Abstract
Background
Different factors influence the clinical outcome of allogeneic transplants, the foremost being good immune recovery.
Materials and methods
The purpose of this study was to evaluate the influence of different factors, such as stem cell source, type of donor, conditioning regimen and acute graft-versus-host disease, on early lymphocyte recovery after transplantation. We then analyzed the impact of early CD4+ cell count on overall survival, transplant-related mortality and disease-related mortality.
Results
Univariate analysis with Spearman’s rho showed a significant correlation between early CD4+ cell recovery and overall survival, transplant-related mortality, stem cell source and type of donor. In multivariate analysis CD4+ cell count was significantly associated with (i) stem cell source, being higher in patients whose haematopoietic progenitor cells were obtained by apheresis than in those whose source of grafted cells was bone marrow, and (ii) type of donor, being higher in patients transplanted from sibling donors than in those whose graft was from an alternative donor. The ROC curve of CD4+ cell count indicated that a cut-off of 115 CD4+ cells/mL could differentiate groups with different outcomes. At 2 years follow-up, patients achieving this CD4+ cell count had significantly lower cumulative transplant-related mortality compared to patients who did not have this count (10%±4% versus 40%±8%, p=0.0026). At the 5-year follow-up, the overall survival rates were 77.5%±0.6% and 36%±7% (p=0.000) in patients with a CD4+ cell count ≥115/mL and in patients with CD4+ cell count ≤ 115/mL, respectively.
Conclusion
Early CD4+ cell recovery after allogeneic transplantation has a relevant impact on overall survival and transplant-related mortality and is influenced by two factors: stem cell source and type of donor.
Keywords: immune reconstitution, allogeneic stem cell transplantation, CD4+ helper T- lymphocytes, clinical outcome
Introduction
Allogeneic haematopoietic progenitor cell transplantation (HPCT) is a curative therapy for patients with several serious disorders, in particular a subgroup of patients with haematological malignancies1. Different factors influence the clinical outcome of transplantation, the foremost being good immune recovery2. Delayed, inadequate or incomplete reconstitution of the immune system after HPCT is related to infectious morbidity3,4 and an increased risk of relapse5,6, both of which are associated with increased mortality7. Immune recovery may depend on several factors: the age of the patient8, pre-transplant residual thymic function9, haematopoietic stem cell source10,11, CD34+ cells12, CD8+ cytotoxic T-cells and CD56+ natural killer (NK) cells13, CD4+CD25hi regulatory T-cell (Treg) doses14, conditioning regimen15,16, incidence or extent of graft-versus-host disease (GVHD)17, cytomegalovirus reactivation and the cytomegalovirus serostatus before HPCT18. In the past, many researchers analysed how long it takes to have good and complete immune reconstitution; in the last years only few researchers have studied the predictive role of early immune recovery on transplant outcome. Kim et al.19 demonstrated a correlation between number of T-helper cells at 3 months after transplantation and clinical outcome of patients. Berger et al.20 found a statistical association between CD4+ cell count at a median time of 35 days after transplantation and transplant-related mortality.
Materials and methods
Aim of the study
The first objective of the study was to evaluate the influence of different factors, such as stem cell source, type of donor, conditioning regimen and acute GVHD, on early lymphocyte recovery after transplantation. We then analysed the impact of early CD4+ cell count on overall survival, transplant-related mortality and disease-related mortality. Finally, a multivariate analysis for overall survival as end point was performed to study factors associated to overall survival.
Patient and graft characteristics
We included 99 patients transplanted in the “A. Neri” Regional Stem Cell Transplant and Cellular Therapy Centre in Reggio Calabria (Italy) between 1993 and 2008 for whom we have all relevant data. The characteristics of the patients and their grafts are presented in Table I. Haematopoietic progenitor cell collected by apheresis (HPC-A) were the stem cells used in 76 cases, whereas bone marrow was the source of the graft (HPC-M) in 23 transplants. The conditioning regimen was myeloablative in 48 patients and reduced intensity in 51 patients. The myeloablative conditioning regimens were mainly cyclophosphamide (60 mg/kg once daily i.v. for 2 consecutive days) followed by total body irradiation 9.9–12 Gy for acute lymphoid leukaemia; busulfan at a dose of 1 mg/kg p.o. or busulfan 0.8 mg/kg i.v. every 6 hours for 4 consecutive days, followed by cyclophosphamide at 60 mg/kg i.v. daily for two doses for acute and chronic myeloid leukaemia. The non-myeloablative conditioning regimens were fludarabine 30 mg/m2 i.v. and cyclophosphamide 300 mg/m2 i.v. for 3 days for multiple myeloma; antithymocyte globulin at 1.5 mg/kg/day i.v. starting 11 days prior to transplantation for 5 consecutive days and total lymphoid irradiation from a 15-MeV linear accelerator with photon beam at a dose of 0.8 Gy/day starting 11 days before transplant with daily doses for 10 total delivered fractions for malignant lymphoid or myeloid disease21; the combination of thiotepa (10 mg/kg i.v. for 1 day) with cyclophosphamide (50 mg/kg once daily i.v. for 2 days) mainly for Hodgkin’s and non-Hodgkin’s lymphomas.
Table I.
Characteristics of the patients and their grafts.
| N. of patients | 99 |
| Sex (male/female) | 48/51 |
| Median age, years | 46 (range 11–67) |
| Diagnosis | |
| Acute myeloid/lymphocytic leukaemia | 38/15 |
| Chronic lymphocytic leukaemia | 8 |
| CML/MDS | 6/2 |
| NHL/HL | 12/7 |
| Multiple myeloma | 10 |
| Aplasia | 1 |
| Disease status (early/advanced) | 66/33 |
| Donor type (matched/mismatched) | 84/15 |
| Donor median age, years | 41 (range 15–65) |
| Donor sex (male/female) | 58/41 |
| ABO compatibility (id/minor/major) | 67/13/19 |
| Cell source (BM/PB) | 23/76 |
| Conditioning (myeloablative/RIC) | 48/51 |
Legend:
CML: chronic myeloid leukaemia; MDS: myelodysplastic syndrome; NHL: non-Hodgkin’s lymphoma; HL: Hodgkin’s lymphoma; BM: bone marrow; PB: peripheral blood; RIC: reduced intensity conditioning).
GVHD prophylaxis mainly consisted in cyclosporine-A (2 mg/kg on day −2, 1 mg/kg from day −1) combined with short-term methotrexate 15 mg/m2 on day +1, and 10 mg/m2 on days +3 and +6 and also on day +11 in case of mild-moderate mucositis. In case of an alternative donor, in vivo T-cell depletion with anti-lymphocytic serum at a total variable dose between 4.5–15 mg/kg was added to cyclosporine A and methotrexate. When the conditioning regimen was total lymphoid irradiation and antithymocyte globulin, methotrexate was replaced with mycophenolate mofetil at a maximum dose of 2 g daily.
Transplants were performed in rooms with positive pressure filtered flow. Antifungal prophylaxis with oral nystatin was administered until the advent of fluconazole. Acylovir was used as antiviral prophylaxis and ciprofloxacin for gut decontamination. Until 2003 total parental nutrition was administered only in the case of severe mucositis, then in all the patients from day +3. All patients received irradiated blood products that had been depleted of leucocytes using filters.
Definitions
The day of HPC infusion was named as day 0. Neutrophil and platelet engraftment was defined as the first of 3 consecutive days with a neutrophil count more than 0.5×109/L and a platelet count more than 30×109/L, respectively. After engraftment, patients surviving more than 14 days and more than 100 days were evaluated for the presence of acute and chronic GVHD, respectively. These complications were diagnosed and graded using established criteria22–24. Overall survival was defined as the time from transplantation until last follow-up or death from any cause. Transplant-related mortality and disease-related mortality were defined as death not related and related to disease recurrence or progression, respectively.
Flow cytometric analysis
We conducted three-colour flow cytometric analyses of the lymphocyte subsets in the peripheral blood at a median time of 20 days after transplant (range, 12–34) using a Facs Calibur (Becton Dickinson). Briefly, 50–100 μL of peripheral blood containing 5×105–1×106 white blood cells were labelled for 20 minutes with each of the 4/8/45 or 3/56/19 reagents or three different antibodies conjugated with fluorescein isothiocyanate, phycoerythrin and phycoerythrin-cyanine 5.1. The samples were further processed with a lysed no-wash procedure, and then measured within 2 hours. The samples were analysed for the content of CD3+ T-cells, CD3+CD4+ helper T-cells, CD3+CD8+ Tc cells, CD19+ B cells and CD3-CD56+ NK cells. Additionally, appropriate isotypic negative controls were set up. The cytometer was calibrated at weekly intervals.
We focused our attention on CD3+CD4+ helper T-lymphocytes The method for CD4+ cell count has been the same over the last 15 years. A double platform was used. The white blood cell count was calculated by Dasit, as was the lymphocyte count: the absolute value for CD4+ was determined in double fluorescence (CD25FITC/CD4PE, CD4FITC/CD8 PE, CD4/CD45RA, CD45RO/CD4, CD4 FITC/CD278 PE) and given by an indirect calculation (multiplying the absolute lymphocyte count by percentage of CD4+ cells).
Statistical analysis
All statistical studies were performed using SPSS software. Univariate analysis was performed using Spearman’s rho. A receiver operating characteristic (ROC) curve was used to find the cut-off value of CD4+ cell count to differentiate groups. A ROC curve is a graphic representation that contains the values of sensitivity and specificity of a test with different cut-off values. The curve has a broken shape where the variation points of decline represent the different cut-offs considered. The optimal cut-off is the one that represents the best compromise between sensitivity and specificity and is given by the value closest to the high left corner of the ROC curve. The closer the area under the ROC curve is to one, the greater the diagnostic power of the test.
Survival curves were analysed using the Kaplan-Meier method and differences between groups were estimated by the log-rank test. Finally, Cox proportional hazard model was used to identify prognostic factors for overall survival.
Results
Statistical analysis for early CD4+ cell recovery as the end-point
Univariate analysis with Spearman’s rho showed a significant correlation between early CD4+ cell recovery and overall survival (R=0.389, p=0.000), transplant-related mortality (R=−0.220 p=0.029), disease-related mortality (R=−0,280 p=0,005), stem cell source (R=−0.369, p=0.000) and type of donor (R=−0.429, p=0.000). No correlation was found between CD4+ cell count and acute GVHD and conditioning regimen. In multivariate analysis T-helper lymphocytes (range, 12–34) were significantly associated with stem cell source, being higher in patients transplated with HPC-A than in those transplanted with HPC-M (p=0.019), and with donor, being higher when the donor was a sibling that when an alternative donor was used (p=0.017).
Clinical outcomes of patients
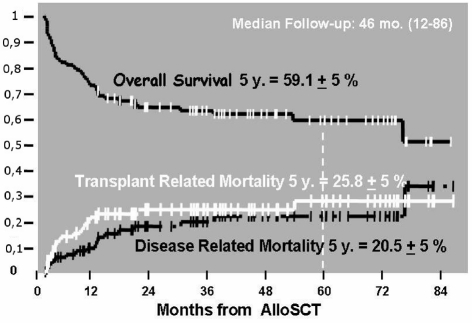
Thirty percent of patients had acute GVHD grade 2 or higher and 9% of patients had acute GVHD of grade three or higher. Limited chronic GVDH was present in 7% of patients and extensive chronic GVHD was present in 19% of the cases. Overall, 25.8% of patients died as a result of transplant complications and 20.5% of patients died as a result of disease relapse (Figure 1). The median follow-up of the patients was 46 months (range, 12–86).
Figure 1.
Clinical outcome of patients.
Transplant-related mortality, disease-related mortality, overall survival and early lymphocyte recovery
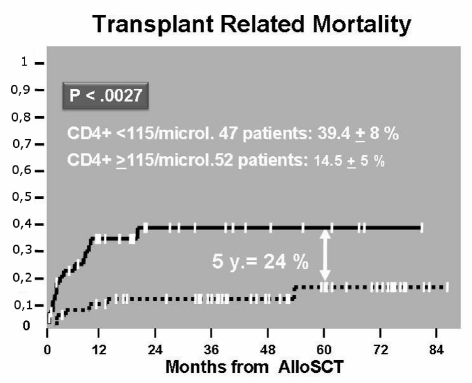
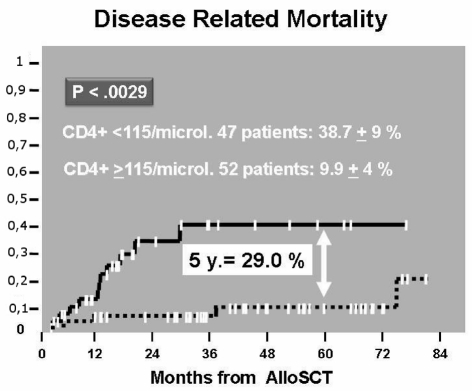
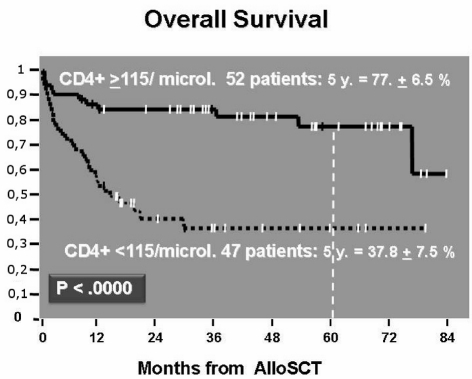
The ROC curve of CD4+ cell count (area=72%, p=0.000) indicated that the best cut-off was 115 CD4+ cells/μL. At 2 years follow-up, patients achieving this CD4+ cell count had a significantly lower cumulative transplant-related mortality compared to patients who did not reach this count (14.5%±5% versus 39.4%±8%, p=0.0027) (Figure 2). As far as concerns cumulative disease-related mortality, this was higher in patients with a CD4+ lymphocyte count less than the cut-off value than in patients with a CD4+ cell count greater than 115/μL (38.7%±9% versus 9.9%±4%, p=0.0029) (Figure 3). At the 5-year follow-up, overall survival was 77.5%±6.5% and 37.8%±7.5% (p=0.000) in patients with CD4+ cell count ≥115/μL and in patients with a CD4+ cell count <115/μL, respectively (Figure 4).
Figure 2.
Cumulative incidence of TRM according to CD4≥ or <than cut off value on day 20.
Figure 3.
Cumulative incidence of DRM according to CD4> or <than cut off value on day 20.
Figure 4.
Survival curve according to CD4≥ or <than 115 μL on day 20.
Multivariate analysis for overall survival as the end-point
We also analysed the other predictive roles of patient and graft factors for overall survival. Using the Kaplan-Meier method, besides early CD4+ cell count, we found a significant correlation between overall survival and donor type, acute GVHD, ABO compatibility, sex of recipient and stem cell source (Table II).
Table II.
Kaplan-Meier for overall survival.
| Variable | P-value |
|---|---|
| Circulating CD4+ | <0.0000 |
| Donor type | <0.0000 |
| Acute GVHD | <0.0000 |
| ABO compatibility | <0.0004 |
| Sex (recipient) | <0.0167 |
| Cell source | ns |
| Conditioning | ns |
| Sex (donor) | ns |
| Disease | ns |
| Disease status | Ns |
| Age (recipient) | Ns |
The Cox regression analysis demonstrated a significant association between overall survival and early CD4+ cell recovery, acute GVHD and donor type. No correlation was found between overall survival and ABO compatibility, conditioning regimen and stem cell source (Table III).
Table III.
Multivariate Cox analysis for overall survival.
| Risk factor | p-value | Hazard ratios | Lower | Upper |
|---|---|---|---|---|
| Circulating CD4+ | 0.012 | 2.686 | 1.241 | 5.813 |
| Acute GVHD | 0.025 | 0.303 | 0.107 | 0.860 |
| Donor type | 0.028 | 0.407 | 0.183 | 0.907 |
| ABO compatibility | 0.083 | 0.491 | 0.220 | 1.098 |
| Conditioning | 0.432 | 0.753 | 0.371 | 1.529 |
| Cell Source | 0.652 | 1.198 | 0.547 | 2.622 |
95.0 % CI
Discussion
Our study examined the impact of rapid T-helper lymphocyte recovery on transplant outcomes after allogeneic HPCT. First, an early reconstitution of CD4+ cell count 20 days after transplant to more than 115/μL strongly correlated with better overall survival and lower disease-related mortality and transplant-related mortality. Second, univariate and multivariate analyses demonstrated that early CD4+ cell recovery depends on stem cell source and donor type. Finally, overall survival is correlated to different patient and graft characteristics but above all to early CD4+ T-cells.
Some of these results confirm data from previous investigations. In particular, Berger et al.20 demonstrated that in a large population of patients who underwent allogeneic bone marrow transplant, a CD4+ cell count on day +35 of more than 86/μL was associated with a lower transplant-related mortality. In that study, early CD4+ cell recovery was influenced, in univariate analysis, by donor type, patient’s age and acute GVHD. In multivariate analysis, the effect of acute GVHD was lost. In our study, early CD4+ cell count was evaluated at a median of 20 days after transplantation, which might be considered a very early time after engraftment and it correlated not only with transplant-related mortality but also with overall survival and disease-related mortality. We analysed transplants performed with bone marrow or peripheral blood as the source of stem cells: the association between rapid CD4+ cell recovery and clinical outcome was confirmed in both cases. We did not find any association between early T-helper cell counts and acute GVHD. Our cut-off value of CD4+ cell count (115/μL) is higher than the cut-off value in Berger’s study but we think that the disparity is due to the different numbers of patients taken into consideration. Kim et al.19, like us, studied patients transplanted with HPC-M and HPC-A and they showed that an early CD4+ T-cell recovery above 200/μL at 3 months strongly predicted a successful transplant outcome after allogeneic HPCT. Novitzky et al.25 obtained different data: they demonstrated that treatment failure was associated with low total CD8+ cell counts, when measured at 6 months, rather than with low CD4+ cells. The same results were described in a paediatric population by Koehl et al.26 and in clinical reports of adult patients by Powles et al.27 We believe that these results are the two faces of the same coin given the central role of CD4+ cells in the generation, maintenance and regulation of both humoral- and cell-mediated immunity28–30.
Many studies have demonstrated better engraftment and immune reconstitution when HPC-A are used as the graft material than when transplants are performed with HPC-M10,11. The differences are attributed to the number of lymphocytes infused with the grafts because HPC-A contain approximately one log more lymphocytes compared with HPC-M31. In our study, both univariate and multivariate analyses confirmed that HPC-A are associated with significantly better early immune recovery in terms of CD4+ cell count than are HPC-M. The impact of donor type in our analysis confirms previous data demonstrating a better CD4+ cell count20 and a better clinical outcome32,33 in case of transplants from matched sibling donors. Our data did not show a correlation between conditioning regimen and early CD4+ cell count, perhaps because reduced intensity regimens are immunosuppressive like myeloablative conditioning. Furthermore, we believe that acute GVHD was not correlated with rapid immune recovery because in the early phase after transplantation it does not affect the immune system.
The most important result of our study is that of the impact of early circulating CD4+ lymphocyte cells on overall survival. In the past, many studies demonstrated that type of donor32,34,35 and acute GVHD36–38 affected overall survival, while the results on a predictive role of ABO compatibility on overall survival are conflicting39–41. In our univariate analysis overall survival was correlated to early T-helper cell count, donor type, acute GVHD, ABO compatibility, sex of recipient and stem cell source. In multivariate analysis a strong correlation remained between overall survival and early CD4+ cell count, acute GVHD and donor type. There was only a trend to an association trend between ABO compatibility and overall survival. Among all these factors, early CD4+ cell count was the most important predictive parameter for overall survival.
In conclusion, we demonstrated that the main independent predictive factor for clinical outcome of patients undergoing allogeneic HPCT is early T-helper cell count. These data are very important because they indicate that patients with low early CD4+ counts need to be followed more carefully to avoid transplant complications and/or disease relapse. In the future, manipulating the graft could improve early immune reconstitution after transplantation and, consequently, overall survival of patients.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411:385–9. doi: 10.1038/35077251. [DOI] [PubMed] [Google Scholar]
- 2.Auletta JJ. Immune restoration following hematopoietic stem cell transplantation: an evolving target. Bone Marrow Transplant. 2005;35:835–7. doi: 10.1038/sj.bmt.1704966. [DOI] [PubMed] [Google Scholar]
- 3.Antin JH. Immune reconstitution: the major barrier to successful stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:43–5. doi: 10.1016/j.bbmt.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Storek J, Gooley T, Witherspoon RP, et al. Infectious morbidity in long-term survivors of allogeneic marrow transplantation is associated with low CD4T cell counts. Am J Hematol. 1997;54:131–8. doi: 10.1002/(sici)1096-8652(199702)54:2<131::aid-ajh6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 5.Maraninchi D, Gluckman E, Blaise D, et al. Impact of T-cell depletion on outcome of allogeneic bone-marrow transplantation for standard-risk leukemias. Lancet. 1987;2:175–8. doi: 10.1016/s0140-6736(87)90763-x. [DOI] [PubMed] [Google Scholar]
- 6.Reddy V, Iturraspe JA, Tzolas AC, et al. Low dendritic cell count after allogeneic hematopoietic stem cell transplantation predicts relapse, death, and acute graft-versus-host disease. Blood. 2004;103:4330–3. doi: 10.1182/blood-2003-09-3325. [DOI] [PubMed] [Google Scholar]
- 7.Shenoy S, Mohanakumar T, Todd G, et al. Immune reconstitution following allogeneic peripheral blood stem cell transplants. Bone Marrow Transplant. 1999;23:335–46. doi: 10.1038/sj.bmt.1701581. [DOI] [PubMed] [Google Scholar]
- 8.Savage1 WJ, Bleesing JJH, Douek D, et al. Lymphocyte reconstitution following non-myeloablative hematopoietic stem cell transplantation follows two patterns depending on age and donor/recipient chimerism. Bone Marrow Transplant. 2001;28:463–71. doi: 10.1038/sj.bmt.1703176. [DOI] [PubMed] [Google Scholar]
- 9.Clave E, Rocha V, Talvensaari K, et al. Prognostic value of pretrasplantation host thymic function in HLA-identical sibling hematopoietic stem cell transplantation. Blood. 2009;105:2608–13. doi: 10.1182/blood-2004-04-1667. [DOI] [PubMed] [Google Scholar]
- 10.Dey BR, Shaffer J, Yee AJ, et al. Comparison of outcomes after transplantation of peripheral blood stem cells versus bone marrow following an identical nonmyeloablative conditioning regimen. Bone Marrow Transplant. 2007;40:19–27. doi: 10.1038/sj.bmt.1705688. [DOI] [PubMed] [Google Scholar]
- 11.Storek J, Dawson MA, Storer B, et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood. 2001;97:3380–9. doi: 10.1182/blood.v97.11.3380. [DOI] [PubMed] [Google Scholar]
- 12.Chen BJ, Cui X, Sempowski GD, et al. Hematopoietic stem cell dose correlates with the speed of immune reconstitution after stem cell transplantation. Blood. 2004;103:4344–52. doi: 10.1182/blood-2003-07-2534. [DOI] [PubMed] [Google Scholar]
- 13.Kim DH, Won D, Lee NY, et al. Non-CD34+ cells, especially CD8+ cytotoxic T cells and CD56+ natural killer cells, rather than CD34 cells, predict early engraftment and better transplantation outcomes in patients with hematologic malignancies after allogeneic peripheral stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:719–28. doi: 10.1016/j.bbmt.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen VH, Shashidhar S, Chang DS, et al. The impact of regulatory T cells on T-cell immunity following hematopoietic cell transplantation. Blood. 2008;111:945–53. doi: 10.1182/blood-2007-07-103895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morecki S, Gelfand Y, Nagler A, et al. Immune reconstitution following allogeneic stem cell transplantation in recipients conditioned by low intensity vs myeloablative regimen. Bone Marrow Transplant. 2001;28:243–9. doi: 10.1038/sj.bmt.1703118. [DOI] [PubMed] [Google Scholar]
- 16.Larosa F, Marmier C, Robinet E, et al. Peripheral T-cell expansion and low infection rate after reduced-intensity conditioning and allogeneic blood stem cell transplantation. Bone Marrow Transplant. 2005;35:859–68. doi: 10.1038/sj.bmt.1704889. [DOI] [PubMed] [Google Scholar]
- 17.Abrahamsen IW, Somme S, Heldal D, et al. Immune reconstitution after allogeneic stem cell transplantation: the impact of stem cell source and graft-versus-host disease. Haematologica. 2005;90:86–93. [PubMed] [Google Scholar]
- 18.Hakki M, Riddel SR, Storek J, et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy and subclinical reactivation. Blood. 2003;102:3060–7. doi: 10.1182/blood-2002-11-3472. [DOI] [PubMed] [Google Scholar]
- 19.Kim DH, Sohn SK, Won DI, et al. Rapid helper T-cell recovery above 200×106/l at 3 months correlates to successful transplant outcomes after allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;37:1119–28. doi: 10.1038/sj.bmt.1705381. [DOI] [PubMed] [Google Scholar]
- 20.Berger M, Figari O, Bruno B, et al. Lymphocyte subsets recovery following allogeneic bone marrow transplantation (BMT): CD4+ cell count and transplant-related mortality. Bone Marrow Transplant. 2008;41:55–62. doi: 10.1038/sj.bmt.1705870. [DOI] [PubMed] [Google Scholar]
- 21.Lowsky R, Takahashi T, Liu YP, et al. Protective conditioning for acute graft-versus-host disease. N Engl J Med. 2005;353:1321–1331. doi: 10.1056/NEJMoa050642. [DOI] [PubMed] [Google Scholar]
- 22.Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 23.Ratanatharathorn V, Ayash L, Lazarus HM, et al. Chronic graft-versus-host disease: clinical manifestation and therapy. Bone Marrow Transplant. 2001;28:121–9. doi: 10.1038/sj.bmt.1703111. [DOI] [PubMed] [Google Scholar]
- 24.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man: a long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–17. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 25.Novitzky N, Davison GM, Hale G, Waldmann H. Immune reconstitution at 6 months following T-cell depleted hematopoietic stem cell transplantation is predictive for treatment outcome. Transplantation. 2002;74:1551–9. doi: 10.1097/00007890-200212150-00012. [DOI] [PubMed] [Google Scholar]
- 26.Koehl U, Bochennek K, Zimmermann SY, et al. Immune recovery in children undergoing allogeneic stem cell transplantation: absolute CD8+CD3+ count reconstitution is associated with survival. Bone Marrow Transplant. 2007;39:269–78. doi: 10.1038/sj.bmt.1705584. [DOI] [PubMed] [Google Scholar]
- 27.Powles R, Singhal S, Treleaven J, et al. Identification of patients who may benefit from prophylactic immunotherapy after bone marrow transplantation for acute leukaemia on the basis of lymphocyte recovery early after transplantation. Blood. 1998;11:3481–6. [PubMed] [Google Scholar]
- 28.Flavell RA. The molecular basis of T cell differentiation. Immunol Res. 1999;19:159–68. doi: 10.1007/BF02786484. [DOI] [PubMed] [Google Scholar]
- 29.Husmann LA, Bevan MJ. Cooperation between helper T cells and cytotoxic T lymphocyte precursors. Ann NY Acad Sci. 1988;532:158–69. doi: 10.1111/j.1749-6632.1988.tb36335.x. [DOI] [PubMed] [Google Scholar]
- 30.Kalams SA, Walker BD. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J Exp Med. 1998;188:2199–204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ottinger HD, Beelen DW, Scheulen B, et al. Improved immune reconstitution after allotransplantation of peripheral blood stem cells instead of bone marrow. Blood. 1996;88:2775–9. [PubMed] [Google Scholar]
- 32.Koh LP, Rizzieri DA, Chao NJ. Allogeneic hematopoietic stem cell transplant using mismatched/haploidentical donors. Biol Blood Marrow Transplant. 2007;13:1249–67. doi: 10.1016/j.bbmt.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Eyrich M, Leiler C, Lang P, et al. A prospective comparison of immune reconstitution in pediatric recipients of positively selected CD34+ peripheral blood stem cells from unrelated donors vs recipients of unmanipulated bone marrow from related donors. Bone Marrow Transplant. 2003;32:379–90. doi: 10.1038/sj.bmt.1704158. [DOI] [PubMed] [Google Scholar]
- 34.Kumar P, Defor TE, Brunstein C, et al. Allogeneic hematopoietic stem cell transplantation in adult acute lymphocytic leukemia: impact of donor source on survival. Biol Blood Marrow Transplant. 2008;14:1394–400. doi: 10.1016/j.bbmt.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crocchiolo R, Ciceri F, Fleischhauer K, et al. HLA matching affects clinical outcome of adult patients undergoing haematopoietic SCT from unrelated donors: a study from the Gruppo Italiano Trapianto di Midollo Osseo and Italian Bone Marrow Donor Registry. Bone Marrow Transplant. 2009;44:571–7. doi: 10.1038/bmt.2009.67. [DOI] [PubMed] [Google Scholar]
- 36.Gratwohl A, Brand R, Apperley J, et al. Graft-versus-host disease and outcome in HLA-identical sibling transplantations for chronic myeloid leukemia. Blood. 2002;100:3877–86. doi: 10.1182/blood.V100.12.3877. [DOI] [PubMed] [Google Scholar]
- 37.Guardiola P, Socié G, Li X, et al. Acute graft-versus-host disease in patients with Fanconi anemia or acquired aplastic anemia undergoing bone marrow transplantation from HLA-identical sibling donors: risk factors and influence on outcome. Blood. 2004;103:73–7. doi: 10.1182/blood-2003-06-2146. [DOI] [PubMed] [Google Scholar]
- 38.Kim DH, Sohn SK, Kim JG, et al. Clinical impact of hyperacute graft-versus-host disease on results of allogeneic stem cell transplantation. Bone Marrow Transplant. 2004;33:1025–30. doi: 10.1038/sj.bmt.1704479. [DOI] [PubMed] [Google Scholar]
- 39.Helming AM, Brand A, Wolterbeek R, et al. ABO incompatible stem cell transplantation in children does not influence outcome. Pediatr Blood Cancer. 2007;49:313–7. doi: 10.1002/pbc.21025. [DOI] [PubMed] [Google Scholar]
- 40.Worel N, Kalhs P, Keil F, et al. ABO mismatch increases transplant-related morbidity and mortality in patients given nonmyeloablative allogeneic HPC transplantation. Transfusion. 2003;43:1153–61. doi: 10.1046/j.1537-2995.2003.00465.x. [DOI] [PubMed] [Google Scholar]
- 41.Stussi G, Seebach L, Muntwyler J, et al. Graft-versus-host disease and survival after ABO-incompatible allogeneic bone marrow transplantation: a single-centre experience. Br J Haematol. 2001;113:251–3. doi: 10.1046/j.1365-2141.2001.02734.x. [DOI] [PubMed] [Google Scholar]