Introduction
In developed countries1, pregnancy-related transfusion accounts for about 6 percent of red blood cell (RBC) units transfused. This means that, for example, in the UK as a whole, approximately 70,000 units of RBC are transfused to obstetric patients each year2. The pattern of blood usage is very different in countries in which diagnostic and treatment options are more limited, with 37 percent of transfusions being given to women with obstetric emergencies1.
Obstetric haemorrhage is a leading cause of maternal and perinatal mortality3. Placental abruption and placenta praevia/accreta can present as antepartum haemorrhage and are risk factors for post-partum haemorrhage (PPH). Uterine atony, inversion, retained products of conception and genital tract trauma all cause PPH and account for the majority of cases of major haemorrhage. Uterine rupture is an infrequent cause of haemorrhage and maternal death and can occur spontaneously or at the site of previous uterine surgery (caesarean section or retained placenta)3.
During the triennium 2006–2008, the rate of deaths from hemorrhage in the UK was 0.39 per 100,000 maternities4.
The incidence of severe bleeding in childbirth has been estimated in various surveys and is approximately one in 200 to 250 deliveries4. In developed countries treatment is generally effective with an approximate case fatality rate between 1 in 600 to 800 cases of obstetric bleeding5–7.
Various blood conservation techniques can reduce exposure to allogeneic blood. These include pre-operative autologous blood donation (PABD), acute normovolaemic haemodilution (ANH) and intra-operative blood salvage (IBS).
PABD may cause anaemia, does not eliminate transfusion risk, cannot be used in an emergency, is not acceptable to Jehovah’s Witnesses and should be reserved for exceptional circumstances8,9.
ANH may induce anaemia and cardiac failure, cannot be used in an emergency and may have a limited role in combination with other techniques9.
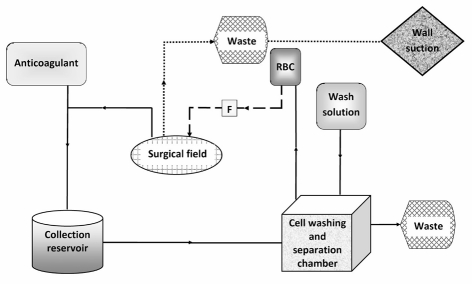
The use of IBS has increased substantially during the last two decades and the re-infusion of shed washed blood is commonplace in many types of surgical operations in which heavy blood loss is anticipated10,11. In this technique, blood shed at the time of surgery is collected and washed, and RBC are returned to the patient as an ongoing process (Figure 1)12,13. In skilled hands, blood salvage can be quickly set up and the final product returned to the patient within minutes of collection. In addition, this technique is also acceptable to some Jehovah’s Witnesses, provided the equipment is set up in continuity with the circulation (Figure 1)14, but consent needs to be obtained on an individual basis.
Figure 1.
Schematic representation of a blood salvage system with a double suction setup to reduce the possible contamination of the blood suctioned from the surgical field138. In this setup, one suction line is connected to the collection reservoir and used for the aspiration of blood to be sent back to the patient, while the other (dotted line) is connected to the regular wall suction and used for the aspiration of the contaminant. For Jehovah’s Witnesses, the machine is set up and operated with an “in-continuity” mode137. This means that the whole circuit is run through with saline and the re-transfusion bag is connected to the intravenous cannula before starting the salvage suction, thereby establishing a continuous circuit between the blood lost and the recipient vein. The dashed line represents infusion sent back to the patient, which is primed with saline before starting to complete continuity of the circuit.
RBC: Processed red blood cells; F: Leucocyte depletion filter.
However, the safety of IBS in obstetrics has been questioned and its introduction in this clinical arena has been delayed because of theoretical concerns stemming from the historical and ongoing dispute focused on the risk of maternal-foetal anti-Rh(D) alloimmunisation and the risk of contamination of the cell-saved blood with traces of amniotic fluid (AF)15,16. The objective of this article is to review the role of autologous blood conservation techniques in obstetrics focusing on the currently available evidence regarding IBS. An overview of current European regulatory requirements on IBS will also be provided.
Autologous blood and obstetrics
Pre-operative autologous blood donation
The last two decades have seen the birth17, development and subsequent gradual decline of PABD18–23. The re-evaluation of its role has recently been emphasised in guidelines that do not recommend this practice unless exceptional clinical circumstances require it and suggest, where suitable, the use of perioperative cell salvage techniques8,9. In the UK, this is reflected in a recent change in National Blood Service policy, such that this service is no longer provided (except on special request)24.
The greatest benefit of PABD is the reduction in risk of infections, although this has already been significantly reduced in recent years thanks to the introduction of molecular biology techniques used for the biological qualification of blood components20,25. Another advantage is the absence of alloantibodies associated with allogeneic red cell transfusion. Nevertheless there are also several potential side effects of PABD26, including a possible higher risk of untoward reactions due to the blood donation itself compared to allogeneic donations26,27, the risk of iatrogenic anaemia19, and as with allogeneic blood, the risk of clerical errors26,28, bacterial contamination26, circulatory overload, possible immune-modulating effects9,23–31 and, last but not least, the fact that autologous donors are more likely to receive any type of transfusion (allogeneic and/or autologous)19,32.
Although PABD was successfully and safely used in the past in obstetrics32–35, it is not recommended in most recent guidelines on the topic. Indeed, it is of very limited value as most cases of major obstetric bleeding cannot be foreseen and often require transfusion support with blood components (RBC, plasma, platelets) that cannot usually be guaranteed by blood donated pre-operatively3,36–38.
A further limitation of PABD is that it must be planned and started weeks before surgery without actually knowing the exact amount of blood that will be needed by the individual patient during surgery. In contrast, IBS can be used only if the need for red cell transfusion during surgery becomes imminent and real39. Clinical data suggest that PABD is most effective if the haematocrit is between 35% and 40% and at least 4 weeks between the (last) donation and surgery are allowed39–45. Otherwise, it will only consist of a simple transfer of red cells from the patient into a plastic bag with a doubtful benefit for the patient. PABD has been shown to be really efficacious in increasing the RBC mass of the patient only following the physiological principles of erythropoiesis39–45: a strong anaemic stimulus and sufficient time for regeneration of the red cell mass.
A comparison of the efficacy, in terms of the increase of red cell mass thanks to the autologous blood saving technique, was carried out in orthopaedic patients: based on a mathematical model, this comparison showed that PABD can be superior to IBS only if there is sufficient time before surgery to regenerate a red cell mass of approximately 400 mL39. To the best of our knowledge, similar data are not available for obstetrics.
Indeed, because of scheduling or other logistic obstacles, “correct” autologous donation in obstetrics might not be easy and other blood saving techniques are, therefore, needed to reduce allogeneic blood transfusion.
PABD can be combined with IBS46 for the management of selected patients with alloantibodies for whom allogeneic blood may be in short supply8,9,38.
Acute normovolaemic haemodilution
ANH is an autologous blood transfusion technique that was introduced in the 1970s47–49. It consists of withdrawing at least 3–4 units of autologous blood immediately before elective surgery. During the haemodilution process, normovolaemia is maintained by the administration of crystalloid and colloid replacement fluids50–54. Colloidal solutions are preferred because they preserve tissue oxygenation better, and crystalloids can result in anaemia and an increased need for transfusions55. ANH is usually performed after the induction of anaesthesia, immediately before surgery.
Patients suitable for ANH should have a haemoglobin concentration that is at least the highest value of the normal range and the same clinical requirements as those needed for PABD9,50,54. The predicted blood loss must be above 50% of the total blood volume or, in any case, not less than 1,500 mL9,51,52,56–58.
The rationale for the use of ANH is that, if the haematocrit level is lowered before the blood loss, fewer RBC will be lost when the patient bleeds54.
All the blood units need to be clearly labelled with the patient’s identification details and the date/time of collection. They should not leave the operating room, can be stored at room temperature and should be transfused within 6 hours of collection.
Blood units are reinfused in the reverse order of collection, because the first unit collected, and therefore the last unit transfused, has the highest haematocrit level and concentration of coagulation factors and platelets9,52,54,59; in addition, if for certain patients and selected surgical procedures ANH is used in conjunction with IBS, it is advisable to re-infuse the IBS red cell units before the ANH units of whole blood. Various formulas and nomograms have been proposed to determine the amount of blood that should be removed to reach the target haematocrit or target haemoglobin concentration52, but the equation published by Gross in 1983 is the simplest one to apply in the acute pre-operative setting60.
There is, however, still some doubt as to whether ANH is really effective in reducing the need for allogeneic blood transfusion61. Several clinical studies, including prospective randomised trials, have shown that ANH can reduce the need for allogeneic transfusion in patients undergoing elective heart, orthopaedic (knee replacement), abdominal, vascular, urological, maxillofacial, and hepatic surgery, as well as patients undergoing surgery following burns62–73.
In pregnancy, despite the large rise in cardiac output and the consequent concern regarding possible cardiac failure or placental insufficiency caused by ANH, this technique was well tolerated in healthy, term parturients undergoing elective caesarean section in whom major haemorrhage was predicted74. It has been used safely in pregnant women at high risk of excessive intra-operative blood loss because of placenta percreta and should be considered for obstetric patients who, because of religious convictions, will not accept blood products75. The successful, combined use of ANH, IBS and pulse cardiac output haemodynamic monitoring for caesarean hysterectomy in a Jehovah’s Witness with placenta percreta was recently reported76.
Studies carried out in non-obstetric clinical settings either did not show any substantial benefit or even revealed an increase in the use of allogeneic blood due to ANH77–79. Doubts regarding the real benefits of the use of this procedure have been confirmed by some meta-analyses80–83, which found little evidence to suggest that ANH alone can save significant amounts of allogeneic blood.
In fact, the relative risk of receiving allogeneic blood in any peri-operative phase is not significantly reduced by resorting to ANH82,84. Similarly, this risk is not reduced even if ANH is compared to other pharmacological blood-saving techniques such as tranexamic acid. Moreover, an increased relative risk of re-intervention because of haemorrhage85, as well as a lack of information regarding the safety of ANH have emerged80,82,83,85.
The routine use of ANH as an allogeneic blood-saving technique is not, therefore, recommended2,9,80–83,86. Likewise, also in obstetrics, ANH should be considered only for selected patients and used with restrictive transfusion threshold or protocols85. It should be part of an integrated and multimodal approach based on local institutional cooperation aiming at reducing the use of allogeneic blood and overcoming the limitations of any single blood-saving intervention55.
Intra-operative blood salvage
Historically, the idea of using IBS in obstetrics can be traced back to James Blundell87 and William Highmore88 who, in 1818 and 1874, respectively, thought of re-infusing shed blood (defibrinated and warmed)88 to treat PPH.
The current increased demand for blood coincides with a significant reduction in blood donations89,90 and strategies to contain allogeneic transfusion requirements are, therefore, attracting growing attention also by specialists in transfusion medicine9. In obstetrics, PABD and ANH have never proven successful for the above-mentioned reasons. In contrast, IBS is being used for an increasing number of obstetric patients.
In the USA, the use of autologous blood salvage devices has been advocated by the American College of Obstetrics and Gynecology for women in whom massive haemorrhage is anticipated such as in the presence of conditions known to be associated with placenta accreta91,92. The American Society of Anesthesiologist Task Force on Obstetric Anesthesia has recommended that IBS should be considered, if available, “in cases of intractable haemorrhage when banked blood is not available or the patient refuses banked blood”93.
In Italy, the recently issued recommendations on “Peri-operative Blood Transfusion” of the Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) suggest that IBS should be used in obstetrics for the emergency management of major bleeding or for cases at increased risk of major haemorrhage, provided a leucodepletion filter can be used for the transfusion of salvaged blood9.
In the UK, the use of blood salvage has been endorsed by the Confidential Enquiry into Maternal and Child Health4 and the National Institute of Clinical Excellence94. The Obstetric Anaesthetists’ Association and the Association of Anaesthetists of Great Britain and Ireland identified emergency use (major obstetric haemorrhage at caesarean section, laparotomy for PPH, genital tract trauma, etc.) and elective use of IBS (expected haemorrhage during caesarean section, e.g. because of placenta praevia, placenta accreta, large fibroid uterus, patients who refuse allogeneic blood, etc.)95,96. The National Blood Service Working Party on Autologous Transfusion has labelled IBS as “the most valuable form of autologous transfusion” and has claimed that UK hospital trust managers should be encouraged to establish its use2. The UK Cell Salvage Action Group suggested that IBS should be available for obstetric cases in which there is the potential for massive haemorrhage97. These cases include: (i) emergency situations, such as ruptured ectopic pregnancy, and intra-partum or post-partum haemorrhage requiring surgical intervention; (ii) elective situations, such as patients with an anticipated blood loss of more than 1,000 mL (e.g. abnormal placentation, large uterine fibroids, and other predictable causes of major haemorrhage), and patients who for religious or other reasons refuse allogeneic blood and have consented to the use of IBS in bleeding situations or in significant anaemia.
In addition, several recent review articles include IBS among the available therapeutic strategies for the management of obstetric haemorrhage3,98–102. Indeed, it should be remembered that uterine blood flow at term (750 mL/min) gives rise to potential sudden massive blood loss103.
Implementation of cell salvage could also be considered when the loss of smaller amounts of blood are anticipated104. Because of the difficulty of accurately predicting substantial blood loss, for the majority of cases it would be appropriate to set up the IBS device in a “stand-by” mode. This “standby” mode is simply the collection system which includes a collection reservoir, a suction line and an anticoagulant. The cost of a “stand-by” set-up is comparable to the cost of reagents for cross-matching two units of allogeneic blood104. If enough blood loss occurs, the expensive components of the system can be used and blood processed13. An in-depth discussion concerning all the financial aspects for the provision of cell salvage services is well beyond the scope of this article, as economic analyses of cell salvage have already been published elsewhere105–109. However, although IBS is an expensive technology, once the capital costs of the machine are accounted for and the personnel trained, its cost compares favourably to that of allogeneic RBC, with the savings increasing as the RBC transfusion volume increases. Assuming that every salvaged blood unit replaces an allogeneic unit, Waters et al. showed that the average saving per unit was $110.54108. The implementation of a cell salvage programme could, therefore, be financially beneficial for a large, tertiary care hospital. In any case, the cost of the cell salvage system can be reduced through the use of a “stand-by” system whether the hospital outsources the IBS to other care providers or provides it through an in-house service.
Clinical data
Indications
In the studies analysed46,76,110–135, the indications for IBS are generally based on well-recognised risk factors for obstetric haemorrhage and circumstances in which significant bleeding could have serious consequences136.
There is now substantial experience with the use of IBS in obstetrics in the UK137. This technique has been prevalently used in elective and emergency caesarean sections114,116,117,120,127,130–134, also for the management a patient with alloantibodies against a high-frequency antigen (Ata)46, a patient with Lub antibodies132, and a patient with β-thalassaemia intermedia and placenta accreta123. Its use in other cases of abnormal placentation (placenta praevia, accreta, percreta) have been reported76,111,112,122,124,125,128–132 and there are also descriptions of IBS in patients with sickle-cell trait129 and in a clinical case in which IBS was coupled with acute normovolaemic haemodilution76. In several circumstances IBS was used for Jehovah’s Witnesses76,119,121,122,124,125,128,132 and/or obstetric bleeding110,119,122,129.
In the series of patients reported by King and co-workers130, the indications for IBS included suspected placental abruption, multiple pregnancy, multiple repeat caesarean sections (three or more), caesarean section at full dilatation, low pre-operative haemoglobin (which was not defined) and cases at the discretion of theatre staff. Therefore, IBS was used in patients undergoing caesarean section whenever the blood loss was likely to necessitate blood transfusion. For some patients multiple indications were recorded including fibroids, high body mass index, pregnancy-induced hypertension, adhesions and low platelet count.
Parry et al. also considered IBS in patients with failed induction of labour and failed instrumental delivery131; while McDonnell et al. also used it for ectopic pregnancies and massive fibroids132.
A recent retrospective study showed that IBS was apparently used more frequently in patients at higher risk of obstetric haemorrhage such as multiparous women and subjects who had undergone a higher number of caesarean sections or multiple pregnancies. Additional risk factors for the use of cell-saving procedures were multiple fibroids, previous myomectomy or PPH, medical conditions (immune thrombocytopenic purpura), and previous ruptured uterus135.
Contraindications
The list of contraindications to blood recovery is extensive138. Anything that results in RBC lysis upon administration of the salvaged blood is defined as a definite or absolute contraindication104. Most contraindications are, however, relative and few data exist to support the possible danger they cause. It is now accepted that three areas exist where IBS needs to be used with caution and following necessary risk-benefit analysis. These include blood aspirated from contaminated or septic wounds or obstetric/surgical fields, and areas of malignancy. Certain procedural measures reduce the amniotic fluid load to the mother to a minimum. These include: (i) the use of two suction devices to avoid aspiration of amniotic fluid into the collection reservoir (Figure 1); (ii) delay of salvage until after removal of the foetus, placenta and amniotic fluid139; (iii) extra washing; (iv) not processing partial bowls, to ensure the best washing efficiency, and (v) the use of leucodepletion filters.
Relative contraindications to IBS include sickle-cell disease and thalassaemia104. The use of IBS in patients with sickle-cell disease has generally been avoided because the hypoxic environment of the reservoir was thought to result in RBC sickling and re-infusion of sickled blood could precipitate a sickle-cell crisis104. However, blood films of salvaged blood from two patients with sickle-cell trait who underwent elective caesarean section showed 15–20 percent of sickled RBC in one patient and 20 percent altered129, but not sickled, in the other: transfusion of autologous salvaged blood was performed in both cases without side effects.
β-thalassemia RBC have increased rigidity and reduced membrane stability which may make them more susceptible to damage from the shear forces they are exposed to during IBS. Nevertheless, increased haemolysis was not evidenced in a case reported by Waters et al.123.
Efficacy
Over 800 IBS procedures have been documented in obstetrics and more than 400 patients have been transfused with salvaged blood (Table I)46,76,110–117,119–135,140, to date. The data available from case reports on transfused salvaged blood refer to amounts ranging from 126 to 12,600 mL (median: 500 mL)46,76,110,113,119,121–125,128,129,134. This is confirmed by six studies including almost 300 patients who were transfused with mean or median amounts of salvaged blood not exceeding 2 units114,117,120,130–132.
Table I.
Clinical studies on cell salvage in obstetrics.
| Year/Author/Publication type | Number of cell-saving procedures (826) | Number of subjects transfused (446) | Re-transfusion rate of salvaged blood (%) | Clinical setting |
|---|---|---|---|---|
| 1983/Keeling115/RS | 43# | Not stated | Not stated | HY, CS, AP |
| 1988/Grimes110/CR | 2 | 2 | 100 | AP/PPH |
| 1990/Zichella116/CR | 8 | 8 | 100 | CS |
| 1993/Jackson117/RS | 119 | 64 | 53 | CS |
| 1996/O’Brien111/RS | 5 | 5 | 100 | Placenta percreta |
| 1998/Rees119/CR | 1 | 1 | 100 | CS/PPH/JW |
| 1998/Rainaldi120/Prospective controlled trial | 34 | 34 | 100 | CS |
| 1998/Rebarber114/Retrospective cohort | 186 | 139 | 74 | CS |
| 1999/Potter113/CR | 1 | 1 | 100 | CS/placenta previa |
| 2000/Oei121/CR | 1 | 1 | 100 | CS/JW/preeclampsia/HELLP |
| 2002/Catling122/CR | 4 | 4 | 100 | Extrauterine placenta/CS PPH CS/JW CS/JW |
| 2003/Waters123/CR | 1 | 1 | 100 | CS/placenta accreta/β-thalassemia intermedia |
| 2003/de Souza124/CR | 1 | 1 | 100 | Placenta praevia/CS/JW |
| 2004/McGurgan125/CR | 1 | 1 | 100 | Placenta praevia/CS/JW |
| 2005/Boonstra46/CR | 1 | 1 | 100 | CS |
| 2007/Teig126/RS* | 182 | 119 | 65 | Not stated |
| 2008/Harkness127/RS** | 7 | 0 | 0 | CS |
| 2008/Nagy76/CR*** | 1 | 1 | 100 | Placenta percreta/JW |
| 2009/Araki128/CR | 1 | 1 | 100 | Placenta accreta |
| 2009/Okunuga129/CR | 2 | 2 | 100 | Placenta praevia/JW/SCT Placenta accreta/SCT |
| 2009/King130/RS | 46 | 19 | 41 | Placenta praevia, suspected placental abruption, multiple pregnancy, multiple repeat CS, previous PPH, refusal of blood transfusion, CS at full dilatation, low preoperative Hb, at the discretion of the theatre team |
| 2009/Eller112/Retrospective cohort | 1 | Not stated | Not stated | Placenta accreta |
| 2010/Parry131/RS | 47 | 17 | 36 | Patients at high risk of haemorrhage (placenta praevia, suspected placental abruption, multiple pregnancy, multiple repeat CS, previous PPH, refusal of blood transfusion, CS at full dilatation, low preoperative Hb, at the discretion of the theatre team, failed induction of labour, failed instrumental delivery) |
| 2010/McDonnell132/Prospective cohort | 51 | 21 | 41 | Placenta praevia, CS, ectopic pregnancy, JW |
| 2010/Sreelakshmi133/CR | 1 | 1 | 100 | CS |
| 2010/Kessack134/CR | 1 | 1 | 100 | CS |
| Year/Author/Publication type | Adverse events | Use of a leucocyte depletion filter | Amount (mL/unit) of autologous blood transfused (T) or yielded (Y) | Units of allogeneic red cells transfused |
|---|---|---|---|---|
| 2010/Hatfield140/CR**** | 1 | 1 | 100 | Placenta accreta |
| 2010/Malik135/RS | 77 | Not stated | Not stated | Placenta praevia, JW |
| 1983/Keeling115/RS | No | No | 700 mL (mean/Y) | Not stated |
| 1988/Grimes110/CR | No | No | AP: 1,700 mL (T) PPH: 2,200 mL (T) |
None |
| 1990/Zichella116/CR | No | No | Not stated | Not stated |
| 1993/Jackson117/RS | No | No | 2 units (mean/T) | Not stated |
| 1996/O’Brien111/RS | Not stated | No | Not stated | Not stated |
| 1998/Rees119/CR | DIC (?) | No | 1,400 mL (T) | None |
| 1998/Rainaldi120/Prospective controlled trial | No | No | 225 mL ±18 (mean/T) | Not stated§ |
| 1998/Rebarber114/Retrospective cohort | Heparin toxicity (n = 1) | No | 250 mL (range: 125–4,750) 543 mL (range: 225–1,160) 450 mL (range: 200–11,250) (median/T) |
Not stated |
| 1999/Potter113/CR | Endomyometritis | No | 250 mL (T) | None |
| 2000/Oei121/CR | Cardiac arrest/death | No | 200 mL (T) | None |
| 2002/Catling122/CR | ARDS/pneumonia | Yes | 1,400 mL (T) | 22 |
| Chest infection | 270 mL (T) | 10 | ||
| Anaemia (n=1) | 350 mL (T) | None | ||
| 245 mL (T) | None | |||
| 2003/Waters123/CR | Anaemia | Yes | 2,250 mL (T) | None |
| 2003/de Souza124/CR | No | No | 350 mL (T) | None |
| 2004/McGurgan125/CR | No | Yes | 400 mL (T) | None |
| 2005/Boonstra46/CR | No | No | 330 mL (T) | None |
| 2007/Teig126/RS* | Not stated | Not stated | Not stated | Not stated |
| 2008/Harkness127/RS** | No | Not stated | 0 | None |
| 2008/Nagy76/CR*** | No | No | 1,769 mL (T) +1,200 mL of whole blood*** |
None |
| 2009/Araki128/CR | No | No | 12,600 mL (T) | 60 |
| 2009/Okunuga129/CR | No | No | 126 mL (T) 1,600 mL (T) |
0 6 |
| 2009/King130/RS | No | Yes | 300 mL (range: 200–800) (median/T) | 2 in 4 patients 24 in 1 patient |
| 2009/Eller112 Retrospective cohort | Not stated | Not stated | Not stated | Not stated |
| 2010/Parry131/RS | No | Not stated | 229 mL (range: 125–534) (mean/T) | Not stated |
| 2010/McDonnell132/Prospective cohort | Hypotension | Yes | 359 mL (range: 60–1,300) (median/T) | 3 (mean) (range: 2–14) |
| 2010/Sreelakshmi133/CR | Hypotension | Yes | 1,000 mL (Y) | 10 |
| 2010/Kessack134/CR | Hypotension | Yes | 600 mL (T) | 9 |
| 2010/Hatfield140/CR**** | Post-bypass bleeding | No | 2,500 mL**** (T) | 5 |
| 2010/Malik135/RS | No | Yes | 95.5 mL (range: 0–1,800) (mean/Y) 13 units (total number/T) |
31 (total number) |
Legend:
includes a not stated number of exploratory laparatomies;
the use of allogeneic blood transfusion was significantly lower in patients submitted to cell salvage;
survey of cell-salvage use (includes 63 episodes of cell-salvage set-up without processing blood);
survey of cell-salvage use;
combined use of acute normovolaemic haemodilution and cell-salvage;
use of standard cardiopulmonary bypass machine and transfusion of unwashed whole blood;
ARDS: adult respiratory distress syndrome; AP: abdominal pregnancy; CR: case report; CS: caesarean section; DIC: disseminated intravascular coagulation; HY: hysterectomy; JW: Jehovah’s witness; PPH: post-partum haemorrhage; RS: retrospective series; SCT: sickle-cell trait.
An exception is represented only by a cohort of six patients who, in a retrospective study, received a median of 543 mL of salvaged packed RBC (Table I)114. In 46 percent of the cell-saving procedures (380/826) not enough blood was lost for it be processed or the salvaged blood was not enough to be transfused (Table I). In seven large studies (Table I) the re-transfusion rates of autologous salvaged blood ranged from 36 to 100%114,117,120,126,130–132.
Data are available on the units of allogeneic RBC transfused in several case reports46,76,110,113,119,121–125, 127–129,133,134,140, but only in three of the larger studies published (Table I)130,132,135.
The efficacy of IBS -defined as avoidance/reduction of allogeneic blood use141,142- has been evaluated in only five studies, in which the percentages of patients who completely avoided allogeneic blood ranged from 6 to 97.1 percent114,120,130–132.
In the three-centre historical cohort study by Rebarber et al. only a small percentage of women (11/186 – 6%) completely avoided allogeneic RBC transfusion thanks to the transfusion of salvaged blood during caesarean section114. The Authors did not provide data to explain the large use of allogeneic blood in their study. Other potential flaws were the different models of cell saver (Haemonetics Corporation, Braintree, MA, USA) used over the 9-year study period and the variables regarding at which point during surgery IBS was started. In addition, each centre had its own method for selecting the medical records of the patients transfused with salvaged blood to be matched to the controls without autotransfusion. This could explain the differences between the two groups. In fact, most of the women who received salvaged blood appeared to be high-risk patients.
A much larger percentage of women avoided allogeneic RBC transfusion (33/34 – 97.1%) in a small prospective, non-blinded, randomised controlled trial assessing IBS carried out with the Dideco Autotrans BT 795 machine (Mirandola, Modena, Italy) during caesarean section120. In fact, a significant difference in allogeneic transfusion rate between the IBS group and the control group (2.9% versus 23.5% - p=0.01) was reported. This major difference is surprising143 as the overall incidence of allogeneic blood transfusion in the control group is much higher than the 1.8 percent reported in a recent retrospective study of 11,781 patients undergoing caesarean section144.
The results also revealed higher post-operative haemoglobin levels and shorter hospitalisation in the study group. The findings may be biased for several reasons: the threshold for post-operative allogeneic transfusion was not pre-defined, the proportion of patients with conditions normally associated with increased intra-operative blood loss was higher in the treatment group, the study was not blinded and there were imbalances in age, weight and haemoglobin level between the groups.
In the series of 46 patients reported by King et al.130, the Haemonetics Cell Saver 5 processed and returned blood to 19 patients: 74 per cent of them (14/19) avoided allogeneic RBC transfusion.
Pre-operative haemoglobin was significantly lower in the 26 percent of women who received both processed and allogeneic blood. Blood loss and pre-operative haemoglobin levels appear to be major factors affecting the re-infusion of salvaged blood. The preliminary data from this study (reduction of the percentage of all theatre cases receiving allogeneic transfusion for the equivalent period in the preceding year) seem to suggest that IBS might have an impact on the number of patients needing allogeneic blood, although the association was not statistically significant.
The percentage of patients who did not receive allogeneic RBC transfusion thanks to IBS was slightly higher (14/17 – 82%) in an audit reported by Parry and collaborators who avoided the use of an estimated 14 units of allogeneic RBC131.
In a prospective cohort study carried out over a 28-month period, blood salvaged with a Cell Saver 5 was re-transfused into 21 women and 33.3 percent of them (7/21) avoided allogeneic RBC132. Also in this study blood loss and pre-operative haemoglobin levels differentiated the patients who did and those who did not receive salvaged blood; 79 percent of the women who did not avoid allogeneic RBC transfusion (11/14) had a pre-operative haemoglobin concentration lower than 120 g/L.
In the above studies, 79 out of 277 (28.5%) patients avoided allogeneic RBC transfusion. However, only limited, not significant conclusions can be drawn on the impact of IBS on the consumption of allogeneic blood supply, due to the small number of patients included in the studies and to their variability.
In 2007, Fong et al. sought to determine to what extent intra-operative salvaged RBC might theoretically reduce exposure to appropriately transfused allogeneic erythrocytes in a retrospective cohort case-control study of almost 12,000 patients who underwent caesarean section144. Though no power analysis was carried out, had IBS been performed, the exposure to allogeneic blood could have been reduced in 48.6 percent of patients. Theoretically, based on best, average, and worst RBC recovery scenarios, 25.1 percent, 21.2 percent, and 14.5 percent of the appropriately transfused patients, respectively, could have completely avoided allogeneic RBC transfusion.
Unfortunately, a randomised controlled trial to establish the efficacy of IBS in caesarean sections still needs to be undertaken and would need to recruit approximately 4,500 caesarean deliveries to be adequately powered to detect a 33 percent proportional reduction of the use of allogeneic blood141,142.
Safety
Despite the concerns on the risks of amniotic fluid embolism (AFE) and Rhesus immunisation, many studies46,76,110–135, mainly retrospective series and case reports, have been carried out on obstetric patients. Some of them were large series of heterogeneous patients including obstetric subjects115,118, other clinical studies combined quality control of shed blood115,116,120,129,132.
The current available literature focuses mainly on safety with limited information about the outcomes of the patients treated114. No complications as a result of salvaged blood were reported in most of the studies.
In 1983, a retrospective review of 725 mainly cardiovascular patients, also included an unstated number of ectopic pregnancies and caesarean sections among the subgroup of 43 miscellaneous patients115. In no case was mortality or morbidity attributed to autologous blood transfusion and no evidence of major coagulopathy, systemic sepsis, air or particulate embolism or renal failure was detected. A retrospective database review of over 36,000 cases of IBS from 1978 to 1996 included an indefinite number of obstetric-gynaecology patients118. Overall, “some bleeding and minor clotting disorders or abnormalities were not uncommon”, though the authors did not specify what they meant by “minor clotting disorder”. The incidence of disseminated intravascular coagulation (DIC) and adult respiratory distress syndrome (ARDS) was 0.05 percent. All the 18 deaths occurred in non-obstetric patients and were not attributed to IBS, but were the result of a complex interaction of shock, hypothermia and massive transfusion.
The retrospective multicenter study by Rebarber et al. was the first to assess the safety of IBS during caesarean section and revealed no statistically significant differences between the autotransfused patients and the group receiving allogeneic blood transfusion only114. The outcome variables analysed were duration of post-partum hospitalisation, need for ventilator support, occurrence of DIC and rate of post-operative infections. No case of ARDS or AFE was identified in either group.
In the cases of abnormal placentation the procedure showed a good level of safety and usefulness in recent retrospective series130 and prospective cohort studies132, and in several case reports76,124,128,129,134. The side effects reported in these patients, who recovered fully, do not seem directly linked to IBS. They included: (i) endomyometritis, attributed to an inability to express the uterus at the end of the surgical procedure113; (ii) post-operative ARDS and staphylococcal pneumonia, in a patient who was also massively transfused with allogeneic blood components122; (iii) moderate anaemia in a patient with β-thalassaemia intermedia123; and (iv) unexplained hypotension during the intra-operative transfusion of salvaged blood during a complex operation for placenta percreta132.
Very recently a case of placenta accreta was successfully managed with caesarean hysterectomy and an unconventional approach of synchronous autotransfusion using a standard cardiopulmonary bypass machine140. The patient was not placed on bypass and the suction devices of the machine aspirated blood from the surgical field, avoiding amniotic fluid. The unwashed blood was filtered through a microaggregate filter prior to reinfusion and no side effect was reported.
Other reported side effects include DIC119, which was described in a case report in which the reinfused blood might have been one of the several factors contributing to the coagulopathy, a presumptive case of heparin toxicity reversed with protamine sulphate administration114, in a case in which a large volume of salvaged blood (45 units) was reinfused, and two additional cases of hypotension associated with the use of leucodepletion filters for the transfusion of salvaged blood133,134. The physical characteristics of the filter might have been implicated in the development of hypotension through the production and release of bradykinin, but, unfortunately, in neither case was transfusion attempted without filtration145.
In 2009, three cases of clinical adverse events, all regarding hypotensive reactions related to re-infusion of blood salvaged intra-operatively, were included in the Serious Hazards Of Transfusion (SHOT) report. Common factors in two cases were the use of acid-citrate-dextrose as an anticoagulant and the use of a leucodepletion filter during re-infusion of the salvaged blood146.
The above case reports and the SHOT seem to provide circumstantial evidence that leucodepletion filters may occasionally cause hypotension. However, more robust data from haemovigilance studies are needed to change the current recommended practice of using these filters to remove foetal contaminants9,94. In the UK, a national survey of obstetric cell salvage availability was endorsed in 2010 by both the Cell Salvage Action Group and the Obstetric Anaesthetists’ Association147. It is to be hoped that further data on the real spread of IBS in obstetric units, on the use of leucodepletion filters and on associated problems will be available in the near future.
Theoretically, the greatest fear accompanying amniotic fluid contamination is its potential to cause iatrogenic AFE96. However, the only fatal obstetric case was published in 2000 and, although clinically attributed to AFE by the authors121, this death has not generally been accepted in the literature as secondary to AFE as the patient was at high risk in terms of obstetric co-morbidity and not enough information was provided to determine whether the salvaged blood, filtered or not, had any causal role16.
The contamination risk of salvaged blood
Salvaged blood in obstetrics can be contaminated by bacteria, amniotic fluid and foetal blood148.
Bacterial contamination
Bacterial contamination of cell salvaged blood appears to be common. Several studies in different fields of surgery have reported bacterial contamination in cases of IBS with a frequency of positive blood cultures ranging from 3 to 97% based on the different kinds of surgery149–154. This indicates that the cell saver itself can be insufficient to remove contaminating bacteria from the processed blood. However, limited or absent clinical signs or laboratory findings of bacteraemia were found in the patients who received the culture-positive autologous blood.
In this regard, several experimental studies have shown the role of some types of leucodepletion filters in reducing bacterial load in deliberately contaminated blood155,157. Waters et al. found a significant (97.6%–100%) removal of inoculated bacteria by cell salvage washing and leucocyte depletion filtration of contaminated blood158.
More recently, it was suggested that the capacity of an additional leucodepletion filter to eliminate bacterial contaminants of shed blood during orthotopic liver transplantation might help to reduce the post-operative bacterial infections159.
Four possible mechanisms have been proposed to explain the removal of bacteria by leucodepletion filters154,160–162: (i) complement-mediated bacterial killing enhanced by filtration, (ii) adherence of bacteria to leucocyte surfaces retained within the filter, (iii) the deformability of infected cells and the disintegration of cells in the filter, and (iv) direct removal of bacteria by the filter media. It is likely that more than one mechanism accounts for the reduction of bacteria in contaminated blood.
At present, the importance of any remaining bacteria is unknown148. Broad-spectrum antibiotics are routinely used to manage the bacteraemia commonly related to most surgical procedures. Several studies have suggested that these drugs can provide additional safety when contaminated blood is re-infused153,163,164.
In obstetrics, bacterial contamination in post-wash, unfiltered samples has been found to be minimal and clinically irrelevant120,165, and leucocyte depletion filtering of cell-salvaged blood obtained from women undergoing caesarean section has been shown to reduce significantly, among the other particulate contaminants, bacterial contamination166.
Amniotic fluid embolism and alloimmunisation
Selecting specific parameters, several laboratory-based studies have focused on quality control of recovered blood in order to address the two main safety concerns regarding the use of salvaged and washed RBC in obstetrics (Table II)115,116,120,132,165–172: AFE and alloimmunisation. Most of the in vitro clearance studies analysed salvaged and processed blood without returning it to the patient as a preliminary process prior to its possible routine use in clinical practice120,132,165–172.
Quality control of the re-infused blood was only performed in three studies and included: (i) haematocrit, haemolysis and bacterial contamination115; (ii) coagulant activity and phosphatidylglycerol116; and (iii) a search for sickled RBC129.
Amniotic fluid contamination
AFE remains one of the greatest enigmas in obstetrics173. The presence of cells of foetal origin in maternal blood was regarded as a marker of AFE, but foetal cells and amniotic fluid material can be commonly found in the maternal circulation166,173–177. AFE is no longer regarded as an embolic disease and seems to be a rare anaphylactoid reaction to the foetal antigen rather than a predictable response to exposure to amniotic fluid178. In 1995, Clark and co-workers suggested that AFE was more of an immunological than embolic phenomenon and recommended that the name of the syndrome should be changed from AFE to “anaphylactoid syndrome of pregnancy”179. Risk factors have now been more clearly identified and include age, ethnic group, induction of labour and caesarean delivery180,181. The pathophysiology of the condition remains controversial, as does the question of which, if any174,175,179, of the components of amniotic fluid might be the primary trigger, and even whether amniotic fluid has a role at all15. However, it has been assumed that in order for “spontaneous” AFE to occur there must be a breach in the physical barriers between the maternal and foetal compartments182. Therefore, rigorous quality control of IBS, as suggested in the recently issued Peri-operative Standards183, is necessary to provide a safe blood product and to reduce the risk to the mother of an “iatrogenic” AFE, caused by the presence of foetal contaminants in re-infused salvaged blood.
The reported incidence of AFE is low and varies 10-fold between 1.3 and 12.5 per 100,000 pregnancies8,180,181,184. It is not an easy task to prove the complete safety of IBS with regards to the theoretical risk of AFE: a prospective randomised study with a power of 80% to show that IBS is not likely to increase this incidence 5-fold would require a population of 27,400 to 264,000 patients. These figures would become, respectively, 35,400 and 340,500 if the power of the designed trial were to be set at 90%. Therefore, to assess the degree of contamination of salvaged blood by possible aetiological triggers of AFE, surrogate markers for the various components of amniotic fluid were measured116,120,132,165–172. However, none of the current methods of detection of elements of amniotic fluid has a 100 percent sensitivity16.
Studies carried out with different cell saver instruments yielded different results. In 1989, Durand et al. were unable to remove all the foetal debris from salvaged blood165. Other studies showed that salvaged blood contained only a small amount of phosphatidylglycerol due to haemolysis and did not have coagulant activity116, or that α-foetoprotein was undetectable in all post-wash samples while squamous cells were reduced but still present168.
In 1996, despite the use of a separate suction device to avoid the aspiration of amniotic fluid, α-foetoprotein and tissue factor were not fully eliminated169. In contrast, different instruments completely eliminated tissue factor activity170, coagulant activity and squamous cells120, and reduced α-fetoprotein to below the lower limits of detection167.
In 1999, the efficacy of a leucodepletion filter (Pall RC 100, Pall Biomedical, Portsmouth, UK) in removing the various components of amniotic fluid from salvaged blood was examined for the first time171. α-foetoprotein was significantly reduced in post-wash samples but unchanged following filtration, while leucocytes and trophoblasts were completely absent in post-filtration samples. Filtration was not effective in removing squamous cells from almost half of the post-filtration samples.
Different results were reported by Waters et al.166 using the same cell saver and a newer generation filter (LeukoGuard RS, Pall Biomedical Products Co., East Hills, NY, USA)185,186. A significant reduction of squamous cells and of lamellar body concentration was obtained. Potassium levels were also significantly lower in the post-filtration samples. The marked difference in the clearance of squamous cells between these studies can be really attributed to the use of different filters, which vary in design features such as fibre diameter and charge15.
The efficiency of the above-mentioned leucodepletion filter was confirmed in 2008, using only one sucker172, and in 2010, using the two-sucker technique, as the vast majority of units currently do132.
The differences between the results of the reported studies may be due to differences between cell saver devices, sizes of processing bowls, amounts of saline wash, and degree of technical expertise in using the devices10. Many of the above reports do not contain sufficient information to evaluate these differences.
Notwithstanding their heterogeneity, most studies reported on consistently/significantly decreased concentrations of contaminants. However, as the non-cellular fraction is greatly reduced in processed salvaged blood, the total content of contaminants is further reduced. Therefore, IBS yields RBC substantially lacking protein elements of amniotic fluid132,167–169,171,172, without significant bacterial contamination115,120,165,166, with a consistent removal of free haemoglobin and potassium120,166,167, and with a debris concentration equivalent to that of maternal venous blood166. Undoubtedly, leucodepletion filtering of salvaged blood prior to transfusion is a consistent and significant step towards safety.
Red blood cell contamination
A combination of cell-salvage washing and filtration appears to produce a blood product comparable to the original maternal blood, with the exception of the contamination by foetal RBC120,132,165–167,171,172. In fact, despite the addition of leucodepletion filters to improve the clearance of amniotic fluid, the cell saver cannot differentiate foetal from maternal RBC, and so maternal alloimmunisation remains a real risk. Any aspirated foetal erythrocyte still present in the final product is re-transfused into the maternal circulation and this could increase the risk of maternal alloimmunisation in cases of RBC antigen incompatibilities between mother and foetus.
An early study by Durand et al. showed little modification of the physical (deformability and morphology) and chemical (adenosine triphosphate and 2,3-diphosphoglycerate) properties of RBC165, but an increase in plasma haemoglobin levels and contamination of foetal erythrocytes in the recovered blood close to 1 percent in seven out of 15 cases. A Kleihauer test was not carried out for haemolysis of samples in the 20 patients studied by Fuhrer and collaborators169. Rainaldi et al. found that the level of foetal haemoglobin was 1.8–2 percent in three of the 15 samples they analysed120. The Kleihauer test was positive in all ten samples analysed by Fong and collaborators167. Similarly, in other studies foetal RBC were still present in the final product132,166,171,172.
The contamination of salvaged blood by foetal Rh-mismatched erythrocytes can be dealt with using anti-D immunoglobulin. The dose of anti-D immunoglobulin required to prevent Rhesus immunisation can be calculated based on the volume of foetal RBC transferred to the mother. The volume of foetal RBC transferred by IBS is 1–5 mL120,166,167. In a study carried out by Catling et al. the maximum foetal blood volume mixed with the maternal cell saved volume was about 19 mL (range, 2–19 mL)171, requiring 500–2,500 IU of anti-D. A dose of 500 IU of anti-D immunoglobulin given to every D-negative woman with no preformed anti-D within 72 hours of delivery of a D-positive baby would be sufficient to prevent sensitisation from a bleed of up to 4 mL of foetal red cells187. It is, therefore, important that the volume of foeto-maternal haemorrhage is promptly and accurately assessed so that, if necessary, a supplementary dose of anti-D immunoglobulin can be administered and maternal alloimmunisation prevented188. In the UK, the techniques most commonly used for the screening and quantification of foeto-maternal haemorrhage are the acid elution method, a modification of the Kleihauer-Betke test, and flow cytometry188. In contrast, flow cytometry services for this function are available in relatively few hospital laboratories in the USA because of logistic and fiscal impediments189. Any confirmed foeto-maternal haemorrhage of 4 mL or more is considered to be significant and a follow-up of maternal sample should be checked for clearance of foetal cells, to confirm that the anti-D immunoglobulin has been given.
Clinically relevant antibodies can be formed to other RBC antigens and be implicated in haemolytic disease of the newborn (e.g. anti-K, anti-c, anti-Fya, and anti-Jka)190. The significance of foetal RBC contamination is still unstudied166, but the risk of alloimmunisation has been estimated to be similar to that present in a normal vaginal delivery3.
ABO mismatch tends to be a minor problem in comparison to Rh mismatch since ABO antigens are not fully developed at birth.
Only some of the studies analysed specifically assessed the degree of foetal RBC contamination of salvaged blood and maternal alloimmunisation was never reported.
The risk of maternal alloimmunisation by allogeneic blood transfusion is much greater than that caused by foetal RBC. Finally, although IBS may increase the total dose of anti-D needed, it is the bleed/surgical intervention, not the salvaged blood, that is the source of the initial exposure142.
European regulatory requirements and intra-operative cell salvage
The Directive 2002/98/EC of the European Parliament and the Council of the European Union set comprehensive standards for the quality and safety for the collection, processing, storage and distribution of human blood and blood components191. The three (daughter) Commission Directives, 2004/33/EC192, 2005/61/EC193 and 2005/62/EC194, implemented Directive 2002/98/EC as regards certain technical requirements for blood and blood components, traceability requirements and notification of serious adverse reactions and events, community standards and specifications relating to a quality system for blood establishments195.
European regulations for peri-operative cell salvage regard only the commercialisation of cell salvage devices196 (Council Directive 93/42/EEC197, amended by directive 2007/47/EC of the European Parliament and of the Council198), the general product safety199, and the liability for defective products (as provided for by Council Directive 85/374/EEC)200.
However, these recent European regulatory acts in the field of blood transfusion do not actually take into consideration the clinical use of blood components as the Community’s legislative framework does not include the therapeutic use of blood components195,201.
The European Directives legislate principally for allogeneic transfusion, but there are several references to “autologous donation” and “autologous transfusion”. Although in general they require the same standards for allogeneic and autologous blood, they do include additional ways of collecting and using autologous blood8.
The use and development of strategies to prevent and reduce bleeding, and the promotion of the use of alternatives to allogeneic transfusion are included among the principles of the European Recommendation Rec (2002) 11202.
According to the Council of Europe Recommendation No. R (95) 15 on the preparation, use and quality assurance of blood components203, autologous transfusion techniques were designed to avoid the risk of alloimmune complications of blood transfusion and reduce the risk of transfusion-associated infectious complications. Red cell salvage during surgery is one of the means of autologous transfusion and can be used in those procedures in which substantial blood loss is expected. It does not allow the storage of the collected blood and is usually performed under the responsibility of anaesthesiologists and/or surgeons203.
In Italy, the “new discipline for blood transfusion activities and national production of blood derivatives”, introduced in 2005 with national law No. 219 of 21 October 2005204, requires that Blood Transfusion Centres (in Italy all public by law) coordinate and set up peri-operative blood salvage activities. In accordance with the law, through the voluntary standards of transfusion medicine205, the Blood Transfusion Centres are given the task of coordinating and organising IBS, while the anaesthetist is responsible for its management in the operating theatre.
This technique can also be used in private health facilities in which responsibility lies with both the anaesthetist and the medical director of the facility, and is regulated under the Health Ministry Decree Law of 1 September 1995206.
Lastly, as far as concerns the prevention and management of bleeding risk in operations in which consistent blood loss is foreseen, the Italian Health Ministry simply suggests adopting “a system for peri-operative blood salvage and utilising a device for the rapid infusion of fluids”207.
In Europe, therefore, the clinical use of allogeneic or autologous blood is more subject to voluntary professional standards, professional judgment and guidelines issued by scientific societies such as the British Society of Haematology8,50,208,209, the Spanish Society for Blood Transfusion210,211, and SIMTI9,212.
There is also a lack of regulations regarding the products yielded by the devices196. In this regard, consideration should be given to the provisions of Directive 2005/62/EC194, stating that “in order to ensure the highest quality and safety for blood and blood components, guidance on good practice should be developed to support the quality system requirements for blood establishments taking fully into account the detailed guidelines referred to in Article 47 of Directive 2001/83/EC213 so as to ensure that the standards required for medicinal products are maintained”.
Although the current standard requirements for RBC units suggested in the recommendations of the European Council include the level of haemolysis (below the threshold of 0.8% of the red cell mass at the end of storage)203, there are no official European quality standards for autologous blood obtained during IBS and this may be a matter of concern for clinicians. In Italy, SIMTI transfusion medicine standards require that “the co-ordination and organisation of autologous transfusion activities except predeposit should also comprise interventions aimed at assessing both the quality and safety of blood products deriving from the peri-operative cell salvage procedures (e.g. excess activation of coagulation factors, excess haemolysis, contaminants from the surgical field)205. Although quality oversight of cell salvage procedures is not currently mandatory, it could represent a strategic tool for both patients’ safety and for understanding and monitoring serious adverse reactions and events. Thus, the adoption of voluntary standards and good practice guidelines for peri-operative cell salvage procedures are priority steps in order to comply with European Directives on transfusion safety, quality and haemovigilance. In this regard, the SHOT Group is continuing to gather all cases related to autologous transfusion, including cell salvage incidents collected in collaboration with the UK Cell Salvage Action Group146.
Conclusions
IBS is the most practical, effective and useful blood conservation technique in obstetrics. PABD and ANH have extremely limited roles in current clinical practice and should really be reserved for exceptional circumstances after a well-conducted risk-benefit analysis.
The role of cell salvage as a blood-saving measure in obstetrics is progressively acquiring relevance thanks to the growing body of evidence regarding its quality and safety coming from over 800 documented procedures and more than 400 patients transfused with salvaged blood. Although information about the outcomes of the patients treated and the allogeneic blood-saving effect is still limited, modern cell savers remove most particulate contaminants and leucodepletion filtering of salvaged blood prior to transfusion adds further safety to this technique. Moreover, AFE is no longer regarded as an embolic disease and it has been suggested that it is a rare anaphylactoid reaction to the foetal antigen. Contamination of salvaged blood by foetal Rh-mismatched erythrocytes can be dealt with by using anti-D immunoglobulin. ABO incompatibility tends to be a minor problem since ABO antigens are not fully developed at birth. Antibodies can be formed against other foetal red cell antigens, but it should also be noted that, although to the best of the knowledge of the authors, evidence from comparative studies is still lacking, there is probably a higher risk of alloimmunisation of the mother from allogeneic transfusion.
Despite potential drawbacks, there is a growing body of evidence to support the safety of cell salvage in obstetrics and the larger studies available show that at least one to two units of autologous blood can be yielded through IBS. Though the overall quality of evidence is poor, there are probably several concomitant reasons for the renewed interest in the use of IBS in obstetrics: research and refinement of equipment15, the growing awareness of possible future shortages of allogeneic blood89,90, and the frequency of the risk factors associated with obstetric haemorrhage and of conditions increasing the risk of bleeding4,214, such as abnormal placentation.
Furthermore, the use of IBS in obstetrics is supported by national bodies and scientific societies and is increasing also thanks to the fact that to date, though large prospective trials of IBS in obstetrics are lacking, no single serious complication leading to poor maternal outcome has been directly attributed to this simple and effective method of blood conservation15.
Given the significant costs and the relatively low likelihood of re-transfusion, where a service for other specialties is already provided, consideration should be given to extending this service to obstetric patients132. In the case of a low rate of blood salvage procedures, operator competency may be an issue and the risk of error is increased. The logistics of providing this infrequent service are, therefore, challenging and may contribute to the risk of error.
The routine use of IBS is not justified in uncomplicated emergency or elective caesarean section. On the contrary, it should be considered for emergency use (major haemorrhage during caesarean section, laparotomy for PPH, genital tract trauma, placental abruption, ruptured ectopic pregnancy) or elective use (expected major haemorrhage during caesarean section [placenta praevia, placenta accreta, placenta percreta], large/multiple uterine fibroids, multiple pregnancies, previous ruptured uterus or PPH, patients with significant pre-operative anaemia or thrombocytopenia and women who do not have allogeneic compatible blood available or refuse it).
All the above indications can be considered relative. The methodological strength of supporting evidence is weak as they are mainly based on results from observational or retrospective studies and case reports and only one randomised controlled trial with important limitations120. Therefore, alternative approaches in managing patients under certain circumstances could be suitable in some cases, and alternative therapeutic options in others.
Although recent evidence shows that blood can safely be salvaged from the beginning of suction, as long as a leucodepletion filter is used172, when practical amniotic fluid should be removed by a separate suction device. A leucodepletion filter should be used for re-transfusion unless the salvaged blood must be reinfused under pressure or there is unexplained hypotension shortly following re-infusion215.
In conclusion, although the use of IBS in combination with a leucodepletion filter appears to be safe in obstetrics, the wide ranges of the percentages of patients avoiding allogeneic blood (6–97.1%) and of the rates of re-infusion of salvaged blood (0–100%) are clear indicators of the need for more appropriate use and shared indications in this clinical setting.
Larger and adequately powered obstetric studies are needed to define the impact of this technique on the consumption of allogeneic blood and on several outcome variables, including alloimmunisation rates in recipients. Furthermore, a good benefit versus risk assessment should be conducted in the individual patient before using IBS and giving salvaged blood and traditional transfusion triggers should be applied132.
Table II.
Overview of laboratory parameters analysed in the studies on cell salvage in obstetrics.
| Year/Author | Quality control of shed blood |
|---|---|
| 1983/Keeling115 | Bacterial contamination, haematocrit, visual haemolysis check |
| 1989/Durand165 | 2,3-DPG, ATP, bacterial contamination, blood and plasma viscosities, erythrocyte deformability and morphology, foetal cells, haemolysis |
| 1990/Zichella116 | Coagulant activity (PT), phosphatidylglycerol |
| 1991/Thornhill168 | α-foetoprotein, foetal squamous cells, lanugo hair, trophoblasts, vernix caseosa |
| 1996/Fuhrer169 | α-foetoprotein, foetal cells, Kleihauer test*, tissue factor |
| 1997/Bernstein170 | Tissue factor |
| 1998/Rainaldi120 | Bacterial contamination, foetal haemoglobin, foetal red blood cells, foeto-placental material, haemolysis, procoagulant activity |
| 1999/Catling171 | α-foetoprotein, foetal squamous cells, fetal red cells, lanugo hair, leucocytes, trophoblasts |
| 1999/Fong167 | α-foetoprotein, haemoglobin, haematocrit, haemolysis, Kleihauer test, lanugo hair, squamous cells, vernix caseosa |
| 2000/Waters166 | Bacterial contamination, foetal haemoglobin, K+, lamellar body, squamous cells |
| 2008/Sullivan172 | α-foetoprotein, foetal red cells, full blood count, heparin, squamous cells |
| 2009/Okunuga129 | Blood film microscopy (search for sickled cells) |
| 2010/McDonnell132 | α-foetoprotein, haematocrit, haemolysis, K+, Kleihauer test |
Legend:
ATP: adenosine triphosphate; 2,3-DPG: 2,3-diphosphoglycerate; PT: prothrombin time; K+: potassium ion;
not performed due to haemolysis of sample.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.WHO. Blood Transfusion Safety. Geneva, Switzerland: WHO; [Last accessed on: 01/22/2011]. Department of Essential Health Technologies. Available from: http://www.who.int/bloodsafety/en/Blood_Transfusion_Safety.pdf. [Google Scholar]
- 2.Catling S. Blood conservation techniques in obstetrics: a UK perspective. Int J Obstet Anesth. 2007;16:241–9. doi: 10.1016/j.ijoa.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Wise A, Clark V. Challenges of major obstetric haemorrhage. Best Pract Res Clin Obstet Gynaecol. 2010;24:353–65. doi: 10.1016/j.bpobgyn.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Cantwell R, Clutton-Brock T, Cooper G, et al. Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006–2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(Suppl 1):1–203. doi: 10.1111/j.1471-0528.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 5.Brace V, Penney G, Hall M. Quantifying severe maternal morbidity: a Scottish population study. BJOG. 2004;111:481–4. doi: 10.1111/j.1471-0528.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- 6.Ronsmans C, Graham WJ. Maternal mortality: who, when, and why. Lancet. 2006;368:1189–200. doi: 10.1016/S0140-6736(06)69380-X. [DOI] [PubMed] [Google Scholar]
- 7.Zhang WH, Alexander S, Bouvier-Colle MH, Macfarlane A the MOMS-B Group. Incidence of severe pre-eclampsia, postpartum haemorrhage and sepsis as a surrogate marker for severe maternal morbidity in a European population-based study: the MOMS-B survey. BJOG. 2005;112:89–96. doi: 10.1111/j.1471-0528.2004.00303.x. [DOI] [PubMed] [Google Scholar]
- 8.Boulton FE, James V. Guidelines for policies on alternatives to allogeneic blood transfusion. 1. Predeposit autologous blood donation and transfusion. British Committee for Standards in Haematology, Transfusion Task Force. Transfus Med. 2007;17:354–65. doi: 10.1111/j.1365-3148.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 9.Liumbruno GM, Bennardello F, Lattanzio A, et al. Raccomandazioni SIMTI sulla Trasfusione Perioperatoria. 1st ed. Milan, Italy: SIMTI Servizi Srl; 2010. [Google Scholar]
- 10.Weiskopf RB. Erythrocyte salvage during cesarean section. Anesthesiology. 2000;92:1519–22. doi: 10.1097/00000542-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Horstmann WG, Ettema HB, Verheyen CC. Dutch orthopedic blood management surveys 2002 and 2007: an increasing use of blood-saving measures. Arch Orthop Trauma Surg. 2010;130:55–9. doi: 10.1007/s00402-009-0910-0. [DOI] [PubMed] [Google Scholar]
- 12.Tawes RL. The basic concepts of an autotransfusor: the cell saver. Semin Vasc Surg. 1994;7:93–4. [PubMed] [Google Scholar]
- 13.Waters JH. The future of blood management. Clin Lab Med. 2010;30:453–65. doi: 10.1016/j.cll.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Waters JH, Potters PS. Cell salvage in the Jehovah’s Witness. Anesth Analg. 2000;90:229–30. doi: 10.1097/00000539-200001000-00053. [DOI] [PubMed] [Google Scholar]
- 15.Allam J, Cox M, Yentis SM. Cell salvage in obstetrics. Int J Obstet Anesth. 2008;17:37–45. doi: 10.1016/j.ijoa.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Catling S. Intraoperative cell salvage in obstetrics. Clin Risk. 2008;14:14–7. [Google Scholar]
- 17.Surgenor DM. The patient’s blood is the safest blood [editorial] N Engl J Med. 1987;316:542–4. doi: 10.1056/NEJM198702263160909. [DOI] [PubMed] [Google Scholar]
- 18.Fergusson D, van Walraven C, Coyle D, Laupacis A. Economic evaluations of technologies to minimize perioperative transfusion: a systematic review of published studies. International Study of Peri-operative Transfusion (ISPOT) investigators. Transfus Med Rev. 1999;13:106–17. doi: 10.1016/s0887-7963(99)80005-4. [DOI] [PubMed] [Google Scholar]
- 19.Henry DA, Carless PA, Moxey AJ, et al. Pre-operative autologous donation for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2002;2:CD003602. doi: 10.1002/14651858.CD003602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brecher ME, Goodnough LT. The rise and fall of preoperative autologous blood donation [editorial] Transfusion. 2002;42:1618–22. doi: 10.1046/j.1537-2995.2002.t01-2-04212.x. [DOI] [PubMed] [Google Scholar]
- 21.Goldman M, Savard R, Long A, et al. Declining value of preoperative autologous donation. Transfusion. 2002;42:819–23. doi: 10.1046/j.1537-2995.2002.00132.x. [DOI] [PubMed] [Google Scholar]
- 22.Rock G, Berger R, Bormanis J, et al. A review of nearly two decades in an autologous blood programme: the rise and fall of activity. Transfus Med. 2006;16:307–11. doi: 10.1111/j.1365-3148.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- 23.Daurat G, Duedari N, Schved JF. The decrease of preoperative autologous transfusion in France has not been linked to an increase of homologous red cell concentrates. Ann Fr Anesth Reanim. 2008;27:141–7. doi: 10.1016/j.annfar.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Walsh TS, Prowse C. BCSH guidelines for policies on alternatives to allogeneic blood transfusion. 1. Predeposit autologous blood donation and transfusion, August 2006 [editorial] Transfus Med. 2007;17:353. doi: 10.1111/j.1365-3148.2007.00751.x. [DOI] [PubMed] [Google Scholar]
- 25.Roth WK, Weber M, Buhr S, et al. Yield of HCV and HIV-1 NAT after screening of 3.6 million blood donations in central Europe. Transfusion. 2002;42:862–8. doi: 10.1046/j.1537-2995.2002.00129.x. [DOI] [PubMed] [Google Scholar]
- 26.Faught C, Wells P, Fergusson D, Laupacis A. Adverse effects of methods for minimizing perioperative allogeneic transfusion: a critical review of the literature. Transfus Med Rev. 1998;12:206–25. doi: 10.1016/s0887-7963(98)80061-8. [DOI] [PubMed] [Google Scholar]
- 27.Popovsky MA, Whitaker B, Arnold NL. Severe outcomes of allogeneic and autologous blood donation: frequency and characterization. Transfusion. 1995;35:734–7. doi: 10.1046/j.1537-2995.1995.35996029156.x. [DOI] [PubMed] [Google Scholar]
- 28.Linden JV, Wagner K, Voytovich AE, Sheehan J. Transfusion errors in New York State: an analysis of 10 years’ experience. Transfusion. 2000;40:1207–13. doi: 10.1046/j.1537-2995.2000.40101207.x. [DOI] [PubMed] [Google Scholar]
- 29.Puppo F, Ghio M, Contini P, et al. Fas, Fas ligand, and transfusion immunomodulation. Transfusion. 2001;41:416–8. doi: 10.1046/j.1537-2995.2001.41030416.x. [DOI] [PubMed] [Google Scholar]
- 30.Ghio M, Contini P, Mazzei C, et al. In vitro immunosuppressive activity of soluble HLA class I and Fas ligand molecules: do they play a role in autologous blood transfusion? Transfusion. 2001;41:988–96. doi: 10.1046/j.1537-2995.2001.41080988.x. [DOI] [PubMed] [Google Scholar]
- 31.Ghio M, Contini P, Ubezio G, et al. Immunomodulatory effects of blood transfusions: the synergic role of soluble HLA Class I free heavy-chain molecules detectable in blood components. Transfusion. 2008;48:1591–7. doi: 10.1111/j.1537-2995.2008.01720.x. [DOI] [PubMed] [Google Scholar]
- 32.Yamada T, Mori H, Ueki M. Autologous blood transfusion in patients with placenta previa. Acta Obstet Gynecol Scand. 2005;84:255–9. doi: 10.1111/j.0001-6349.2005.00698.x. [DOI] [PubMed] [Google Scholar]
- 33.Kruskall MS, Leonard S, Klapholz H. Autologous blood donation during pregnancy: analysis of safety and blood use. Obstet Gynecol. 1987;70:938–41. [PubMed] [Google Scholar]
- 34.Herbert WN, Owen HG, Collins ML. Autologous blood storage in obstetrics. Obstet Gynecol. 1988;72:166–70. [PubMed] [Google Scholar]
- 35.McVay PA, Hoag RW, Hoag MS, Toy PT. Safety and use of autologous blood donation during the third trimester of pregnancy. Am J Obstet Gynecol. 1989;160:1479–86. doi: 10.1016/0002-9378(89)90873-9. [DOI] [PubMed] [Google Scholar]
- 36.James AH, Paglia MJ, Gernsheimer T, et al. Blood component therapy in postpartum hemorrhage. Transfusion. 2009;49:2430–3. doi: 10.1111/j.1537-2995.2009.02318.x. [DOI] [PubMed] [Google Scholar]
- 37.Fuller AJ, Bucklin B. Blood component therapy in obstetrics. Obstet Gynecol Clin North Am. 2007;34:443–58. doi: 10.1016/j.ogc.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Fuller AJ, Bucklin BA. Blood product replacement for postpartum hemorrhage. Clin Obstet Gynecol. 2010;53:196–208. doi: 10.1097/GRF.0b013e3181cc42a0. [DOI] [PubMed] [Google Scholar]
- 39.Singbartl G, Schreiber J, Singbartl K. Preoperative autologous blood donation versus intraoperative blood salvage: intraindividual analyses and modeling of efficacy in 1103 patients. Transfusion. 2009;49:2374–83. doi: 10.1111/j.1537-2995.2009.02291.x. [DOI] [PubMed] [Google Scholar]
- 40.Erslev AJ, Caro J, Miller O, Silver R. Plasma erythropoietin in health and disease. Ann Clin Lab Sci. 1980;10:250–7. [PubMed] [Google Scholar]
- 41.Erslev AJ, Wilson J, Caro J. Erythropoietin titers in anemic, nonuremic patients. J Lab Clin Med. 1987;109:429–33. [PubMed] [Google Scholar]
- 42.Weisbach V, Corbière C, Strasser E, et al. The variability of compensatory erythropoiesis in repeated autologous blood donation. Transfusion. 2001;41:179–83. doi: 10.1046/j.1537-2995.2001.41020179.x. [DOI] [PubMed] [Google Scholar]
- 43.Singbartl G. Preoperative autologous blood donation-part I. Only two clinical parameters determine efficacy of the autologous predeposit. Minerva Anestesiol. 2007;73:143–51. [PubMed] [Google Scholar]
- 44.Singbartl G, Malgorzata S, Quoss A. Preoperative autologous blood donation-part II. Adapting the predeposit concept to the physiological basics of erythropoiesis improves its efficacy. Minerva Anestesiol. 2007;73:153–60. [PubMed] [Google Scholar]
- 45.Singbartl G. Pre-operative autologous blood donation: clinical parameters and efficacy. Blood Transfus. 2011;9:10–8. doi: 10.2450/2010.0088-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boonstra JG, Overbeeke MA, de Rijke YB, Duvekot JJ. A pregnant woman with irregular erythrocyte antibodies for whom no compatible packed red blood cells were available. Ned Tijdschr Geneeskd. 2005;149:2633–6. [PubMed] [Google Scholar]
- 47.Messmer K, Lewis DH, Sunder-Plassmann L, et al. Acute normovolemic hemodilution. Changes of central hemodynamics and microcirculatory flow in skeletal muscle. Eur Surg Res. 1972;4:55–70. doi: 10.1159/000127600. [DOI] [PubMed] [Google Scholar]
- 48.Laks H, O’Connor NE, Pilon RN, et al. Acute normovolemic hemodilution: effects on hemodynamics, oxygen transport, and lung water in anesthetized man. Surg Forum. 1973;24:201–2. [PubMed] [Google Scholar]
- 49.Bauer H, Pichlmaier H, Ott E, et al. Autotransfusion through acute, preoperative hemodilution: 1st clinical experiences. Langenbecks Arch Chir. 1974;(Suppl):185–9. [PubMed] [Google Scholar]
- 50.Napier JA, Bruce M, Chapman J, et al. Guidelines for autologous transfusion. II. Perioperative haemodilution and cell salvage. British Committee for Standards in Haematology Blood Transfusion Task Force. Autologous Transfusion Working Party. Br J Anaesth. 1997;78:768–71. doi: 10.1093/bja/78.6.768. [DOI] [PubMed] [Google Scholar]
- 51.Pape A, Habler O. Alternatives to allogeneic blood transfusions. Best Pract Res Clin Anaesthesiol. 2007;21:221–39. doi: 10.1016/j.bpa.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Kreimeier U, Messmer K. Perioperative hemodilution. Transfus Apher Sci. 2002;27:59–72. doi: 10.1016/s1473-0502(02)00027-7. [DOI] [PubMed] [Google Scholar]
- 53.Shander A, Rijhwani TS. Acute normovolemic hemodilution. Transfusion. 2004;44(2 Suppl):26S–34S. doi: 10.1111/j.0041-1132.2004.04293.x. [DOI] [PubMed] [Google Scholar]
- 54.Monk TG. Acute normovolemic hemodilution. Anesthesiology Clin N Am. 2005;23:271–81. doi: 10.1016/j.atc.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Shander A, Goodnough LT. Objectives and limitations of bloodless medical care. Curr Opin Hematol. 2006;13:462–70. doi: 10.1097/01.moh.0000245692.32085.bd. [DOI] [PubMed] [Google Scholar]
- 56.Goodnough LT, Monk TG, Brecher ME. Acute normovolemic hemodilution should replace the preoperative donation of autologous blood as a method of autologous-blood procurement. Transfusion. 1998;38:473–6. doi: 10.1046/j.1537-2995.1998.38598297217.x. [DOI] [PubMed] [Google Scholar]
- 57.Billote DB, Abdoue AG, Wixson RL. Comparison of acute normovolemic hemodilution and preoperative autologous blood donation in clinical practice. J Clin Anesth. 2000;12:31–5. doi: 10.1016/s0952-8180(99)00129-4. [DOI] [PubMed] [Google Scholar]
- 58.Weiskopf RB. Efficacy of acute normovolemic hemodilution assessed as a function of fraction of blood volume lost. Anesthesiology. 2001;94:439–46. doi: 10.1097/00000542-200103000-00013. [DOI] [PubMed] [Google Scholar]
- 59.Messmer K. Hemodilution. Surg Clin North Am. 1975;55:659–78. doi: 10.1016/s0039-6109(16)40641-9. [DOI] [PubMed] [Google Scholar]
- 60.Gross JB. Estimating allowable blood loss: corrected for dilution. Anesthesiology. 1983;58:277–80. doi: 10.1097/00000542-198303000-00016. [DOI] [PubMed] [Google Scholar]
- 61.Cardone D, Klein AA. Perioperative blood conservation. Eur J Anaesthesiol. 2009;26:722–9. doi: 10.1097/EJA.0b013e32832c5280. [DOI] [PubMed] [Google Scholar]
- 62.Kochamba GS, Pfeffer TA, Sintek CF, Khonsari S. Intraoperative autotransfusion reduces blood loss after cardiopulmonary bypass. Ann Thorac Surg. 1996;61:900–3. doi: 10.1016/0003-4975(95)01155-2. [DOI] [PubMed] [Google Scholar]
- 63.Helm RE, Klemperer JD, Rosengart TK, et al. Intraoperative autologous blood donation preserves red cell mass but does not decrease postoperative bleeding. Ann Thorac Surg. 1996;62:1431–41. doi: 10.1016/0003-4975(96)00755-2. [DOI] [PubMed] [Google Scholar]
- 64.Olsfanger D, Fredman B, Goldstein B, et al. Acute normovolaemic haemodilution decreases postoperative allogeneic blood transfusion after total knee replacement. Br J Anaesth. 1997;79:317–21. doi: 10.1093/bja/79.3.317. [DOI] [PubMed] [Google Scholar]
- 65.Terada N, Arai Y, Matsuta Y, et al. Acute normovolemic hemodilution for radical prostatectomy: can it replace preoperative autologous blood transfusion? Int J Urol. 2001;8:149–52. doi: 10.1046/j.1442-2042.2001.00272.x. [DOI] [PubMed] [Google Scholar]
- 66.Matot I, Scheinin O, Jurim O, Eid A. Effectiveness of acute normovolaemic haemodilution to minimize allogeneic blood transfusion in major liver resections. Anesthesiology. 2002;97:794–800. doi: 10.1097/00000542-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 67.Wong JC, Torella F, Haynes SL, et al. ATIS Investigators. Autologous versus allogeneic transfusion in aortic surgery: a multicenter randomized clinical trial. Ann Surg. 2002;235:145–51. doi: 10.1097/00000658-200201000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Habler O, Schwenzer K, Zimmer K, et al. Effects of standardized acute normovolemic hemodilution on intraoperative allogeneic blood transfusion in patients undergoing major maxillofacial surgery. Int J Oral Maxillofac Surg. 2004;33:467–75. doi: 10.1016/j.ijom.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 69.Bennet J, Haynes S, Torella F, et al. Acute normovolemic hemodilution in moderate blood loss surgery: a randomized controlled trial. Transfusion. 2006;46:1097–103. doi: 10.1111/j.1537-2995.2006.00857.x. [DOI] [PubMed] [Google Scholar]
- 70.Takayanagi A, Masumori N, Kobayashi K, et al. Acute normovolemic hemodilution for radical retropubic prostatectomy and radical cystectomy. Urology. 2008;72:401–5. doi: 10.1016/j.urology.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 71.Imai R, Matsumura H, Uchida R, Watanabe K. Perioperative hemodilutional autologous blood transfusion in burn surgery. Int J Care Injured. 2008;39:57–60. doi: 10.1016/j.injury.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 72.Parkin IR, Chiu GA, Schwarz PA, Hodder SC. Acute perioperative normovolaemic haemodilution in major maxillofacial surgery. Br J Oral Maxillofac Surg. 2008;46:387–90. doi: 10.1016/j.bjoms.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 73.Jarnagin WR, Gonen M, Maithel SK, et al. A prospective randomized trial of acute normovolemic hemodilution compared to standard intraoperative management in patients undergoing major hepatic resection. Ann Surg. 2008;248:360–9. doi: 10.1097/SLA.0b013e318184db08. [DOI] [PubMed] [Google Scholar]
- 74.Grange CS, Douglas MJ, Adams TJ, Wadsworth D. The use of acute hemodilution in parturients undergoing cesarean section. Am J Obstet Gynecol. 1998;178:156–60. doi: 10.1016/s0002-9378(98)70644-1. [DOI] [PubMed] [Google Scholar]
- 75.Estella NM, Berry DL, Baker BW, et al. Normovolemic hemodilution before cesarean hysterectomy for placenta percreta. Obstet Gynecol. 1997;90:669–70. doi: 10.1016/s0029-7844(97)00394-3. [DOI] [PubMed] [Google Scholar]
- 76.Nagy CJ, Wheeler AS, Archer TL. Acute normovolemic hemodilution, intraoperative cell salvage and PulseCO hemodynamic monitoring in a Jehovah’s Witness with placenta percreta. Int J Obstet Anesth. 2008;17:159–63. doi: 10.1016/j.ijoa.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 77.Kahraman S, Altunkaya H, Celebioğlu B, et al. The effect of acute normovolemic hemodilution on homologous blood requirements and total estimated red blood cell volume lost. Acta Anaesthesiol Scand. 1997;41:614–7. doi: 10.1111/j.1399-6576.1997.tb04752.x. [DOI] [PubMed] [Google Scholar]
- 78.Hohn L, Schweizer A, Licker M, Morel DR. Absence of beneficial effect of acute normovolemic hemodilution combined with aprotinin on allogeneic blood transfusion requirements in cardiac surgery. Anesthesiology. 2002;96:276–92. doi: 10.1097/00000542-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 79.Svenmarker S, Engstrom KG. The inflammatory response to recycled pericardial suction blood and the influence of cell-saving. Scand Cardiovasc J. 2003;37:158–64. doi: 10.1080/14017430310001465. [DOI] [PubMed] [Google Scholar]
- 80.Bryson GL, Laupacis A, Wells GA. Does acute normovolemic hemodilution reduce perioperative allogeneic transfusion? A meta-analysis. The International Study of Perioperative Transfusion. Anesth Analg. 1998;86:9–15. doi: 10.1213/00000539-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 81.Gillon J, Desmond M, Thomas MJ. Acute normovolemic haemodilution. Transfus Med. 1999;9:259–64. doi: 10.1046/j.1365-3148.1999.00205.x. [DOI] [PubMed] [Google Scholar]
- 82.Segal JB, Blasco-Colmenares E, Norris EJ, Guallar E. Preoperative acute normovolemic hemodilution: a meta-analysis. Transfusion. 2004;44:632–44. doi: 10.1111/j.1537-2995.2004.03353.x. [DOI] [PubMed] [Google Scholar]
- 83.Carless P, Moxey A, O’Connell DO, Henry D. Autologous transfusion techniques: a systematic review of their efficacy. Transfus Med. 2004;14:123–44. doi: 10.1111/j.0958-7578.2004.0489.x. [DOI] [PubMed] [Google Scholar]
- 84.Zohar E, Fredman B, Ellis M, et al. A comparative study of the postoperative allogeneic blood sparing effect of tranexamic acid versus acute normovolemic hemodilution after total knee replacement. Anesth Analg. 1999;89:1382–7. doi: 10.1097/00000539-199912000-00010. [DOI] [PubMed] [Google Scholar]
- 85.Davies L, Brown TJ, Haynes S, et al. Cost-effectiveness of cell salvage and alternative methods of minimizing perioperative allogeneic blood transfusion: a systematic review and economic model. Health Technol Assess. 2006;10:1–210. doi: 10.3310/hta10440. [DOI] [PubMed] [Google Scholar]
- 86.Society of Thoracic Surgeons Blood Conservation Guideline Task Force. Ferraris VA, Ferraris SP, Saha SP, et al. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007;83(5 Suppl):S27–86. doi: 10.1016/j.athoracsur.2007.02.099. [DOI] [PubMed] [Google Scholar]
- 87.Blundell J. Experiments on the transfusion of blood by the syringe. Med Chir Trans. 1818;9:56–92. doi: 10.1177/09595287180090p107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Highmore W. Practical remarks on an overlooked source of blood supply for transfusion in postpartum haemorrhage. Lancet. 1874;1:89. [Google Scholar]
- 89.Sullivan MT, Cotten R, Read EJ, Wallace EL. Blood collection and transfusion in the United States in 2001. Transfusion. 2007;47:385–94. doi: 10.1111/j.1537-2995.2007.01128.x. [DOI] [PubMed] [Google Scholar]
- 90.Seifried E, Klueter H, Weidmann C, et al. How much blood is needed? Vox Sang. 2011;100:10–21. doi: 10.1111/j.1423-0410.2010.01446.x. [DOI] [PubMed] [Google Scholar]
- 91.Committee on Obstetric Practice. ACOG committee opinion. Placenta accreta. Number 266, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 2002;77:77–8. doi: 10.1016/s0020-7292(02)80003-0. [DOI] [PubMed] [Google Scholar]
- 92.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin: Clinical Management Guidelines for Obstetrician-Gynecologists. Number 76, October 2006: postpartum hemorrhage. Obstet Gynecol. 2006;108:1039–47. doi: 10.1097/00006250-200610000-00046. [DOI] [PubMed] [Google Scholar]
- 93.American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Practice guidelines for obstetric anesthesia: an updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Anesthesiology. 2007;106:843–63. doi: 10.1097/01.anes.0000264744.63275.10. [DOI] [PubMed] [Google Scholar]
- 94.National Institute for Health and Clinical Excellence. Interventional Procedure Guidance 144. London, UK: National Institute for Health and Clinical Excellence; 2005. [Last accessed on: 01/22/2011]. Intraoperative Blood Cell Salvage In Obstetrics. Available from: http://www.nice.org.uk/nicemedia/live/11038/30690/30690.pdf. [Google Scholar]
- 95.The Association of Anaesthetists of Great Britain and Ireland, Obstetric Anaesthetists’ Association. OAA/AAGBI Guidelines For Obstetric Anaesthetic Services. Revised edition 2005. London, UK: The Association of Anaesthetists of Great Britain and Ireland; 2005. [Last accessed on: 01/22/2011]. p. 25. Available from: http://www.aagbi.org/publications/guidelines/docs/obstetric05.pdf. [Google Scholar]
- 96.The Association of Anaesthetists of Great Britain and Ireland. Intra-Operative Cell Salvage. London, UK: The Association of Anaesthetists of Great Britain and Ireland; 2009. [Last accessed on: 01/22/2011]. Blood Transfusion And The Anaesthetist; p. 11. Available from: http://www.aagbi.org/publications/guidelines/docs/cell%20_salvage_2009_amended.pdf. [Google Scholar]
- 97.UK Cell Salvage Action Group; UK Cell Salvage Action Group. Intraoperative Cell Salvage: Education Workbook. UK: UK Cell Salvage Action Group; 2008. [Last accessed on: 01/22/2011]. Indications and contraindications; pp. 50–2. Available from: http://www.transfusionguidelines.org.uk/docs/pdfs/bbt-03_icsag_workbook-0812_all.pdf. [Google Scholar]
- 98.Fuller AJ, Bucklin BA. Blood product replacement for postpartum hemorrhage. Clin Obstet Gynecol. 2010;53:196–208. doi: 10.1097/GRF.0b013e3181cc42a0. [DOI] [PubMed] [Google Scholar]
- 99.Lombaard H, Pattinson RC. Common errors and remedies in managing postpartum haemorrhage. Best Pract Res Clin Obstet Gynaecol. 2009;23:317–26. doi: 10.1016/j.bpobgyn.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 100.Gallos G, Redai I, Smiley RM. The role of the anesthesiologist in management of obstetric hemorrhage. Semin Perinatol. 2009;33:116–23. doi: 10.1053/j.semperi.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 101.Mercier FJ, Van de Velde M. Major obstetric hemorrhage. Anesthesiol Clin. 2008;26:53–66. doi: 10.1016/j.anclin.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 102.Wise A, Clark V. Strategies to manage major obstetric haemorrhage. Curr Opin Anaesthesiol. 2008;21:281–7. doi: 10.1097/ACO.0b013e3282f8e257. [DOI] [PubMed] [Google Scholar]
- 103.Clark V. Facilities for blood salvage (cell saver technique) must be available in every obstetric theatre. Int J Obstet Anesth. 2005;14:50–2. doi: 10.1016/j.ijoa.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 104.Esper SA, Waters JH. Intraoperative cell salvage: a fresh look at the indications and contraindications. Blood Transfus. 2011;9:139–47. doi: 10.2450/2011.0081-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davies L, Brown TJ, Haynes S, et al. Cost-effectiveness of cell salvage and alternative methods of minimizing perioperative allogeneic blood transfusion: a systematic review and economic model. Health Technol Assess. 2006;10:1–210. doi: 10.3310/hta10440. [DOI] [PubMed] [Google Scholar]
- 106.Szpisjak DF, Potter PS, Capehart BP. Economic analysis of an intraoperative cell salvage service. Anesth Analg. 2004;98:201–5. doi: 10.1213/01.ANE.0000093231.53663.DD. [DOI] [PubMed] [Google Scholar]
- 107.Phillips SD, Maguire D, Deshpande R, et al. A prospective study investigating the cost effectiveness of intraoperative blood salvage during liver transplantation. Transplantation. 2006;81:536–40. doi: 10.1097/01.tp.0000199318.17013.c5. [DOI] [PubMed] [Google Scholar]
- 108.Waters JR, Meier HH, Waters JH. An economic analysis of costs associated with development of a cell salvage program. Anesth Analg. 2007;104:869–75. doi: 10.1213/01.ane.0000258039.79028.7c. [DOI] [PubMed] [Google Scholar]
- 109.King M, Wrench I, Whiting P. Cost effectiveness of using a cell saver in a large obstetric unit. Int J Obstet Anesth. 2007;16(Suppl 1):S5. [Google Scholar]
- 110.Grimes DA. A simplified device for intraoperative autotransfusion. Obstet Gynecol. 1988;72:947–50. doi: 10.1097/00006250-198812000-00030. [DOI] [PubMed] [Google Scholar]
- 111.O’Brien JM, Barton JR, Donaldson ES. The management of placenta percreta: conservative and operative strategies. Am J Obstet Gynecol. 1996;175:1632–8. doi: 10.1016/s0002-9378(96)70117-5. [DOI] [PubMed] [Google Scholar]
- 112.Eller AG, Porter TF, Soisson P, Silver RM. Optimal management strategies for placenta accreta. BJOG. 2009;116:648–54. doi: 10.1111/j.1471-0528.2008.02037.x. [DOI] [PubMed] [Google Scholar]
- 113.Potter PS, Waters JH, Burger GA, Mraović B. Application of cell-salvage during cesarean section. Anesthesiology. 1999;90:619–21. doi: 10.1097/00000542-199902000-00038. [DOI] [PubMed] [Google Scholar]
- 114.Rebarber A, Lonser R, Jackson S, et al. The safety of intraoperative autologous blood collection and autotransfusion during cesarean section. Am J Obstet Gynecol. 1998;179:715–20. doi: 10.1016/s0002-9378(98)70070-5. [DOI] [PubMed] [Google Scholar]
- 115.Keeling MM, Gray LA, Jr, Brink MA, et al. Intraoperative autotransfusion. Experience in 725 consecutive cases. Ann Surg. 1983;197:536–41. doi: 10.1097/00000658-198305000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zichella L, Gramolini R. Autotransfusion during cesarean section. Am J Obstet Gynecol. 1990;162:295. doi: 10.1016/0002-9378(90)90880-g. [DOI] [PubMed] [Google Scholar]
- 117.Jackson SH, Lonser RE. Safety and effectiveness of intracesarean blood salvage. Transfusion. 1993;33:181. doi: 10.1046/j.1537-2995.1993.33293158054.x. [DOI] [PubMed] [Google Scholar]
- 118.Tawes RL, Jr, Duvall TB. Is the “salvaged-cell syndrome” myth or reality? Am J Surg. 1996;172:172–4. doi: 10.1016/S0002-9610(96)00144-4. [DOI] [PubMed] [Google Scholar]
- 119.Rees SG, Boheimer NO. Autologous blood transfusion. Br J Anaesth. 1998;80:563. doi: 10.1093/bja/80.4.563. [DOI] [PubMed] [Google Scholar]
- 120.Rainaldi MP, Tazzari PL, Scagliarini G, et al. Blood salvage during caesarean section. Br J Anaesth. 1998;80:195–8. doi: 10.1093/bja/80.2.195. [DOI] [PubMed] [Google Scholar]
- 121.Oei SG, Wingen CBM, Kerkkamp HEM. Cell salvage: how safe in obstetrics? Int J Obstet Anesth. 2000;9:143. [Google Scholar]
- 122.Catling SJ, Freites O, Krishnan S, Gibbs R. Clinical experience with cell salvage in obstetrics: 4 cases from one UK centre. Int J Obstet Anesth. 2002;11:128–34. doi: 10.1054/ijoa.2001.0914. [DOI] [PubMed] [Google Scholar]
- 123.Waters JH, Lukauskiene E, Anderson ME. Intraoperative blood salvage during cesarean delivery in a patient with beta thalassemia intermedia. Anesth Analg. 2003;97:1808–9. doi: 10.1213/01.ANE.0000087046.91072.E8. [DOI] [PubMed] [Google Scholar]
- 124.de Souza A, Permezel M, Anderson M, et al. Antenatal erythropoietin and intra-operative cell salvage in a Jehovah’s Witness with placenta praevia. BJOG. 2003;110:524–6. [PubMed] [Google Scholar]
- 125.McGurgan P, Maouris P, Hart R, et al. En caul delivery of the fetus to facilitate cell salvage. Aust N Z J Obstet Gynaecol. 2004;44:585. doi: 10.1111/j.1479-828X.2004.00316.x. [DOI] [PubMed] [Google Scholar]
- 126.Teig M, Harkness M, Catling S, Clark V. Survey of cell-salvage use in obstetrics in the UK. Int J Obstet Anesth. 2007;16(Suppl 1):S30. [Google Scholar]
- 127.Harkness M, Clark V. The use of cell salvage during obstetric procedures: an audit of Scotland’s maternity units. Scott Med J. 2008;53:24–7. doi: 10.1258/RSMSMJ.53.3.24. [DOI] [PubMed] [Google Scholar]
- 128.Araki Y, Fukuda I, Kamiya I, et al. Case of caesarean section using Cell Savers5+ in a patient with the placenta accreta associated with massive hemorrhage. Masui. 2009;58:499–502. [PubMed] [Google Scholar]
- 129.Okunuga A, Skelton VA. Use of cell salvage in patients with sickle cell trait. Int J Obstet Anesth. 2009;18:90–1. doi: 10.1016/j.ijoa.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 130.King M, Wrench I, Galimberti A, Spray R. Introduction of cell salvage to a large obstetric unit: the first six months. Int J Obstet Anesth. 2009;18:111–7. doi: 10.1016/j.ijoa.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 131.Parry N, Junghans C, Skelton V, Okunuga A. Audit of cell salvage use in obstetric patients: adding experience. Int J Obstet Anesth. 2010;19:238–9. doi: 10.1016/j.ijoa.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 132.McDonnell NJ, Kennedy D, Long LJ, et al. The development and implementation of an obstetric cell salvage service. Anaesth Intensive Care. 2010;38:492–9. doi: 10.1177/0310057X1003800313. [DOI] [PubMed] [Google Scholar]
- 133.Sreelakshmi TR, Eldridge J. Acute hypotension associated with leucocyte depletion filters during cell salvaged blood transfusion. Anaesthesia. 2010;65:742–4. doi: 10.1111/j.1365-2044.2009.06190.x. [DOI] [PubMed] [Google Scholar]
- 134.Kessack LK, Hawkins N. Severe hypotension related to cell salvaged blood transfusion in obstetrics. Anaesthesia. 2010;65:745–8. doi: 10.1111/j.1365-2044.2010.06301.x. [DOI] [PubMed] [Google Scholar]
- 135.Malik S, Brooks H, Singhal T. Cell saver use in obstetrics. J Obstet Gynaecol. 2010;30:826–8. doi: 10.3109/01443615.2010.511727. [DOI] [PubMed] [Google Scholar]
- 136.Catling S, Thomas D. Intraoperative autologous blood transfusion. In: Lynch CB, Keith L, Lalonde A, Karoshi M, editors. A Textbook Of Postpartum Haemorrhage. Duncow, UK: Sapiens Publishing; 2006. pp. 421–6. [Google Scholar]
- 137.Coker A, Oliver R. Definitions and classifications. In: Lynch CB, Keith L, Lalonde A, Karoshi M, editors. A Textbook Of Postpartum Haemorrhage. Duncow, UK: Sapiens Publishing; 2006. pp. 11–7. [Google Scholar]
- 138.Waters JH, Dyga RM, Yazer MH, editors. Guidelines For Blood Recovery And Reinfusion In Surgery And Trauma. Bethesda, MD, USA: AABB; 2010. [Google Scholar]
- 139.Waters JH, Santrach PJ. Pro/con debate: is cell salvage a safe technique for the obstetric patient? SOAP Newsletter. 2005 Fall;:7–9. [Google Scholar]
- 140.Hatfield T, Kraus H, McConnell D, Nageotte M. Synchronous autotransfusion during cesarean hysterectomy. Am J Obstet Gynecol. 2010;202:e15–6. doi: 10.1016/j.ajog.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 141.Geoghegan J, Daniels JP, Moore PA, et al. Cell salvage at caesarean section: the need for an evidence-based approach. BJOG. 2009;116:743–7. doi: 10.1111/j.1471-0528.2009.02129.x. [DOI] [PubMed] [Google Scholar]
- 142.Catling S, Wrench I. Cell salvage at Caesarean section: the need for an evidence-based approach. BJOG. 2010;117:122–3. doi: 10.1111/j.1471-0528.2009.02394.x. [DOI] [PubMed] [Google Scholar]
- 143.McDonald JM, Sethi B. Blood transfusion for Caesarean section. Br J Anaesth. 1998;81:825–6. doi: 10.1093/bja/81.5.825-a. [DOI] [PubMed] [Google Scholar]
- 144.Fong J, Gurewitsch ED, Kang HJ, et al. An analysis of transfusion practice and the role of intraoperative red blood cell salvage during cesarean delivery. Anesth Analg. 2007;104:666–72. doi: 10.1213/01.ane.0000253232.45403.e5. [DOI] [PubMed] [Google Scholar]
- 145.Hussain S, Clyburn P. Cell salvage-induced hypotension and London buses. Anaesthesia. 2010;65:659–63. doi: 10.1111/j.1365-2044.2010.06428.x. [DOI] [PubMed] [Google Scholar]
- 146.Taylor C, Cohen H, Mold D, et al., editors. on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group. The 2009 Annual SHOT Report 2010. Manchester, UK: SHOT; 2010. [Last accessed on: 01/22/2011]. Available from: http://www.shotuk.org/wp-content/uploads/2010/07/SHOT2009.pdf. [Google Scholar]
- 147.Obstetric Anaesthetists’ Association. National survey of obstetric cell salvage availability. London, UK: Obstetric Anaesthetists’ Association; 2010. [Last accessed on: 01/22/2011]. Available from: http://survey.oaa-anaes.ac.uk/PublicDisplay.aspx?id=90. [Google Scholar]
- 148.Waters JH. Indications and contraindications of cell salvage. Transfusion. 2004;44(12 Suppl):40S–4S. doi: 10.1111/j.0041-1132.2004.04176.x. [DOI] [PubMed] [Google Scholar]
- 149.Sugai Y, Sugai K, Fuse A. Current status of bacterial contamination of autologous blood for transfusion. Transfus Apher Sci. 2001;24:255–9. doi: 10.1016/s1473-0502(01)00067-2. [DOI] [PubMed] [Google Scholar]
- 150.Schwieger IM, Gallagher CJ, Finlayson DC, et al. Incidence of Cell-Saver contamination during cardiopulmonary bypass. Ann Thorac Surg. 1989;48:51–3. doi: 10.1016/0003-4975(89)90175-6. [DOI] [PubMed] [Google Scholar]
- 151.Ezzedine H, Baele P, Robert A. Bacteriologic quality of intraoperative autotransfusion. Surgery. 1991;109:259–64. [PubMed] [Google Scholar]
- 152.Locher MC, Sailer HF. The use of the Cell Saver in transoral maxillofacial surgery: a preliminary report. J Craniomaxillofac Surg. 1992;20:14–7. doi: 10.1016/s1010-5182(05)80189-1. [DOI] [PubMed] [Google Scholar]
- 153.Wollinsky KH, Oethinger M, Büchele M, et al. Autotransfusion-bacterial contamination during hip arthroplasty and efficacy of cefuroxime prophylaxis. A randomized controlled study of 40 patients. Acta Orthop Scand. 1997;68:225–30. doi: 10.3109/17453679708996689. [DOI] [PubMed] [Google Scholar]
- 154.Bland LA, Villarino ME, Arduino MJ, et al. Bacteriologic and endotoxin analysis of salvaged blood used in autologous transfusions during cardiac operations. J Thorac Cardiovasc Surg. 1992;103:582–8. [PubMed] [Google Scholar]
- 155.Holden F, Foley M, Devin G, et al. Coagulase-negative staphylococcal contamination of whole blood and its components: the effects of WBC reduction. Transfusion. 2000;40:1508–13. doi: 10.1046/j.1537-2995.2000.40121508.x. [DOI] [PubMed] [Google Scholar]
- 156.Siblini L, Lafeuillade B, Ros A, et al. Reduction of Yersinia enterocolitica load in deliberately inoculated blood: the effects of blood prestorage temperature and WBC filtration. Transfusion. 2002;42:422–7. doi: 10.1046/j.1525-1438.2002.00066.x. [DOI] [PubMed] [Google Scholar]
- 157.Siblini L, Lafeuillade B, Ros A, et al. Influence of blood prestorage conditions and white blood cell filtration on the bacterial load of blood deliberately inoculated with Gram-positive and Gram-negative pathogens. Vox Sang. 2004;87:241–9. doi: 10.1111/j.1423-0410.2004.00565.x. [DOI] [PubMed] [Google Scholar]
- 158.Waters JH, Tuohy MJ, Hobson DF, Procop G. Bacterial reduction by cell salvage washing and leukocyte depletion filtration. Anesthesiology. 2003;99:652–5. doi: 10.1097/00000542-200309000-00021. [DOI] [PubMed] [Google Scholar]
- 159.Liang TB, Li JJ, Li DL, et al. Intraoperative blood salvage and leukocyte depletion during liver transplantation with bacterial contamination. Clin Transplant. 2010;24:265–72. doi: 10.1111/j.1399-0012.2009.01091.x. [DOI] [PubMed] [Google Scholar]
- 160.Steneker I, van Luyn MJ, van Wachem PB, Biewenga J. Electronmicroscopic examination of white cell reduction by four white cell-reduction filters. Transfusion. 1992;32:450–7. doi: 10.1046/j.1537-2995.1992.32592327720.x. [DOI] [PubMed] [Google Scholar]
- 161.Pietersz RN, van der Meer PF, Seghatchian MJ. Update on leucocyte depletion of blood components by filtration. Transfus Sci. 1998;19:321–8. doi: 10.1016/s0955-3886(98)00056-3. [DOI] [PubMed] [Google Scholar]
- 162.Dzik W. Use of leukodepletion filters for the removal of bacteria. Immunol Invest. 1995;24:95–115. doi: 10.3109/08820139509062765. [DOI] [PubMed] [Google Scholar]
- 163.Smith RN, Yaw PB, Glover JL. Autotransfusion of contaminated intraperitoneal blood: an experimental study. J Trauma. 1978;18:341–4. doi: 10.1097/00005373-197805000-00008. [DOI] [PubMed] [Google Scholar]
- 164.Bowley DM, Barker P, Boffard KD. Intraoperative blood salvage in penetrating abdominal trauma: a randomised, controlled trial. World J Surg. 2006;30:1074–80. doi: 10.1007/s00268-005-0466-2. [DOI] [PubMed] [Google Scholar]
- 165.Durand F, Duchesne-Gueguen M, Le Bervet JY, et al. Rheologic and cytologic study of autologous blood collected with Cell Saver 4 during cesarean. Rev Fr Transfus Hemobiol. 1989;32:179–91. doi: 10.1016/s1140-4639(89)80039-5. [DOI] [PubMed] [Google Scholar]
- 166.Waters JH, Biscotti C, Potter PS, Phillipson E. Amniotic fluid removal during cell salvage in the cesarean section patient. Anesthesiology. 2000;92:1531–6. doi: 10.1097/00000542-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 167.Fong J, Gurewitsch ED, Kump L, Klein R. Clearance of fetal products and subsequent immunoreactivity of blood salvaged at cesarean delivery. Obstet Gynecol. 1999;93:968–72. doi: 10.1016/s0029-7844(98)00559-6. [DOI] [PubMed] [Google Scholar]
- 168.Thornhill ML, O’Leary AJ, Lussos SA, et al. An in-vitro assessment of amniotic fluid removal from human blood through cell saver processing. Anesthesiology. 1991;75(Suppl 3):A830. [Google Scholar]
- 169.Fuhrer Y, Bayoumeu F, Boileau S, et al. Evaluation of the blood quality collected by cell-saver during cesarean section. Ann Fr Anesth Reanim. 1996;15:1162–7. doi: 10.1016/s0750-7658(97)85873-x. [DOI] [PubMed] [Google Scholar]
- 170.Bernstein HH, Rosenblatt MA, Gettes M, Lockwood C. The ability of the Haemonetics 4 Cell Saver System to remove tissue factor from blood contaminated with amniotic fluid. Anesth Analg. 1997;85:831–3. doi: 10.1097/00000539-199710000-00021. [DOI] [PubMed] [Google Scholar]
- 171.Catling SJ, Williams S, Fielding AM. Cell salvage in obstetrics: an evaluation of the ability of cell salvage combined with leucocyte depletion filtration to remove amniotic fluid from operative blood loss at caesarean section. Int J Obstet Anesth. 1999;8:79–84. doi: 10.1016/s0959-289x(99)80002-8. [DOI] [PubMed] [Google Scholar]
- 172.Sullivan I, Faulds J, Ralph C. Contamination of salvaged maternal blood by amniotic fluid and fetal red cells during elective Caesarean section. Br J Anaesth. 2008;101:225–9. doi: 10.1093/bja/aen135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Clark S. Amniotic fluid embolism. Clin Obstet Gynecol. 2010;53:322–8. doi: 10.1097/GRF.0b013e3181e0ead2. [DOI] [PubMed] [Google Scholar]
- 174.Benson MD. A hypothesis regarding complement activation and amniotic fluid embolism. Med Hypotheses. 2007;68:1019–25. doi: 10.1016/j.mehy.2006.09.052. [DOI] [PubMed] [Google Scholar]
- 175.Clark SL, Pavlova Z, Greenspoon J, et al. Squamous cells in the maternal pulmonary circulation. Am J Obstet Gynecol. 1986;154:104–6. doi: 10.1016/0002-9378(86)90402-3. [DOI] [PubMed] [Google Scholar]
- 176.Kuhlman K, Hidvegi D, Tamura R, Depp R. Is amniotic fluid material in the central circulation of peripartum patients pathologic? Am J Perinatol. 1985;2:295–9. doi: 10.1055/s-2007-999974. [DOI] [PubMed] [Google Scholar]
- 177.Lee W, Ginsburg K, Cotton DB, Kaufman RH. Squamous and trophoblastic cells in the maternal pulmonary circulation identified by invasive hemodynamic monitoring during the peripartum period. Am J Obstet Gynecol. 1986;155:999–1001. doi: 10.1016/0002-9378(86)90334-0. [DOI] [PubMed] [Google Scholar]
- 178.Gist RS, Stafford IP, Leibowitz AB, Beilin Y. Amniotic fluid embolism. Anesth Analg. 2009;108:1599–602. doi: 10.1213/ane.0b013e31819e43a4. [DOI] [PubMed] [Google Scholar]
- 179.Clark SL, Hankins GD, Dudley DA, et al. Amniotic fluid embolism: analysis of the national registry. Am J Obstet Gynecol. 1995;172:1158–67. doi: 10.1016/0002-9378(95)91474-9. [DOI] [PubMed] [Google Scholar]
- 180.Knight M, Tuffnell D, Brocklehurst P, et al. UK Obstetric Surveillance System. Incidence and risk factors for amniotic-fluid embolism. Obstet Gynecol. 2010;115:910–7. doi: 10.1097/AOG.0b013e3181d9f629. [DOI] [PubMed] [Google Scholar]
- 181.Tuffnell D, Knight M, Plaat F. Amniotic fluid embolism - an update. Anaesthesia. 2011;66:3–6. doi: 10.1111/j.1365-2044.2010.06597.x. [DOI] [PubMed] [Google Scholar]
- 182.Conde-Agudelo A, Romero R. Amniotic fluid embolism: an evidence-based review. Am J Obstet Gynecol. 2009;201:445.e1–13. doi: 10.1016/j.ajog.2009.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ilstrup SJ, Dyga RM, Rios Yazer MH, editors. Standards For Perioperative Autologous Blood Collection And Administration. 4th ed. Bethesda, MD, USA: AABB; 2010. [Google Scholar]
- 184.Gilbert WM, Danielsen B. Amniotic fluid embolism: decreased mortality in a population-based study. Obstet Gynecol. 1999;93:973–7. doi: 10.1016/s0029-7844(99)00004-6. [DOI] [PubMed] [Google Scholar]
- 185.Dzick S. Leukodepletion blood filters: filter design and mechanisms of leukocyte removal. Transf Med Rev. 1993;7:65–77. doi: 10.1016/s0887-7963(93)70125-x. [DOI] [PubMed] [Google Scholar]
- 186.UK Cell Salvage Action Group; UK Cell Salvage Action Group. Intraoperative Cell Salvage: Education Workbook. UK: UK Cell Salvage Action Group; 2008. [Last accessed on: 01/22/2011]. Practicalities - reinfusion; p. 81. Available from: http://www.transfusionguidelines.org.uk/docs/pdfs/bbt-03_icsag_workbook-0812_all.pdf. [Google Scholar]
- 187.Parker J, Wray J, Gooch A, et al. Guidelines for the use of prophylactic anti-D immunoglobulin. London, UK: British Society for Haematology; 2006. [Last accessed on: 01/22/2011]. British Committee for Standards in Haematology. Available from: http://www.bcshguidelines.com/documents/Anti-D_bcsh_07062006.pdf. [Google Scholar]
- 188.Austin E, Bates S, de Silva M, et al. Working Party of the British Committee for Standards in Haematology, Transfusion Taskforce. Guidelines for the estimation of fetomaternal haemorrhage. London, UK: British Society for Haematology; 2009. [Last accessed on: 01/22/2011]. Available from: http://www.bcshguidelines.com/documents/BCSH_FMH_bcsh_sept2009.pdf. [Google Scholar]
- 189.Sandler SG, Sathiyamoorthy S. Laboratory methods for Rh immunoprophylaxis: a review. Immunohematology. 2010;26:92–103. [PubMed] [Google Scholar]
- 190.Murphy M, Pamphilon D, Weatherall D. Prenatal and childhood transfusions. In: Murphy M, Pamphilon D, editors. Practical Transfusion Medicine. 2nd ed. Oxford, UK: Blackwell Publishing; 2005. pp. 97–118. [Google Scholar]
- 191.Directive 2002/98/EC of the European Parliament and of the Council of 27 January 2003 setting standards of quality and safety for the collection, testing, processing, storage and distribution of human blood and blood components and amending Directive 2001/83/EC. Official Journal of the European Union. 08/2/2003;L 33:30–40. [Google Scholar]
- 192.Commission Directive 2004/33/EC of 22 March 2004 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards certain technical requirements for blood and blood components. Official Journal of the European Union. 30/03/2004;L 91:25–39. [Google Scholar]
- 193.Commission Directive 2005/61/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards traceability requirements and notification of serious adverse reactions and events. Official Journal of the European Union. 01/10/2005;L 256:32–40. [Google Scholar]
- 194.Commission Directive 2005/62/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards Community standards and specifications relating to a quality system for blood establishments. Official Journal of the European Union. 01/10/2005;L 256:41–48. [Google Scholar]
- 195.Robinson EAE. The European Union Blood Safety Directive and its implications for blood services. Vox Sang. 2007;93:122–30. doi: 10.1111/j.1423-0410.2007.00942.x. [DOI] [PubMed] [Google Scholar]
- 196.Muñoz M, Slappendel R, Thomas D. Laboratory characteristics and clinical utility of post-operative cell salvage: washed or unwashed blood transfusion? Blood Transfus. 2011;9:248–61. doi: 10.2450/2010.0063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Council Directive 93/42/EEC of 14 June 1993 concerning medical devices. Official Journal of the European Union. 12/07/1993;L 169:1–51. [Google Scholar]
- 198.Directive 2007/47/EC of the European Parliament and of the Council of 5 September 2007 amending Council Directive 90/385/EEC on the approximation of the laws of the Member States relating to active implantable medical devices, Council Directive 93/42/EEC concerning medical devices and Directive 98/8/EC concerning the placing of biocidal products on the market. Official Journal of the European Union. 21/09/2007;L 247:21–55. [Google Scholar]
- 199.Directive 2001/95/EC (Directive 2001/95/EC of the European Parliament and of the Council of 3 December 2001 on general product safety. Official Journal of the European Communities. 15/01/2002;L 11:4–17. [Google Scholar]
- 200.Council Directive 85/374/EEC of 25 July 1985 on the approximation of the laws, regulations and administrative provisions of the Member States concerning liability for defective products. Official Journal of the European Union. 07/08/1985;L 210:29–33. [Google Scholar]
- 201.Grazzini G. Clinical appropriateness of blood component transfusion: regulatory requirements and standards set by the Scientific Society in Italy. Blood Transfus. 2008;6:186–90. doi: 10.2450/2008.0049-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Recommendation Rec (2002) 11 of the Committee of Ministers to member states on the hospital’s and clinician’s role in the optimal use of blood and blood products. (Adopted by the Committee of Ministers on 10 October 2002 at the 811th meeting of the Ministers’ Deputies) [Last accessed on: 01/22/2011]. Available at: https://wcd.coe.int/ViewDoc.jsp?id=312229&BackColorInternet=B9BDEE&BackColorIntranet=FFCD4F&BackColorLogged=FFC679.
- 203.European Directorate for the Quality of Medicines & HealthCare of the Council of Europe. Recommendation no R (95) 15. 16th ed. Strasbourg: Council of Europe; 2011. Guide to the Preparation, Use and Quality Assurance of Blood Components. [Google Scholar]
- 204.Italian Republic. Law n. 219 of October 21st, 2005. New discipline of transfusion medicine activities and national production of blood derivatives. Gazzetta Ufficiale della Repubblica Italiana n. 251, October 27th, 2005.
- 205.Società Italiana di Medicina Trasfusionale e Immunoematologia (SIMTI) Standard di Medicina Trasfusionale. 2nd ed. Milano, Italia: SIMTI Servizi srl; 2010. [Last accessed on: 01/22/2011]. Available at: http://www.transfusionmedicine.org/linee_guida.aspx?ok=1. [Google Scholar]
- 206.Decreto del Ministro della Sanità 1 settembre 1995. Disciplina dei rapporti tra le strutture pubbliche provviste di servizi trasfusionali e quelle pubbliche e private, accreditate e non accreditate, dotate di frigoemoteche. Gazzetta Ufficiale della Repubblica Italiana, Serie Generale, n. 240, 13 ottobre 1995.
- 207.Ministero del Lavoro, della Salute e delle Politiche Sociali. Dipartimento della Qualità, Direzione Generale della Programmazione Sanitaria, dei Livelli di Assistenza e dei Principi Etici di Sistema, Ufficio III. Manuale Per La Sicurezza In Sala Operatoria: Raccomandazioni E Checklist. [Last accessed on: 01/22/2011]. Available at: http://www.ministerosalute.it/imgs/C_17_pubblicazioni_1119_allegato.pdf.
- 208.Murphy MF, Wallington TB, Kelsey P, et al. British Committee for Standards in Haematology, Blood Transfusion Task Force. Guidelines for the clinical use of red cell transfusions. Br J Haematol. 2001;113:24–31. doi: 10.1046/j.1365-2141.2001.02701.x. [DOI] [PubMed] [Google Scholar]
- 209.Stainsby D, MacLennan S, Thomas D, et al. British Committee for Standards in Haematology. Guidelines on the management of massive blood loss. Br J Haematol. 2006;135:634–41. doi: 10.1111/j.1365-2141.2006.06355.x. [DOI] [PubMed] [Google Scholar]
- 210.Ortiz P, Mingo A, Lozano M, et al. Sociedad Española de Transfusión Sanguínea. Guía sobre la transfusión de componentes sanguíneos. Med Clin (Barc) 2005;125:389–96. doi: 10.1157/13079172. [DOI] [PubMed] [Google Scholar]
- 211.Leal-Noval R, Muñoz M, Páramo JA, García-Erce JA for the Spanish Expert Panel on Alternatives to Allogeneic Blood Transfusion. Spanish consensus statement on alternatives to allogeneic transfusions: the ‘Seville document’. Transfus Altern Transfus Med. 2006;8:178–202. [Google Scholar]
- 212.Liumbruno G, Bennardello F, Lattanzio A, et al. Recommendations for the transfusion of red blood cells. Blood Transfus. 2009;7:49–64. doi: 10.2450/2008.0020-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use. Official Journal of the European Union. 28/11/2004;L 311:67–128. [Google Scholar]
- 214.Knight M, Spark P, Pierce M, Kayem G, Kurinczuk JJ, Brocklehurst P, editors. on behalf of UKOSS. United Kingdom Obstetric Surveillance System (UKOSS) Annual Report 2010. Oxford UK: National Perinatal Epidemiology Unit; 2010. [Last accessed on: 01/22/2011]. Available from: https://www.npeu.ox.ac.uk/downloads/ukoss/UKOSS_annual_report_2010.pdf. [Google Scholar]
- 215.Tanqueray T, Allam J, Norman B, Cox M. Leucocyte depletion filter and a second suction circuit during intra-operative cell salvage in obstetrics [letter] Anaesthesia. 2010;65:207–8. doi: 10.1111/j.1365-2044.2009.06212_1.x. [DOI] [PubMed] [Google Scholar]