Abstract
Objective
Early-life stress (ES) such as adoption, change of caregiver, or experience of emotional neglect [0]may influence the way in which affected individuals respond to emotional stimuli of positive or negative valence. These modified responses may stem from a direct alteration of how emotional stimuli are coded, and/or the cognitive function implicated in emotion modulation, such as self-regulation or inhibition. These ES effects have been probed on tasks either targeting reward and inhibitory function. Findings revealed deficits in both reward processing and inhibitory control in ES youths. However, no work has yet examined whether incentives can improve automatic response or inhibitory control in ES youths.
Method
To determine whether incentives would only improve self-regulated voluntary actions or generalize to automated motoric responses, participants were tested on a mixed eye movement task that included reflex-like prosaccades and voluntary controlled antisaccade eye movements. Seventeen adopted children (10 females, mean age 11.3 years) with a documented history of neglect and 29 typical healthy youths (16 females, mean age 11.9 years) performed the mixed prosaccade/antisaccade task during monetary incentive conditions or during no-incentive conditions.
Results
Across both saccade types, ES adolescents responded more slowly than controls. As expected, control participants committed fewer errors on antisaccades during the monetary incentive condition relative to the no-incentive condition. By contrast, ES youths failed to show this incentive-related improvement on inhibitory control. No significant incentive effects were found with prepotent prosaccades trials in either group. Finally, co-morbid psychopathology did not modulate the findings.
Conclusions
These data suggest that youths with experience of early stress exhibit deficient modulation of inhibitory control by reward processes, in tandem with a reward-independent deficit in preparation for both automatic and controlled responses. These data may be relevant to interventions in ES youths.
Keywords: reward, antisaccade, cognitive control, early adversity, stress
Introduction
Early-life stress (ES), in the form of physical or sexual abuse, or emotional neglect, confers risk for various forms of psychopathology (Green et al., 2010; Kilpatrick et al., 2003; Stein et al., 1996). ES is also experienced when toddlers and children are given up for adoption by their biological parents or are removed from their original caregivers because of maltreatment and placed into foster care (Nelson et al., 2007). Experience of these different types of maltreatment or stress during change of caregiver may significantly impact cognitive and motivational functioning. Cognitive functions, on the one hand, include diverse processes such as visual attention, self-regulatory control, or short and long-term memory. Motivation, on the other hand, can be defined as the energy invested to complete an action (or cognitive function). Positive incentives provided by reward or negative incentives provided by punishment can significantly enhance motivation. Within the context of maltreatment and early-stress, negative experiences with caregivers may alter sensitivity to rewarding or punishing incentives. Such ES-related changes in response to incentives may increase vulnerability for psychopathology. Indeed, psychopathologies associated with ES such as mood and anxiety disorders, are characterized by impaired responses to incentives (Eshel & Roiser, 2010; Figee et al., 2011).
Deficits in reward-related processing have been reported in adolescents (Guyer et al., 2006) and adults (Dillon et al., 2009) with a history of ES. For example, when given the opportunity to win money on a wheel-of-fortune task, one would expect participants to respond faster on reward trials than on trials where no such gains could be made. However, while this is indeed the case for healthy adolescents, ES youths fail to show speeded response times during incentive conditions (Guyer et al., 2006). Such motivational deficits are mirrored by changes in the underlying brain regions that subserve processing of incentives. A study in adults with a history of abuse has documented changes in basal ganglia reactivity to positive incentive (Dillon et al., 2009).
In parallel, executive function and associated self-regulatory control are critical cognitive skills required in everyday life. At a developmental level, cognitive control may refer to the ability to delay gratification of an immediate reward for a larger reward later on (Mischel, Shoda, & Rodriguez, 1989) or the ability to regulate and inhibit impulsive behavior and emotional outbursts (Fox & Calkins, 2003). Thus, one important question is whether providing positive or negative incentive in ES could facilitate self-regulatory inhibitory control in this group. A related question is whether a change in sensitivity to positive and negative incentives during the developmental period increases risk for later psychopathology. Developmental studies have suggested that typically developing children and adolescents can improve their performance on tasks that require strong inhibitory control when presented with the opportunity to gain a reward (Geier, Terwilliger, Teslovich, Velanova, & Luna, 2010; Jazbec et al., 2006). By contrast, cognitive control deficits have been reported in children and adolescents with ES (Bos, Fox, Zeanah, & Nelson, 2009; Carrion, Garrett, Menon, Weems, & Reiss, 2008; Lewis, Dozier, Ackerman, & Sepulveda-Kozakowski, 2007). For example, in a functional Magnetic Resonance Imaging (fMRI) study, we recently showed that ES adolescents responded slower than controls when they were required to inhibit a prepotent response and execute a less familiar response instead (Mueller, Maheu, et al., 2010). Again, in ES youths this impairment in inhibitory control was mirrored by changes in neural circuitry commonly associated with such processes including the striatum and prefrontal cortex.
The association of early adversity with deficits in both reward processing (Guyer et al., 2006) and cognitive control (Carrion et al., 2008; Lewis et al., 2007; Mueller, Maheu, et al., 2010) would suggest that a positive influence of incentive on cognitive control typically seen in unaffected controls might be impaired in children with a history of ES. An ideal way to examine whether positive or negative incentives as measured by potential monetary gains or losses influences inhibitory control is the antisaccade task. This task requires the inhibition of a prepotent eye movement to a peripherally appearing target (the prosaccade response), and the generation of an eye movement to the opposite direction (the antisaccade) (Hallett & Adams, 1980). Several advantages of the antisaccade task over other common cognitive control tasks that are performed with manual responses deserve mention.
First, the neural circuitry of pro- and antisaccades has been extensively investigated in human and non-human primates and the underlying neurobiology of this task is well-understood (Munoz & Everling, 2004). This knowledge permits us to formulate predictions about the seat of neural dysfunction associated with performance impairment on this task. Second, the saccade task provides the opportunity to compare the integrity of automatic responses (prosaccade) to that of cognitively-controlled responses (antisaccades). Such comparison informs the extent to which impairments on this task might comprise pure motoric, cognitive control abilities, or both. Third, saccade tasks have been extensively used in clinical settings for the study of a range of psychopathologies (Biscaldi, Fischer, & Aiple, 1994; Klein, Raschke, & Brandenbusch, 2003; Mostofsky, Lasker, Singer, Denckla, & Zee, 2001; Mueller, Jackson, Dhalla, Datsopoulos, & Hollis, 2006; Rommelse, Van der Stigchel, & Sergeant, 2008), and in the developmental studies of children, adolescents, and adults (Luna et al., 2001). Fourth, studies using this task have demonstrated utility in assessing the influence of incentives on inhibitory control. For example, studies have shown that antisaccade performance improves under (monetary) reward conditions relative to no reward (Duka & Lupp, 1997; Jazbec et al., 2006). This performance enhancement is deficient in youths with psychopathology such as anxiety disorder (Hardin et al., 2009; Jazbec, McClure, Hardin, Pine, & Ernst, 2005) or bipolar disorder (Mueller, Ng, et al., 2010). In addition, previous studies did not find an effect of maltreatment on motor processing in manual tasks (De Bellis et al., 2010). By contrast, antisaccade performance in youths with ES has not yet been investigated.
This study examines the impact of incentives on cognitive control using a validated version of the monetary incentive antisaccade task (Jazbec et al., 2005). We predicted that children with ES, much like children with mood and anxiety disorders, would show impaired inhibitory control (Carrion et al., 2008; Lewis et al., 2007; Mueller, Maheu, et al., 2010) and reward processing (Dillon et al., 2009; Guyer et al., 2006). Thus, we expected that the ES group would present (a) impaired inhibitory control, indexed by higher antisaccade error rates, relative to the control group, and (b) the failure of incentives to improve inhibitory control, in contrast to the expected improvement seen in typical children (Jazbec et al., 2006). By comparison, no such modulatory effect of ES would be present for prepotent prosaccade responses. Collectively, these predictions would be reflected in a significant three-way interaction between group, reward, and saccade type.
Method
Participants
Seventeen youths who experienced early life stress (M = 11.32 years, SD = 1.89 years, 10 females) and 29 healthy control participants (M = 11.93 years, SD = 2.36 years, 16 females), completed the study (see Table 1). Groups differed on IQ, t(44) = −3.32, p < .05, but not age, t(44) = −0.89, ns, sex distribution, x2(1)=0.06, ns, or socioeconomic status; education: t(44) = −0.44, ns; annual family income: t(42) = −0.23, ns. Of note, ES youths exhibited an average IQ of M = 104.65 (SD = 13.23), while controls exhibited a higher than average IQ of M = 116.07 (SD = 9.98). Thus, IQ was covaried in all subsequent analyses. We chose to recruit ~60% more controls than ES subjects for adequate matching on sex and age and in order to reduce inter-subject variance, increase statistical power, and to obtain a truer representation of the mean for typical adolescents, given variability in developmental trajectories among adolescents. The study was approved by the Institutional Review Boards of the National Institute of Mental Health (NIMH) and the University of Delaware. The parents of participants provided written informed consent while the adolescents provided written assent.
Table 1.
Demographic and clinical characteristics for the ES and control group. Unless indicated all data are Mean (SD).
| Demographic information | Adopted youths (n =17) | Unaffected controls (n = 29) | P-value |
|---|---|---|---|
| Age | 11.3 (1.9) | 11.9 (2.4) | ns |
| IQ | 104.7 (13.2) | 116.1 (9.9) | <.05 |
| SES income | 7.4 (1.7) | 7.3 (1.0) | ns |
| SES education | 6.2 (1.2) | 6.1 (0.8) | ns |
| Female (N) | 10 | 16 | ns |
| State anxiety (STAI)* | 29.5 (4.5)/29.8 (4.1) | 27.6 (4.5) | ns/ns |
| Trait anxiety (STAI)* | 29.5 (5.9)/29.8 (6.1) | 28.0 (7.0) | ns/ns |
| Depression (CDI)* | 44.8 (9.2)/44.0 (8.5) | 40.3 (5.3) | <.05/ns |
| Co-morbid psychopathology | |||
| Anxiety disorder | 3 | - | |
| Bipolar Disorder | 1 | - | |
| Medication | 1 | - | |
| Enuresis psychiatric disorder removed. | 1 | - |
Means (SD) for all participants in the ES group/means (SD) for participants in ES group with the four subjects with diagnosis of
Recruitment and inclusion and exclusion criteria
ES adolescents were recruited from a cohort of a larger, ongoing study, the Infant Caregiver Project. Subjects from this cohort have also participated in previous fMRI studies (Maheu et al., 2010; Mueller, Maheu, et al., 2010). The mean age of adoption among ES youths was around 24 1/2 months of age (~2 years, +/−20 months) after being given up for adoption by their biological parents (N = 13) or being removed from their parents because of severe chronic neglect (N = 4). None of the invited participants dropped out of the study. However, one participant was excluded from the study due to a very low IQ (IQ = 64). All other participants (ES youths and controls) had an IQ > 80. Participants for the healthy control group were recruited by advertisement in the local newspapers. Inclusion criteria for this group required participants to reside with their biological parents and an absence of medical or psychiatric problems and no history of maltreatment as established by the Trauma section of the K-SADS (see below).
Measures
Kiddie Schedule for Affective Disorders and Schizophrenia for School age children
Psychiatric history was examined using the Kiddie Schedule for Affective Disorders and Schizophrenia for School age children (K-SADS-PL; (Kaufman et al., 1997), a semi-structured diagnostic interview, which assesses present and lifetime episodes of child and adolescent psychopathology. Interviews were administered to both children and parents by experienced clinicians with established inter-rater reliability (k > 0.90). Given the nature of the study, the Trauma section of the K-SADS was also administered to all participants. Parents (adoptive parents for the ES group, biological parents for the control group) were also interviewed about the adolescent participant’s behaviors. Four adolescents from this ES group suffered from co-morbid psychopathology, two of whom suffered from specific phobias, one from social phobia and generalized anxiety disorder and one from bipolar disorder. Only the last patient was on medication at the time of testing (see Table 1).
Wechsler Abbreviated Scale of Intelligence
Intelligence Quotient was scored using the Wechsler Abbreviated Scale of Intelligence (WASI; (Wechsler, 1999) using the full-scale IQ 2 subtests (FSIQ-2) consisting of Vocabulary and Matrix Reasoning abilities. The reliability of these subtests in children has been shown to range from .86 to .93 for the Vocabulary test and from .86 to .96 for the Matrix Reasoning test (Wechsler, 1999).
State Trait Anxiety Inventory
Levels of anxiety were assessed with the State Trait Anxiety Inventory (STAI-C; (Spielberger, Gorsuch, & Lushene, 1970). The STAI is a widely-used 40-item self-report questionnaire that measures levels of state (20 items) and trait (20 items) anxiety. Higher scores on this factor indicate higher levels of anxiety. The child version of the inventory has been normed on 1551 elementary school children grades 4–6 (MHS Inc, North Tonawanda, NY) and shown to possess good psychometric properties (Spielberger et al., 1970).
Child Depression inventory
Depressive mood state was assessed with the Child Depression Inventory (CDI; (Helsel & Matson, 1984). This widely-used self-report inventory is a 27-item scale for children and adolescents aged 7–17. The CDI assesses ineffectiveness, negative mood, negative self-esteem, anhedonia, and interpersonal problems and has been normed on 1100 children (PsychCorp, San Antonio, TX). The CDI has been reported to possess internal consistency in both clinical and nonclinical samples (Helsel and Matson, 1984) and acceptable test–retest reliability (Finch, Saylor, Edwards, & McIntosh, 1987).
Apparatus
Eye movements were recorded from the left eye using a remote mounted eye tracker with a sampling rate of 240Hz (Applied Science Laboratories; ASL Inc., Bedford, MA) and were analyzed off-line with the Ilab toolbox (Gitelman, 2002) in Matlab (The Mathworks Inc, Natick, MA, United States). Participants were required to make one of two eye movements: either an eye movement towards (prosaccades) or an eye movement away (antisaccades) from a white target asterisk that was subtending 0.5 deg in visual angle (Figure 1). In addition, each eye movement was paired with one of three incentive conditions. Prior to the target, a cue indicated the type of incentive and the type of saccade participants had to perform. If the color of the cue was grey, an antisaccade was required; if white, a prosaccade was required. The cue itself consisted of a ‘+’, a ‘−’, or a ‘0’. A ‘+’ sign indicated that participants could win $1 if they executed a correct eye movement and a ‘−’ sign indicated that they would lose $1 for an incorrect eye movement; a ‘0’ indicated no gain or loss regardless of whether the eye movement was correct or incorrect (no incentive condition). Participants were given immediate feedback as to whether they had won $1 (displayed in green) or lost $1 (displayed in red). Stimuli were presented on a black background. Each trial started with the cue, which was presented for 1250 – 1750 msec and which indicated the type of incentive and the type of saccade, both of which were randomized. Once the cue disappeared, the white target asterisk was displayed for 1850 msec on either the left hand or right hand side approximately 6.15 deg to the centrally presented cue. Then, the feedback display (subtending roughly 1.8 deg in visual angle) was presented for 1000 msec at the location where the correct eye movement should have occurred.
Figure 1.
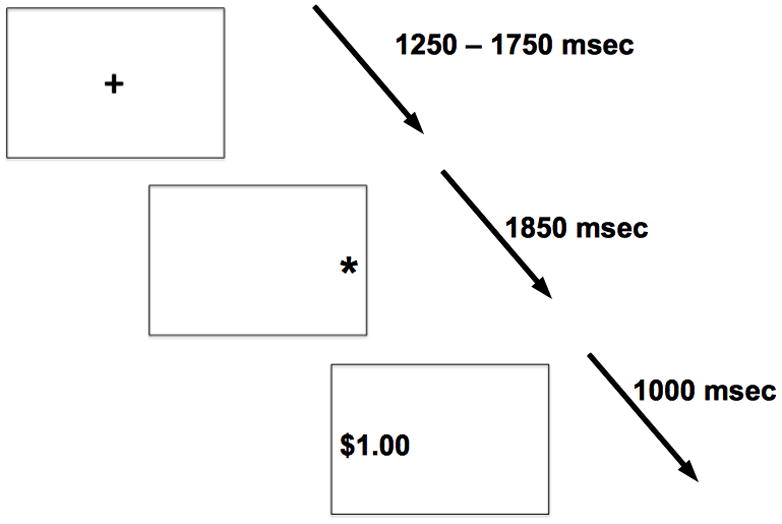
Example of a trial in which an antisaccade is required during the reward condition (grey plus sign) followed by the white target asterisk and the correct feedback location with positive feedback assuming a correct eye movement. Please note that for easier illustration, black and white have been inverted.
Procedure
The experiment was conducted in an illuminated room with standard fluorescent lights. Children were seated in a chair about 26 inches away from the screen and stabilized in a chin-rest to minimize movement. Participants completed 144 trials presented in 3 runs of 48 randomly intermixed trials. Subjects started with $0.00 and could win up to $4.80 per run. If necessary, subjects were re-calibrated in-between runs. After the experiment, participants were thanked and debriefed and sent a check for the amount of their winnings.
Statistical analysis
In eye movement research, two saccade parameters are frequently analyzed. First, accuracy refers to the overall level of performance, as measured by percent of trials that were performed incorrectly. On these trials an eye movement was executed in the wrong direction. Latency refers to the time when the saccade is initiated and is equivalent to reaction time of correct responses in (measured in msec). A correct response was defined by its direction and latency. Anticipatory (latency < 80 msec) or late (latency >700 msec) saccades, i.e., judged to not be in response to the target, were discarded. Saccade threshold criterion was set at 30 deg/sec. Each saccade parameter was entered separately into a three-way repeated measures analysis of co-variance (ANCOVA), with Group (Controls vs. ES) as the between-subjects factor and with two within-subjects factors: Saccade type (prosaccades vs. antisaccades) and Incentive (reward, loss, no incentive). Given a significant group difference in IQ, this factor served as a covariate. Our hypothesis that incentives would enhance performance in controls, but not ES youths, selectively on cognitively controlled antisaccades but not prosaccades, would be confirmed by the presence of a significant three-way interaction for our two dependent variables of interest (accuracy and latency). In addition, to account for potential impact of co-morbid psychopathology, the four subjects with diagnosis of psychopathology were removed and the ANCOVA on each variable of interest was re-run. Finally, Pearson Product Moment correlations (r) were conducted to examine any potential modulations of current mood state (STAI-C/CDI) on the findings.
Results
Latency
A significant effect of Group, F(1,43) = 6.89, p = .01, was present on latency (Figure 2) reflecting slower responses by ES adolescents than controls for both prosaccades, F(1,43) = 6.01, p < .05 and antisaccades, F(1,43) = 5.62, p < .05. No other main effects or interactions were significant.
Figure 2.
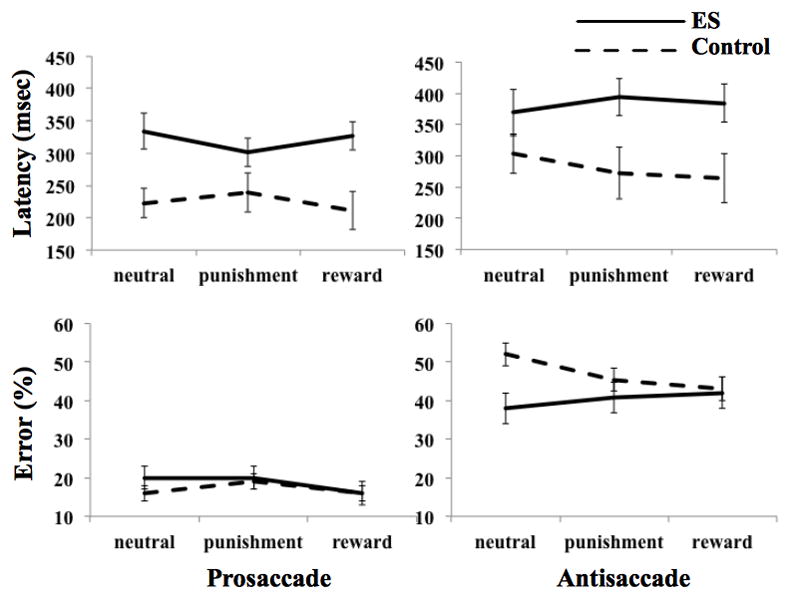
Figure shows the performance means in controls (dashed lines) and ES group (solid lines) for prosaccades (left panel) and antisaccades (right panel) split by incentive type (upper panel: latencies in msec; lower panel error rates in %). Error bars denote SEM.
Accuracy
As predicted, the three-way interaction of Group × Saccade × Incentive on accuracy was significant, F(2,86) = 4.38, p = .02. In addition, a main effect of saccade, F(1,43) = 14.42, p < .01, showed that participants committed significantly fewer errors on prosaccades, M = 18%, SEM = 1.35, than antisaccades, M = 44%, SEM = 2.06. The reward by saccade interaction was also significant, F(2,86) = 3.43, p < .05. The main effects of Group, F(1,43) = .56, p = .46, incentive, F(2,86) = 2.47, p = .09, or IQ, F(1,43) = 2.59, p = .12 were not significant. No other effects were significant.
To follow up the significant three-way interaction, we examined separately pro-and anti-saccades, using a repeated 2 (Group) × 3 (Incentive type) ANCOVA for each saccade type (Figure 2). In antisaccades, the Group × Incentive interaction was significant, F(2,86) = 3.35, p < .05. In prosaccades, no significant findings emerged.
We then followed up the significant Incentive × Group interaction on antisaccade trials and examined the main effect of incentive on antisaccade separately in ES and control groups. The findings indicated a significant effect of incentive in controls, F(2,54) = 4.54, p < .05, but not in ES, F(2,30) = 1.35, p = .27. Paired t-test comparisons revealed that, in controls, error rates on reward trials were significantly reduced relative to neutral trials, t(28) = 2.15, p < .05. This was not the case for the ES group, t(16) = 0.00, p = 1.00. No other comparisons were significant.
Associations with co-morbid illnesses or mood and anxiety symptoms
When the four subjects with co-morbid mood and anxiety disorders, including one patient who was on medication at the time of testing, were removed from analyses, the critical three-way interaction of Group × Incentive × Saccade remained significant, F(2,78) = 5.07, p < .01, suggesting that the findings were not impacted by co-morbid psychopathology.
The correlations of performance with mood state (CDI; STAIC-State; STAIC-Trait) in each group revealed a significant negative correlation between antisaccade punishment error rates and state anxiety, r(28) = −.39, p < .05, and a marginal negative correlation with CDI, r(26) = −.38, p = .056 in controls, i.e., higher accuracy with lower anxiety and lower depressive scores. The corresponding correlations in the ES group were not significant; STAIC-State: r(17) = .26, p = .31; CDI: r(16) = .32, p = .22. Importantly, the correlation coefficients of antisaccade punishment error rates with STAIC-State differed significantly between groups, F(1,41) = 4.76, p < .05. Similar differences between groups for the CDI and antisaccade punishment accuracy were trending toward significance, F(1,37) = 3.50, p = .07. Corresponding measures on the STAI-Trait were not significant for either controls: r(26) = −.10, p = .61 or ES: r(16) = .29, p = .27.
Discussion
We predicted that ES would be associated with changed performance on a monetary incentive saccade task. The data analyses resulted in three major findings. First, as expected, the ES group failed to show the reward-related improvement on antisaccade accuracy that characterized the control group. However, in contrast to hypotheses, performance on inhibitory control (antisaccades) per se did not differ between groups. Second, the ES group had longer reaction time (latency) on both pro- and anti-saccades compared to the control group. Third, the ES group showed no correlations between punishment antisaccade accuracy and state anxiety and depression, in contrast to the control group, which exhibited a correlation showing that lower accuracy was associated with higher scores on anxiety and depressive mood.
With regards to the first finding, as expected, ES youths did not improve their performance as measured by error rates of either pro- or antisaccade by incentives. By comparison, controls significantly decreased their error rates during the difficult antisaccade task when presented with incentives. These data are consistent with previous findings in healthy participants and youths with mood and anxiety disorders (Duka & Lupp, 1997; Hardin, Schroth, Pine, & Ernst, 2007; Jazbec et al., 2005; Mueller, Ng, et al., 2010). Two aspects of this result deserve comment. First, the absence of a modulation of prosaccades by incentives in both groups suggest that incentives do not modulate performance in conditions with strong prepotency but only when strong cognitive control is required. Alternatively, it could be argued that the absence of an incentive effect in prepotent prosaccades could be driven by a floor effect seen in too low error rates. However, this is unlikely because ES youths, when compared to controls, exhibited lengthened latency to prosaccades, testifying to the possible modulation of prosaccade performance. Attending to the task instructions - identify the cue and prepare for the correct response - was an attentional process common to both pro- and antisaccades. Since incentives affected only anti-saccades, it can be deduced that incentive did not affect attention capacity, but rather inhibitory processes.
The weaker effect of reward on performance in the ES group is consistent with previous behavioral work in children temporally removed from their home because of abuse and/or neglect (Guyer et al., 2006). This previous study used a wheel of fortune task involving probabilistic gains, and showed that maltreated children failed to vary response speed as a function of reward level, in contrast to the speeded response with increased potential reward in the control group. These findings are also consistent with the work by Dillon et al. (2009) that compared adults with a history of childhood trauma to typical adults on a monetary-rewarded reaction time task. Although no differential effect of reward was detected between groups at the behavioral level, early-trauma adults showed weaker neural response to reward within the basal ganglia compared to controls. This was not true for punishment conditions. Collectively, these studies suggest that trauma during development may lead to specific changes in reward processes. Such changes in reward sensitivity may herald risk for later psychopathology including substance use problems or anxiety disorders.
Regarding the latter, studies of anxious or depressed children, using a similar reward-saccade task as here, also showed reward-related dysfunction in children with an internalizing disorder compared to controls. These studies found reduction in performance improvement under reward conditions compared to control children (Jazbec et al., 2005; Hardin et al., 2007). These incentive-driven effects were also seen with punishments, suggesting a more pervasive effect of anxiety on reward systems compared to the reward-selective effects of ES. Longitudinal studies could clarify the potential role of responses to punishment for the development of anxiety disorders in at-risk individuals by virtue of ES.
The second finding of slowed latencies across both automatic and controlled saccades in ES youths compared to control youths suggests a deficit in response execution. However, previous studies have reported intact motor skills in children with early psychosocial deprivation (Bos et al., 2009; De Bellis, Hooper, Woolley, & Shenk, 2010), in contrast to the presence of deficits in visual attention (De Bellis et al., 2010; Pollak et al., 2010). As discussed above, the selective effect of incentive on performance appeared to affect inhibitory control. Here, the finding of slowed latencies across saccade types may indicate general difficulties in attentional processing rather than motor control. Previous work indicates mixed findings on the integrity of inhibitory control in children with early stress by virtue of early institutionalization. One study by Colvert et al (2008) reported clear deficits on the Stroop task, a prototypical inhibitory control task, and another study by Pollak et al (2010) failed to find deficits on the less specific Wisconsin-like task, which nonetheless requires intact inhibitory control. A major difference in the present sample and the Colvert et al’s sample concerns the amount of early stress. In the current study, youths were given up for adoption by their biological parents or adopted after being removed from their biological parents because of neglect, while in Colvert’s study, youths were orphans experiencing the additional psychological and temporal burden of early institutional deprivation by being placed as infants in Romanian orphanages. In point of fact, children adopted into Romanian families also performed significantly better on the Stroop task relative to children left in institutional care (Colvert et al., 2008). The different levels of stress exposure between both studies may account for the lack of pure inhibitory deficit in the present work. However, future studies could aim to further characterize inhibitory and attentional deficits in ES.
The third finding suggests that anxiety state, and to a lesser degree depressive state, modulates performance under negative incentive (punishment) in healthy youths, but not in ES youths. This effect may be driven by an absence of variability in performance during punishment conditions in ES youths compared to control youths. Such a finding would be consistent with the failure of positive or negative incentives to improve performance in ES youths. However, together with the differential impact of punishment on performance in ES and anxious youths, as alluded to above, cognitive changes in response to punishment may represent a tipping factor that contributes to the emergence of pathological anxiety in ES individuals.
Limitations and future directions
Some strengths and limitations of the present study require discussion. With regards to internal and external validity of the present findings, a frequent limitation of studies with ES youths is the comparatively small sizes and heterogeneity of these samples. The present study suffers from a relatively small sample size as well, however, participants from this sample were recruited from a cohort that was followed longitudinally and for which consistent differences relative to healthy populations have been documented in other tasks (Maheu et al., 2010; Mueller, Maheu, et al., 2010). Prior studies of childhood adversity document differences in findings attributable to the type of maltreatment (Bruce, Fisher, Pears, & Levine, 2009). Due to difficulties in recruitment, study samples are frequently heterogenous with regards to multiple types of maltreatment (Carrion et al., 2008; Dillon et al., 2009) or sampling population (i.e., different orphanages) (Chugani et al., 2001; Eluvathingal et al., 2006). Interestingly, across these differences in ES characteristics, recent epidemiological work has not found a specificity of early adversity for risk for later psychopathology (Green et al., 2010). Instead, the stress response is expected to be a common denominator setting off common neurobiological responses in these ES individuals, such as changes in stress-related hormones (e.g., cortisol) and neurotransmitters (e.g., serotonin) (Carpenter et al., 2007; Tyrka et al., 2009; Tyrka et al., 2008). Future research efforts will need to further investigate possible differentiations among maltreatment subtypes. In the current study, however, all participants had been adopted within the first two years of life and experienced comparatively moderate forms of maltreatment such as ES by virtue of a change of caregiver. Although later negative experiences in the adoptive families or a contribution of other environmental factors between the time of adoption and participation of the study cannot be excluded, none of the participants showed symptoms of PTSD at the time of testing. In addition, participants were well-matched on annual family income and socio-economic status in their new adoptive homes reducing a possible environmental impact. Although these data suggest that ES adolescents are insensitive to rewarding stimuli, replication of the current findings is needed from studies that include participants who experienced other types of maltreatment such as physical or sexual abuse. Therefore, with regards to generalizability and external validity, longitudinal studies in larger cohorts are needed to specify the relevance of the current findings for the type of experienced stress, type of maltreatment, and psychiatric diagnosis. Such a study design would additionally provide valuable information on the predictability of impaired incentive processing on future mental health outcomes or development of psychopathology.
Of note, although the Trauma section of the K-SADS is sufficient to establish DSM-IV criteria for PTSD, it is limited in its ability to identify different subtypes of traumatic experiences. Future research with similar goals could utilize the Clinician Administered PTSD Scale for Children and Adolescents (CAPS-CA, Western Psychological Services, Los Angeles, CA) or the University of California Posttraumatic Stress Disorder Reaction Index (UCLA PTSD Reaction Index, UCLA Trauma Psychiatric Service, Los Angeles, CA) for more refined assessment.
Clinical implications
Finally, the findings of the current study may bear implications for clinical intervention models that aim to mitigate the impact of experienced maltreatment and removal from the biological home after placement into the foster care system. Studies have shown that positive, i.e., rewarding, parenting strategies of foster parents improved children’s behavioral adjustments (Fisher, Gunnar, Chamberlain, & Reid, 2000). Developments of such interventions could examine the impact of specific types of reward sensitivity of individuals in foster care. Additionally, interventions could investigate whether this disturbed reward sensitivity can be re-established using positive parenting strategies. The current study controlled for differences in IQ. However, an intriguing question in relation to future interventions is whether differences in individual learning abilities may predict sensitivity to such reward modification. The monetary antisaccade task may serve as a sensitive tool to test reward sensitivities before and after intervention for example.
Summary
This is the first study, to our knowledge, to test antisaccade performance in a sample of ES youths. The results revealed that ES youths do not improve their performance with incentive when inhibitory control is required. They also suggest that attention capacity for response execution in general is impaired in these youths. Future functional neuroimaging studies could aim to capture the neural correlates of impaired reward processing in ES youths.
Acknowledgments
Role of the funding source
This work was supported by the intramural program of the NIMH, NIH, by NIMH grants 52135, 74374, 84135 and by a National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award and a postdoctoral fellowship from the Fonds de la recherche en santé du Québec (FRSQ) to FSM.
Footnotes
Disclosure Statement
None of the authors has a conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Biscaldi M, Fischer B, Aiple F. Saccadic eye movements of dyslexic and normal reading children. Perception. 1994;23:45–64. doi: 10.1068/p230045. [DOI] [PubMed] [Google Scholar]
- Bos KJ, Fox N, Zeanah CH, Nelson CA. Effects of early psychosocial deprivation on the devvelopment of memory and executive function. Frontiers in Behavioral Neuroscience. 2009;3:1–7. doi: 10.3389/neuro.08.016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool-aged foster children:differential effects of maltreatment type. Developmental Psychobiology. 2009;51:14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry. 2007;62(10):1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Garrett A, Menon V, Weems CF, Reiss AL. Posttraumatic stress symptoms and brain function during a response-inhibition task: an fMRI study in youth. Depression & Anxiety. 2008;25(6):514–526. doi: 10.1002/da.20346. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage. 2001;14(6):1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- Colvert E, Rutter M, Kreppner J, Beckett C, Castle J, Groothues C, Sonuga-Barke EJ. Do theory of mind and executive function deficits underlie the adverse outcomes associated with profound early deprivation?: findings from the English and Romanian adoptees study. Journal of Abnormal Child Psychology. 2008;36(7):1057–1068. doi: 10.1007/s10802-008-9232-x. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Hooper SR, Woolley DP, Shenk CE. Demographic, maltreatment, and neurobiological correlates of PTSD symptoms in children and adolescents. Journal of Pediatric Psychology. 2010;35(5):570–577. doi: 10.1093/jpepsy/jsp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological Psychiatry. 2009;66(3):206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Lupp A. The effects of incentive on antisaccades: is a dopaminergic mechanism involved? Behavioural Pharmacology. 1997;8(5):373–382. [PubMed] [Google Scholar]
- Eluvathingal TJ, Chugani HT, Behen ME, Juhasz C, Muzik O, Maqbool M, Makki M. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics. 2006;117(6):2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- Eshel N, Roiser JP. Reward and punishment processing in depression. Biological Psychiatry. 2010;68(2):118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Figee M, Vink M, de Geus F, Vulink N, Veltman DJ, Westenberg H, Denys D. Dysfunctional Reward Circuitry in Obsessive-Compulsive Disorder. Biological Psychiatry. 2011;69(9):867–874. doi: 10.1016/j.biopsych.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Finch AJ, Saylor CF, Edwards GL, McIntosh JA. Children’s Depression Inventory: Reliability over repeated adminsitrations. Journal of Clinical Child Psychology. 1987;16(4):339–341. [Google Scholar]
- Fisher PA, Gunnar M, Chamberlain P, Reid JB. Preventive Intervention for Maltreated Preschool Children: Impact on Children’s Behavior, Neuroendocrine Activity, and Foster Parent Functioning. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(11):1356–1364. doi: 10.1097/00004583-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Fox NA, Calkins SD. The Development of Self-Control of Emotion: Intrinsic and Extrinsic Influences. Motivation and Emotion. 2003;27(1):7–26. [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20(7):1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR. ILAB: a program for postexperimental eye movement analysis. Behavior Research Methods Instruments & Computers. 2002;34(4):605–612. doi: 10.3758/bf03195488. [DOI] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Archives General Psychiatry. 2010;67(2):113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Kaufman J, Hodgdon HB, Masten CL, Jazbec S, Pine DS, Ernst M. Behavioral alterations in reward system function: the role of childhood maltreatment and psychopathology. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(9):1059–1067. doi: 10.1097/01.chi.0000227882.50404.11. [DOI] [PubMed] [Google Scholar]
- Hallett PE, Adams BD. The predictability of saccadic latency in a novel voluntary oculomotor task. Vision Research. 1980;20(4):329–339. doi: 10.1016/0042-6989(80)90019-x. [DOI] [PubMed] [Google Scholar]
- Hardin MG, Mandell D, Mueller SC, Dahl RE, Pine DS, Ernst M. Inhibitory control in anxious and healthy adolescents is modulated by incentive and incidental affective stimulli. Journal of Child Psychology and Psychiatry. 2009;50(12):1550–1558. doi: 10.1111/j.1469-7610.2009.02121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin MG, Schroth E, Pine DS, Ernst M. Incentive-related modulation of cognitive control in healthy, anxious, and depressed adolescents: development and psychopathology related differences. Journal of Child Psychology and Psychiatry. 2007;48(5):446–454. doi: 10.1111/j.1469-7610.2006.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsel WJ, Matson JL. The assessment of depression in children: the internal structure of the Child Depression Inventory (CDI) Behavior Research and Therapy. 1984;22(3):289–298. doi: 10.1016/0005-7967(84)90009-3. [DOI] [PubMed] [Google Scholar]
- Jazbec S, Hardin MG, Schroth E, McClure E, Pine DS, Ernst M. Age-related influence of contingencies on a saccade task. Experimental Brain Research. 2006;174(4):754–762. doi: 10.1007/s00221-006-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazbec S, McClure E, Hardin M, Pine DS, Ernst M. Cognitive control under contingencies in anxious and depressed adolescents: an antisaccade task. Biological Psychiatry. 2005;58(8):632–639. doi: 10.1016/j.biopsych.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Ruggiero KJ, Acierno R, Saunders BE, Resnick HS, Best CL. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: results from the National Survey of Adolescents. Journal of Consult Clinical Psychology. 2003;71(4):692–700. doi: 10.1037/0022-006x.71.4.692. [DOI] [PubMed] [Google Scholar]
- Klein CH, Raschke A, Brandenbusch A. Development of pro- and antisaccades in children with attention-deficit hyperactivity disorder (ADHD) and healthy controls. Psychophysiology. 2003;40(1):17–28. doi: 10.1111/1469-8986.00003. [DOI] [PubMed] [Google Scholar]
- Lewis EE, Dozier M, Ackerman J, Sepulveda-Kozakowski S. The effect of placement instability on adopted children’s inhibitory control abilities and oppositional behavior. Developmental Psychology. 2007;43(6):1415–1427. doi: 10.1037/0012-1649.43.6.1415. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13(5):786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Maheu F, Dozier M, Mandell D, Peloso E, Poeth K, Jenness J, Ernst M. Enhanced amygdala and hippocampal responses to fear faces among Youth with a History of Early Caregiver Deprivation. Cognitive, Affective, & Behavioral Neuroscience. 2010;10(1):34–49. doi: 10.3758/CABN.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244(4907):933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Lasker AG, Singer HS, Denckla MB, Zee DS. Oculomotor Abnormalities in Boys With Tourette Syndrome With and Without ADHD. Journal of the American Academy of Child Adolescent Psychiatry. 2001;40(12):1464–1472. doi: 10.1097/00004583-200112000-00018. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Jackson GM, Dhalla R, Datsopoulos S, Hollis CP. Enhanced Cognitive Control in Young People with Tourette’s syndrome. Current Biology. 2006;16(6):570–573. doi: 10.1016/j.cub.2006.01.064. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, Leibenluft E, Ernst M. Early-life stress is associated with impairment in cognitive control in adolescence: An fMRI study. Neuropsychologia. 2010;48(10):3037–3044. doi: 10.1016/j.neuropsychologia.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Ng P, Hardin M, Pine DS, Leibenluft E, Ernst M. Perturbed reward processing in pediatric bipolar disorder: an antisaccade study. Journal of Psychopharmacology. 2010;24(12):1779–1784. doi: 10.1177/0269881109353462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nature Reviews Neuroscience. 2004;5(3):218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Nelson CA, 3rd, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science. 2007;318(5858):1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Nelson CA, Schlaak MF, Roeber BJ, Wewerka SS, Wiik KL, Gunnar MR. Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child Development. 2010;81(1):224–236. doi: 10.1111/j.1467-8624.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelse NNJ, Van der Stigchel S, Sergeant JA. A review on eye movement studies in childhood and adolescent psychiatry. Brain and Cognition. 2008;68(3):391–414. doi: 10.1016/j.bandc.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Stein MB, Walker JR, Anderson G, Hazen AL, Ross CA, Eldridge G, Forde DR. Childhood physical and sexual abuse in patients with anxiety disorders and in a community sample. American Journal of Psychiatry. 1996;153(2):275–277. doi: 10.1176/ajp.153.2.275. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic-pituitary-adrenal axis reactivity. Biological Psychiatry. 2009;66(7):681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, Carpenter LL. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biological Psychiatry. 2008;63(12):1147–1154. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, Tx: Harcourt Assessment; 1999. [Google Scholar]


