Abstract
Objective
The objective of this study was to compare the tensile bond strength of metal brackets bonding to glazed ceramic surfaces using three various surface treatments.
Materials and Methods
Forty two glazed ceramic disks were assigned to three groups. In the first and second groups the specimens were etched with 9.5% hydrofluoric acid (HFA). Subsequently in first group, ceramic primer and adhesive were applied, but in second group a bonding agent alone was used. In third group, specimens were treated with 35% phosphoric acid followed by ceramic primer and adhesive application. Brackets were bonded with light cure composites. The specimens were stored in distilled water in the room temperature for 24 hours and thermocycled 500 times between 5°C and 55°C. The universal testing machine was used to test the tensile bond strength and the adhesive remenant index scores between three groups was evaluated. The data were subjected to one-way ANOVA, Tukey and Kruskal-Wallis tests respectively.
Results
The tensile bond strength was 3.69±0.52 MPa forfirst group, 2.69±0.91 MPa for second group and 3.60±0.41 MPa for third group. Group II specimens showed tensile strength values significantly different from other groups (P<0.01).
Conclusion
In spite of limitations in laboratory studies it may be concluded that in application of Scotch bond multipurpose plus adhesive, phosphoric acid can be used instead of HFA for bonding brackets to the glazed ceramic restorations with enough tensile bond strength.
Keywords: Glazed Ceramic, Dental Bonding, Orthodontics, Tensile Strength
INTRODUCTION
Ceramic is a common restorative material in dentistry being available in different forms of veneers, crown jackets, crown and ceramic bridges. These are used to repair severely destructed teeth or to replace lost teeth in order to restore dental health and esthetics, particularly in adult patients. Different types of ceramics varying greatly in the chemical composition, method of manufacture and physical properties have been developed.
The demands for orthodontic treatment in adults have been considerably increased together with the increase of patients’ knowledge and change in the modern life style [1, 2]. As a result, orthodontists may attach orthodontic attachments or fixed retainers to teeth using ceramic restorations such as crowns or veneers. As ceramic is an inert material, it does not adhere chemically to any of the currently available bonding resins [3]. Therefore, ceramic surfaces preparation is an essential step prior to the bonding process. Numerous approches have been suggested in this regard [4–10]. As glazed ceramic surfaces are not amenable to resin penetration during orthodontic bonding [4] mechanical or chemical pre-treatment of the surface is crucial for successful direct bonding. However, as the conventional acid-etching technique is not effective in pre-treatment of non-enamel surfaces [Smith et al., 1988], four types of surface-conditioning techniques have been commonly used: using diamond drills or sandpaper discs in order to roughen the porcelain surface, sandblasting via aluminum oxide particles, chemical methods using hydrofluoric acid or acidulated phosphate fluoride and utilizaion of silanes which increase the wettability of the porcelain surface by providing chemical links between porcelain and the composite [11].
Most bonding techniques to ceramics are associated with the potential risk for the crown, veneer or hazards to oral tissues due to the use of hydrofluoric acid (HFA) which is the conventional accepted surface treatment of ceramic. Hydrofluoric acid to etch porcelain surface is a potential risk for oral tissue health [12]. The conventional ceramics surface treatment methods for increasing retention of orthodontic attachments have not provide optimal quality [13–14]. Glazing layer plays an important role in ceramic resistance as its resistance and strength possibly reduce to half values after removing this layer [15]. Scotch bond multi-purpose plus adhesive (3M Unitek, Monovoria, CA) is a newly developed bonding system that provide phosphoric acid instead of HF for surface preparation before bonding. Increasing demands of adults for orthodontic treatment and contaversy of the results in efficient methods of bonding to porcelain required more investigations.
Moreover some of the studies reported that HFA can be substituted by phosphoric acid in non-glazed porcelain bonding procedures [16]. The aim of this study was to evaluate tensile bond strength of metal brackets bonded to the glazed ceramic surfaces using three different surfaces conditionings.
MATERIALS AND METHODS
Forty-two glazed ceramic disks (10 mm in diameter and 2 mm in width) were fabricated using Feldespatic porcelain (Noritake super porcelain EX-3, Noritake Co., Inc., Nagoya, Japan). The disks were examined to be free of cracks and defects. They were washed with water and then randomly assigned into three groups, each containing 14 specimens.
In the first group, the surfaces of the glazed ceramics were etched with 9.5% HFA (Ultra-dent, USA) for 2 minutes, rinsed with water and dried with oil-free air. Then, a silane layer (Scotch bond ceramic primer, 3M, USA) was applied on the ceramic surfaces and dried with air flow.
Scotch bond multipurpose adhesive layer (3M, USA) was applied on the ceramic surface afterwards, thinned using gentle air flow and cured with a light curing device for 10 seconds. Metal brackets of the maxillary central incisors (Dynalock, Standard Edgewise 0.18, 3M-Unitek, USA) were bonded to the surface of treated ceramics followed by placing the composite on the surface beyond.
The brackets were compressed to the ceramic surface by 250 gm force applied from a gauge.
The composite excess were removed from the bracket periphery and light cured for 40 seconds (10 seconds from each side) and light guide were placed against tooth surface at an angle of 45 with an output of 500 mW/cm2 for polymerization of the composite.
In the second group, the glazed ceramics were exposed by 9.5% HFA for 2 minutes, washed by water spray for 20 second and dried using gentle air flow. Then an unfilled resin layer (3M Unitek) was used on the ceramic surface, thinned with gentle air stream and light cured for 10 seconds. The bracket bonding and other procedures were performed similar to first group.
In third group, 35% phosphoric acid was used for surface treatment of the ceramic surfaces for 15 seconds as recommended by the Scotch bond multipurpose plus adhesive (3M Unitek, Monovoria, CA) brochure. The surfaces were washed with water and dried by gentle air flow. A layer of adhesion promotor, Scotch bond ceramic primer (3M Unitek, Monorovia. CA) was applied on the ceramic surfaces and dried gently.
Afterwards, Scotch bond multipurpose adhesive was utilized on the surface, thinned by gentle air flow and light cured for 10 seconds.
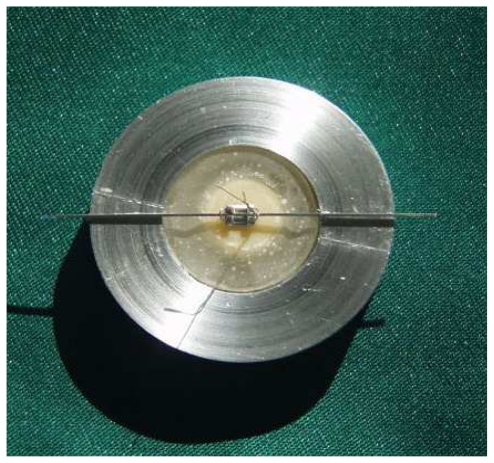
The bracket bonding and other procedures were performed similar to the first group. Following the bonding process, the specimens were stored in distilled water in room temperature for 24 hours and then subjected to thermo-cycling procedure for 500 times in a bath between 5°C and 55 °C. The dwelling time in the bath was 60 seconds while the specimens were transferred between two bathes in 8 seconds. All samples were transferred to a specimen prepared as described previously [16] and subsequently embedded in acrylic resin (Fig 1). Zwick universal testing machine (Z/100, Germany) was used to determine the tensile bond strength of the ceramic specimens in different groups. For this purpose, acrylic specimens were positioned in the lower part of the device and a steel wire which was connected to the upper part of the device was placed beneath the bracket wings (Fig 2). The device was calibrated to apply tensile force with 0.5 mm/min crosshead speed on the brackets until debonding occurred. Tensile bond strength was calculated by Newton being converted into mega-pascal (MPa) by dividing the force to the bracket base area (mm2) (MPa=N/mm2).
Fig 1.
Specimens were mounted in acrylic resin in a special jig.
Fig 2.
Specimen setup for testing the tensile bond strength in a universal testing machine
The bracket base area was 16.52 mm2 as informed by the manufacturer.
After the debonding procedure, the specimen surfaces were analyzed for the calculation of Adhesive Remnant Index (ARI) under ×2 magnification using the following measurements [17]:
The entire composite remained on the ceramic surface,
More than 90% of the composite remained on the ceramic surface,
More than 10% and less than 90% of the composite remained on the ceramic surface,
Less than 10% of the composite remained on the ceramic surface,
No composite remained on the ceramic surface.
Bond strength of the three groups was calculated. One-way analysis of variance and Tukey post-hoc tests were used for statistical analysis of SBS. Adhesive remnant index (ARI) was analyzed by the Kruskal-Wallis test.
RESULTS
One of the first group specimens, three of the second group specimens and two of the third group specimens were lost during acrylic resin embedment and calibration of the debonding device.
The mean tensile bond strength values of specimens of groups first,second and third were 3.69 MPa, 2.69 MPa and 3.6 MPa respectively. Descriptive indices of tensile bond strength of three groups were shown in Table 1.
Table 1.
Tensile Bond Strength of Tested Specimens
| Surface condition | N | Mean (MPa) | Standard Deviation | Min. (MPa) | Max. (MPa) |
|---|---|---|---|---|---|
| I | 13 | 3.69 | 0.52 | 2.53 | 4.59 |
| II | 11 | 2.69 | 0.91 | 1.26 | 4.04 |
| III | 12 | 3.60 | 0.41 | 2.98 | 4.26 |
The one way ANOVA showed that there was a statistical significant difference in tensile bond strength between three groups. According to Tukey HSD test, significant differences were observed between second (without using scotch bond multipurpose bond) and the other groups using scotch bond multipurpose adhesive (P<0.01). However, no significant differences were noted between first and second groups (P=0.937).
Significant differences were observed regarding ARI index of three different surface treatment modalities (P<0.001). In first group, the entire composite remained on the ceramic surface on 92.3% of the specimens (ARI index=1). In second group, no composite remained on the ceramic surface on 45.5% of the specimens and in third group, the entire composite remained on the ceramic surface on 91.7% of the specimens.
DISCUSSION
Conventional bond strength tests include shear and tensile experiments, although torsional experiment results have been reported in some cases [18, 19]. Both shear and tensile tests are valid methods [20].
In the shear bond strength test, complex stress distribution is developed making the exact stress calculation impossible in the interfacial area; so failure may occur due to the higher concentrations of local stresses.
Therefore, shaer bond stress test is not able to show the accurate characteristics of the adhesive in the surface/interface areas [19]. The tensile bond strength test provides a specimen design with a more unified stress distribution across the interface area [21].
In the present study, long thin wires were used beneath the bracket wings for the calculation of tensile forces. This modification was recommended by Katone and Chen [22]. As stated by Newman [23] 14 kg/cm2 (≈1.5 MPa) is the maximum load which may be entered by an orthodontic appliance to a human tooth. The tensile bond strength of three surface treatment modalities in the present study was much more than this value.
In a laboratory study, Olsen. [24] assessed shear bond strength of metal brackets to the tooth surfaces using scotch bond multipurpose adhesive together with 37% phosphoric acid and 10% maleic acid. They calculated 13.1±4.8 MPa and 10.3±3.1 MPa shear bond strength to the tooth surface for phosphoric acid and maleic acid conditioning respectively. Thermocycling of specimens before assessment of bonding strength and the type of bonding strength test can be justified the differences with our results. Furthermore, it has been shown that shear bond strength results are higher than tensile bond strength values in orthodontic bonding [18, 25, 26].
Thurmond et al [27] demonstrated a higher shear bond strength following HFA and silane application compared to phosphoric acid and-ceramic primer use after 24 hours or 3 months storage of the specimens in water.
However, some studies suggested that HFA surface conditioned cannot increase bond strength [28]. In addition, Aida et al [29] concluded that acid etching procedure with HFA could be replaced with phosphoric acid in addition with using an appropriate silane. Kussano et al [30] demonstrated no significant differences between phosphoric acid application or HFA etching plus silane regarding bond strength. The present study showed similar tensile bond strength values following phosphoric acid or HFA etching in the first and third groups. Major et al [31] concluded most failures occur on the ceramic-adhesive interfaces in the cases of lower bond strength while together with the increased bond strength, failure sites tend to occur at the bracket/adhesive interface or cohesive within the composite resin. As shown by the present study, 100% of first group and 91.7% of third group specimens had more than 90% composite resin remaining on the ceramic surface. But in second group with lower bond strength values than other groups, 54.6% of the specimens had less than 10% composite resin remaining on the ceramic surface.
The tensile bond strength to the ceramic surface demonstrated by Cochran et al [32], Kocadereli et al [33] and Harari et al [34] are higher than our results despite using a similar methodology to ours. The differences may be justified regarding specimen thermocycling which was not done by these researchers. Thermocycling is a laboratory prosses that simulate thermal conditions of the oral cavity and seems to have a significant effect on bond strength values [35]. Although in vitro bond strength studies are useful to provide information about new adhesive materials and bonding techniques, in vitro bond strength data should be interpreted with caution [3]. An important negative aspect of in vitro bond strength studies is that complete simulation of oral environment including temperature, humidity, PH, forces and microbial flora is almost impossible. Another comparison made between tensile bond strength of the glazed and non-glazed ceramics showed no significant differences between similar groups [16] which was similar to the findings of Eustaquio et al [36], Zelos et al [26], Kocadereli et al [33] and Sant’ Anna et al [37]. However, Barbosa et al [7] and Schmage et al [10] recommended removal of the glazed layer necessary for obtaining adequate bond strength.
CONCLUSION
In spite of limitations in laboratory studies it may be concluded that in application of Scotch bond multipurpose plus adhesive, phosphoric acid can be used instead of HFA for bonding brackets to the glazed ceramic restorations with enough tensile bond strength.
Table 2.
Adhesive Remnant Index (ARI) Scores for the Groups
| Group | ARI | Total | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| I | Count | 12 | 1 | 0 | 0 | 0 | 13 |
| % Within Group | 92.3% | 7.7% | 0% | 0% | 0% | 100% | |
| II | Count | 2 | 0 | 3 | 1 | 5 | 11 |
| % Within Group | 18.2% | 0% | 27.3% | 9.1% | 45.5% | 100% | |
| III | Count | 11 | 1 | 0 | 0 | 0 | 12 |
| % Within Group | 91.7% | 8.3% | 0% | 0% | 0% | 100% | |
AKNOWLEDGMENTS
This investigation was supported by Dental Research Center, Tehran University of Medical Sciences, grant No. 132/5708.
REFERENCES
- 1.Robb SI, Sandowsky C, Schneider BJ, BeGole EA. Effectiveness and duration of orthodontic treatment in adults and adolescents. Am J Orthod Dentofac Orthop. 1998 Oct;114(4):383–6. doi: 10.1016/s0889-5406(98)70182-9. [DOI] [PubMed] [Google Scholar]
- 2.Graber TM, Vanarsdall RL. Orthodontics: Current principles and practice. 3rd ed. St. Louise: Mosby; 2000. pp. 840–1. [Google Scholar]
- 3.Zachrisson YO, Zachrisson BU, Buyukyilmaz T. Surface preparation for orthodontic bonding to porcelain. Am J Orthod Dentofacial Orthop. 1996 Apr;109(4):420–30. doi: 10.1016/s0889-5406(96)70124-5. [DOI] [PubMed] [Google Scholar]
- 4.Smith GA, McInnes-Ledoux P, Ledoux WR, Wenberg R. Orthodontic bonding to porcelain bond strength and refinishing. Am J Orthod Dentofacial Orthop. 1988 Sep;94(3):245–52. doi: 10.1016/0889-5406(88)90034-0. [DOI] [PubMed] [Google Scholar]
- 5.Diaz-Arnold AM, Aquilino SA. An evaluation of the bond strengths of four organosilane materials in response to thermal stress. J Prosthet Dent. 1988 Sep;62(3):257–60. doi: 10.1016/0022-3913(89)90327-2. [DOI] [PubMed] [Google Scholar]
- 6.Rezk-Lega F, Ogaard B. Tensile bond force of glass ionomer cements in direct bonding of orthodontic brackets: an in vitro study. Am J Orthod Dentofacial Orthop. 1991 Oct;100(4):357–61. doi: 10.1016/0889-5406(91)70074-7. [DOI] [PubMed] [Google Scholar]
- 7.Barbosa VL, Almeida MA, Chevitarese O, Keith O. Direct bonding to porcelain. Am J Orthod Dentofacial Orthop. 1995 Feb;107(2):159–64. doi: 10.1016/s0889-5406(95)70131-1. [DOI] [PubMed] [Google Scholar]
- 8.Chen JH, Matsumura H, Atsuta M. Effect of different etching periods on the bond strength of a composite resin to a machinable porcelain. J Dent. 1998 Jan;26(1):53–8. doi: 10.1016/s0300-5712(96)00078-4. [DOI] [PubMed] [Google Scholar]
- 9.Kitayama Y, Komori A, Nakahara R. Tensile and shear bond strength of resin reinforced glass ionomer cement to glazed porcelain. Angle Orthod. 2003 Aug;73(4):451–56. doi: 10.1043/0003-3219(2003)073<0451:TASBSO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Schmage P, Nergiz I, Herrmann W, Ozcan M. Influence of various surface conditioning methods on the bond strength of metal brackets to ceramic surfaces. Am J Orthod Dentofacial Orthop. 2003 May;123(5):540–6. doi: 10.1067/mod.2003.S0889540602569110. [DOI] [PubMed] [Google Scholar]
- 11.Türkkahraman H, Küçükesmen HC. Porcelain surface-conditioning techniques and the shear bond strength of ceramic brackets. Eur J Orthod. 2006 Oct;28(5:):440–3. doi: 10.1093/ejo/cjl026. Epub 2006 Aug 17. [DOI] [PubMed] [Google Scholar]
- 12.Moore PA, Manor RC. Hydrofluoric acid burns. J Prosthet Dent. 1982 Mar;47(3):338–9. doi: 10.1016/0022-3913(82)90165-2. [DOI] [PubMed] [Google Scholar]
- 13.Zachrisson BU. Bonding in orthodontics. In: Graber TM, Vanarsdall RL, editors. Orthodontics: current principles and practice. 3rd ed. St. Louise: Mosby; 2000. pp. 840–1. [Google Scholar]
- 14.Zachrisson BU. Orthodontic bonding to artificial tooth surfaces: clinical versus laboratory findings. Am J Orthod Dentofacial Orthop. 2000 May;117(5):592–4. doi: 10.1016/s0889-5406(00)70211-3. [DOI] [PubMed] [Google Scholar]
- 15.Anusavice KJ. Phillip’s science of dental materials. 10th ed. Philadelphia: WB Saunders; 1996. [Google Scholar]
- 16.Ahmad Akhoundi M, Rahmati Kamel M, Hooshmand T, Harririan I, Kharazi Fard M, Noroozi H. Assessment of Bond Strength between Metal Brackets and Non-Glazed Ceramic in Different Surface Treatment Methods. J Dent (Tehran) 2010 Spring;7(2):64–70. Epub 2010 Jun 30. [PMC free article] [PubMed] [Google Scholar]
- 17.Bishara SE, Soliman MM, Oonsombat C, Laffoon JF, Ajlouni R. The effect of variation in mesh-base design on the shear bond strength of orthodontic brackets. Angle Orthod. 2004 Jun;74(3):400–4. doi: 10.1043/0003-3219(2004)074<0400:TEOVIM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Merrill SW, Oesterle LJ, Hermesch CB. Ceramic bracket bonding: a comparison of shear, tensile, and torsional bond strengths of ceramic brackets. Am J Orthod Dentofacial Orthop. 1994 Sep;106(3):290–7. doi: 10.1016/S0889-5406(94)70049-4. [DOI] [PubMed] [Google Scholar]
- 19.Eliades T, Brantley WA. The inappropiatness conventional orthodontic bond strength assessment protocols. Eur J Orthod. 2000;22(1):13–23. doi: 10.1093/ejo/22.1.13. [DOI] [PubMed] [Google Scholar]
- 20.Van Meerbeek B, Peumans M, Poitevin A, Mine A, Van Ende A, Neves A, De Munck J. Relationship between bond-strength tests and clinical outcomes. Dent Mater. 2010 Feb;26(2):e100–21. doi: 10.1016/j.dental.2009.11.148. [DOI] [PubMed] [Google Scholar]
- 21.Hooshmand T, van Noort R, Keshvad A. Bond durability of the resin-bonded and silane treated ceramic surface. Dent Mater. 2002 Mar;18(2):179–88. doi: 10.1016/s0109-5641(01)00047-1. [DOI] [PubMed] [Google Scholar]
- 22.Katona TR, Chen J. Engineering and experimental analysis of the tensile loads applied during strength testing of direct bonded orthodontic brackets. Am J Orthod Dentofacial Orthop. 1994 Aug;106(2):167–74. doi: 10.1016/S0889-5406(94)70035-4. [DOI] [PubMed] [Google Scholar]
- 23.Newman GV. Epoxy adhesives for orthodontic attachments: Progress report. Am J Orthod. 1965 Dec;51(12):901–2. doi: 10.1016/0002-9416(65)90203-4. [DOI] [PubMed] [Google Scholar]
- 24.Olsen ME, Bishara SE, Damon P, Jakobsen JR. Evaluation of Scotch bond Multipurpose and maleic acid as alternative methods of bonding orthodontic brackets. Am J Orthod Dentofacial; Orthop. 1997 May;111(5):498–501. doi: 10.1016/s0889-5406(97)70286-5. [DOI] [PubMed] [Google Scholar]
- 25.Ghassemi-Tary B. Direct bonding to porcelain: an in vitro study. Am J Orthod. 1979 Jul;76(1):80–3. doi: 10.1016/0002-9416(79)90301-4. [DOI] [PubMed] [Google Scholar]
- 26.Zelos L, Bevis RR, Keenan KM. Evaluation of the ceramic/ceramic interface. Am J Orthod Dentofacil Orthop. 1994 Jul;106(1):10–21. doi: 10.1016/S0889-5406(94)70016-8. [DOI] [PubMed] [Google Scholar]
- 27.Thurmond JW, Barkmeier WW, Wilwerding TM. Effect of porcelain surface treatments on bond strengths of composite resin bonded to porcelain. J Prosthet Dent. 1994 Oct;72(4):355–9. doi: 10.1016/0022-3913(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 28.Saygili G, Sahmali S. Effect of ceramic surface treatment on the shear bond strengths of two resin luting agents to all-ceramic materials. J Oral Rehabil. 2003 Jul;30(7):758–64. doi: 10.1046/j.1365-2842.2003.01027.x. [DOI] [PubMed] [Google Scholar]
- 29.Aida M, Hayakawa T, Mizukawa K. Adhesion of composite to porcelain with various surface conditions. J Prosthet Dent. 1995 May;73(5):464–70. doi: 10.1016/s0022-3913(05)80076-9. [DOI] [PubMed] [Google Scholar]
- 30.Kussano CM, Bonfante G, Batista JG, Pinto H. Evaluation of shear bond strength of composite to porcelain according to surface treatment. Braz Dent J. 2003;14(2):132–5. doi: 10.1590/s0103-64402003000200011. Epub 2003 Oct 3. [DOI] [PubMed] [Google Scholar]
- 31.Major PW, Koehler JR, Manning KE. 24-hour shear bond strength of metal orthodontic brackets bonded to porcelain using various adhesion promoters. Am J Orthod Dentofacial Orthop. 1995 Sep;108(3):322–9. doi: 10.1016/s0889-5406(95)70028-5. [DOI] [PubMed] [Google Scholar]
- 32.Cochran D, O’Keefe KL, Turner DT, Powers JM. Bond strength of orthodontic composite cement to treated porclain. Am J Orthod Dentofacial Orthop. 1997 Mar;111(3):297–300. doi: 10.1016/s0889-5406(97)70188-4. [DOI] [PubMed] [Google Scholar]
- 33.Kocadereli I, Canay S, Akca K. Tensile bond strength of ceramic orthodontic brackets bond strength of ceramic orthodontic brackets bonded to porcelain surfaces. Am J Orthod Orthofacial Orthop. 2001 Jun;119(6):619–20. doi: 10.1067/mod.2001.113655. [DOI] [PubMed] [Google Scholar]
- 34.Harari D, Shapira-Davis S, Gillis I, Roman I, Redlich M. Tensile bond strength of ceramic brackets bonded to porcelain facets. Am J Or thod Dentofacial Orthop. 2003 May;123(5):551–4. doi: 10.1067/mod.2003.S0889540602569134. [DOI] [PubMed] [Google Scholar]
- 35.Bishara SE, Ajlouni R, Laffon JF. Effect of thermocycling on the shear bond strength of a cyanoacrilate orthodontic adhesive. Am J Orthod Dentofacial Orthop. 2003;123(1):21–24. doi: 10.1067/mod.2003.1. [DOI] [PubMed] [Google Scholar]
- 36.Eustaquio R, Garner LD, Moore BK. Comparative tensile strengths of brackets bonded to porcelain with orthodontic adhesive and porcelain repair systems. Am J Orthod Dentofacial Orthop. 1988 Nov;94(5):421–5. doi: 10.1016/0889-5406(88)90132-1. [DOI] [PubMed] [Google Scholar]
- 37.Sant’ Anna EF, Monnerat ME, Chevitarese O, Stuanni MB. Bonding brackets to porcelain--in vitro study. Braz Dent J. 2002;13(3):191–6. doi: 10.1590/s0103-64402002000300010. [DOI] [PubMed] [Google Scholar]