Introduction
For 150 years, since Paul Erhlich discovered basophils by light microscopy1, these cells have not been given the attention paid for years to lymphocytes or other blood cells, although in the last few years they have been given some slight recognition2. Recent studies have defined previously unrecognised roles for basophils in both allergic responses and immune regulation, and have markedly altered our image of these cells, from a neglected minority to key players in the immune system. Research on basophils was hampered for a long time because of the paucity of these cells in peripheral blood, which often resulted in scant yields and difficulty in their isolation, forcing researchers to use time-consuming and quite expensive approaches for their physical purification. Furthermore, the lack of genetically homogeneous cell lines and of a true genetic knock-out animal model (only mice depleted of basophils by anti-receptor antibodies and a few transgenic animals are available), the existence of a relatively high response variability among the healthy population and the difficulty of purifying the cells from blood have made it very difficult to study this subgroup of leucocytes. With the respectable exception of allergists, it seems that most immunologists and immuno-haematologists have given up. All this has shifted attention to mast cells, which are easier to handle. Nevertheless, in clinical settings, basophils are a practical and convenient alternative for the diagnosis of allergy because they are much more accessible than tissue-resident mast cells and it is sufficient to take a blood sample in order to obtain them.
Basophils are circulating blood granulocytes that are mainly involved in hypersensitivity (atopic) and anaphylactic reactions2–5; they stem from CD34+ hematopoietic progenitor cells in bone marrow (lineage-negative CD34+FcɛRIhic-Kit−), for which differential expression of the key transcription factors C/EBPα and GATA-2 appears to play the major role in lineage determination leading to mast cell and basophil differentiation6. Basophilic cells are able to promote chronic allergy inflammation2,4, to regulate Th2 cell function7,8 and immune cell memory9,10 and even to behave as antigen-presenting cells11. When activated, basophils degranulate to release histamine, proteoglycans (e.g. chondroitin and chondroitin sulphate), and several proteolytic enzymes (e.g. elastase and lysophospholipase). They also secrete lipid mediators such as leukotrienes and prostaglandins (LTC4, PGD2), express cysteinyl leukotriene (LTD4, LTE 4) receptors12, produce several important cytokines (IL-3, IL-4, IL-6, IL-9, IL-13, IL-25), chemokines (RANTES, MIP-1α, MIP-1β, MCP-1) and GM-CSF but not IFN-γ, IL-17 or IL-513–18 and express activation-related membrane markers19, many of which are useful for dissecting basophil function and diagnosing allergies20,21. Histamine and proteoglycans are pre-stored within cellular granules while the other secreted substances are newly generated. Each of these substances contributes to the onset of inflammation16. Table I lists the functions of basophils while Table II presents the mediators released and the receptors expressed by these cells.
Table I.
Function and properties of basophils and diagnostic cellular markers.
| Properties | Functions in the immune system | Phenotypic and activation markers |
|---|---|---|
| Metachromatic granulocytes Size: 7–11 μm Multilobed nucleus High concentration of intracellular anionic granules Contents of granules: GAG, herapan sulphate, major basic protein. Charcot-Leyden crystals Tryptase: only type B Location: blood, tissue, lymph nodes Lifespan: a few days Development and maturation: bone marrow Common progenitor with mast cell Activation by IgE-mediated and IgE-non mediated mechanisms Release of vasoactive amines (histamine) and other mediators Degranulate when activated Scant yields in blood specimens Easily activated spontaneously (autocrine IL-3) No pure cell lines existing Few transgenic mouse models as knock out animals Time consuming and quite expensive purification procedures Used as cellular probes to diagnose allergy (basophil activation test) in flow cytometry Basophils are both effector and regulatory cells Population: 10–15% possess no responder (syk deficient) basophils |
Effector cells in hypersensitivity reactions Main innate cell producing IL-4 Production of IL-13 Up-regulation of CD63, CD203c and other molecules Degranulation Production of histamine and LTC4 Release of other inflammatory mediators Anaphylaxis by IgE/histamine mechanism Anaphylaxis by IgG/PAF mechanism Rolling and diapedesis with selectins and integrins Transmigration to inflamed tissue Down-regulation by allergens/IgE/FcɛRI Down-regulation by histamine (H2) Effector cells in innate defence Able to be activated by non-IgE-mediated mechanisms Production of IL-4 (towards Th2) Production of IL-3, GM-CSF and IL-6 Activation by PAMP, TLR and PAR Expression of MHC class I Regulatory cells in acquired immunity Antigen presenting cell (APC) Expressing MHC class II molecules Role of Th0 → Th2 maturation: production of TLSP and IL-4 Presenting CD40, CD80 and CD86 Aid dendritic cells in producing IL-4 Enhancing B-cell memory Production of IL-6 through FcɛRI regulation by Fcγ receptors Regulation of Th2/Th1 balance with histamine Regulation of B-cell survival and function by IgD Participating in Treg down-regulation of allergy |
Phenotypic membrane markers Anti-IgE CD45 CD203c CD193 (CCR3) CD13 CD123 CRTH2 HLADR Membrane markers of activation CD63 CD203c CD193 (CCR3) CD164 CD107a CD13 CD69 Main intracellular markers of activation Cytokines MAPK-38p Spleen tyrosine kinase (syk) Calcium Other kinases/proteases Flow cytometry gating protocols Anti-IgE-FITC or anti-IgE-PE CD45dim anti-IgEbright HLADRneg CD123bright CD45dim HLADRneg CD123bright CD3neg CRTH2pos CD3negCCR3pos SSC/CD203pos CD14dimCD13pos |
Table II.
Main molecules secreted and expressed by basophils.
| Mediators | Role | Main membrane receptors | Ligands | Main membrane receptors | Ligands |
|---|---|---|---|---|---|
| Histamine PAF (highly expressed) Granzyme B β-hexosaminidase Chondroitin sulphate Elastase Lysophospholipase Tryptase B LTC4 |
These compounds are released during basophil activation and degranulation (exocytosis through degranulation). They are immune mediators (histamine and PAF), vaso-active amines and endothelial interacting substances. | Receptors for chemokines | Complement receptors | ||
| CCR1 | MIP-1α | CD11b (Mac-1) | CD54, iC3b | ||
| CCR2 | MCP-1 | CD11c | Fibrinogen | ||
| CD193 (CCR3) | Eotaxin | C3bi, C3d, C3g | |||
| CD195 (CCR5) | CD21 | EBV, C3d, C3g | |||
| CXCR1 | IL-8 | CD35 | C3b, C4b | ||
| CXCR2 | GRO-α | CD46 | C3b, C4b | ||
| CD170 (CXCR4) | SDF-1 | CD55 | C4b/2a, C3b/Bb | ||
| CRTH2 | PGD2 | CD59 | C5b-8, C5b-9, CD2 | ||
| CD88 | C5a | ||||
|
Main cytokines IL-4 (highly expressed) IL-13 IL-3 IL-5 IL-6 IL-25 |
Main cytokines are IL-4 (which drives to Th2 and B-cell maturation, IgE production), IL-13 which promotes allergy inflammation, IL-5, which is a chemoattractant for eosinophils, IL-6 and IL-25 as immune modulators. (for other information see text) | Receptors for cytokines | |||
| CD25 | IL-2 | Immunoglobulin receptors | |||
| CD116 | GM-CSF | ||||
| CD123/CD131 | IL-3 | LIR | |||
| CD124 | IL-4 | FcɛRI | |||
| CD125 | IL-5 | FcγRIIA, FcγRIIB | |||
| CD128 | IL-8 | ||||
| IL-18R | IL-18 | Selectins and integrins | P- and L- selectins | ||
| IL-25R | IL-25 (IL-17E) | CD15s (Lex) | MADCAM, CD34 | ||
| IL-33R | IL-33 | CD62L | P- and L- selectins | ||
|
Growth factors GM-CSF TLSP VEGF |
TLSP is the main growth factor, together with IL-4, for Th0 (naïve) maturation to Th2. GM-CSF and IL-3 are basophil growth factors and survival cytokines, VEGF (mostly produced by mast cells) contributes to endothelial tissue repair and growth during the inflammatory response | CD162 | ICAM-1, 2 and 3 | ||
| Toll-like receptors | CD11a (LFA-1) | (CD54) | |||
| TLR-2 | PDG | ICAMs | |||
| TLR-4 | LPS | CD18 | Laminin, collagen | ||
| TLR-9, TLR-10 | CD29 | Hyaluronic acid | |||
| CD44 | Laminin, collagen | ||||
| Other receptors | VLA-1 | Fibronectin, VCAM-1 | |||
| HLA class I | VLA-4 | ||||
| HLA class II (low expr.) | |||||
| CD40 | CD40L | ||||
|
Chemokines MIP-5 Eotaxin, RANTES IL-8, MPC-1 Leptin CCL22 (MDC) MIP-1α, MIP-1β CCL3 CCL5 |
Most of the chemokines attract neutrophils and monocytes towards the site of inflammation | CD40L | CD40 | ||
| Basophilin, 212H6 | Unknown Substance P, NGF | ||||
| Somatostatin | Unknown Substance P, NGF | ||||
| Substance P, NGF-R |
Since their first description1, basophils have been recognised as unique, white-blood cells with metachromatic-staining properties. The most outstanding characteristics of basophils, including expression of high-affinity receptors for IgE, histamine content, and metachromatic staining, are also the prerogative of tissue-dwelling mast cells, with which basophils share a common haematopoietic lineage6. This seems to justify why mast cells often replace basophils in cellular investigations of allergy. Mast cells and basophils are both granulated cells which appear to possess complex and partially overlapping roles in acquired and innate immunity, including both effector and regulatory activities. Basophils have, however, recently gained new consideration given their strategic role in immunity, as these cells regulate many pathways linking innate to acquired immunity and the differences between these cells and their tissue "relatives", mast cells, have been widely highlighted2. Like other granulocytes basophils are motile cells; along with the progression of allergic reactions, basophils migrate from the blood compartment to inflamed tissues and act as allergic, inflammatory cells. Mast cells and basophils cooperate in exacerbating22 and/or modulating inflammation as well as in mediating subsequent tissue repair23. During inflammation mast cells release a series of potent pro-angiogenic molecules that stimulate both vessel sprouting and new vessel formation. Recently reported data suggest that basophils may play a role in inflammation-related angiogenesis, mainly through the expression of several forms of vascular endothelial growth factors and their receptors23.
New roles for basophils in the immune system
Most physicians believe that basophils are only loose cannons ready to burst vasoactive substances into the micro-environment when, once sensitised, they encounter an allergen or when they are triggered by a stimulating factor, giving rise to hypersensitivity reactions. Although they do have this property, basophils play an important role against many parasites, as well24. Given their similarities to mast cells, basophils have often been neglected and considered as a "redundant" mast cell-like circulating population25; notwithstanding, several differences between mast cells and basophils were recently outlined18. Basophils are predominantly found in peripheral blood but they also enter lymph nodes and spleen and are recruited to the site of inflammation following exposure to allergens or helminth parasites26. These cells are directly activated by nematodes proteins: at least one glycoprotein from Schistosoma genus parasites, IPSE/α-1, is thought to activate basophils to produce large amounts of interleukin (IL)-4 by non-specifically interacting with IgE and/or with the high affinity receptor for IgE, FcɛRI, inducing receptor cross-linking, unlike various other helminth antigens27,28. Indeed, several reports have confirmed the role of basophils in eradicating helminths, for example Trichuris muris29,30. Besides Th2 CD4+ lymphocytes, basophils are the major innate cells producers of IL-4 during primary helminth infection, as for instance with the nematode Necator americanus. During parasitosis, IL-4-producing basophils were detected systemically, and tissue recruitment occurred independently of IL-4/STAT6 signaling31. In fact, the innate immune response in basophils is regulated by the existence of a large network of cell surface receptors and of pathogen-derived molecules. The main innate receptors are able to promote IL-4 production, bypassing the FcɛRI/IgE/allergen cross-linking when respective ligands activate them: Toll-like receptors (TLR2 and TLR4, whose ligands are bacterial peptidoglycan and lipopolysaccharide, respectively)32, CpG receptors (whose ligands are small nucleic acid sequences mostly from viruses), protease activated receptors (PAR), formylated peptides receptors (FPR)29, many soluble cytokines which have their receptors on the basophil membrane: granulocyte-monocyte colony-stimulating factor (GM-CSF), IL-18, IL-33 and IL-329,33–35 and the so-called "superallergens" or "superantigens"36, which are represented by viral, bacterial or other pathogen patterns that are able to cross-link FcɛRI in the absence of specific IgE37. A few years ago, debate was raised about the role of superallergens as superantigens in dermatological diseases, such as atopic dermatitis, in humans38. Some cell surface receptors, such as CD200R3, a member of the family of CD200 receptors39 and leucocyte immunoglobin-like receptors (LIR: LIR2, LIR3 and LIR7)40 are able to cross-link the IgE/receptor complex and to elicit IL-4 production. The role of IL-4, together with IL-13, is fundamental for allergic disease and for the onset of the inflammatory response in which innate and acquired immunity may play their defensive potential5. IL-4 is the main cytokine that links innate immunity with Th2 maturation and acquired immunity: actually, basophils are initiators, regulators and effectors of type 2 inflammation and have been called "innate Th2 cells"24.
Basophils contribute to the regulation of acquired immune response, by acting as innate immunoregulatory cells18,41, although a possible role in promoting allergic inflammation and in inducing the onset of chronic allergy has been also suggested2,4. Basophils are indeed important IL-4-providing inducers of Th28,13. Dendritic cells play a central role in the initiation of Th1- and Th17-mediated immunity and the differentiation of these cells requires a combination of both cellular surface and secreted signals in order to activate and differentiate these effector lymphocytes from naïve CD4+ T cells. Until recently it was thought that dendritic cells played an analogous role in Th2 differentiation, despite the fact that dendritic cells are unable to produce the Th2-inducing IL-4. Dendritic cells are capable of inducing Th2 differentiation through surface molecules such as Jagged and OX-40L8. When a non-T-cell source has been suggested as necessary to provide the IL-4 needed for the generation of functional Th2-mediated immune responses, basophils are the main possible candidates as IL-4-producing innate cell42,43. The importance of basophil-derived IL-4 during in vivo Th2 immunity does, however, remain to be assessed, as basophils produce other factors able to promote Th2 immunity in lymph nodes, such as thymic stromal lymphopoietin (TLSP)43. Researchers have not yet reached a general consensus about the role of basophils in Th2 cell differentiation but evidence has been reported concerning the involvement of basophils in T-cell destiny in the lymph node44. According to the results of studies performed in mouse models, the role of basophils in initiating Th2 cell differentiation could be described as follows: (i) basophils are transiently recruited via blood vessels to the T-cell zone in lymph nodes, provide IL-4 to naïve CD4+ T cells while dendritic cells, recruited via lymphatic vessels, work as antigen-presenting cells and co-operate in Th2 differentiation; (ii) basophils themselves act as antigen-presenting cells, as they express MHC class II antigens and co-stimulatory molecules (CD80, CD86 or CD40)44. Recent studies demonstrated that basophils, rather than dendritic cells, are the critical antigen-presenting cells for driving Th2 cell differentiation44–46. However, some controversy remains, as the level of MHC II expression in basophils is much lower than that in other classic antigen-presenting cells and because HLA-DR+ basophils have not been reported, although there is recent evidence that a fraction of basophils expresses HLA-DR in response to IL-3 stimulation46. Further insights are required to elucidate this issue, although data were reported suggesting that dendritic cells may not be essential for the development of Th2 responses and that basophils should play a leading role47.
A synoptic model about the possible involvement of basophils and dendritic cells in Th2-differentiation was proposed by Karasuyama and colleagues quite recently: (i) dendritic cells behave as antigen-presenting cells and rule all Th2 differentiation; (ii) dendritic cells and basophils co-operate; (iii) basophils are able to replace dendritic cell function completely (both as antigen-presenting cells and as IL-4-providing cells). Moreover, only for Th2 cell differentiation, the existence of a late-activator antigen-presenting cell (LAPC), able to do everything and involving GATA-3 expression, has also been discovered in the mouse44,48. Certainly, the role of basophils in inducing a Th2 immune response is a hallmark of these leucocytes, in addition to their involvement in hypersensitivity reactions and chronic allergy: this appears to be supported by the fact that even non immune-related factors, such as retinoic acid produced by IL-3-stimulated basophils, are able to promote Th2 differentiation, at least in vitro, thus enlarging the panoply of immuno-modulators related to basophil function49.
While a newly discovered role of basophils in immunity appears to be related to Th2-differentiation, the involvement of these cells in connecting innate to acquired immunity seems to be more substantial than previously thought. The immuno-modulatory property of basophils is represented mainly by these cells' ability to skew Th2 responses towards allergens and parasites18. This is an ability of basophils more than of mast cells. Circulating basophils exert a regulatory role in that they express MHC-II molecules, possess the cellular machinery to take up, process and present antigens to T cells on MHC-II molecules and release IL-4 that leads to T-cell skewing to a Th2 phenotype.
Basophils express MHC class I molecules and CD86 on their cell surface and, therefore, have the potential to present antigens to CD8+ cells, then altering CD8+ T-cell differentiation into IL-10-producing phenotypes50, whereas CD8+ cells activated by dendritic cells produce IFN-γ. At least in the mouse, basophils are able to produce IL-6: the production of IL-4 and of IL-6 by antigen-exposed basophils requires the Fc receptor γ-chain (FcγR): FcγRI, FcγRIIB and FcγRIII are common and important regulatory signalling components of FcɛRI51, as well as many other basophil membrane receptors52. IL-6 is involved in the mechanism of basophil-mediated amplification of humoral memory response9,10. When activated by IL-3 or FcɛRI/IgE cross-linking, basophils induce B-cell proliferation and the production of IgM and IgG1 in the presence of activated CD4+ T-cells: this B-cell proliferation requires IL-4, IL-6 and cell contact. Both IL-6 release and interactions between CD40 and its ligand CD40L were recognised as a prerogative of activated basophils (CD40L is expressed in basophils); in vitro evidence indicates that activated basophils enhance the humoral memory response both by secreting IL-6 and by altering the CD4+ T-cell phenotype to help B cells better by producing IL-4, IL-5, IL-10, IL-13 and the transcription factor GATA-3 and also by down-regulating the production of IFN-γ and IL-210. Basophils support humoral memory immune responses by increasing B-cell proliferation and Ig production as well as by inducing a Th2 and B helper phenotype in T cells. There is evidence from mouse models that in the absence of basophils, plasma cells of naïve or immunised mice rapidly undergo apoptosis in vitro and produce only low amounts of immunoglobulins. In contrast, in the presence of basophils and, even more, in the presence of activated basophils, the survival of plasma cells is markedly increased and continuous production of immunoglobulins is enabled: basophils are important for the survival of plasma cells in vitro and in vivo53.
In the case of an allergic reaction, antigen-presenting cells introduce processed allergens to T-helper lymphocytes, where a decision to develop different types of T-cell immunity is made under the influence of several cytokines, chemokines, co-stimulatory signals and regulatory T cells. Among Th2-type cytokines, IL-4 and IL-13 are responsible for class switching in B cells, which results in the production of allergen-specific IgE antibodies that bind to specific receptors on mast cells and basophils. These two cytokines are the main interleukins produced by basophils54.
Basophils in allergy and in inflammation
So, what can basophils do in immunity and during inflammation?
Unlike mast cells, basophils are dispensable for IgE-mediated systemic anaphylaxis. Instead, basophils play the major role in IgG-mediated systemic anaphylaxis. In vivo depletion of basophils protects mice from anaphylactic death. Upon capture of IgG-allergen complexes, basophils release platelet-activating factor, which increases vascular permeability, leading to anaphylactic shock. Thus, there are two major, distinct pathways to allergen-induced systemic anaphylaxis: one mediated by basophils, IgG and platelet-activating factor, and the other 'classical' pathway mediated by mast cells, IgE and histamine55. As already discussed above, basophils, as typical innate response leucocytes, can be activated by the well-known allergic FcɛRI/IgE-mediated pathway but also by many non-IgE mediated signals, including cytokines (IL-3, IL-18, IL-33), proteases, parasite antigens and bacterial and viral molecules otherwise indicated as pathogen-activated molecular patterns (PAMP), recognised by Toll-like receptors and pattern recognition receptors (PRR); although previous studies showed that basophils do express CD1456, this marker is weakly expressed on polymorphonuclear leucocytes, including basophils. Basophil preparations constitutively express mRNA of several Toll-like receptors, including TLR2, TLR4, TLR9 and TLR10. TLR mRNA expression in basophils is generally less prominent than that in neutrophils and monocytes, but basophils expressed significantly higher levels of TLR2 and TLR4 mRNA than did eosinophils. Toll-like receptor activation related to up-regulation of integrins, such as CD11b molecule, is elicited by IFN-γ: treatment with this cytokine enables basophils to respond to bacterial lipolysaccharide through TLR4 and up-regulates CD11b expression. However, the surface levels of TLR2 and TLR4 on basophils are not apparently affected by IFN-γ. These results suggest that TLR4 on basophils may be involved in the pathogenesis of infection-induced exacerbation of allergic inflammation by modulating basophil functions57.
Activated basophils secrete cytokines that support the development of IL-4-producing CD4+ T cells and of IgE-secreting B cells associated with the Th2 immune response. The impact of allergens and microbial antigens on effector cells and antigen-presenting cells in allergic diseases is usually described as follows: allergens bind specifically to IgE linked to the high-affinity receptor for IgE (FcɛRI) and stimulate a cascade of cellular events. In contrast, microbial antigens are recognised by pattern-recognition receptors of the innate immune system, to which Toll-like receptors belong. Given the high number of microbial antigens, allergens and other soluble ligands in the cellular microenvironment in vivo, it is very likely that not only separate, but also concomitant stimulation of both receptor types, i.e. FcɛRI and Toll-like receptors, occurs frequently under physiological conditions and in particular in the context of allergic and infectious disorders58. In allergic inflammation, basophils produce IL-4 and IL-13 and express CD40L, events that come into play during B-cell activation and immunoglobulin class switching. After proper stimulation, basophils, in contrast to mast cells, are able to target B cells and to induce their production of IgE and regulatory IgG4 antibodies29.
The main players involving and recruiting basophils in inflammation are cytokines, chemokines, tissue factors and molecules, as well as other leucocytes. Basophils are able to migrate into the inflamed tissue when they are recruited by cytokines such as GM-CSF, IL-3 and IL-5 or by the chemokines MCP-1, eotaxin, TARC, MDC for which these cells possess related receptors, chemokine receptor CCR2, CCR3 and CCR4, respectively and CCR1 for RANTES. Eotaxin/chemokine ligand CCL11 induces the most potent basophil migration into the tissue and, together with RANTES/CCL5 the strongest trans-endothelial migration, while IL-3, IL-8 and RANTES contribute to migration across the basement membrane, where a role is played by metallo-proteinase 959,60. During allergic inflammation, basophils, attracted by soluble mediators from tissues and other leucocytes, migrate from the blood compartment to inflammatory sites, where they act as effector cells in concert with eosinophils. Trans-endothelial migration is an essential step in the extravasation of cells; adhesion molecules such as beta2-integrins, P-selectins, CD49d and the L-selectin CD62L, whose ligands are CD34 and MADCAM-1, are involved in basophil rolling and play the primary role in basophil trans-endothelial migration, but beta1 integrins are also involved, especially in trans-endothelial migration of cytokine/chemokine-stimulated basophils61,62. With the exception of the activation mediated by MCP-1/CCR2, the trans-endothelial migration of basophils is comparable to that of eosinophils62. Many newly discovered cytokines, such as IL-18, IL-17, IL-33 and IL-25 and histamine itself, are involved in the regulation of basophils that have reached the inflammatory site. Basophils exert effector functions during allergy and cause most of the typical symptoms through several compounds produced and released upon cross-linking of IgE-high affinity receptor in the course of immediate or late phase reactions following allergen exposure. In this context, a long-standing paradigm is that antigen-specific Th2 cells and their cytokines such as IL-4, IL-5, and IL-13 orchestrate the characteristic features of atopic allergy. Recently, the discovery of a role for IL-17-producing (Th17) and IL-22-producing (Th22) T helper cells in inflammatory diseases has added an additional layer of complexity to the understanding of the pathogenesis of allergic diseases, mostly in allergic asthma and atopic dermatitis63. Recently the Th1/Th2 concept has been revised and Th17 cells have been implicated in allergy; however, despite clear correlative evidence, the cellular and molecular bases for the connection between increased IL-17A and IgE in allergy have not yet been elucidated64. A study by Milovanovich et al. showed that IL-17A+ cells promote IgE production and that IL-17A exerts its pro-allergic effect directly at the level of B cells65. IL-25 has been related recently with atopic dermatitis, a common skin disease associated with a Th2 response and increased levels of Th2-associated cytokines and IgE. The mechanism resulting in skewing the immune response in a Th2 direction in atopic dermatitis are not fully elucidated; however, such skewing has recently been associated with IL-25 in a murine model of allergic airway disease. IL-25 produced by dendritic cells could have a dual role as both an inducer of the Th2 response and as an inhibitor of filaggrin synthesis, thereby directly affecting skin barrier function in patients with atopic dermatitis66. IL-25, otherwise known as IL-17E, also enhances the expansion of TSLP- dendritic cell-activated Th2 memory cells67.
IL-18 has pleiotropic effects in inflammatory foci and also plays a role in the onset of asthma68. In association with IL-12, this cytokine stimulates various cells to produce significant amounts of IFN-γ, which in turn activates macrophages to produce nitric oxide, leading to the eradication of intracellular pathogens, such as the protozoan Leishmania major. However, IL-18 alone is also able to promote the production of Th2 cytokines by T cells and basophils. In this context, IL-18 shows the ability to regulate both Th1 and Th2 responses depending on its cytokine milieu69. As the expulsion of some types of gastrointestinal nematodes depends on Th2 responses, this has raised speculation about the protective roles of IL-18 against helminth infection70. IL-33, a member of the IL-1 cytokine family which includes IL-1 and IL-18, is also considered to be crucial for induction of Th2-type cytokine-associated immune responses such as host defence against nematodes and allergic diseases by inducing production of Th2-type cytokines such as IL-5 and IL-13 by Th2 cells, mast cells, basophils and eosinophils. IL-33 is involved in the induction of non-Th2-type acute and chronic inflammation as a pro-inflammatory cytokine, similar to IL-1 and IL-1871.
Some author has investigated basophils and dendritic cells for their critical role in Th2 induction and suggested that newly identified cell populations inducing cytokines IL-25, IL-33 and thymic stromal lymphopoietin are also involved in the development of Th2 responses in asthma72,73. IL-33, which is produced by epithelial and endothelial cells, has gained much interest in recent years due to its similarity to IL-18 and its role in inflammatory mechanisms71 and in B1-cell activation exacerbating contact sensitivity74.
Basophils as effector and immuno-regulatory cells
Understanding of the role of basophils in the immune system has changed dramatically. Although basophils are well-known effector cells in hypersensitivity reactions and chronic allergy, in addition to resident mast cells, their role in building up those regulating relationships between innate and acquired immunity is becoming increasing clear. The latest evidence concerning the role of basophils as immuno-modulatory cells is being gained from assessment of their strategic function in the immune system29,75. Some substances released from basophils play a role in this scenario. Histamine itself has an immuno-regulatory function. Several studies have indicated the many functional roles of histamine in immunity and hematopoiesis76. For example, histamine has an important role in the complex cytokine network, regulating cytokine production by immune cells through distinct receptor signalling and biological effects, a type of regulation which is particularly relevant in the context of Th1/Th2 differentiation76. Broadly speaking, histamine should not only be considered the major mediator of acute inflammatory and immediate hypersensitivity responses, but also be appreciated as affecting chronic inflammation and regulating several essential events in the immune response77,78. Dendritic cells express all four types of histamine receptors (H1R to H4R), while basophils express only H2R and Th-lymphocytes also H1R. In Th1 cells the expression of H1R is predominant, but not exclusive, whereas Th2 cells and basophils show mainly up-regulation of H2R. Histamine produced by basophils and tissue mast cells up-regulates IL-10 and down-regulates IL-12 in antigen-presenting cells79. IL-10 is a suppressor cytokine and one of the major regulatory agents of inflammatory responses. The modulatory response of histamine is also exerted by down-regulating the inflammatory response acting on IL-12 production by antigen-presenting cells, on CD86 up-regulation, on self-induction of IL-1α and by interacting with basophil H2R, so suppressing histamine production. At the same time histamine shifts the Th1/Th2 balance towards Th1, by down-regulating Th2 proliferation and IL-4 and IL-13 production and by up-regulating Th1 proliferation and IFN-γ release. It is possible that IL-3 amplifies this negative-feedback by increasing H1R expression on Th1 cells80.
Besides histamine and a panoply of cytokines and chemokines, a newly discovered immuno-regulatory task of basophils involves its relationship with IgD81. There is recently reported evidence that IgD is an important immuno-modulator that drives an ancestral surveillance system at the interface between immunity and inflammation. Basophils are able to bind circulating IgD. Basophils exposed to IgD-reactive antigens migrate to systemic and mucosal lymphoid organs, possibly in response to chemotactic factors released by IgD-stimulated mast cells. Tissue-recruited basophils enhance immune protection by releasing B-cell-stimulating, chemotactic pro-inflammatory factors, as well as antimicrobial and opsonising mediators, such as cathelicidin, IL-1b, IL-4, IL-8, IL-13, CD40L, B-cell activating factor (BAFF), a proliferation-inducing ligand (APRIL), tumour necrosis factor-alpha (TNF-α), and CXCL10, but they not release histamine. Finally, IgD-armed basophils may regulate B-cell homeostasis through tonic release of the obligatory B-cell survival factor BAFF in response to IgD-reactive foreign or autologous antigens81. Another immuno-modulatory role played by basophils regards their participation in T-reg involvement in allergy82,83. Skewing of allergen-specific effector T cells to T-reg cells appears to be a crucial event in the control of healthy immune responses to allergens and for successful allergen-specific immunotherapy. The increased levels of IL-10 and transforming growth factor-beta (TGF-β) that are produced by T-reg cells, potently suppress IgE production, while simultaneously increasing the production of non-inflammatory isotypes IgG4 and IgA, respectively. Moreover, T-reg cells directly or indirectly suppress effector cells of allergic inflammation such as mast cells, basophils, and eosinophils83. On the other hand, Th17 cells are characterised by their expression of IL-17 (or IL-17A), IL-17E (or IL-25), IL-6, TNF-α and IL-22, which coordinates local tissue inflammation through up-regulation of pro-inflammatory cytokines and chemokines56.
T-cell subsets have recently been reported to be involved in the regulation of atopy: in fact, T-reg cells, such as Th17 and Th9, play a central role in the maintenance of peripheral homeostasis, they act in the establishment of controlled immune responses, and the inhibition of allergen-specific effector cells and control local tissue inflammation through the up-regulation of pro-inflammatory cytokines and chemokines84.
As regulatory cells in immunity, basophils interact with several immuno-competent cells, often in a bidirectional fashion. Besides their relationship with CD8+ T-cell and B lymphocytes, basophils participate in the skewing of macrophage differentiation towards the M2 phenotype, together with IL-3 and GM-CSF85, play a role during angiogenesis by producing several vascular endothelial growth factors (VEGF) and their receptors86, interact and regulate IL-3 and GM-CSF producing invariant NKT (iNKT) cells87 and finally participate in the regulatory basophil/plasmacytoid dendritic cell axis by IFN-α and histamine88. Invariant NKT are very interesting in chronic allergy, too. It has been demonstrated that TLR7, which recognises single strand RNA (viruses), when stimulated triggers not only antiviral responses, but also alleviates experimental asthma. Invariant NKT cells express TLR7 and respond to ligands by producing high levels of IFN-γ in the presence of IL-12, findings consistent with the conclusion that their contribution to the alleviation of allergic inflammation upon treatment with TLR7 ligands is mediated through IFN-γ89. The immuno-regulatory property of basophils has suggested the utility of new pharmacological substances, for example plant flavonoids90,91 while clinicians are calling for the search for new drugs able to modulate basophil function and for allergy therapy, even in autoimmune diseases29. Figure 1 summarises the main functions of basophils in the immune system.
Figure 1.
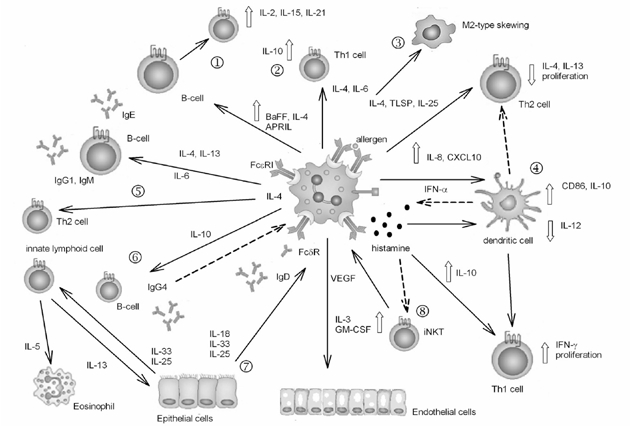
Cartoon illustrating the effector functions and immune regulatory actions involving basophils. Dashed lines show inhibitory and feedback pathways. ➀Basophil participation in B-cell survival, maturity and apoptosis: IgD, binding to basophils, contribute to immuno-regulation towards B cells (by IL-4, B➀AFF and APRIL) and towards dendritic cells in which it up-regulates IL-8 and CCL10 production. ➁IL-6 induction of IL-10 by Th1 cells: basophil regulates the role of dendritic cells in balancing Th1/Th2 responses, participates in the skewing of IFN-γ producing Th1 to IL-10-producing cells, regulates basophil activation by IFN-α and inhibits IL-3 and GM-CSF producing iNKT. ➂Macrophage type 2 skewing; ➃Participation in dendritic cells' regulation of Th2/Th1 balance; ➄Activation of Th2 and B-cell humoral response: the role of basophil is central in inducing Th2 differentiation, B-cell production of IgE and of B-cell memory and regulates allergy by inducing IL-10 production and IgG4; ➅Down-regulation of allergic inflammation by IgG4 and IL-10; ➆Inflammatory response mediated by IL-18, IL-25 and IL-33: the basophil is linked to tissue function by producing VEGF and by responding to several factors, such as IL-18, IL-25 and IL-33, which in turn promote IL-5 production by Th2-cells able to recruit eosinophils. ➇Production of histamine as an effector molecule and immune modulator. For further information see the text.
How to study basophil function
Precisely because of the extraordinary importance of basophils in immunology, given their strategic role at the crossroads of innate and acquired immunity, researchers endeavouring to understand the role of these unusual and fascinating cells, are asking whether the claimed 21st century renaissance of basophils has finally arrived92. Despite their importance, the methods for investigating basophils are limited and relatively poor, hindering attempts to understand the biology of these cells. Indeed, basophils are poorly represented in peripheral blood and have a short lifespan, there is a lack of primary basophilic cell lines, no knock-out animals, few markers to characterise the presence and/or functions of the cells and few agonists and/or antagonists able to elicit or counteract their cellular response.
Research on basophil function has long been hampered by the absence of immortalised cell lines equivalent to primary basophils, with the exception of KU-812 (KU-812F), a human leukaemia cell line with spontaneous erythroid terminal maturation93 and the rat leukemia cell lines (RBL-2H3 and RBL-703/21), which encompass both mast cell than basophil features94. Immune or transgenic animals would represent novel tools for investigation. Basophil-depleted mice are very important for research on these cells: for example, the transgenic mouse Mcpt8DTR, lacking basophils, enabled it to be determined that basophils, more than mast cells, are responsible for the allergen/IgE-mediated resistance to tick infection95. Most common phenotypes are obtained by using the anti-basophil monoclonal antibodies Ba103 and MAR-1, which recognise CD200R and FcɛRIα, respectively, and are the surrogate for knock-out animals for basophil research44; however, this antibody-based approach has deleterious effects on mast cells, causing side effects such as mast cell activation and their partial depletion. Basophil-depleted mice might suffer from several disorder due to allergic reactions or parasite infections if they are not maintained in a completely germ-free environment. Mice depleted by antibodies are also deprived of most mast cells, while some phenotypes are markedly and specifically mast-cell depleted96. Furthermore, the strategy of antibody-mediated depletion causes several problems related to the low specificity of these functional knock-out models and animals suffer from depletion of γδT lymphocytes in the gastrointestinal tract, anaemia, sterility, a high incidence of dermatitis, papillomas and other malignancies. An alternative was suggested by observation of animals bearing c-kit mutations. The c-kit mutations in Kit(W/W-v) mice usually impair melanogenesis and result in anaemia, sterility, and markedly reduced levels of tissue mast cells. However, an improved model was recently obtained: Kit(W-sh/W-sh) mice, which bear the W-sash (W(sh)) inversion mutation and have mast cell deficiency but are not anaemic and not sterile. Adult kit(W-sh/W-sh) mice have a profound deficiency in mast cells in all tissues examined but normal levels of major classes of other differentiated haematopoietic and lymphoid cells. Kit(W-sh/W-sh) mice have normal numbers of intraepithelial γδT lymphocytes in the intestines and do not exhibit a high incidence of idiopathic dermatitis, ulcers, or squamous papillomas of the stomach, but like Kit(W/W-v) mice, they lack interstitial cells of Cajal in the gut and exhibit bile reflux into the stomach. For the reconstitution of systemic or local mast cell populations non-irradiated adult Kit(W-sh/W-sh) mice are treated by intravenous, intraperitoneal, or intradermal injection of wild-type bone marrow-derived cultured mast cells96.
In order to overcome the limitations related to antibody-mediated basophil depletion, a transgenic mouse Mcpt8DTR was recently generated97. This mouse model harbours a cDNA-encoding diphtheria toxin receptor fused to green fluorescent protein (GFP) and an internal ribosome entry site, inserted into the 3′ untranslated region of a gene encoding a granzyme-like protease (mast cell protease-8 or mMCP-8) which is stored in the secretory granules of basophils: GFP is expressed only in basophils of Mcpt8DTR mice. Diphtheria toxin injection causes a transient depletion of basophils, lasting 5 days about, in this transgenic mice. This model was useful, for example, in clarifying basophil behavior during tick infestation97. An alternative to GFP was generated by Ohnmacht et al. by using a bacterial artificial chromosome construct in which the coding sequence of Cre-recombinase gene is inserted behind the start codon of the Mcpt8 gene (Mcpt8 Bac transgenic mouse)98. In this phenotype, however, more than 90% basophils were spontaneously deleted, due to the high level of Cre expression44. Basophil depletion is a good way of studying the role of these cells in immunity. In humans, very few pathologies appear to be related to basophils. We are, however, unable to assess whether this depends on how much we know about these leucocytes. Basophils, like other leucocytes, can be involved in leukaemia. Acute basophilic leukaemia has been described recently and can be distinguished from acute leukaemia with basophilia by several haematological aspects99. In humans the main functional defects involving basophils are related to genetic loss of some signalling protein that induce a non-responder (non-releaser) phenotype. The high-affinity IgE receptor is unresponsive on mast cells and basophils in about 10–15% subjects in several populations through a still unknown mechanism. Similarly, FcɛRI-positive basophils from 'non-releasers' are IgE-unresponsive and lack the tyrosine kinase Syk100.
Most in vitro studies concerning basophils, however, deal with peripheral blood separation of these cells. In mice, basophils can be purified after staining with specific antibodies giving the complex phenotype DX5+(CD49b+)FcɛRIα+c-kit-CD3-CD11b+NK1.1Th1.2+CD11c-2B4+B220-Gr1-29. Numerous attempts to isolate and purify basophils from other blood cells have been made but in most cases they proved to be expensive and time-consuming approaches, taking into account a gradient separation and a purification step with selective antibodies on magnetic beads101–103. Physical separation of basophilic cells from whole blood or, better still, from blood-derived leucocyte-enriched buffy coats, needs, therefore, immuno-selection by monoclonal antibodies. This is a general technique used also in flow cytometry. Basophils can be physically separated from other cells by a cell sorter104 or electronically captured by a flow cytometer21: in both cases, basophils are targeted with monoclonal antibodies able to identify (phenotype) them, in order to be able to differentiate them from other cells. Basophils are not physically separated from other leucocytes by routine flow cytometry, but they can be clearly identified in vitro. Flow cytometry can identify these cells and follow their behaviour upon stimulation or inhibition: they are "captured" as electronic events and plotted as an "almost purified" population in the so-called dot plot diagram105. Few phenotypic markers are used to identify basophils: basophils are typical CD45dim cells in the lymphocyte area21, express IL-3 receptor α-chain (CD123bright), do not express HLA-DR. Figure 2 shows basophils separated from other leucocytes using a CD123bright/HLADRnon expressing gating protocol21. Many other strategies have been suggested by several authors. There has been recent debate about the feasibility and reliability of IgE+, CD3-/CRTH2+; CD3-/CCR3+; CD14-/CD13+, CD45+/CD203c+ in tracing basophil biology106. Once basophils have been gated, they can be examined for their response to several agonists by following the up-regulation or down-regulation of various membrane molecules: CD63, CD203c, CD193 (CCR3), CD164, CD107a, CD13, CD6919,107 but intracellular molecules, such as signalling proteins or cytokines108–110, can also be checked by targeting them with fluorochrome monoclonal antibodies and intracellular calcium can be scanned by fluorescent probes111. The evaluation of basophil activation upon allergen challenge by flow cytometry is the basic principle of the basophil activation test, a laboratory tool widely used for the diagnosis of allergy108,114. This in vitro cellular assay is able to detect antigen-dependent cellular processes without any risk for the patient. After antigen stimulation, flow cytometric basophil activation assays by determining surface activation markers (CD63, CD203c), represent an accepted model for in vivo basophil or mast cell stimulation particularly in immediate-type IgE-dependent reactions110. Obviously, the value of these assays should be estimated considering the type of antigen and further diagnostic options. However, because of the logistic effort, cellular assays are often available only in specialised laboratories.
Figure 2.
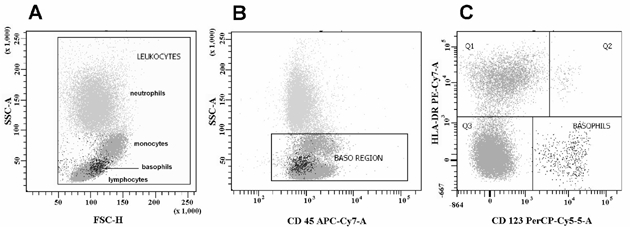
Electronic separation of basophils as CD123bright/HLADRnegative cells. A: morphological dot plot: a first gate is made taking into account only leucocytes, which are plotted using the side scatter/forward scatter (SSC/FSC) method. Starting from the top it is possible to distinguish three areas, neutrophils (with eosinophils), monocytes, lymphocytes with basophils (on the left); B: immunological dot plot: all leucocytes express CD45. Expression of CD45 in basophils is quite low (CD45dim). Basophils are plotted in the same area as lymphocytes: the gate of this area is called the "Baso Region". This gate would include all basophils in the sample. C: a third gate is made to investigate expression of CD123 and HLA-DR of all cells gated in the baso region. Basophils are captured as a separate cloud, which is then shown by a blue colour in the dot plot to facilitate their identification in the graph as a CD123bright/HLADRnegative population. Q1: monocytes; Q2: plasmacytoid dendritic cells; Q3: lymphocytes. The procedure was performed with a BD FACScanto cytometer equipped with two lasers (see references 21 and 101 for more detailed explanations).
In daily routine they are important in cases with a clear-cut history but negative results in conventional diagnostic procedures, in case of rare allergens (drugs, exotic food), as well as when there are contraindications to skin and/or provocation tests (hymenoptera venom allergy, anaphylaxis).
The basophil in transfusion medicine
Table III summarises the role of basophils in transfusion medicine. Most reports concerning basophils in transfusion medicine deal with non-haemolytic transfusion reactions. Non-haemolytic transfusion reactions represent the most common transfusion reactions and include transfusion-related acute lung injury (TRALI) and allergic and febrile reactions. In these adverse effects of transfusion, white blood cell antibodies against human leucocyte antigens and human neutrophil antigens in blood components are frequently implicated in non-haemolytic transfusion reactions, especially in TRALI.
Table III.
Role of basophils in blood transfusion.
| Basophils: roles and issues in transfusion medicine | References |
|---|---|
| Anaphylaxis associated with non-haemolytic transfusion reactions (NHTRs): | |
| a. anaphylaxis to IgA; | Sandler, Transf Med Hemother, 2003 |
| b. anaphylaxis to haptoglobin; | Shimada et al., Transfusion, 2002 |
| c. anaphylaxis to blu methylene-treated fresh-frozen plasma. | Gilstad, Curr Opin Hematol, 2003 |
| Febrile non-haemolytic transfusion reactions due to cytokines production | Dewachter et al, Br J Anaesth, 2011 |
| Activation of basophils by BRMs: | Addas-Carvalho et al., Transf Med, 2006 |
| a. cytokines; | |
| b. bacterial products (PAMP, PAR, fMLP, LPS, etc.); | Matsuyama et al., Transf Med, 2005 |
| c. activated complement factors; | |
| d. chemokines. | |
| Passive IgE sensitisation following blood transfusion | |
| Usefulness in diagnosing allergy to blood transfusion: | Johansson et al., Allergy, 2005 |
| a. basophil activation test; | |
| b. flow cytometry of basophil function (intracellular markers). |
The basophil activation test, which was originally developed to identify allergens in the field of allergic diseases, might be useful in transfusion medicine in this context115. Reactions elicited by basophils and other leucocytes, possibly present in blood components used in transfusion medicine, are a matter of fact in transfusion safety. Transfusion reactions are more frequent with platelet transfusions than with red cell transfusions. Most allergic reactions are urticarial and febrile reactions following platelet transfusion. The clinical characteristics of platelet transfusion reactions vary from febrile non-haemolytic transfusion reactions and allergic reactions to chills, discomfort, tachycardia, and respiratory difficulties. A febrile non-haemolytic transfusion reaction is conventionally defined as a rise in temperature of +1 °C or more in association with a transfusion. Allergic reactions include hives, urticaria, pruritus, erythema, bronchospasm, and hypotension. Anaphylactic reactions may occur in IgA-deficient patients. TRALI, a rare but acute respiratory distress syndrome, can also occur. The relatively lower rate of febrile reactions may be due to the increased use of leucodepletion filters, which effectively prevents most febrile reactions in the transfused population, while the allergic or urticarial reactions may likely be due to sensitisation to plasma constituents that cannot be filtered out. Anaphylactic reactions following transfusion are rare events116. Nevertheless, the incidence of allergic reactions to blood products is similar to incidence of allergic reactions to drugs, such as beta-lactams antibiotics, and such reactions are, therefore, worthy of proportionate attention.
In this regard, comprehensive reviews and guidelines on the management of anaphylaxis currently do not include much information on blood products. Current guidelines for the specific management of anaphylactic transfusion reactions are contradictory as to the utility of anti-IgA testing and incomplete by not offering suggestions for the management of non-IgA related reactions. Most reactions do not derive from anti-IgA in blood components but are related mainly to platelet transfusions117. Specific IgE haptoglobin antibodies detected in the sera of Japanese patients were suggested to play a role in inducing anaphylactic non-haemolytic transfusion reactions in these haptoglobin-deficient patients undergoing blood transfusion118. Plasma transfusions have also been associated with anaphylactic reactions. Methylene blue-treated, fresh-frozen plasma is mainly used in Europe: the advantage is that units can be treated individually and the combined action of methylene blue and illumination prevents viral RNA and DNA replication.
A recent report has described a case of anaphylaxis following methylene blue-treated plasma transfusion through the application of routine diagnostic tests for allergy, such as the basophil activation test119,120. As the role of basophils in these adverse reactions is associated with the response of the blood transfusion recipient, any molecular component, such as immunoglobulins, platelet products, antigens and other non-IgE activating factors may elicit an anaphylactic reaction. However, these adverse events are often considered negligible and the basophil activation test is not commonly considered in transfusion medicine.
Together with other contaminating leucocytes, basophils are able to produce inflammatory cytokines such as IL-6 and induce other white blood cells to release pro-inflammatory cytokines which may cause febrile non-haemolytic transfusion reactions121,122. Allergic reactions are the most common adverse effects in blood transfusion123. Although allergens in blood components may include food-derived molecules or drugs ingested by the donor immediately before the blood was collected, these molecules cannot be identified. Patients suffering from plasma deficiencies of C4, haptoglobin and IgA may be identified as possible target of anaphylaxis. However, the so-called biological response modifiers, such as bacterial products, chemokines and complement factors, can activate basophils present in blood components, through those receptors (PAR, TLR, formylated peptide receptors) that are not related directly to IgE/FcɛRI activation123. For example, the chemokine RANTES can accumulate in platelets concentrates and can activate contaminating basophils, leading to histamine production and non-haemolytic transfusion reactions124. Given that the only anaphylaxis-causing population in whole blood is formed by basophils and the particular difficulty in separating basophils from whole blood, their presence as contaminants in cellular blood components would need more attention. Furthermore, activating factors in fresh frozen plasma, able to trigger recipient's basophils, represent another possible cause of non-haemolytic transfusion reactions in transfusion medicine and suggest the usefulness of the basophil activation test in this field.
Conclusion
This review of the literature on basophils has shown the large number of reports on the regulatory functions of these cells in the immune system, an intriguing and encouraging finding given the severe obstacles faced by even the most enthusiastic researcher. Is the time right to treat these cells with the respect they deserve? Perhaps, the classical paradigm of innate and acquired immunity requires a profound review. Innate immunity cells play key functions in the complex network of self maintenance. Cells which were for a long time exclusively relegated to hypersensitivity and to an unrefined inflammatory response are now being understood to be sophisticated handmaids devoted to immuno-regulation, with vital roles in the central system of immunity.
Basophils are well known effector cells in allergy: they secrete several mediators, such as histamine and LTC4 but to a lesser extent than mast cells, which have the true task of atopic cells in inflamed tissues and produce many other vasoactive mediators. Basophils participate in hypersensitivity reactions but mostly as innate cells that, while producing IL-4, drive the Th2 skewing of the immune response and so participating actively in Th2-B-cell mechanisms underlying IgE. They are important components of IgE-histamine mediated anaphylaxis but, unlike mast cells, they can promote a IgG-PAF mediated anaphylactic reaction. The role of basophils as effector cells in allergy seems to be bring allergic reactions to their completion and down-regulation.
Basophils are no longer insignificant cells capable only of discharging histamine and creating uncomfortable over-reactions. Perhaps they deserve the same attention given, for example, to lymphocytes, even though they appear less sophisticated than main cells of acquired immunity. Enough evidence has already been produced in this century to induce a clear change in our consideration and understanding of basophil biology and it may be time to bring these cells into the spotlight.
Footnotes
The Author declare no conflicts of interest.
References
- 1.Ehrlich P. Beiträge zur Kenntnis der granulierten Bindegewebszellen und der eosinophilen Leukocythen. Arch Anat Physiol. 1879;3:166–9. [Google Scholar]
- 2.Karasuyama H, Mukai K, Tsujimura Y, Obata K. Newly discovered roles for basophils: a neglected minority gains new respect. Nat Rev Immunol. 2009;9:9–13. doi: 10.1038/nri2458. [DOI] [PubMed] [Google Scholar]
- 3.Simons FE. Anaphylaxis. J Allergy Clin Immunol. 2008;121:S402–S407. doi: 10.1016/j.jaci.2007.08.061. [DOI] [PubMed] [Google Scholar]
- 4.Mukai K, Obata K, Tsujimura Y, Karasuyama H. New insights into the roles for basophils in acute and chronic allergy. Allergol Int. 2009;58:11–9. doi: 10.2332/allergolint.08-RAI-0059. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder JT. Basophils beyond effector cells of allergic inflammation. Adv Immunol. 2009;101:123–61. doi: 10.1016/S0065-2776(08)01004-3. [DOI] [PubMed] [Google Scholar]
- 6.Arinobu Y, Iwasaki H, Akashi K. Origin of basophils and mast Cells. Allergol Int. 2009;58:21–8. doi: 10.2332/allergolint.08-RAI-0067. [DOI] [PubMed] [Google Scholar]
- 7.Gibbs BF, Streatfield C, Falcone FH. Basophils as critical orchestrators of Th2-type immune responses. Expert Rev Clin Immunol. 2009;5:725–34. doi: 10.1586/eci.09.47. [DOI] [PubMed] [Google Scholar]
- 8.Sokol CL, Medzhitov R. Role of basophils in the initiation of Th2 responses. Curr Opin Immunol. 2010;22:73–7. doi: 10.1016/j.coi.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denzel A, Maus UA, Gomez MR, et al. Basophils enhance immunological memory responses. Nature. 2008;9:733–42. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 10.Kawakami T. Basophils now enhance memory. Nat Immunol. 2008;9:720–1. doi: 10.1038/ni0708-720. [DOI] [PubMed] [Google Scholar]
- 11.Maddur MS, Kaveri SV, Bayry J. Basophils as antigen presenting cells. Trends Immunol. 2010;31:45–8. doi: 10.1016/j.it.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Gauvreau GM, Plitt JR, Baatjes A, MacGlashan DW. Expression of functional cysteinyl leukotriene receptors by human basophils. J Allergy Clin Immunol. 2005;116:80–7. doi: 10.1016/j.jaci.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Min B, Paul WE. Basophils and type 2 immunity. Curr Opin Hematol. 2008;15:59–63. doi: 10.1097/MOH.0b013e3282f13ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125:S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaguchi M, Koketsu R, Suzukawa M, et al. Human basophils and cytokines/chemokines. Allergol Int. 2009;58:1–10. doi: 10.2332/allergolint.08-RAI-0056. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder JT, Chichester KL, Bieneman AP. Human basophils secrete IL-3: evidence of autocrine priming for phenotypic and functional responses in allergic disease. J Immunol. 2009;182:2432–8. doi: 10.4049/jimmunol.0801782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knol EF, Gibbs BF. Basophil survival and immuno-modulatory function are uniquely regulated by a novel MyD88-dependent pathway. J Leuk Biol. 2009;86:753–5. doi: 10.1189/jlb.0409248. [DOI] [PubMed] [Google Scholar]
- 18.Knol EF, Olszewski M. Basophils and mast cells: underdog in immune regulation? Immunol Lett. 2011;138:28–31. doi: 10.1016/j.imlet.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Hennersdorf F, Florian S, Jakob A, et al. Identification of CD13, CD107a, and CD164 as novel basophil-activation markers and dissection of two response patterns in time kinetics of IgE-dependent upregulation. Cell Res. 2005;15:325–35. doi: 10.1038/sj.cr.7290301. [DOI] [PubMed] [Google Scholar]
- 20.de Weck AL, Sanz ML, Gamboa PM, et al. Diagnostic tests based on human basophils: more potentials and perspectives than pitfalls. Int Arch Allergy Immunol. 2008;146:177–89. doi: 10.1159/000115885. [DOI] [PubMed] [Google Scholar]
- 21.Chirumbolo S, Vella A, Ortolani R, et al. Differential response of human basophil activation markers: a multi-parameter flow cytometry approach. Clin Mol Allergy. 2008;6:12–25. doi: 10.1186/1476-7961-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marone G, Triggiani M, de Paulis A. Mast cells and basophils: friends as well as foes in bronchial asthma? Trends Immunol. 2005;26:25–31. doi: 10.1016/j.it.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Crivellato E, Travan L, Ribatti D. Mast cells and basophils: a potential link in promoting angiogenesis during allergic inflammation. Int Arch Allergy Immunol. 2010;151:89–97. doi: 10.1159/000235998. [DOI] [PubMed] [Google Scholar]
- 24.Siracusa MC, Comeau MR, Artis D. New insights into basophil biology: initiators, regulators, and effectors of type 2 inflammation. Ann N Y Acad Sci. 2011;1217:166–77. doi: 10.1111/j.1749-6632.2010.05918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falcone FH, Haas H, Gibbs BF. The human basophil: a new appreciation of its role in immune responses. Blood. 2000;96:4028–38. [PubMed] [Google Scholar]
- 26.Siracusa MC, Perrigone JG, Corneau MR, Artis D. New paradigms in basophil development, regulation and function. Immunol Cell Biol. 2010;88:275–84. doi: 10.1038/icb.2010.1. [DOI] [PubMed] [Google Scholar]
- 27.Schramm G, Mohrs K, Wodrich M, et al. Cutting edge: IPSE/alpha-1, a glycoprotein from Schistosoma mansoni eggs, induces IgE-dependent, antigen-independent IL-4 production by murine basophils in vivo. J Immunol. 2007;178:6023. doi: 10.4049/jimmunol.178.10.6023. [DOI] [PubMed] [Google Scholar]
- 28.Mitre E, Nutman TB. Basophils, basophilia and helminth infections. Chem Immunol All. 2006;90:141. doi: 10.1159/000088886. [DOI] [PubMed] [Google Scholar]
- 29.Schneider E, Thieblemont N, De Moares ML, Dy M. Basophils: new players in the cytokine network. Eur Cytok Netw. 2010;21:142–53. doi: 10.1684/ecn.2010.0197. [DOI] [PubMed] [Google Scholar]
- 30.Karasuyama H, Wada T, Yoshikawa S, Obata K. Emerging roles of basophils in protective immunity against parasites. Trends Immunol. 2011;32:125–30. doi: 10.1016/j.it.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Van Pahuys N, Prout M, Forbes E, et al. Basophils are the major producers of IL-4 during primary helminth infection. J Immunol. 2011;186:2719–28. doi: 10.4049/jimmunol.1000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komiya A, Nagase H, Okugawa S, et al. Expression and function of toll-like receptors in human basophils. Int Arch Allergy Immunol. 2006;140(s1):23–7. doi: 10.1159/000092707. [DOI] [PubMed] [Google Scholar]
- 33.Yoshimoto T, Nakanishi K. Roles of IL-18 in basophils and mast cells. Allergol Int. 2006;55:105–13. doi: 10.2332/allergolint.55.105. [DOI] [PubMed] [Google Scholar]
- 34.Kroeger KM, Sullivan BM, Locksley RM. IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88- and p38alpha-dependent pathway. J Leukoc Biol. 2009;86:769–78. doi: 10.1189/jlb.0708452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oboki K, Ohno T, Kajiwara N, et al. IL-33 and IL-33 receptors in host defense and diseases. Allergol Int. 2010;59:143–60. doi: 10.2332/allergolint.10-RAI-0186. [DOI] [PubMed] [Google Scholar]
- 36.Stow NW, Douglas R, Tantilipikorn P, Lacroix JS. Superantigens. Otolaryngol Clin North Am. 2010;43:489–502. doi: 10.1016/j.otc.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Marone G, Rossi FW, Detoraki A, et al. Role of superallergens in allergic disorders. Chem Immunol Allergy. 2007;93:195–213. doi: 10.1159/000100896. [DOI] [PubMed] [Google Scholar]
- 38.Solanki LS, Srivastava N, Singh S. Superantigens: a brief review with special emphasis on dermatologic diseases. Dermatol Online J. 2008;14:3. [PubMed] [Google Scholar]
- 39.Kojima T, Obata K, Mukai K, et al. Mast cells and basophils are selectively activated in vitro and in vivo through CD200R3 in an IgE-independent manner. J Immunol. 2007;179:7093–100. doi: 10.4049/jimmunol.179.10.7093. [DOI] [PubMed] [Google Scholar]
- 40.Sloane DE, Tedla N, Awoniyi M, et al. Leukocyte immunoglobulin-like receptors: novel innate receptors for human basophil activation and inhibition. Blood. 2004;104:2832–9. doi: 10.1182/blood-2004-01-0268. [DOI] [PubMed] [Google Scholar]
- 41.Min B. Basophils induce Th2 immunity. Is this the final answer? Virulence. 2010;1:399–401. doi: 10.4161/viru.1.5.12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corthay A. A three-cell model for activation of naïve T helper cells. Scand J Immunol. 2006;64:93–6. doi: 10.1111/j.1365-3083.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 43.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nature Immunol. 2008;9:310–8. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karasuyama H, Mukai K, Obata K, Tsujimura Y, et al. Nonredundant roles of basophils in immunity. Annu Rev Immunol. 2011;29:45–69. doi: 10.1146/annurev-immunol-031210-101257. [DOI] [PubMed] [Google Scholar]
- 45.Sokol CL, Chu NQ, Yu S, et al. Basophils function as antigen presenting cells for an allergen-induced T helper type 2 response. Nature Immunol. 2009;10:713–20. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshimoto T, Yasuda K, Tanaka H, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nature Immunol. 2009;10:706–12. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 47.Perrigoue JG, Saenz SA, Siracusa MC, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nature Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoo JK, Calligan CL, Virtanen C, Fish EN. Identification of a novel antigen-presenting cell population modulating antiinfluenza type 2 immunity. J Exp Med. 2010;207:1435–51. doi: 10.1084/jem.20091373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spiegl N, Didichenko S, McCaffey P, et al. Human basophils activated by mast cell-derived IL-3 express retinaldehyde dehydrogenase-II and produce the immunoregulatory mediator retinoic acid. Blood. 2009;112:3762–71. doi: 10.1182/blood-2008-01-135251. [DOI] [PubMed] [Google Scholar]
- 50.Kim S, Shen T, Min B. Basophils can directly present or cross-present antigen to CD8 lymphocytes and alter CD8 T cell differentiation into IL-10-producing phenotypes. J Immunol. 2009;183:3033–9. doi: 10.4049/jimmunol.0900332. [DOI] [PubMed] [Google Scholar]
- 51.Uermösi C, Beerli RR, Bauer M, et al. Mechanisms of allergen-specific desensitization. J Allergy Clin Immunol. 2010;126:375–83. doi: 10.1016/j.jaci.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 52.Katz HR. Inhibitory receptors and allergy. Curr Opin Immunol. 2002;14:698–704. doi: 10.1016/s0952-7915(02)00400-4. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez Gomez M, Talke Y, Goebel N, et al. Basophils support the survival of plasma cells in mice. J Immunol. 2010;185:7180–5. doi: 10.4049/jimmunol.1002319. [DOI] [PubMed] [Google Scholar]
- 54.Ozdemir C, Akdis M, Akdis CA. T-cell response to allergens. Chem Immunol Allergy. 2010;95:22–44. doi: 10.1159/000315936. [DOI] [PubMed] [Google Scholar]
- 55.Karasuyama H, Tsujimura Y, Obata K, Mukai K. Role for basophils in systemic anaphylaxis. Chem Immunol Allergy. 2010;95:85–97. doi: 10.1159/000315939. [DOI] [PubMed] [Google Scholar]
- 56.Terstappen LW, Hollander Z, Meiners H, Loken MR. Quantitative comparison of myeloid antigens on five lineages of mature peripheral blood cells. J Leuk Biol. 1990;48:138–48. doi: 10.1002/jlb.48.2.138. [DOI] [PubMed] [Google Scholar]
- 57.Komiya A, Nagase H, Okugawa S, et al. Expression and function of toll-like receptors in human basophils. Int Arch Allergy Immunol. 2006;140(Suppl 1):23–7. doi: 10.1159/000092707. [DOI] [PubMed] [Google Scholar]
- 58.Novak N, Bieber T, Peng WM. The immunoglobulin E-Toll-like receptor network. Int Arch Allergy Immunol. 2010;151:1–7. doi: 10.1159/000232565. [DOI] [PubMed] [Google Scholar]
- 59.Suzukawa M, Komiya A, Iikura M, et al. Trans-basement membrane migration of human basophils: role of matrix-metalloproteinase-9. Int Immunol. 2006;18:1575–83. doi: 10.1093/intimm/dxl090. [DOI] [PubMed] [Google Scholar]
- 60.Yamaguchi M, Koketsu R, Suzukawa M, et al. Human basophils and cytokines/chemokines. Allergol Int. 2009;58:1–10. doi: 10.2332/allergolint.08-RAI-0056. [DOI] [PubMed] [Google Scholar]
- 61.Kim S, Prout M, Ramshaw H, et al. Cutting edge: basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J Immunol. 2010;184:1143–7. doi: 10.4049/jimmunol.0902447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iikura M, Ebisawa M, Yamaguchi M, et al. Transendothelial migration of human basophils. J Immunol. 2004;173:5189–95. doi: 10.4049/jimmunol.173.8.5189. [DOI] [PubMed] [Google Scholar]
- 63.Souwer Y, Szegedi K, Kapsenberg ML, de Jong EC. IL-17 and IL-22 in atopic allergic disease. Curr Opin Immunol. 2010;22:821–6. doi: 10.1016/j.coi.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 64.Lee FE, Georas SN, Beck LA. IL-17: important for host defense, autoimmunity, and allergy? J Invest Dermatol. 2010;130:2540–2. doi: 10.1038/jid.2010.295. [DOI] [PubMed] [Google Scholar]
- 65.Milovanovic M, Drozdenko G, Weise C, et al. Interleukin-17A promotes IgE production in human B cells. J Invest Dermatol. 2010;130:2621–8. doi: 10.1038/jid.2010.175. [DOI] [PubMed] [Google Scholar]
- 66.Hvid M, Vestergaard C, Kemp K, et al. IL-25 in atopic dermatitis: a possible link between inflammation and skin barrier dysfunction? J Invest Dermatol. 2011;131:150–7. doi: 10.1038/jid.2010.277. [DOI] [PubMed] [Google Scholar]
- 67.Wang YH, Angkasekwinai P, Lu N, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–47. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kamiński M, Kłoda K, Pawlik A. The role of interleukin-18 in the pathogenesis of bronchial asthma and other allergic diseases and in activation of basophils and mastocytes. Pneumonol Alergol Pol. 2008;76:432–6. [PubMed] [Google Scholar]
- 69.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–74. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 70.Yoshimoto T, Nakanishi K. Roles of IL-18 in basophils and mast cells. Allergol Int. 2006;55:105–13. doi: 10.2332/allergolint.55.105. [DOI] [PubMed] [Google Scholar]
- 71.Oboki K, Ohno T, Kajiwara N, et al. IL-33 and IL-33 receptors in host defense and diseases. Allergol Int. 2010;59:143–60. doi: 10.2332/allergolint.10-RAI-0186. [DOI] [PubMed] [Google Scholar]
- 72.Kaiko GE, Foster PS. New insights into the generation of Th2 immunity and potential therapeutic targets for the treatment of asthma. Curr Opin Allergy Clin Immunol. 2011;11:39–45. doi: 10.1097/ACI.0b013e328342322f. [DOI] [PubMed] [Google Scholar]
- 73.Borish L, Steinke JW. Interleukin-33 in asthma: how big of a role does it play? Curr Allergy Asthma Rep. 2011;11:7–11. doi: 10.1007/s11882-010-0153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Komai-Koma M, Gilchrist DS, McKenzie AN, et al. IL-33 activates B1 cells and exacerbates contact sensitivity. J Immunol. 2011;186:2584–91. doi: 10.4049/jimmunol.1002103. [DOI] [PubMed] [Google Scholar]
- 75.Cadman ET, Lawrence RA. Granulocytes: effector cells or immunomodulators in the immune response to helminth infection? Parasite Immunol. 2010;32:1–19. doi: 10.1111/j.1365-3024.2009.01147.x. [DOI] [PubMed] [Google Scholar]
- 76.Dy M, Schneider E. Histamine-cytokine connection in immunity and hematopoiesis. Cytokine Growth Factor Rev. 2004;15:393–410. doi: 10.1016/j.cytogfr.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Jutel M, Blaser K, Akdis CA. The role of histamine in regulation of immune responses. Chem Immunol Allergy. 2006;91:174–87. doi: 10.1159/000090280. [DOI] [PubMed] [Google Scholar]
- 78.Akdis CA, Jutel M, Akdis M. Regulatory effects of histamine and histamine receptor expression in human allergic immune responses. Chem Immunol Allergy. 2008;94:67–82. doi: 10.1159/000154858. [DOI] [PubMed] [Google Scholar]
- 79.Jutel M, Watanabe T, Akdis M, et al. Immune regulation by histamine. Curr Opin Immunol. 2002;14:735–40. doi: 10.1016/s0952-7915(02)00395-3. [DOI] [PubMed] [Google Scholar]
- 80.Schneider E, Rolli-Derkinderen M, Arock M, Dy M. Trends in histamine research: new functions during immune responses and hematopoiesis. Trends Immunol. 2002;23:255–63. doi: 10.1016/s1471-4906(02)02215-9. [DOI] [PubMed] [Google Scholar]
- 81.Chen K, Cerutti A. New insights into the enigma of immunoglobulin D. Immunol Rev. 2010;237:160–79. doi: 10.1111/j.1600-065X.2010.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burchell JT, Strickland DH, Stumbles PA. The role of dendritic cells and regulatory T cells in the regulation of allergic asthma. Pharmacol Ther. 2010;125:1–10. doi: 10.1016/j.pharmthera.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 83.Akdis M, Blaser K, Akdis CA. T regulatory cells in allergy. Chem Immunol Allergy. 2006;91:159–73. doi: 10.1159/000090279. [DOI] [PubMed] [Google Scholar]
- 84.Jutel M, Akdis CA. T-cell subset regulation in atopy. Curr Allergy Asthma Rep. 2011;11:139–45. doi: 10.1007/s11882-011-0178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leite-de-Moraes MC, Lisbonne M, Arnould A, et al. Ligand-activated natural killer T lymphocytes promptly produce IL-3 and GM-CSF in vivo: relevance to peripheral myeloid recruitment. Eur J Immunol. 2002;32:1897–904. doi: 10.1002/1521-4141(200207)32:7<1897::AID-IMMU1897>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 86.de Paulis A, Prevete N, Fiorentino I, et al. Expression and functions of the vascular endothelial growth factors and their receptors in human basophils. J Immunol. 2006;177:7322–31. doi: 10.4049/jimmunol.177.10.7322. [DOI] [PubMed] [Google Scholar]
- 87.Leite-de-Moraes MC, Diem S, Michel ML, et al. Cutting edge: histamine receptor H4 activation positively regulates in vivo IL-4 and IFN-gamma production by invariant NKT cells. J Immunol. 2009;182:1233–6. doi: 10.4049/jimmunol.182.3.1233. [DOI] [PubMed] [Google Scholar]
- 88.Schroeder JT, Bieneman AP, Chichester KL, et al. Pulmonary allergic responses augment interleukin-13 secretion by circulating basophils yet suppress interferon-alpha from plasmacytoid dendritic cells. Clin Exp Allergy. 2010;40:745–54. doi: 10.1111/j.1365-2222.2010.03456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grela F, Aumeunier A, Bardel E, et al. The TLR7 agonist R848 alleviates allergic inflammation by targeting invariant NKT cells to produce IFN-gamma. J Immunol. 2011;186:284–90. doi: 10.4049/jimmunol.1001348. [DOI] [PubMed] [Google Scholar]
- 90.Chirumbolo S. The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm Allergy Drug Targets. 2010;9:263–85. doi: 10.2174/187152810793358741. [DOI] [PubMed] [Google Scholar]
- 91.Chirumbolo S, Marzotto M, Conforti A, et al. Bimodal action of the flavonoid quercetin on basophil function: an investigation of the putative biochemical targets. Clin Mol Allergy. 2010;8:13–24. doi: 10.1186/1476-7961-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Falcone FH, Zillikens D, Gibbs BF. The 21st century renaissance of the basophil? Current insights into its role in allergic responses and innate immunity. Exp Dermatol. 2006;15:855–64. doi: 10.1111/j.1600-0625.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- 93.Nakazawa M, Mitjavila MT, Debili N, et al. KU 812: a pluripotent human cell line with spontaneous erythroid terminal maturation. Blood. 1989;73:2003–13. [PubMed] [Google Scholar]
- 94.Passante E, Frankish N. The RBL-2H3 cell line: its provenance and suitability as a model for the mast cell. Inflamm Res. 2009;58:737–45. doi: 10.1007/s00011-009-0074-y. [DOI] [PubMed] [Google Scholar]
- 95.Wada T, Ishiwata K, Koseki H, et al. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest. 2010;120:2867–75. doi: 10.1172/JCI42680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grimbaldeston MA, Chen CC, Piliponsky AM, et al. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–48. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Min B. Mice that "conditionally" lack basophils, AT LAST. J Clin Invest. 2010;120:2648–51. doi: 10.1172/JCI44058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ohnmacht C, Schwartz C, Panzer M, et al. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–74. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 99.Lichtman MA, Segel GB. Uncommon phenotypes of acute myelogenous leukemia: basophilic, mast cell, eosinophilic, and myeloid dendritic cell subtypes: a review. Blood Cells Molec Dis. 2005;35:370–83. doi: 10.1016/j.bcmd.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 100.Kepley CL. Antigen-induced reduction in mast cell and basophil functional responses due to reduced Syk protein levels. Int Arch Allergy Immunol. 2005;138:29–39. doi: 10.1159/000087355. [DOI] [PubMed] [Google Scholar]
- 101.Metcalfe DD. Isolation of human basophils. Curr Protoc Immunol. 2001;Chapter 7(Unit 7):24. doi: 10.1002/0471142735.im0724s06. [DOI] [PubMed] [Google Scholar]
- 102.Reimer JM, Magnusson S, Juremalm M, et al. Isolation of transcriptionally active umbilical cord blood-derived basophils expressing Fc epsilon RI, HLA-DR and CD203c. Allergy. 2006;61:1063–70. doi: 10.1111/j.1398-9995.2006.01149.x. [DOI] [PubMed] [Google Scholar]
- 103.Gibbs BF, Papenfuss K, Falcone FH. A comparison of procedures for purifying human basophils to near homogeneity. Inflamm Res. 2008;57:S11–2. doi: 10.1007/s00011-007-0604-4. [DOI] [PubMed] [Google Scholar]
- 104.Yang L, Xu WG, Xu YP, et al. Method for umbilical cord blood-derived basophils by FCM. Hybridoma (Larchmt) 2010;29:367–70. doi: 10.1089/hyb.2010.0004. [DOI] [PubMed] [Google Scholar]
- 105.Triggiani M, Marone G. Basophil's secrets revealed by flow cytometry. Allergy. 2006;61:1025–7. doi: 10.1111/j.1398-9995.2006.01217.x. [DOI] [PubMed] [Google Scholar]
- 106.Chirumbolo S, Ortolani R, Vella A. CCR3 as a single selection marker compared to CD123/HLADR to isolate basophils in flow cytometry: some comments. Cytometry A. 2011;79:102–6. doi: 10.1002/cyto.a.21008. [DOI] [PubMed] [Google Scholar]
- 107.Florian S, Sonneck K, Czerny M, et al. Detection of novel leukocyte differentiation antigens on basophils and mast cells by HLDA8 antibodies. Allergy. 2006;61:1054–62. doi: 10.1111/j.1398-9995.2006.01171.x. [DOI] [PubMed] [Google Scholar]
- 108.Verweij MM, De Knop KJ, Bridts CH, et al. P38 mitogen-activated protein kinase signal transduction in the diagnosis and follow up of immunotherapy of wasp venom allergy. Cytometry B Clin Cytom. 2010;78:302–7. doi: 10.1002/cyto.b.20531. [DOI] [PubMed] [Google Scholar]
- 109.Scherer K, Bircher AJ, Heijnen IA. Diagnosis of stinging insect allergy: utility of cellular in-vitro tests. Curr Opin Allergy Clin Immunol. 2009;9:343–50. doi: 10.1097/ACI.0b013e32832dd1f5. [DOI] [PubMed] [Google Scholar]
- 110.Foster B, Prussin C, Liu F, et al. Detection of intracellular cytokines by flow cytometry. Curr Protoc Immunol. 2007;Chapter 6(Unit 6):24. doi: 10.1002/0471142735.im0624s78. [DOI] [PubMed] [Google Scholar]
- 111.June CH, Moore JS. Measurement of intracellular ions by flow cytometry. Curr Protoc Immunol. 2004;Chapter 5(Unit 5):5. doi: 10.1002/0471142735.im0505s64. [DOI] [PubMed] [Google Scholar]
- 112.De Week AL, Sanz ML, Gamboa PM, et al. Diagnostic tests based on human basophils: more potentials and perspectives than pitfalls. II. Technical issues. J Investig Allergol Clin Immunol. 2008;18:143–55. [PubMed] [Google Scholar]
- 113.Chirumbolo S. The use of IL-3 in basophil activation tests is the real pitfall. Cytometry B Clin Cytom. 2011;80:137–8. doi: 10.1002/cyto.b.20570. [DOI] [PubMed] [Google Scholar]
- 114.Wedi B, Kapp A. Cellular in-vitro assays. Applicability in daily routine. Hautarzt. 2010;61:954–60. doi: 10.1007/s00105-010-1968-x. [DOI] [PubMed] [Google Scholar]
- 115.Hirayama F. Recent advances in laboratory assays for nonhemolytic transfusion reactions. Transfusion. 2010;50:252–63. doi: 10.1111/j.1537-2995.2009.02388.x. [DOI] [PubMed] [Google Scholar]
- 116.Yu H, Sandler SG. IgA anaphylactic transfusion reactions. Transus Med Hemother. 2003;30:214–20. [Google Scholar]
- 117.Gilstad CW. Anaphylactic transfusion reactions. Curr Opin Hematol. 2003;10:419–23. doi: 10.1097/00062752-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 118.Shimada E, Tadokoro K, Watanabe Y, et al. Anaphylactic transfusion reactions in haptoglobin-deficient patients with IgE and IgG haptoglobin antibodies. Transfusion. 2002;42:766–73. doi: 10.1046/j.1537-2995.2002.00117.x. [DOI] [PubMed] [Google Scholar]
- 119.Dewachter P, Castro S, Nicaise-Roland P, et al. Anaphylactic reaction after methylene-blue treated plasma transfusion. Br J Anaesth. 2011;106:687–9. doi: 10.1093/bja/aer009. [DOI] [PubMed] [Google Scholar]
- 120.Nubret K, Delhoume M, Orsel I, et al. Anaphylactic shock to fresh-frozen plasma inactivated with methylene blue. Transfusion. 2011;51:125–8. doi: 10.1111/j.1537-2995.2010.02800.x. [DOI] [PubMed] [Google Scholar]
- 121.Addas-Carvalho M, Salles TS, Saad ST. The association of cytokine gene polymorphisms with febrile non-hemolytic transfusion reaction in multi-transfused patients. Transf Med. 2006;16:184–91. doi: 10.1111/j.1365-3148.2006.00665.x. [DOI] [PubMed] [Google Scholar]
- 122.Yazer MH, Podlosky L, Clarke G, Nahimiak SM. The effect of prestorage WBC reduction on the rates of febrile nonhemolytic transfusion reactions to platelet concentrates and RBC. Transfusion. 2004;44:10–5. doi: 10.1046/j.0041-1132.2003.00518.x. [DOI] [PubMed] [Google Scholar]
- 123.Matsuyama N, Hirayama F, Wakamoto S, Yasui K. Application of the basophil activation test in the analysis of the allergic transfusion reactions. Transf Med. 2005;19:274–7. doi: 10.1111/j.1365-3148.2009.00939.x. [DOI] [PubMed] [Google Scholar]
- 124.Wakamoto S, Fujihara M, Kuzuma K, et al. Biologic activity of RANTES in apheresis PLT concentrates and its involvement in non hemolytic transfusion reactions. Transfusion. 2003;43:1038–46. doi: 10.1046/j.1537-2995.2003.00458.x. [DOI] [PubMed] [Google Scholar]


