Abstract
Culex pipiens quinquefasciatus Say fed blood containing 6.8 ± 0.3 logs (mean ± SE) plaque-forming units of West Nile virus (WNV)/ml were maintained at 28°C for incubation periods (IP) of 7, 14, or 21 d. Several attributes of vector competence were determined at each IP using quantitative real-time reverse transcriptase polymerase chain reaction to estimate plaque forming unit equivalents including: infection rate (WNV-positive abdomens), dissemination rate (WNV-positive legs or thoraces), combined dissemination rate (WNV-positive legs and thoraces), transmission rate (WNV-positive saliva), and WNV titers in abdomens, legs, thoraces, and saliva. Each rate increased or was equivalent with increasing IP. Mosquitoes transmitting WNV in saliva also had significantly higher IP-dependent WNV titers in abdomens, legs, and thoraces. Titers of WNV in abdomens were significantly correlated with titers in legs and thoraces, but the degree of association changed with IP. However, titers of abdomens, legs, and thoraces were not correlated with WNV presence or titer in the saliva. The results show that WNV presence or titer in the saliva of infected Cx. p. quinquefasciatus was not directly influenced by processes involved in WNV replication in other tissues. The processes controlling midgut infection and escape are, in part, independent from the infection processes in other tissues. The relationship between infection, dissemination, and transmission varied over time. The infection and replication of WNV in different tissues is likely influenced by different barriers encountered during the extrinsic incubation period. The significance of these observations for understanding vector competence is discussed.
Keywords: West Nile virus transmission, Culex, vector competence
West Nile virus (WNV; family Flaviviridae: genus Flavivirus) emerged in the northeastern United States in 1999 and has been observed in 49 continental states (Centers for Disease Control [CDC] 2010). Although 62 mosquito species from 11 genera can become infected with WNV upon feeding on a viremic host (CDC 2007, Brault 2009), less than half of these species have been shown to transmit WNV (Turell et al. 2005). Investigations to understand how biological and environmental factors influence vector competence of the primary vectors are essential to determine the relative importance of different vectors in the epidemiology of West Nile (Hayes et al. 2005, Kramer et al. 2007, Blitvitch 2008).
Vector-virus interactions that are important to vector competence mechanisms are complex. The virus must overcome midgut infection (MIB) and midgut escape (MEB) barriers, as well as salivary gland infection (SIB) and escape (SEB) barriers during the extrinsic incubation period (EIP) for subsequent transmission to a host (Hardy et al. 1983).
Culex pipiens quinquefasciatus Say is one of the primary vectors of WNV in the United States (Sardelis et al. 2001). This species likely contributes to the maintenance, amplification, and early transmission of WNV in Florida as it feeds on both birds and mammals (Zinser et al. 2004) and is abundant during winter and spring (Provost 1969, O’Meara and Evans 1983). Though previous studies of WNV infection (virus-positive body), dissemination (virus-positive legs), and transmission (virus-positive saliva) rates in Cx. p. quinquefasciatus have been described at the end of a 12–21 d incubation period (IP) (Vanlandingham et al. 2004, Jansen et al. 2008), studies dealing with the temporal progression of the suite of characteristics contributing to vector competence for WNV are scarce. Studies on the vector competence of Culex tarsalis Coquillett and selected species for several viruses illustrate the complexity of the influences of the different aspects of vector competence on one another and that the dynamics of these relationships are influenced by environmental factors (reviewed in Hardy and Reeves 1990). Anderson et al. (2010a) showed that WNV escaped from the midgut of Cx. p. quinquefasciatus at a greater rate with increasing IP, regardless of extrinsic incubation temperature (EIT) or initial virus dose. The same study evaluated infection rates over time and showed the relationship between infection and escape from the midgut (i.e., dissemination) was not consistent in different environments and at different IPs (range, 4–12 d postinfection). Elsewhere we have reported on the complexity of vector competence for WNV because of effects of biological and environmental factors and interactions between them (Richards et al. 2007, 2009, 2010). The current study expands on this by characterizing the relationships between several different attributes of Cx. p. quinquefasciatus vector competence for WNV over a range of IPs.
For instance, if the midgut escape barrier was not present, we would expect infection in tissues outside of the midgut (e.g., legs, thorax) to be similar or increase over time. If the midgut infection and/or escape barriers did not exist, but salivary gland infection and/or escape barriers were present, then we would expect WNV presence or titer in saliva to be lower than other tissues such as the abdomen, legs, and thorax. We would expect these patterns to vary between individuals, species, or populations because of variation in the efficiency of the barriers (i.e., in dose needed to overcome a barrier or degradation of barriers over time).
The goal of this research was to assess the relationships between infection and WNV titer in different tissues over time to determine whether knowledge of WNV infection and virus titer in one tissue provides information about relationships to other tissues. Of particular interest was whether virus presence or titer in saliva could be predicted by infection levels in other tissues, because virus in saliva is directly related to transmission. Knowledge about the relationships between different attributes of vector competence at different IPs is essential for understanding the progression of arbovirus infectiousness in the vector and the basis for inter- and intraspecific refractoriness or susceptibility of vectors. This information is necessary to identify the importance of specific vectors in different environments and epidemiological cycles.
Materials and Methods
Mosquitoes
Cx. p. quinquefasciatus from a colony established from Alachua County, FL (generation F >56) were reared at 28°Cand maintained under a 14:10 (light: dark) cycle as previously described (Richards et al. 2009). Twenty four hours before experiments, 100 adult females were transferred to each of five 1-liter cardboard cages with mesh screening on top, and water provided ad libitum.
Blood Meal Preparation
AT-75 cm2 flask of African green monkey (Vero) cells was inoculated with 0.25 ml of WNV strain WN-FL03p2–3 (GenBank accession number DQ983578) (passaged four times in Vero cells and one time in baby hamster kidney cells). Then, 12 ml of Medium 199 (with EarleÕs salts, 10% fetal bovine serum [FBS], penicillin/streptomycin, mycostatin) was added to the flask and incubated at 35°C in an atmosphere of 5% CO2. At 48 h postinoculation, one part of the supernatant was harvested and mixed with nine parts of citrated bovine blood. This experiment was conducted once and two 0.1 ml samples of the blood meal were each added to separate tubes containing 1.0 ml BA-1 diluent and stored at −80°C until tested to determine blood meal titer.
Mosquito Infection
Pledgets were soaked in WNV-infected blood and warmed (35°C) for 10 min. Mosquitoes were allowed to feed on pledgets for 30 min. Subsequent to feeding, fully engorged mosquitoes were determined visually (ca. 350 mosquitoes) and transferred to four 1-liter cardboard cages (ca. 80–100 mosquitoes/cage) with mesh screening on top and maintained in incubators at 28°C for the duration of the experiment with 20% sugar solution ad libitum. Unfed or partially engorged mosquitoes were discarded. All mosquitoes were fed on the same day from pledgets soaked in the same WNV-blood solution to ensure a consistent dose was fed.
Blood Meal and Mosquito Processing
Incubation periods of 7, 14, and 21 d were used to represent time points that were early, intermediate, and late stages of the EIP (as defined by tissue-specific infections) for this mosquito species, virus dose, and EIT (28°C) (Anderson et al. 2010a). All mosquito dissections used aseptic techniques described previously to avoid contamination between individual tissues and mosquitoes (Richards et al. 2009). At each IP, ≈100 mosquitoes were collected randomly across cages and their legs and wings were detached using forceps and legs transferred to separate sample tubes containing 1.0 ml BA-1. Saliva was then collected from these mosquitoes in capillary tubes with immersion oil using methods described by Anderson et al. (2010b). Subsequently, for each mosquito, thoraces were precisely separated from abdomens using a razorblade and transferred to separate tubes containing 1.0 ml BA-1. Forceps and razorblades were sterilized between each mosquito (Richards et al. 2009).
WNV RNA titers in abdomens were used to measure the vector competence attribute of midgut infection. Titers in the legs and thoraces were used to represent virus dissemination out of the midgut. Virus RNA in saliva was used as a measure of the attribute of transmission.
Viral Nucleic Acid Detection
Total nucleic acids were extracted from tissues and saliva (Richards et al. 2009) and the presence of WNV RNA was determined and quantified via quantitative real-time reverse transcriptase polymerase chain reaction (qPCR) with specifications described previously to estimate plaque forming unit equivalents (PFUeq) (Lanciotti et al. 2000, Richards et al. 2007). The resulting titers are expressed in PFUeq/ml of mosquito homogenate and therefore do not take into account significant differences in tissue volume such as between abdomens and legs. We conducted additional analyses to test for live virus in thoraces and legs of 12 mosquito samples to verify lack of infection when sample crossing points were >35 cycles. For these 12 instances, infectivity was also tested by inoculating 0.1 ml of samples into Vero cell culture.
Statistical Analysis
Vector competence was measured as follows: rates of infection (number with WNV-positive abdomens/number tested), dissemination (number with WNV-positive legs or thoraces/number with WNV-positive abdomens), combined dissemination (number with both WNV-positive legs and thoraces/number with WNV-positive abdomens), and transmission (number with WNV-positive saliva/number with WNV-positive abdomens), as well as WNV titer (PFUeq) in abdomens, legs, thoraces, and saliva. Mosquitoes may exhibit dissemination to both leg and thorax tissues or to only one of these tissues. Hence, we refer to dissemination as infection of either the legs or thorax because infection of either tissue represents the virus disseminating out of the midgut. Rates of dissemination and transmission were determined using only mosquitoes that contained WNV in the tissue that is essential to WNV exposure to another tissue. For example, the presence of WNV in the legs, thorax, or saliva can only occur if virus is present in the abdomen, that is, virus infection of the midgut. Legs may or may not be infected for WNV to be present in saliva, because leg infection is not essential for virus transmission. The thorax must be infected for WNV to be present in saliva, because the salivary glands are located here. Hence, we use rates that describe the biological characteristic of the particular tissue. The rate described for any tissue is based on the number of mosquitoes tested with virus in another tissue that is essential for infection of the subsequent tissue. Mosquitoes that did not contain virus in a tissue required for subsequent infection of another tissue are not included in the total number tested to assess the rate.
SAS statistical software was used for all analyses (SAS Institute, Cary, NC).χ2 tests were used to test for significant differences (P < 0.05) in infection, dissemination, combined dissemination, and transmission rates at each IP and between IPs. Titers of abdomens, thoraces, legs, and saliva were log-transformed [log (x + 1)] before analysis. Analysis of variance (ANOVA) (PROC GLM) was used to determine significant differences (P < 0.05) in titers of virus-positive abdomens, thoraces, legs, or saliva between IPs. When significant differences were observed, Duncan means comparison tests were used to determine which means were significantly different (P < 0.05).
Relationships between titers in different tissues were visualized using scatter plots, followed by correlation and regression analyses. Correlation analyses were conducted for all biologically relevant relationships using a Bonferroni correction (Curtin and Schulz 1998) to correct for multiple comparisons. Regression analyses were performed only for factors where significant correlations were observed and where information about a predictive relationship may be useful in future experiments or field studies. Only mosquitoes with infected (titer >0) abdomens, thoraces, and legs were included in the correlation and regression analyses, as our goal was to identify predictive relationships between infected tissues. Significance and parameter estimates in correlation or regression analyses will be influenced by the clustering of the data, and may reflect differences between the groups rather than relationships within each group. To determine if there were relationships between variables within the clusters as well as between them, we divided the data into high and low titer groups. We used a threshold of three log10 PFUeq WNV/ml in abdomens or thoraces identified by visual inspection to determine natural clustering of the data. Mosquitoes below this threshold were considered “low titer” and those above “high titer.” The low titer group was too small to permit separate analysis, so only the high titer group was analyzed separately. The correlation and regression analyses were repeated for the high titer group. If a mosquito fell below the threshold in either the thorax or abdomen titer, it was classed as low titer for all analyses. Leg titers exhibited a continuous distribution so the high and low titer grouping was not used for leg titer. Correlation and regression analyses are presented both with and without the low titer group for abdomens and thoraces.
Individual mosquitoes were categorized as having saliva infection or no saliva infection. At each IP, Pearson correlation coefficients (PROC CORR) were used to determine significant correlations (P value with Bonferroni correction <0.002) between vector competence variables, that is, titers in abdomen, legs, thorax, and saliva, as well as WNV presence in saliva.
Significant correlations between attributes of vector competence were followed by regression analyses to determine if predictive relationships could be identified. Simple linear regression analyses (PROC REG) were used to determine if the titer of one tissue could predict the titer of subsequently infected tissues.
We conducted t-tests to determine if there were differences in virus titers in the abdomens, legs, and thoraces between mosquitoes transmitting (virus in saliva) or not transmitting the virus (no virus in saliva).
Results
Virus Titer of Blood Meal
Mosquitoes were fed a blood meal containing 6.8 ± 0.3 log10 (mean ± SE) PFUeq WNV/ml. The results for WNV infection and titer at different IPs for each tissue type and saliva are presented in Table 1.
Table 1.
The mean titers (log10 PFUeq WNV/ml) ± SE and rates of infection (% with WNV-positive abdomens), dissemination (% infected with WNV-positive legs or thoraces), and transmission (% infected with WNV-positive saliva) for Cx. p. quinquefasciatus fed a WNV-infected blood meal and held at 28°C for IPs of 7, 14, or 21 d
| Incubation period |
No. tested |
No. abdomen infection (%)a |
No. thorax infection (%)a |
No. leg infection (%)a |
No. combined leg and thorax infection (%)a |
No. saliva infection (%)a |
Abdomen titerb |
Thorax titerb |
Leg titerb |
Saliva titerb |
|---|---|---|---|---|---|---|---|---|---|---|
| 7 | 100 | 100 (100)a | 86 (86)b | 89 (89)b | 78 (78)b | 2 (2)b | 6.3 ± 0.1b | 4.9 ± 0.1b | 3.0 ± 0.1c | 1.7 ± 0.6a |
| 14 | 100 | 100 (100)a | 99 (99)a | 100 (100)a | 99 (99)a | 29 (29)a | 6.8 ± 0.1a | 7.0 ± 0.1a | 4.8 ± 0.1b | 2.8 ± 0.2a |
| 21 | 99 | 99 (100)a | 99 (100)a | 97 (98)a | 97 (98)a | 23 (23)a | 6.7 ± 0.04a | 6.9 ± 0.1a | 5.1 ± 0.05a | 2.3 ± 0.2a |
Treatment groups with the same letter in each column are not significantly different between incubation periods by χ2.
Treatment groups with the same letter in the same column are not significantly different by means comparisons.
Our qPCR procedure detected 12 individuals that had no WNV RNA in the thorax, but WNV RNA-positive legs (using a threshold of detection ≤35 cycles). These 12 thoraces were retested via qPCR and cell culture and found to be negative for WNV RNA and WNV. In view of the questionable observation of these mosquitoes, we omitted these individuals from the statistical analyses.
WNV Infection, Dissemination, and Transmission Rates Between IPs
All (100%) of the mosquitoes had infected abdomens at all IPs. However, we observed that virus dissemination to the thorax and legs, as well as transmission in the saliva was significantly different between IPs, with significantly lower rates at 7 d compared with 14 and 21 d (dissemination to thorax: χ2 = 25.55, df = 2, P < 0.0001; dissemination to legs: χ2 = 16.48, df = 2, P = 0.0003; combined dissemination:χ2 = 17.72, df = 2,P < 0.0001; transmission in saliva:χ2 = 24.82, df = 2, P < 0.0001). We observed 12 individuals with uninfected thoraces (titer = 0) but infected legs (N = 11 at 7 d; N = 1 at 14 d).
WNV Infection Rates Between Different Tissues and Saliva
There were significant differences in WNV infection rates between different tissues (abdomen, thorax, legs,combined thorax and leg, and saliva) at 7d (χ2 = 313.14, df = 4, P < 0.0001), 14 d (χ2 = 318.98, df = 4, P < 0.0001), and 21 d (χ2 = 335.77, df = 4, P < 0.0001). At each IP, the rate of mosquitoes showing a saliva infection was consistently lower than rates for abdomen, thorax, leg, or combined thorax and leg infection.
Effects of IP on WNV Titer in Different Tissues and Saliva
ANOVA showed that WNV titers varied significantly between IPs for abdomens (F = 21.77; df = 2, 296; P < 0.0001), thoraces (F = 109.94; df = 2, 283; P < 0.0001), and legs (F = 128.55; df = 2, 283; P < 0.0001), but not saliva (F = 1.95; df = 2, 52; P = 0.153). Titers in abdomens and thoraces at IP ≥ 14 d were significantly higher than respective WNV titers in abdomens and thoraces at 7 d. Leg titers showed a different pattern, with titers increasing significantly at each subsequent IP from 7–21 d.
Scatter Plots
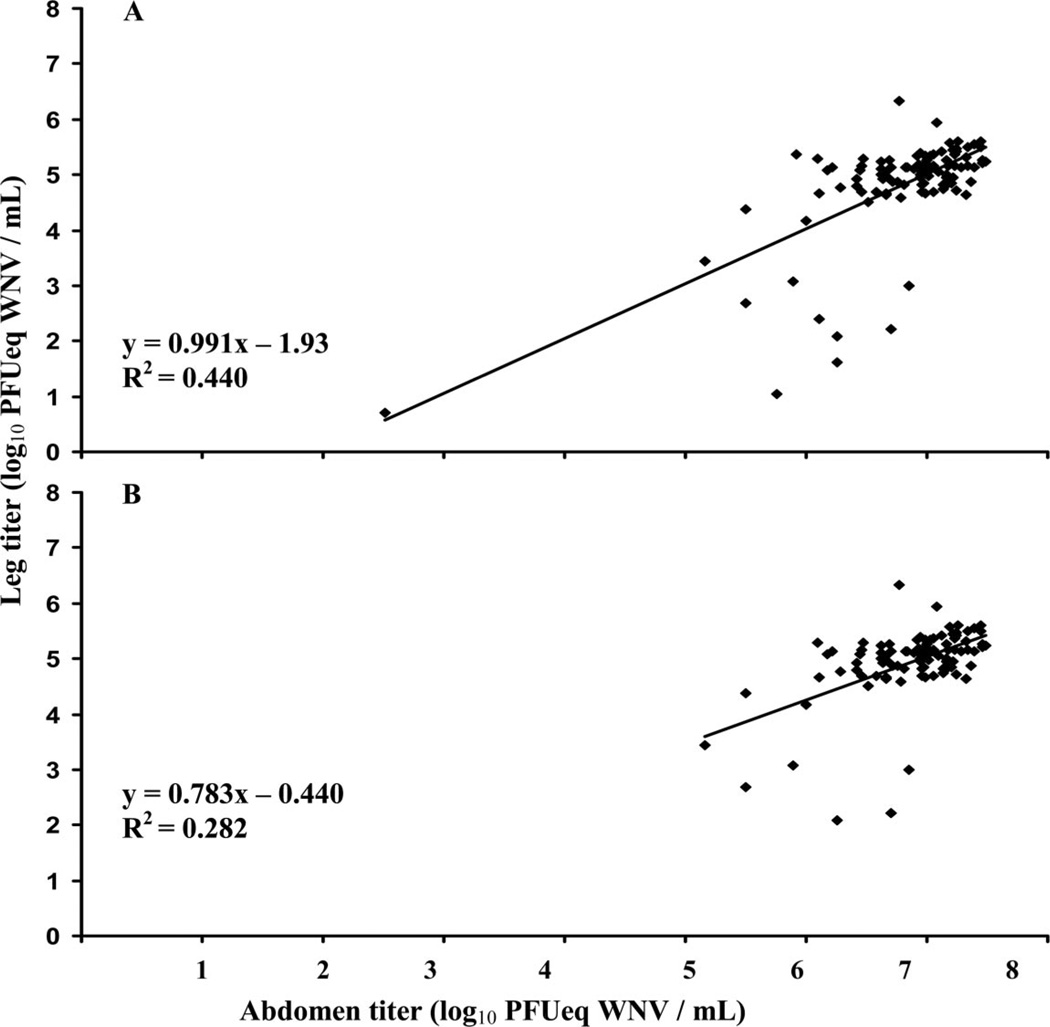
Scatter plots (e.g., Figure 1A) showed a small percentage of individuals with abdomens (N = 2, 0.6% of total N) or thoraces (N = 26, 9% of total N) with titers <3 log10 PFUeq WNV/ml leading to bimodal distributions. These individuals (N = 28) were classified in the low titer group and the correlation analyses were done both with and without this group (e.g., Figure 1B). The regression equations and trend lines for Fig. 1A and B show that the strength of association between abdomen titer and leg titer decreased when mosquitoes in the low titer group for abdomens and thoraces were excluded from the analysis. This indicates that the clustering in the data had a substantial effect on the association between abdomen and leg titers over the entire range of titers (see below for analysis within high titer group).
Fig. 1.
Example of scatter plots used for data exploration. This figure shows the relationship between abdomen titer and leg titer of Cx. p. quinquefasciatus at 14 d postinfection with WNV; (A) All individuals included in analysis, (B) Low titer mosquitoes removed (individuals with abdomen or thorax titer <3 log10 PFUeq WNV/ml removed from analysis). Regression equation and trend line included for each plot.
Of the individuals in the low titer group, 22 had low titers (<3 log10PFUeq WNV/ml)in thoraces, but high titers (>3 log10 PFUeq WNV/ml) in the abdomen. In addition, 12 mosquitoes with no WNV RNA in the thorax, but WNV-positive legs generally had high abdomen titers (average 6.4 log10 PFUeq WNV/ml).
Correlations of WNV Titer Between Tissues and With WNV Presence in Saliva
Because a large number (N = 27) of correlation tests were made, a Bonferroni correction was applied for all correlation analyses so that correlations were significant when P < 0.002. When all virus-positive individuals were included, significant correlations between tissue titers were most prevalent at 14 and 21 d (Table 2). Saliva titer and the presence of WNV in saliva were not significantly correlated with the titer of any of the other tissues (Table 2).
Table 2.
Pearson correlation coefficients (r) for vector competence parameters at different IPs
| Abdomen titer | Leg titer | Thorax titer | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 7 d | 14 d | 21 d | 7 d | 14 d | 21 d | 7 d | 14 d | 21 d | |
| Leg titer | r = 0.133 | r = 0.664 | r = 0.425 | — | — | — | — | — | — |
| P = 0.186 | P < 0.0001 | P < 0.0001 | |||||||
| Thorax titer | r = 0.116 | r = 0.473 | r = 0.720 | r = 0.389 | r = 0.572 | r = 0.509 | — | — | — |
| P = 0.957 | P < 0.0001 | P < 0.0001 | P < 0.0001 | P < 0.0001 | P < 0.0001 | ||||
| Saliva titer | r = 0.090 | r = 0.224 | r = 0.026 | r = 0.201 | r = 0.139 | r = 0.055 | r = 0.157 | r = 0.208 | r = 0.071 |
| P = 0.376 | P = 0.025 | P = 0.796 | P = 0.044 | P = 0.169 | P = 0.590 | P = 0.119 | P = 0.038 | P = 0.488 | |
| WNV present in saliva | r = 0.099 | r = 0.238 | r = 0.031 | r = 0.220 | r = 0.152 | r = 0.059 | r = 0.170 | r = 0.224 | r = 0.066 |
| P = 0.329 | P = 0.017 | P = 0.762 | P = 0.028 | P = 0.130 | P = 0.562 | P = 0.092 | P = 0.025 | P = 0.517 | |
All individuals are included in analyses. Significant P values (P < 0.002 calculated using Bonferroni correction) in bold.
Removing the low titer mosquitoes from the analysis changed the correlation pattern, most notably at 7 and 21 d (Table 3). The titers in abdomens, thoraces, and legs were significantly correlated at 14 d, but correlations varied at 7 and 21 d. Saliva titer and the presence of WNV in saliva were still not significantly correlated with the titer in the other tissues.
Table 3.
Pearson correlation coefficients (r) for vector competence parameters at different IPs
| Abdomen titer | Leg titer | Thorax titer | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 7 d | 14 d | 21 d | 7 d | 14 d | 21 d | 7 d | 14 d | 21 d | |
| Leg titer | r = 0.273 | r = 0.531 | r = 0.303 | — | — | — | — | — | — |
| P = 0.015 | P < 0.0001 | P = 0.003 | |||||||
| Thorax titer | r = 0.287 | r = 0.726 | r = 0.631 | r = 0.679 | r = 0.626 | r = 0.275 | — | — | — |
| P = 0.010 | P < 0.0001 | P < 0.0001 | P < 0.0001 | P < 0.0001 | P = 0.007 | ||||
| Saliva titer | r = 0.132 | r = 0.227 | r = −0.028 | r = 0.211 | r = 0.087 | r = 0.014 | r = 0.263 | r = 0.241 | r = −0.028 |
| P = 0.243 | P = 0.027 | P = 0.783 | P = 0.060 | P = 0.399 | P = 0.895 | P = 0.018 | P = 0.018 | P = 0.785 | |
| WNV present in saliva | r = 0.146 | r = 0.235 | r = −0.029 | r = 0.231 | r = 0.092 | r = 0.013 | r = 0.290 | r = 0.247 | r = −0.064 |
| P = 0.196 | P = 0.022 | P = 0.776 | P = 0.039 | P = 0.377 | P = 0.901 | P = 0.009 | P = 0.016 | P = 0.537 | |
Outliers have been removed (individuals with abdomen or thorax titers < 3 log10 PFUeq WNV/ml are not included in analyses). Significant P values (P < 0.002 calculated using Bonferroni correction) in bold.
Simple Linear Regression Analysis to Determine the Association of Abdomen Titer With Titers of Legs and Thoraces
Saliva titer and the presence of WNV RNA in saliva were not included in regression analyses because neither was correlated with the titer in the other tissues. When all mosquitoes with infected abdomens, legs, and thoraces were included in regression analyses, abdomen titer predicted the titers of legs and thoraces at 14 and 21 d (Table 4). However, when low titer mosquitoes were excluded from analyses, abdomen titer predicted the titers of thoraces at both 14 and 21 d, but leg titers at only 14 d (Table 5). Hence, the regressions with infected mosquitoes were influenced by the two clusters and may be showing relationships between these clusters rather than a continuous relationship between the variables. The analyses excluding low titer mosquitoes show the relationship between the variables in the region of more continuous variation.
Table 4.
Simple linear regression analyses for determining if WNV RNA titers of thoraces or legs (dependent variables) can be predicted by abdomen titer (independent variable) at each IP
| Incubation period |
Dependent variable |
Independent variable |
B | SE β | t | df | P | Model R2 |
|---|---|---|---|---|---|---|---|---|
| 14 d | ||||||||
| Thorax titer | Abdomen titer | 0.93 | 0.18 | 5.31 | 1 | <0.0001 | 0.22 | |
| Constant | 0.56 | 1.20 | 0.47 | 1 | 0.639 | |||
| Leg titer | Abdomen titer | 0.99 | 0.11 | 8.78 | 1 | <0.0001 | 0.44 | |
| Constant | −1.93 | 0.77 | −2.51 | 1 | 0.014 | |||
| 21 d | ||||||||
| Thorax titer | Abdomen titer | 1.81 | 0.18 | 10.22 | 1 | <0.0001 | 0.518 | |
| Constant | −5.30 | 1.20 | −4.43 | 1 | <0.0001 | |||
| Leg titer | Abdomen titer | 0.90 | 0.19 | 4.63 | 1 | <0.0001 | 0.181 | |
| Constant | −1.05 | 1.31 | −0.80 | 1 | 0.425 |
Regressions were only carried out for relationships where correlations were significant. All individuals with infected abdomens, legs, and thoraces are included in analyses. Significant P values (P < 0.05) in bold.
Table 5.
Simple linear regression analyses for determining if WNV RNA titers of thoraces or legs (dependent variables) can be predicted by abdomen titer (independent variable) at each IP
| Incubation period |
Dependent variable |
Independent variable |
B | SE β | t | df | P | Model R2 |
|---|---|---|---|---|---|---|---|---|
| 14 d | ||||||||
| Thorax titer | Abdomen titer | 0.894 | 0.09 | 10.19 | 1 | <0.0001 | 0.53 | |
| Constant | 1.00 | 0.60 | 1.66 | 1 | 0.101 | |||
| Leg titer | Abdomen titer | 0.78 | 0.13 | 6.05 | 1 | <0.0001 | 0.28 | |
| Constant | −0.44 | 0.89 | −0.49 | 1 | 0.622 | |||
| 21 d | ||||||||
| Thorax titer | Abdomen titer | 0.73 | 0.09 | 7.89 | 1 | <0.0001 | 0.40 | |
| Constant | 2.15 | 0.62 | 3.45 | 1 | 0.001 |
Regressions were only carried out for relationships where correlations were significant. Low titer mosquitoes (individuals with abdomen or thorax titers < 3 log10 PFUeq WNV/ml) are not included in analyses. Significant P values (P < 0.05) in bold.
Comparison of WNV Titer in Tissues for Mosquitoes With Virus-Positive or -Negative Saliva
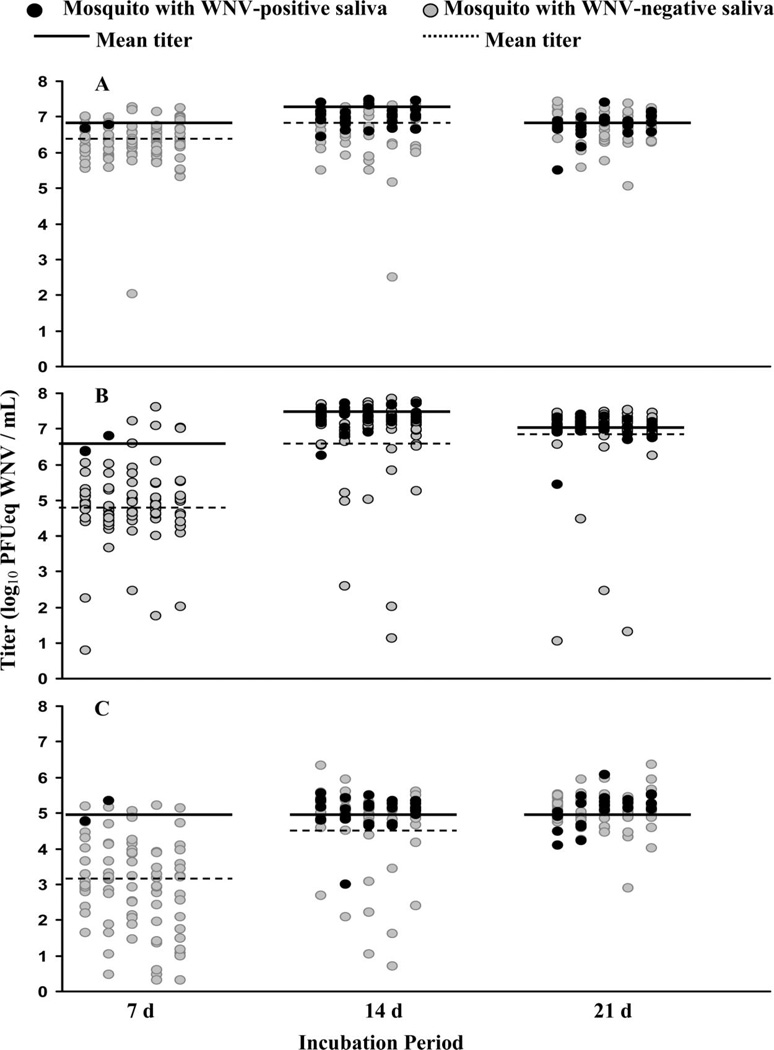
The relationships between WNV titer in mosquito abdomens, legs, and thoraces with WNV in saliva (transmitters) compared with mosquitoes that did not have WNV in their saliva (nontransmitters) changed depending on the IP (Fig. 2). Because there were only two mosquitoes at 7 d with concurrent abdomen, thorax, leg, and saliva infections, this IP was not analyzed further. Abdomen WNV titers (7.0 ± 0.1 log10 PFUeq/ml) of mosquitoes that transmitted WNV were significantly higher than abdomen titers of nontransmitters (6.7 ± 0.1 log10 PFUeq/ml; t = 2.86; P = 0.003) at 14 d, but not at 21 d (6.7 ± 0.1 log10 PFUeq/ml and 6.7 ± 0.1 log10 PFUeq/ml, respectively; t = 0.367; P = 0.358).
Fig. 2.
Titers of abdomen (A), thorax (B), and legs (C) in mosquitoes with WNV-positive versus WNV-negative saliva at 7, 14, and 21 d postinfection with WNV. For each IP, individuals are separated randomly into five groups for better visualization. No analyses were conducted at the 7 d IP; however, these individuals are included in the figure for reference. Note that at in some cases (21 d, abdomens and legs) the means were similar and thus only one line is visible.
Leg titers showed a similar pattern to abdomen titers at 14 d with significantly higher titers (5.0 ± 0.1 log10 PFUeq/ml) in mosquitoes that transmitted WNV, compared with leg titers of nontransmitters (4.7 ± 0.1 log10 PFUeq/ml; t = 1.98; P = 0.025). At 21 d, leg titers of transmitters (5.1 ± 0.1 log10 PFUeq/ml) were not different from nontransmitters (5.0 ± 0.1 log10 PFUeq/ml; t = 0.86; P = 0.196).
At 14 d, thorax titers were significantly higher (t = 3.43; P = 0.0005) in mosquitoes that transmitted WNV (7.3 ± 0.1 log10 PFUeq/ml), compared with nontransmitters (6.7 ± 0.2 log10 PFUeq/ml). However, no differences were observed between thorax titers in WNV transmitters (7.0 ± 0.1 log10 PFUeq/ml) and nontransmitters (6.9 ± 0.1 log10 PFUeq/ml) at 21 d (t = 0.945; P = 0.174).
Discussion
Our results demonstrated that relationships between different attributes of Cx. p. quinquefasciatus competence for WNV change depending on the IP. Other studies on Cx. p. quinquefasciatus (Richards et al. 2007, 2009, 2010) have shown that the environment has significant effects on vector competence. Here, we show that there are periods during the EIP when WNV infection and/or titer in one tissue, for example, abdomens and/or thoraces, is correlated with infection and/or virus titer in another tissue. However, the titer and the presence of WNV RNA in the saliva showed no such relationship with these other tissues.
Viral RNA quantified via qPCR measures both infectious and noninfectious virus particles. Previous studies have shown that PCR can detect viral RNA in vertebrate blood that is not infectious to a vector arthropod upon blood feeding (Tabachnick et al. 1996). Portions of the viral RNA detected in some of our mosquitoes may have been noninfectious particles. If some mosquitoes reported as disseminated infections by qPCR were not infectious virus this would explain why some mosquitoes in our studies with disseminated infection did not transmit. We do not believe this is likely because: (1) if noninfectious viral RNA was significant then both infectious replicating particles and noninfectious viral RNA accumulates in infected mosquitoes resulting in increasing accumulation over time because of remnant RNA and viral replication. There is no evidence for this because WNV RNA in thoraces plateaued at 14 d. However, it is difficult to prove or disprove this because of the dynamic relationship between RNA production and degradation over time that was not tested here. (2) WNVRNA in legs increased from 7 to 21 d, consistent with the possibility of the accumulation of both live virus and remnant virus RNA. Further, mean leg titers at 14 d were higher in transmitters compared with nontransmitters consistent with the possibility that the nontransmitting mosquitoes did not have live virus in their legs and may not be disseminated infections of live virus. However, at both 14 and 21 d, there were individual nontransmitting mosquitoes where the leg titers were as high as or higher than transmitters supporting the presence of live WNV.
Others have shown that mosquitoes with disseminated infections do not always transmit the arbovirus (e.g., Ortiz et al. 2008, Kramer et al. 2011). In our study, we hypothesize several explanations for the failure of mosquitoes with disseminated infections to transmit WNV. These include: (1) noninfectious particles interfered with the ability of the infectious particles to enter and escape the salivary glands, (2) there were differences between mosquitoes in the salivary gland infection and/or escape barriers that prevented mosquitoes with thorax infections to transmit, and/or (3) bits of thorax tissue contaminated the leg samples so these were not true leg infections. Our studies cannot discriminate between the first two explanations. The third explanation is less likely because care was taken to ensure this did not occur, the coxa (basal segment of the leg) is not removed during leg dissections, and similar studies by others show little indication that this is a major concern (e.g., Tiawsirisup et al. 2005, Styer et al. 2007). However, it remains possible and would have to be considered for all similar experiments characterizing virus in diverse tissues. It is not possible to compare the titers between the different tissues because there is variation in the amounts of tissue that influences the amount of virus in the sample. Hence, we report results in PFUeq ml per tissue homogenate. Our analysis focused on examining potential relationships between titers in different tissues, which would include such calibration factors in the regression parameters.
Mosquitoes with low or zero WNV titer in their thoraces with high abdomen titers is consistent with the possibility of a barrier to infection and/or virus replication to the thorax tissues in these mosquitoes. A threshold of midgut WNV infection may be necessary in Culex for subsequent virus dissemination (Girard et al. 2004) or transmission (Reisen et al. 2006) to occur. The lowest body, leg, and thorax titers occurred most frequently at the shortest IP, 7 d, which had 71% of the total of low titer mosquitoes and hence the lowest dissemination, combined dissemination, and transmission rates compared with the other IPs. This is consistent with temporal progression of virus in the vector reported in other vector-virus systems (Mellor 2000, Kuno and Chang 2005).
The observation of 12 mosquitoes with no WNV-RNA in the thorax and WNV in the legs is puzzling and requires further investigations to determine if: (1) The 12 leg samples are false positives, (2) The observed data are correct, or (3) The 12 thoraces are false negatives. Alternatively, for some unknown reason, the virus may have degraded to a greater extent in the thorax compared with legs, thereby rendering it undetectable in the thoraces.
Abdomen titer consistently predicted thorax titer at the 14 and 21 d IPs, suggesting that virus replication in abdomens and thoraces progressed similarly once each tissue was infected. Once WNV overcame the MIB and dissemination occurred, there was a predictive relationship between replication in the abdomen and replication in the thorax. Some of the thorax infections could be because of the anterior portion of the midgut that is contained in the thorax region. However, this is unlikely because the relationship between abdomen and thorax infection was only apparent at the later 14 and 21 d IPs that is understandable because thoraces are infected later than abdomens so that replication in thoraces is delayed.
A few studies have investigated a predictive relationship between the vector competence attributes of infection, dissemination, and transmission. Studies with Cx. p. pipiens Linnaeus (Turell et al. 2000, 2001, Anderson et al. 2008) showed that a large proportion of mosquitoes with disseminated infection to the legs transmitted WNV to animals after a 12 d IP. These same studies demonstrated that there was little evidence for salivary gland infection and escape barriers under these conditions. Conversely, only 25% of Cx. p. pipiens fed virus similar to our virus dose (6.5 logs CID50 (50% chicken infectious doses)/ml) and with disseminated infections (i.e., one mosquito with virus-infected saliva/four mosquitoes with virus infected legs) transmitted WNV to capillary tubes after a 14 d IP (Tiawsirisup et al. 2005, recalculated from their Table 2). This is similar to our observations with Cx. p. quinquefasciatus saliva. Reisen et al. (2006) showed that female Cx. tarsalis with body WNV titers <3 log10 PFU WNV/ml did not transmit WNV to capillary tubes. These studies collectively show evidence of a salivary gland infection and/or escape barrier. It is possible that the differences between the latter studies and those reporting no salivary gland infection and escape barriers using animals are because of using capillary tubes compared with an animal to assess transmission. Cx. tarsalis and Cx. p. pipiens transmitted more WNV to animals compared with the capillary tube method (Styer et al. 2007). Conversely, Aedes albopictus Skuse and Ochlerotatus (Aedes) taeniorhynchus Wiedemann transmitted more Venezuelan equine encephalitis virus using the capillary method compared with animals (Smith et al. 2006). However, there were also methodological differences between these studies with 1:1 FBS:sucrose in capillary tube and WNV detected by plaque assay (Styer et al. 2007) compared with immersion oil in the capillary tubes and Venezuelan equine encephalitis virus detected by qPCR (Smith et al. 2006). The low transmission to capillary tubes by Cx. p. pipiens may be because of the use of FBS:sucrose in the tubes because Culex spp. have a hydrophobic proboscis and are more likely to salivate into immersion oil, while saliva can be collected from Aedes spp. and Ochlerotatus spp. using either oil or water-based media (Colton et al. 2005, Colton and Nasci 2006). Hence, different media in capillary tubes could affect the amount of saliva excreted. Furthermore, plaque assay detects only live virions while qPCR detects live and dead virions and degraded viral RNA. A greater number of virus particles may die during the longer salivation period (ca. 30–45 min) required in the capillary tube method compared with the animal method (ca. 3–11 min) and these dead virions in the capillary tube would go undetected using plaque assay. Hence, our system using qPCR and capillary tube collection would overestimate WNV detection. The absence of WNV in saliva transmitted to capillary tubes is not likely because of our method. It remains possible that host factors elicit more virions in saliva than capillary feeding, or more likely, that the differences observed using different species and viruses are the result of variation between mosquitoes and viruses. This should be studied further.
The Cx. p. quinquefasciatus we studied did not have either a MIB or MEB because ≥98% of the mosquitoes had WNV RNA in their abdomens, thoraces, and legs at ≥ 14 d. However, the increasing prevalence of WNV RNA in leg and thorax tissues over time showed that virus replication in these tissues was still increasing at 7 d. While transmission rates significantly increased between the 7 d IP (2%) and later IPs (≥23%), saliva titers did not change with IP, indicating that WNV titer in the saliva of this colony of Cx. p. quinquefasciatus under the test conditions may plateau quickly after infection of the salivary gland. Girard et al. (2007) also observed that WNV titers in Cx. p. quinquefasciatus saliva did not change between 7–28 d at 28°C. However, significant differences were observed in WNV titers in Cx. p. quinquefasciatus saliva between IPs of 14 and 21 d at 26°C (Vanlandingham et al. 2004) and in Cx. tarsalis between IPs ranging from 2 to 35 d and EITs ranging from 14–30°C (Reisen et al. 2006). These observations collectively provide evidence for a SIB and/or SEB that varies between populations, species, and environmental conditions. These barriers affect both virus presence and viral titers at different IPs.
Mosquitoes with WNV RNA in saliva had higher average PFUeq titers in abdomens, legs, and thoraces than mosquitoes without WNV RNA in saliva, but only at 14 d. Similar results were reported in WNV-infected Cx. tarsalis where higher body titers were observed in transmitters compared with nontransmitters and this relationship changed with time (Reisen et al. 2006). Although this observation may be the result of specific conditions during the current study, further work will be needed to determine if this IP may be useful in predicting transmission rates from titers of other mosquito tissues. Higher average titers in the tissues of transmitters were not seen at 21 d and titers in abdomens and thoraces began to decrease from 14 to 21 d, consistent with virus-induced apoptosis occurring with time reported elsewhere (Girard et al. 2007). Some individual mosquitoes that did not transmit had WNV RNA titers as high as transmitters. Further work should be done to evaluate these differences and explore the barriers to transmission by the salivary glands.
The primary goal of this study was to assess the relationships between different attributes of Cx. p. quinquefasciatus vector competence for WNV at different periods of the infection process. Information about these relationships is important to improve laboratory characterizations of vector competence and may also be used to better understand mosquito vector competence in nature. For example, does the WNV titer of one mosquito tissue provide information about the titer or presence of WNV in another tissue or saliva and is this influenced by biological and environmental factors? Certainly, the presence of WNV in the abdomen and thorax is a prerequisite for infection of the saliva. This does not necessarily mean a quantitative relationship between virus titers in different tissues, at one time point, are likely or can be identified. However, the Cx. p. quinquefasciatus in this study also had SIBs or SEBs so that WNV presence in saliva was not influenced by the titer of another tissue. Even some mosquitoes with high titer in the thorax did not have WNV in the saliva. Further work should evaluate the relationship between infection of thorax tissues (MEB), the salivary glands (SIB), and saliva (SEB).To better understand these relationships, future studies should consider the entire EIP and investigate if there is a time-lagged predictive relationship between infections of different tissues. We expect these relationships to differ between different populations and under different experimental conditions. However, if some trends in relationships between the infections of different tissues are evident under a range of conditions, then predictive relationships will follow.
Knowledge of the relationships between attributes of vector competence is essential for understanding the process of vector competence and how vector-virus interactions contribute to epidemiology. We have shown that the relationships between infection, dissemination, and transmission vary through the infection cycle and this will affect our understanding of vector competence and perception of natural infections. It is generally accepted that not all virus-infected mosquitoes can transmit (e.g., Rutledge et al. 2003, Girard et al. 2004, Vanlandingham et al. 2004) and studies that focus only on vector infection rates may not provide useful information for understanding epidemiology (Bustamante and Lord 2010). Here, we show that assessing transmission in the laboratory is a complex undertaking. The WNV transmission potential of Cx. p. quinquefasciatus, though dependent or related to the virus titer in the thorax in particular, may not be dependent or consistently predicted by the virus titer in other tissues. The processes of salivary gland infection and escape of WNV into saliva are not directly influenced by the amount of virus in tissues surrounding the gland. We have no doubt that there will be variation in these features between species, populations, and in different environments, only emphasizing the complexity of these processes as we have reported previously (Richards et al. 2007, 2009, 2010). Further studies are needed to elucidate the diverse and complex mechanisms involved in vector-virus interactions that comprise vector competence. Studies are needed on the influence of variation in these mechanisms and the environmental factors that influence the mechanisms, as well as studies on the temporal dynamics of these processes in natural vector populations. Mosquitoes play a significant role in arboviral transmission cycles and understanding vector-virus dynamics is essential for protecting public health.
Acknowledgments
We thank Samantha Yost and Heather Robinson for laboratory assistance and James Colee for statistical advice. We also thank Jonathan Day and Carol Thomas for critically reviewing earlier versions of the manuscript. We greatly appreciate the thoughtful comments of two anonymous reviewers who provided suggestions that substantially improved this manuscript. This research was supported by the National Institutes of Health (grant AI-42164) to Cynthia Lord and Walter Tabachnick. Sheri Anderson was supported by a University of Florida Graduate Alumni Award.
References Cited
- Anderson JF, Main AJ, Delroux K, Fikrig E. Extrinsic incubation periods for horizontal and vertical transmission of West Nile virus by Culex pipiens pipiens (Diptera: Culicidae) J. Med. Entomol. 2008;45:445–451. doi: 10.1603/0022-2585(2008)45[445:eipfha]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Anderson SL, Richards SL, Smartt CT, Tabachnick WJ. The effects of West Nile virus dose on temporal progression of vector competence in Culex pipiens quinquefasciatus Say (Diptera: Culicidae) J. Am. Mosq. Control Assoc. 2010a;26:103–107. doi: 10.2987/09-5926.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SL, Richards SL, Smartt CT. A simple method for examining arbovirus transmission in mosquitoes. J. Am. Mosq. Control Assoc. 2010b;26:108–111. doi: 10.2987/09-5935.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitvitch BJ. Transmission dynamics and changing epidemiology of West Nile virus. Anim. Health Res. Rev. 2008;9:71–86. doi: 10.1017/S1466252307001430. [DOI] [PubMed] [Google Scholar]
- Brault A. Changing patterns of West Nile virus transmission: altered vector competence and host susceptibility. Vet. Res. 2009;40:43. doi: 10.1051/vetres/2009026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante DM, Lord CC. Sources of error in the estimation of mosquito infection rates used to assess risk of arbovirus transmission. Am. J. Trop. Med. Hyg. 2010;82:1172–1184. doi: 10.4269/ajtmh.2010.09-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (CDC) Centers for Disease Control. West Nile virus activity – United States, 2006. M.M.W.R. 2007;56:556–559. [PubMed]
- (CDC) Centers for Disease Control. West Nile virus. 2010 http://www.cdc.gov/ncidod/dvbid/westnile/
- Colton L, Biggerstaff BJ, Johnson A, Nasci RS. Quanti�cation of West Nile virus in vector mosquito saliva. J. Am. Mosq. Control Assoc. 2005;21:49–53. doi: 10.2987/8756-971X(2005)21[49:QOWNVI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Colton L, Nasci RS. Quanti�cation of West Nile virus in the saliva of Culex species collected from the southern United States. J. Am. Mosq. Control Assoc. 2006;22:57–63. doi: 10.2987/8756-971X(2006)22[57:QOWNVI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Curtin F, Schulz P. Multiple correlations and Bonferroni’s correction. Biol. Psychiatry. 1998;44:775–777. doi: 10.1016/s0006-3223(98)00043-2. [DOI] [PubMed] [Google Scholar]
- Girard AY, Klinger KA, Higgs S. West Nile virus dissemination and tissue tropisms in orally infected Culex pipiens quinquefasciatus. Vector-Borne and Zoon. Dis. 2004;4:109–122. doi: 10.1089/1530366041210729. [DOI] [PubMed] [Google Scholar]
- Girard AY, Schneider BS, McGee CE, Wen J, Han V, Popov PW, Mason V, Higgs S. Salivary gland morphology and virus transmission during long-term cytopathologic West Nile virus infection in Culex mosquitoes. Am. J. Trop. Med. Hyg. 2007;76:118–128. [PubMed] [Google Scholar]
- Hardy JL, Houk EJ, Kramer LD, Reeves WC. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu. Rev. Entomol. 1983;28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- Hardy JL, Reeves WC. Experimental studies on infection in vertebrate hosts. In: Reeve WC, editor. Epidemiology and control of mosquitoborne arboviruses in California, 1943–1987. Sacramento, CA: California Mosquito Vector Control Association; 1990. pp. 66–127. [Google Scholar]
- Hayes EB, Komar N, Nasci RS, Montgomery SP, O’Leary DR, Campbell GL. Epidemiology and transmission dynamics of West Nile virus disease. Emerg. Infect. Dis. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen CC, Webb CE, Northhill JA, Ritchie SA, Russell RC, Van Den Hurk AF. Vector competence of Australian mosquito species for a North American strain of West Nile virus. Vector-Borne Zoon. Dis. 2008;8:805–811. doi: 10.1089/vbz.2008.0037. [DOI] [PubMed] [Google Scholar]
- Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Annu. Rev. Entomol. 2007;53:61–81. doi: 10.1146/annurev.ento.53.103106.093258. [DOI] [PubMed] [Google Scholar]
- Kramer LD, Chin P, Cane RP, Kauffman EB, Mackereth G. Vector competence of New Zealand mosquitoes for selected arboviruses. Am J. Trop. Med. Hyg. 2011;85:182–189. doi: 10.4269/ajtmh.2011.11-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno G, Chang GJ. Biological transmission of arboviruses: Reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin. Microbiol. Rev. 2005;18:608–637. doi: 10.1128/CMR.18.4.608-637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, et al. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan Reverse Transcriptase-PCR assay. J. Clin. Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor PS. Replication of arboviruses in insect vectors. J. Comp. Path. 2000;123:231–247. doi: 10.1053/jcpa.2000.0434. [DOI] [PubMed] [Google Scholar]
- O’Meara G, Evans F. Seasonal patterns of abundance among three species of Culex mosquitoes in a South Florida wastewater lagoon. Ann. Entomol. Soc. Am. 1983;76:130–133. [Google Scholar]
- Ortiz DI, Kang W, Weaver SC. Susceptibility of Ae. aegypti (Diptera: Culicidae) to infection with epidemic (subtype IC) and enzootic (subtypes ID, IIIC, IIID) Venezuelan equine encephalitis complex alphaviruses. J. Med. Entomol. 2008;45:1117–1125. doi: 10.1603/0022-2585(2008)45[1117:soaadc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Provost M. The natural history of Culex nigripalpus. In: Sawder WT, editor. St. Louis encephalitis in Florida. Jacksonville, FL: Florida State Board of Health Monogr.; 1969. pp. 46–62. (no. 12) [Google Scholar]
- Reisen WK, Fang Y, Martinez VM. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae) J. Med. Entomol. 2006;43:309–317. doi: 10.1603/0022-2585(2006)043[0309:EOTOTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Richards SL, Mores CN, Lord CC, Tabachnick WJ. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens quinquefasciatus (Diptera: Culicidae) for West Nile virus. Vector Borne Zoon. Dis. 2007;7:629–636. doi: 10.1089/vbz.2007.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards SL, Lord CC, Pesko KA, Tabachnick WJ. Environmental and biological factors influencing Culex pipiens quinquefasciatus Say (Diptera: Culicidae) vector competence for Saint Louis encephalitis virus. Am. J. Trop. Med. Hyg. 2009;81:264–272. [PMC free article] [PubMed] [Google Scholar]
- Richards SL, Lord CC, Pesko KA, Tabachnick WJ. Environmental and biological factors influencing Culex pipiens quinquefasciatus Say (Diptera: Culicidae) vector competence for West Nile virus. Am. J. Trop. Med. Hyg. 2010;83:126–134. doi: 10.4269/ajtmh.2010.09-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge CR, Day JF, Lord CC, Stark LM, Tabachnick WJ. West Nile virus transmission by Florida Culex mosquitoes: Transmission rates are different from infection rates. J. Med. Entomol. 2003;40:253–258. doi: 10.1603/0022-2585-40.3.253. [DOI] [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ, Dohm DJ, O’Guinn ML. Vector competence of selected North American Culex and Coquilletidia mosquitoes for West Nile virus. Emerg. Infect. Dis. 2001;7:1018–1022. doi: 10.3201/eid0706.010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, Aguilar PV, Coffey LL, Gromowski GD, Wang E, Weaver SC. Venezuelan equine encephalitis virus transmission and effect on pathogenesis. Emerg. Infect. Dis. 2006;12:1190–1196. doi: 10.3201/eid1208.050841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styer LM, Kent KA, Albright RG, Bennett CJ, Kramer LD, Bernard KA. Mosquitoes inoculate high doses of West Nile virus as they probe and feed live hosts. PLoS Pathog. 2007;3:1262–1270. doi: 10.1371/journal.ppat.0030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick WJ, MacLachlan NJ, Thompson LH, Hunt GJ, Patton JF. Infection of Culicoides variipennis using PCR detectable bluetongue virus in cattle blood. Am. J. Trop. Med. Hyg. 1996;54:481–485. doi: 10.4269/ajtmh.1996.54.481. [DOI] [PubMed] [Google Scholar]
- Tiawsirusup S, Platt KB, Evans RB, Rowley WA. A comparison of West Nile virus transmission by Ochlerotatus trivittatus (COQ.), Culex pipiens (L.), and Aedes albopictus (Skuse) Vector Borne Zoon. Dis. 2005;5:40–47. doi: 10.1089/vbz.2005.5.40. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Dohm DJ, Sardelis MR, O’Guinn ML, Andreadis TG, Blow JA. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J. Med. Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O’Guinn M, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J. Med. Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O’Guinn M, Oliver J. Potential for New York mosquitoes to transmit West Nile virus. Am. J. Trop. Med. Hyg. 2000;62:413–414. doi: 10.4269/ajtmh.2000.62.413. [DOI] [PubMed] [Google Scholar]
- Vanlandingham DL, Schneider BS, Klinger K, Fair J, Beasley D, Huang J, Hamilton P, Higgs S. Realtime reverse transcriptase-polymerase chain reaction quantification of West Nile virus transmitted by Culex pipiens quinquefasciatus. Am. J. Trop. Med. Hyg. 2004;71:120–123. [PubMed] [Google Scholar]
- Zinser M, Ramberg F, Willot E. Culex quinquefasciatus (Diptera: Culicidae) as a potential West Nile virus vector in Tucson, Arizona: blood meal analysis indicates feeding on both humans and birds. J. Insect Sci. 2004;4:20–22. doi: 10.1093/jis/4.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]