Abstract
Amphetamines have rewarding and aversive effects. Relative sensitivity to these effects may be a better predictor of vulnerability to addiction than sensitivity to one of these effects alone. We tested this hypothesis in a dose-response study in a second replicate set of mouse lines selectively bred for high vs low methamphetamine (MA) drinking (MADR). Replicate 2 high (MAHDR-2) and low (MALDR-2) MA drinking mice were bred based on MA consumption in a two-bottle choice procedure, and examined for novel tastant drinking. Sensitivities to the rewarding and aversive effects of several doses of MA (0.5, 2, and 4 mg/kg) were measured using a place conditioning procedure. After conditioning, mice were tested in a drug-free and then drug-present state for time spent in the saline- and MA-paired contexts. Similar to the first set of MADR lines, by the end of selection, MAHDR-2 mice consumed about 6 mg MA/kg/18 h, compared to nearly no MA in MALDR-2 mice, but had similar taste preference ratios. MAHDR-2 mice exhibited place preference in both the drug-free and drug-present tests, and no significant place aversion. In contrast, MALDR-2 mice exhibited no place preference or aversion during the drug-free test, but robust place aversion in the drug-present test. These data extend our preliminary findings from the first set of MADR lines, and support the hypothesis that the combination of greater sensitivity to the rewarding effects of MA and insensitivity to the aversive effects of MA is genetically associated with heightened risk for MA consumption.
Keywords: CPP, CPA, reward, aversion, selective breeding, drug abuse, methamphetamine consumption
Introduction
Methamphetamine (MA) has a high potential for abuse (http://www.nida.nih.gov/ResearchReports/methamph/methamph3.html), with devastating health and social consequences (Gonzales et al. 2010). MA is known to induce rewarding effects, such as prolonged euphoria and high energy, and aversive effects, such as anxiety, dysphoria and headaches (Cruickshank & Dyer 2009; Sheridan et al. 2009). A similar duality of appetitive and avoidance behaviors that reflect motivational drug effects has also been shown in animal studies (Spyraki et al. 1982; Fudala & Iwamoto 1990; Bardo et al. 1999; Ricoy & Martinez 2009; Davis & Riley 2010). Individual differences in the balance of sensitivity to these effects could explain why only a fraction of individuals who experiment with MA escalate their use and ultimately develop an addiction.
With genetic animal models, the question of whether some of the same genes influence avidity for MA and sensitivity to MA can be directly studied. We recently reported the results for the first of two sets of lines from a bidirectional selective breeding project that was based on voluntary oral self-administration of MA in mice (Wheeler et al. 2009). The oral route was chosen because an intravenous (IV) self-administration model was not tractable for a selective breeding study involving hundreds of mice. Furthermore, the oral route is relevant to human addiction (Galloway et al. 2010). To validate the high (MAHDR) and low (MALDR) MA drinking lines as a model of genetically-determined differential sensitivity to the motivational effects of MA, preliminary studies examined their responses using two conditioning procedures, place and taste conditioning. The former utilized tactile cue associations with MA, and the latter utilized a novel taste cue association with MA. Results for a single dose of MA showed significant MA-induced conditioned place preference (CPP) in MAHDR, but not MALDR, mice (Wheeler et al. 2009). Results for two modest doses of MA showed significant MA-induced conditioned taste aversion (CTA) in MALDR mice that was absent in MAHDR mice (Wheeler et al. 2009).
To further investigate the relationship between level of MA intake and sensitivity to the rewarding and aversive effects of MA, we replicated the selection, extended our examination of place conditioning to higher doses of MA and included an evaluation of state-dependent effects of MA on CPP. The goals were to determine the reliability of the selection and of the genetically correlated CPP differences, and to determine whether there was a relative shift in the dose-response curve for MA reward and aversion in the selected lines, versus a complete lack of sensitivity to either of these effects in either of the lines. In addition, we predicted that CPP measured in the presence of MA treatment would reveal conditioned effects not apparent during the drug-free test, and might reveal additional selected line differences. Other studies have found conditioned place aversion (CPA) displayed under this condition at doses for which CPA is not expressed in a drug-free test (Cunningham & Noble 1992).
Methods
Animals
The F2 cross of the C57BL/6J and DBA/2J inbred strains (B6D2F2) and lines of mice bred for differences in MA consumption from this F2 were used. Experimental procedures using animals were consistent with guidelines from the National Institutes of Health and approved by the Portland VA Institutional Animal Care and Use Committee. Mice were group housed (2-4 per cage) with same sex littermates in standard acrylic plastic shoebox cages (28.5 × 17.5 × 12 cm) with Bed-O-Cob™ bedding (Animal Specialties Inc., Hubbard, OR, USA) and free access to rodent chow (Purina 5001™, 4.5% fat content; Animal Specialties Inc., Hubbard, OR, USA) and water, except during two-bottle choice testing, when they were isolate housed. The animal room was maintained at 21 ± 1°C on a 12:12-h light:dark schedule (lights on 0600 h).
Drugs and tastants
(+)Methamphetamine hydrochloride (MA), saccharin sodium salt, quinine hemisulfate salt, and potassium chloride (KCl) were purchased from Sigma (St Louis, MO, USA). For injection, MA was dissolved in sterile physiological saline (0.9% NaCl; Baxter Healthcare Corporation, Deerfield, IL, USA). All injections were given intraperitoneally (IP) at a volume of 10 ml/kg. For consumption, MA was dissolved in tap water. Drinking solutions of saccharin, quinine and KCl were also prepared by dissolving each tastant in tap water.
Selective breeding for MA consumption
Mass selection procedures were used to create the second replicate set of MA drinking (MADR-2) mice from the B6D2F2 cross, exactly as previously described (Wheeler et al. 2009). The founding population of 124 F2 mice (half of each sex; 54-64 d old) was tested for amount of MA consumed in a two-bottle choice test. Briefly, mice were isolate-housed and given access to two water-filled 25-ml tubes for two consecutive days, and then during the next 8 days, one tube was filled with tap water and one with MA in tap water (20 mg/l for 4 days, then 40 mg/l for 4 days). Water was available 24-h per day, but mice had access to the MA-filled tubes for 18 h/day; the MA solution bottle was removed from the cage during hours 3-9 of the light phase. The MA and water tube positions were switched every 2 days, and body weight was measured every 4 days and used for calculation of MA consumption in mg/kg. Volume changes were used to calculate consumption and preference ratio (ml MA:total ml from both tubes during the same 18 hr period) values by averaging the values for the second and fourth days for each concentration of MA, the second day after a tube side switch. F2 parents chosen for breeding to establish the MAHDR-2 and MALDR-2 lines (n=13 male and 13 female mice per line) were selected based on their consumption of the 40 mg/l solution. Selection continued in this fashion, within-line, for each subsequent generation. The numbers and ages of the offspring tested in each of the S1 to S4 generations are given in the Figure 1 legend.
Figure 1.
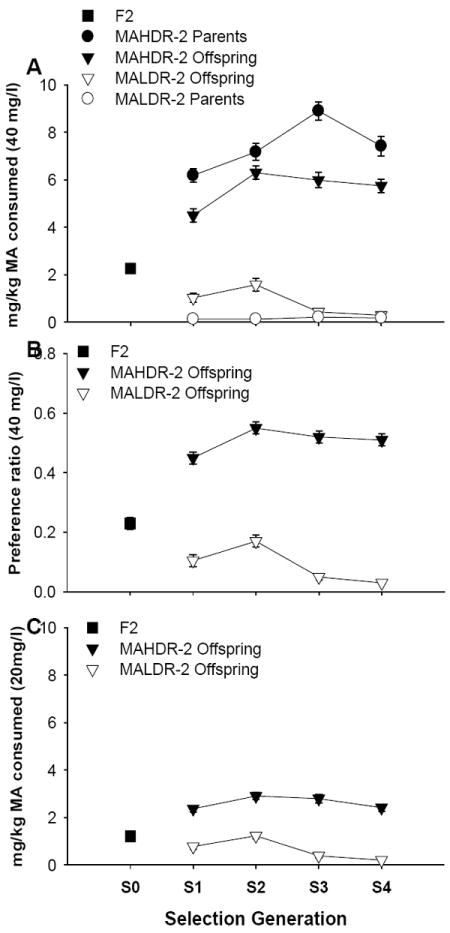
Bidirectional selective breeding for MA consumption is replicable and also produces differences in methamphetamine (MA) preference ratio. (A) mg/kg of MA consumed by the originating B6D2F2 (F2; S0), and the parent and offspring mice across selection generations, when MA was offered in a 40 mg/l solution in tap water vs plain tap water. (B) MA preference ratio for the same animals, calculated as the ratio of the volume of the MA solution consumed to total fluid consumed. (C) mg/kg of MA consumed when MA was offered in a 20 mg/l solution vs water (prior to offering the higher concentration) for the same mice. All data are averages of days 2 and 4 from the two-bottle choice procedure. n = 120 for F2, 50-80 per line for offspring mice, and 26 per line for parent mice of each generation. Symbols and error bars represent means ± SEM. MAHDR-2, MA high drinking replicate 2; MALDR-2, MA low drinking replicate 2.
Two-bottle choice test for novel tastants
MA has a bitter taste (www.nida.nih.gov/Infofacts/methamphetamine.html) that could influence its consumption, so it is important to determine whether the MA consumption lines possess differences in taste preference (or aversion) that could account for their differences in MA consumption. Second to fourth litter offspring (n=10-14 per line, sex and dose) from the S2 generation of MADR-2 mice were used to measure quinine, saccharin and KCl consumption and preference ratios as done previously (Wheeler et al. 2009). Mice (74-81 days old) were exposed to two, 4-day tests (24 h/day) for each tastant (quinine, 0.015 and 0.03 mM; saccharin, 0.033 and 0.066%; and KCl, 100 and 200 mM) in which the two concentrations of the same tastant were presented consecutively versus tap water and the lower concentration was presented first. The order of tastant presentation was counterbalanced to account for the possible influence of consumption of one tastant on consumption of another. Tastant consumption and preference ratios were calculated as described for MA drinking. Mice in the current tastant study had participated in an ethanol drinking study, which ended 5 days before the initiation of tastant testing.
Place conditioning
An unbiased place conditioning procedure (Cunningham et al. 2006) was used to measure sensitivities of the F2 and the MADR-2 lines to the rewarding and aversive effects of several doses of MA. An initial study in drug- and experiment-naïve B6D2F2 mice (n=15-16 per sex and MA dose; 73-77 days old) was completed in two passes. In a second experiment, drug- and experiment-naïve second to fourth litter offspring (n=10-14 per line, sex and dose; 56-94 days old) from the S3 generation of MADR-2 mice were tested in three passes. In both experiments, passes included equal numbers of mice with regard to sex, MA dose and genotype. Conditioning and testing occurred between 0800 and 1500 h and mice of different types and in different dose groups were counterbalanced across the time period.
The procedure and equipment were identical to those used in our previous study (Wheeler et al. 2009). Conditioning boxes (San Diego Instruments, San Diego, CA, USA) in illuminated and ventilated sound attenuating chambers were equipped with a removable black plastic wall that could be used to confine animals to the right or left half. The removable floor was comprised of two half panels of three possible types: black acrylic plastic, ‘grid’ (2.3 mm stainless-steel rods mounted 6.4 mm apart) and ‘hole’ (stainless steel with 6.4 mm round holes on 9.5 mm staggered centers). Activity level and animal position were measured automatically by photocell interruptions. In the F2 study, 6 conditioning trials (one per day) with 0.5, 1, or 2 mg/kg of MA were interspersed with 6 saline trials (one per day). The choice of these doses was based on previous pilot and published studies (Wheeler et al. 2009; Cunningham & Noble 1992; Niwa et al. 2007). We made an a priori decision to include the 0.5 mg/kg MA dose so that results for this dose could be independently compared to those for the initial set of MADR lines that had been tested only with this dose. After evaluating the data from the F2 mice, a higher 4 mg/kg dose was chosen for the selected line study, in addition to the 0.5 and 2 mg/kg doses, based on the prediction that this dose would be more likely to reveal significant line differences.
In an initial habituation session, mice received saline and were exposed to the whole conditioning box with the smooth plastic floor for 5 min. On conditioning days, which began the next day, mice were confined to one half of the apparatus for 15 min after saline or MA treatment (left or right side, with grid and hole floors counterbalanced for position) for 12 consecutive days, excluding weekend days. Saline or MA treatment was alternated and each was paired with a specific floor cue (grid or hole) for each animal. Animals of group “G+” received MA on the grid floor and saline on the hole floor; animals of group “G-” received saline on the grid floor and MA on the hole floor. A 30-min drug-free preference test was conducted one day after the last conditioning trial, during which mice received saline, and then had access to both floor cues. Two days later, a drug-present preference test was performed; instead of saline, mice were injected with the dose of MA they had received during conditioning. For both tests, floor cues for individual mice were consistently placed to match their position in the apparatus during their conditioning days. Note: a subset of MAHDR-2 (n = 6; 2 per dose) and MALDR-2 (n = 6; 2 per dose) mice from the first pass of the study were randomly selected to receive saline on the drug-present preference test day for another study, and thus they did not contribute data for this test (final n for the drug-present preference test = 7-13 per line, sex and dose).
Data Analysis
Data were analyzed with Statistica (StatSoft, Tulsa, OK, USA) using factorial analysis of variance (ANOVA) or covariance (ANCOVA) with repeated measures as appropriate. Sex was included as a factor in all initial analyses and excluded from subsequent analyses, if no significant effects involving sex were found. Sex is mentioned in the results only when it had a significant effect. Three-way interactions were further examined by two-way ANOVA within each level of a relevant factor (e.g., for effects of dose and conditioning group within each selected line). Two-way interactions were examined for significant simple effects. Post hoc mean comparisons (Newman-Keuls) were applied only when the simple effect was significant or to examine group differences for main effects. Mean comparisons were restricted to those appropriate for evaluating specific hypotheses, rather than testing for all possible differences, to reduce Type I error. Correlations were determined using Pearson’s r. Statistical results were considered significant at p < 0.05. Finally, the selection response realized heritability calculation was based on the ratio of R, the response to selection, over S, the selection differential (Falconer & Mackay 1996).
The dependent variable used for place conditioning was indexed in sec/min, which was calculated by dividing the number of seconds spent on the grid floor by the total duration of the test session in minutes. The advantage of this simple transformation is that results are easily compared to the full range of possible outcomes (e.g., 0 sec/min indicates complete aversion to grid; 60 sec/min indicates complete preference for grid). This measure also facilitates comparison between experiments that involve different test durations. Because there are only two floors, the amount of time spent on the hole floor can be determined simply by subtracting the mean sec/min on grid from 60. In the unbiased, fully counterbalanced design used here, place preference (or aversion) is defined by between-group comparison of time spent on the grid floor by the G+ and G- conditioning subgroups. The expectation is that time spent on the grid floor will be greater in G+ mice than in G- mice when MA is rewarding, but less when MA is aversive. These outcomes are reflected statistically as a main effect of conditioning group (G+ vs. G-); drug dose effects are indicated by significant interactions with conditioning group. Although other dependent variables and statistical strategies are sometimes used to draw conclusions about place conditioning, this between-groups approach is preferred because it provides an appropriate control for drug-induced learning (Cunningham 1993) and because it exposes performance in each of the counterbalanced subgroups, which is typically ignored in most alternative approaches (Cunningham et al. 2003a). A more complete discussion of this issue and a comparison of various dependent variables in both a biased and unbiased place conditioning procedure can be found elsewhere (Cunningham 1993; Cunningham et al. 2003a). We have also included the data expressed as percent time on drug-paired floor, to show that the findings were comparable for the two measures.
Data were examined for both the first 15-min of the test, a time period comparable to the duration of conditioning trials, and for the entire 30-min period of the test. Outcomes were virtually identical; thus, data for only the entire 30-min test are presented, consistent with our previous examination (Wheeler et al. 2009).
Results
Response to selection
The response to selection was bidirectional in the MADR-2 lines (Figure 1), similar to the response seen in the first set of MADR lines (Wheeler et al. 2009). The difference between the lines was maximal by S3, with the MAHDR-2 mice consuming about 6 mg/kg MA compared to near 0 mg/kg in the MALDR-2 mice. Analysis of MA consumption data for S1-S4 offspring grouped on generation, line and sex, identified significant two-way interactions [generation × line (F3,507=7.0, p<0.001), and sex × line (F1,507=17.0, p<.001); Figure 1A], but no 3-way interaction. There was increasing divergence in MA consumption between the lines across generations; the line difference was significant in every generation (p values<.001). There was a sex difference in the MAHDR-2 line, with higher MA consumption for female (mean ± SE = 6.2 ± 0.2; n = 132) than male mice (mean ± SE=5.0±0.2; n=135), but no difference for MALDR-2 mice (mean ± SE = 0.8 ± 0.1; n = 132 and 1.0 ± 0.2; n = 124 for female and male mice, respectively). Because there was no change in the sex × line interaction across generations, data are presented in Figure 1 for the sexes combined.
The bidirectional response to selection was statistically supported by inclusion of data from the F2 (S0 generation) mice in two separate ANOVAs that also included either MALDR-2 or MAHDR-2 S1-S4 data. There was a significant main effect of generation in both cases [F1,386=45.4, p<.001 for MAHDR-2; F1,375=17.0, p<.001 for MALDR-2]. Post-hoc mean comparisons indicated significant differences between the F2 and S1-S4 mice for both lines. Therefore, there was a significant change in MA consumption from the consumption of the originating F2 in both lines, starting in the first generation of selection.
Preference ratio results for the 40 mg/L solution (Figure 1B) and for consumption of 20 mg/l MA (Figure 1C) were similar to those for consumption of the 40 mg/l solution (see supplementary materials). There was a significant line × generation interaction for total fluid consumption (F3,513=10.3, p<.05); S1 MAHDR-2 mice consumed 0.5 ml less fluid than MALDR-2 mice, but this difference reversed in subsequent generations by about the same amount. A difference of this magnitude was found in the first set of MADR lines as well, with MAHDR mice consuming about 0.5 ml more fluid than MALDR mice.
In a selective breeding experiment, divergence of the selected lines indicates that the behavioral trait subjected to selection is heritable; that is that genetic variability within the population of individuals accounts for some portion of the trait variance (Palmer & Phillips 2000). The realized heritability calculation indicates what portion of the trait variance can be accounted for by genetic factors. For the MADR-2 lines, the heritability was 0.35, nearly identical to that for the first set of MADR lines, for which the heritability was 0.34. Thus, genetic differences accounted for 35% of the variance in MA consumption in the MADR-2 lines.
Escalated intake of MA has been shown to occur over repeated periods of long access (Kitamura et al. 2006). We examined our data for evidence of escalation by determining whether MA intake changed significantly across days of 18-h access. Escalated intake was seen in MAHDR-2, but not MALDR-2 or F2 mice for both the 20 mg/l (Figure 2A) and 40 mg/l (Figure 2B) MA concentrations. Repeated measures ANOVAs revealed significant interactions of genotype × day (4 days for each MA concentration) for both 20 mg/l (F6,1929=9.8, p<.001) and 40 mg/l (F6,1920=36.1, p<.001) MA. Simple effects analysis indicated that there were significant differences in MA consumption for all genotypes across days. To determine the nature of the change across days MA intake on day 1 was compared to intake on days 2-4. Significant results are presented in Figure 2. MAHDR-2 mice showed significant increases in MA intake across days, whereas significant reductions in intake were seen on some days in F2 and MALDR-2 mice.
Figure 2.
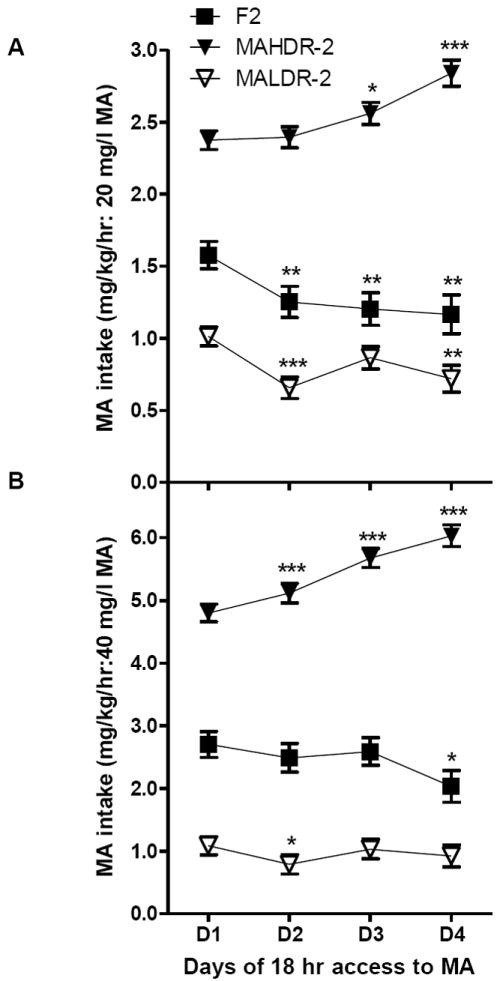
Escalation of MA intake in MAHDR-2, but not F2 or MALDR-2, mice. Shown are means ± SEM mg/kg of MA consumed by the originating B6D2F2 (F2) and the MADR-2 mice (collapsed on generation) for the 20 mg/l (A) and 40 mg/l (B) concentrations across days 1-4 (D1 – D4). *p<.05, **p<0.005, ***p<.001 for the comparison between day 1 and day 2, 3, or 4, within each genotype. MAHDR-2, MA high drinking replicate 2; MALDR-2, MA low drinking replicate 2.
Novel Tastants
There were no significant differences between the lines for quinine or saccharin consumption or preference ratio (Figure 3A and 3B). Significant sex × concentration interactions (see details in supplementary materials) did not interact with line. For KCl consumption (Figure 3A), although there was a significant interaction of line, sex and concentration (F1,44=5.1, p<.05), further examination at each concentration and within each sex yielded no significant line differences. No significant line differences were found for KCl preference ratio (Figure 3B). Overall, these data suggest that taste differences likely played little role in the MA consumption differences between the MADR-2 lines.
Figure 3.
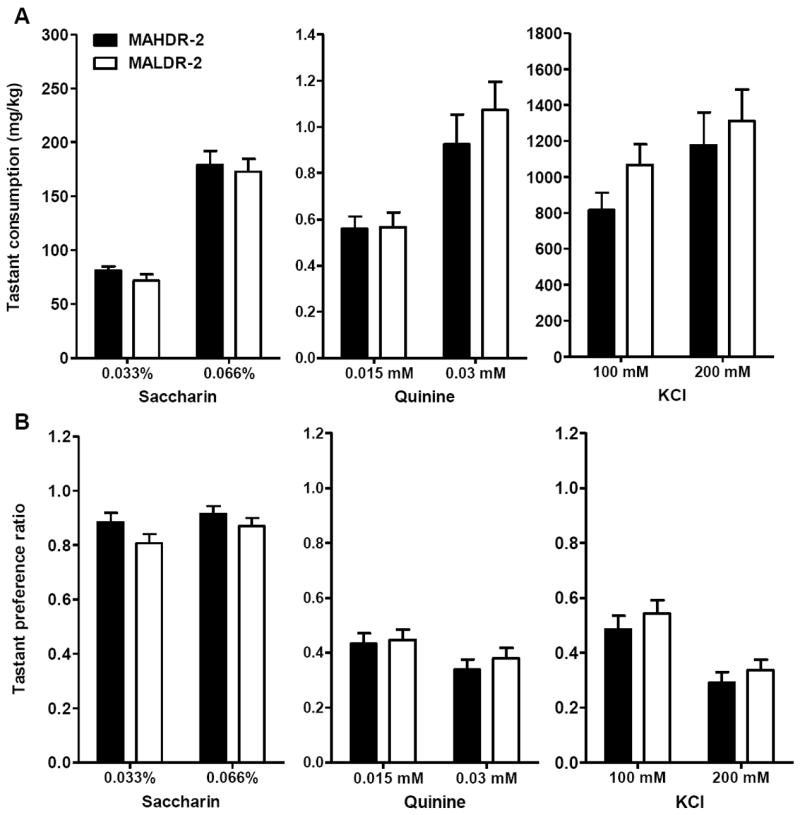
Consumption and preference ratios for unfamiliar sweet (saccharin), bitter (quinine) and salty (KCl) tastes in methamphetamine (MA) drinking line mice of the S2 generation. (A) Tastant consumption in mg/kg. (B) Tastant preference ratio for the same animals, calculated as the ratio of the volume of the tastant solution consumed to total fluid consumed. All data are averages of day 2 and 4 from the two-bottle choice procedure. n = 12 per line and sex. Bars and error bars are means ± SEM. MAHDR-2, MA high drinking replicate 2; MALDR-2, MA low drinking replicate 2.
Place conditioning in F2 mice
There was a significant interaction of conditioning group (G+ or G-) × MA dose (F2,88=3.7, p<.05), indicating that MA effects were dose-dependent. For the 0.5 mg/kg MA dose only, the G+ group spent more time on the grid floor than did the G- group (Figure 4A), indicating significant CPP at this dose (p<.001). To show differences in pharmacological effects with this procedure, a common practice is to analyze percent time on drug-paired floor (Cunningham et al. 2003a). Percent time on MA-paired floor was calculated by dividing time on MA-paired floor with time on both floors and multiplying by 100 (Figure 4B). ANOVA identified a significant dose effect (F2,91=3.7, p<.05). The 0.5 mg/kg MA group spent a significantly greater percentage of their time on the MA-paired floor, compared to the other two doses. There were no differences in locomotor activity level on this test day (see Figure S1 in supplementary materials).
Figure 4.
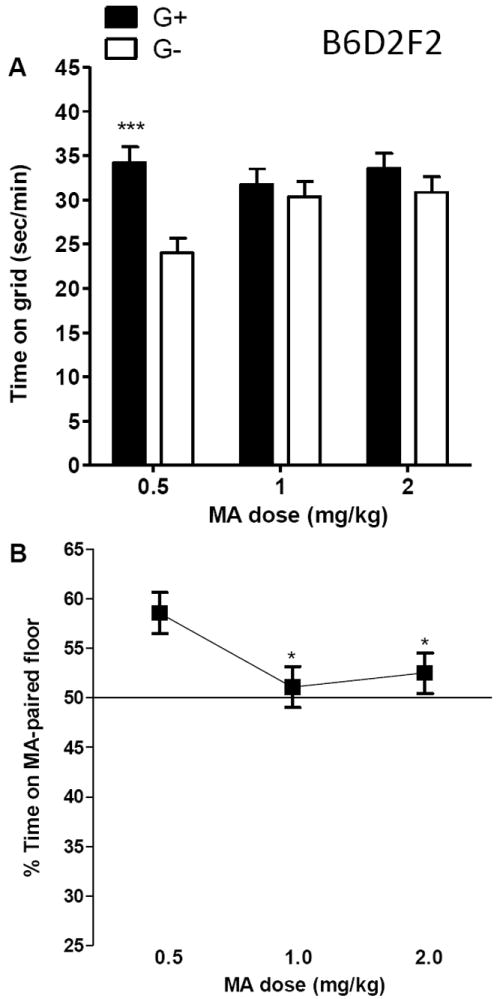
B6D2F2 mice exhibit a conditioned place preference (CPP) to a low, but not to higher, doses of methamphetamine (MA). Data for the 30-min drug-free preference test in mice conditioned with MA on the grid (G+) or on the hole (G-) floor were analyzed as: (A) sec/min on the grid floor for each group (n = 15-17 per group and dose), and (B) percent time on the MA-paired floor for the two groups combined (n = 31-32 per dose). Bars and error bars are means ± SEM. ***p<.001 for the comparison of G+ and G- at 0.5 mg/kg MA. *p<.05 for the comparison between 0.5 mg/kg MA and either 1.0 or 2.0 mg/kg MA.
Locomotor activity on conditioning days
During the conditioning trials, MA exposure was associated with acute stimulation and with locomotor sensitization (Figure 5). Repeated measures ANOVA performed on first trial data only revealed a significant interaction of trial type (saline vs MA) × MA dose (F2,92=8.6, p<.001). MA had significant stimulant effects, compared to saline, at every dose (p<.01); the 1 and 2 mg/kg MA doses were significantly more stimulating than 0.5 mg/kg (p<.05). Locomotor sensitization was supported by results of a repeated measures ANOVA that identified a significant MA dose × trial interaction (F10,460=3.4, p<.001). For saline conditioning sessions, there was a significant main effect of trial (F5,460=9.1, p<.001), associated with reduced levels of activity across sessions. Significant mean differences between trial 1 and subsequent trial responses for each dose are indicated in Figure 5.
Figure 5.
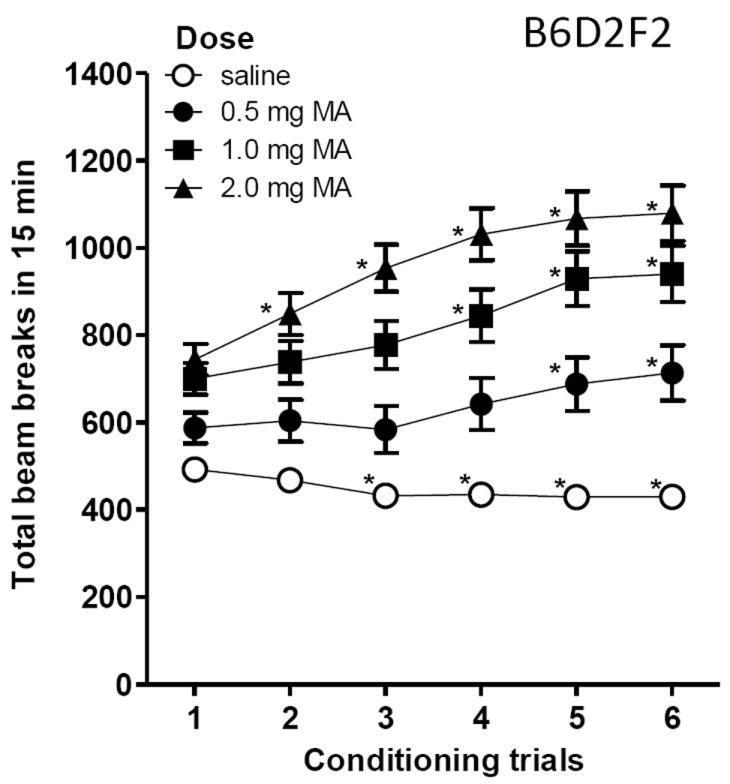
Locomotor activity levels in B6D2F2 mice during the 6 conditioning trials for saline and methamphetamine (MA). Dose groups differed in the number of MA treatments required to induce locomotor sensitization. Locomotor activity was measured as photocell beam breaks during the 15-min conditioning sessions. Conditioning sessions were alternated between saline and MA in a counterbalanced order across animals. MA dose groups were independent, so there were separate saline conditioning trials for each group. Since there were no significant MA dose group effects on activity levels during the saline trials, these data are presented collapsed on MA dose group. n = 31-32 per dose. *p<.05 for the comparison of trial 1 and the indicated subsequent trial. Bars and error bars are means ± SEM. Note: some error bars are hidden by the symbols.
Place conditioning in MADR-2 mice
Drug-free place preference test
MAHDR-2 mice showed a significant MA-induced CPP, but MALDR-2 mice did not (Figure 6A). ANOVA for data grouped on line, dose and conditioning group identified significant interactions of conditioning group × line (F1,127=19.1, p<.001) and of conditioning group × dose (F2,127 = 4.2, p<.02), but no significant 3-way interaction. When the effect of conditioning group was examined within each line, there was significant preference found only for MAHDR-2 mice, with G+ mice spending more time on the grid floor than G- mice (p<.001). This indicates significant CPP, regardless of dose. When data from only the 0.5 mg/kg conditioned mice were analyzed for comparison to results in the first set of MADR lines, a significant interaction of conditioning group × line was found (F1,44=5.8, p<.02), and significant CPP was present in MAHDR-2 (p<.001), but not MALDR-2, mice. Similar results were found for percent time on MA-paired floor. There was a significant effect of line (F1,133=15.7, p<.001), with a greater percentage of time spent on the drug-paired floor by MAHDR-2, compared to MALDR-2, mice (Figure 6B). Locomotor activity measured during the drug-free test was not affected by any of the independent variables (see Figure S2 in supplementary materials).
Figure 6.
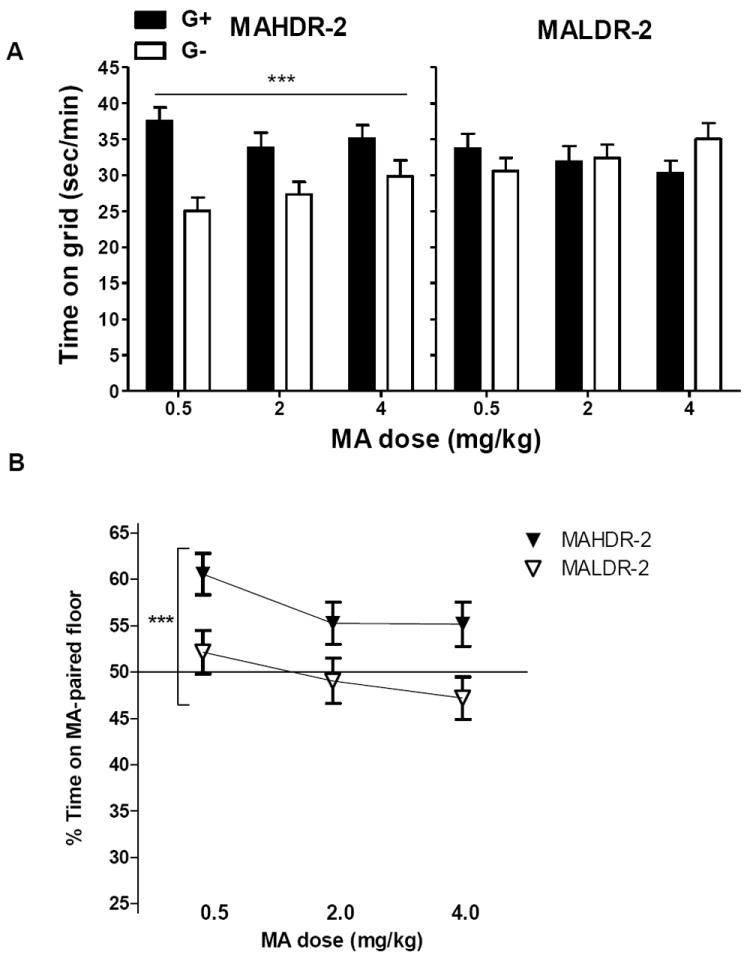
In the drug-free preference test, MAHDR-2 mice exhibit MA-induced conditioned place preference (CPP), whereas MALDR-2 mice do not. Shown are means ± SEM sec/min on the grid floor measured during 30-min place preference tests for animals conditioned with MA on the grid (G+) or hole (G-) floor. Measurements were taken in a standard drug-free preference test (A), in which animals (n = 9-15 per line, group and dose) were administered saline before the test. Differences in pharmacological effects of MA during the same place preference test (B) are also presented as, percent (%) time on MA-paired floor. ***p<.001 for the main effect of conditioning group (A), or for the main effect of line (B). MAHDR-2, MA high drinking replicate 2; MALDR-2, MA low drinking replicate 2.
Drug-present place preference test
When MA was injected before the preference test, MAHDR-2 and MALDR-2 mice displayed markedly different behavioral effects compared to each other and to results seen for the drug-free test. For time on grid (Figure 7A), there were significant interactions of conditioning group × line (F1,115=35.6, p<.001) and conditioning group × dose (F2,115=7.6, p<.006), but no three-way interaction. When the effect of conditioning group was examined within each line, there was a significant effect only for MALDR-2 mice, with G+ mice spending less time on the grid floor than G- mice (p<.001). This indicates significant CPA, regardless of dose. When data for the 0.5 mg/kg dose of MA were examined separately, a significant interaction of conditioning group × line was found (F1,40=27.7, p<.001). Significant conditioning group differences indicated that MAHDR-2 mice showed CPP (p<.001), whereas MALDR-2 mice showed CPA (p<.005), supporting opposite motivational effects of MA in the two lines. Similar results were found for percent time on drug-paired floor (Figure 7C). There were significant effects of line (F1,121=39.0, p<.001) and dose (F2,121= 7.4, p<.001), with no interaction of these variables. MALDR-2 mice spent a significantly smaller percentage of their time on the MA-paired floor, compared to MAHDR-2 mice, and overall, higher MA doses were associated with lower percentage times on the MA-paired floor.
Figure 7.
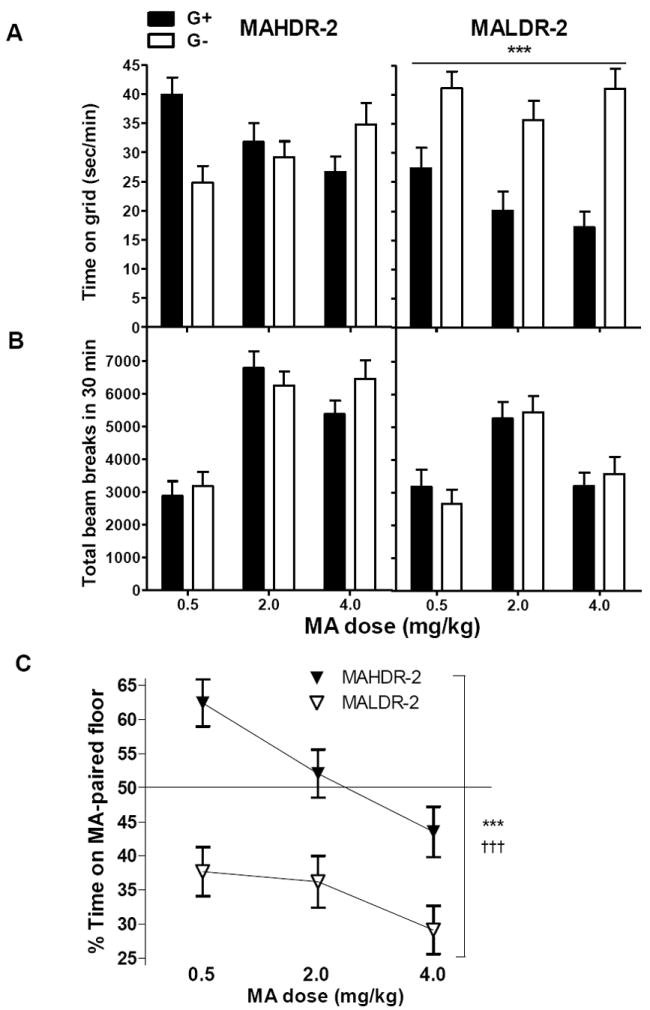
In the drug-present preference test, MALDR-2 mice exhibit CPA, regardless of dose. Shown are mean ± SEM sec/min on the grid floor (A) and beam breaks (B) measured during 30-min place preference tests for animals conditioned with MA on the grid (G+) or hole (G-) floor. Measurements were taken in a drug-present preference test in which animals (n = 7-13 per line, group and dose; see text for explanation of reduced n) were administered MA before the test. Differences in pharmacological effects of MA during the same place preference test (C) are also presented as, percent (%) time on MA-paired floor. ***p<.001 for the main effect of conditioning group (A) and, for the main effect of line (C). †††p<.001 for the main effect of dose (C). MAHDR-2, MA high drinking replicate 2; MALDR-2, MA low drinking replicate 2.
For locomotor activity on the preference test day (Figure 7B), there was a significant line × dose interaction (F2,115=6.8, p<.005), but no significant results involving conditioning group. MAHDR-2 mice exhibited higher levels of activity after treatment with 2 and 4 mg/kg MA, compared to 0.5 mg/kg (p values<.001), whereas MALDR-2 mice exhibited higher levels of activity after 2, but not 4, mg/kg MA compared to 0.5 mg/kg (p<.001). MAHDR-2 mice were more active than MALDR-2 mice after treatment with 2 (p<.05) and 4 (p<.001) mg/kg MA.
Locomotor activity on conditioning days
Conditioning trial locomotor activity data (Figure 8) were first examined for acute drug effects on conditioning Trial 1. There was a significant interaction of trial type (saline vs MA) and MA dose group (F2,133=26.0, p<.001), but no significant line difference. Locomotor activity levels were significantly higher after MA treatment than after saline treatment for all MA doses groups (p values<.001), and activity levels were significantly higher after a higher MA dose than a lower dose (p values<0.05).
Figure 8.
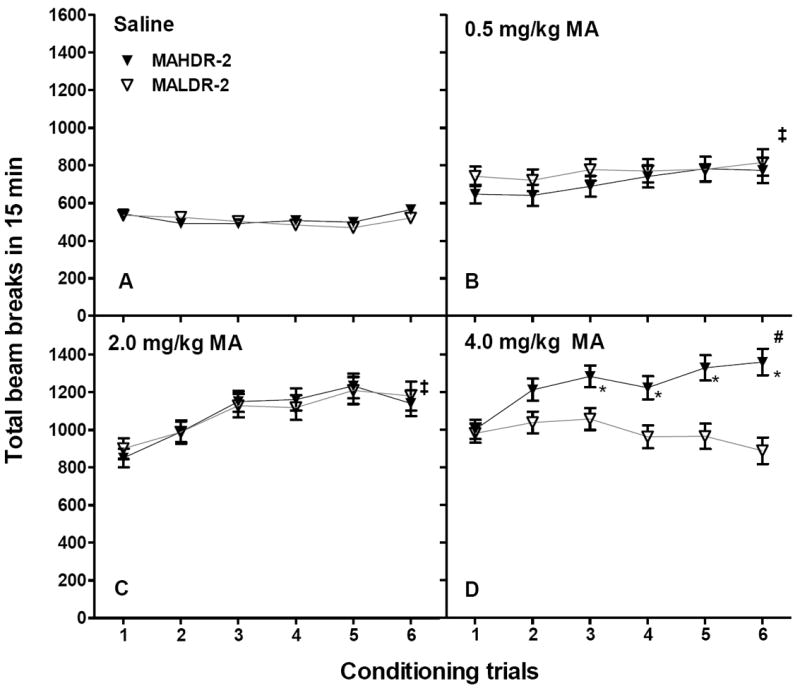
Locomotor activity levels in methamphetamine (MA) drinking line mice during the 6 conditioning trials for (A) saline and (B-D) MA. Locomotor activity was measured as photocell beam breaks during the 15-min conditioning sessions. Shown are means ± SEM total beam breaks. Conditioning sessions were alternated between saline and MA in a counterbalanced order across animals. MA dose groups were independent, so there were separate saline conditioning trials for each group. Since there were no significant MA dose group effects on activity levels during the saline trials, these data are presented collapsed on MA dose group. ‡p<.05 significant mean difference between trial 1 and 6, indicating locomotor sensitization (data were collapsed on line for these comparisons, because there were no significant line differences). #p<.05 significant mean differences between trial 1 and all subsequent trials for MAHDR-2 mice. *p<.05 for the comparison of MAHDR-2 and MALDR-2 line means. n = 20-25 per line and dose group. MAHDR-2, MA high drinking replicate 2; MALDR-2, MA low drinking replicate 2.
Repeated measures ANOVA including all MA test trials identified a significant interaction of trial × line × dose (F10,665=3.2, p<.001). There were no significant line, dose or trial effects on saline conditioning days (Figure 8A). For the 0.5 and 2 mg/kg MA doses, there were significant effects of trial (p values<.05) that did not interact with line, indicating similar levels of sensitization in the two lines (Figures 8B and 8C). However, for the 4 mg/kg dose, there was a significant interaction of trial and line (F5,220=9.2, p<.001), associated with significant sensitization in the MAHDR-2 line only (Figure 8D).
Relationship between locomotor activity and expression of MA-induced place conditioning
Because the effects of MA on locomotor activity might interfere with the expression of preference or aversion during a drug-present test (Cunningham et al. 2006; Gremel & Cunningham 2007), we examined the association between these two variables. The relationship of locomotor activity with conditioned preference and aversion were examined independently, by using scores for G+ and G- mice for percent time on their MA-paired floor during the drug-present test, and considering those with numerical values below 50% (representing no preference through aversion), separately from those with numerical values above 50% (representing no preference through preference). The values above and below 50% were then separately correlated with locomotor activity scores during the test (Figure 9). Locomotor activity score was significantly associated with percent time spent on the MA-paired floor for both sets of scores. In both cases, preference scores approached 50% at higher locomotor activity levels (>7000 beam breaks), suggesting that higher activity levels may interfere with the expression of preference or aversion. Analysis of covariance (ANCOVA) was used to determine the proportion of variance in percent time spent on the MA-paired floor that was accounted for by locomotor activity score. MA dose and line were included in the analysis as independent variables. Locomotor activity accounted for a significant proportion of variance (F1,117 =9.6, p<.005). However, when the effect of locomotor activity was controlled for, a significant line × dose interaction (F2,117=3.4, p<.05) was present. MAHDR-2 mice spent a significantly larger percentage of their time on the MA-paired floor, compared to MALDR-2 mice after treatment with MA doses of 0.5 (p<.001) and 2 (p<.05) mg/kg, but not 4 mg/kg (p=.26). This line difference is apparent in Figure 9, which shows that the majority of MAHDR-2 mice had preference scores above 50%, whereas the majority of MALDR-2 mice had preference scores below 50%.
Figure 9.
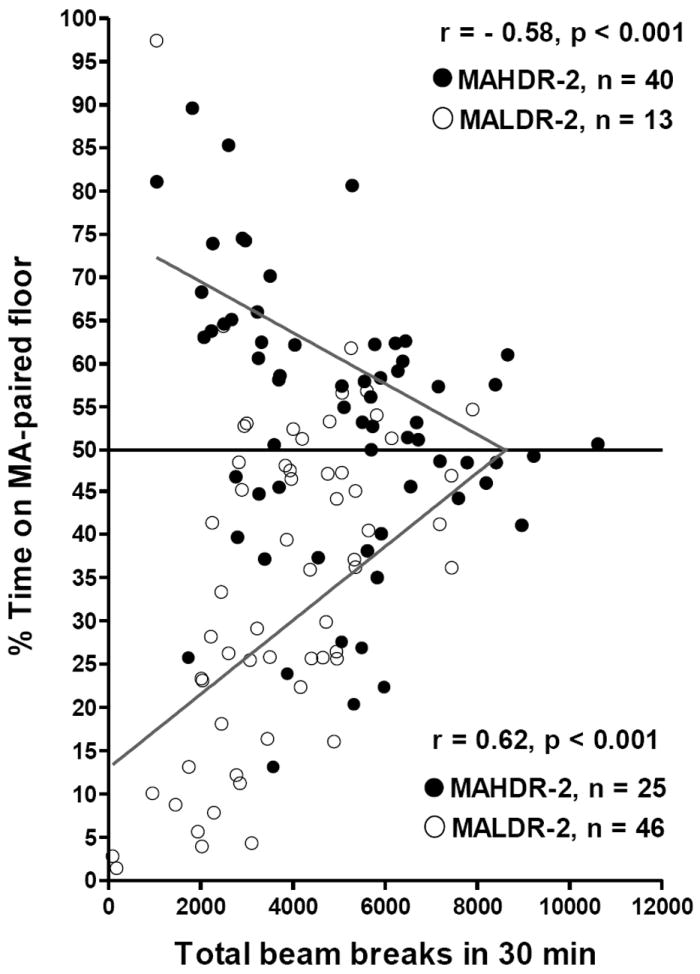
Correlation between locomotor activity level (total beam breaks in 30 min) and scores above (A) or (B) below 50% time on MA-paired floor are indicated separately. Locomotor activity was associated significantly with preference (those above 50%; graph A) and aversion (those below 50%; graph B) scores, but in opposite directions. Data points are differentiated by line only to indicate that the majority of MAHDR-2 mice had preference scores above 50%, whereas the majority of MALDR-2 mice had preference scores below 50%.
Discussion
Our data indicate that genetic factors associated with heightened MA consumption have opposite effects on sensitivity to the rewarding and aversive effects of MA. Mice bred for high MA consumption not only consumed significantly greater amounts of MA than either the originating population or the oppositely selected low MA consuming line, but were also the only ones that escalated their MA intake over days. MA doses across a larger range produced rewarding effects in MAHDR-2 mice, compared to the founding F2 mice, and no rewarding effects in MALDR-2 mice. When mice were treated with MA prior to the preference test, the line difference was accentuated, with MALDR-2 mice displaying robust aversion for the conditioned cues, but MAHDR-2 mice displaying no significant aversion. Overall, these data indicate that a line of mice bred for high MA intake has pronounced sensitivity to the rewarding effects and weakened or no sensitivity to the aversive effects of MA, whereas mice bred for low MA intake have the opposite pattern of sensitivities. These data are consistent with the view that individual vulnerability to drug use may be best explained by the balance in sensitivity to these opposing factors (Davis & Riley 2010; Ettenberg 2004).
Results for this replicated set of lines both extend and closely mimic those for the first set of lines selected for the same trait (Wheeler et al. 2009). Heritability calculations were comparable, MA consumption and preference diverged in the first generation of selection in both sets of lines, and the extent of divergence was not explained by differences in either total fluid intake or avidity for novel tastants. To reduce animal usage, we typically obtain tastant data following a washout period in animals that have been used to measure drug consumption (e.g., Kamens et al., 2005; Wheeler et al. 2009). We examined tastant consumption and preference ratios after a washout period following measurement of ethanol drinking in the replicate 2 lines, and after a washout period following the measurement of cocaine drinking in the first set of MADR lines (Wheeler et al. 2009). We cannot say with certainty that our results would be the same in experimentally naïve mice; however, we did obtain the same results in the two sets of lines despite prior exposure to different drugs.
Taste stimuli can become conditioned stimuli during drinking procedures (Meisch 2001). This conditioning occurs because, although pharmacological effects are delayed, the taste stimulus provides an immediate stimulus feedback that overlaps with pharmacological effects in prolonged oral route procedures. In fact, in the first replicate of MADR lines, only MALDR mice developed a strong, dose-dependent MA-induced conditioned taste aversion (CTA) to a novel NaCl solution (Wheeler et al. 2009). In that study, the pharmacological effects of an IP injection of MA would have been much more rapid than after oral MA, allowing for a strong association of the taste stimulus (from the NaCl) with the immediate effects of MA treatment. Thus, a parsimonious explanation for the divergence in MA consumption is a difference in sensitivity to the rewarding and aversive effects of MA. In addition, the current data showing a strong MA-induced CPA, indicate that heightened sensitivity to the aversive effects of MA can be seen in mice that avoid consuming MA, using a test that does not involve taste.
Over time and depending upon dose, an animal can develop either a conditioned preference or aversion to exteroceptive cues associated with the drug (Cunningham et al. 2003b; Reicher & Holman 1977). Our data show that these biphasic effects of MA were strongly influenced by genetic differences. In a drug-free preference test, F2, MAHDR and MAHDR-2 mice expressed a CPP after conditioning with the lowest dose of MA, whereas MALDR and MALDR-2 mice did not. While CPP was dose-dependent in the F2 mice and seen only at 0.5 mg/kg MA, CPP was not dose-dependent in MAHDR-2 mice, which indicates that MAHDR-2 mice were sensitive to conditioned rewarding effects of higher doses of MA. It is notable that DBA/2J mice, one of the two progenitor strains of the MADR lines, were found in a previous study to express a CPP after conditioning with 0.5 mg/kg MA, but not higher doses (Cunningham & Noble 1992). Although we have not yet published data showing that MALDR mice can learn a conditioned approach response, they have been shown here and previously (Wheeler et al. 2009) to be capable of learning two conditioned avoidance responses (CTA and CPA). Treatment with MA before the preference test enhanced the expression of aversion for the MA-paired cues in MALDR-2 mice. This effect was also seen in a previous study in DBA/2J mice at 8 mg/kg, though not at doses ranging from 0.25 to 2 mg/kg (Cunningham & Noble 1992). After MA treatment, MAHDR-2 mice continued to express a preference for the 0.5 mg/kg MA-paired cues, suggesting reduced sensitivity to aversive effects of MA, compared to MALDR-2 mice. At higher MA doses, no significant preference or aversion was seen in the MAHDR-2 line. Locomotor stimulant effects at these doses could have interfered with the expression of preference. In addition, drug treatment could have had initial aversive effects that impacted the expression of preference in MAHDR-2 mice, for example, as animals experienced the transition from a sober to an intoxicated state.
Although there was a relationship of locomotor activity with the extent of aversion expressed (see Figure 9), significant CPA was still seen in MALDR-2 mice in the drug-present test. In addition, activity levels of MAHDR-2 and MALDR-2 mice were comparable after treatment with 0.5 mg/kg MA, yet they exhibited opposite motivational responses. A parsimonious interpretation of our results is that the immediate unconditioned aversive effects of MA induced aversive/avoidance responses to MA-conditioned cues consequently reducing approach and thus, time in contact with those cues. In rodents and humans, doses between 0.1 and 0.5 mg/kg MA or amphetamine are associated with positive/rewarding effects, but higher doses with negative/aversive effects (Cruickshank & Dyer 2009; Grilly & Loveland 2001). In those individuals most sensitive to such aversive effects of MA, cues previously associated with MA may be more likely to elicit aversive responses in the presence of MA than in its absence. Results for the drug-free and drug-present preference tests also underscore the fact that both high and low MA consuming mice are able to discriminate MA-conditioned cues, though conditioned responses are state-dependent. Of course, it is possible that results would be different if an independent group of mice had been tested in the drug-present condition without prior drug-free testing.
The opposite sensitivities to the motivational effects of MA in the selected lines nicely parallel their differences in MA consumption. For example, S3 MAHDR-2 mice (the generation used for CPP testing) consumed approximately 6 mg/kg of MA in an 18-h period. If one divides this evenly, this would be 0.33 mg/kg/hr, which is close to the dose found to be rewarding in MAHDR-2 mice. We acknowledge that the amount of MA consumed over the 18-h period is not likely to be this systematic and may vary among animals. However, in humans, MA consumed orally at a dose as low as 0.125 mg/kg, induced arousing and euphoric effects (Cruickshank & Dyer 2009; Perez-Reyes et al. 1991). In contrast, MALDR-2 mice consume virtually no MA, and display no conditioned preference. Similarly, sensitivity to these opposing properties of cocaine appears to affect self-administration in rats. Lewis rats showed stronger cocaine-induced CPP than Fischer rats (Kosten et al. 1994) and acquired operant IV self-administration of cocaine faster than Fischer rats in a 2-h access procedure (Kosten et al. 1997). When given 18-h access to IV cocaine, only Lewis rats escalated their intake (Picetti et al. 2010). In Wistar rats given 24-h free-access to oral d-amphetamine in a two-bottle choice paradigm over a period of 42 weeks, escalated intake from less than 0.5 mg/kg/day to approximately 2.5 mg/kg/day was seen in weeks 40 and 42 (Galli & Wolffgramm 2004). Our MAHDR lines, show much higher MA consumption (~6.0 mg/kg/18hr when given access to 40 mg MA/l) than that reported in rats, and faster escalation of MA intake (i.e., days as opposed to weeks in rats). This difference between MAHDR mice and rats may be explained by the selective breeding of the mice for high MA consumption and differences in the concentrations of solutions offered to the rats (100-400 mg d-amphetamine/l), compared to mice (20-40 mg MA/l).
Genetically-determined differences in MA consumption also contributed to differences in locomotor sensitization, but not acute stimulation, in the current study. This increased responsiveness to MA after repeated administration is considered to be a behavioral reflection of underlying neuroadaptation (Pierce & Kalivas 1997; Phillips et al. 2008). The MADR-2 mice exhibited similar levels of sensitization to the 0.5 and 2 mg/kg MA doses, but only the MAHDR-2 line exhibited sensitization to the 4 mg/kg MA dose. This difference between the lines was also apparent when the mice received MA just prior to the CPP test. We have recently completed a study using these and higher doses of MA and the results indicate that the line difference in sensitization cannot be explained by a difference in susceptibility to MA-induced stereotypy. These results indicate that selective breeding for increased MA consumption increased susceptibility to the sensitizing effects of a higher dose of MA. We cannot yet rule out a possible role for pharmacokinetic or pharmacodynamic differences between the lines in the locomotor sensitization differences or in other line differences reported here.
The current genetic model of differential voluntary MA consumption provides evidence that MA consumption is strongly influenced by genetic factors, and provides an opportunity to identify these genetic factors. Quantitative trait locus (QTL) mapping studies, coordinating behavioral and expression QTLs, have been initiated. Initial expression results for nucleus accumbens tissue from the first set of MADR lines, both naïve and those treated with saline or 2 mg/kg MA, have been published (Wheeler et al. 2009). With regard to MA response, these lines showed differences in the expression of genes in neurotoxicity pathways that, at the dose administered, may be related to sensitivity to the stressful, rather than neurotoxic, effects of MA. In addition, in the absence of MA treatment, the lines showed differences in the expression of the serotonin and noradrenaline transporter genes, such that MAHDR mice showed higher expression than MALDR mice (Wheeler et al. 2009), suggesting possible mechanisms of risk for MA consumption. The noradrenaline transporter has been implicated in the aversive effects of cocaine (Uhl et al. 2002; Jones et al. 2009; Jones et al. 2010), and is one mechanism being considered for future work for its possible role in the selected line difference in sensitivity to the aversive effects of MA.
Supplementary Material
Acknowledgments
We thank Sue Burkhart-Kasch, Na Li and Harue Baba for their assistance with selective breeding of the MADR-2 lines. We also thank John Harkness for conducting the two-bottle choice study for novel tastants. This work was supported by a grant from the Department of Veterans Affairs, NIDA T32 DA07262, and NIDA Center grant P50 DA018165.
Footnotes
All authors report no conflicts of interest or biomedical financial interests
References
- Bardo MT, Valone JM, Bevins RA. Locomotion and conditioned place preference produced by acute intravenous amphetamine: role of dopamine receptors and individual differences in amphetamine self-administration. Psychopharmacology (Berl) 1999;143:39–46. doi: 10.1007/s002130050917. [DOI] [PubMed] [Google Scholar]
- Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- Cunningham CL. Pavlovian drug conditioning. In: van Haaran F, editor. Methods in behavioral pharmacology. Elsevier; Amsterdam: 1993. pp. 349–381. [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003a;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Noble D. Methamphetamine-induced conditioned place preference or aversion depending on dose and presence of drug. Ann N Y Acad Sci. 1992;654:431–433. doi: 10.1111/j.1749-6632.1992.tb25989.x. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Smith R, McMullin C. Competition between ethanol-induced reward and aversion in place conditioning. Learn Behav. 2003b;31:273–280. doi: 10.3758/bf03195988. [DOI] [PubMed] [Google Scholar]
- Davis CM, Riley AL. Conditioned taste aversion learning: implications for animal models of drug abuse. Ann N Y Acad Sci. 2010;1187:247–275. doi: 10.1111/j.1749-6632.2009.05147.x. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. Opponent process properties of self-administered cocaine. Neurosci Biobehav Rev. 2004;27:721–728. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to quantitative trait genetics. Pearson; Essex, England: 1996. [Google Scholar]
- Fudala PJ, Iwamoto ET. Conditioned aversion after delay place conditioning with amphetamine. Pharmacol Biochem Behav. 1990;35:89–92. doi: 10.1016/0091-3057(90)90209-z. [DOI] [PubMed] [Google Scholar]
- Galli G, Wolffgramm J. Long-term voluntary D-amphetamine consumption and behavioral predictors for subsequent D-amphetamine addiction in rats. Drug Alcohol Depend. 2004;73:51–60. doi: 10.1016/j.drugalcdep.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Galloway GP, Singleton EG, Buscemi R, Baggott MJ, Dickerhoof RM, Mendelson JE Methamphetamine Treatment Project Corporate Authors. An examination of drug craving over time in abstinent methamphetamine users. Am J Addict. 2010;19:510–514. doi: 10.1111/j.1521-0391.2010.00082.x. [DOI] [PubMed] [Google Scholar]
- Gonzales R, Mooney L, Rawson RA. The methamphetamine problem in the United States. Annu Rev Public Health. 2010;31:385–398. doi: 10.1146/annurev.publhealth.012809.103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL. Role of test activity in ethanol-induced disruption of place preference expression in mice. Psychopharmacology (Berl) 2007;191:195–202. doi: 10.1007/s00213-006-0651-5. [DOI] [PubMed] [Google Scholar]
- Grilly DM, Loveland A. What is a “low dose” of d-amphetamine for inducing behavioral effects in laboratory rats? Psychopharmacology (Berl) 2001;153:155–169. doi: 10.1007/s002130000580. [DOI] [PubMed] [Google Scholar]
- Jones JD, Hall FS, Uhl GR, Rice K, Riley AL. Differential involvement of the norepinephrine, serotonin and dopamine reuptake transporter proteins in cocaine-induced taste aversion. Pharmacol Biochem Behav. 2009;93:75–81. doi: 10.1016/j.pbb.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Hall FS, Uhl GR, Riley AL. Dopamine, norepinephrine and serotonin transporter gene deletions differentially alter cocaine-induced taste aversion. Pharmacol Biochem Behav. 2010;94:580–587. doi: 10.1016/j.pbb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ. Sensitivity to psychostimulants in mice bred for high and low stimulation to methamphetamine. Genes Brain Behav. 2005;4:110–125. doi: 10.1111/j.1601-183X.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl) 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Chi S, Nestler EJ. Fischer and Lewis rat strains show differential cocaine effects in conditioned place preference and behavioral sensitization but not in locomotor activity or conditioned taste aversion. J Pharmacol Exp Ther. 1994;269:137–144. [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Haile CN, DeCaprio JL, Jatlow PI, Nestler EJ. Acquisition and maintenance of intravenous cocaine self-administration in Lewis and Fischer inbred rat strains. Brain Res. 1997;778:418–429. doi: 10.1016/s0006-8993(97)01205-5. [DOI] [PubMed] [Google Scholar]
- Meisch RA. Oral drug self-administration: an overview of laboratory animal studies. Alcohol. 2001;24:117–128. doi: 10.1016/s0741-8329(01)00149-5. [DOI] [PubMed] [Google Scholar]
- Niwa M, Nitta A, Mizoguchi H, Ito Y, Noda Y, Nagai T, Nabeshima T. A novel molecule “shati” is involved in methamphetamine-induced hyperlocomotion, sensitization, and conditioned place preference. J Neurosci. 2007;27:7604–7615. doi: 10.1523/JNEUROSCI.1575-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AA, Phillips TJ. Quantitative trait locus (QTL) mapping in mice. In: Liu Y, Lovinger DM, editors. Methods in alcohol-related neuroscience research. CRC Press; Boca Raton, FL: 2000. pp. 1–30. [Google Scholar]
- Perez-Reyes M, White WR, McDonald SA, Hill JM, Jeffcoat AR, Cook CE. Clinical effects of methamphetamine vapor inhalation. Life Sci. 1991;49:953–959. doi: 10.1016/0024-3205(91)90078-p. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Kamens HM, Wheeler JM. Behavioral genetic contributions to the study of addiction-related amphetamine effects. Neurosci Biobehav Rev. 2008;32:707–759. doi: 10.1016/j.neubiorev.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picetti R, Ho A, Butelman ER, Kreek MJ. Dose preference and dose escalation in extended-access cocaine self-administration in Fischer and Lewis rats. Psychopharmacology (Berl) 2010;211:313–323. doi: 10.1007/s00213-010-1899-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Reicher MA, Holman EW. Location preference and flavor aversion reinforced by amphetamine in rats. Animal Learning & Behavior. 1977;5:343–346. [Google Scholar]
- Ricoy UM, Martinez JL., Jr Local hippocampal methamphetamine-induced reinforcement. Front Behav Neurosci. 2009;3:47. doi: 10.3389/neuro.08.047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan J, Butler R, Wheeler A. Initiation into methamphetamine use: qualitative findings from an exploration of first time use among a group of New Zealand users. J Psychoactive Drugs. 2009;41:11–17. doi: 10.1080/02791072.2009.10400670. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Fibiger HC, Phillips AG. Dopaminergic substrates of amphetamine-induced place preference conditioning. Brain Res. 1982;253:185–193. doi: 10.1016/0006-8993(82)90685-0. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Hall FS, Sora I. Cocaine, reward, movement and monoamine transporters. Mol Psychiatry. 2002;7:21–26. doi: 10.1038/sj.mp.4000964. [DOI] [PubMed] [Google Scholar]
- Wheeler JM, Reed C, Burkhart-Kasch S, Li N, Cunningham CL, Janowsky A, Franken FH, Wiren KM, Hashimoto JG, Scibelli AC, Phillips TJ. Genetically correlated effects of selective breeding for high and low methamphetamine consumption. Genes Brain Behav. 2009;8:758–771. doi: 10.1111/j.1601-183X.2009.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


