Abstract
Background
The effects of HIV infection and antiretroviral therapy (ART) on usual lipid levels have been reported. The effects of initiating versus deferring ART on high- and low-density lipoprotein particle concentrations (HDL-P and LDL-P) and apolipoprotein (Apo) levels are not well described.
Methods
In a subgroup of participants not taking ART at study entry who were randomized in the Strategies for Management of Antiretroviral Therapy (SMART) to immediately initiate ART (‘VS group’) or to defer it (‘DC group’), lipoprotein particle concentrations and ApoA1 and ApoB levels were measured at baseline and at 2 and 6 months following randomization.
Results
Compared to DC group (n=126), HDL-P and ApoA1 levels increased among VS participants (n=128) after starting ART. At 6 months, VS participants had 13% higher total HDL-P (p < 0.001) and 9% higher ApoA1 (p < 0.001). LDL-P, VLDL-P, and ApoB did not differ significantly between the VS and DC groups. Among VS participants, predictors of HDL-P and ApoA1 increases included baseline levels of hsCRP and IL-6, but not HIV RNA level, CD4 count or traditional CVD risk factors. The effect of starting ART on changes in HDL-P and ApoA1 was greater for those with higher versus lower baseline levels of IL-6 (p=0.001 and 0.08, respectively, for interaction) or hsCRP (p=0.01 and 0.04, respectively, for interaction).
Conclusion
HDL-P and ApoA1 increase following ART initiation, to a degree that depends on the degree of inflammation present at entry. These findings suggest that activation of inflammatory pathways contribute to HIV-associated changes in HDL.
Keywords: HIV infection, antiretroviral therapy, high-density lipoprotein, apolipoprotein A1, inflammation
Introduction
Pro-atherogenic changes in blood lipids and lipoproteins, both as a consequence of HIV infection and exposure to antiretroviral therapy (ART), are associated with an increased risk of cardiovascular disease (CVD) among HIV-infected individuals [1]. Serum lipid levels uniformly decline following HIV sero-conversion [2]. Among untreated HIV-infected participants, high-density lipoprotein cholesterol (HDL-C) levels are inversely correlated with HIV RNA levels [3]. HIV treatment with ART leads to a rise in total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) that typically exceeds pre-infection levels, whereas the recovery of high-density lipoprotein cholesterol (HDL-C) following ART initiation may be incomplete [2]. The effect of HIV infection on HDL-C is, in part, a consequence of a non-specific inflammatory response as well as a direct effect of HIV itself on HDL metabolism and reverse cholesterol transport [4–6].
Methods utilizing NMR (nuclear magnetic resonance) spectroscopy are able to ‘count’ numbers of lipoprotein particles based on their size, which provides an assessment of lipoprotein particle structure beyond traditional lipid fraction measures of the cholesterol content within those particles (e.g., HDL-C) [7, 8]. Estimates of lipoprotein particle size and concentration, as well as measures of lipid-associated apolipoproteins (Apo), may have advantages over traditional cholesterol measures in terms of predicting CVD risk [8–13]. ApoB and ApoA1 are the signature proteins of LDL and HDL particles, respectively, and the ratio of ApoB to ApoA1 is one means of assessing the balance of cholesterol transport and atherogenic potential [12]. Importantly, LDL-P, HDL-P, ApoB and ApoA1 can been reliably measured with non-fasting specimens [7].
In the Strategies for Management of AntiRetroviral Therapy (SMART) trial, some participants were not taking ART at entry. For these participants, the study design allowed a randomized comparison between immediately initiating versus deferring ART. We compare these two treatment groups for changes in lipoprotein particle concentrations and apolipoproteins, and determine baseline predictors of changes in these measures following the initiation of ART. Our general aim was to further characterize HIV-associated dyslipidemia and assess how ART modified it.
Methods
The methods and results of the SMART trial, including the subgroup not taking ART at entry, have been reported [14–16].
Study Population
Of the 5,472 randomized participants in SMART, 477 had never taken ART or had not used ART for at least 6 months prior to randomization (henceforth referred to as the ‘no-ART’ subgroup). To reduce the likelihood of recent ART exposure, participants with low HIV RNA levels (<10,000 copies/mL) during the 6 months before randomization were not included in this no-ART subgroup. Among the 477 participants in this no-ART subgroup, 254 consented to have plasma specimens stored every 2 months during the first year following randomization, and form the basis of this report. For these participants the randomized intervention in SMART of DC (drug conservation) or VS (viral suppression) strategy was a comparison of immediate versus deferred (until CD4 counts declined to 250 cells/mm3) initiation of ART, respectively [15].
The SMART study protocol was approved by the institutional review board (IRB) or ethics committee (EC) at each clinical site and at the University of Minnesota, which served as the Statistical and Data Management Center. The IRB at the University of Minnesota also approved plans for analysis of stored specimens for consenting participants.
Lipids, Lipoprotein Particles, Apolipoproteins and Inflammatory Biomarkers
Traditional serum lipids were measured at baseline by Quest Diagnostics, Inc. (Madison, NJ) using standard enzymatic methods. LDL-C was directly measured. CD4 count and HIV RNA levels were measured at clinical sites. For consenting participants in SMART, plasma specimens were collected using EDTA tubes, processed within 4 hours of collection, frozen at -70°C, and shipped to a central repository where they continued to be stored at −70°C. Samples were not required to be fasting specimens.
Lipoprotein particle size and concentration was estimated by an automated proton nuclear magnetic resonance (NMR) spectroscopic assay at LipoScience, Inc. (Raleigh, NC), as previously described [7]. Lipoprotein class was categorized by particle diameter as VLDL (27–200 nm), LDL (18–27 nm), and HDL (7.3–13 nm). Lipoprotein particle subclasses were further categorized by HDL particle diameter as large (8.8–13.0 nm), medium (8.2–8.8 nm) and small (7.3–8.2 nm), and by LDL particle diameter as large (21.3–23 nm) and small (18.3–21.2 nm). Lipoprotein particle concentrations (μmol/L for HDL and nmol/L for other measures) were then determined from the measured amplitudes of NMR signals, and are denoted by ‘P’ (e.g., HDL-P).
Levels of the HDL-associated apolipoprotein A1 (ApoA1), and the LDL-associated apolipoprotein B (ApoB), were measured by the Laboratory for Clinical Biochemistry Research at the University of Vermont. ApoA1 antigen and ApoB antigen were measured using the BNII nephelometer (N Antiserum to Human Apolipoprotein AI and B, respectively; Dade Behring Inc., Deerfield, IL). The amount of ApoA1 and ApoB present in the sample is quantitatively determined by assessing the intensity of light scattered by antigen-antibody molecules. Expected values for ApoA1 in normal, healthy individuals range from 1.25–2.15 g/L in women and 1.10–2.05 g/L in men. Expected values for ApoB in normal, healthy individuals range from 55 – 125 mg/dl in women and 55 – 140 mg/dl in men. The average inter-assay coefficient of variation for this project was 4.2% for ApoA1 and 3.6% for ApoB. Two inflammatory biomarkers (hsCRP and IL-6) were also measured at the University of Vermont using methods previously reported [17]. All samples were analyzed blinded to treatment group.
Statistical Methods
Unless otherwise stated, comparisons between the VS and DC groups are intent-to-treat (all randomized participants in the no-ART subgroup consenting to the storage of specimens are included in the analysis). An analysis is also carried out in which one VS participant who did not initiate ART and 14 DC participants who initiated ART before 6 months are excluded. Simple descriptive statistics are used for baseline cross-sectional analyses. Analysis of covariance with the baseline level of the biomarker included as a covariate was used to compare treatments for changes in total VLDL-P, total LDL-P, total HDL-P, and HDL-P subclass concentrations and ApoA1 and ApoB levels at 2 and 6 months. Analysis of covariance models that included interaction terms between treatment group and baseline variables were used for subgroup analyses. Multiple regression analysis is used to study predictors of change after 6 months for VS participants. The following predictors were: age, gender, race, hepatitis (B or C) co-infection, smoking, blood-pressure lowering medication, lipid-lowering medication, body mass index (BMI), diabetes, CD4+ cell count, HIV RNA level (log10 transformed), naïve to all ART (or not), prior AIDS, LDL-C, triglycerides, inerleukin-6 (IL-6) and high sensitivity C-reactive protein (hsCRP). Values of lipid measures, lipoprotein particle concentrations and inflammatory biomarkers were loge-transformed prior to analysis. Estimates of the percentage difference between treatment groups in lipid biomarkers at the 2 and 6 month visits were estimated by exponentiating the loge-transformed treatment differences. Analyses were performed using SAS (Version 9.1). All reported p-values are 2-sided.
Results
Study Sample Characteristics
Baseline characteristics of the DC and VS groups were similar and are given in Table 1. Median level of TC was 161 mg/dL; 84% had a TC < 200 mg/dL. Approximately 7% of participants were taking lipid-lowering medication.
Table 1.
Baseline Characteristics and Lipid Measures for DC and VS Participants in SMART Who Were Not Taking ART at Entry
| Total (N = 254)
|
DC Group (N = 126)
|
VS Group (N = 128)
|
|
|---|---|---|---|
| Demographics | |||
| Age, median years (IQR) | 42 (37,48) | 43 | 42 |
| Gender (% female) | 27.6 | 23.8 | 31.3 |
| Race (% black) | 50.4 | 50.0 | 50.8 |
| Clinical characteristics | |||
| Nadir CD4 Count, median cells/mm3) (IQR) | 362 (299, 429) | 361 | 362 |
| CD4 Count, median cells/mm3 (IQR) | 447 (391, 550) | 464 | 431 |
| HIV RNA Level, mean log10copies/mL (IQR) | 4.6 (4.1, 4.9) | 4.6 | 4.5 |
| Prior AIDS (%) | 14.2 | 11.9 | 16.4 |
| ART Naive (%) | 51.2 | 51.6 | 50.8 |
| Co-infection with hepatitis B or C (%) | 22.8 | 21.4 | 24.2 |
| Current smoker (%) | 48.4 | 50.0 | 46.9 |
| Diabetes (%) | 7.5 | 9.5 | 5.5 |
| Blood pressure lowering drugs (%) | 20.5 | 22.2 | 18.8 |
| Lipid lowering drugs (%) | 7.1 | 7.9 | 6.3 |
| Prior CVD (%) | 3.5 | 2.4 | 4.7 |
| Body Mass Index (BMI), median kg/m2 (IQR) | 25.9 (23.1, 30.4) | 25.3 | 26.2 |
| Started NNRTI-based ART (%)** | -- | -- | 53.1 |
| Started PI-based ART (%)** | -- | -- | 30.5 |
| Started other ART (%)** | -- | -- | 15.6 |
| Blood Lipids | |||
| Traditional serum lipid measures, median (IQR) | |||
| Total cholesterol (mg/dL) | 161 (140, 184) | 158 | 161 |
| Triglycerides (mg/dL) | 132 (91, 194) | 124 | 139 |
| LDL cholesterol (mg/dL) | 94 (76, 116) | 94 | 95 |
| HDL cholesterol (mg/dL) | 36 (28, 44) | 37 | 35 |
| Total/HDL cholesterol ratio | 4.5 (3.5, 5.8) | 4.4 | 4.7 |
| Lipid particle concentrations, median (IQR) | |||
| Total VLDL-P (nmol/L) | 60.3 (39.9, 85.9) | 57.5 | 62.9 |
| Total LDL-P (nmol/L) | 1096 (894, 1393) | 1106 | 1084 |
| Total HDL-P (nmol/L) | 25.9 (22.6, 29.3) | 25.9 | 25.9 |
| Large HDL-P (nmol/L) | 4.7 (3.1, 7.1) | 4.9 | 4.4 |
| Medium HDL-P (nmol/L) | 3.1 (0.8, 5.7) | 2.1 | 4.1 |
| Small HDL-P (nmol/L) | 17.2 (12.5, 20.7) | 17.7 | 17.0 |
| Lipid-associated proteins, median (IQR) | |||
| ApoA1 (g/l)* | 1.12 (0.98, 1.24) | 1.12 | 1.12 |
| ApoB (g/l)* | 0.75 (0.60, 0.89) | 0.73 | 0.75 |
| ApoB/ ApoA1 ratio | 0.68 (0.52, 0.86) | 0.67 | 0.69 |
| Inflammatory Biomarkers | |||
| hsCRP (μg/mL) | 1.32 (0.59, 3.60) | 1.72 | 1.16 |
| IL-6 (pg/mL) | 2.68 (1.59, 4.36) | 2.72 | 2.68 |
ApoA1 and ApoB available for n=117 DC and n=116 VS participants
1 participant in VS group did not start ART and was not included in the ART groups
Correlations among baseline lipid and lipoprotein measures, inflammatory markers, CD4+ count and HIV RNA level are given in Table 2. LDL-C, LDL-P and ApoB were positively correlated (range of correlations 0.74–0.75); HDL-C, total HDL-P, and ApoA1 were also positively correlated (range: 0.56 to 0.61). HIV RNA levels were negatively correlated with HDL measures (range: −0.15 to −0.07); correlations of HIV RNA levels with LDL measures were smaller and not significantly differ from zero (range: −0.09 to 0.01). CD4+ cell count was not significantly associated with any of the lipid and lipoprotein measures. hsCRP and IL-6 were significantly negatively correlated with the HDL measures. The correlations of hsCRP and IL-6 with LDL measures tended to be smaller and correlations were not consistent for the two inflammatory markers.
Table 2.
Correlation Between Log-Transformed Lipid and Lipoprotein Measures with CD4 Count, HIV RNA Level and Biomarkers at Baseline: SMART Participants Not Taking ART at Study Entry.
| Correlation Coefficient (p-value) | ||||||||
|---|---|---|---|---|---|---|---|---|
| HDL Measures | HDL-P (nmol/L) | ApoA1 (g/L) | LDL-P (nmol/L) | ApoB (g/L) | CD4 Count (cells/mm3) | HIV RNA (copies/mL) | hsCRP (μg/mL) | IL-6 (pg/mL) |
| HDL-C (mg/dL) | 0.57 (<0.001) | 0.56 (<0.001) | −0.17 (0.007) | −0.11 (0.095) | 0.02 (0.74) | −0.14 (0.026) | −0.20 (0.001) | −0.12 (0.073) |
| HDL-P (nmol/L) | -- | 0.61 (<0.001) | 0.20 (0.002) | 0.28 (<0.001) | 0.01 (0.89) | −0.07 (0.26) | −0.19 (0.003) | −0.35 (<0.001) |
| ApoA1 (g/L) | 0.61 (<0.001) | -- | 0.07 (0.32) | 0.36 (<0.001) | −0.01 (0.86) | −0.15 (0.023) | −0.26 (<0.001) | −0.20 |
|
| ||||||||
|
LDL Measures
| ||||||||
| LDL-C(mg/dL) | 0.40 (<0.001) | 0.26 (<0.001) | 0.74 (<0.001) | 0.74 (<0.001) | −0.05 (0.43) | −0.09 (0.17) | 0.07 (0.30) | −0.20 (0.001) |
| LDL-P (nmol/L) | 0.20 (0.002) | 0.07 (0.32) | -- | 0.75 (<0.001) | 0.05 (0.39) | 0.01 (0.92) | 0.19 (0.003) | −0.10 (0.12) |
| ApoB (g/L) | 0.28 (<0.001) | 0.36 (<0.001) | 0.75 (<0.001) | -- | 0.01 (0.90) | −0.04 (0.59) | 0.02 (0.78) | −0.12 (0.068) |
|
| ||||||||
|
VLDL Measures
| ||||||||
| Triglycerides (mg/dL) | 0.02 (0.69) | −0.03 (0.66) | 0.37 (<0.001) | 0.45 (<0.001) | 0.05 (0.43) | 0.09 (0.16) | 0.03 (0.65) | 0.03 (0.59) |
| VLDL-P (nmol/L) | 0.12 (0.049) | 0.00 (0.94) | 0.43 (<0.001) | 0.52 (<0.001) | 0.04 (0.49) | 0.07 (0.29) | 0.02 (0.79) | −0.11 (0.075) |
Lipoprotien particle concentrations, lipid levels and biomarkers were loge, and HIV RNA log10, transformed prior to comparisons. HDL-C and HDL-P = high-density lipoprotein cholesterol and particles, respectively; LDL-C and LDL-P = low-density lipoprotein cholesterol and particles, respectively; VLDL-P = very low-density lipoprotein particles; ApoA1 = apolipoprotein A1; ApoB = apolipoprotein B.
Changes in Lipids and Lipoproteins after 2 and 6 Months: Immediate (VS) versus Deferred (DC) ART
One hundred and twenty seven of 128 participants in the VS group initiated ART following randomization, and at 6 months 118 (92%) remained on ART. The most frequent nucleoside reverse transcriptase inhibitor combination used was zidovudine and lamivudine (n=40, 31%), and 68 participants (53%) started a non-nucleoside reverse transcriptase inhibitor based regimen. At 6 months 78 participants (62%) had a suppressed HIV RNA level. The most common reason for VS participants stopping ART was toxicity (33%).
For the VS group, HIV RNA levels were 2.07 log10 copies/mL lower at 2 months (p<0.001) and 2.13 log10 copies/mL lower at 6 months (p<0.001), compared to DC group. CD4+ count declines in the DC group and increases in the VS group resulted in a difference between groups of 117 cells/mm3 at 2 months and 191 cells/mm3 at month 6 (p<0.001).
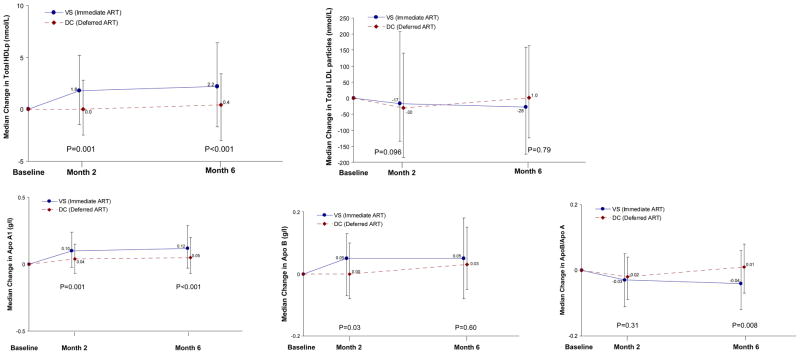
Changes in HDL-related measures (HDL-P concentrations and ApoA1 levels) over follow-up by treatment group are presented in Figure 1 and Table 3. When compared with the DC group, those in the VS group had an 11.8% higher total HDL-P concentration and 8.0% higher ApoA1 levels at month 2, which persisted at month 6. At month 6 the median change in total HDL-P was 0.4 nmol/L for the DC group and 2.2 nmol/L for the VS group (a 12.9% difference; 95% CI: 7.1 to 19.1%); the median change in ApoA1 was 0.05 g/L for the DC group and 0.12 g/L for the VS group (8.8% difference; 95% CI: 3.7 to 14.1%). After adjusting for traditional CVD risk factors (see methods) and baseline hsCRP and IL-6 levels, differences in total HDL-P and ApoA1 levels remained significant at 6 months (data not shown).
Figure 1. Change in Lipoprotein Particle Concentrations and Apolipoproteins.
Change in total HDL-P concentration, ApoA1 level, ApoB level, and ApoB / ApoA1 is presented at months 2 and 6 for VS (immediate ART) and DC (deferred ART initially) groups. Point estimates represent median change from baseline, error bars represent the inter-quartile range (IQR) for change, and p-values are included for the relative differences for VS compared to DC participants at month 2 (11.8% for HDL-P, 8.0% for ApoA1, and 5.8% for ApoB) and month 6 (12.9% for HDL-P; 8.8% for ApoA1; and −7.0% for ApoB / ApoA1).
Table 3.
HDL Particle Subclasses by Treatment Group
| DC Median Change (IQR) | VS Median Change (IQR) | VS-DC % difference* (95%CI) | p-value | |
|---|---|---|---|---|
| Total HDL-P (nmol/L) | ||||
| 2 Months | 0.0 (−2.5, 2.8) | 1.8 (−1.5, 5.2) | 11.8 (4.6–19.5) | 0.001 |
| 6 Months | 0.4 (−3.0, 2.3) | 2.2 (−1.7, 6.4) | 12.9 (7.1–19.1) | <0.001 |
| Large HDL-P (nmol/L) | ||||
| 2 Months | 0.1 (−1.0, 1.1) | 1.0 (−0.5, 2.6) | 16.7 (1.8–33.8) | 0.03 |
| 6 Months | 0.0 (−1.4, 1.0) | 1.0 (0.0, 2.5) | 28.7 (10.8–49.5) | 0.001 |
| Medium HDL-P (nmol/L) | ||||
| 2 Months | 0.0 (−0.9, 1.4) | 0.3 (−1.9, 2.7) | 16.3 (−25.5–81.7) | 0.51 |
| 6 Months | 0.3 (−0.9, 2.1) | 0.9 (−1.4, 3.2) | 72.2 (12.0–164.9) | 0.01 |
| Small HDL-P (nmol/L) | ||||
| 2 Months | −0.6 (−3.5, 2.8) | 0.4 (−3.0, 3.6) | 6.2 (−5.9–19.8) | 0.33 |
| 6 Months | −0.2 (−3.3, 2.2) | 0.9 (−3.4, 4.8) | 6.2 (−3.8–17.2) | 0.24 |
Difference is adjusted for baseline level of corresponding HDL-P measure, and, thus, represents the change in these lipoprotein measures attributable to starting ART
At months 2 and 6 months, there were no significant differences between VS and DC groups in total LDL-P or VLDL-P concentrations (at month 6, 95% CI for % difference between groups were -6.1 to 5.0% for LDL-P and -6.3 to 16.4% for VLDL-P). ApoB levels were 5.8% higher for the VS compared to DC group (p=0.03) at month 2 but did not differ at month 6 (95% CI for percentage difference: −3.6 to 6.5%). As a consequence of the increase in ApoA1 levels, the ratio of ApoB / ApoA1 was 7.0% lower for the VS compared to the DC group at month 6 (p=0.008; Figure 1).
When VS participants that did not initiate ART (n=1) and DC participants that initiated ART before 6 months (n=14) are excluded, the corresponding differences at month 6 were 12.0% for total HDL-P concentrations (p < 0.001), 8.1% for ApoA1 (p = 0.002), −0.4% for total LDL-P (p = 0.90), and 2.1% for ApoB (p = 0.42). When comparisons restricted to participants with no prior ART exposure, differences at month 6 were smaller for total HDL-P concentrations (4.7%; p = 0.17) and but similar for ApoA1 (7.9%; p = 0.03).
The median change (IQR) and percent differences (VS compared to DC group) for large, medium and small HDL-P subclasses over follow-up are presented in Table 3. The increase in total HDL-P among VS participants at month 6 is accounted for primarily by increases in large and medium HDL-P concentrations. There was no treatment effect at 6 months for small LDL-P (95% CI for VS-DC difference was −23.0 to 13.4), but an increase in large LDL-P was seen (20.6%; 95% CI: 0.5 to 44.8; p=0.04).
Predictors of HDL Particle and ApoA1 Changes after 6 Months in the VS Group
Among VS participants, increases in total HDL-P concentration and ApoA1 levels at 6 months were inversely correlated with the decline in levels of HIV RNA (r = −0.28, p=0.002 and r = −0.20, p=0.04, respectively), hsCRP (r = −0.41, p <0.001 and r = −0.34, p < 0.001, respectively), and IL-6 (r = −0.28, p = 0.003 and r = −0.19, p = 0.05, respectively). Consistent with this, VS participants with an HIV RNA level <400 copies/mL at 6 months demonstrated larger improvements in total HDL-P (16.1%; p <0.001) and ApoA1 (19.1%; p <0.001) than those with HIV RNA levels that were persistently detectable at month 6 (4.8% for HDL-P, p=0.15; 9.1% for ApoA1, p=0.01). Descriptions of changes in hsCRP and IL-6 levels among this sample have been previously published [16].
Baseline predictors of change in total HDL-P and ApoA1 at 6 months for VS participants are summarized in web appendix Tables A1 and A2. In univariate models, higher levels of hsCRP and IL-6, but not CD4+ count or HIV RNA level or any other baseline covariate considered (with the exception of baseline level of LDL-C and HDL-P), were associated with greater increases in total HDL-P (p<0.001 and p=0.008, respectively). Findings were similar for baseline hsCRP levels predicting increases in ApoA1 levels, though baseline IL-6 did not reach significance (p=0.002 and p=0.15, respectively). In multiple regression models, baseline hsCRP and IL-6 were independently associated with subsequent changes in total HDL-P (p = 0.004 and 0.05, respectively) and ApoA1 (p = 0.002 and 0.05, respectively).
Subgroup Comparisons: Differences in HDL Changes between Treatment Groups According to Level of HIV RNA, hsCRP and IL-6 at Baseline
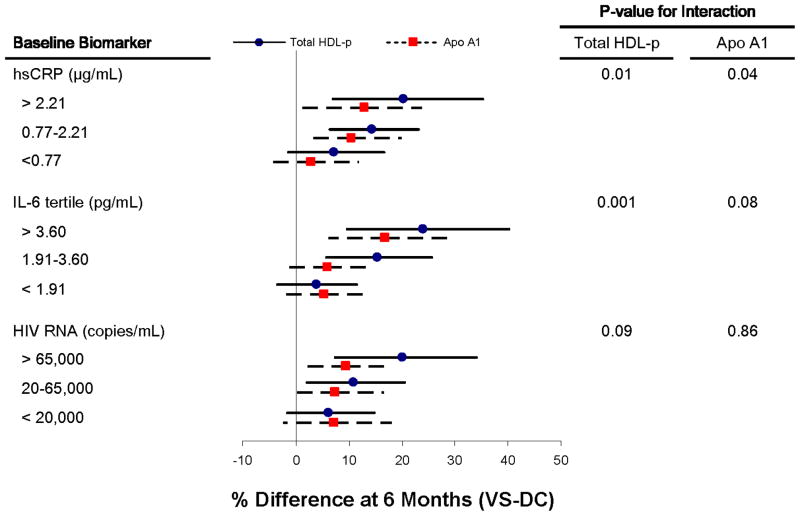
The findings indicating that hsCRP and IL-6 predicted changes in HDL-P and ApoA1 at 6 months for VS participants motivated subgroup analyses which are depicted in Figure 2. When subgroups defined by tertile of baseline levels of hsCRP and IL-6 are examined, an interaction is present – that is among those with higher levels of inflammation at entry, the effect of treatment (VS-DC differences) on HDL measures at 6 months is greater than for those with lower levels of these inflammatory markers at entry (Figure 2). The interaction between treatment group and HIV RNA level at baseline was not significant. When HDL subclasses were examined separately, participants with higher baseline IL-6 levels demonstrated greater improvements in large HDL-P (p=0.05 for interaction) and small HDL-P (p=0.05 for interaction), but not for medium HDL-P.
Figure 2. Effect of ART Treatment on Total HDL Particles and ApoA1 Levels when Stratified by Baseline Level of Inflammatory Biomarkers or HIV RNA.
The percent difference (VS-DC groups) at 6 months in total HDL-P and ApoA1 is presented separately for subgroups defined by baseline levels (in tertiles) of hsCRP, IL-6, and HIV RNA. The percent difference is adjusted for baseline level of HDL-P and ApoA1, respectively, and thus represents the change in these lipoprotein measures 6 months after starting ART. P-values represent the interaction term of baseline biomarker level and treatment effect at 6 months. The degree of improvement in total HDL-P and ApoA1 was dependent on the degree of inflammation (reflected in hsCRP and IL-6 levels), but not the HIV RNA level, at baseline.
Discussion
We describe longitudinal changes in lipoprotein particle concentrations and levels of ApoB and ApoA1, which may more accurately reflect pro- and anti-atherogenic potential than traditional cholesterol indices, among participants with untreated HIV infection randomized to immediate verses deferred ART initiation. We found that starting ART led to increases in the number of HDL particles (HDL-P), levels of the HDL-associated protein ApoA1, and an improvement in the ApoB / A1 ratio. The effect of immediate versus deferred ART on HDL-P and ApoA1 varied according to the degree of inflammation (reflected in IL-6 and hsCRP levels) at baseline, and increases in HDL measures were inversely correlated with changes in hsCRP and IL-6 for those initiating ART. Taken together with other reports, these data suggest that HIV-associated dyslipdiemia is, in part, related to chronic inflammation, and that the effect of ART on HDL changes could be mediated by suppressing inflammation.
The association between HIV infection, or HIV replication, and low HDL is well established [2, 3, 18]. On a molecular level, the HIV-nef protein blocks ATP-binding cassette transporter A1 (ABCA1)-dependent cholesterol efflux from monocyte or macrophages to HDL particles, resulting in intracellular accumulation of lipids (e.g., foam cells) [5, 6]. The ability of HDL to promote cholesterol efflux from lipid-laden macrophages (so called ‘cholesterol efflux capacity’) is an independent predictor of carotid intima-media thickness and obstructive coronary lesions by angiography, even independent of the plasma HDL-C level itself [19]. In the later stages of reverse cholesterol transport HIV infection up-regulates cholesteryl ester transfer protein (CETP) activity, which facilitates the transfer of cholesterol to apoB-containing lipoproteins (e.g., VLDL and LDL) in exchange for triglycerides [20]. Coupled with HIV-related increases in triglycerides [21], the net effect is a preferential delivery of cholesterol to extra-hepatic cells including the vessel wall in the context of HIV infection.
Recent findings by Feeney and colleagues have further demonstrated that intracellular cholesterol accumulation in monocytes from HIV-infected participants is inversely correlated with traditional HDL-C levels (but not with LDL-C), whereas cholesterol accumulation in HIV negative controls is correlated with LDL-C levels (but not with HDL-C) [22]. This difference highlights the unique features of HIV-associated dyslipidemia, and this effect on reverse cholesterol transport appears to have clinical implications. In SMART, HDL-C and HDL-P were stronger predictors of CVD risk than the corresponding LDL measures [23]. Our findings build on these data and show that improvements in HDL-P after starting ART primarily reflect increases in large HDL-P, which would be consistent with improved cholesterol efflux from monocytes and macrophages into nascent smaller HDL particles.
An acute phase inflammatory response, independent of HIV infection, also results in lower levels of HDL-C and ApoA1 and impaired cholesterol efflux from macrophages [4, 24]. For example, low HDL-C levels have been described in patients with acute infections and rheumatologic diseases, and cholesterol efflux to HDL particles is impaired in animal models of sepsis [25–27]. As with HIV infection, HDL changes in the context of other infections are, to some extend, a non-specific consequence of inflammation [28]. Consistent with this, we describe increases in HDL measures that were greatest for those with the most inflammation at baseline (reflected in hsCRP and IL-6 levels). This despite that hsCRP and IL-6 levels did not significantly improve among these same participants after starting ART [16]. The differential effect of ART on HDL measures based on the degree of inflammation we describe was present even after controlling for the contribution of HIV RNA levels.
Elevations in traditional measures of TC, LDL-C and triglycerides with ART exposure are well characterized in the literature, and tend to be more pronounced after starting PI-based regimens [29, 30]. The lack of significant increases in LDL-P and VLDL-P concentrations in this study may be due to the high proportion of participants in SMART who used an NNRTI-based regimen (where LDL changes are less drastic). Several trials have reported increases in HDL-C that are greater with NNRTI- versus PI-based ART [31, 32]. In SMART, any approved ART regimen could be used and therefore comparisons between specific regimens were not randomized.
Taken cumulatively, the consequences of HIV infection and exposure to ART contribute to a clinical phenotype that resembles the metabolic syndrome (i.e., low HDL, high triglycerides, increased waist circumference, insulin resistance, elevated blood pressure) [33–36]. Inflammation is also a key component of the metabolic syndrome that may both contribute to, and be a consequence of, lipoprotein changes, insulin resistance and visceral obesity both in HIV-infected and uninfected populations [37–40]. While effective treatments exist for elevated cholesterol levels (e.g., LDL and triglycerides), strategies to raise HDL are fewer and pharmacologic associated changes in HDL have failed to demonstrate a clear clinical benefit [41, 42]. Thus, anti-inflammatory therapies warrant further study as a CVD prevention strategy particularly in the context of HIV infection.
We focused these analyses on changes in novel measures of lipoprotein particle concentrations and apolipoproteins, which may be more informative for CVD risk than traditional measures of cholesterol content (e.g. HDL-C) [8–13]. Lower HDL-P concentration and size has been independently associated with higher coronary heart disease risk in the general population [43], and in the Cardiovascular Health Study the large HDL-P subclass was inversely related to incident coronary heart disease [9]. In SMART, large HDL-P was the only HDL subclass significantly associated with CVD risk and the ApoB / ApoA1 ratio was a stronger predictor of CVD risk than any of the individual cholesterol indices measured [23, 44]. Our findings specifically describe ART-related improvements in large HDL-P concentration and ApoB / ApoA1 ratio. Longer-term clinical data are needed to determine the most useful lipoprotein measure for assessing and modifying clinical CVD risk among individuals with HIV infection.
Strengths of this study include use of a randomized control group of participants deferring ART when characterizing ART-related changes in lipoprotein measures. A limitation is the lack of traditional lipid measures (TC, LDL-C, HDL-C and triglycerides) at 2 and 6 months of follow-up, though changes in these parameters after starting ART have been extensively reported [2, 45]. Finally, unlike VLDL-P, measures of LDL-P or HDL-P subclasses and levels of ApoB and ApoA1 are more reliable with non-fasting specimens [7].
In summary, ART initiation improves HDL-P number and ApoA1 levels probably as a result of reduced inflammation. It remains unclear whether the increases in HDL-P and ApoA1 following ART initiation reduce clinical CVD risk.
Supplementary Material
Acknowledgments
Support provided by NIH grants: 5 K12 RR023247, 5UO1AIO68641
We would like to sincerely thank the participants who participated in SMART, the SMART study team (see N Engl J Med, 2006:355:2294-2295 for list of investigators), and the INSIGHT Executive Committee.
Role of the Funding Source: The study was funded in part by the National Institute of Allergy and Infectious Disease (NIAID) and in part by the National Heart, Lung, and Blood Institute (NHLBI). The funding sources had no role in data collection, data analysis, or the decision to publish the results.
Footnotes
Disclosures and Conflicts of Interest: J. Baker reported research support from the U.S. National Institutes of Health, the U.S. Center for Disease Control and Prevention, the American Heart Association, Gilead Sciences, Tibotec, and ViiV Healthcare. D. Duprez reported research support from U.S. National Institutes of Health, Roche and Novartis. J. Hoy reported research support from the Australian National Health and Medical Research Council, Gilead Sciences, and Merck Sharp & Dohme. R. Tracy reported the following activities: Honoraria or grant support from Aviir, Abbott, Merck, GlaxoSmithKline/diaDexus, Celera Diagnostics; external advisory board for Wake Forest University Pepper Center on Aging, Johns Hopkins University Pepper Center on Aging, University of Florida Pepper Center on Aging; Haematologic Technologies—owner; thrombosis and fibrinolysis biochemical reagents and blood collection tubes; contract research in this area; and Ashcraft & Gerel Attorneys at Law—consulting on mechanisms in inflammation, atherosclerosis and thrombosis.
Clinical Trials.gov identifier: NCT00027352
References
- 1.Baker JV, Henry WK, Neaton JD. The consequences of HIV infection and antiretroviral therapy use for cardiovascular disease risk: shifting paradigms. Curr Opin HIV AIDS. 2009;4:176–182. doi: 10.1097/COH.0b013e328329c62f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riddler SA, Smit E, Cole SR, Li R, Chmiel JS, Dobs A, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 3.El-Sadr WM, Mullin CM, Carr A, Gibert C, Rappoport C, Visnegarwala F, et al. Effects of HIV disease on lipid, glucose and insulin levels: results from a large antiretroviral-naïve cohort. HIV Med. 2005;6:114–121. doi: 10.1111/j.1468-1293.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 4.Feingold KR, Grunfeld C. The acute phase response inhibits reverse cholesterol transport. J Lipid Res. 2010;51:682–684. doi: 10.1194/jlr.E005454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mujawar Z, Rose H, Morrow MP, Pushkarsky T, Dubrovsky L, Mukhamedova N, et al. Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol. 2006;4:e365. doi: 10.1371/journal.pbio.0040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asztalos BF, Mujawar Z, Morrow MP, Grant A, Pushkarsky T, Wanke C, et al. Circulating Nef Induces Dyslipidemia in Simian Immunodeficiency Virus-Infected Macaques by Suppressing Cholesterol Efflux. J Infect Dis. 2010 doi: 10.1086/654817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clinics in laboratory medicine. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Otvos JD, Collins D, Freedman DS, Shalaurova I, Schaefer EJ, McNamara JR, et al. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density LipoproteinIntervention Trial. Circulation. 2006;113:1556–1563. doi: 10.1161/CIRCULATIONAHA.105.565135. [DOI] [PubMed] [Google Scholar]
- 9.Kuller L, Arnold A, Tracy R, Otvos J, Burke G, Psaty B, et al. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the cardiovascular health study. Arterioscler Thromb Vasc Biol. 2002;22:1175–1180. doi: 10.1161/01.atv.0000022015.97341.3a. [DOI] [PubMed] [Google Scholar]
- 10.Rosenson RS, Otvos JD, Freedman DS. Relations of lipoprotein subclass levels and low-density lipoprotein size to progression of coronary artery disease in the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) trial. Am J Cardiol. 2002;90:89–94. doi: 10.1016/s0002-9149(02)02427-x. [DOI] [PubMed] [Google Scholar]
- 11.Sniderman AD, Furberg CD, Keech A, Roeters van Lennep JE, Frohlich J, Jungner I, et al. Apolipoproteins versus lipids as indices of coronary risk and as targets for statin treatment. Lancet. 2003;361:777–780. doi: 10.1016/s0140-6736(03)12663-3. [DOI] [PubMed] [Google Scholar]
- 12.Walldius G, Jungner I. Rationale for using apolipoprotein B and apolipoprotein A-I as indicators of cardiac risk and as targets for lipid-lowering therapy. European heart journal. 2005;26:210–212. doi: 10.1093/eurheartj/ehi077. [DOI] [PubMed] [Google Scholar]
- 13.McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372:224–233. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 14.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 15.Emery S, Neuhaus J, Phillips A, Babiker A, Cohen CJ, Gatell JM, et al. Major clinical outcomes in antiretroviral therapy (ART)-naïve participants and in those not receiving ART at baseline in the SMART study. JID. 2008;197:1133–1144. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 16.Baker JV, Neuhaus J, Duprez D, Kuller LH, Tracy R, Belloso W, et al. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. JAIDS. 2011;56:36–43. doi: 10.1097/QAI.0b013e3181f7f61a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riddler SA, Li X, Otvos J, Post W, Palella F, Kingsley L, et al. Antiretroviral therapy is associated with an atherogenic lipoprotein phenotype among HIV-1-infected men in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2008;48:281–288. doi: 10.1097/QAI.0b013e31817bbbf0. [DOI] [PubMed] [Google Scholar]
- 19.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. The New England journal of medicine. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose H, Hoy J, Woolley I, Tchoua U, Bukrinsky M, Dart A, et al. HIV infection and high density lipoprotein metabolism. Atherosclerosis. 2008;199:79–86. doi: 10.1016/j.atherosclerosis.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74:1045–1052. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 22.Feeney E, McAuleyN Halloran JO, Low J, Satchell C, Mallon P. Correlations between low HDL-C and monocyte intracellular cholesterol accumulation in HIV infected ptients reflect disturbances in reverse cholesterol transport. 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA, USA. 2011. [Google Scholar]
- 23.Duprez DA, Kuller LH, Tracy R, Otvos J, Cooper DA, Hoy J, et al. Lipoprotein particle subclasses, cardiovascular disease and HIV infection. Atherosclerosis. 2009;207:524–529. doi: 10.1016/j.atherosclerosis.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khovidhunkit W, Memon RA, Feingold KR, Grunfeld C. Infection and inflammation-induced proatherogenic changes of lipoproteins. The Journal of infectious diseases. 2000;181 (Suppl 3):S462–472. doi: 10.1086/315611. [DOI] [PubMed] [Google Scholar]
- 25.McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, et al. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–1145. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarkissian T, Beyene J, Feldman B, McCrindle B, Silverman ED. Longitudinal examination of lipid profiles in pediatric systemic lupus erythematosus. Arthritis and rheumatism. 2007;56:631–638. doi: 10.1002/art.22332. [DOI] [PubMed] [Google Scholar]
- 27.Sammalkorpi K, Valtonen V, Kerttula Y, Nikkila E, Taskinen MR. Changes in serum lipoprotein pattern induced by acute infections. Metabolism: clinical and experimental. 1988;37:859–865. doi: 10.1016/0026-0495(88)90120-5. [DOI] [PubMed] [Google Scholar]
- 28.Mostaza JM, Camino N, Gerique JG, Pena R, Baquero M, Lahoz C. C-reactive protein levels and prevalence of chronic infections in subjects with hypoalphalipoproteinemia. Metabolism: clinical and experimental. 2005;54:33–37. doi: 10.1016/j.metabol.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Young J, Weber R, Rickenbach M, Furrer H, Bernasconi E, Hirschel B, et al. Lipid profiles for antiretroviral-naïve patients starting PI-and NNRTI-based therapy in the Swiss HIV cohort study. Antivir Ther. 2005;10:585–591. [PubMed] [Google Scholar]
- 30.Asztalos BF, Schaefer EJ, Horvath KV, Cox CE, Skinner S, Gerrior J, et al. Protease inhibitor-based HAART, HDL, and CHD-risk in HIV-infected patients. Atherosclerosis. 2006;184:72–77. doi: 10.1016/j.atherosclerosis.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 31.Shlay JC, Bartsch G, Peng G, Wang J, Grunfeld C, Gibert C, et al. Long-term body composition and metabolic changes in antitretroviral naïve persons randomized to protease inhibitor-, nonnucleoside reverse transcriptase inhibitor-, or protease inhibitor plus nonnucleoside reverse transcriptase inhibitor-based strategy. JAIDS. 2007;45:506–517. doi: 10.1097/QAI.0b013e31804216cf. [DOI] [PubMed] [Google Scholar]
- 32.van der Valk M, Kastelein JJ, Murphy RL, van Leth F, Katlama C, Horban A, et al. Nevirapine-containing antiretroviral therapy in HIV-1 infected patients results in an anti-atherogenic lipid profile. AIDS. 2001;15:2407–2414. doi: 10.1097/00002030-200112070-00008. [DOI] [PubMed] [Google Scholar]
- 33.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 34.Grinspoon S, Carr A. Cardiovascular risk and body-fatabnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 35.Morse CG, Kovacs JA. Metabolic and skeletal complications of HIV infection: the price of success. Jama. 2006;296:844–854. doi: 10.1001/jama.296.7.844. [DOI] [PubMed] [Google Scholar]
- 36.Bonfanti P, Giannattasio C, Ricci E, Facchetti R, Rosella E, Franzetti M, et al. HIV and metabolic syndrome: a comparison with the general population. Journal of acquired immune deficiency syndromes. 2007;45:426–431. doi: 10.1097/QAI.0b013e318074ef83. [DOI] [PubMed] [Google Scholar]
- 37.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA : the journal of the American Medical Association. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 38.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arteriosclerosis, thrombosis, and vascular biology. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 39.Frohlich M, Imhof A, Berg G, Hutchinson WL, Pepys MB, Boeing H, et al. Association between C-reactive protein and features of the metabolic syndrome: a population-based study. Diabetes care. 2000;23:1835–1839. doi: 10.2337/diacare.23.12.1835. [DOI] [PubMed] [Google Scholar]
- 40.Boger MS, Shintani A, Redhage LA, Mitchell V, Haas DW, Morrow JD, et al. Highly sensitive C-reactive protein, body mass index, and serum lipids in HIV-infected persons receiving antiretroviral therapy: a longitudinal study. J Acquir Immune Defic Syndr. 2009;52:480–487. doi: 10.1097/qai.0b013e3181b939e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Briel M, Ferreira-Gonzalez I, You JJ, Karanicolas PJ, Akl EA, Wu P, et al. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ. 2009;338:b92. doi: 10.1136/bmj.b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh IM, Shishehbor MH, Ansell BJ. High-density lipoprotein as a therapeutic target: a systematic review. JAMA : the journal of the American Medical Association. 2007;298:786–798. doi: 10.1001/jama.298.7.786. [DOI] [PubMed] [Google Scholar]
- 43.El Harchaoui K, Arsenault BJ, Franssen R, Despres JP, Hovingh GK, Stroes ES, et al. High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med. 2009;150:84–93. doi: 10.7326/0003-4819-150-2-200901200-00006. [DOI] [PubMed] [Google Scholar]
- 44.Duprez D, Neuhaus J, Baker JV for the INSIGHT SMART Study Group. Prognostic value of apolipoprotein B/A1 ratio, total cholesterol/HDL ratio and small LDL/HDL particle concentrations for coronary heart disease in HIV-infected participants: a nested case-control study. 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA, USA. 2011. [Google Scholar]
- 45.Kotler DP. HIV and antiretroviral therapy: lipid abnormalities and associated cardiovascular risk in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;49 (Suppl 2):S79–85. doi: 10.1097/QAI.0b013e318186519c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.