cell wall assembly: still an attractive antibacterial target. Trends in Biotechnology. 2011;29:167–173. doi: 10.1016/j.tibtech.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Llarrull LI, Testero SA, Fisher JF, Mobashery S. The future of the β-lactams. Current Opinion in Microbiology. 2010;13:551–557. doi: 10.1016/j.mib.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3*.Schneider T, Sahl H-G. An oldie but a goodie – cell wall biosynthesis as antibiotic target pathway. International Journal of Medical Microbiology. 2010;300:161–169. doi: 10.1016/j.ijmm.2009.10.005. This recent article describes the mechanism of action of many cell wall reactive antimicrobial agents and discusses the future of targeting the bacterial cell wall as a therapeutic strategy. [DOI] [PubMed] [Google Scholar]
- 4*.Vollmer W, Blanot D, De Pedro MA. Peptidoglycan structure and architecture. FEMS Microbiology Reviews. 2008;32:149–167. doi: 10.1111/j.1574-6976.2007.00094.x. This review article describes the chemical structure of PG in a variety of species, and considers the macromolecular architecture of the cell wall. [DOI] [PubMed] [Google Scholar]
- 5**.Swoboda JG, Campbell J, Meredith TC, Walker S. Wall Teichoic Acid Function, Biosynthesis, and Inhibition. ChemBioChem. 2010;11:35–45. doi: 10.1002/cbic.200900557. This article gives a comprehensive overview of WTA and describes recent work that they and others have performed on targeting WTA as an antibacterial strategy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt RR, Pedersen CM, Qiao Y, Zahringer U. Chemical synthesis of bacterial lipoteichoic acids: An insight on its biological significance. Organic & Biomolecular Chemistry. 2011;9:2040–2052. doi: 10.1039/c0ob00794c. [DOI] [PubMed] [Google Scholar]
- 7**.Yother J. Capsules of Streptococcus pneumoniae and Other Bacteria: Paradigms for Polysaccharide Biosynthesis and Regulation. Annual Review of Microbiology. 2011;65:563–81. doi: 10.1146/annurev.micro.62.081307.162944. This recent paper gives a thorough overview of what is known regarding synthesis and regulation of CPS production for a variety of bacterial species, with a focus on S. pneumoniae. [DOI] [PubMed] [Google Scholar]
- 8.Sutcliffe I, Russell R. Lipoproteins of gram-positive bacteria. J Bacteriol. 1995;177:1123–1128. doi: 10.1128/jb.177.5.1123-1128.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reffuveille F, Leneveu C, Chevalier S, Auffray Y, Rincé A. Lipoproteins of Enterococcus faecalis: bioinformatic identification, expression analysis and relation to virulence. Microbiology. 2011 doi: 10.1099/mic.0.053314-0. [DOI] [PubMed] [Google Scholar]
- 10*.Kovacs-Simon A, Titball RW, Michell SL. Lipoproteins of Bacterial Pathogens. Infect Immun. 2011;79:548–561. doi: 10.1128/IAI.00682-10. A recent overview of lipoprotein biosynthesis and the relationship of lipoproteins to bacterial pathogenesis, covering important topics outside the scope of this review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.Marraffini LA, DeDent AC, Schneewind O. Sortases and the Art of Anchoring Proteins to the Envelopes of Gram-Positive Bacteria. Microbiology and Molecular Biology Reviews. 2006;70:192–221. doi: 10.1128/MMBR.70.1.192-221.2006. This review comprehensively covers the covalent attachment of proteins to PG and how this relates to bacterial physiology and pathogenesis, which are important topics outside the scope of this review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paterson GK, Mitchell TJ. The biology of Gram-positive sortase enzymes. Trends in Microbiology. 2004;12:89–95. doi: 10.1016/j.tim.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Ton-That H, Marraffini LA, Schneewind O. Protein sorting to the cell wall envelope of Gram-positive bacteria. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2004;1694:269–278. doi: 10.1016/j.bbamcr.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Vollmer W, Seligman SJ. Architecture of peptidoglycan: more data and more models. Trends in Microbiology. 2010;18:59–66. doi: 10.1016/j.tim.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Chatfield CH, Koo H, Quivey RG., Jr The putative autolysin regulator LytR in Streptococcus mutans plays a role in cell division and is growth-phase regulated. Microbiology. 2005;151:625–631. doi: 10.1099/mic.0.27604-0. [DOI] [PubMed] [Google Scholar]
- 16.Johnsborg O, Havarstein LS. Pneumococcal LytR, a Protein from the LytR-CpsA-Psr Family, Is Essential for Normal Septum Formation in Streptococcus pneumoniae. The Journal of Bacteriology. 2009;191:5859–5864. doi: 10.1128/JB.00724-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Over B, Heusser R, McCallum N, Schulthess B, Kupferschmied P, Gaiani JM, Sifri CD, Berger-Bächi B, Stutzmann Meier P. LytR-CpsA-Psr proteins in Staphylococcus aureus display partial functional redundancy and the deletion of all three severely impairs septum placement and cell separation. FEMS Microbiology Letters. 2011;320:142–151. doi: 10.1111/j.1574-6968.2011.02303.x. [DOI] [PubMed] [Google Scholar]
- 18.Miller J. Large Scale Screen Highlights the Importance of Capsule for Virulence in the Zoonotic Pathogen, Streptococcus iniae. Infection and Immunity. 2005 doi: 10.1128/IAI.73.2.921-934.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Davis KM, Weiser JN. Modifications to the Peptidoglycan Backbone Help Bacteria To Establish Infection. Infection and Immunity. 2011;79:562–570. doi: 10.1128/IAI.00651-10. This recent paper describes modifications of PG by bacterial pathogens that leads to resistance to lysozyme and immune evasion and how these modification systems are regulated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Jordan S, Hutchings MI, Mascher T. Cell envelope stress response in Gram-positive bacteria. FEMS Microbiology Reviews. 2008;32:107–146. doi: 10.1111/j.1574-6976.2007.00091.x. This paper reviews regulation of the cell envelope stress response and its implications for physiology and homeostasis of Gram-positive bacteria. [DOI] [PubMed] [Google Scholar]
- 21.Wang G, Olczak A, Forsberg LS, Maier RJ. Oxidative Stress-induced Peptidoglycan Deacetylase in Helicobacter pylori. Journal of Biological Chemistry. 2009;284:6790–6800. doi: 10.1074/jbc.M808071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aziz R. In silico analysis of a family of extracellular polysaccharide deacetylases involved in virulence of pathogenic gram-positive cocci. BMC Bioinformatics. 2010;11 :P8. [Google Scholar]
- 23.Doran KS, Engelson EJ, Khosravi A, Maisey HC, Fedtke I, Equils O, Michelsen KS, Arditi M, Peschel A, Nizet V. Blood-brain barrier invasion by group B Streptococcus depends upon proper cell-surface anchoring of lipoteichoic acid. The Journal of Clinical Investigation. 2005;115:2499–2507. doi: 10.1172/JCI23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheen T, Ebrahimi C, Hiemstra I, Barlow S, Peschel A, Doran K. Penetration of the blood–brain barrier by Staphylococcus aureus contribution of membrane-anchored lipoteichoic acid. Journal of Molecular Medicine. 2010;88:633–639. doi: 10.1007/s00109-010-0630-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atilano ML, Pereira PM, Yates J, Reed P, Veiga H, Pinho MG, Filipe SR. Teichoic acids are temporal and spatial regulators of peptidoglycan cross-linking in Staphylococcus aureus. Proceedings of the National Academy of Sciences. 2010;107:18991–18996. doi: 10.1073/pnas.1004304107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Oku Y, Kurokawa K, Matsuo M, Yamada S, Lee B-L, Sekimizu K. Pleiotropic Roles of Polyglycerolphosphate Synthase of Lipoteichoic Acid in Growth of Staphylococcus aureus Cells. The Journal of Bacteriology. 2009;191:141–151. doi: 10.1128/JB.01221-08. Work described in this article identified permissive conditions for an LTA deficient S. aureus mutant, and characterizes the role of LTA in cell division and control of autolysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peschel A, Vuong C, Otto M, Gotz F. The D-Alanine Residues of Staphylococcus aureus Teichoic Acids Alter the Susceptibility to Vancomycin and the Activity of Autolytic Enzymes. Antimicrobial Agents and Chemotherapy. 2000;44:2845–2847. doi: 10.1128/aac.44.10.2845-2847.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schirner K, Marles-Wright J, Lewis RJ, Errington J. Distinct and essential morphogenic functions for wall- and lipo-teichoic acids in Bacillus subtilis. EMBO J. 2009;28:830–842. doi: 10.1038/emboj.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer W. Physiology of lipoteichoic acids in bacteria. Adv Microb Physiol. 1988;29:233–302. doi: 10.1016/s0065-2911(08)60349-5. [DOI] [PubMed] [Google Scholar]
- 30.Xia G, Kohler T, Peschel A. The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus. International Journal of Medical Microbiology. 2010;300:148–154. doi: 10.1016/j.ijmm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Yamada S, Sugai M, Komatsuzawa H, Nakashima S, Oshida T, Matsumoto A, Suginaka H. An autolysin ring associated with cell separation of Staphylococcus aureus. The Journal of Bacteriology. 1996;178:1565–1571. doi: 10.1128/jb.178.6.1565-1571.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlag M, Biswas R, Krismer B, Kohler T, Zoll S, Yu W, Schwarz H, Peschel A, Götz F. Role of staphylococcal wall teichoic acid in targeting the major autolysin Atl. Molecular Microbiology. 2010;75:864–873. doi: 10.1111/j.1365-2958.2009.07007.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim JO, Weiser JN. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J Infect Dis. 1998;177:368–377. doi: 10.1086/514205. [DOI] [PubMed] [Google Scholar]
- 34.Candela T, Fouet A. Bacillus anthracis CapD, belonging to the γ-glutamyltranspeptidase family, is required for the covalent anchoring of capsule to peptidoglycan. Molecular Microbiology. 2005;57:717–726. doi: 10.1111/j.1365-2958.2005.04718.x. [DOI] [PubMed] [Google Scholar]
- 35.Deng L, Kasper DL, Krick TP, Wessels MR. Characterization of the linkage between the type III capsular polysaccharide and the bacterial cell wall of group B Streptococcus. J Biol Chem. 2000;275:7497–7504. doi: 10.1074/jbc.275.11.7497. [DOI] [PubMed] [Google Scholar]
- 36.Tanabe S, Bonifait L, Fittipaldi N, Grignon L, Gottschalk M, Grenier D. Pleiotropic effects of polysaccharide capsule loss on selected biological properties of Streptococcus suis. Can J Vet Res. 2010;74:65–70. [PMC free article] [PubMed] [Google Scholar]
- 37.Moscoso M, Domenech M, García E. Vancomycin tolerance in clinical and laboratory Streptococcus pneumoniae isolates depends on reduced enzyme activity of the major LytA autolysin or cooperation between CiaH histidine kinase and capsular polysaccharide. Molecular Microbiology. 2010;77:1052–1064. doi: 10.1111/j.1365-2958.2010.07271.x. [DOI] [PubMed] [Google Scholar]
- 38.Hammerschmidt S, Wolff S, Hocke A, Rosseau S, Muller E, Rohde M. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect Immun. 2005;73:4653–4667. doi: 10.1128/IAI.73.8.4653-4667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Weidenmaier C, Peschel A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat Rev Micro. 2008;6:276–287. doi: 10.1038/nrmicro1861. This article provides an instructive overview of cell-wall glycopolymers in Gram-positive organisms, some of which are outside the scope of this article, and the functions they exact during pathogenesis. [DOI] [PubMed] [Google Scholar]
- 40.Barendt SM, Land AD, Sham L-T, Ng W-L, Tsui H-CT, Arnold RJ, Winkler ME. Influences of Capsule on Cell Shape and Chain Formation of Wild-Type and pcsB Mutants of Serotype 2 Streptococcus pneumoniae. The Journal of Bacteriology. 2009;191:3024–3040. doi: 10.1128/JB.01505-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barendt SM, Sham L-T, Winkler ME. Characterization of Mutants Deficient in the L, D-Carboxypeptidase (DacB) and WalRK (VicRK) Regulon, Involved in Peptidoglycan Maturation of Streptococcus pneumoniae Serotype 2 Strain D39. The Journal of Bacteriology. 2011;193:2290–2300. doi: 10.1128/JB.01555-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cieslewicz MJ, Kasper DL, Wang Y, Wessels MR. Functional analysis in type Ia group B Streptococcus of a cluster of genes involved in extracellular polysaccharide production by diverse species of streptococci. J Biol Chem. 2001;276:139–146. doi: 10.1074/jbc.M005702200. [DOI] [PubMed] [Google Scholar]