Abstract
This one-arm pilot study investigated the effect of tai chi on cognition in elders with cognitive impairment. Although no significant difference existed between pre- and post-test performance on all cognition measures, a dose-response relationship was demonstrated between attendance and some cognition measures.
Introduction
Approximately 5.4 million people (22.2%) ages 71 or older in the United States have cognitive impairment (CI) without dementia (Plassman et al., 2008), and another 3.4 million individuals (13.9%) have CI with dementia (Plassman et al., 2007). Cognitive impairment is a major risk factor for dependency in activities of daily living (Cigolle, Langa, Kabeto, Tian, & Blaum, 2007; McConnell, Pieper, Sloane, & Branch, 2002). Pharmacological interventions are used commonly to treat impaired cognition. However, the medications have side effects in about 25% of patients (Arsland et al., 2003). Non-pharmacological interventions, such as physical exercise, have been shown to have effect sizes similar to pharmacological interventions in improving cognitive function, but without the side effects (Brotons & Koger, 2000; Graf et al., 2001; Luijpen, Scherder, Van Someren, Swaab, & Sergeant, 2003). The purpose of this study is to test tai chi, a mild aerobic exercise, on cognition in elders with CI.
Literature Review
Exercise generally has been shown to improve cognitive functioning. In an early study, Dustman and colleagues (1984) found aerobic exercise improved scores of community-dwelling elders (N=43) on the digit symbol test (p< 0.001), the Stroop test (p< 0.05) and simple reaction time (p< 0.05). Other early researchers reported similar findings (Hill, Storandt, & Malley, 1993; Rikli & Edwards, 1991). Elders (N=87) who participated in a long-term aerobic exercise program significantly improved their psychomotor/cognitive processing speed and memory, as measured by the digit symbol test and the Wechsler Memory Scale Logical Memory subset (p< 0.01) (Hill et al., 1993). After participating in a 3-year aerobic exercise program, older women (N=48) demonstrated significantly improved attention and cognitive processing speed, as measured by simple reaction time (p<0.05) and choice reaction time (p<0.001), respectively (Rikli & Edwards, 1991).
Findings from the earlier studies were supported by several large-scale longitudinal studies. For example, physical activities in a community sample of 9,008 randomly selected elders in Canada were associated with lower risk of CI (odds ratio: 0.50 for Alzheimer’s type of CI; 0.63 for other types of CI) (Laurin, Verreault, Lindsay, MacPherson, & Rockwood, 2001). In a study of the effects of walking on cognition in 5,925 women without CI, more walking was associated with less cognitive decline during 6–8 years of follow up (Yaffe, Barnes, Nevitt, Lui, & Covinsky, 2001). Similarly, a high level of exercise was associated with less decline in Mini Mental State Exam (MMSE) scores after 2-year follow up (odds ratio=0.39) of 1,146 community-dwelling elders (Lytle, Vander Bilt, Pandav, Dodge, & Ganguli, 2004).
A number of studies reported exercise also benefited cognition in elders with CI. For instance, 11 elders with MMSE scores of 0–29 participated in a 6-month group strengthening exercise program. Scores on the MMSE improved by 3.1 points after the exercise intervention, with an effect size of 0.54 (Baum, Jarjoura, Polen, Faur, & Rutecki, 2003). Similarly, a 6-month exercise program consisting of range of motion exercises, strengthening exercises, and walking maintained scores on the MMSE (p=0.18) in the exercise group but the scores of a non-exercise comparison group decreased significantly (p<0.001) (Bastone Ade & Jacob Filho, 2004). Finally, the logical memory test score (p< 0.05) and the MMSE score (p< 0.05) improved significantly in exercisers who participated in one-time 45-minute group or individual exercises than in controls in 15 elders with CI. Other tests, such as the digit span, recognition, and the digit symbol test, however, were not associated with any improvement (Molloy, Beerschoten, Borrie, Crilly, & Cape, 1988).
Even though exercise appears effective in maintaining cognitive function for elders, many community-dwelling elders with CI do not exercise because they have little physical strength and multiple medical conditions (Bynum et al., 2004). They require exercise programs tailored to their frail physical conditions. Tai chi (TC) may be an excellent intervention for elders with CI because it is a safe, gentle form of exercise that can be performed while standing or sitting. It is appropriate for different levels of mobility, and requires no special equipment or special clothing. Because TC is not expensive, it is feasible in a wide variety of settings in the community, including senior centers, churches, adult day centers, and continuing care communities (Li et al., 2001; Tsai et al., 2009).
Authors speculated that group exercises such as TC can help to prevent the cognitive decline caused by normal and pathological aging because exercises may assist with neurongenesis and angiogenesis in the brain, decrease chronic inflammation; and provide elders with cognitive stimulation and social interactions A defect in the dentate gyrus of the hippocampus area is associated with cognitive decline, including impairments in learning and short-term memory, during aging (Patrylo & Williamson, 2007; Rosenzweig & Barnes, 2003). Importantly, animal studies indicate voluntary exercise (wheel running) can enhance generation of neurons (neurogenesis) in the dentate gyrus (Eadie, Redila, & Christie, 2005; van Praag, Kempermann, & Gage, 1999), which enhances learning in aged animals (van Praag, Shubert, Zhao, & Gage, 2005).
An increase of blood vessel growth in the brain (angiogenesis) is important to maintain the health of newly generated neurons. Using magnetic resonance imaging (MRI), a recent study found good correlations among exercise, neurogenesis in the dentate gyrus, cerebral blood volume, and cognition in mice and in humans (Pereira et al., 2007). An earlier study using the same MRI technique reported similar findings (Colcombe et al., 2003). Such findings suggest these observations could be made in elders who participate in TC exercise regularly.
Aside from normal physiological aging, pathological brain conditions caused by inflammation, such as Alzheimer’s disease accelerate neuronal cell death and dysfunction (Akiyama et al., 2000; Mrak & Griffin, 2001). Exercise may slow the decline of cognitive function in elders with these conditions because it reduces pro-inflammatory cytokines in the periphery (Woods, Vieira, & Keylock, 2006). Convincing evidence indicates alteration of cytokine levels in the periphery also will affect the central nervous system (Wilson, Finch, & Cohen, 2002a) because circulating pro-inflammatory cytokines can be transported into the central nervous system across the blood-brain barrier (Maier, 2003). Alternatively, circulating cytokines can interact with receptors on the endothelial cells of the blood-brain barrier and trigger the release of related cytokines inside the central nervous system (Banks, Farr, & Morley, 2002; Maier, 2003). A reduction in pro-inflammatory cytokines in the periphery caused by exercise may lead to decreased pro-inflammatory cytokines in the brain, and thus slow the decline of cognitive function in elders with pathological brain conditions caused by inflammation.
Cognitive stimuli and social interactions are also important for the preservation of cognitive health in older adults. A study of older Catholic nuns, priests, and brothers (N=801) found frequent participation in cognitively stimulating activities (e.g., reading newspapers, playing card games, visiting museums) was associated with a lower risk of Alzheimer’s disease (Wilson et al., 2002b). The Kungsholmen Project, a longitudinal population-based study of aging and dementia in Sweden, found both social interaction and intellectual stimulation were important to prevent cognitive decline (Wang, Karp, Winblad, & Fratiglioni, 2002). A follow-up analysis by the same research group concluded physical, cognitive, and social components contributed equally to the prevention of cognitive decline (Karp et al., 2006).
Theoretical framework
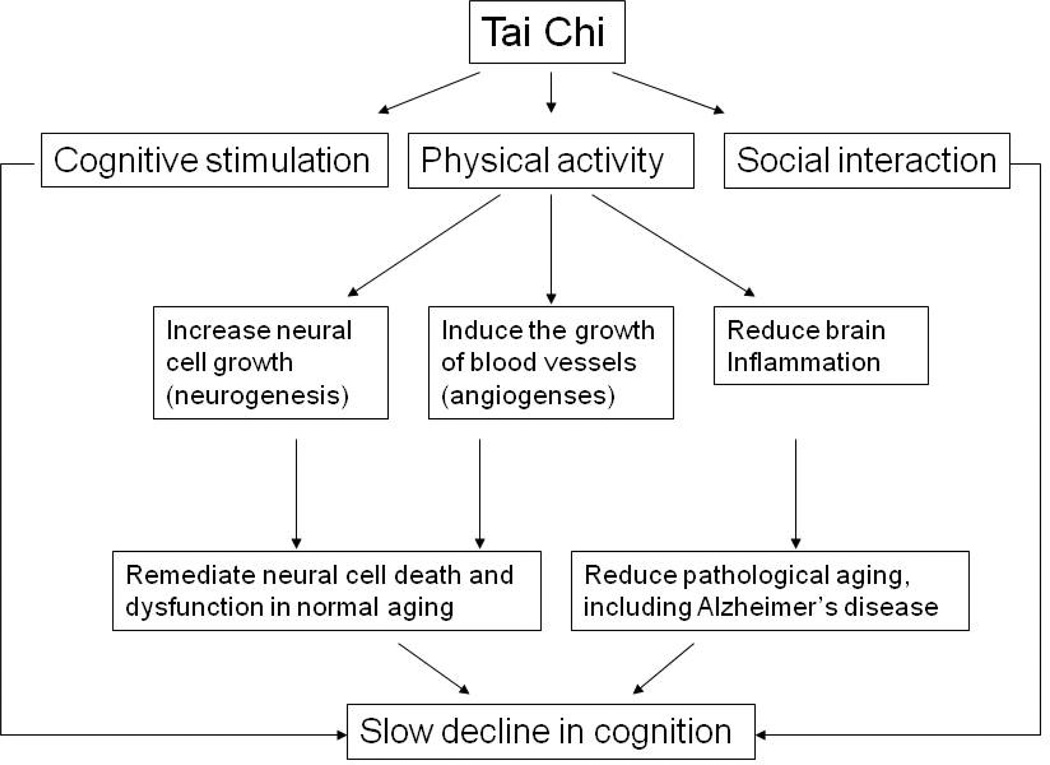
Based on the past studies, the expected links between TC and cognition in elders with CI are shown in Figure 1. Cognitive decline in elders may result from two major mechanisms. First, the normal aging process leads to neuronal cell death and dysfunction in the brain, causing impairment in cognition. Second, pathological brain conditions caused by inflammation, such as Alzheimer’s Disease, accelerate neuronal cell death and dysfunction (Akiyama et al., 2000; Mrak & Griffin, 2001). Physical exercise may slow the decline of cognition by mediating these situations-increasing new growth of neuronal cells (neurogenesis), providing more blood to the brain by new blood vessel growth (angiogenesis) and reducing the inflammatory state of the brain. Cognitive stimuli and social interactions are also important for the preservation of cognitive health in older adults although the mechanism is less clear (Karp et al., 2006; Wilson et al., 2002b).
Figure 1.
Links between Tai Chi and Cognitive Functioning
An exercise program with all three components (physical, cognitive, social) conceivably could slow the decline of cognition in elders with CI. TC is an exercise program that accommodates all these requirements. Physically, it is a gentle, dance-like exercise characterized by continuous, slow, fluid movements appropriate for elders. Concentration and active cognitive engagement are required to perform TC because each form may involve movements of various body parts, and a series of forms are practiced in a particular sequence. Finally, TC provides social interactions when practiced in a group setting. As a result, TC should be beneficial for maintaining cognition in elders, which was demonstrated in a few recent studies with healthy elderly sample (Matthews & Williams, 2008; Taylor-Piliae et al., 2010).
Study aim
Studies investigating the effects of TC on health outcomes often excluded elders with CI (Brismee et al., 2005; Hartman et al., 2000; Li et al., 2001; Song, Lee, Lam, & Bae, 2003; Wolf et al., 2003). Additionally, no research has investigated the effect of TC on cognition in elders with CI and inconsistent findings were reported in elders without CI (Hall, Miszko, & Wolf, 2009; Matthews & Williams, 2008; Taylor-Piliae et al.,2010). Therefore, this study pilot tested the effect of TC on cognition in elders with CI. The current pilot study was part of a larger study investigating the effect of TC on pain and cognition in elders with CI. Only data related to cognition are reported in this article.
Methods
In this one-group pre-post test pilot study, elders participated in a 15-week “Sun-style TC for Arthritis” program twice a week for 20–40 minutes per session. With its high stance and follow-steps, the Sun-style 12-form TC developed by Lam (2004) is especially suitable for older adults with weak muscles, bodily pain, and risk of falling (Song et al., 2003).
The Committee on the Conduct of Human Research of the University of Arkansas for Medical Sciences approved the study. To participate in the study, elders had to show impairment in at least one area of cognition to allow room for improvement after participating in TC. MMSE scores from 15–27, a Digit Symbol-Coding score on the WAIS-III (Psychological Corporation, 1997) of 6 or less, a Digit Span on the WAIS-III (Psychological Corporation, 1997) of 6 or less, a Stroop Color and Word test score (Golden, 2002) of 39 or less, or a Hopkins Verbal Learning Test – Revised score (Benedict, Schretlen, Groninger, & Brandt, 1998) of 39 or less was considered impaired. In addition, elderly participants could have no depressive symptoms as measured by the Geriatric Depression Scale (Vinkers, Gussekloo, Stek, Westendorp, & Van Der Mast, 2004) and have chronic pain measured by the Bodily Pain scale of the Medical Outcomes Study short form (SF-36) (Ware, 2000) to participate.
The five cognitive screening tools listed above also were used to measure outcomes. SF-36 was used to measure participants’ physical and mental function (Ware, 2000). TC accuracy was assessed using Rosengren’s method (Rosengren, Christou, Yang, Kass, & Boule, 2003). After the TC program was completed, the elders followed the instructor to perform the 12-form TC twice while a research assistant videotaped their TC performance. The tape was sent to co-author Rosengren’s lab for coding and evaluation of the accuracy of TC performance. His coding method was published elsewhere (Rosengren et al., 2003). Descriptive statistics were used to describe the sample. Paired t-tests, independent t-tests, and Spearman’s rho were used for data analysis.
Findings
Eleven elders participated in the study, with the majority female (N=10). The average age was 85 and MMSE score was 23. Average scores on the SF-36 physical and mental component summary were 45% and 56% respectively, indicating they achieved only about half of the maximum physical and mental function. The first analysis compared attendance with accuracy of TC performance. The average attendance in minutes was 768 ± 458; average attendance in sessions was 20 ± 12, and mean performance score was 28% ± 22%. Results from this part of the study indicated attendance in minutes was related significantly to accuracy of performance (Spearman’s rho= 0.83, p< 0.01). The second analysis compared pre- and post-test scores on all cognitive function tests. Results showed no significant differences on the five cognition measures before and after the TC intervention [MMSE: t(df)=1.31 (10), p=.22; Digit Symbol-Coding: t(df)=.32 (10), p=.76; Digit Span: t(df)=1.08 (10), p=.31; Stroop Color and Word: t(df)=.89 (9), p=.40; Hopkins Verbal Learning Immediate Recall: t(df)=1.06 (10), p=.31; Hopkins Verbal Learning Delay Recall : t(df)=.95 (10), p=.36].
While no significant difference in cognition was found when pre- and post-test scores were compared, detailed analysis of data revealed a dose-response relationship. Based on attendance over the study period, three elders who participated for four or fewer sessions were categorized as the low-dose group. The eight elders who participated in 24–29 sessions were categorized as the regular-dose group. Table 1 summarizes the attendance, the accuracy of their performance, and changes in three cognitive function scores that are significant or approaching significant differences between groups.
Table 1.
Accuracy of performance, attendance and changes in cognition
| Group* | ID | Accuracy of Performance |
Attendance | Change in cognitive function scores | |||
|---|---|---|---|---|---|---|---|
| Session | Minute | MMSE | DSC | DS | |||
| Regular does group |
1 | 25% | 27 | 1130 | 2 | 0 | 0 |
| 2 | 45% | 25 | 1120 | 1 | 0 | 0 | |
| 3 | 45% | 27 | 1199 | −2 | 1 | 2 | |
| 4 | 66% | 24 | 1099 | 0 | 1 | 2 | |
| 5 | 53% | 29 | 1065 | −1 | −1 | −2 | |
| 6 | 21% | 27 | 975 | 0 | 0 | −3 | |
| 7 | 19% | 27 | 888 | 0 | 0 | −1 | |
| 8 | 17% | 28 | 708 | 0 | 1 | 2 | |
| Low dose group |
9 | 0% | 4 | 190 | −4 | −1 | −3 |
| 10 | 15% | 2 | 77 | −6 | −2 | −2 | |
| 11 | 0% | 0 | 0 | 0 | 0 | −2 | |
Regular-dose group participated 24 or more sessions of TC. Low-dose group participated 4 or less sessions of TC.
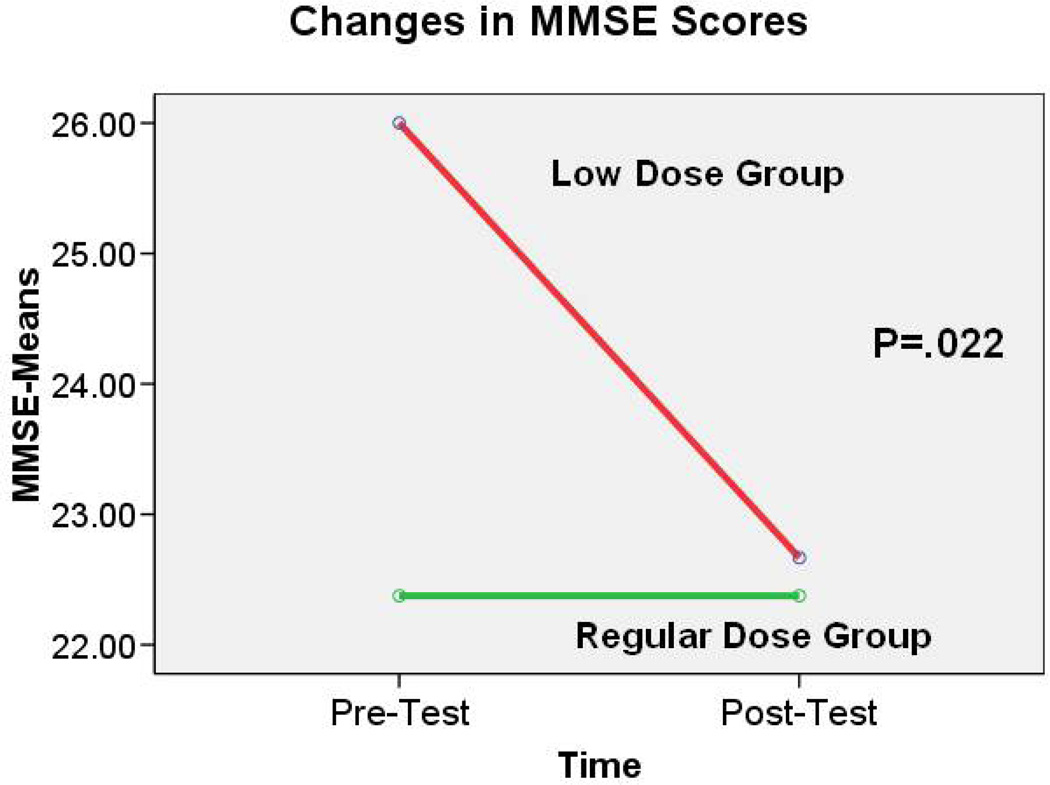
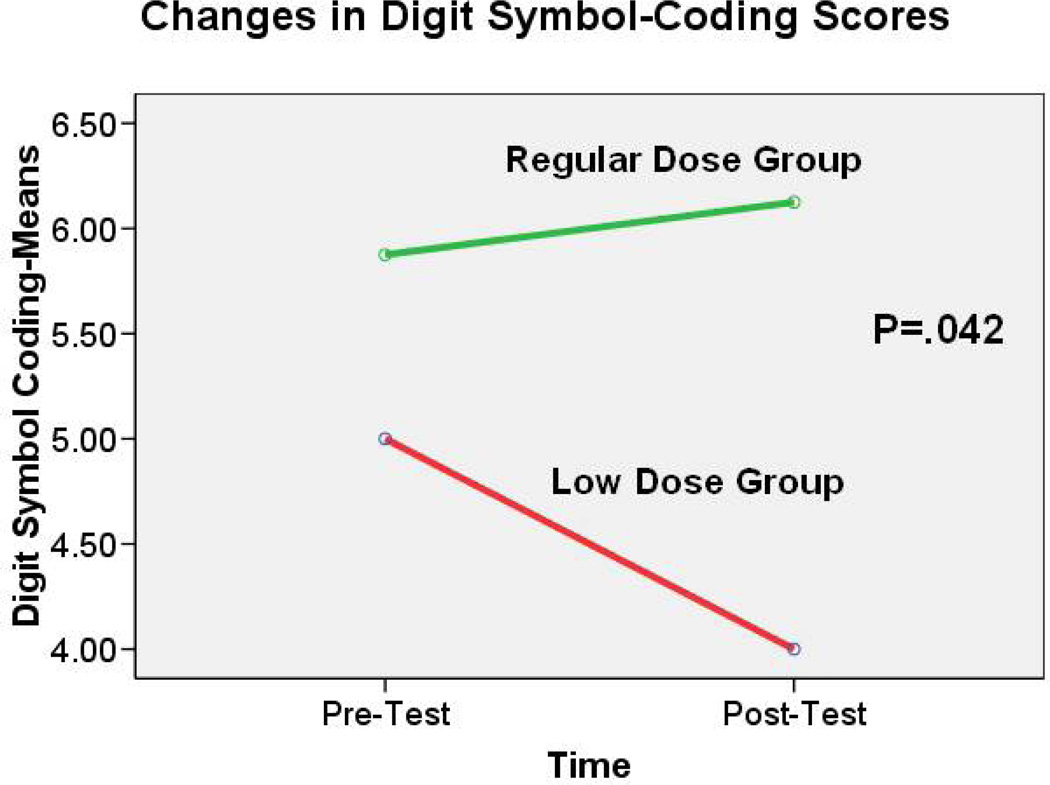
Comparison of these two groups showed significant differences in the change of MMSE score [t(df)=2.76 (9), p< 0.05] and in the change of Digit Symbol-Coding score [t(df)=2.36 (9), p<0.05] between pre- and post-test. The MMSE scores remained almost the same from pre- to post-test in the regular exercise group (mean change=0.00 ± 1.20). However, a decline in MMSE scores occurred among those in the low-dose group (mean change=− 3.33 ±3.06) (Fig. 2). Analysis of the Digit Symbol-Coding scores indicated a slight increase in the scores of those in the regular-dose group (mean change=0.25 ± 0.71). In contrast, scores in the low-dose group decreased (mean change=−1.00 ± 1.00) (see Figure 3).
Figure 2.
Figure 3.
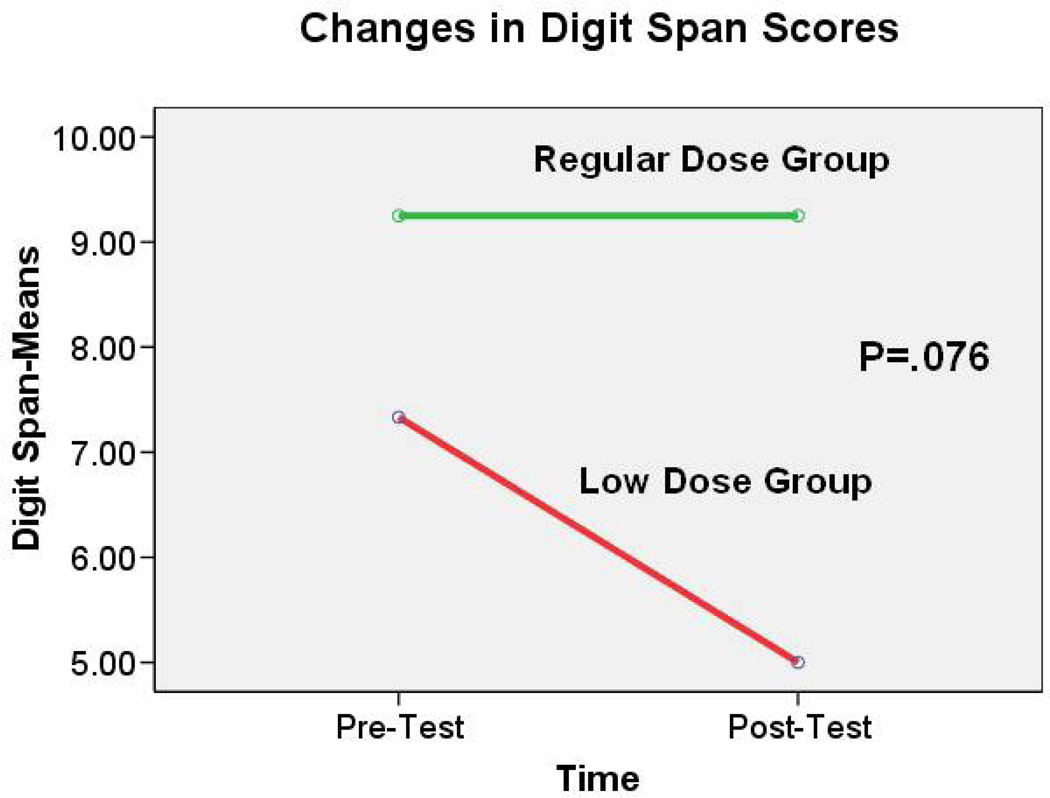
Although it was not significant at the 0.05 level, a similar trend was noted in the change of Digital Span score [t(df)=2.00 (9), p=.076]. Almost no change occurred in Digit Span scores among elders in the regular-dose group (mean change=0.00 ± 1.93). However, scores of elders in the low-dose group dropped (mean change=−2.33 ± 0.58) (see Figure 4). No significant difference was found in the change scores on the Stroop Color and the Word test (t(df)= −.22 (8), p=.83) and Hopkins Verbal Learning Test – Revised [Immediate Recall (t(df)= .17 (9), p=.87); Delayed Recall (t(df)= −.47 (9), p=.68)] between the regular-dose and low-dose groups.
Figure 4.
Discussion
Results from this small-scale study indicated the feasibility of teaching cognitively impaired elders to practice TC. In addition, authors found CI elders who attended TC sessions regularly preserved MMSE and Digit Symbol-Coding more than persons with low attendance. Furthermore, participants who attended TC sessions regularly tended to have better Digit Span score than those with low attendance. These results allow the speculation that TC had some impact on these cognitive measures. Alternatively, elders with declining cognitive functioning tended to miss TC classes. Without a randomized controlled trial involving more participants, these two possibilities cannot be distinguished.
Findings indicate TC has some impact on global cognitive functioning (MMSE) and psychomotor/cognitive processing speed (Digit Symbol-Coding) but not attention (Stroop Color and Word test and Digit Span) and Memory (Hopkins Verbal Learning Test – Revised). Because MMSE is a global measure of cognition, it is possible that TC will have some effects on every measure of cognition, including attention and memory but in varying levels of strength. The sample size in the current study is small and thus might not have enough power to detect the effect of TC on these specific cognitive functions. A larger study is needed to confirm this observation. As noted earlier, this pilot study is part of a larger study in which participants had to have both chronic pain and impaired cognition to be included in the study. Thus, having a chronic pain condition may have influenced the results of this study. For example, study showed that self-reported pain experience was positively related to performance on cognitive function tests (Oosterman, de Vries, Dijkerman, de Haan, & Scherder, 2009). If elders took analgesics, such as propoxyphene, the patients may have side effect, such as confusion and drowsiness, which may affect the cognitive tests (Desai & Chibnall, 2004),
With intent-to-treat analysis, the paired t-test did not reveal the difference between pre- and post-tests. However, analysis of the dose-response relationship between attendance and cognition measures reveals the important trends. This dose-response pattern should be made part of the study aims or pre-planned subgroup endpoint analyses in a future randomized controlled trial in addition to the intent to treat analysis.
Conclusion
Up to 78% of elders in the United States are not physically active enough to receive health benefits (Agency for Healthcare Research and Quality & the Centers for Disease Control, 2002). Elders may not exercise because they do not have the physical strength or because exercise can worsen their existing pain. With its gentle and slow motions, TC could be an ideal exercise for the elders. It can offer many benefits, such as increased mobility, balance, and flexibility; reduced risk of falls; and provision of a feeling of accomplishment and well-being. These benefits lead to enhanced independence and self-confidence for elders. Results of this pilot study suggested elders with CI also can learn and perform TC in a group setting. In addition, regular participation in TC may slow the progression of cognitive decline in elders with CI. If this observation can be confirmed in a large-scale, randomized controlled study, TC may be used to slow cognitive decline in elders with CI.
Nursing implication
This is the first study to test TC’s efficacy in slowing cognitive decline in elders with CI. If we find that TC has effects in this population, the next study will test TC’s effectiveness in slowing cognitive decline in community-dwelling elders with CI and test the sustainability of the TC intervention. If TC shows efficacy and effectiveness in slowing cognitive decline in elders with CI, we can adopt it as a routine cost-effective means to promote physical fitness and slow cognitive decline for this population.
Acknowledgement
This study was supported by the Beverly HealthCare Corporation and the National Institute on Aging funded Alzheimer’s Disease Center (5 P30 AG019606-05). It was also partially supported by the John A. Hartford Foundation under the Building Academic Geriatric Nursing Capacity Scholar Program to the corresponding author. Without the research participants, nursing home staff, Marye Ann Boyd, and Nola Ballinger, this study could not have been completed.
Contributor Information
Jason Y. Chang, Department of Neurobiology and Developmental Sciences, College of Medicine, University of Arkansas for Medical Sciences.
Pao-Feng Tsai, College of Nursing, University of Arkansas for Medical Sciences, Office Address: 4301 West Markham, Slot 529, Little Rock, AR, 72205, tsaipaofeng@uams.edu, Office Phone: 501-2961999, Home Phone: 501-2192521, Fax number: 501-6868350.
Cornelia Beck, Department of Geriatrics, College of Medicine, University of Arkansas for Medical Sciences.
Jody Hagen, Department of Geriatrics, College of Medicine, University of Arkansas for Medical Sciences; Private Practice - Haas Psychiatric Services, PA.
Debbie Cooley Huff, College of Nursing, University of Arkansas for Medical Sciences.
K. J. S. Anand, Morris & Hettie Oakley Endowed Chair of Critical Care Medicine; Department of Pediatrics, Anesthesiology, Pharmacology, Neurobiology, College of Medicine, University of Arkansas for Medical Sciences; Pain Neurobiology Laboratory, Arkansas Children's Hospital Research Institute.
Paula K. Roberson, Department of Biostatistics, Colleges of Medicine and Public Health, University of Arkansas for Medical Sciences.
Karl Rosengren, Department of Psychology, Northwestern University.
Linda Beuscher, School of Nursing, Vanderbilt University.
Reference
- Agency for Healthcare Research and Quality & the Centers for Disease Control. Physical activity and older americans: Benefits and strategies. 2002 Retrieved from http://www.ahrq.gov/ppip/activity.htm.
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Wyss-Coray T. Inflammation and alzheimer's disease. Neurobiology of Aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsland D, Engedal KA, Nygaard HA, Louhija J, Ulstein I, Holm M. cholinesterase inhibitors in norway--effectiveness and side effects in clinical practice. Tidsskrift for Den Norske Lægeforening. 2003;123(11):1500–1503. [PubMed] [Google Scholar]
- Banks WA, Farr SA, Morley JE. Entry of blood-borne cytokines into the central nervous system: Effects on cognitive processes. Neuroimmunomodulation. 2002;10(6):319–327. doi: 10.1159/000071472. [DOI] [PubMed] [Google Scholar]
- Bastone Ade C, Jacob Filho W. Effect of an exercise program on functional performance of institutionalized elderly. Journal of Rehabilitation Research and Development. 2004;41(5):659–668. doi: 10.1682/jrrd.2003.01.0014. [DOI] [PubMed] [Google Scholar]
- Baum EE, Jarjoura D, Polen AE, Faur D, Rutecki G. Effectiveness of a group exercise program in a long-term care facility: A randomized pilot trial. Journal of the American Medical Directors Association. 2003;4(2):74–80. doi: 10.1097/01.JAM.0000053513.24044.6C. [DOI] [PubMed] [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins verbal learning test – revised: Normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12(1):43–55. [Google Scholar]
- Brismee J, Boatright JD, Hagar JM, McCaleb JA, Quintela MM, Chyu M, Shen C. Effect of tai chi on the status of elderly subjects with knee osteoarthritis: A prospective randomized controlled trial. Medicine and Science in Sports and Exercise. 2005;37(5):S256. [Google Scholar]
- Brotons M, Koger SM. The impact of music therapy on language functioning in dementia. Journal of Music Therapy. 2000;37(3):183–195. doi: 10.1093/jmt/37.3.183. [DOI] [PubMed] [Google Scholar]
- Bynum JP, Rabins PV, Weller W, Niefeld M, Anderson GF, Wu AW. The relationship between a dementia diagnosis, chronic illness, medicare expenditures, and hospital use. Journal of the American Geriatrics Society. 2004;52(2):187–194. doi: 10.1111/j.1532-5415.2004.52054.x. [DOI] [PubMed] [Google Scholar]
- Cigolle CT, Langa KM, Kabeto MU, Tian Z, Blaum CS. Geriatric conditions and disability: The health and retirement study. Annals of Internal Medicine. 2007;147(3):156–164. doi: 10.7326/0003-4819-147-3-200708070-00004. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. The Journals of Gerontology. Series A Biological Sciences and Medical Sciences. 2003;58(2):176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Desai AK, Chibnall JT. Propoxyphene use in the elderly. Journal of the American Geriatrics Society. 2004;52(7):1227. doi: 10.1111/j.1532-5415.2004.52327_13.x. [DOI] [PubMed] [Google Scholar]
- Dustman RE, Ruhling RO, Russell EM, Shearer DE, Bonekat HW, Shigeoka JW, Bradford DC. Aerobic exercise training and improved neuropsychological function of older individuals. Neurobiol Aging. 1984;5(1):35–42. doi: 10.1016/0197-4580(84)90083-6. [DOI] [PubMed] [Google Scholar]
- Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. The Journal of Comparative Neurology. 2005;486(1):39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Galasko D, Kershaw PR, Schneider L, Zhu Y, Tariot PN. Galantamine maintains ability to perform activities of daily living in patients with alzheimer's disease. Journal of the American Geriatrics Society. 2004;52(7):1070–1076. doi: 10.1111/j.1532-5415.2004.52303.x. [DOI] [PubMed] [Google Scholar]
- Golden CJ. Stroop color and word test: A manual for clinical and experimental uses. Wood Dale, IL: Stoelting; 2002. [Google Scholar]
- Graf A, Wallner C, Schubert V, Willeit M, Wlk W, Fischer P, Neumeister A. The effects of light therapy on mini-mental state examination scores in demented patients. Biological Psychiatry. 2001;50(9):725–727. doi: 10.1016/s0006-3223(01)01178-7. [DOI] [PubMed] [Google Scholar]
- Hall CD, Miszko T, Wolf SL. Effects of tai chi intervention on dual-task ability in older adults: A pilot study. Archives of Physical Medicine and Rehabilitation. 2009;90(3):525–529. doi: 10.1016/j.apmr.2008.09.566. [DOI] [PubMed] [Google Scholar]
- Hartman CA, Manos TM, Winter C, Hartman DM, Li B, Smith JC. Effects of tai chi training on function and quality of life indicators in older adults with osteoarthritis. Journal of the American Geriatrics Society. 2000;48(12):1553–1559. doi: 10.1111/j.1532-5415.2000.tb03863.x. [DOI] [PubMed] [Google Scholar]
- Hill RD, Storandt M, Malley M. The impact of long-term exercise training on psychological function in older adults. Journal of Gerontology. 1993;48(1):P12–P17. doi: 10.1093/geronj/48.1.p12. [DOI] [PubMed] [Google Scholar]
- Karp A, Paillard-Borg S, Wang HX, Silverstein M, Winblad B, Fratiglioni L. Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dementia and Geriatric Cognitive Disorders. 2006;21(2):65–73. doi: 10.1159/000089919. [DOI] [PubMed] [Google Scholar]
- Lam P. Tai chi for arthritis. Narwee, Australia: East Action Publishing Pty Ltd.; 2004. [Google Scholar]
- Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Archives of Neurology. 2001;58(3):498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- Li F, Harmer P, McAuley E, Duncan TE, Duncan SC, Chaumeton N, Fisher KJ. An evaluation of the effects of tai chi exercise on physical function among older persons: A randomized controlled trial. Annals of Behavioral Medicine. 2001;23(2):139–146. doi: 10.1207/S15324796ABM2302_9. [DOI] [PubMed] [Google Scholar]
- Luijpen MW, Scherder EJ, Van Someren EJ, Swaab DF, Sergeant JA. Non-pharmacological interventions in cognitively impaired and demented patients--a comparison with cholinesterase inhibitors. Reviews in the Neurosciences. 2003;14(4):343–368. doi: 10.1515/revneuro.2003.14.4.343. [DOI] [PubMed] [Google Scholar]
- Lytle ME, Vander Bilt J, Pandav RS, Dodge HH, Ganguli M. Exercise level and cognitive decline: The movies project. Alzheimer Disease and Associated Disorders. 2004;18(2):57–64. doi: 10.1097/01.wad.0000126614.87955.79. [DOI] [PubMed] [Google Scholar]
- Maier SF. Bi-directional immune-brain communication: Implications for understanding stress, pain, and cognition. Brain, Behavior, and Immunity. 2003;17(2):69–85. doi: 10.1016/s0889-1591(03)00032-1. [DOI] [PubMed] [Google Scholar]
- Matthews MM, Williams HG. Can tai chi enhance cognitive vitality? A preliminary study of cognitive executive control in older adults after a tai chi intervention. Journal of the South Carolina Medical Association. 2008;104(8):255–257. [PubMed] [Google Scholar]
- McConnell ES, Pieper CF, Sloane RJ, Branch LG. Effects of cognitive performance on change in physical function in long-stay nursing home residents. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2002;57(12):M778–M784. doi: 10.1093/gerona/57.12.m778. [DOI] [PubMed] [Google Scholar]
- Molloy DW, Beerschoten DA, Borrie MJ, Crilly RG, Cape RD. Acute effects of exercise on neuropsychological function in elderly subjects. Journal of the American Geriatrics Society. 1988;36(1):29–33. doi: 10.1111/j.1532-5415.1988.tb03430.x. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Griffin WS. Interleukin-1, neuroinflammation, and alzheimer's disease. Neurobiology of Aging. 2001;22(6):903–908. doi: 10.1016/s0197-4580(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Oosterman JM, de Vries K, Dijkerman HC, de Haan EH, Scherder EJ. Exploring the relationship between cognition and self-reported pain in residents of homes for the elderly. International Psychogeriatrics. 2009;21(1):157–163. doi: 10.1017/S1041610208007941. [DOI] [PubMed] [Google Scholar]
- Patrylo PR, Williamson A. The effects of aging on dentate circuitry and function. Progress in Brain Research. 2007;163:679–696. doi: 10.1016/S0079-6123(07)63037-4. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(13):5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Wallace RB. Prevalence of cognitive impairment without dementia in the united states. Annals of Internal Medicine. 2008;148(6):427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Wallace RB. Prevalence of dementia in the united states: The aging, demographics, and memory study. Neuroepidemiology. 2007;29(1–2):125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychological Corporation. WAIS-III technical manual. 3rd ed. New York: Psychological Corporation; 1997. [Google Scholar]
- Rikli RE, Edwards DJ. Effects of a three-year exercise program on motor function and cognitive processing speed in older women. Research Quarterly for Exercise and Sport. 1991;62(1):61–67. doi: 10.1080/02701367.1991.10607519. [DOI] [PubMed] [Google Scholar]
- Rosengren KS, Christou E, Yang Y, Kass D, Boule A. Quantification of taiji learning in older adults. Journal of the American Geriatrics Society. 2003;51(8):1186–1187. doi: 10.1046/j.1532-5415.2003.51376.x. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: Plasticity, network dynamics, and cognition. Progress in Neurobiology. 2003;69(3):143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Song R, Lee EO, Lam P, Bae SC. Effects of tai chi exercise on pain, balance, muscle strength, and perceived difficulties in physical functioning in older women with osteoarthritis: A randomized clinical trial. The Journal of Rheumatology. 2003;30(9):2039–2044. [PubMed] [Google Scholar]
- Taylor-Piliae RE, Newell KA, Cherin R, Lee MJ, King AC, Haskell WL. Effects of tai chi and western exercise on physical and cognitive functioning in healthy community-dwelling older adults. Journal of Aging and Physical Activity. 2010;18(3):261–279. doi: 10.1123/japa.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai P, Beck C, Chang JY, Hagen J, Anand S, Kuo Y, Beuscher L. The feasibility of implementing tai chi for nursing home residents with knee osteoarthritis and cognitive impairment. Activities Directors' Quarterly for Alzheimer's & Other Dementia Patients. 2009;10(1):9–17. [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. The Journal of Neuroscience. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkers DJ, Gussekloo J, Stek ML, Westendorp RG, Van Der Mast RC. The 15-item geriatric depression scale (gds-15) detects changes in depressive symptoms after a major negative life event. The leiden 85-plus study. International Journal of Geriatric Psychiatry. 2004;19(1):80–84. doi: 10.1002/gps.1043. [DOI] [PubMed] [Google Scholar]
- Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: A longitudinal study from the kungsholmen project. American Journal of Epidemiology. 2002;155(12):1081–1087. doi: 10.1093/aje/155.12.1081. [DOI] [PubMed] [Google Scholar]
- Ware JE., Jr Sf-36 health survey update. Spine. 2000;25(24):3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition--the case for a head-to-toe inflammatory paradigm. Journal of the American Geriatrics Society. 2002a;50(12):2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident alzheimer disease. JAMA. 2002b;287(6):742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Sattin RW, Kutner M, O'Grady M, Greenspan AI, Gregor RJ. Intense tai chi exercise training and fall occurrences in older, transitionally frail adults: A randomized, controlled trial. Journal of the American Geriatrics Society. 2003;51(12):1693–1701. doi: 10.1046/j.1532-5415.2003.51552.x. [DOI] [PubMed] [Google Scholar]
- Woods JA, Vieira VJ, Keylock KT. Exercise, inflammation, and innate immunity. Neurologic Clinics. 2006;24(3):585–599. doi: 10.1016/j.ncl.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: Women who walk. Archives Internal Medicine. 2001;161(14):1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]