Abstract
Background
There is evidence that percutaneous dilatational tracheotomy (PDT) can be safely performed in patients with severe coagulation disorders if these are carefully corrected immediately before the procedure. However, it is currently unclear whether PDT can be performed safely in patients in an Intensive Care Unit (ICU) with uncorrected mild coagulation disorders.
Materials and methods
In a randomised controlled trial we determined the effect of correction of mild coagulation disorders on bleeding during and after PDT. ICU patients planned for bedside PDT with: (i) a prothrombin time (PT) between 14.7–20.0 seconds, (ii) a platelet count between 40–100×109/L and/or (iii) active treatment with acetylsalicylic acid were randomised to receive infusion with fresh-frozen plasma (FFP) and/or platelets (“correction”) versus no transfusion (“no correction”) before PDT.
Results
We randomised 35 patients to the “correction” group and 37 patients to the “no correction” group. In patients who received FFP, the decrease in PT was marginal (mean decrease 0.40±0.56 seconds); the median increase in platelet counts after transfusion of platelets was 35 [11–47]x109/L. The median blood loss was 3 [IQR: 1–6] grams in the “correction” group and 3 [IQR: 2–6] grams in the “no correction” group (P=0.96).
Discussion
Bleeding during and after bedside PDT in ICU patients with mild coagulation disorders is rare in our setting. Correction of subclinical coagulation disorders by transfusion of FFP and/or platelets does not affect bleeding.
Keywords: percutaneous tracheostomy, coagulation disorders, transfusion
Introduction
Percutaneous dilatational tracheotomy (PDT) is a common surgical procedure in mechanically ventilated patients in an intensive care unit (ICU), especially in those patients who have an (expected) prolonged duration of mechanical ventilation1,2. One feared complication of PDT is peri-procedural bleeding3–5. Although PDT is a simple surgical procedure, the rate of peri-procedural bleeding is reported to be as high as 5%6–8.
Many ICU patients have coagulation abnormalities, varying from slight lengthening of coagulation test values and/or mild thrombocytopenia, to severe coagulation disorders9,10. In addition, a substantial number of ICU patients receive active treatment with acetylsalicylic acid. There is evidence that PDT can be safely performed in patients with severe coagulation disorders if these are carefully corrected by infusion of fresh-frozen plasma (FFP) and/or platelets immediately before the procedure11–16. However, it is currently unclear whether PDT can be performed safely in ICU patients with uncorrected mild coagulation disorders. A recent postal survey in the Netherlands showed that the opinions regarding which coagulation disorders should be corrected before PDT varied greatly17. Notably, it is common practice in many ICU to correct even mild coagulations disorders.
We performed a randomised controlled trial to determine the effect of correction of mild coagulation disorders on bleeding during and after PDT. We hypothesised that correction of mild coagulation disorders before PDT via transfusion of FFP and/or platelets decreases the rate of bleeding.
Materials and methods
Study design and setting
This was an open-label, randomised controlled trial in a 32-bed, mixed medical-surgical ICU of a university-affiliated hospital in the Netherlands. The study was approved by the local ethics committees. Each patient or a legal representative gave written informed consent to participation in the study.
Inclusion and exclusion criteria
Patients planned for bedside PDT with mild coagulation disorders (prothrombin time, PT, 14.7–20.0 seconds, and/or platelet counts 40–100×109/L) and/or active treatment with acetylsalicylic acid at any dose were eligible.
Exclusion criteria were: (i) age <18 years; (ii) need for surgical tracheotomy; (iii) contraindications to transfusion of blood products; (iv) use of clopidogrel. A patient would also be excluded from participation in this trial if the attending physician insisted on the need for transfusion of FFP and/or platelets.
Study groups
Patients with a prolonged PT (normal values are between 11.0–14.7 seconds) assigned to the “correction group” received one or two units of FFP (1 unit contains 300 mL of FFP: if the PT was between 14.7–18.0 seconds the patient received 1 unit of FFP; if the PT was between 18.0–20.0 seconds the patient received 2 units of FFP). Patients with a low platelet count and/or active use of acetylsalicylic acid assigned to the correction group received five units of platelet concentrates prepared from five pooled buffy coats. Patients assigned to the “no correction” group received neither plasma nor platelets. However, FFP and/or platelets were made available for immediate transfusion in case bleeding occurred during or after PDT.
Percutaneous dilatational tracheotomy procedure
The local policy for tracheotomy included all ICU patients with respiratory failure expected to require mechanical ventilation for more than 10 days. Other indications for tracheotomy included a persisting Glasgow Coma Score of less than 8, (suspected) critical illness polyneuromyopathy (CIPNM) and/or muscle weakness, inability of the patient to maintain a patent airway, sputum retention, failed tracheal extubation, insufficient swallowing or cough reflex, indication for home ventilation and obstruction of the upper airways. Contraindications included severe coagulation disorders (e.g., disseminated intravascular coagulation) and/or anticoagulation (e.g., infusion of activated protein C), complex or abnormal anatomy, need for prone ventilation, haemodynamic instability and ventilatory instability.
PDT (Ciaglia Blue Rhino, Cook, Son, the Netherlands) was the method of choice unless the abovementioned contraindications required a surgical approach. PDT was performed by trained ICU physicians under fibre-optic bronchoscopy guidance. Enteral feeding was stopped 2 hours before the procedure. During PDT the patient’s blood pressure, heart rate, respiratory rate, oxygen saturation, and cardiac rhythm strip was monitored. Mechanical ventilation was maintained in a mandatory mode while the inspired oxygen fraction was increased to 100%. In the event of hypotension due to anaesthetics and opioids, 500 mL of 6% hydroxyethyl starch 130/0.4 in 0.9% sodium chloride injection (Voluven®, Fresenius Kabi, Den Bosch, the Netherlands) could be administered. In the event that the blood pressure remained low, noradrenaline was started or the dose adjusted.
Data collection
The volume of peri-procedural blood loss was calculated by measuring the difference in weight of the gauzes before and after the procedure. The intensity of intra-tracheal periprocedural bleeding was scored by the endoscopist (“none”, “mild, but not requiring intra-tracheal suction”, or “severe, requiring intra-tracheal suction”). After the procedure the trachea was suctioned every hour (for a maximum of 12 hours) and the duration that blood was visible in the tracheal aspirates was documented.
Other data to be collected included the patient’s demographics, type of admission, reason for admission, age, gender, APACHE II score, time from ICU admission until tracheotomy, reason for mechanical ventilation, reason for tracheotomy, duration of ICU stay, ICU and hospital mortality.
Definitions
Peri-procedural bleeding was defined as bleeding during or within the first 12 hours after the procedure. Clinically irrelevant bleeding was defined as procedural bleeding of less than 100 g of blood which could be controlled with the application of local pressure and did not require re-exploration or transfusion of packed red cells. Minor bleeding was defined as blood loss greater than 100 g which could be controlled with the application of local pressure and did not require re-exploration or transfusion of packed red cells. Major bleeding was defined as the presence of blood in the airways requiring repeated suction post-procedure, emergency surgery and/or transfusion of packed red cells.
Randomisation
A computer-generated randomisation scheme was used. Each assignment (“correction” or “no correction”) was recorded on a piece of paper folded three times and enclosed in a consecutively numbered, opaque, sealed envelope.
Cost analysis
Costs were analysed from a provider’s perspective. The differences in costs of transfusion of FFP, platelets and packed red cells were calculated between the study groups.
Power analysis
The power calculation was based on reviews of complications during or after PDT6,7,18, in which the rate of serious bleeding was estimated to be approximately 5%. However, these series included patients without coagulation disorders and blood products were freely used. There was a large variation in the incidence of blood loss mentioned in individual studies which could be explained by differences in definitions of blood loss between studies. In one study of a series of patients the incidence of blood loss was found to be up to 20%8. We expected a higher rate of bleeding of 20% in patients with uncorrected mild coagulation disorders, and thus hypothesised a 15% absolute decrease following the correction of subclinical bleeding disorders. We calculated that in order to detect a difference, with a two-sided level of significance of 0.05 and a power of 80%, 76 patients had to be included in each group.
Statistical analysis
Data were analysed according to intention-to-treat analysis. Continuous normally distributed variables are expressed as means and standard deviations, or as medians and interquartile ranges, where appropriate. Categorical variables are expressed as numbers and percentages. A Student’s t-test was used to test groups of continuous normally distributed variables. If continuous data were not normally distributed the Mann-Whitney U test was used. Categorical variables were compared with the chi-square test or Fisher’s exact test when appropriate. A P-value less than 0.05 was considered statistically significant. Data were analyzed using SPSS version 16.0.
Results
Early termination of the study
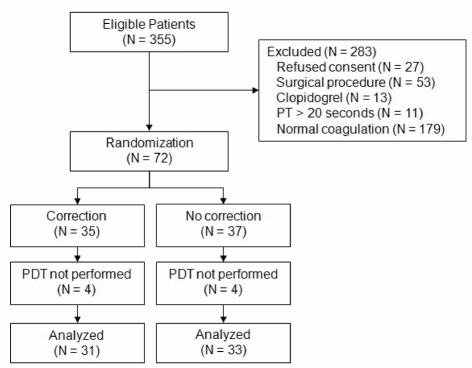
Between July 2007 and October 2009, 355 patients underwent PDT, of whom 72 met the inclusion criteria (see Figure 1). Although a sample size of 152 patients was considered necessary to find a difference of 15% in bleeding between groups, the study was prematurely terminated. Recognition of the small amount of observed blood loss even in the “no correction” group resulted in an increasing resistance of the physicians to transfuse FFP and/or platelets in the “correction” group. With a yearly incidence of approximately 150 PDT we would have need approximately another 3 years to complete this study. We considered these arguments valid and decided to terminate the study prematurely.
Figure 1.
Patients’ disposition in the study.
Patients
Table I shows the baseline characteristics of the study groups. Thirty-five patients were randomized to the “correction” group, and 37 patients to the “no correction” group. After randomisation PDT was not performed in seven patients because of unexpected difficulties in the recognition of anatomical landmarks. These patients subsequently underwent surgical tracheotomy. In one patient tracheotomy was not performed because of logistical problems. There were no complications of the procedure.
Table I.
Baseline characteristics of the study groups.
| Correction N=35 |
No correction N=37 |
P-value | |
|---|---|---|---|
| Demographics | |||
| Male, N (%) | 22 (63) | 16 (43) | 0.10 |
| Age (years), median [IQR] | 64 [56–72] | 68 [60–76] | 0.21 |
| APACHE II score, mean±SD | 22±8 | 21±7 | 0.49 |
| Time from admission to tracheotomy (days), mean±SD | 9±6 | 10±6 | 0.62 |
| Reason for mechanical ventilation | |||
| Post-surgical, N (%) | 5 (14) | 9 (24) | 0.28 |
| Coma, N (%) | 3 (9) | 4 (11) | 0.75 |
| Cardiac arrest, N (%) | 4 (11) | 4 (11) | 0.93 |
| Acute respiratory failure, N (%) | 19 (51) | 18 (49) | 0.63 |
| Trauma, N (%) | 2 (6) | 1 (3) | 0.48 |
| Other, N (%) | 2 (6) | 1 (3) | 0.48 |
| Reason for tracheotomy | |||
| Complicated weaning, N (%) | 10 (29) | 17 (46) | 0.13 |
| Expected prolonged duration of mechanical ventilation, N (%) | 12 (34) | 4 (11) | 0.02 |
| Need for frequent airway suctioning, N (%) | 3 (9) | 2 (5) | 0.47 |
| Low GCS score, N (%) | 5 (14) | 6 (16) | 0.37 |
| CIPNM, N (%) | 4 (11) | 5 (14) | 0.54 |
| Other, N (%) | 1 (3) | 3 (8) | 0.61 |
| Possible reasons for coagulation disorders, N (%) | |||
| Acute renal failure | 9 (26) | 3 (8) | 0.45 |
| Chronic renal failure | 2 (6) | 2 (5) | 0.95 |
| Sepsis | 7 (20) | 9 (24) | 0.66 |
| Massive transfusion | 5 (14) | 5 (14) | 0.93 |
| Liver cirrhosis | 0 | 0 | - |
| Haematological malignancy | 2 (6) | 3 (8) | 0.69 |
| Duration of mechanical ventilation (days), median (IQR) | 11 (7–24) | 16 (10–21) | 0.16 |
| Length of stay ICU (days), median [IQR] | 15 [8–29] | 21 [14–26] | 0.21 |
| ICU mortality, N (%) | 4 (11) | 11 (31) | 0.06 |
| Hospital mortality, N (%) | 11 (31) | 15 (41) | 0.42 |
Legend: GCS, Glasgow Coma Scale; CIPNM, critical illness polyneuromyopathy.
Correction of mild coagulation disorders
Haematological values and transfusion requirements are presented in Table II. Of all patients, 56.9% had a prolonged PT, 31.9% had a low platelet count, 31.9% received acetylsalicylic acid and in 20.8% more than one coagulation disorder was present. A total of 19 units of FFP and 23×5 units of platelets were transfused. Twelve patients were transfused with FFP alone, 17 patients received only platelets, and 6 patients received both blood products. No transfusions were given in the “no correction” group.
Table II.
Haematological values.
| Correction N=35 |
No correction N=37 |
P-value | |
|---|---|---|---|
| Prolonged PT, N (%) | 19 (54) | 22 (59) | 0.66 |
| PT, seconds, mean±SD* | 16.0±1.2 | 16.6±1.1 | 0.39 |
| Low platelet count, N (%) | 13 (37) | 10 (27) | 0.36 |
| Platelet count, ×109/L, median (IQR)* | 81 [63–85] | 56 [47–70] | 0.03 |
| Active treatment with acetylsalicylic acid, N (%) | 11 (31) | 12 (32) | 0.93 |
| >1 coagulation disorder present, N (%) | 8 (23) | 7 (19) | 0.68 |
| Decrease in PT after transfusion, seconds, mean±SD | |||
| 1 unit of plasma (N=17) | 0.40±0.56 | - | - |
| 2 units of plasma (N=1) | 1.4 (−) | - | - |
| Increase in platelet count after transfusion,x109/L, median [IQR] | |||
| 5 units of platelets (N=23) | 35 [11–47] | - | - |
Legend: PT, Prothrombin time;
Only patients who had a prolonged PT or low platelet count are included in the calculation of means/medians
Blood loss with percutaneous dilatational tracheotomy
The median blood loss during PDT was similar in the two groups (Table III). In the “correction” group mild intra-tracheal bleeding occurred once in every 1.3 patients and in the “no correction” group once in every 1.7 patients (P=0.16). One in every ten patients in the “correction” group experienced a severe intra-tracheal bleed, compared to one in every 16 patients in the “no correction” group. The duration that blood was visible in the tracheal aspirates was not affected by correction (Table III).
Table III.
Bleeding outcomes.
| Correction N=35 |
No correction N=37 |
P-value | |
|---|---|---|---|
| Blood loss (grams), median [IQR] | 3.0 [1.0–6.0] | 3.0 [2.0–6.0] | 0.96 |
| Intratracheal bleeding during procedure, N (%) | |||
| None, N (%) | 5 (14) | 12 (32) | 0.07 |
| Mild, N (%) | 23 (66) | 19 (51) | 0.16 |
| Severe, N (%) | 3 (9) | 2 (5) | 0.67 |
| Hours until no blood aspirated (hours), median [IQR] | 2.0 [0–3.0] | 1 [0.5–3.0] | 0.99 |
In one patient on active acetylsalicylic acid treatment in the no-correction group bleeding was observed directly after skin incision, but before opening the airway. Local compression was applied and the wound was sutured. Total blood loss was 17 grams which classifies as clinically irrelevant. There was no need for transfusion of packed red cells due to blood loss during or after PDT.
Cost analysis
The cost of one FFP and five units of platelet concentrates in our hospital is €172 ($252) and €484 ($709), respectively. A surplus of €14,423 ($21,104) was spent in the “correction” group compared to the “no correction” group. Consequently, a procedure that is not preceded by correction of mild coagulation disorders saves, on average, €465 per patient.
Discussion
ICU patients undergoing PDT frequently have mild coagulation disorders which may increase their risk of bleeding during or after invasive procedures. In this study we compared bleeding in patients after correction of mild coagulation disorders to those without prior correction and found no differences. Notably, clinically significant bleeding was not present in either of the two study groups.
This trial has some important limitations. Firstly, this was an open-label study. Hypothetically, physicians who disbelieved that correction of mild coagulation disorders would benefit patients undergoing PDT may have tried to perform PDT in such a way that blood loss was kept to a minimum in the “no correction” group, or vice versa may have acted imprudently in the “correction” group. Secondly, the intended inclusion of 152 patients turned out to be unrealistic and the study was terminated prematurely. During conduct of the trial there was growing resistance of the ICU-physicians to infuse blood products, including platelet concentrates and plasma, because blood loss with PDT was found to be minimal. Given the early termination of this trial, the results should be interpreted with care. In addition, since the definition and hence, reported incidences of blood loss differ between studies, the number of patients needed for inclusion would have changed when different expected incidences were used for the power calculation. With hindsight, we might have overestimated the chance of bleeding and the effect size of blood products. However, we feel that the amount of bleeding we powered for was clinically relevant. Also, since there were several inclusion and treatment arms, the expected blood loss may differ between groups and even more patients may be needed to provide sufficient power to exclude a type II error definitely. Finally, although we report on possible aetiologies of the coagulation disorders, we did not report on feeding. Vitamin K shortage could have been an underestimated cause of mild coagulation disorders.
Inclusion was restricted to patients with mild coagulation disorders and/or active treatment with acetylsalicylic acid. Severe coagulation disorders were judged as an absolute contraindication to PDT unless haemostasis was carefully corrected18. Patients treated with clopidogrel were also excluded because our national guideline on percutaneous tracheostomy advises that combinations of platelet aggregation inhibitors are best avoided, and many patients use clopidogrel in addition to acetylsalicylic acid. This may have resulted in a far lower rate of bleeding than assumed beforehand, and additional studies are needed to evaluate the safety of PDT in the case of combination treatment. It is worth noting that the PDT was performed by physicians with extensive experience with the technique and only the Blue Rhino technique was used although earlier studies did not show higher bleeding with one specific percutaneous technique6.
It should be noted that this study was not designed according to current transfusion guidelines in which prophylactic transfusion of FFP is discouraged in ICU patients with mild coagulation disorders19–22. However, in a recent survey on peri-operative management of PDT in the Netherlands, we found that it is common practice to correct for mild prolongation of PT before the procedure17. Indeed, prophylactic transfusion of FFP before an invasive procedure in non-bleeding patients may often be given, even though the benefits with regards to preventing bleeding may be low19,20. One could argue that the dose of FFP used in this study was too low, as transfusion of one unit of FFP hardly influenced the PT. International guidelines advise that in the case of major bleeding normal coagulation requires a correction of coagulation factors to 30% of normal. This requires at least four units of FFP when the circulating volume is 6 L. In addition, transfusion guidelines suggest that platelet transfusion may be indicated only when the platelet count is below 50×109/L21. However, prophylactic infusion with these amounts of plasma and platelets was in accordance with our hospital blood bank policy. Also, many ICU patients may suffer from thrombocytopathy (apart from thrombocytopenia), which could decrease the threshold for transfusion.
Transfusion of blood products bears the risk of transfusion-related morbidity, such as infectious diseases and transfusion-associated acute lung injury (TRALI)23,24. In light of these insights one can speculate whether a transfusion policy such as that used in our ICU is even ethical. Unnecessary transfusion of FFP and platelets may occur too often. Education regarding the indications for transfusion and improved identification of active bleeding may reduce transfusion rates and costs20. This study supports the policy of restrictive use of blood products by showing that transfusion of blood products before PDT in the case of mild coagulation disorders is not indicated and is an unnecessary expense.
Our results are in line with those of a large, prospective, observational study that evaluated risk factors associated with bleeding during and after PDT25. That study showed no correlation between acute bleeding and coagulation disorders. In contrast to our results though, a correlation was found between chronic bleeding and coagulation disorders. Notably, the risk of bleeding was mostly present in patients with more severe bleeding disorders than those in the patients included in our study. In a recently published retrospective study a very low bleeding risk (1%) was found in patients with various coagulation disorders and it was concluded that, with strict adherence to a transfusion protocol and an experienced surgical team, PDT can be safely performed in these patients26. However, patients with mild coagulation disorders were also transfused, which was probably unnecessary. We suggest that a standardised transfusion protocol be applied only to patients with more severe coagulation disorders.
Although one patient on active treatment with acetylsalicylic acid needed subcutaneous sutures to stop bleeding (which, it should be noted, was not clinically significant according the study definitions), we did not find a higher risk of bleeding in other patients using acetylsalicylic acid, even in those with an additional prolonged PT. The attitude towards withholding acetylsalicylic acid peri-operatively has been changing over the years. Most guidelines advise the continuation of acetylsalicylic acid for non-cardiac surgery, except in patients with a low risk of thrombosis when bleeding may occur in closed spaces, or when excessive blood loss is expected27,28. The same recommendations are made for patients taking clopidogrel or dual anti-platelet therapy.
The continuously rising costs of blood donation and transfusion in the current economic environment stress the need for studies that challenge our current practices. We should aim to abolish superfluous infusion of blood products especially in critically ill patients.
Conclusion
Bleeding during or after bedside PDT in ICU patients with mild coagulation disorders is rare. Correction of subclinical coagulation disorders by transfusion of FFP and/or platelets does not affect bleeding either during or after PDT.
Footnotes
Authors’ contributions
Denise P. Veloo participated in the design of the study, inclusion of patients and drafting the manuscript. Alexander P. Vlaar and Frederique Paulus participated in the inclusion of patients and drafting the manuscript. Dave A. Dongelmans, Marcel Levi and Fenny Berends participated in the design of the study. Jan M. Binnekade participated in drafting the manuscript. Marcus J. Schultz conceived the study, participated in its design and coordination, and helped to draft the manuscript. All authors have read and approved the final manuscript.
Trial registration: Current Controlled Trials ISRCTN31808827.
The Authors declare no conflicts of interest.
References
- 1.Fischler L, Erhart S, Kleger GR, Frutiger A. Prevalence of tracheostomy in ICU patients. A nation-wide survey in Switzerland. Intensive Care Med. 2000;26:1428–33. doi: 10.1007/s001340000634. [DOI] [PubMed] [Google Scholar]
- 2.Esteban A, Anzueto A, Alía I. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med. 2000;161:1450–8. doi: 10.1164/ajrccm.161.5.9902018. [DOI] [PubMed] [Google Scholar]
- 3.Heffner JE, Miller KS, Sahn SA. Tracheostomy in the intensive care unit. Part 1: Indications, technique, management. Chest. 1986;90:269–74. doi: 10.1378/chest.90.2.269. [DOI] [PubMed] [Google Scholar]
- 4.Heffner JE, Miller KS, Sahn SA. Tracheostomy in the intensive care unit. Part 2: Complications. Chest. 1986;90:430–6. doi: 10.1378/chest.90.3.430. [DOI] [PubMed] [Google Scholar]
- 5.Sollid SJM, Strand K, Søreide E. Percutanous dilatational tracheotomy in the ICU: a Norwegian survey focusing on perceived risk and safety attitudes. Eur J Anaesthesiol. 2008;25:925–32. doi: 10.1017/S0265021508004791. [DOI] [PubMed] [Google Scholar]
- 6.De Leyn P, Bedert L, Delcroix M, et al. Belgian Association of Pneumology and Belgian Association of Cardiothoracic Surgery. Tracheotomy: clinical review and guidelines. Eur J Cardiothorac Surg. 2007;32:412–21. doi: 10.1016/j.ejcts.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Bradley P. Bleeding around a tracheostomy wound: what to consider and what to do? J Laryngol Otol. 2009;34:103–22. doi: 10.1017/S002221510900526X. [DOI] [PubMed] [Google Scholar]
- 8.Fikkers BG, Staatsen M, Lardenoije SG, et al. Comparison of two percutaneous tracheostomy techniques, guide wire dilating forceps and Ciaglia Blue Rhino: a sequential cohort study. Crit Care. 2004;8:R299–R305. doi: 10.1186/cc2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byhahn C, Lischke V, Westphal K. Translaryngeal tracheostomy in highly unstable patients. Anaesthesia. 2000;55:678–82. doi: 10.1046/j.1365-2044.2000.01467.x. [DOI] [PubMed] [Google Scholar]
- 10.Vanderschueren S, De Weerdt A, Malbrain M, et al. Thrombocytopenia and prognosis in intensive care. Crit Care Med. 2000;28:1871–6. doi: 10.1097/00003246-200006000-00031. [DOI] [PubMed] [Google Scholar]
- 11.Kluge S, Baumann HJ, Nierhaus A, et al. Safety of percutaneous dilational tracheostomy in hematopoietic stem cell transplantation recipients requiring long-term mechanical ventilation. J Crit Care. 2008;23:394–8. doi: 10.1016/j.jcrc.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Kluge S, Meyer A, Kühnelt P, et al. Percutaneous tracheostomy is safe in patients with severe thrombocytopenia. Chest. 2004;126:547–51. doi: 10.1378/chest.126.2.547. [DOI] [PubMed] [Google Scholar]
- 13.Blankenship DR, Kulbersh BD, Gourin CG, et al. High-risk tracheostomy: exploring the limits of the percutaneous tracheostomy. Laryngoscope. 2005;115:987–9. doi: 10.1097/01.MLG.0000163107.80668.12. [DOI] [PubMed] [Google Scholar]
- 14.Auzinger G, O’Callaghan GP, Bernal W, et al. Percutaneous tracheostomy in patients with severe liver disease and a high incidence of refractory coagulopathy: a prospective trial. Crit Care. 2007;11:R110. doi: 10.1186/cc6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordin A, Netzer A, Joachims HZ, Golz A. Percutaneous tracheotomy in bone marrow transplant patients with severe thrombocytopenia. Otolaryngol Head Neck Surg. 2005;133:377–80. doi: 10.1016/j.otohns.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Al Dawood A, Haddad S, Arabi Y, et al. The safety of percutaneous tracheostomy in patients with coagulopathy or thrombocytopenia. Middle East J Anesthesiol. 2007;19:37–49. [PubMed] [Google Scholar]
- 17.Veelo DP, Dongelmans DA, Phoa KN, et al. Tracheostomy: current practice on timing, correction of coagulation disorders and peri-operative management - a postal survey in the Netherlands. Acta Anaesthesiol Scand. 2007;51:1231–6. doi: 10.1111/j.1399-6576.2007.01430.x. [DOI] [PubMed] [Google Scholar]
- 18.NVIC guideline:tracheostomy on the intensive care unit for adult patients [Internet] [updated november 2007; cited 27th August, 2009]. Available at: http://www.nvic.nl/richtlijnen_geaccordeerd.php?id=45&titel=Tracheostomy-on-the-intensive-care-unit-for-adult-patients.
- 19.Segal JB, Dzik WH Transfusion Medicine/Hemostasis Clinical Trials Network. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion. 2005;45:1413–25. doi: 10.1111/j.1537-2995.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 20.Vlaar APJ, in der Maur AL, Binnekade JM, et al. A survey of physicians’ reasons to transfuse plasma and platelets in the critically ill: a prospective single-centre cohort study. Transfus Med. 2009;19:207–12. doi: 10.1111/j.1365-3148.2009.00928.x. [DOI] [PubMed] [Google Scholar]
- 21.American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies Practice Guidelines for Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 22.O’Shaughnessy DF, Atterbury C, Bolton Maggs P, et al. British Committee for Standards in Haematology, Blood Transfusion Task Force. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004;126:11–28. doi: 10.1111/j.1365-2141.2004.04972.x. [DOI] [PubMed] [Google Scholar]
- 23.Gajic O, Rana R, Winters JL, et al. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med. 2007;176:886–91. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gajic O, Dzik WH, Toy P. Fresh frozen plasma and platelet transfusion for nonbleeding patients in the intensive care unit: benefit or harm? Crit Care Med. 2006;34:S170–S173. doi: 10.1097/01.CCM.0000214288.88308.26. [DOI] [PubMed] [Google Scholar]
- 25.Beiderlinden M, Eikermann M, Lehmann N, et al. Risk factors associated with bleeding during and after percutaneous dilational tracheostomy. Anaesthesia. 2007;62:342–6. doi: 10.1111/j.1365-2044.2007.04979.x. [DOI] [PubMed] [Google Scholar]
- 26.Pandian V, Vaswani RS, Mirski MA, et al. Safety of percutaneous dilational tracheostomy in coagulopathic patients. Ear Nose Throat J. 2010;89:387–95. [PubMed] [Google Scholar]
- 27.Di Minno MND, Prisco D, Ruocco AL, et al. Perioperative handling of patients on antiplatelet therapy with need for surgery. Intern Emerg Med. 2009;4:279–88. doi: 10.1007/s11739-009-0265-0. [DOI] [PubMed] [Google Scholar]
- 28.Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery: Executive Summary. Circulation. 2007;116:1971–96. [Google Scholar]