Abstract
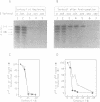
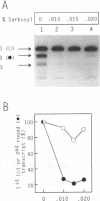
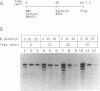
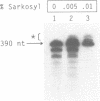
There are indications that different concentrations of Sarkosyl can block transcription initiation by RNA polymerase II in vitro at different functional steps [Hawley and Roeder (1985) J. Biol. Chem. 260, 8163-8172]. Consequently, this reagent could be a very useful tool for mechanistic studies. So far, however, evidence for the selectivity of Sarkosyl effects on RNA polymerase II transcription has been only indirect. To directly investigate the effect of Sarkosyl on transcription initiation and reinitiation by RNA polymerase II, we employed the reinitiation assay based on utilization of templates containing G-free cassettes (colliding polymerases reinitiation assay, or CoPRA). These experiments showed unambiguously that, under the appropriate conditions, Sarkosyl can be used to block transcription reinitiation by RNA polymerase II while allowing a first round of initiations from preassembled initiation complexes. This inhibition is not due to a disruption of the SII-dependent elongation of the reinitiated transcripts, and the levels of Sarkosyl that prevent transcription reinitiation coincide with the levels that block preinitiation complex assembly. However, Sarkosyl addition to transcription reactions reconstituted with partially purified transcription factors was found to have several undesirable side effects. The usefulness and limitations of the Sarkosyl-based and CoPRA assays for measurements of transcription reinitiation are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S., Bunick D., Zandomeni R., Weinmann R. RNA polymerase II ternary transcription complexes generated in vitro. Nucleic Acids Res. 1983 Sep 10;11(17):6041–6064. doi: 10.1093/nar/11.17.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnosti D. N., Merino A., Reinberg D., Schaffner W. Oct-2 facilitates functional preinitiation complex assembly and is continuously required at the promoter for multiple rounds of transcription. EMBO J. 1993 Jan;12(1):157–166. doi: 10.1002/j.1460-2075.1993.tb05641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenhagen D. F., Wormington W. M., Brown D. D. Stable transcription complexes of Xenopus 5S RNA genes: a means to maintain the differentiated state. Cell. 1982 Feb;28(2):413–421. doi: 10.1016/0092-8674(82)90359-2. [DOI] [PubMed] [Google Scholar]
- Buratowski S. The basics of basal transcription by RNA polymerase II. Cell. 1994 Apr 8;77(1):1–3. doi: 10.1016/0092-8674(94)90226-7. [DOI] [PubMed] [Google Scholar]
- Carcamo J., Lobos S., Merino A., Buckbinder L., Weinmann R., Natarajan V., Reinberg D. Factors involved in specific transcription by mammalian RNA polymerase II. Role of factors IID and MLTF in transcription from the adenovirus major late and IVa2 promoters. J Biol Chem. 1989 May 5;264(13):7704–7714. [PubMed] [Google Scholar]
- Conaway J. W., Conaway R. C. Initiation of eukaryotic messenger RNA synthesis. J Biol Chem. 1991 Sep 25;266(27):17721–17724. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariglio P., Buss J., Green M. H. Sarkosyl activation of RNA polymerase activity in mitotic mouse cells. FEBS Lett. 1974 Aug 30;44(3):330–333. doi: 10.1016/0014-5793(74)81170-1. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., Roeder R. G. Functional steps in transcription initiation and reinitiation from the major late promoter in a HeLa nuclear extract. J Biol Chem. 1987 Mar 15;262(8):3452–3461. [PubMed] [Google Scholar]
- Hawley D. K., Roeder R. G. Separation and partial characterization of three functional steps in transcription initiation by human RNA polymerase II. J Biol Chem. 1985 Jul 5;260(13):8163–8172. [PubMed] [Google Scholar]
- Izban M. G., Luse D. S. Transcription on nucleosomal templates by RNA polymerase II in vitro: inhibition of elongation with enhancement of sequence-specific pausing. Genes Dev. 1991 Apr;5(4):683–696. doi: 10.1101/gad.5.4.683. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Gralla J. D. Uncoupling of initiation and reinitiation rates during HeLa RNA polymerase II transcription in vitro. Mol Cell Biol. 1993 Aug;13(8):4572–4577. doi: 10.1128/mcb.13.8.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T. Assembly and disassembly of the Drosophila RNA polymerase II complex during transcription. J Biol Chem. 1990 Feb 15;265(5):2624–2631. [PubMed] [Google Scholar]
- Kash S. F., Innis J. W., Jackson A. U., Kellems R. E. Functional analysis of a stable transcription arrest site in the first intron of the murine adenosine deaminase gene. Mol Cell Biol. 1993 May;13(5):2718–2729. doi: 10.1128/mcb.13.5.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovelman R., Roeder R. G. Sarkosyl defines three intermediate steps in transcription initiation by RNA polymerase III: application to stimulation of transcription by E1A. Genes Dev. 1990 Apr;4(4):646–658. doi: 10.1101/gad.4.4.646. [DOI] [PubMed] [Google Scholar]
- Kunkel G. R., Danzeiser D. A. Formation of a template committed complex on the promoter of a gene for the U6 small nuclear RNA from the human requires multiple sequence elements, including the distal region. J Biol Chem. 1992 Jul 15;267(20):14250–14258. [PubMed] [Google Scholar]
- Laspia M. F., Wendel P., Mathews M. B. HIV-1 Tat overcomes inefficient transcriptional elongation in vitro. J Mol Biol. 1993 Aug 5;232(3):732–746. doi: 10.1006/jmbi.1993.1427. [DOI] [PubMed] [Google Scholar]
- Reines D., Chamberlin M. J., Kane C. M. Transcription elongation factor SII (TFIIS) enables RNA polymerase II to elongate through a block to transcription in a human gene in vitro. J Biol Chem. 1989 Jun 25;264(18):10799–10809. [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Factors involved in specific transcription by human RNA polymerase II: analysis by a rapid and quantitative in vitro assay. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4394–4398. doi: 10.1073/pnas.82.13.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Schultz M. C., Choe S. Y., Reeder R. H. In vitro definition of the yeast RNA polymerase I enhancer. Mol Cell Biol. 1993 May;13(5):2644–2654. doi: 10.1128/mcb.13.5.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- Szentirmay M. N., Sawadogo M. Synthesis of reinitiated transcripts by mammalian RNA polymerase II is controlled by elongation factor SII. EMBO J. 1993 Dec;12(12):4677–4684. doi: 10.1002/j.1460-2075.1993.tb06156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmay M. N., Sawadogo M. Transcription factor requirement for multiple rounds of initiation by human RNA polymerase II. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10691–10695. doi: 10.1073/pnas.88.23.10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Maniatis T. Transcriptional activation: a complex puzzle with few easy pieces. Cell. 1994 Apr 8;77(1):5–8. doi: 10.1016/0092-8674(94)90227-5. [DOI] [PubMed] [Google Scholar]
- Tolunay H. E., Yang L., Anderson W. F., Safer B. Isolation of an active transcription initiation complex from HeLa cell-free extract. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5916–5920. doi: 10.1073/pnas.81.19.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke M. W., Roeder R. G., Sawadogo M. Physical analysis of transcription preinitiation complex assembly on a class II gene promoter. Science. 1988 Sep 9;241(4871):1335–1338. doi: 10.1126/science.3413495. [DOI] [PubMed] [Google Scholar]
- White J., Brou C., Wu J., Lutz Y., Moncollin V., Chambon P. The acidic transcriptional activator GAL-VP16 acts on preformed template-committed complexes. EMBO J. 1992 Jun;11(6):2229–2240. doi: 10.1002/j.1460-2075.1992.tb05282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest D. K., Hawley D. K. In vitro analysis of a transcription termination site for RNA polymerase II. Mol Cell Biol. 1990 Nov;10(11):5782–5795. doi: 10.1128/mcb.10.11.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest D. K., Wang D., Hawley D. K. Mechanistic studies of transcription arrest at the adenovirus major late attenuation site. Comparison of purified RNA polymerase II and washed elongation complexes. J Biol Chem. 1992 Apr 15;267(11):7733–7744. [PubMed] [Google Scholar]
- Zawel L., Reinberg D. Initiation of transcription by RNA polymerase II: a multi-step process. Prog Nucleic Acid Res Mol Biol. 1993;44:67–108. doi: 10.1016/s0079-6603(08)60217-2. [DOI] [PubMed] [Google Scholar]