Abstract
The mammalian HIRA/UBN1/CABIN1/ASF1a (HUCA) histone chaperone complex deposits the histone H3 variant H3.3 into chromatin, and is linked to gene activation, repression and chromatin assembly in diverse cell contexts. We recently reported that a short N-terminal fragment of UBN1 containing amino acids 1–175 is necessary and sufficient for interaction with the WD repeats of HIRA, and attributed this interaction to a region from residues 120–175 that is highly conserved in a yeast ortholog Hpc2 and so termed the HRD for Hpc2-Related Domain. In this report, through a more comprehensive and refined biochemical and mutational analysis, we identify a smaller and more moderately conserved region within residues 41–77 of UBN1, that we term the NHRD, that is essential for interaction with the HIRA WD repeats; we further demonstrate that the HRD is dispensable for this interaction. We employ analytical ultracentrifugation studies to demonstrate that the NHRD of UBN1 and the WD repeats of HIRA form a tight 1:1 complex with a dissociation constant in the nanomolar range. Mutagenesis experiments identify several key residues in the NHRD that are required for interaction with the HIRA WD repeat domain, stability of the HUCA complex in vitro and in vivo and changes in chromatin organization in primary human cells. Together, these studies implicate the NHRD domain of UBN1 as being an essential region for HIRA interaction and chromatin organization by the HUCA complex.
Keywords: HIRA, UBN1, Histone Deposition, Chromatin Regulation
In higher eukaryotes, the incorporation of different histone variants into chromatin is linked to distinct DNA functions (1). In the case of histone H3, the canonical variant H3.1 and the replacement variant H3.3 differ by only 5 amino acids but are associated with DNA synthesis-coupled chromatin assembly and DNA synthesis-independent assembly, respectively (2). H3.1 expression peaks during S phase to ensure efficient DNA synthesis-coupled chromatin assembly (3). In contrast, H3.3 is constantly expressed throughout the cell cycle and in quiescence to provide a continuous H3 source for DNA synthesis-independent chromatin assembly (4, 5). These H3 variants are deposited by distinct histone chaperone complexes (6, 7). The trimeric CAF-1 complex promotes histone H3.1 deposition during replication and DNA-damage repair (8, 9) and HIRA is associated with histone H3.3 deposition independently of DNA synthesis (10). Specifically, the trimeric HIRA/UBN1/CABIN1 complex has inherent affinity for naked DNA and so HIRA-containing complexes are thought to promote assembly of H3.3-containing nucleosomes via a "gap filling" mechanism (11). HIRA also participates in large-scale deposition of H3.3 on the paternal pronucleus after fertilization in Drosophila (12), at various stages of mouse development and HIRA−/− embryonic stem cells exhibit defective H3.3 accumulations at promoters and active gene bodies in mouse (13–15).
Single amino-acid mutation in HIRA causes fly infertility (12), while HIRA knockout is embryonic lethal in mouse (16), indicating essential roles for HIRA. In mammals, HIRA is implicated in both gene activation and repression. Specifically, HIRA is required for activation of viral gene expression upon host cell infection and during angiogenesis and myogenesis (17–20). Conversely, HIRA is linked to transcription silencing and/or heterochromatin formation during meiotic sex chromosome inactivation in mice, latency of the HIV viral genome and in senescent human cells (13, 21, 22). Ectopic expression of HIRA accelerates formation of specialized domains of heterochromatin called Senescence Associated Heterochromatin Foci (SAHF) in senescing primary human diploid fibrloblasts (21). Conversely, inactivation of HIRA with a dominant negative mutant blocks formation of SAHF (23).
Human HIRA can deposit H3.3 in the context of a multi-component complex that also contains Ubinuclein 1 (UBN1), the Calcineurin-binding protein 1 (CABIN1), and Anti-Silencing Factor 1a (ASF1a) (15, 24), although HIRA-mediated H3.3 deposition activity is likely not strictly dependent on ASF1a (11, 25). S. cerevisiae has only a single H3 that resembles H3.3, but its DNA-replication independent deposition also involves a multi-component complex that contains Hir1p, Hir2p, Hir3p, Hpc2p and Asf1p (26, 27). HIRA has high sequence similarity to both Hir1p and Hir2p and shares orthologous functions with them (26–28). ASF1a, and its paralog ASF1b, are also highly homologous to Asf1p in sequence (29) but only ASF1a is part of the HIRA-containing complex (21, 24). CABIN1 was initially identified to inhibit calcineurin-mediated signal transduction (30) and acts as a transcriptional co-repressor of MEF-2 (31) and p53 (32). Sequence homology between CABIN1 and Hir3p is more limited (33) but we have recently reported that CABIN1 and Hir3p are functional orthologs (34). UBN1 is a nuclear protein that interacts with cellular and viral transcription factors (35) and is associated with tight junctions in epithelial cells (36). Although the overall sequences are quite divergent between UBN1 and Hpc2p, UBN1, and its paralog UBN2, have sequence motifs at their N-termini that resemble those in the C-terminus of Hpc2, suggesting that they are also functional orthologs (33, 37). We refer to the HIRA, UBN1, CABIN1 and ASF1a assembly as the HUCA complex (34).
Among the HUCA components, it appears that HIRA serves as the scaffolding protein. We have shown that a central B-domain region of HIRA interacts with ASF1a through one distinct surface (38), while ASF1a employs another surface to interact with a H3/H4 heterodimer substrate (39, 40). We have also more recently showed that CABIN1 physically interacts with the C-terminal portion of HIRA (34). Through bioinformatics and biochemical studies, we previously reported that the N-terminal WD repeat region of HIRA (aa 1–405) mediates a direct protein-protein interaction with the N-terminal region of UBN1 (aa 1–175) (37). This N-terminal region of UBN1contains the evolutionarily conserved Hpc2-related domain (HRD), spanning UBN1 residues 120–175, that is highly conserved with the C-terminal portion of yeast Hpc2 (33, 37). Together, these biochemical and structural studies suggest that HIRA recruits the other HUCA components to specify H3.3 deposition. It is therefore important to delineate the protein-protein interactions of the HUCA complex that are important for its biological functions.
In this report, we focus on the further characterization of the interaction between the N-terminal portion of UBN1 and the N-terminal WD repeats of HIRA (37). In our initial characterizations that focused on the UBN1 HRD domain, the observation that mutations in the most obviously conserved HRD domain of UBN1 affected HIRA interaction led us to suggest that the UBN1 HRD is responsible for direct HIRA WD repeat interaction (37). However, by employing a more comprehensive series of deletions and point-mutations, we have now found that a less conserved region in UBN1 spanning residues 41–77, just N-terminal to the HRD region (termed NHRD), is necessary and sufficient for interaction with the HIRA WD repeats. Additional studies indicate that the UBN1 HRD itself does not mediate direct interaction with HIRA and is dispensable for UBN1-HIRA interaction. We further demonstrated that the UBN1 NHRD and HIRA WD repeats form a stable 1:1 complex that is sensitive to specific amino acid substitutions in the NHRD and important for the stability of the HUCA complex in vitro, chromatin organization in vivo and instigation of cell senescence. Together, these studies implicate that the NHRD domain of UBN1 is an essential element for HIRA-UBN1 association and chromatin organization by the HUCA complex.
MATERIALS AND METHODS
Cloning and Plasmid constructions
Plasmid DNA constructs containing sequences that encode the HIRA WD repeats (HIRA(1–472) and HIRA(1–405)) were amplified by PCR from a HIRA cDNA template (X81844) and cloned into the BamHI/XhoI sites of pFASTBacHTB (Invitrogen) to make 6His-tagged fusions. To prepare untagged HIRA(1–405), mutagenesis PCR was performed using primers listed below to remove the N-terminal 6His tag from the 6His-HIRA(1–405) plasmid.
HIRA_N1_untag_F: gcgcggatctcggtccgaaaccAtgaagctcctgaagccgacctg,
HIRA_N1_untag_R: caggtcggcttcaggagcttcaTggtttcggaccgagatccgcgc.
A Thrombin-cleavable UBN1(41–175)113LVPR114 plasmid was engineered by using mutagenesis primers:
UBN1_113LVPR114_F; gaagaaaaatacggtctggtacctcgtggaaagaaacgtaga
UBN1_113LVPR114_R; tctacgtttctttccacgaggtaccagaccgtatttttcttc
The UBN1_del(90–120) construct was engineered by using mutagenesis primers:
UBN1_del(90–120)_F: aagaagaaagatctgtcagatcgaatacaggacttgatcgat
UBN1_del(90–120)_R: atcgatcaagtcctgtattcgatctgacagatctttcttctt
UBN1(41–175) and selected N-terminal or C-terminal deletion mutants were amplified by PCR from a cDNA template (NM_016936) and cloned into the BamHI/XhoI sites of pFASTBacHTB to prepare 6His-UBN1 fusion proteins. Site-directed mutagenesis was performed on the pFastBacHTB-UBN1(41–175) plasmid to introduce nucleotide substitutions by standard QuikChange mutagenesis (Stratagene) protocols that result in codon changes and amino acid substitutions. Selected UBN1 sequences were subcloned into a customized pFASTBac vector that encodes TEV-cleavable GST fusion proteins. All clones were verified by DNA sequencing.
Expression of recombinant proteins in baculovirus infected insect cells
Sequence-confirmed pFastBac transfer vectors containing sequences for human HIRA and UBN1 were transformed into DH10Bac cells. Proper recombination of the HIRA and UBN1 sequences into the baculovirus genome was determined by PCR and positive bacmid DNAs were transfected into Sf9 cells. Passage 1 (P1) virus stocks were recovered 96 hours post-transfection. A high-titer P2 virus stock was generated by infecting Sf9 at an MOI (Multiplicity Of Infection) of ~0.1, followed by incubation for 120 hours. For productions, 1 × 106 Sf9 cells/ml in Sf900-III medium (Invitrogen) were co-infected with each virus at an MOI of 1. Infected cells were harvested 48 hours post-infection.
Purification of recombinant proteins
To purify recombinant proteins from baculovirus infected insect cells, cell pellets were lysed by Dounce homogenization in 20 mM Hepes pH 8.0, 150 mM NaCl, and 10 mM Imidazole, supplemented with 5 mM 2-mercaptoethanol and protease inhibitors (PMSF, Aprotenin, Leupeptin, Pepstatin). Clarified supernatants were incubated with Ni-NTA (Thermo Scientific) for 1 hour. Bound protein(s) were washed with 60 column volumes of lysis buffer prior to elution with 250 mM of imidazole. For immunopurification of FLAG-tagged proteins, cell lysates were prepared in 20 mM Hepes pH 8.0, 500 mM NaCl, 5 mM 2-mercaptoethanol, and protease inhibitors (PMSF, Aprotenin, Leupeptin, Pepstatin). Clarified cell lysates were incubated with anti-FLAG (M2) affinity resin (Sigma) for 1 hour at 4°C. The column was then washed with 30 column volumes of lysis buffer and bound proteins were eluted with 1 column volume of lysis buffer supplemented with 800 mg/ml of FLAG peptide (Sigma). Protein samples (supernatant, flow-through, eluates, and protein remaining on the resin after elution) were analyzed on a 4–12% NuPAGE Bis-Tris gel (Invitrogen) run in 1X MOPS running buffer and stained with R250 Coomassie Blue stain. Western Blot analyses were performed on selected co-infections to verify protein expression and solubility using anti-His (GE Healthcare Sciences) and anti-HIRA (Santa Cruz Biotechnology) antibodies.
Sedimentation Equilibrium
Sedimentation equilibrium analysis of HIRA(1–405) in complex with UBN1(41–175) or UNB1(41–119) was performed at 4°C with absorbance optics at 280 nm using a Beckman Optima XL-I analytical ultracentrifuge employing a 4-hole rotor. The partial specific volume and viscosity of the complexes were estimated using Sedenterp (41). Analysis was performed using six-channel centerpieces with quartz windows, spinning at 14,000, 22,000, and 32,000 RPM. Protein samples were analyzed at concentrations of OD280 = 0.6, 0.4 and 0.2 in a 25 mM HEPES pH7.0, 150 mM NaCl, and 5 mM 2-mercaptoethanol buffer. Data for each speed were collected in quadruplicate, and the most representative runs were included in the global fit performed using three protein concentrations and three centrifugation speeds with the program HeteroAnalysis (42). The data for both complexes fit best to a single species A:B complex model, but also fit well to the equilibrium model A+B ↔A•B with A as a HIRA monomer and B as a UBN1 monomer. The quality of the fit was assessed by analysis of the RMS deviation.
Immunofluorescence and SAHF
Two-color indirect immunofluorescence and Senescence-Associated Heterochromatin Foci (SAHF) assays were performed as described previously (23).
Retrovirus infections
Retrovirus-mediated gene transfer was performed as described previously (21) using Phoenix cells to make the infectious viruses (Gary Nolan, Stanford University). Cells infected with viruses encoding resistance to puromycin were selected in 1mg/ml of the selection agent.
Antibodies
anti-HIRA (43), anti-FLAG (Sigma F3165 and F7425), anti-PML (Santa Cruz sc-966 and sc-5621), anti-cyclin A (Santa Cruz sc-751), anti-p16INK4a (Santa Cruz sc-56330), anti-Actin (Sigma AC-15) and anti-His (GE Healthcare 27471001).
RESULTS
HIRA-UBN1 association is required for solubility and stability of the recombinant proteins
We have previously shown that an N-terminal fragment of human UBN1, spanning amino acid residues 1–175, is necessary and sufficient for interaction with the WD repeats (residues 1–405) of human HIRA (37). This region of UBN1 contains a highly conserved HRD (Hpc2-related domain), spanning residues 120–170, a less conserved region N-terminal to the HRD defined by residues 41–74 and a largely charged putative linker region spanning residues 75–119 (Figure 1). Strikingly, we found that the expression of the recombinant HIRA(1–405) or UBN1(1–175) proteins on their own in either bacteria or insect cell expression systems yielded either insoluble protein or soluble aggregates (data not shown and (37)). In contrast, we found that coexpression of the proteins in insect cells produced a soluble complex that remains stably associated through Ni-NTA, anion exchange, and gel filtration chromatography (Figure 2 and (37)). This observation suggested that formation of the HIRA/UBN1 complex facilitates stability of the HIRA and UBN1 proteins, as confirmed by a Western Blot examining the HIRA(1–405)/UBN1 (41–175) complex (Figure 2D). Because of this observation, coupled with the inability to produce individual recombinant HIRA or UBN1 protein in the absence of the other binding partner, we used the insect cell co-expression assay to define the minimal region of UBN1 that was necessary and sufficient for interaction with the HIRA WD repeats.
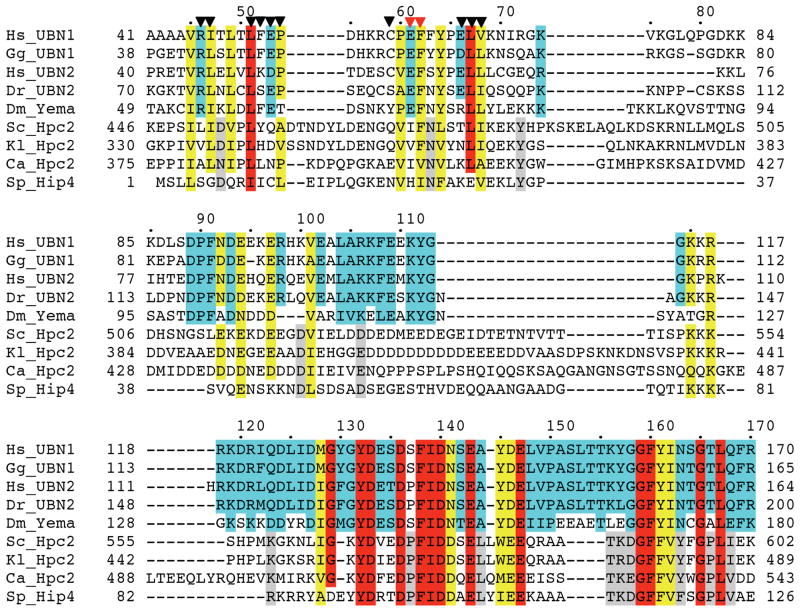
Figure 1.
A UBN1 region N-terminal to the previously defined HRD (Hpc2-related Domain) shows modest yet significant sequence homology among UBN1/UBN2 and, to a lesser extent, Hpc2. Sequence conservation is shaded as: red (identical), yellow (highly conserved), cyan (highly conserved among UBN1/UBN2 only), and grey (highly conserved among Hpc2 only). Residue numbers are given along the sequences, with human UBN1 residue numbers also marked on top of the alignment. Triangles are used to label UBN1 residues that are mutationally sensitive (red), or not sensitive (black), for HIRA binding. Sequences are from organisms Hs (homo sapiens), Gg (Gallus gallus), Dr (Danio rerio), Dm (Drosophila Melanogaster), Sc (Saccharomyces cerevisiae), Kl (Kluyveromyces lactis), Ca (Candida albicans), and Sp (Schizosaccharomyces pombe). “yema" stands for yemanuclein-α.
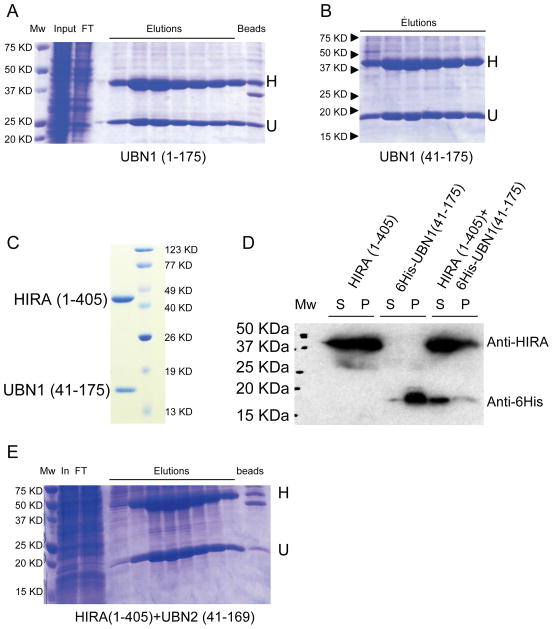
Figure 2.
Co-expression of UBN1 with HIRA WD repeat region enhances the solubility of both proteins. A) 6His-UBN1(1–175)/HIRA(1–405) co-expression, B) 6His-UBN1(41–175)/HIRA(1–405) co-expression, C) Peak of 6His-UBN1(41–175)/HIRA(1–405) on size exclusion chromatography D) Western Blot of 6His-UBN1(41–175)/HIRA(1–405) co-expression and proteins alone, and E) 6His-UBN2(41–169)/HIRA(1–405) co-expression. H–HIRA; U–UBN1/UBN2; Mw–molecular weight markers; In–Ni-NTA input; FT–NI-NTA flow-through; Elution–Ni-NTA fractions at increasing Imidazole concentration; beads–Ni-NTA resin after elution; S–soluble fraction; P–insoluble fraction. Anti-HIRA (Santa Cruz Biotechnology) and anti-6His (GE Healthcare Sciences) were used to detect HIRA(1–405) and 6His-UBN1, respectively. The same labeling applies to figures 3 and 5.
A region N-terminal to the HRD of UBN1 is required for interaction with the HIRA WD repeats
Within the N-terminal UBN1(1–175) fragment, residues 1–40 are very diverged between UBN2 and Hpc2 proteins across species. We therefore hypothesized that this N-terminal portion of UBN1 was dispensable for HIRA interaction. Indeed, co-expression of untagged-HIRA(1–405) with 6His-UBN1(41–175) resulted in the retention of an apparent stoichiometric complex between UBN1 and HIRA on the Ni-NTA resin, revealing that residues 41–175 of UBN1 were sufficient for this interaction (Figures 2A and 2B). This protein complex could be further purified to homogeneity using anion exchange and size exclusion chromatography (Figure 2C). Similarly, expression of the paralogue UBN2(41–169) also formed a stable complex with the HIRA WD repeats (Figure 2E). These two experiments collectively indicate that the nonconserved N-terminal 40 residues of UBN1/UBN2 are dispensable for HIRA interaction.
Using the baculovirus co-expression assay in insect cells, we mapped the amino acids of UBN1 that were necessary for binding to the HIRA WD repeats. Specifically, we co-expressed a series of N-terminal UBN1 deletions that were each tested for complex formation with HIRA(1–405) (Figure 3). Ni-NTA affinity purification from these co-expressions showed that N-terminal deletion beyond UBN1 residue 41 reduced complex formation with HIRA(1–405) (Figures 3A, top row). Specifically, larger deletions compromise the ability of the proteins to form a stable soluble HIRA/UBN1 complex that can be captured and detected on Ni-NTA, with the HIRA(1–405) coexpression with UBN1(81–175) revealing no detectable complex. Western Blot analysis showed that HIRA protein expression and solubility levels were similar across all co-expressions, while increased N-terminal deletion on UBN1 decreased the expression and solubility of UBN1 (Figure 3B). This data suggests that, in this assay, a region N-terminal to the HRD, within residues 41–80, is essential for the stability of UBN1 and its interaction with the HIRA WD repeats.
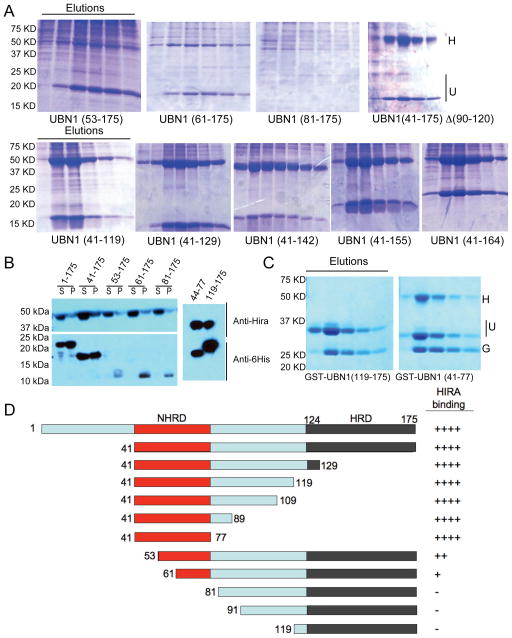
Figure 3.
The UBN1 NHRD is necessary and sufficient for HIRA WD repeat interaction. A) HIRA (residues 1–405), was coexpressed with 6His-UBN1 deletion constructs in Sf9 cells and complexes purified using Ni-agarose chromatography. H = HIRA(1–405), U = UBN1 constructs. B) Western Blots of whole cell soluble protein extracts from Sf9 cells co-infected with HIRA(405) and 6His-UBN1 deletion mutants (left) or GST-UBN1(44–77) and GST-UBN1(119–175) (right-side). UBN1 deletion constructs are indicated above the gel. C) HIRA (residues 1–405) coexpressed with GST-UBN1 deletion constructs in Sf9 cells and complexes purified using GSH-sepharose chromatography. G=GST.. D) Schematic diagram that summarizes the binding of UBN1 to the HIRA WD repeats, as scored to the right.
The UBN1 HRD is dispensable for interaction with the HIRA WD repeats
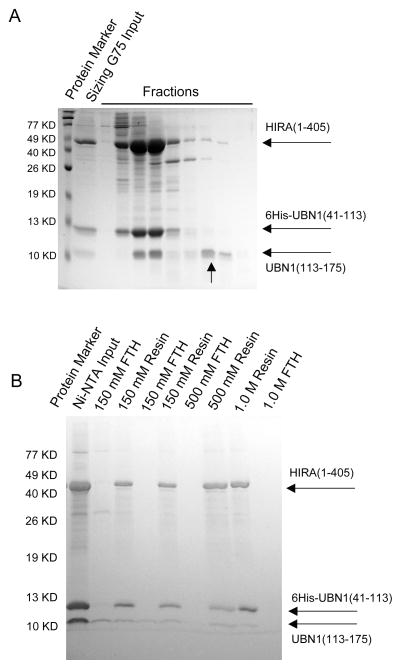
Having identified the N-terminal boundary of the HIRA-binding domain in UBN1, we set out to map the C-terminal boundary of the minimal UBN1 fragment able to bind to the HIRA WD repeats. Specifically, we prepared a series of C-terminal deletions of UBN1 spanning from residue 41 to residues 164, 155, 142, 129 and 119, respectively (as schematized in Figure 3D). As shown in Figure 3A, bottom row, a UBN1 fragment as short as 41–119 retained HIRA-binding activity similar to that of UBN1(41–175) and the UBN1(41–119)/HIRA(1–405) complex could be purified to homogeneity through ion exchange and size exclusion chromatography (Figure S1). We observed near identical results from co-expression studies with a UBN2 protein that lacks the HRD (data not shown). This data together demonstrates that the HRD region of UBN1/UBN2 is dispensable for interaction with the HIRA WD repeats. Indeed, a co-expression of HIRA(1–405) with the minimal UBN1 HRD, GST-UBN1(119–175), was insufficient to reconstitute the HIRA-UBN1 interaction in vitro (Figure 3C).
A UBN1 region N-terminal to the HRD is necessary and sufficient for HIRA interaction
Inspection of the amino acids in the UBN1 fragment 41–119 that we found to be sufficient for HIRA interaction identified an N-terminal region defined by residues 41–74 that shows conservation across UBN1 orthologs and a putative linker region (residues 75–119) that is largely charged (Figure 1). To investigate the role of the linker region in HIRA WD repeat interaction, we prepared a protein, UBN1(41–175)del(90–120), which has amino acids 90–120 within the putative linker region deleted. As shown in Figure 3A, top row, coexpression of this protein with the HIRA WD repeats had no impact on the ability to reconstitute the interaction in vitro, suggesting that the linker region is dispensable for HIRA interaction. Knowing that both the HRD and the linker region were dispensable for HIRA interaction, we designed GST fusions with fragments of UBN1 encoding amino acids 41–119, 41–109, 41–89, and 41–77 to test for HIRA binding (Figure S2). As shown in Figure 3C,, stable complex formation with HIRA could be readily observed with a UBN1 fragment as short as residues 41–77, indicating that this N-terminal conserved region is necessary and sufficient for binding to the HIRA WD repeats. In light of the sequence conservation and significance in HIRA binding, we term residues 41–77 of UBN1 as the NHRD (N-terminal to the HRD).
The UBN1 NHRD forms a tightly associated 1:1 complex with the HIRA WD repeats
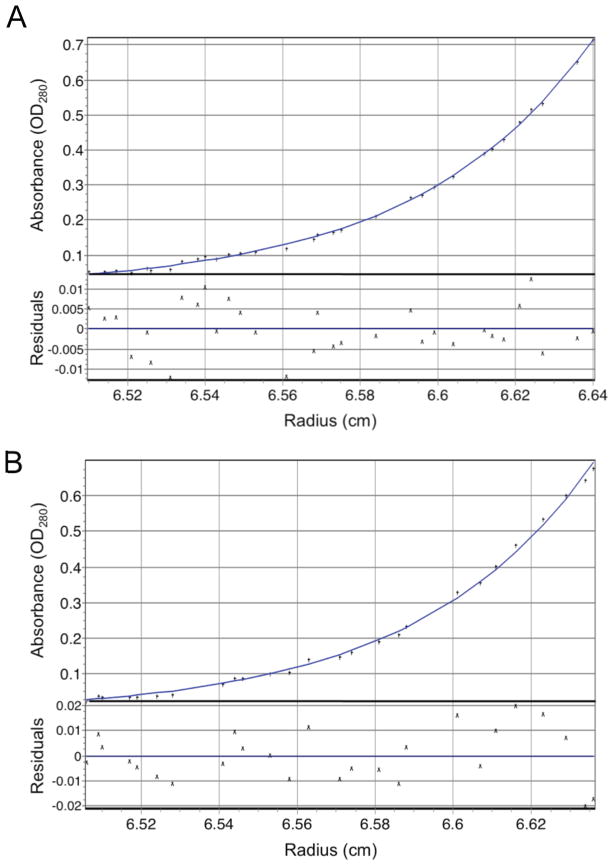
To more quantitatively assess the UBN1 NHRD interaction with HIRA, we used sedimentation equilibrium analysis to derive the binding affinity and stoichiometry of the HIRA/UBN1 complex. Specifically, a UBN1(41–119) fragment containing the NHRD, but not the HRD, was co-expressed with HIRA(1–405) to form the UBN1(41–119)/HIRA(1–405) complex that was purified to homogeneity through gel filtration chromatography. The protein complex was prepared at three different concentrations (OD280 = 0.6, 0.4, and 0.2) and spun at three different centrifugation speeds (14,000, 22,000, and 32,000 RPM). The data were best fit to a single-species model containing one molecule of HIRA and one molecule of UBN1 (Figure 4A and S3A), with an RMS deviation of 0.00923 for the fit. The data could also be fit reasonably well into an equilibrium model of A+B ↔A•B, with derived Kd = 6.5 nM (data not shown). These sedimentation equilibrium data demonstrates that the UBN1 NHRD and HIRA WD repeats mediate a tight 1:1 complex with a dissociation constant in the nanomolar range and is consistent with formation of a similar 1:1 complex in the context of the full-length proteins.
Figure 4.
The UBN1 NHRD stoichiometrically binds the HIRA WD repeats. Representative sedimentation equilibrium data for two HIRA/UBN1 complexes collected at a centrifugation speed of 32,382 rpm and a protein OD280 of 0.2. A) Representative curve for the HIRA(1–405)/UBN1(41–119) complex. B) Representative curve for the HIRA(1–405)/UBN1(41–175) complex. The global fits with three centrifugation speeds and three protein concentrations are shown in Figure S3.
Although the UBN1 HRD is dispensable for HIRA binding, it is possible that it may still contribute to HIRA interaction in the context of the NHRD. To address this question, a UBN1 fragment that contains both the HRD and NHRD, UBN1(41–175), was co-expressed with HIRA(1–405) to form the UBN1(41–175)/HIRA(1–405) complex. The same sedimentation equilibrium protocol as above was applied to this complex. As shown in Figure 4B and S3B, UBN1(41–175)/HIRA(1–405) complex is a tight 1:1 complex with a RMSD of 0.00986 for a single-species model fit and a Kd=13.3 nM for a equilibrium model fit, very similar to the UBN1(41–119)/HIRA(1–405) complex above that contains only the NHRD, but not the HRD. This data suggests that the UBN1 HRD does not contribute to HIRA binding in the context of NHRD.
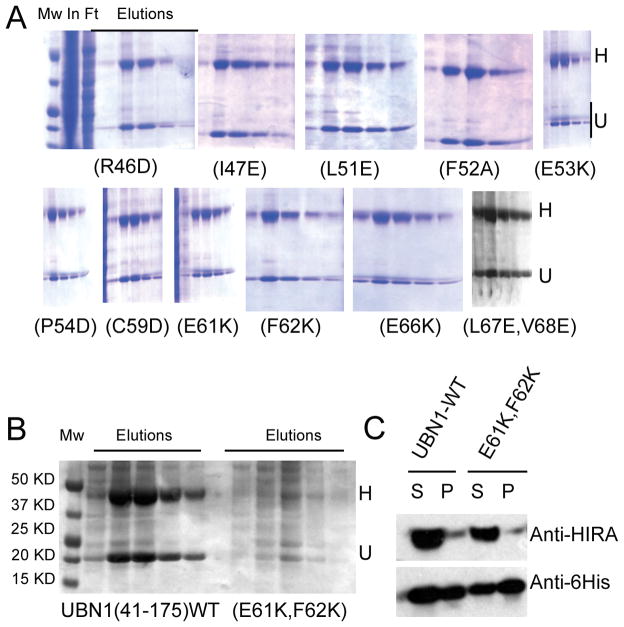
Point mutations in the NHRD disrupt interaction with the HIRA WD repeats
Having established that the UBN1 NHRD alone is likely the major driver of the interaction between UBN1 and the HIRA WD repeats, we next set out to more precisely define the residues of the NHRD that mediate the interaction with the HIRA WD repeats. We introduced amino acid substitutions in the His-UBN1(41–175) protein at NHRD residues that are most conserved among UBN1, UBN2 and Hpc2 across various species (Figure 1, marked with triangles). Specifically, UBN1 mutants (R46D), (I47E), (L51E), (F52A), (E53K), (P54D), (C59D), (E61K), (F62K), (E66K) and (L67E, V68E) were prepared and assayed for complex formation with HIRA(1–405) (Figure 5). As shown in Figure 5A, all of these mutants appear to interact with HIRA as avidly as wild-type UBN1(41–175). However, a double mutant, (E61K, F62K), that combines two non-defective mutants, was found to significantly impair this interaction (Figure 5B). Western blot analysis (Figure 5C) confirmed that both UBN1(41–175)(E61K, F62K) and HIRA(1–405) were expressed in a soluble form, suggesting that this double mutation either directly disrupts the HIRA-UBN1 interaction, or alters the conformation of the UBN1 protein in such a way that it loses HIRA-binding capacity. Taken together, these experiments have identified critical amino acid residues (i.e. E61 and F62) within the newly defined NHRD that are required to maintain stable complex formation between UBN1 and HIRA.
Figure 5.
The UBN1 NHRD mutation (E61K, F62K) abrogates HIRA WD repeat interaction. A) Co-expression of HIRA(1–405) with UBN1 harboring single amino acid substitutions that do not disrupt the HIRA-UBN1 interaction. B) Co-expression of HIRA(1–405) with either His-UBN1(41–175) WT (left) or the (E61K, F62K) mutant (right), respectively, C) Western Blots of HIRA/UBN1 co-infections of either wild-type His-UBN1(41–175) or the (E61K, F62K) mutation. Labels are as in Figure 2.
The UBN1 NHRD/HIRA WD repeats interaction is required for stability of the HUCA complex
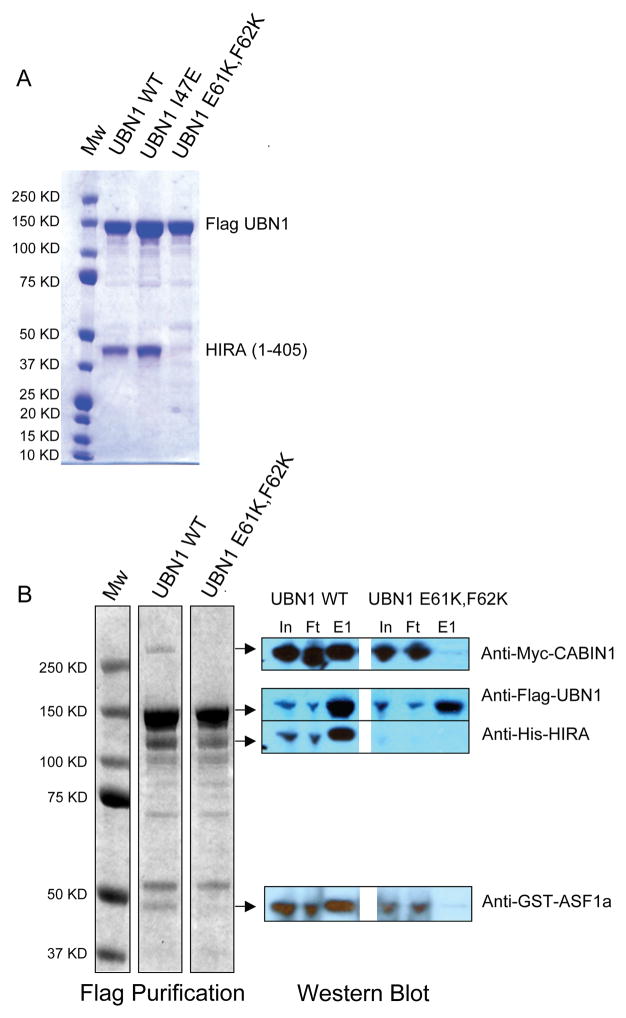
To assess whether the UBN1 NHRD domain is necessary for interaction with the HIRA WD repeats in the context of the full length UBN1 protein, we first assayed the effect of the UBN1(E61K, F62K) mutant on the binding of full-length UBN1 to HIRA(1–405). Specifically, the (E61K, F62K) mutations were introduced into full-length Flag-tagged UBN1 and tested for complex formation with HIRA(1–405) in the insect cell co-expression assay. We also introduced a control (I47E) mutation that had no effect on the interaction between UBN1(41–175) and the HIRA WD repeats (Figure 5A, top row). Flag affinity purifications of full-length Flag-UBN1 WT and Flag-UBN1(I47E) retained the ability to interact with HIRA(1–405). However, Flag-UBN1(E61K, F62K) failed to pull down any HIRA(1–405), suggesting that the NHRD is the only domain in UBN1 that interacts with the HIRA WD repeats (Figure 6A),
Figure 6.
The UBN1 NHRD (E61K, F62K) mutant disrupts UBN1 interaction with HIRA(1–405) and the assembly of the HUCA complex. A) Anti-Flag purification of full-length Flag-UBN1, either WT, (I47E), or (E61K, F62K) mutants, that are co-expressed with HIRA(1–405). B) Anti-Flag purification of Flag-UBN1 (WT or E61K, F62K) that was coexpressed with full-length HUCA components (HIRA, CABIN1, ASF1a) in insect cells. Left–coomassie blue R250 stain of FLAG peptide eluates; Right–Western blot analysis of anti-Flag column input (In), flow through (FT), eluate (E1). MW–molecular weight markers.
To investigate the role of the UBN1 NHRD in the assembly and stability of the HUCA complex, we coexpressed full-length His-HIRA, Myc-CABIN1, GST-ASF1a with full-length Flag-UBN1 WT, Flag-UBN1(I47E), or Flag-UBN1(E61K, F62K), respectively, in insect cells (34). Expression of Flag-UBN1(E61K, F62K) failed to retain the other components (i.e. HIRA, CABIN, and ASF1a) of the HUCA complex following immunopurification (Figure 6B). In addition, Western blots of the HUCA components reveals extremely low levels of HIRA in the presence of the UBN1(E61K, F62K) (Figure 6B, right side), which is consistent with the possibility that UBN1 association with HIRA contributes significantly to HIRA stability. So in the absence of a HIRA/UBN1 interaction HIRA is either not expressed or insoluble.. Taken together, these results further highlight the importance of the HIRA-UBN1 interaction for maintaining the integrity of the HUCA complex.
The UBN1 HRD may physically interact with the NHRD
In this report, we used various techniques to establish that UBN1 utilizes a small and relatively poorly conserved domain N-terminal to the HRD (termed NHRD), but not the more conserved HRD, to mediate its physical interaction with the WD repeats of HIRA. Moreover, this interface is the only one mediating interaction between full-length UBN1 and HIRA (Figure 6). Paradoxically, we found earlier that point mutations within the UBN1 HRD also negatively affect the UBN1/HIRA interaction (37). Given the observations reported here that HRD itself is not directly involved in the interaction with HIRA, the HIRA binding defect caused by HRD mutations suggests that the NHRD and HRD of UBN1 form an intramolecular interaction, and that this interaction may stabilize the NHRD for optimal HIRA interaction. To test such a model, an UBN1 construct, 6His-UBN1(41–175)113LVPR114, was engineered to introduce a thrombin cleavage motif (LVPR) in between residues 113 and 114 to facilitate the subsequent cleavage of this UBN1 construct into separate NHRD and HRD domains. This UBN1 variant was co-expressed with HIRA(1–405) to produce a binary complex that was subsequently subjected to thrombin cleavage to introduce a nick between UBN1 residues 113 and 114 (Figure S4). The end product was then subjected to size exclusion chromatography. As shown in Figure 7A, in a buffer that contains 250 mM NaCl, while some of the UBN1 HRD (114–175) was found to dissociate from the UBN1(41–113)/HIRA(1–405) complex, the majority of UBN1 HRD(114–175) was still retained in the complex. To further evaluate the association of the UBN1 HRD to the UBN1(41–113)/HIRA(1–405) complex, thrombin-cleaved 6His-UBN1(41–175)113LVPR114/HIRA(1–405) was retained on Ni-NTA resins and then subjected to multiple washes using buffers containing increasing NaCl concentration. As shown in Figure 7B, a substoichiometric amount of the HRD still remains associated with the UBN1(41–113)/HIRA(1–405) complex after as high as a 1.0 M NaCl wash. Since we have shown that the HRD region itself does not associate with HIRA(1–405) (Figures 3A and S2), this data suggests that the UBN1 HRD is associated to the HIRA WD repeats indirectly through its interaction with the NHRD. It is therefore possible that in vivo the UBN1 NHRD and HRD function as an entity (potentially as a globular domain) with the NHRD providing a binding surface for the HIRA WD repeats. Mutations within the HRD (37), therefore, may affect the stability of such a domain and compromise HIRA binding. In addition to stabilizing UBN1 NHRD, it is possible that HRD may also mediate other HIRA-associated functions that are yet to be characterized.
Figure 7.
The HRD is associated with the UBN1-NHRD/HIRA(1–405) complex. A) Thrombin-cleaved 6His-UBN1(41–175)113LVPR114/HIRA(1–405) complex was subjected to size exclusion chromatography and resolved on an SDS-PAGE. Dissociated HRD is indicated with an arrow. B) Samples prepared as described in (A) were retained on Ni-NTA resin and then subjected to multiple washes with increasing amount of NaCl as indicated. Equal aliquots of the flow-through (FTH) and resins after wash (Resin) were prepared and resolved on SDS-PAGE.
The NHRD/WD interaction is required for SAHF formation in vivo
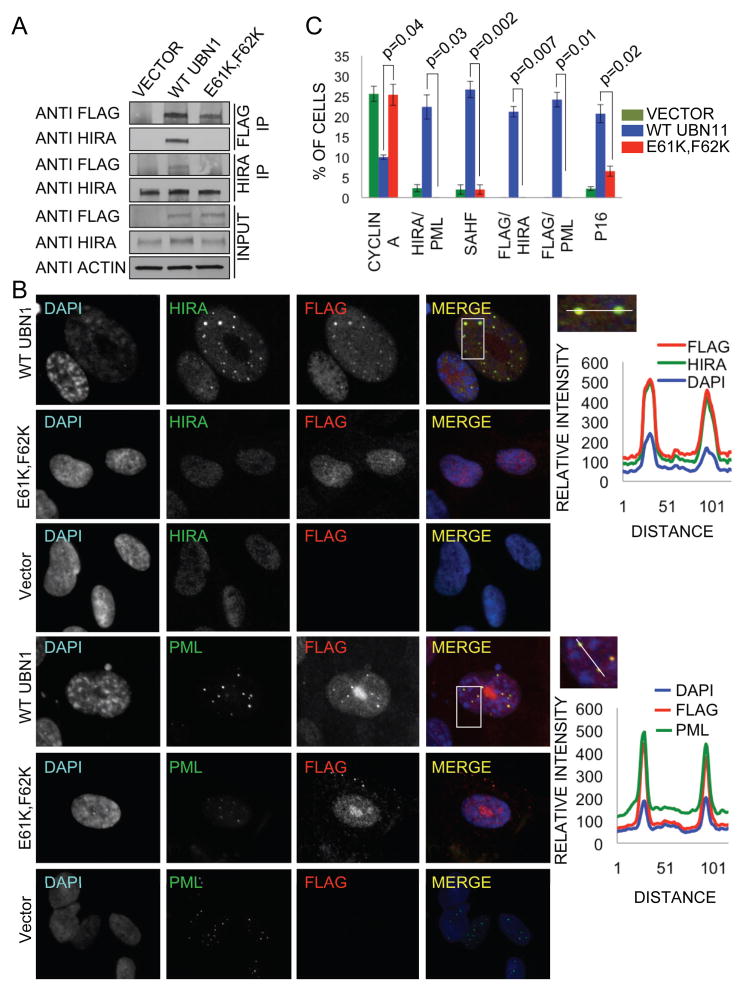
Next, we wanted to assess the functional significance of the HIRA/UBN1 interaction in vivo. More specifically, we set out to probe the role of the interaction between the UBN1 NHRD and HIRA WD repeats. Previously, we have shown that ectopic expression of HUCA proteins (HIRA, UBN1, CABIN1 and ASF1a) in primary human fibroblasts induces chromatin reorganization, manifest in formation of SAHF, and premature cell senescence (21, 37). This activity is thought to reflect the role of the endogenous HUCA complex in chromatin reorganization in senescent cells. Mutations that disrupt the interaction between HIRA and ASF1a block the induction of SAHF in this assay (21). To similarly test the importance of the HIRA/UBN1 interaction, primary human fibroblasts were infected with retroviruses encoding flag-tagged wild type UBN1 (flag-UBN1), flag-UBN1 (E61K, F62K) or empty vector as a control. Both the wild type and mutant UBN1 proteins were comparably expressed (Figure 8A). Although immunoprecipitation of wild-type flag-UBN1 efficiently co-precipitated endogenous HIRA, the mutant protein failed to bind to HIRA in this assay (Figure 8A). This confirms that the (E61K, F62K) mutation disrupts the interaction between HIRA and UBN1 in mammalian cells, just as it does in vitro. As shown previously, ectopic expression of wild type flag-UBN1 induced formation of SAHF (Figures 8B, C) and associated markers of senescence-associated proliferation arrest (decreased expression of cyclin A and increased expression of cell cycle inhibitor, p16INK4a (Figures S5A)) (21, 37). In addition, wild type flag-UBN1 efficiently localized to PML nuclear bodies that are thought to be important structures in the SAHF assembly process (21, 23, 37), and also induced recruitment of HIRA to these subnuclear bodies (Figures 8B, C and S5B). Strikingly, the mutant flag-UBN1 protein that failed to bind to HIRA was also markedly impaired in each of these assays. Flag-UBN1 (E61K, F62K) failed to localize to PML bodies and recruit HIRA to PML bodies, suggesting that these proteins enter PML bodies as a complex (Figures 8B, C and S5B). Most importantly, the mutant UBN1 failed to induce formation of SAHF and markers of senescence-associated proliferation arrest, specifically repression of cyclin A and expression of p16INK4a (Figures 8B, C and S5A), confirming the functional importance in vivo of the HIRA/UBN1 interaction, and particularly the UBN1 NHRD/HIRA WD repeats interaction.
Figure 8.
Ectopically expressed UBN1 WT, but not the (E61K, F62K) NHRD mutant, interacts with endogenous HIRA. Proliferating IMR90 cells were infected with retroviruses encoding wild-type flag-UBN1, flag-UBN1 (E61K, F62K) mutant or empty vector, as indicated. A) 5 days later, lysates were prepared and physical interactions between ectopically expressed wild type or mutant UBN1 and endogenous HIRA detected by immunoprecipitation-western blot analysis. B) 28 days later, cells were stained with DAPI and anti-HIRA (mouse monoclonal IgG1 WC cocktail) or anti-PML (Santa Cruz sc-966, mouse monoclonal IgG1) and anti-flag (Sigma F7425, rabbit polyclonal) antibodies and visualized by immunofluorescence. Upper panel on the right represents relative intensity of DAPI, HIRA, and Flag fluorescence along a straight line through the HIRA/Flag foci. Bottom panel on the right represents relative intensity of DAPI, PML body, and Flag fluorescence along a straight line through the PML/Flag foci. C) Quantitation of results from B and Figure S5. More than 100 cells were scored from 3 independent biological replicates. p values were calculated using two-tailed t-test with Bonferroni correction.
DISCUSSION
In this report, we identify an N-terminal region of the UBN1 subunit (residues 41–77) of the mammalian HUCA (HIRA/UBN1/CABIN1/ASF1a) histone H3.3 deposition complex that is important for interaction with the WD repeats of HIRA in vitro and stability and functional activity of the HUCA complex both in vitro and in vivo. This region of UBN1, that we term the NHRD (for N-terminal to the Hpc2-Related Domain), forms a 1:1 complex with HIRA WD repeats and likely also forms an intramolecular interaction with the highly conserved HRD region. Together, these studies implicate the NHRD domain of UBN1 as an essential region for integrity of the HUCA complex and for its functional properties in cells.
One outstanding question that arises from this study relates to the sequence and functional conservation of the UBN1 NHRD among other species. As is evident in Figure 1, unlike the high sequence conservation of the HRD in human UBN1/UBN2 and budding yeast Hpc2 (discussed below), the NHRD is only modestly conserved among the UBN1/UBN2 paralogs and appears more diverged in various Hpc2 species. We had previously established that the C-terminal portion of Hpc2, containing both the HRD and the NHRD, physically interacts with Hir1 (37). Very recently, Prochasson and colleagues described that in budding yeast, a so called Conserved Domain II (CD II) in Hpc2, which corresponds to the motif that best aligns with the UBN1 NHRD in Figure 1, is as essential as the CD III region (corresponding to UBN1 HRD) for the association and integrity of the HIR complex (44). This data is consistent with our findings, suggesting that there is functional conservation between the NHRD and CD II regions in human and budding yeast. However, unlike the findings or Prochasson and colleagues, our studies suggest a greater importance of the NHRD/CD II region relative to the HRD/CD III region suggesting some subtle differences between the two species. A complex analogous to the S. cerevisiae HIR complex has also been characterized in S. pombe (45). Interestingly, Hip4, the Hpc2 homolog in S. pombe, is a much smaller protein and has its putative NHRD-HRD fragment located at its extreme N-terminus (Figure 1). The characterization of the physical interaction of Hip4 with its binding partner, presumably Hip1, should also provide insights into whether the HIRA/UBN1-NHRD interaction is conserved among these species.
Importantly, we have shown that the HRD region of UBN1 does not directly interact with HIRA and is not required for UBN1-HIRA interaction, yet it appears to associate with HIRA through the NHRD of UBN1. This observation, coupled with the fact that the HRD is the most highly conserved region within the UBN1 orthologs from various species (Figure 1), suggests that the HRD might play some other role in the HUCA complex. Aravind and colleagues had proposed that a set of highly conserved aspartic acid and glutamic acid residues within the HRD form one face of a predicted helix that might interact with the highly basic histone tails (33). If this is the case, the HRD might recruit histones into the HUCA complex for histone deposition, and possibly specify H3.3 association. If the HRD interacts with both histones and the HIRA-binding surface (NHRD) of UBN1, this points to mechanistic interactions that shed light on the histone delivery function of the complex. For example, the HRD might bind histones and the NHRD in mutually exclusive fashion, allowing the NHRD to expel histones from the HUCA complex. Alternatively, the HRD might participate in other activities of the HUCA complex. For a small domain capable of specific protein-protein recognition, the small histone CENP-A recognition domain in HJURP is a good example (46).
We have shown here that mutations of UBN1 that prevent HIRA/UBN1 complex formation also block UBN1-induced chromatin changes and proliferation arrest in primary human cells. Previously, we showed that mutations in HIRA or ASF1a which disrupt the HIRA/ASF1a interaction similarly prevent induction of chromatin changes and proliferation arrest in primary human cells (21). Significantly, we have previously shown that an N-terminally truncated HIRA mutant, HIRA(520–1017), is a dominant negative inhibitor of senescence-associated chromatin changes (23). Since HIRA(520–1017) lacks the UBN1 and ASF1a binding domains, but retains only the CABIN1 binding domain (21, 34, 37, 38), it seems likely that the dominant negative activity of this fragment depends, at least in part, on its ability to titrate CABIN1 away from ASF1a and UBN1. Together, these results underscore the requirement for an intact complex for HUCA to mediate its functions.
Regarding specific functions of the HUCA complex, we have shown here that a mutant of UBN1 that does not bind to HIRA, UBN1(E61K, F62K), is not recruited to PML bodies when ectopically expressed in primary human cells. Interestingly, wild type UBN1, but not UBN1(E61K, F62K), also recruited its binding partner, HIRA, to PML bodies. PML bodies are thought to be important for HUCA function, because HIRA, CABIN1 and UBN1 are all recruited to PML bodies in senescent cells and disruption of PML bodies blocks formation of SAHF in senescent cells (21, 23, 37). Thus, we have previously proposed that PML bodies are a "staging ground" for proper assembly, activation or modification of HUCA complexes prior to their formation of SAHF. Results reported here suggest that one specific function of the HIRA/UBN1 interaction is to facilitate recruitment of the HUCA complex to PML bodies. As discussed above, the HIRA/UBN1 interaction must also play a direct role in nucleosome assembly, through a mechanism that remains to be defined. Dissection of the functional domains, as performed here, is an important step towards this more detailed mechanistic understanding.
Supplementary Material
Acknowledgments
This work was supported by a grant from the NIH to R.M., D.S.C. and P.D.A. (AG 031862). We thank the Wistar Protein Expression and Molecular Screening Core Facility funded by NIH grant CA 010815 for help with the protein expression analysis presented in this study.
Footnotes
SUPPORTING INFORMATION AVAILABLE
The supporting information contains 5 supplemental figures. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Talbert PB, Henikoff S. Histone variants--ancient wrap artists of the epigenome. Nat Rev Mol Cell Biol. 2010;11:264–275. doi: 10.1038/nrm2861. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad K, Henikoff S. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol Cell. 2002;9:1191–1200. doi: 10.1016/s1097-2765(02)00542-7. [DOI] [PubMed] [Google Scholar]
- 3.Osley MA. The regulation of histone synthesis in the cell cycle. Annu Rev Biochem. 1991;60:827–861. doi: 10.1146/annurev.bi.60.070191.004143. [DOI] [PubMed] [Google Scholar]
- 4.Wu RS, Tsai S, Bonner WM. Patterns of histone variant synthesis can distinguish G0 from G1 cells. Cell. 1982;31:367–374. doi: 10.1016/0092-8674(82)90130-1. [DOI] [PubMed] [Google Scholar]
- 5.Frank D, Doenecke D, Albig W. Differential expression of human replacement and cell cycle dependent H3 histone genes. Gene. 2003;312:135–143. doi: 10.1016/s0378-1119(03)00609-7. [DOI] [PubMed] [Google Scholar]
- 6.De Koning L, Corpet A, Haber JE, Almouzni G. Histone chaperones: an escort network regulating histone traffic. Nat Struct Mol Biol. 2007;14:997–1007. doi: 10.1038/nsmb1318. [DOI] [PubMed] [Google Scholar]
- 7.Ray-Gallet D, Almouzni G. Nucleosome dynamics and histone variants. Essays Biochem. 2010;48:75–87. doi: 10.1042/bse0480075. [DOI] [PubMed] [Google Scholar]
- 8.Gaillard PH, Martini EM, Kaufman PD, Stillman B, Moustacchi E, Almouzni G. Chromatin assembly coupled to DNA repair: a new role for chromatin assembly factor I. Cell. 1996;86:887–896. doi: 10.1016/s0092-8674(00)80164-6. [DOI] [PubMed] [Google Scholar]
- 9.Smith S, Stillman B. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell. 1989;58:15–25. doi: 10.1016/0092-8674(89)90398-x. [DOI] [PubMed] [Google Scholar]
- 10.Ray-Gallet D, Quivy JP, Scamps C, Martini EM, Lipinski M, Almouzni G. HIRA is critical for a nucleosome assembly pathway independent of DNA synthesis. Mol Cell. 2002;9:1091–1100. doi: 10.1016/s1097-2765(02)00526-9. [DOI] [PubMed] [Google Scholar]
- 11.Ray-Gallet D, Woolfe A, Vassias I, Pellentz C, Lacoste N, Puri A, Schultz DC, Pchelintsev NA, Adams PD, Jansen LE, Almouzni G. Dynamics of histone H3 deposition in vivo reveal a nucleosome gap-filling mechanism for H3.3 to maintain chromatin integrity. Mol Cell. 2011;44:928–941. doi: 10.1016/j.molcel.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Loppin B, Bonnefoy E, Anselme C, Laurencon A, Karr TL, Couble P. The histone H3.3 chaperone HIRA is essential for chromatin assembly in the male pronucleus. Nature. 2005;437:1386–1390. doi: 10.1038/nature04059. [DOI] [PubMed] [Google Scholar]
- 13.van der Heijden GW, Derijck AA, Posfai E, Giele M, Pelczar P, Ramos L, Wansink DG, van der Vlag J, Peters AH, de Boer P. Chromosome-wide nucleosome replacement and H3.3 incorporation during mammalian meiotic sex chromosome inactivation. Nat Genet. 2007;39:251–258. doi: 10.1038/ng1949. [DOI] [PubMed] [Google Scholar]
- 14.Torres-Padilla ME, Bannister AJ, Hurd PJ, Kouzarides T, Zernicka-Goetz M. Dynamic distribution of the replacement histone variant H3.3 in the mouse oocyte and preimplantation embryos. Int J Dev Biol. 2006;50:455–461. doi: 10.1387/ijdb.052073mt. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg AD, Banaszynski LA, Noh KM, Lewis PW, Elsaesser SJ, Stadler S, Dewell S, Law M, Guo X, Li X, Wen D, Chapgier A, DeKelver RC, Miller JC, Lee YL, Boydston EA, Holmes MC, Gregory PD, Greally JM, Rafii S, Yang C, Scambler PJ, Garrick D, Gibbons RJ, Higgs DR, Cristea IM, Urnov FD, Zheng D, Allis CD. Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell. 2010;140:678–691. doi: 10.1016/j.cell.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts C, Sutherland HF, Farmer H, Kimber W, Halford S, Carey A, Brickman JM, Wynshaw-Boris A, Scambler PJ. Targeted mutagenesis of the Hira gene results in gastrulation defects and patterning abnormalities of mesoendodermal derivatives prior to early embryonic lethality. Mol Cell Biol. 2002;22:2318–2328. doi: 10.1128/MCB.22.7.2318-2328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutta D, Ray S, Home P, Saha B, Wang S, Sheibani N, Tawfik O, Cheng N, Paul S. Regulation of angiogenesis by histone chaperone HIRA-mediated incorporation of lysine 56-acetylated histone H3.3 at chromatin domains of endothelial genes. J Biol Chem. 2010;285:41567–41577. doi: 10.1074/jbc.M110.190025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang JH, Song Y, Seol JH, Park JY, Yang YJ, Han JW, Youn HD, Cho EJ. Myogenic transcriptional activation of MyoD mediated by replication-independent histone deposition. Proc Natl Acad Sci U S A. 2011;108:85–90. doi: 10.1073/pnas.1009830108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Placek BJ, Huang J, Kent JR, Dorsey J, Rice L, Fraser NW, Berger SL. The histone variant H3.3 regulates gene expression during lytic infection with herpes simplex virus type 1. J Virol. 2009;83:1416–1421. doi: 10.1128/JVI.01276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang JH, Choi JH, Jang H, Park JY, Han JW, Youn HD, Cho EJ. Histone chaperones cooperate to mediate Mef2-targeted transcriptional regulation during skeletal myogenesis. Biochem Biophys Res Commun. 2011;407:541–547. doi: 10.1016/j.bbrc.2011.03.055. [DOI] [PubMed] [Google Scholar]
- 21.Zhang R, Poustovoitov MV, Ye X, Santos HA, Chen W, Daganzo SM, Erzberger JP, Serebriiskii IG, Canutescu AA, Dunbrack RL, Pehrson JR, Berger JM, Kaufman PD, Adams PD. Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev Cell. 2005;8:19–30. doi: 10.1016/j.devcel.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Gallastegui E, Millan-Zambrano G, Terme JM, Chavez S, Jordan A. Chromatin reassembly factors are involved in transcriptional interference promoting HIV latency. J Virol. 2011;85:3187–3202. doi: 10.1128/JVI.01920-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye X, Zerlanko B, Zhang R, Somaiah N, Lipinski M, Salomoni P, Adams PD. Definition of pRB- and p53-dependent and -independent steps in HIRA/ASF1a-mediated formation of senescence-associated heterochromatin foci. Mol Cell Biol. 2007;27:2452–2465. doi: 10.1128/MCB.01592-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tagami H, Ray-Gallet D, Almouzni G, Nakatani Y. Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell. 2004;116:51–61. doi: 10.1016/s0092-8674(03)01064-x. [DOI] [PubMed] [Google Scholar]
- 25.Bonnefoy E, Orsi GA, Couble P, Loppin B. The essential role of Drosophila HIRA for de novo assembly of paternal chromatin at fertilization. PLoS Genet. 2007;3:1991–2006. doi: 10.1371/journal.pgen.0030182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green EM, Antczak AJ, Bailey AO, Franco AA, Wu KJ, Yates JR, 3rd, Kaufman PD. Replication-independent histone deposition by the HIR complex and Asf1. Curr Biol. 2005;15:2044–2049. doi: 10.1016/j.cub.2005.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prochasson P, Florens L, Swanson SK, Washburn MP, Workman JL. The HIR corepressor complex binds to nucleosomes generating a distinct protein/DNA complex resistant to remodeling by SWI/SNF. Genes Develop. 2005;19:2534–2539. doi: 10.1101/gad.1341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorain S, Demczuk S, Lamour V, Toth S, Aurias A, Roe BA, Lipinski M. Structural Organization of the WD repeat protein-encoding gene HIRA in the DiGeorge syndrome critical region of human chromosome 22. Genome Res. 1996;6:43–50. doi: 10.1101/gr.6.1.43. [DOI] [PubMed] [Google Scholar]
- 29.Sillje HH, Nigg EA. Identification of human Asf1 chromatin assembly factors as substrates of Tousled-like kinases. Curr Biol. 2001;11:1068–1073. doi: 10.1016/s0960-9822(01)00298-6. [DOI] [PubMed] [Google Scholar]
- 30.Sun L, Youn HD, Loh C, Stolow M, He W, Liu JO. Cabin 1, a negative regulator for calcineurin signaling in T lymphocytes. Immunity. 1998;8:703–711. doi: 10.1016/s1074-7613(00)80575-0. [DOI] [PubMed] [Google Scholar]
- 31.Jang H, Cho EJ, Youn HD. A new calcineurin inhibition domain in Cabin1. Biochem Biophys Res Commun. 2007;359:129–135. doi: 10.1016/j.bbrc.2007.05.066. [DOI] [PubMed] [Google Scholar]
- 32.Jang H, Choi SY, Cho EJ, Youn HD. Cabin1 restrains p53 activity on chromatin. Nat Struct Mol Biol. 2009;16:910–915. doi: 10.1038/nsmb.1657. [DOI] [PubMed] [Google Scholar]
- 33.Balaji S, Iyer LM, Aravind L. HPC2 and ubinuclein define a novel family of histone chaperones conserved throughout eukaryotes. Mol Biosyst. 2009;5:269–275. doi: 10.1039/b816424j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rai TS, Puri A, McBryan T, Hoffman J, Tang Y, Pchelintsev NA, van Tuyn J, Marmorstein R, Schultz DC, Adams PD. Human CABIN1 Is a Functional Member of the Human HIRA/UBN1/ASF1a Histone H3.3 Chaperone Complex. Mol Cell Biol. 2011;31:4107–4118. doi: 10.1128/MCB.05546-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aho S, Buisson M, Pajunen T, Ryoo YW, Giot JF, Gruffat H, Sergeant A, Uitto J. Ubinuclein, a novel nuclear protein interacting with cellular and viral transcription factors. J Cell Biol. 2000;148:1165–1176. doi: 10.1083/jcb.148.6.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aho S, Lupo J, Coly PA, Sabine A, Castellazzi M, Morand P, Sergeant A, Manet E, Boyer V, Gruffat H. Characterization of the ubinuclein protein as a new member of the nuclear and adhesion complex components (NACos) Biol Cell. 2009;101:319–334. doi: 10.1042/BC20080072. [DOI] [PubMed] [Google Scholar]
- 37.Banumathy G, Somaiah N, Zhang R, Tang Y, Hoffmann J, Andrake M, Ceulemans H, Schultz D, Marmorstein R, Adams PD. Human UBN1 is an ortholog of yeast Hpc2p and has an essential role in the HIRA/ASF1a chromatin-remodeling pathway in senescent cells. Mol Cell Biol. 2009;29:758–770. doi: 10.1128/MCB.01047-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang Y, Poustovoitov MV, Zhao K, Garfinkel M, Canutescu A, Dunbrack R, Adams PD, Marmorstein R. Structure of a human ASF1a-HIRA complex and insights into specificity of histone chaperone complex assembly. Nat Struct Mol Biol. 2006;13:921–929. doi: 10.1038/nsmb1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.English CM, Adkins MW, Carson JJ, Churchill ME, Tyler JK. Structural basis for the histone chaperone activity of Asf1. Cell. 2006;127:495–508. doi: 10.1016/j.cell.2006.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Natsume R, Eitoku M, Akai Y, Sano N, Horikoshi M, Senda T. Structure and function of the histone chaperone CIA/ASF1 complexed with histones H3 and H4. Nature. 2007;446:338–341. doi: 10.1038/nature05613. [DOI] [PubMed] [Google Scholar]
- 41.Laue TM, Shah BD, Ridgeway TM, Pelletier SL. Analytical Ultracentrifugation in Biochemistry and Polymer Science. In: Harding SE, Horton JC, Rowe AJ, editors. Royal Society of Chemistry; London, United Kingdom: 1992. pp. 90–125. [Google Scholar]
- 42.Cole JL. Analysis of heterogeneous interactions. Methods Enzymol. 2004;384:212–232. doi: 10.1016/S0076-6879(04)84013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall C, Nelson DM, Ye X, Baker K, DeCaprio JA, Seeholzer S, Lipinski M, Adams PD. HIRA, the human homologue of yeast Hir1p and Hir2p, is a novel cyclin-cdk2 substrate whose expression blocks S-phase progression. Mol Cell Biol. 2001;21:1854–1865. doi: 10.1128/MCB.21.5.1854-1865.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vishnoi N, Flaherty K, Hancock L, Ferreira M, Amin AD, Prochasson P. Separation-of-function mutation in HPC2, a member of the HIR complex in S. cerevisiae, results in derepression of the histone genes but does not confer cryptic TATA phenotypes. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbagrm.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson HE, Kagansky A, Wardle J, Rappsilber J, Allshire RC, Whitehall SK. Silencing mediated by the Schizosaccharomyces pombe HIRA complex is dependent upon the Hpc2-like protein, Hip4. PLoS One. 2010;5:e13488. doi: 10.1371/journal.pone.0013488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu H, Liu Y, Wang M, Fang J, Huang H, Yang N, Li Y, Wang J, Yao X, Shi Y, Li G, Xu RM. Structure of a CENP-A-histone H4 heterodimer in complex with chaperone HJURP. Genes Develop. 2011;25:901–906. doi: 10.1101/gad.2045111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.