Abstract
Regeneration requires initiation of programs tailored to the identity of missing parts. Head-versus-tail regeneration in planarians presents a paradigm for study of this phenomenon. Following injury, Wnt signaling promotes tail regeneration. We report that wounding elicits expression of the Wnt inhibitor notum preferentially at anterior-facing wounds. This expression asymmetry occurs at essentially any wound, even if the anterior pole is intact. notum(RNAi) animals regenerate an anterior-facing tail instead of a head, and double-RNAi experiments indicate notum inhibits Wnt signaling to promote head regeneration. notum expression is itself controlled by Wnt signaling, suggesting regulation of feedback inhibition controls the binary head-tail regeneration outcome. We conclude that local detection of wound orientation with respect to tissue axes results in distinct signaling environments that initiate appropriate regeneration responses.
How an organism determines what cells or tissues are missing for regeneration is poorly understood. Planarians are freshwater flatworms that can regenerate from nearly any injury (1). The head-versus-tail regeneration decision in planarians, known as regeneration polarity, is a paradigm for studying appropriate regeneration program specification (2). Wnt signaling controls regeneration polarity, with pathway components β-catenin-1 (3–5) and wnt1 (formerly called wntP-1) (6–8) required to prevent head regeneration and promote tail regeneration at posterior-facing wounds. wnt1 expression is upregulated near both anterior- and posterior-facing wounds (6, 8, 9). Therefore, how wnt1 and β-catenin act to promote tail formation only at appropriate wounds is unknown.
We sought factors that inhibit Wnt signaling at anterior-facing wounds to promote head regeneration, and identified a planarian homolog of Drosophila notum (Smednotum, Figure S1). Notum proteins are secreted α/β-hydrolase-family members (10, 11), cleave GPI anchors of cell-surface proteins (12), and can act on glypicans to modulate Drosophila Wnt signaling (10, 11, 13, 14). Glypicans are cell-surface, heparan-sulfate proteoglycans that participate in several signaling pathways (15). The roles of Notum proteins in development are unknown outside of Drosophila.
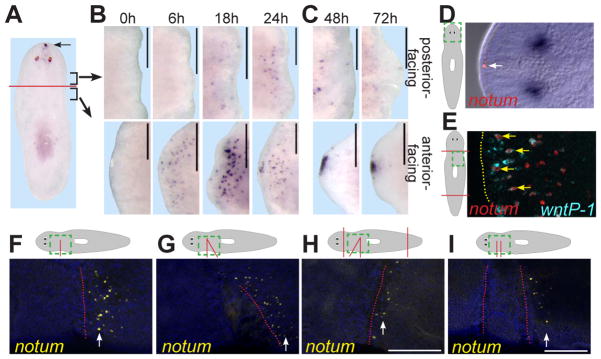
notum was expressed at the planarian anterior pole (Figure 1A). wnt1, by contrast, is expressed oppositely, at the posterior pole (3, 6). Early following head and tail amputation (6–24 hours, h), notum expression was highly upregulated preferentially near anterior-facing wounds (Figure 1B, Figure S2). notum expression was weaker, and initiated later, at posterior-facing wounds. Later following amputation (48–72h), anterior notum expression was coalesced at the pole, while posterior expression remained low (Figure 1C, Figure S2). notum was expressed in subepidermal cells (Figure 1D) that, at wounds, resemble wnt1-expressing cells (6). Indeed, notum and wnt1 were co-expressed in some cells at anterior-facing wounds (Figure 1E).
Figure 1. notum is expressed at anterior-facing wounds.
(A-C) notum in situ hybridizations: intact animals (A); regenerating head and trunk fragments over time (hours, h) (B, C). (A) Brackets: regions imaged in (B, C). (D) notum is expressed in anterior-pole, subepidermal cells (arrow). (D, E) Dotted green line is enlarged in right panels. (E) Double-fluorescence in situ hybridization (FISH); notum (red) and wnt1 (blue) are co-expressed (arrows) at an anterior-facing wound (dotted line) 18h after amputation. (F-I) FISH; notum expression 6h after incisions. Above, green box depicts region imaged. Red lines depict incisions. Below, red line shows sealed wound location. In (G, H) triangular tissue was removed and the wound allowed to seal; tissue between incision sites (~200 microns apart) in (I) was not removed. Anterior, left (B-I) or top (A). Dorsal view (A, 72h in C, D), or ventral view (all others). Images represent ≥4 of 5 animals per panel. Bars, 200 microns.
To test whether wound-site notum expression is specific to head amputation, we incised animal sides without tissue removal. notum expression was detected specifically on the anterior-facing side of these sealed incisions (Figure 1F). Therefore, asymmetric notum expression following wounding (greater at anterior-facing than at posterior-facing wounds) does not require loss of large tissue regions, such as the anterior pole. Asymmetric wound expression also occurred at sealed incisions diagonal to the main body axis (Figure 1G) and was independent of anterior or posterior pole presence (Figure 1H), indicating local cues rather than signals from poles control notum expression asymmetry at wounds. We conclude that wounding elicits notum expression, dependent on wound-edge orientation with respect to the polarized primary body axis.
Posterior-facing wounds could be non-permissive, and/or anterior-facing wounds could be specifically instructive, for notum expression. We therefore examined notum expression between two closely opposed wounds. Regions neighboring only an anterior-facing wound had more notum-expressing cells than did regions bordering both anterior- and posterior-facing wounds (11.0 +/- 6.9 versus 1.6 +/- 2.4 cells, respectively, n=8 animals; Figure 1I). These data suggest posterior-facing wounds suppress wound-induced notum expression, providing expression asymmetry.
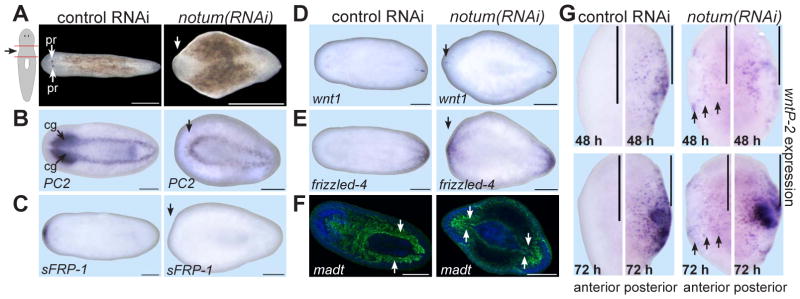
The specificity of strong notum expression for anterior-facing wounds suggested notum might control regeneration polarity. Following head and tail amputation, notum(RNAi) animals failed to regenerate a head with photoreceptors (47%, n=113) and regenerated posterior-facing tails apparently normally (Figure 2A). notum(RNAi) animals that did regenerate at least one photoreceptor did so aberrantly, possibly reflecting a weakly expressive notum(RNAi) phenotype (Figure S3). To characterize notum(RNAi) anterior blastemas lacking photoreceptors, we assessed axial marker expression (Figure 2B-F). notum(RNAi) anterior blastemas lacked cephalic ganglia and anterior-pole marker expression (sFRP-1) (Figure 2B-C). By contrast, notum(RNAi) anterior blastemas expressed the posterior markers wnt1 and frizzled-4 (Figure 2D-E). Furthermore, notum(RNAi) animals regenerated an anterior gut with posterior-specific morphology (two main branches; Figure 2F). We conclude that notum inhibition caused regeneration of an anterior-facing second tail following head and tail amputation. notum dsRNA delivery only after amputation also resulted in a regeneration polarity reversal (Figure S4), indicating a requirement for new notum expression following wounding.
Figure 2. notum is required for head-tail regeneration polarity.
(A) notum(RNAi) fragments failed to regenerate a head by 14 days following amputation (47%, n=133; controls were normal, 100%, n=101). (B-F) Control or notum(RNAi) regenerating animals lacking photoreceptors were probed for expression of (B) PC2 (prohormone convertase 2, CNS marker), (C) sFRP-1 (anterior-pole marker), (D) wnt1 and (E) fzd-4 (posterior markers), and (F) madt (gut marker, green). Blue, Hoechst. Arrows, lack of anterior marker (B-C), posterior marker presence (D-E), or posterior gut morphology (F), in notum(RNAi) animals. cg, cephalic ganglia; pr, photoreceptors. Images are representatives: (B) 9/11, (C) 8/25, (D) 7/24, and (E) 11/38 notum(RNAi) animals; other panels, 100%, n ≥ 7. (G) Anterior- or posterior-facing wounds, probed for wntP-2 expression. wntP-2 has been proposed to be Wnt11-related with name wnt11-5 (8). Arrows, ectopic wntP-2 expression. Images represent ≥5/6 animals per panel. Anterior, left. Bars, 500 microns (A) and 200 microns.
wntP-2 expression is upregulated at posterior- and not anterior-facing wounds, and this requires wnt1 and β-catenin-1 (6). Therefore, wntP-2 expression reflects an early readout (i.e., prior to significant tissue formation) of wnt1/β-catenin-mediated polarity specification. notum(RNAi) fragments expressed wntP-2 ectopically at anterior-facing wounds by 48 hours after injury (Figure 2G), indicating notum normally prevents activation of β-catenin targets at anterior-facing wounds.
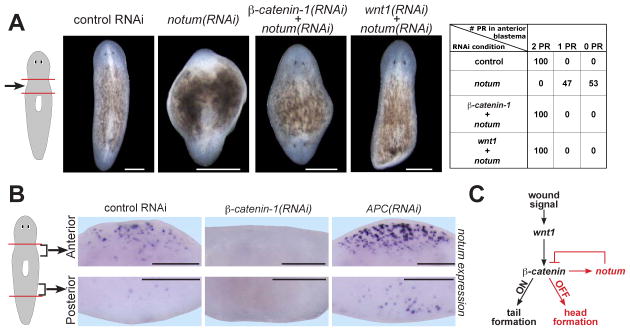
The notum(RNAi) phenotype is similar to that caused by inactivation of APC, an intracellular β-catenin inhibitor (4). Additionally, Drosophila notum inhibits Wnt signaling in imaginal discs (10, 11). Therefore, we performed double-RNAi experiments to assess the candidate pathway of action involving notum, β-catenin-1, and wnt1. β-catenin-1 and notum double RNAi resulted in a polarity phenotype identical to that of β-catenin-1 RNAi alone—anterior- and posterior-facing head regeneration (Figure 3A). Similarly, wnt1 inhibition suppressed the polarity phenotype caused by notum RNAi (Figure 3A). notum RNAi efficiency was not reduced in double-RNAi animals (Figure S5), indicating that suppression of the notum phenotype by wnt1 and β-catenin-1 dsRNA is unlikely simply due to competition with notum dsRNA for RNAi. These data suggest that the notum(RNAi) phenotype requires wnt1 and β-catenin genes, supporting a model in which notum normally inhibits wnt1 and β-catenin-1 function to allow head regeneration.
Figure 3. notum is a Wnt signaling-dependent Wnt inhibitor that controls regeneration polarity.
(A) Double RNAi between notum and Wnt signaling components. Chart shows phenotypes (PR, photoreceptors) as animal percentages. wnt1 RNAi can cause tail regeneration failure and/or head regeneration at posterior-facing wounds (6–8). Competition between wnt1 and notum dsRNA likely accounts for tail regeneration failure rather than ectopic head regeneration in wnt1(RNAi);notum(RNAi) animals. (B) β-catenin-1(RNAi) reduced, and APC(RNAi) enhanced, notum expression 18h after amputation. notum-expressing cell numbers at anterior-facing wounds: controls, 102+/−17 cells; β-catenin-1(RNAi), 17+/−23 cells (p=6.5x10−8); APC(RNAi), 186+/−37 cells (p=8.1x10−6). Number of notum-expressing cells at posterior-facing wounds: controls, 9+/−5 cells; β-catenin-1(RNAi), 1+/−3 cells (p=0.003); APC(RNAi), 30+/−24 cells (p=0.014). Errors, standard deviations; p-values, 2-tailed T-tests. Anterior, top (A), or left (B). Bars, 200 microns. (C) Proposed pathway: selective feedback inhibition of wound-induced Wnt signaling by notum at anterior-facing wounds controls switch-like behavior of regeneration polarity.
Hedgehog signaling impacts planarian regeneration polarity (9, 16), so we tested whether notum requires or influences Hedgehog signaling. patched RNAi overactivates Hedgehog signaling and increases wnt1 wound expression; hedgehog inhibition reduces wnt1 wound expression (9, 16). By contrast, notum(RNAi) animals displayed normal wnt1 expression following amputation (Figure S6A), suggesting notum does not act in polarity by influencing Hedgehog activity. Second, patched(RNAi) animals regenerated anterior tails (n=3/10), but had normal asymmetric notum expression at wounds (n=8/8, Figure S6B), suggesting Hedgehog signaling does not act in regeneration polarity to drive asymmetric notum expression at wounds.
notum can function as a wingless (Wnt) feedback inhibitor in Drosophila (10, 11), so we tested whether Wnt signaling is required for wound-induced notum expression. β-catenin-1 inhibition prior to amputation robustly reduced notum expression levels near wounds (Figure 3B, S7). Conversely, APC RNAi caused notum upregulation near wounds (Figure 3B, S7A-C). Therefore, Wnt signaling is necessary and can be sufficient at wounds for notum expression. Whether Smed-notum is a direct or indirect β-catenin transcriptional target is unknown, but notum is a direct target of Wnt signaling in cultured Drosophila and mammalian cells (17, 18). Wnt signaling perturbation impacted notum expression regardless of wound orientation (Figure S7A-B), so we propose that some other process ensures asymmetric notum expression at wounds. Specifically, in APC(RNAi) animals, notum was upregulated at wounds, but expression asymmetry remained. Because Smed-notum inhibits β-catenin-1 activity and requires β-catenin-1 for its effects, these results suggest regulation of feedback inhibition controls the regeneration polarity decision (Figure 3C).
Wnt signaling is used broadly in regeneration (19–23), and our results suggest that Notum proteins can be an important determinant of the outcome of Wnt expression in regeneration. Additionally, primary body axis development involves anterior Wnt inhibition in many animals (24); notum is an ancient gene present in many metazoans (Fig. S1), making it a candidate for controlling anterior identity broadly. Feedback inhibitors operate in many signaling pathways (25), and frequently simply attenuate pathway output. Here we present evidence that a target and inhibitor of Wnt signaling, the secreted hydrolase NOTUM, controls the switch-like behavior of the head-versus-tail regeneration decision in planarians. These results raise the possibility that control of whether feedback inhibition occurs could in general be used to mediate binary developmental decisions.
In principle, decisions of which tissues to regenerate could be accomplished only by sensing the absence of particular structures. By contrast, our results indicate that regeneration programs elicited at wounds can involve local responses to tissue orientation regardless of the identity of missing tissue. We conclude that initiation of correct regeneration programs involves responses to wounding that depend on local tissue polarization, such as along a body axis.
Supplementary Material
Acknowledgments
We thank the Reddien Lab for discussions and S. Lapan for hedgehog constructs. P.W.R. is a Howard Hughes Medical Institute early career scientist. We acknowledge support by NIH R01GM080639, ACS RSG-07-180-01-DDC, the Keck Foundation, and an American Cancer Society postdoctoral fellowship to C.P.P.
References
- 1.Reddien PW, Sánchez Alvarado A. Ann Rev Cell Dev Bio. 2004;20:725–57. doi: 10.1146/annurev.cellbio.20.010403.095114. [DOI] [PubMed] [Google Scholar]
- 2.Morgan TH. Arch Entw Mech Org. 1898;7:364–397. [Google Scholar]
- 3.Petersen CP, Reddien PW. Science. 2008;319:327–30. doi: 10.1126/science.1149943. [DOI] [PubMed] [Google Scholar]
- 4.Gurley KA, Rink JC, Sánchez Alvarado A. Science. 2008;319:323–7. doi: 10.1126/science.1150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iglesias M, Gomez-Skarmeta JL, Saló E, Adell T. Development. 2008;135:1215–21. doi: 10.1242/dev.020289. [DOI] [PubMed] [Google Scholar]
- 6.Petersen CP, Reddien PW. PNAS. 2009;106:17061–6. doi: 10.1073/pnas.0906823106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adell T, Saló E, Boutros M, Bartscherer K. Development. 2009;136:905–10. doi: 10.1242/dev.033761. [DOI] [PubMed] [Google Scholar]
- 8.Gurley KA, et al. Dev Biol. 2010;347:24–39. doi: 10.1016/j.ydbio.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rink JC, Gurley KA, Elliott SA, Sánchez Alvarado A. Science. 2009;326:1406–10. doi: 10.1126/science.1178712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerlitz O, Basler K. Genes Dev. 2002;16:1055–9. doi: 10.1101/gad.991802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giraldez AJ, Copley RR, Cohen SM. Dev Cell. 2002;2:667–76. doi: 10.1016/s1534-5807(02)00180-6. [DOI] [PubMed] [Google Scholar]
- 12.Traister A, Shi W, Filmus J. Biochem J. 2007 doi: 10.1042/BJ20070511. [DOI] [PubMed] [Google Scholar]
- 13.Han C, Yan D, Belenkaya TY, Lin X. Development. 2005;132:667–79. doi: 10.1242/dev.01636. [DOI] [PubMed] [Google Scholar]
- 14.Kreuger J, Perez L, Giraldez AJ, Cohen SM. Dev Cell. 2004;7:503–12. doi: 10.1016/j.devcel.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Yan D, Lin X. Cold Spring Harb Perspect Biol. 2009;1:a002493. doi: 10.1101/cshperspect.a002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yazawa S, Umesono Y, Hayashi T, Tarui H, Agata K. PNAS. 2009;106:22329–34. doi: 10.1073/pnas.0907464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker DS, Ni YY, Chang JL, Li J, Cadigan KM. Mol Cell Biol. 2008;28:1815–28. doi: 10.1128/MCB.01230-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torisu Y, et al. Cancer Sci. 2008;99:1139–46. doi: 10.1111/j.1349-7006.2008.00814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokoyama H, Ogino H, Stoick-Cooper CL, Grainger RM, Moon RT. Dev Biol. 2007;306:170–8. doi: 10.1016/j.ydbio.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoick-Cooper CL, et al. Development. 2007;134:479–89. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- 21.Goessling W, et al. Dev Biol. 2008;320:161–74. doi: 10.1016/j.ydbio.2008.05.526. [DOI] [PubMed] [Google Scholar]
- 22.Lin G, Slack JM. Dev Biol. 2008;316:323–35. doi: 10.1016/j.ydbio.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 23.Kim JB, et al. J Bone Miner Res. 2007;22:1913–23. doi: 10.1359/jbmr.070802. [DOI] [PubMed] [Google Scholar]
- 24.Petersen CP, Reddien PW. Cell. 2009;139:1056–68. doi: 10.1016/j.cell.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 25.Brandman O, Meyer T. Science. 2008;322:390–5. doi: 10.1126/science.1160617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.