Abstract
Most studies of cancer stem cells (CSC) involve the inoculation of cells from human tumors into immunosuppressed mice, preventing an assessment on the immunological interactions and effects of CSCs. In this study, we examined the vaccination effects produced by CSC-enriched populations from histologically distinct murine tumors after their inoculation into different syngeneic immunocompetent hosts. Enriched CSCs were immunogenic and more effective as an antigen source compared with unselected tumor cells in inducing protective anti-tumor immunity. Immune sera from CSC-vaccinated hosts contained high levels of IgG which bound to cancer stem cells, resulting in CSC lysis in the presence of complement. CTLs generated from PBMCs or splenocytes harvested from CSC-vaccinated hosts were capable of killing CSCs in vitro. Mechanistic investigations established that CSC-primed antibodies and T cells were capable of selective targeting CSCs and conferring anti-tumor immunity. Together, these proof of concept results provide a rationale for a new type of cancer immunotherapy based on the development of CSC vaccines that can specifically target CSCs.
Keywords: Cancer stem cell (CSC), antibody, T Cells, antitumor immunity
Introduction
Clinical trials to treat cancer patients utilizing adoptively transferred T cells (1–3) or dendritic cells (DCs) (4–6) have shown therapeutic efficacy for patients with advanced diseases. However, the clinical responses to such immunotherapeutic approaches have been confined to a limited percentage of treated patients. To date, bulk tumor masses with heterogeneous populations of cancer cells have been used as a source of antigen either to generate effector T cells or to prime DC vaccines. Human tumors are composed of heterogeneous tumor cell clones that differ with respect to proliferation, differentiation, and ability to initiate daughter tumors. The inability to target cancer stem cells with current immune approaches may be a significant factor for treatment failures.
The identification of human cancer stem cells (7–17) presents a new paradigm for the development of cancertreatments. These stem cells have been shown to be relatively resistant to conventional chemotherapeutic regimens and radiation (18, 19) and are postulated to be the cells responsible for the relapse and progression of cancers after such therapies. In an analogous fashion, the cancer stem cell phenomenon may adversely affect the development of effective immunotherapies for cancer. These therapies have involved targeting cells that express differentiated tumor antigens. However, such antigens may be selectively expressed on differentiated tumor cells. Cancer stem cells that do not express these antigens may thus escape these immunologic interventions.
While a few studies have evaluated the resistance of cancer stem cells to the cytotoxic effects of chemotherapy (18, 20–23) and low-dose radiation treatment (19), the immunogenicity of cancer stem cells and their susceptibility to immune-based therapy have not been determined. So far, the majority of cancer stem cell studies have been conducted using human tumors inoculated into severely immunosuppressed hosts (e.g. SCID mice). These hosts represent very useful models for the studies of the biology, tumorigenicity, and signaling pathways of human cancer stem cells as well as for the screening of small molecules which may lead to the development of new drugs that target cancer stem cells. A very recent report described the isolation of cancer-initiating cells (CICs) using ALDEFLUOR/ALDH as a marker from human head and neck, breast, and pancreas carcinoma cell lines, and the generation of ALDH1A1-specific CD8 T cells in vitro (24). These T cells eliminated CICs in vivo by adoptive transfer to immunodeficient (SCID) mice bearing human tumor xenografts. However, the absence of adaptive immune responses in the SCID mouse precludes the ability to investigate the host immune response to cancer stem cells. Although normal mouse mammary stem cells have been isolated (25), there is a need to develop model systems where cancer stem cells can be isolated in the immunocompetent host in order to evaluate the immunogenicity of cancer stem cells.
In this study, we isolated and assessed the tumorigenicity of murine CSCs in two histologically different tumors from two genetically distinct immunocompetent hosts. From there, we evaluated the immunogenicity induced by purified cancer stem cells used as a source of antigen to prime dendritic cells (DC) as a vaccine. We found that CSC-based vaccines conferred effective protective anti-tumor immunity which was associated with the induction of humoral and cellular responses that directly targeted cancer stem cells via complement-dependent cytotoxicity (CDC) and cytotoxic T lymphocytes (CTLs), respectively.
Materials and Methods
Mice
Female C57BL/6 (B6) and C3H/HeNCrMTV- (C3H) mice were from Charles River Laboratories. All the animals were maintained in a pathogen-free environment and used at age 8 weeks or older. The University of Michigan Laboratory of Animal Medicine approved all the animal protocols.
Murine tumors
D5 is a clone which our laboratory produced (26) from the B16-BL6 tumor line that is a poorly immunogenic melanoma of spontaneous origin syngeneic to B6 mice (27, 28). SCC7 is a spontaneously arising squamous cell cancer syngeneic to C3H mice also described in our previous report (29).
ALDEFLUOR assay
The ALDEFLUOR kit (StemCell Technologies, Durham, NC) labels the ALDEFLUOR+/ALDHhigh population including the stem/progenitor cells (30–33). The ALDEFLUOR assay uses a fluorescent substrate of the enzyme (BAAA) freely diffusible across cell membranes. Polar fluorescent products (BAA) accumulate when this substrate is oxidized in cells that express aldehyde dehydrogenase (ALDH). Consequently, cells with high levels of ALDH enzymatic activity stain more brightly (ALDEFLUOR+ also referred to as ALDH+ or ALDHhigh) than cells with lower ALDH (ALDEFLUOR− also referred to as ALDH− or ALDHlow). The fluorescent product BAA is trapped in the cells, due to its negative charges. In each experiment, a sample of cells was stained under identical conditions with specific ALDH inhibitor diethylaminobenzaldehyde (DEAB) as negative control. Flow cytometry based sorting is conducted using a FACStarPLUS. The sorting gates are established using as negative controls the PI stained cells for viability and the ALDEFLUOR stained cells treated with DEAB.
Test of tumorigenicity of ALDEFLUOR+ cells
Equal number of ALDEFLUOR+ or ALDEFLUOR− tumor cells mixed with Matrigel (BD Biosciences, Bedford, MA) (1:1) were injected into the opposite side of the syngeneic mice. Tumor size was measured every 3–4 days.
Vaccination
To examine the protective antitumor immunity induced by vaccination with DCs pulsed with the lysate of ALDEFLUOR+ cells (CSC-TPDC), ALDEFLUOR+/ALDHhigh and ALDEFLUOR−/ALDHlow cells were isolated as described above either from cultured D5 and SCC7 cells or from freshly harvested growing tumors from initial respective ALDEFLUOR+ D5 or SCC cell injection. ALDEFLUOR+, ALDEFLUOR− and unsorted cells were frozen and thaw 3 times to make cell lysate. Bone-marrow derived DCs were cultured in IL-4 and GM-CSF as previously described in our lab (5, 27), and were pulsed with tumor lysate to generate tumor lysate-pulsed DCs (TPDC). After 24 hr co-culture, normal animals were vaccinated with CSC-TPDC or DC pulsed with lysate from unsorted heterogeneous tumor cells (H-TPDC), or DCs pulsed with sorted ALDEFLUOR− cell lysate (ALDHlow-TPDC) at the same DC to tumor cell lysate ratio as CSC-TPDC.
Tumor challenge
After vaccine, the B6 mice were challenged with the heterogeneous D5 tumor cells i.v and the lungs harvested 20 days later to enumerate lung metastases. In SCC7 model, the C3H mice were challenged with the heterogeneous SCC7 tumor cells s.c on the opposite side of the vaccine and the tumor size was monitored.
Antibody production
To test systematic immune responses conferred by CSC-based vaccine, spleens were harvested at the end of experiments. Spleen B cells were activated with LPS plus anti-CD40 (FGK45) mAb ascites as previously described (28). After activation, supernatants were collected and analyzed for IgG production.
Cancer stem cell binding by immune plasma
Plasma was collected from vaccinated hosts at the end of the experiments. IgG level was tested using ELISA. ALDEFLUOR+ cells were washed with FACS buffer; blocked with anti-CD16/CD32 (BD biosciences), and incubated with the plasma with equal quantity of IgG for 60 min on ice. Cells were washed again and incubated with FITC anti-mouse IgG (0.5μg/106 cells) for 30 min on ice. Cells were then washed, and their binding to plasma IgG was detected using flow cytometry.
Antibody and complement-mediated cytotoxicity
Cancer stem cell lysis mediated by antibodies in plasma was assessed by incubation 105 viable ALDH+ CSCs or ALDH− non-CSCs, (serving as control) with plasma in test tubes on ice for 1 h followed by culture in the presence of rabbit complement (Calbiochem, La Jolla, CA) in a 37°C water bath for another hour. Viable cells were then counted after trypan blue staining to calculate cancer stem cell lysis. % of viable cells = viable cells counted after plasma and complement incubation /105. Lower % of viable cells at the end of incubation indicates more cell lysis.
CTL cytotoxicity
CTLs were generated from the PBMCs or splenocytes harvested from vaccinated animals by anti-CD3/CD28 activation and IL-2 expansion, which consistently results in >90% of CD3+ T cells (data not shown). CTL-mediated CSC cytotoxicity was tested using the LDH-release assay (CytoTox 96 Non-Radioactive Cytotoxicity Assay, Promega, Madison, WI) according to the manufacturer's protocol. The following formula was used to calculate cytotoxicity:
Statistical Analysis
The significance of difference in tumorigenicity; metastatic nodules; tumor size; the concentration of IgG, and cancer stem cell lysis by antibodies or CTLs was determined using unpaired t test. P values of <0.05 were considered statistically significant between the experiment groups.
Results
1: Identification of cancer stem cells in two syngeneic immunocompetent animal models
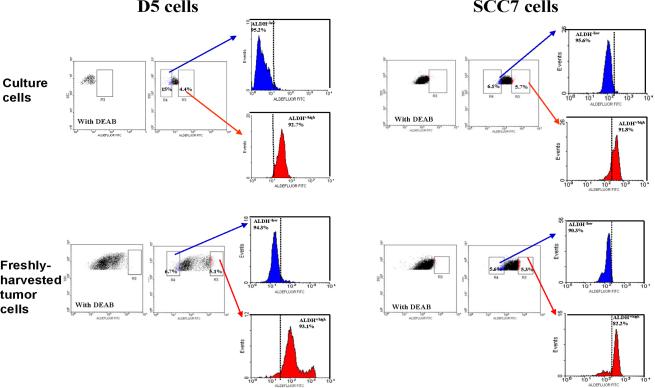
We have previously described the isolation of stem cell-enriched populations using ALDEFLUOR/ALDH as a marker (30, 33, 34). Using this technique, we identified cancer stem cell-enriched populations in two different immunocompetent murine tumor models. As indicated in Figure 1, approximately 4–6% of the cultured murine melanoma D5 and squamous cancer SCC7 cells are ALDEFLUOR+/ALDH+/high; with the rest (~95%) being ALDEFLUOR−. The existence of a small percentage of ALDEFLUOR+ cells in established murine tumors was confirmed by analyzing freshly harvested tumor cells. Processed tumor cells from in vivo established D5 and SCC7 murine tumors also revealed approximately 5% of the ALDEFLUOR+ cells (Figure 1). To determine the purity of the sorted cells, the whole ALDEFLUOR+/ALDH+/high cells and an approximately equal number (5–15%) of the ALDEFLUOR− cells used for the subsequent immunogenicity analyses (ALDH−/low cells) were collected and re-stained with ALDEFLUOR using the same staining protocol. High percentages (>90% in 7 out of the 8 restains) of the ALDH−/low cells (in Blue) and ALDH+/high cells (in Red) after restaining confirmed the purity of originally sorted cells (Figure 1).
Figure 1.
Existence of ALDEFLUOR+ /ALDH+/high populations in murine D5 melanoma and SCC7 squamous cell tumors. The ALDEFLOUR kit labels the ALDEFLUOR positive population including the stem/progenitor cells. The ALDEFLOUR assay isolates the population with a high ALDH enzymatic activity. As negative control, an aliquot of each sample of cells was treated with 50 mmol/l diethylaminobenzaldehyde (DEAB), a specific ALDH inhibitor. To test the purity of the sorted cells, the whole ALDEFLUOR+/ALDH+/high cells (4–6%) and an approximately equal number (5–15%) of the ALDEFLUOR− cells used for the subsequent immunogenicity analyses (ALDH−/low cells) were collected and re-stained with ALDEFLOUR using the same staining protocol. The percentages of ALDH+/high and ALDH−/low cells are listed with the purity of 7 out of the 8 restains being higher than 90%.
The tumorigenicity of sorted D5 melanoma ALDEFLUOR+ cells was evaluated in the syngeneic immunocompetent C57BL/6 host. ALDEFLUOR+ D5 cells (5,000 per inoculum) generated large size tumors in 19 days (Figure 2A), while equal numbers of ALDEFLUOR− cells injected into the opposite side of the same mouse failed to generate tumors. Separate mice were injected with much lower numbers of ALDEFLUOR+ D5 cells. In 4 weeks, 500 injected ALDEFLUOR+ cells formed tumors (Figure 2A). In contrast, the curve in Figure 2A shows that as many as 50,000 ALDEFLUOR− D5 cells did not grow. The tumorigenicity of sorted SCC7 ALDEFLUOR+ population was evaluated in the syngeneic immunocompetent C3H host. As was the case for the D5 model, only the ALDEFLUOR+ (as few as 2,000) cells grew into tumors while equal numbers or even much greater numbers (as many as 200,000) of ALDEFLUOR− cells failed to generate tumor (Figure 2B). These results indicate that the ALDEFLUOR− tumor cells are less tumorigenic than ALDEFLUOR+ cells.
Figure 2.
Testing of tumorigenicity of ALDEFLUOR+ populations in D5 and SCC7 tumor models. Equal numbers of D5 (A) or SCC7 (B) ALDEFLUOR+ and ALDEFLUOR− cells were injected into the opposite side of the same mouse. Tumor growth was then observed. ALDEFLUOR+ cells can form tumors more efficiently than ALDEFLUOR− cells.
Collectively, these data indicate that ALDEFLUOR/ALDH can serve as a reliable marker for the enrichment of murine D5 and SCC7 CSCs. This has allowed us to characterize CSC-induced anti-tumor immunity in the immunocompetent murine host in the subsequent experiments.
2: Cancer stem cell vaccination confers significant protective anti-tumor immunity
Dendritic cells (DCs) pulsed with whole tumor lysate have been reported to be an effective vaccine for cancer both in animal studies (35) and in clinical trials (4) including the findings reported by our own group (5, 27). To examine if DCs pulsed with the lysate of cancer stem cells are more effective in inducing antitumor immunity, we evaluated the protective antitumor immunity induced by vaccination with DCs pulsed with the lysate of ALDEFLUOR+ cells (CSCTPDC), and used DCs pulsed with the lysate of whole unsorted heterogeneous tumor cells (HTPDC) as a positive control. In the D5 melanoma model, we used D5 CSCs as a source of antigen. D5 subcutaneous tumors were established and used as a source of CSC by sorting for ALDEFLUOR+ cells. DCs were pulsed with tumor lysate to generate tumor lysate-pulsed DCs (TPDC) to be used as a vaccine. Normal immunocompetent B6 mice were immunized with D5 CSC-TPDC or D5 H-TPDC (at the same lysate: DC ratio as D5 CSC-TPDC). Control groups received PBS. The TPDCs were inoculated s.c. for two doses (106/each) given 1 week apart. Seven days after the last vaccine, the mice were challenged with the heterogeneous D5 tumor cells intravenously (i.v.) and the lungs harvested 20 days later to enumerate lung metastases. The study scheme and results are illustrated in Figures 3. Compared with non-vaccinated, PBS-injected animals (PBS), H-TPDC induced protective immunity against tumor growth, which corroborates with previous observations (27, 35). Of note, the pulsing of tumor cell lysate to DC to generate H-TPDC was suboptimal compared to what has been reported in the past (4, 5, 27, 34), which may partially explain why H-TPDCs did not immunize effectively (Figure 3). In these experiments, DCs were pulsed with lysate of ALDEFLUOR+ cells to generate CSC-TPDC at the ratio of DCs to ALDEFLUOR+ cells 3:1 (D5) and 10:1 (SCC7), respectively. This ratio is much lower compared with the DC: unsorted tumor cell ratio (1:3) as previously described by our group (4, 5, 27). We used fewer tumor cells to pulse DC to generate CSC-TPDC due to the following reasons: 1). The number of ALDEFLUOR+ cells obtained was limited, and 2) we wanted to see the antitumor potential of DCs pulsed with this limited number of ALDEFLUOR+ cells compared with the DCs pulsed with the same number of unsorted cells. Importantly, mice that received CSC-TPDC prepared at an identical low lysate to DC ratio resulted in significantly fewer lung metastases than the PBS control group as well as the H-TPDC vaccine group. These results suggested that D5 CSCs are immunogenic and can induce an immune response, even under a suboptimal CSC to DC pulsing condition, which led to decreased lung colonization upon tumor challenge. Significantly, these experiments demonstrated a not yet recognized gain and beneficial effect against tumor growth mediated by CSC-TPDC vs. H-TPDC (P=0.018 in Expt.1 and P=0.001 in Expt.2).
Figure 3.
Vaccination of DC pulsed with ALDELFUOR+ D5 cell lysate (CSC-TPDC) induced significantly higher protective immunity against tumor than whole D5 tumor cell lysate pulsed DCs (H-TPDC). (A) CSC-induced protective antitumor immunity in the D5 melanoma model syngenaic to B6 host in a pulmonary metastatic model. The mean numbers of lung metastases (SEM) are depicted in the graph. (B) Pictures of tumors from representative animals in (A). Two of the two independently performed experiments are shown.
In the SCC7 model, subcutaneous SCC7 tumors were established and used as a source of CSC by sorting for ALDEFLUOR+ cells. Normal C3H animals were vaccinated with CSC-TPDC (DCs pulsed with ALDEFLUOR+ SCC7 cells) or DC pulsed with lysate from unsorted heterogeneous SCC7 cells (H-TPDC). The TPDCs were inoculated subcutaneously for three doses given 1 week apart on days −14, −7, and 0 in the right flank of C3H mice. CSC-TPDC or H-TPDC vaccinated mice were challenged with unsorted SCC7 tumor cells subcutaneously into the left flank on day 0 and tumor growth monitored. Compared with non-vaccinated control animals, H-TPDC induced protective immunity against tumor growth to a modest extent (Figure 4 A, B, C). By contrast, mice that received CSC-TPDC showed significant inhibition of tumor growth compared to the no treatment group (P=0.003) (Figure 4A), and the tumors were much smaller than those growing in the H-TPDC-treated hosts (Figure 4B). These results confirmed our previously observed CSC-induced protective immunity against D5 melanoma in a second tumor model syngeneic to a different immunocompetent host, as well as in a different tumor setting (s.c tumor growth) that enriched CSCs are immunogenic and more effective as an antigen source than unsorted heterogeneous tumor cells in inducing immunity of the host to reject the challenge of tumor cells.
Figure 4.
CSC-induced protective antitumor immunity was confirmed in the second tumor model (SCC7) syngeneic to a different immunocompetent host (C3H) in a subcutaneous (s.c). tumor setting. The mean tumor sizes (SEM) for the groups are depicted in the graph (A). Data are representative of three experiments performed. (B) Pictures of tumors from representative animals used in (A). (C) Experiments repeating (A) with one additional control group: DCs pulsed with sorted ALDEFLUOR− SCC7 cell lysate (ALDHlow-TPDC). (D) CSC-induced protective antitumor immunity was verified in D5 tumor model with a subcutaneous (s.c). tumor challenge using the ALDEFLUOR+ and ALDEFLUOR− cells isolated from freshly harvested growing D5 tumor.
We repeated the experiment as shown in Fig. 4A, but added one control group: ALDHlowTPDC e.g. DCs pulsed with sorted ALDH−/low SCC7 cell lysate. As shown in Fig. 4C, vaccination with ALDHhighTPDC (CSC-TPDC) induced significantly higher protective immunity against tumor than H-TPDC as well as ALDHlowTPDC. Furthermore, we have performed additional experiments with the D5 tumor model by evaluating the efficacy of CSC-TPDC in the protection of hosts against s.c. tumor challenge. As shown in Fig. 4D, vaccination of DC pulsed with ALDH+/high D5 cell lysate (ALDHhighTPDC) induced significantly higher protective immunity against s.c. tumor challenge than H-TPDC as well as ALDHlowTPDC vaccination. We analyzed the phenotype of the tumors growing from ALDFLUOR+ cells. The ALDEFLUOR+ population regenerated the initial heterogeneity of the tumor by reconstitution of both ALDEFLUOR+ and ALDEFLUOR− cell populations. To verify our findings using the ALDELFUOR+/ALDH+/high cells isolated directly from the cultured cell lines, we separated ALDELFUOR+ cells isolated from freshly harvested growing tumor in murine hosts which resulted from previous ALDELFUOR+ cell injection. The stem cell nature of the ALDELFUOR+ cells isolated from freshly harvested growing tumors was verified both in vitro and in vivo; in both D5 and SCC7 models (Supplemental data Figure S1, Table S1 and Figure S2S2). The data shown in Fig. 4D was generated by pulsing DCs with lysates from ALDELFUOR+/ALDH+/high cells isolated freshly from the growing D5 tumor followed by D5 s.c. challenge. Consistent with our early findings using cultured tumor cells as a source of CSCs, these experiments demonstrated that vaccination of DC pulsed with the lysate of ALDELFUOR+/ALDH+/high cells isolated from growing tumor induced significantly higher protective immunity against tumor than H-TPDC as well as DC pulsed with the lysate of ALDH−/low cells also isolated from growing tumors (Fig. 4D). It is worth noting that the p values of the difference between PBS vs. H-TPDC and PBS vs. ALDHlowTPDC growth curves in Fig.4C using SCC7 was <0.05 at all time points except for Day 11 (p=0.523 and 0.308, respectively) after tumor inoculation. In contract, there was no significant difference between PBS vs. H-TPDC or PBS vs. ALDHlowTPDC growth curves in Fig.4D using D5. However, Fig. 4C and 4D both demonstrated that vaccination of DC pulsed with the lysate of ALDH+/high cells induced significantly higher antitumor immunity than PBS, H-TPDC as well as DC pulsed with the lysate of ALDH−/low cells in two tumor models. These data further highlight the advantage of using CSCs in vaccination versus the traditional vaccine strategy using bulk unsorted tumor cells (H-TPDC) or using ALDH−/low cells.
3: Systemic humoral and cellular responses in CSC-TPDC vaccinated hosts and direct targeting of cancer stem cells by antibody and CTLs
To define possible mechanisms underlying CSC-induced protective antitumor immunity, we harvested the splenocytes from the animals subjected to H-TPDC and CSC-TPDC vaccination. These cells were secondarily activated in vitro and the culture supernatants collected for antibody detection. We found significantly higher IgG production by LPS/anti-CD40 activated splenocytes isolated from the animals vaccinated with D5 CSC-TPDC or SCC7 CSC-TPDC compared with D5 H-TPDC (P=0.004) or SCC7 H-TPDC (P=0.031) (Figure 5A). These data demonstrated systemic humoral responses in CSC-TPDC vaccinated immunocompetent hosts.
Figure 5.
A. Systemic humoral responses in CSC-TPDC vaccinated immunocompetent host. Splenocytes were harvested from the animals subjected to H-TPDC or CSC-TPDC vaccination, and were activated with anti-CD3/anti-CD28/IL-2 for T cells, or with LPS/anti-CD40 for B cells. The culture supernatants were then collected for IgG detection using ELISA. Data are representative of two experiments performed. B. Plasma harvested from D5 CSC TPDC-treated or SCC7 CSC TPDC-treated animals binds to ALDH+ D5 CSCs and ALDH+ SCC7 CSCs respectively. Data were repeated in a second experiment for the D5 model, and are representative of three experiments performed for the SCC7 model.
In the experiments shown in Figures 3 and 4, the enhanced CSC-induced protective anti-tumor immunity is postulated to occur by inhibiting the growth of CSCs present in the unsorted tumor inoculums. To examine this hypothesis, we harvested the sera and PBMCs from the animals subjected to vaccination to determine the specificity of the immune responses to CSCs. Using flow cytometry (Figure 5B), we observed that immune sera from D5 CSC-TPDC vaccinated hosts bound to D5 CSCs (>80%) much more efficiently than the binding of the sera from D5 H-TPDC vaccinated hosts or sera from PBS injected hosts to D5 CSCs (30.6% and 30.1%, respectively). Similarly, immune sera from SCC7 CSC-TPDC vaccinated hosts bound to SCC7 CSCs (26.4%) significantly more than the binding of the sera from SCC7 H-TPDC vaccinated hosts (4.0%) or the background binding by sera from PBS injected hosts (0.9%) to SCC7 CSCs (Figure 5B).
To evaluate the immunological significance of the binding of CSC-primed antibody to CSCs, we examined antibody and complement-dependent cytotoxicity (CDC) of cancer stem cells. Immune sera from D5 CSC-TPDC vaccinated hosts mediated significantly more efficient D5 CSC lysis than the sera collected from D5 H-TPDC vaccinated (P=0.002) or PBS treated (P=0.004) hosts (Figure 6A). Such complement-dependent cytotoxicity mediated by CSC-primed antibody was CSC specific, because sera from the same D5 CSC-TPDC vaccinated hosts resulted in minimal lysis of ALDH− D5 cells (Figure 6A). Of note, this enhanced D5 CSC lysis correlated with the significantly increased protective antitumor immunity induced by D5 CSC-TPDC vaccination (Figures 3). Similarly, immune sera from SCC7 CSC-TPDC vaccinated hosts mediated significantly increased SCC7 CSCs lysis compared with the sera collected from SCC7 H-TPDC vaccinated hosts (P=0.001) or from PBS treated hosts (P= 0.001), but not the ALDH− SCC7 cells (Figure 6B), indicating again the relative specificity of the CSC-TPDC-induced humoral response towards ALDH+ cancer stem cells. This enhanced SCC7 CSC destruction correlated with the increased protective antitumor immunity induced by SCC7 CSC-TPDC vaccination (Figures 4).
Figure 6.
Targeting of cancer stem cells by cancer stem cell-primed antibody and complement-dependent cytotoxicity (CDC). Plasma antibody and complement-mediated CSC lysis was assessed by incubating 105 viable ALDH+ CSCs or ALDH− non-CSCs (as control) with plasma harvested from animals subjected to PBS, H-TPDC, or CSC-TPDC treatment in test tubes for 1 h followed by cell culture in the presence of rabbit complement for another hour. Viable cells were then counted under a microscope after trypan blue staining to calculate cell lysis. % of viable cells = viable ALDH+ or ALDH− cells after plasma and complement incubation /105. Lower % of viable cells at the end of incubation indicates more cell lysis. Data of CDC were replicated in a second experiment for both the D5 and SCC7 tumor models.
To provide further evidence that the enhanced CSC-induced anti-tumor immunity is due to direct targeting of CSCs, we harvested PBMCs from D5 or SCC7 CSC-TPDC-vaccinated animals to generate CTLs by activation in vitro with anti-CD3/CD28 mAb in the presence of IL-2. These activated cells were assessed for cytotoxicity against ALDH+ or ALDH− tumor cells. D5 CSC-TPDC primed CTLs killed D5 CSCs efficiently (approximately 60%) and significantly more than D5 H-TPDC primed CTLs (approximately 20%) (Figure 7A, P=0.003). Concurrently, the killing of ALDH− D5 cells by D5 CSC-TPDC primed CTLs was significantly less effective (approximately 20%) (P=0.005). In contrast, D5 H-TPDC primed CTLs killed ALDH− D5 cells (approximately 40%) more than D5 CSC-TPDC primed CTLs (P=0.03). SCC7 CSC-TPDC primed CTLs killed SCC7 CSCs efficiently (approximately 60%) and significantly more than their killing of unsorted SCC7 cells (approximately 20%) (P=0.001), or ALDH− SCC7 cells (approximately 25%) (P=0.002) (Figure 7A). We also performed CTL experiments using the effector cells generated from the splenocytes harvested from the vaccination experiments shown in Figure 3. As revealed in Figure 7B, D5 CSC-TPDC primed CTLs killed ALDH+ D5 CSCs significantly higher (p<0.01 at all E: T ratios) than ALDH− D5 non-CSCs. Additionally, we have observed a reduction of residual ALDHhigh CSCs within the tumors growing in the hosts subjected to ALDHhigh-TPDC vaccination compared with ALDHlow-TPDC vaccination (Supplemental Data Table S2).
Figure 7.
Targeting of cancer stem cells by cancer stem cell-primed CTLs. Cytotoxicities of CSCs mediated by CTLs generated from the PBMCs (A) harvested from the CSC TPDC-vaccinated animals are shown. The killing of CSCs mediated by the CTLs was measured by an LDH release assay as described in the Materials and Methods. Higher % of cytotoxicity indicates more cell lysis. Data were replicated in a second experiment for both the D5 and SCC7 tumor models. Cytotoxicity of ALDH+ vs. ALDH− D5 cells by effector cells generated from the splenocytes (B) of mice vaccinated with D5 CSC-TPDC was also measured by the LDH release assay as in A. Data are representative of two experiments performed.
Together, these results provide direct experimental evidence that cancer stem cells can be destroyed by CSC vaccine-primed antibodies and T cells. The immunological targeting of cancer stem cells may provide a novel approach for the development of more effective cancer immunotherapies.
Discussion
ALDEFLUOR/ALDH has been successfully used as a marker to isolate stem cell-enriched populations in human cancers (30–33). Utilizing this marker, we enriched CSCs from two histologically distinct murine tumors in order to investigate immunological strategies which may specifically target cancer-initiating cells in two genetically different immunocompetent hosts. Schatton et. al recently identified a subpopulation enriched for human malignant-melanoma-initiating cells (MMIC) defined by expression of the chemoresistance mediator ABCB5 (17). We have reported that ALDH is a CSC marker for human head and neck squamous cell cancers (36). Controversy exists regarding the existence of CSCs in human melanomas (37) since tumorigenicity of non-CSCs is evident when extreme immunocompromised hosts (NOD/SCID/IL-2rγnull) are used. However, Civenni et al. recently found that the avoidance of trypsin for isolating CSCs from human melanomas which leaves intact surface epitopes on tumor cells results in the ability to isolate cells with genuine CSC properties (38). In our murine studies, we have avoided the use of trypsin to isolate CSCs from freshly harvested growing tumors. These reports that CSCs have been identified in human melanoma and squamous cell cancers provide a rationale to explore CSCs as a target for immune therapies in our murine models.
Studies have demonstrated that cancer stem cells are resistant to chemotherapy (18, 20–23). These studies highlight the limitation of current cancer chemotherapies that are unable to target CSC populations. Novel therapeutic strategies are needed, particularly by targeting the CSCs. We have recently reported that CXCR1 blockade can selectively target human breast CSCs in vitro and in xenografts (34). CXCR1 is a receptor for IL-8 which stimulates the self-renewal of CSCs. In the present study, we have identified a different method to selectively target CSCs utilizing the host immune system. We have been able to demonstrate that CSCs can be selectively targeted by both B and T cell mechanisms in an active immunization protocol. This was done in two genetically distinct immunocompetent hosts using a pulmonary metastasis model and a subcutaneous tumor model respectively. Cancer immunotherapy utilizing DNA vaccine (39); adoptively transferred T cells (40) or DCs (27, 41) has demonstrated the involvement and modulation of host immune effector cells. T and B cell depletion in the recipient mice to demonstrate absent effects on tumor behavior would confirm that CSC vaccination induces cellular and humoral anti-CSC responses, and underscore the relative role of host T and B cells in the immune response induced by CSC-based vaccines.
Several reports have described the killing of CSCs via non-specific immune effector cells, such as NK cells (42). Contag et al. reported a modified approach to kill CSCs which involved cytokine-induced killer cells (IFNγ and anti-CD3 activated) that were used as cellular vehicles to deliver oncolytic virus to CSCs, but no CSC specific antibodies or T cells were identified, even though immune components necessary for targeting the virus itself were examined and identified (43). Interestingly, Todaro et. al described killing of human colon cancer stem cells by γδ T lymphocytes in vitro (44), but no in vivo protective or therapeutic experiments were performed to correlate the immunological significance of the in vitro CSC killing with potential in vivo antitumor immunity against tumor growth or development.
Our immune monitoring studies revealed direct targeting of cancer stem cells by antibody CTLs. This was evident by the production of IgG by the splenocytes isolated from the hosts subjected to CSC-TPDC vaccination, and the binding of the antibody to the CSCs which resulted in the CSC lysis via complement-dependent cytotoxicity. Additionally, CTLs generated from the PBMCs and splenocytes obtained from CSC-vaccinated hosts selectively killed CSCs. Pellegatta et al. used a murine brain tumor cell line GL261 and reported that neurospheres (NS) from glioblastoma multiforme are enriched in CSCs, and that DC loaded with GL261-NS protected mice (45). However, no evidence was described for the direct targeting of CSCs, and no immunological evaluation was performed to elucidate the mechanisms potentially involved in the protection. Xu et.al. reported a human glioblastoma-derived stem-like cell study in vitro (46). They used a rat 9L CSC brain tumor model, and found that neurosphere-pulsed DCs prolonged survival in rats bearing 9L tumors and induced IFNγ mRNA expression in CD8 cells. Nevertheless, no direct and specific targeting and killing of the CSCs, either by antibody-mediated cytotoxicity or by T cell-mediated cytotoxicity was examined. Our studies provide the first direct experimental evidence that cancer stem cells can be selectively targeted and destroyed by CSC vaccine-primed antibodies and T cells, and such immunological targeting of cancer stem cells is associated with enhanced in vivo CSC vaccine-conferred antitumor immunity.
The immunogenic response elicited against bulk tumor cells may be skewed towards differentiation antigens expressed on these cells. This might mask immunologic responses to CSCs which represent only a minor percentage of tumor cells. Our results suggest that CSCs, which may not express immunogenic differentiation antigens (47), can elicit an anti-CSC response when presented as a vaccine. Furthermore, since this immune response is specifically directed against the CSC populations, it may have a greater biological effect than one directed at bulk tumor cell populations. Schatton et al. reported that human ABCB5+ melanoma initiating cells manifested immunomodulatory functions that were immunosuppressive to T cell activation in culture (48). Instead of an in vitro system, we utilized an in vivo approach to activate host T cell and antibody responses which resulted in the induction of specific anti-CSC immunity. Furthermore, we used CSC lysate for vaccine preparation instead of using intact CSCs. Identification of the respective murine melanoma D5 and SCC7 CSC antigens responsible for the observed effects in this study warrants further investigation. We have shown herein preferential induction by ALDH+ cell-based DC vaccines of a humoral response to ALDH+ CSCs. Use of polyclonal sera from CSC-vaccinated recipients in immunoprecipitation assays may lead to the identification of differentially expressed candidate antigens in CSCs that can be validated experimentally in vaccination studies.
It is important to determine the therapeutic efficacy of established tumors with CSC–based vaccines. Since established tumors are comprised of a very small percentage of CSCs, we postulate that the use of CSC-based vaccines alone will have minimal effect on established tumors and will require other adjunctive therapies. Furthermore, CSCs isolated from highly passaged cell lines or from freshly harvested tumors after inoculation may possess immune activity that is not a feature of CSCs obtained directly from spontaneously arising tumors. The lack of using spontaneous tumors remains one of the limitations of the findings in this study. Nevertherless, evaluation of the immunogenicity of CSCs from tumors derived from immunocompetent hosts is novel, and the use of CSCs in such syngeneic hosts permits the assessment of immune responses against these cells. Our animal models will allow us to explore combinatorial approaches for the therapy of more established tumors by selectively targeting cancer stem cells.
Supplementary Material
Acknowledgments
This work was supported by The Will and Jeanne Caldwell Endowed Research Fund of the University of Michigan Comprehensive Cancer Center, and in part by NIH grant CA82529 and the Gillson Longenbaugh Foundation.
Reference
- 1.Chang AE, Li Q, Jiang G, Sayre DM, Braun TM, Redman BG. Phase II trial of autologous tumor vaccination, anti-CD3-activated vaccine-primed lymphocytes, and interleukin-2 in stage IV renal cell cancer. J Clin Oncol. 2003;21:884–90. doi: 10.1200/JCO.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 2.Chang AE, Li Q, Bishop DK, Normolle DP, Redman BD, Nickoloff BJ. Immunogenetic therapy of human melanoma utilizing autologous tumor cells transduced to secrete granulocyte-macrophage colony-stimulating factor. Hum Gene Ther. 2000;11:839–50. doi: 10.1089/10430340050015455. [DOI] [PubMed] [Google Scholar]
- 3.Prieto PA, Durflinger KH, Wunderlich JR, Rosenberg SA, Dudley ME. Enrichment of CD8+ cells from melanoma tumor-infiltrating lymphocyte cultures reveals tumor reactivity for use in adoptive cell therapy. J Immunother. 2010;33:547–56. doi: 10.1097/CJI.0b013e3181d367bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redman BG, Chang AE, Whitfield J, Esper P, Jiang G, Braun T, et al. Phase Ib trial assessing autologous, tumor-pulsed dendritic cells as a vaccine administered with or without IL-2 in patients with metastatic melanoma. J Immunother. 2008;31:591–8. doi: 10.1097/CJI.0b013e31817fd90b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang AE, Redman BG, Whitfield JR, Nickoloff BJ, Braun TM, Lee PP, et al. A phase I trial of tumor lysate-pulsed dendritic cells in the treatment of advanced cancer. Clin Cancer Res. 2002;8:1021–32. [PubMed] [Google Scholar]
- 6.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–33. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–51. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 9.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–83. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 11.Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–33. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 12.Patrawala L, Calhoun-Davis T, Schneider-Broussard R, Tang DG. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67:6796–805. doi: 10.1158/0008-5472.CAN-07-0490. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 14.Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5:738–43. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- 15.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 16.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–9. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shafee N, Smith CR, Wei S, Kim Y, Mills GB, Hortobagyi GN, et al. Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors. Cancer Res. 2008;68:3243–50. doi: 10.1158/0008-5472.CAN-07-5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nandi S, Ulasov IV, Tyler MA, Sugihara AQ, Molinero L, Han Y, et al. Low-dose radiation enhances survivin-mediated virotherapy against malignant glioma stem cells. Cancer Res. 2008;68:5778–84. doi: 10.1158/0008-5472.CAN-07-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naka K, Hoshii T, Muraguchi T, Tadokoro Y, Ooshio T, Kondo Y, et al. TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature. 2010;463:676–80. doi: 10.1038/nature08734. [DOI] [PubMed] [Google Scholar]
- 21.Zhao C, Chen A, Jamieson CH, Fereshteh M, Abrahamsson A, Blum J, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–9. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dallas NA, Xia L, Fan F, Gray MJ, Gaur P, vanBuren G, 2nd, et al. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009;69:1951–7. doi: 10.1158/0008-5472.CAN-08-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamauchi K, Yang M, Hayashi K, Jiang P, Yamamoto N, Tsuchiya H, et al. Induction of cancer metastasis by cyclophosphamide pretreatment of host mice: an opposite effect of chemotherapy. Cancer Res. 2008;68:516–20. doi: 10.1158/0008-5472.CAN-07-3063. [DOI] [PubMed] [Google Scholar]
- 24.Visus C, Wang Y, Lozano-Leon A, Ferris RL, Silver S, Szczepanski MJ, et al. Targeting ALDH(bright) human carcinoma-initiating cells with ALDH1A1- specific CD8+ T cells. Clin Cancer Res. 2011;17:6174–84. doi: 10.1158/1078-0432.CCR-11-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–8. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 26.Arca MJ, Krauss JC, Aruga A, Cameron MJ, Shu S, Chang AE. Therapeutic efficacy of T cells derived from lymph nodes draining a poorly immunogenic tumor transduced to secrete GM-CSF. Cancer Gene Ther. 1996;3:39–47. [PubMed] [Google Scholar]
- 27.Ito F, Li Q, Shreiner AB, Okuyama R, Jure-Kunkel MN, Teitz-Tennenbaum S, et al. Anti-CD137 monoclonal antibody administration augments the antitumor efficacy of dendritic cell-based vaccines. Cancer Res. 2004;64:8411–9. doi: 10.1158/0008-5472.CAN-04-0590. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Teitz-Tennenbaum S, Donald EJ, Li M, Chang AE. In vivo sensitized and in vitro activated B cells mediate tumor regression in cancer adoptive immunotherapy. J Immunol. 2009;183:3195–203. doi: 10.4049/jimmunol.0803773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moyer JS, Li J, Wei S, Teitz-Tennenbaum S, Chang AE. Intratumoral dendritic cells and chemoradiation for the treatment of murine squamous cell carcinoma. J Immunother. 2008;31:885–95. doi: 10.1097/CJI.0b013e3181880f1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carpentino JE, Hynes MJ, Appelman HD, Zheng T, Steindler DA, Scott EW, et al. Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer Res. 2009;69:8208–15. doi: 10.1158/0008-5472.CAN-09-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.den Hoogen C v, der Horst G v, Cheung H, Buijs JT, Lippitt JM, Guzmán-Ramírez N, et al. High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer. Cancer Res. 2010;70:5163–73. doi: 10.1158/0008-5472.CAN-09-3806. [DOI] [PubMed] [Google Scholar]
- 33.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–131. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120:485–97. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirk CJ, Hartigan-O'Connor D, Nickoloff BJ, Chamberlain JS, Giedlin M, Aukerman L, et al. T cell-dependent antitumor immunity mediated by secondary lymphoid tissue chemokine: augmentation of dendritic cell-based immunotherapy. Cancer Res. 2001;61:2062–70. [PubMed] [Google Scholar]
- 36.Clay MR, Tabor M, Owen JH, Carey TE, Bradford CR, Wolf GT, et al. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck. 2010;32:1195–201. doi: 10.1002/hed.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–8. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Civenni G, Walter A, Kobert N, Mihic-Probst D, Zipser M, Belloni B, et al. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011;71:3098–109. doi: 10.1158/0008-5472.CAN-10-3997. [DOI] [PubMed] [Google Scholar]
- 39.Jacob JB, Kong YC, Nalbantoglu I, Snower DP, Wei WZ. Tumor regression following DNA vaccination and regulatory T cell depletion in neu transgenic mice leads to an increased risk for autoimmunity. J Immunol. 2009;182:5873–81. doi: 10.4049/jimmunol.0804074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Q, Carr AL, Donald EJ, Skitzki JJ, Okuyama R, Stoolman LM, et al. Synergistic effects of IL-12 and IL-18 in skewing tumor-reactive T-cell responses towards a type 1 pattern. Cancer Res. 2005;65:1063–70. [PubMed] [Google Scholar]
- 41.Kjaergaard J, Wang LX, Kuriyama H, Shu S, Plautz GE. Active immunotherapy for advanced intracranial murine tumors by using dendritic cell-tumor cell fusion vaccines. J Neurosurg. 2005;103:156–64. doi: 10.3171/jns.2005.103.1.0156. [DOI] [PubMed] [Google Scholar]
- 42.Castriconi R, Daga A, Dondero A, Zona G, Poliani PL, Melotti A, et al. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J Immunol. 2009;182:3530–9. doi: 10.4049/jimmunol.0802845. [DOI] [PubMed] [Google Scholar]
- 43.Contag CH, Sikorski R, Negrin RS, Schmidt T, Fan AC, Bachireddy P, et al. Definition of an enhanced immune cell therapy in mice that can target stem-like lymphoma cells. Cancer Res. 2010;70:9837–45. doi: 10.1158/0008-5472.CAN-10-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Todaro M, D'Asaro M, Caccamo N, Iovino F, Francipane MG, Meraviglia S, et al. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol. 2009;182:7287–96. doi: 10.4049/jimmunol.0804288. [DOI] [PubMed] [Google Scholar]
- 45.Pellegatta S, Poliani PL, Corno D, Menghi F, Ghielmetti F, Suarez-Merino B, et al. Neurospheres enriched in cancer stem-like cells are highly effective in eliciting a dendritic cell-mediated immune response against malignant gliomas. Cancer Res. 2006;66:10247–52. doi: 10.1158/0008-5472.CAN-06-2048. [DOI] [PubMed] [Google Scholar]
- 46.Xu Q, Liu G, Yuan X, Xu M, Wang H, Ji J, et al. Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens. Stem Cells. 2009;27:1734–40. doi: 10.1002/stem.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133–7. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schatton T, Schütte U, Frank NY, Zhan Q, Hoerning A, Robles SC, et al. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 2010;70:697–708. doi: 10.1158/0008-5472.CAN-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.