Abstract
Maintaining brain health promotes successful aging. The main determinants of brain health are the preservation of cognitive function and remaining free from structural and metabolic abnormalities, including loss of neuronal synapses, atrophy, small vessel disease and focal amyloid deposits visible by neuroimaging. Promising studies indicate that these determinants are to some extent modifiable, even among adults seventy years and older. Converging animal and human evidence further suggests that inflammation is a shared mechanism, contributing to both cognitive decline and abnormalities in brain structure and metabolism. Thus, inflammation may provide a target for intervention. Specifically, circulating inflammatory markers have been associated with declines in cognitive function and worsening of brain structural and metabolic characteristics. Additionally, it has been proposed that older brains are characterized by a sensitization to neuroinflammatory responses, even in the absence of overt disease. This increased propensity to central inflammation may contribute to poor brain health and premature brain aging. Still unknown is whether and how peripheral inflammatory factors directly contribute to decline of brain health. Human research is limited by the challenges of directly measuring neuroinflammation in vivo. This review assesses the role that inflammation may play in the brain changes that often accompany aging, focusing on relationships between peripheral inflammatory markers and brain health among well-functioning, community-dwelling adults seventy years and older. We propose that monitoring and maintaining lower levels of systemic and central inflammation among older adults could help preserve brain health and support successful aging. Hence, we also identify plausible ways and novel experimental study designs of maintaining brain health late in age through interventions that target the immune system.
Keywords: Brain health, Central inflammatory processes, Aging
Maintaining brain health and preserving cognitive functioningas we age are critical for promoting autonomy and protecting against disability, dementia, and mortality [1, 2]. Brain health is affected by several modifiable factors, including cardiometabolic parameters and aspects of our lifestyles. Emerging evidence shows that these risk factors for brain health decline are also related to systemic levels of inflammation. Moreover, there is comparable evidence indicating that increases in levels of peripheral markers of inflammation are associated with age-related declines in brain health. Such evidence parallels longstanding findings from neurocognitive studies in populations of older adults living in the community, consistently indicating an association between higher inflammatory levels and lower cognitive levels and higher risk of cognitive impairment over time. (see Gorelick for a review [3])
Considering the above in aggregate, it is important to note that an association of heightened peripheral inflammatory markers with structural and functional brain changes in association with neurocognitive changes over the course of life is biologically plausible, because the CNS is intimately and continuously engaged in a two- way communication with the peripheral immune system (Figure 1).
Figure 1.
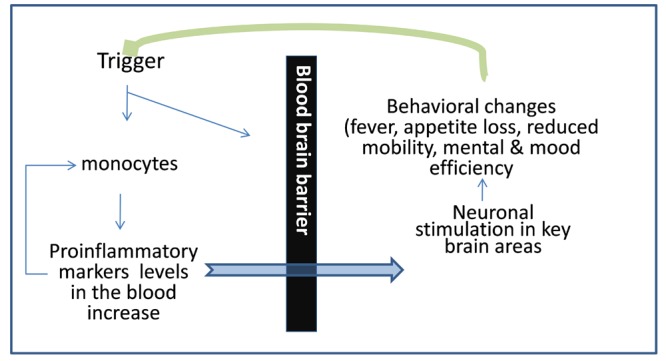
Communication between central nervous system and immune system. Triggers (cardiometabolic and lifestyle factors, or injuries of other nature) occurring anywhere in the body are communicated to the CNS via release of proinflammatory factors. An inflammatory response is initiated when monocytes/macrophages are activated by pathogens or tissue damage to release pro-inflammatory cytokines, including interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α, and chemokines, such as IL-8. These chemical mediators coordinate a local inflammatory response, resulting in the recruitment and activation of leukocytes to the site of invasion/injury. They also enter peripheral circulation to stimulate a systemic response, which includes the synthesis and release of acute phase proteins, such as C-reactive protein and fibrinogen. The CNS responds to this information by initiating behaviors to adapt to such triggers (e.g., fever, reduced activity) and by releasing immune mediators to respond to the peripheral stimulus (green arrow). In normal circumstances, peripheral inflammatory responses are terminated quickly by the action of anti-inflammatory factors released locally and systemically to shut off the inflammatory response, leading to the adaptive restoration of a lower inflammatory state.
To elaborate, multiple behavioral and disease processes can lead to heightened levels of systemic inflammation. In the case of acute illness, the inflammatory response is tightly controlled by a diverse set of regulatory mechanisms to handle the extent of the precipitating infection or injury, resolve quickly and restore health. In contrast, in older age these regulatory processes seem to be impaired, resulting in augmented and/or longer inflammatory response with subsequent tissue damage. For example, aging is associated with a chronic increase in the level of systemic inflammation [4, 5], that is relatively stable over extended periods [6], and predicts risk for a range of age-associated diseases, including cardiovascular disease, type 2 diabetes, frailty and general functional decline [7–10].
Additionally, recent evidence shows that older brains mount exaggerated central inflammatory responses to peripheral inflammatory stimuli even in the absence of clinically overt neuropathology [11, 12]. Thus, prolonged exposure to inflammatory triggers, higher levels of peripheral pro-inflammatory mediators that communicate with the CNS, lower levels of anti-inflammatory factors, and the “priming” of immune target cells in the brain can all contribute to heightened or prolonged neuroinflammation (Figure 2).
Figure 2.
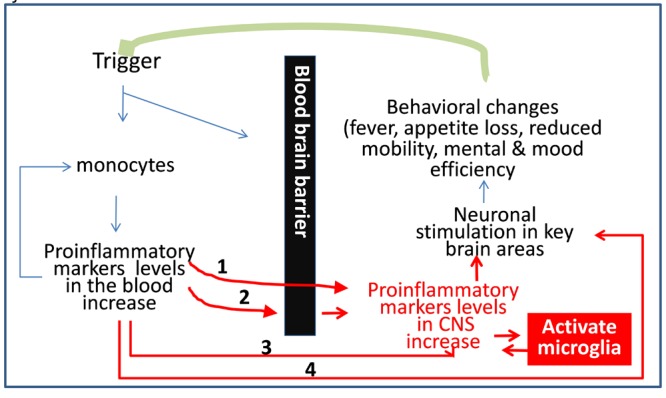
Biological rationale for a relationship between peripheral inflammatory factors and brain health. Proinflammatory cytokines cause greater endothelial permeability and adhesively of the blood brain barrier (BBB) and may reach the CNS through several pathways: 1 = through the post-inflammatory dysfunctional endothelium of the BBB; 2 = through binding to specific receptors on the BBB cell, and subsequently triggering mRNA production of pro-inflammatory factors; 3 = via diffusion through “nude” areas. 4. Via stimulation of the adrenergic system through the vagal nerve. Upon stimulation, the microglia in the CNS is also capable of producing cytokines directly, thus cytokines levels can increases in the CNS and can trigger brain inflammation even if they are not transferred from the peripheral blood into the brain parenchyma.
This review assesses evidence for, and potential mechanisms of, the contribution of peripheral inflammatory markers to brain health and the potential for prevention and intervention. Specifically, it remains to be determined whether and to what extent higher levels of peripheral inflammation reflect the presence and the severity of brain abnormalities and of brain inflammation in community-dwelling older adults. The answer to this question is critical to understand whether serum measurements of inflammatory markers could serve as biomarkers of underlying brain health. The potential use of these markers is very attractive because these measures present low risk, they are reasonably easy to be obtained, inexpensive, and reliable. We caution in this review, however, that researchers cannot assume that circulating levels of inflammatory mediators provide one-to-on measures of immune-derived inflammatory processes, as many cells other than immune cells produce these signaling proteins, including adipocytes and endothelial cells [13, 14]. Indeed, adipose tissue is a key source of peripheral IL-6, with adipocytes (particularly those residing in visceral adipose tissue) producing 10–35% of circulating levels [13]. Thus, this review will also consider the possible contribution of obesity to neuroinflammatory processes.
This review is organized into three sections: Section One describes and defines the determinants of brain health and proposes a model for potential inflammatory mechanisms; Section Two assesses evidence for the contribution of peripheral inflammatory markers to declining brain health; Section Three identifies opportunities to help maintain brain health late in age, through interventions targeting the immune system.
The complexity of the inflammatory and immune systems will not be addressed in detail in this review. The reader is invited to consult the many excellent reviews published on these topics [15–24].
1. Brain health in older age: measurements and potential inflammatory mechanisms
This section provides the background for a relationship between inflammation and brain health decline in older adults. We first summarize the main measurements of brain health that is brain function and structure using cognitive testing and neuroimaging methods, and brain indicators of inflammation (1.1.). We then briefly review the main cardiometabolic and lifestyle factors related to brain health (1.2.) and their relationship with inflammatory factors (1.3). Lastly, we describe potential mechanisms that may contribute to age-related declines in brain health (1.4), considering the role of cardiometabolic and lifestyle factors, and related systemic inflammation.
1.1. Overview of measurements of brain function and structure in older adults
Cognitive decline generally begins in the late 20s and progresses at a consistent rate across adulthood [25]. Some domains, including working memory, attention, and executive functioning, are more affected than others in older age, and age-related declines are predominant in processing speed [26, 27]. Compared with younger individuals, healthy elderly also perform less well on measures of delayed recall and recognition, and mental flexibility [28–30].
Overall, declines in brain function among older adults are associated with brain atrophy, hyperintensities in the white matter, and lacunae, as well as with amyloid deposits and metabolic changes [2, 31, 32]. However, these neuroimaging findings accompany numerous conditions and are not disease-specific. For example, global brain atrophy and hippocampal atrophy commonly accompany a variety of memory impairment syndromes, including Alzheimer’s, Vascular, and Parkinson dementias [33], as well as memory impairment in healthy elderly adults [34]. They are also frequently seen in those who have suffered severe hypoxia and traumatic brain injury [35]. Observational studies have also shown associations of higher prevalence of infarcts, white matter hyperintensities, and atrophy with inflammatory markers in older adults in the absence of overt clinical disease [36–43].
Applications of advanced neuroimaging methods that quantify micro-structure and metabolism with increased spatial resolution and regions-of interest approaches have begun to change our understanding of brain aging processes. For example, atrophy in older adults free from dementia primarily affects the prefrontal cortex and the subcortical regions, including the basal ganglia and the hippocampus [44–50]. Similarly, the very recent application of the Pittsburgh-Compound B to study amyloid deposition in cognitively normal older adults has shown focal amyloid deposition within fronto-parietal and posterior cingulated networks, known to be related to executive control function [51, 52]. Additionally, Diffusion Tensor Imaging (DTI) has uncovered the presence of microstructural abnormalities that remain otherwise invisible on conventional MRI [53, 54] Specifically, higher mean diffusivity and lower fractional anisotropy from DTI indicate loss of homogeneity of brain tissue and are observed in brain abnormalities that develop with aging [55] and are associated with slower processing speed [56]. Overall, these neuroimaging findings support the clinical observations that older adults who are non-demented primarily display executive and memory dysfunction.
Recent methodological developments in neuroimaging are also beginning to provide markers of brain inflammation in vivo. For example, MRI combined with Gadolinium as a contrast agent can provide images of brain vascular permeability, a correlate of blood-brain barrier alterations and thus a potential inflammatory marker. Notably, however, this newer method has a limited spatial resolution. Additionally, greater vascular permeability occurs in advanced stages of inflammation; thus, this method would not yet appear to capture subtle signs or earlier stages of inflammation. Ultra high-field neuroimaging combined with injections of iron oxide nanoparticles is emerging as a neuroimaging method to visualize inflammatory phenomena at the cellular level by marking activated microglia in early inflammatory stages. This method shows greater regional binding of contrast agent even in the absence of frank disruptions to blood-brain barrier permeability and with greater spatial resolution than Gadolinium contrast approaches (see references [57–60] for reviews). Magnetic resonance molecular imaging has been used in acute ischemic stroke to identify endothelial activation by targeting biomolecular agents [61]. Although very promising, to date these methods have been applied mostly in patient populations and in animal disease models [62–69].Moreover, compared with many of the other modalities reviewed here, these neuroimaging approaches are more invasive and less feasible for large-scale and longitudinal epidemiological studies.
1.2. Association of cardiometabolic and lifestyle factors with brain health
Although the biological bases for the selective spatial distribution of brain abnormalities accompanying aging remain unclear, growing evidence points to the vascular anatomy and hemorrheological characteristics of the flow within these regions as predisposing factors. Fronto-subcortical networks, deep striato-capsular and lenticular regions, and finally the deep white matter are localized within the watershed areas of the cerebral blood supply system, and thus have poor collateral vascularization as well as low cerebral perfusion pressure. These predisposing conditions make the fronto-parietal and subcortical regions more susceptible to disturbances of cerebral perfusion [70].
Several factors related to aging, in particular hypertension, arteriosclerosis and glucose dysregulation, contribute to disturbances of cerebral perfusion, and are associated with cognitive decline and related changes in brain structure and metabolism [71–73]. Among adults 75 years and older, hypertension is the most common risk factor longitudinally associated with increased white matter hyperintensities, along with arteriosclerosis, and hemodynamic dysregulation [44, 74–77]. A role of cerebral amyloid angiopathy in altering the blood brain barrier permeability and contributing to small vessel disease has also been proposed [78, 79]. Pulse pressure (the difference between systolic and diastolic blood pressure, which is associated with vessel stiffening and vascular aging) has also been cross-sectionally associated with white matter hyperintensities in older adults [80]. Glucose-related disturbances have also been associated with brain structural changes [81–83].
Among lifestyle factors, attention has recently focused on the role of diet and physical activity as well as to social and cognitive engagement in brain aging [84]. This review focuses on these modifiable factors because they may be amenable to cost-effective, non-pharmacological interventions that promote other aspects of whole-body and brain health, including lower cardiovascular risk and mood improvements [85]. Higher levels of adiposity are associated with higher circulating levels of inflammatory factors [86], and predict temporal lobe and global brain tissue atrophy, a reduced integrity of white matter tracts, cognitive decline, and even incident dementia [87–91]. Corroborating these human epidemiological findings, experimental and animal studies further demonstrate that obesity predicts deficits in hippocampal-dependent learning and memory [92–94]. We have shown [95, 96] that greater percent body fat is associated with poorer cognitive function, and lower hippocampal grey matter volume among relatively healthy mid-life adults, 30–54 years of age, suggesting that the cognitive decline associated with adiposity begins well before clinically significant deficits. It is plausible that long-term exposure to adiposity could presage accelerated brain and cognitive aging. In addition to adiposity, atherogenic diet has been associated with higher levels of brain inflammation in animal models [97].
Several lines of evidence suggest that level of social engagement and other social behaviors related to lifestyle predict cognitive aging, independently of other known risk factors (e.g., depressive symptoms, frailty, and cardiovascular and cerebrovascular health status). For example, higher scores on a social engagement scale (assessing membership in diverse social groups and frequency of social interactions) predicted less of a 12-year decline in global mental status among 2,812 community dwelling adults older than 65 yrs [98]. Such findings have been corroborated and extended in studies of social participation [99–101] and social-network size in particular, as well as in studies of social engagement and working memory and related executive control functions [102].
Recent studies indicate that interventions that decrease cardiometabolic risk in older adults [75, 103–108] and improve lifestyle parameters such as diet, social engagement, and exercise [109, 103, 110, 111]can delay cognitive decline and slow the progression of structural brain abnormalities, specifically signs of subclinical cerebral disease [75], white matter hyperintensities [112], and brain tissue atrophy. Critically, there is also evidence that human brains retain substantial plasticity, or the potential to change in function and structure, and several reserve capacities even in their late eighties [85]. Therefore, it is very important to understand the mechanisms underlying the relationship between these factors and brain health.
1.3. Association of cardiometabolic and lifestyle factors with inflammation
Many of the risk factors for brain health decline, including higher homocysteine, oxidized lipids, free radicals, and Angiotensin II are directly toxic to blood-brain barrier permeability, and can trigger vascular inflammatory events [19, 71]. Of relevance to the current review, activated immune cells involved in the inflammatory response are a primary source of oxidative stress [113, 114]. Indeed, evidence shows a positive association of systemic markers of inflammation with oxidative burden [115–117], which may play a role in the pathogenesis of neurodegeneration. Additionally, small vessel abnormalities, occlusions and/or lower blood flow can cause an inflammatory response in the vascular districts via poor oxygenation and lower glucose delivery to neurons, which in turn trigger subsequent cellular modifications and changes in beta protein metabolism. Specifically, higher levels of beta fragments, due either to a genetic predisposition (APO-e4) and/or to neurodegeneration following vascular insults, can function as “irritants” and trigger an inflammatory response [118, 119]. These findings suggest that a main mechanism linking cardiometabolic factors with inflammation may be localized to the vascular district, specifically, an induced state of vascular inflammation with release of cytokines and chemokines locally and systemically across the blood brain barrier.
It is well established that adipose tissue is a potent source of peripheral inflammatory mediators, with adipocytes producing 10–35% of circulating IL-6 levels [92]. Further, mononuclear cells of obese people are “primed” to express pro-inflammatory cytokines [92]. Thus, obesity can reasonably be viewed as an inflammatory condition, with circulating levels of IL-6 and other inflammatory mediators covarying positively with adiposity. Conversely, greater social engagement and related social factors (e.g., social support) are inversely related to expression of inflammatory markers [24]. Animal evidence indicates that manipulation of the social environment, particularly social isolation, increases pro-inflammatory cytokine expression (specifically IL-1beta) in the hippocampus, and decreases brain-derived neurotrophic factor (BDNF) expression [120]. Moreover, social isolation impairs hippocampal-dependent memory, and this effect is blocked by intra-hippocampal injection of an IL-1 receptor antagonist, which also prevents BDNF decreases [120]. However, we are unaware of studies linking social engagement with indicators of brain morphology and related aspects of cognition that co-vary with inflammation, as would be predicted by converging lines of epidemiological, animal, and neuroimaging work.
1.4. Potential mechanisms underlying decline in brain health
The pathways linking pro-inflammatory levels in the circulating blood with brain health have been examined in detail in many excellent reviews and are only briefly summarized here (Figure 2) [15–17, 21–24, 105, 121–125]. Direct pathways include: a) active passage across the blood brain barrier; and b) passive diffusion from the plexus choriodeus and other blood brain barrier-nude regions into other brain regions. Cytokines can also affect the CNS through indirect pathways that include: a) stimulation of the vagal nerve, and b) inflammation of the endothelial cells, which in turn produce inflammatory factors.
Regardless of the nature and location of the originating trigger, increased levels of inflammatory factors cause loss of vascular anti-adhesive and impermeability properties of the blood brain barrier — thus allowing adherence and entrance of inflammatory cells into the CNS parenchyma, oxidation of LDL and accumulation of an inflammatory locus within the vascular wall. Furthermore, inflammatory factors in the CNS and peripheral pro-inflammatory factors stimulate the production of central pro-inflammatory factors by microglial cells in discrete brain regions (Figure 2). The production and release of pro-inflammatory factors from the microglia ultimately contribute to neurotoxicity and brain parenchyma abnormalities. These in turn can sustain the inflammatory reaction, thus maintaining a vicious cycle.
It has been suggested that increases in low-grade systemic inflammation, such as those that accompany cardiometabolic risk and aging as described above, sensitize the brain response to inflammatory factors. Specifically, in vivo and in vitro animal studies, as well as post-mortem human studies, indicate that older brains are in a native heightened inflammatory state even in the absence of overt disease [16, 17, 22–24, 97, 121, 126]. In addition to the chronic exposure to low-grade systemic inflammation, this state has also been attributed to an age-related change in microglia to a phenotype that release inflammatory factors and respond excessively to stimulation. Another possible cause of this primed state is the life-time exposure to cellular debris from pruning and remodeling as well as neuronal senescence processes that act as local “irritants” and trigger inflammatory reactions. It has been shown that there is a relative increase in expression of pro-inflammatory genes in older mice brains [21]. Since peripheral pro-inflammatory cytokines stimulate the production of central pro-inflammatory cytokines by microglial cells, a sensitization of neuroinflammatory responses may play a role in the premature brain and cognitive aging that accompany increased systemic inflammation.
1.5. Implications for mechanisms linking inflammation and brain health
In summary, risk factors for declining brain health in older adults include numerous cardiometabolic conditions and lifestyle factors that each relate to inflammation. Prolonged exposure to these conditions and risk factors might trigger and then sustain a systemic inflammatory response as well as a focal response in the brain. The increasing levels of pro-inflammatory factors in the blood may in turn affect the inflammatory state of the CNS through direct and/or indirect pathways as described above. Since the CNS and the immune system are in constant communication, a heightened inflammatory state in the periphery can plausibly occur concurrently with a heightened inflammatory state in the brain and vice versa. For example, cardiometabolic risk factors may trigger vascular inflammation in multiple vascular districts concurrently, including the peripheral and cerebral districts. Once these factors cause vascular inflammation in the cerebro-vascular district, then endothelial cells of the blood brain barrier may produce and release cytokines within the CNS. The preferential genetic expression of pro- versus anti-inflammatory factors in older age might also facilitate either the start and/or the maintenance of an inflammatory state. In addition to the biological plausibility of an association between inflammation and brain health, there is recent consistent evidence of associations between peripheral cytokines and brain health as described in the next section.
2. Contribution of peripheral inflammatory markers to declining brain health
A number of peripheral blood markers of inflammation have been examined in studies of brain aging. Key markers include interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α, and chemokines, such as IL-8 [127]. In contrast to IL-1β and TNF-α, which decay rapidly, levels of IL-6 and C-Reactive Protein can be reliably detected in peripheral circulation and are widely assumed to reflect systemic levels of inflammation. Consistent evidence from animal studies and disease models indicates that peripheral inflammatory mediators, such as interleukin (IL)-6, modulate central inflammatory processes that affect cognitive function [24, 128–130]. Notably, receptors for IL-6 are concentrated in the hippocampus and prefrontal cortex [130–132].We have recently shown that higher IL-6 levels are associated with smaller hippocampal and prefrontal gray matter volume and with lower executive function and memory performance in otherwise healthy middle-aged community dwelling adults [95, 96]. It is likely that other peripheral proinflammatory mediators, such as IL-1 beta and TNF-alpha, also play a role in the induction of central neuroinflammation; however it is harder to reliably assess these factors due to their low circulating levels and short half-lives. Research suggests that IL-1β, IL-6, and TNFα are involved in neurophysiological processes that subserve cognitive function and brain health [133]. Animal studies indicate that IL-1 affects long-term potentiation and possibly neurogenesis, IL-6 is an important regulator of neurogenesis, and TNFα influences cognitive function through direct effects on long term potentiation and synaptic scaling [133]. Experimental studies using animal models demonstrate that long-term exposure to IL-6, as seen in normal aging and certain neurodegenerative diseases, can interfere with neurocognitive functioning by impairing adult neurogenesis [21, 134].
2.1. Evidence from patients with primary neurodegenerative disorders
Studies in patients with ischemia and stroke indicate that peripheral levels of the inflammatory markers IL-6 and TNF-α provide markers of the severity of the underlying lesions as well as predict patient prognosis [135–140]. One study showed that patients with recurrent chronic brain infarctions had higher levels of monocyte chemoattractant proteins and C-reactive protein [141].
Studies of patients with Alzheimer’ and Parkinson’s diseases show an association between blood levels of inflammatory markers and severity of brain functional impairment [142–144]. Still unknown is whether higher levels of peripheral inflammatory markers indicate underlying declining brain health in community-dwelling older adults. The majority of longitudinal studies examining the relationship of inflammation with brain health in older adults have focused on dementia populations, with comparatively few examining community-dwelling older adults [145].
2.2. Evidence from patients with primary chronic peripheral inflammatory diseases
Studies of rheumatoid arthritis and lupus strongly support the presence of immune-to-brain communication pathways [146–148]. Pioneering neuroimaging studies in humans report brain metabolic alterations and volumetric reductions in patients relative to controls, suggestive of underlying brain degeneration [147, 149–151]. It is not clear whether these inflammatory diseases affect the brain via increased levels of peripheral inflammatory factors (e.g., via the mechanisms described in 1.3 and illustrated in Figure 2) or by pain-mediated mechanisms, which have also been suggested [152]. One recent study of patients with rheumatoid arthritis suggests an association between TNF alpha blocking agents and worse micro-structural brain characteristics [153].
A review of seven studies of patients with arthritis taking anti-inflammatory medications (NSAIDs and steroids) found strong evidence for decreased risk for Alzheimer disease [154]. However, a similar association was not observed in patients with rheumatoid arthritis taking corticosteroids [71], raising the possibility that the effects are specific to NSAIDs. In this regard, two reviews of observational studies of non-demented populations [155, 156] concluded that NSAIDs intake is associated with higher cognitive function, slower progression of cognitive decline and lower risk of developing Alzheimer’s disease. However, NSAID intake in demented adults appears to have no or little effect on progression of cognitive decline. These preliminary results should spur further research to test the hypothesis that NSAID intake is most beneficial early, rather than late in the course of the neurodegenerative process. The mechanisms underlying the effects of NSAIDs have not been examined. It is not clear whether NSAIDs’ neuroprotective effects result from the dampening of inflammatory processes or from the direct lowering of Aβ levels. Mediating effects on pain have also been suggested as potential mechanisms.
2.3. Evidence from experimental studies
Experimental studies inducing higher blood levels of pro-inflammatory markers via peripheral immune stimuli in young adults have shown a slowing of information processing and negative mood changes within 12 hours [157–159]. Related functional neuroimaging data obtained within this timeframe show concurrent changes in patterns of neuronal activation and heightened response to emotional-related stressors. This is consistent with the loop linking peripheral stressors with adaptive neuronal responses (Figure 1). Initial experimental studies using peripheral administration of cytokine-blocking agents also show rapid and short-term changes in the CNS [160–162]. The work by Hess et al [161], suggests that TNF neutralization in a mouse model of rheumatoid arthritis has rapid effects on neuronal activity in regions related to pain perception six hours after drug intake, without detectable changes in peripheral cytokines levels. Another study of rheumatoid arthritis patients taking naproxen [162] found a reduced hypothalamic-pituitary-adrenal (HPA) axis response in the short, though not long term. These initial findings suggest that inflammatory factors may affect the CNS directly.
While we take stock of the mounting evidence that cytokines are associated with brain function and structure, we also notice that most studies are cross-sectional, leaving open the debate as to whether raised peripheral inflammatory markers’ levels are the cause or consequence of brain health decline [163]. Initial evidence indicates that early interventions on factors that initiate inflammatory processes may also decrease risk for brain health decline. Future work is needed to understand the exact mechanisms of the relationship between peripheral inflammation and brain health decline (see Implications for future studies, below).
3. Opportunities for preventative and therapeutic intervention
The central notions that the aging brain becomes increasingly subject to systemic inflammation in later life, and retains a remarkable level of plasticity well into late life, each provide a basis for understanding how several lifestyle factors and interventions can have preventative and therapeutic benefits.
Perhaps the most salient lifestyle factor amenable to intervention is physical activity. It is well established that a sedentary lifestyle confers risk for several syndromes associated with heightened levels of inflammation—including obesity, diabetes, cardio-vascular disease, depression, and dementia. Even moderate levels of physical activity are associated with decreased levels of inflammation [84, 164–168], which could plausibly slow the progression of functional and cognitive decline in aging [169, 170]. For example, aerobic exercise regimens involving regular and brisk walking among older adults (approximately 30 minutes per day/most days of the week) have been associated with improvements in cognitive performance and with changes in patterns of brain activation in prefrontal and parietal cortices that more closely resemble those seen in younger individuals [171, 172]. Moreover, regular physical activity has been associated with an increase in the volume of gray matter and related cognitive functions [111, 173, 174]. An open research question is whether physical activity may improve or maintain aspects of brain health in aging by directly down-regulating inflammatory processes. The ongoing intervention trials SMART [175] and LIFE-Main [176] may provide answers to these questions because they will monitor longitudinal changes in physical activity and in brain function, and are also collecting and storing sera that might be employed to quantify levels of peripheral inflammation.
Weight gain in mid- and later life (possibly related to low levels of physical activity), is associated with reduced brain tissue volume and associated cognitive changes ([177], also see above). One recent study also reports that weight loss is associated with decreased levels of systemic inflammation and concurrent increases in brain activity within memory-related regions [178]. Most of these studies have focused on adults in their sixties or younger. Weight changes in very old adults follow differential patterns and are linked with frailty and other co-morbidities; their role in improving brain health requires further investigation.
It is noteworthy that particular dietary aspects of energy balance and food choices throughout life could plausibly affect brain aging and be targeted by intervention. In their recent review, Jang and Johnson [179] suggest that a diet rich in flavonoids- that is fruits and vegetables- might reduce the age-related priming of microglia and/or the magnitude of peripheral inflammatory reactions and consequently assuage the severity of cognitive decline. Additionally, there is emerging evidence that higher intake of key ω-3 fatty acids – eicosapentaenoic and docosahexaenoic acids (EPA, DHA), found primarily in cold water fatty fish and in fish oil supplements –is associated with a reduction in systemic inflammation, decreased risk for premature cognitive impairments, and higher cognitive function and regional brain tissue volumes. Interestingly, whereas EPA and DHA comprise about 4% of the fatty acids in plasma and red blood cells, 14–30% of the fatty acids in the phospholipids of human brain tissue, particularly gray matter tissue, are either EPA or DHA [180, 181]. Hence, the types and relative concentrations of fatty acids in the diet may, over time, affect brain tissue composition and integrity—specifically through known diffusion or active transport pathways [182, 183]. Finally, it is notable here that ω-3 and ω-6 polyunsaturated fatty acids are precursors to prostaglandins and leukotrienes. EPA is a substrate for anti-inflammatory prostaglandins and leukotrienes, whereas the principal ω-6 fatty acid, arachidonic acid, is a substrate for pro-inflammatory mediators [184]. Viewed as inflammatory precursors, it has been recommended that dietary intake of fatty acids be regarded as preventative strategy to decrease levels of systemic inflammation and thereby promote physical and mental health [185].
In addition to the functions described above, EPA and DHA have functional and morphological roles in the brain, being associated with complexity of dendritic branching, synaptic plasticity, and long-term potentiation within the hippocampus; ω-3 fatty acid deficiency also simplifies dendritic arborization in the cortex [186, 187]. Moreover, increasing ω-3 fatty acid intake by oral DHA or EPA administration increases dendritic spine expression [188, 189]. Finally, ω-3 fatty acid deprivation has been shown to reduce brain-derived neurotrophic factor and neuron size in the hippocampus, hypothalamus, and parietal cortex [186, 190–192]. Consistent with work in animals, we found that self-reported dietary intake of EPA and DHA was associated with greater gray matter volumes in the prefrontal and medial temporal lobes [193]. The recent OmegAD Study indicates that treatment with ω-3FAs in AD patients did not substantially modify inflammatory markers’ levels in the blood or spinal fluid, suggesting that fatty acids protective effects might be more evident among non-demented adults [194].
In the aggregate, promising evidence regarding habits of lifestyle amendable to intervention, namely physical activity, weight maintenance, and intake of flavonoids and ω-3 fatty acids, suggests that brain aging and brain health could be preserved and positively affected by preventative behavioral changes and programs. However, it remains unclear precisely how much flavonoids or fatty acid individuals should consume on a daily or weekly basis, over what period of time, in what form (e.g., from food or supplement), and beginning at what age to achieve improvements or maintenance of brain and cognitive health in later life. Also unclear is how EPA and DHA consumption interacts with other factors, such as physical activity or use of NSAIDs, in affecting inflammatory processes linked to aging. In this regard, epidemiological studies in ethnic groups with life-time exposure to high levels of fatty acids, specifically Japanese, Icelandic and Norwegian cohorts may provide insights into the effectiveness of diet in different genetic populations [195].
4. Implications for future studies
It is possible that low-grade chronic inflammatory and neurodegenerative conditions contribute to a recursive cycle of damage that may worsen brain health over time. The greatest challenge in efforts to maintain brain health is to identify how to interrupt this inflammatory cycle. While we have described several targets for low cost interventions, we propose that advancements in this field require the integrated application of novel methods, longitudinal designs and unique populations that move beyond maintenance of a favorable profile of cardiometabolic and lifestyle factors.
First, the rapid advances in neuroimaging technology can now provide direct measures of brain inflammation and blood brain barrier permeability which were not available until only a few years ago. (see references in Section one). Multimodal neuroimaging measures of the blood brain barrier, concurrent with brain micro-structure and quantification of amyloid deposition can quantify early abnormalities at the micro-structural and metabolic level. Addressing these abnormalities before they build into brain macro-structural damage, disease and overt inflammation may have a substantial impact on maintaining cognitive function in older persons. There is also the need to conduct studies in community dwelling older adults to relate markers inflammation in the blood to markers of inflammation in the cerebrospinal fluid (CSF). Values of inflammatory markers in the CSF of older adults in the absence of overt disease are unknown. To date, only few reports have documented levels of inflammatory factors concurrently in the blood and the CSF. Most of these studies have examined patients populations [194, 196–198] and report a significant correlation between cytokines’ levels in the blood and in the CSF. Overall, these results suggest that plasma levels of inflammatory factors might be candidate biomarkers of underlying neuroinflammatory processes in patients’ population. We are aware of one normative study in healthy volunteers [199] 20–90 years old, without history of acute or chronic inflammatory disease. The study showed that IL-6,IL-8, IL-10 and the soluble TNF receptor are detectable both in the blood and CSF, and that age did not significantly affect the plasma to CSF ratio of inflammatory markers. However, this study did not report the strength of the correlation between markers in the two compartments. Longitudinal study designs with repeated CSF and blood levels measurement of inflammatory factors are warranted to quantify the repeatability and reliability of blood levels of inflammatory markers in predicting CSF markers. These studies could also clarify whether inflammatory levels increase concurrently in the blood and spinal fluid or are independent of each other. Specifically, studies applying serial and integrated measurements of levels of inflammatory markers in these two compartments, together with multimodal neuroimaging and cognitive testing can help clarify whether there is a causal pathway between peripheral inflammatory factors and declining brain health and higher brain inflammation.
It is not clear whether inflammatory levels increase concurrently in the blood and spinal fluid or are independently of each other and values in the absence of overt disease are also unknown. Studies applying serial and integrated measurements of levels of inflammatory markers in these two compartments, together with multimodal neuroimaging and cognitive testing can help clarify whether there is a causal pathway between peripheral inflammatory factors and declining brain health and higher brain inflammation. Novel study designs applying international epidemiological collaborative approaches would be particularly helpful to understand the impact of ethnicity, lifestyle and ecological variations on the interplay between inflammation and brain health.
Secondly, there are new drugs with potent anti-inflammatory effects that appear to have an effect on the brain. The application of cytokine blocking agents has revolutionized the treatment of peripheral inflammatory diseases, specifically of patients with rheumatoid arthritis. However, with the exception of one study, the effect on the brain has not been directly quantified concurrently with symptomatology of the main disease. Ongoing trials to test TNFα receptor antagonists in patients with rheumatoid arthritis should include the measurements described above to quantify CNS changes related to drug intake. If these drugs do affect the brain and slow the progression of cognitive decline among patients with chronic inflammatory conditions, then there will be new avenues for exploration of their application in the prevention of brain declines that accompany normal aging.
A unique opportunity to study the course of inflammatory diseases and cognitive decline in aging adults is presented by the cohort of patients with multiple sclerosis and HIV who are now living into older age. Studies applying the measurements described above can clarify the interaction between disease- and age-related brain changes, and can provide biomarkers to quantify the effects of pharmacologic interventions on either and/or both processes. These findings can be particularly important among those who convert to dementia.
Further, the application of advanced neuroimaging methods can model personalized medicine approaches. Neuroimaging may assist in identifying the optimal window of time for administration of NSAIDs. Preliminary evidence suggests that NSAIDs may be most useful in delaying cognitive decline if taken before the onset of dementia. However, it is not clear how “early” would be too early. NSAIDs lowering effects of the inflammatory response even when clinically indicated may cause harm, because the inflammatory response is at its core an adaptive response aimed at restoring health. Specifically, preliminary studies indicate that NSAIDs might harm the inflammatory response to Aβ amyloid depositions [155]. Thus, neuroimaging markers of brain inflammation, structure, and metabolism may serve to identify the window of time to maximize the therapeutic effects of NSAIDs.
In conclusion, addressing the existing empirical gaps and pursuing the lines of basic and clinical inquiry discussed in this review will help to better characterize the complex interplay between the central nervous and immune systems in the context of brain and cognitive aging. Understanding the mechanisms and causal pathways linking peripheral inflammatory markers with brain health and neuroinflammation will help understand whether inflammatory markers can serve as surrogate markers of declining brain health. To date, the application of these markers to quantify risk of recurrent brain vascular events has been shown in patient populations. While it is biologically plausible that peripheral inflammatory markers reflect underlying brain health, the validity of peripheral inflammatory factors as prognostic/diagnostic markers of brain health will need to be tested in community-dwelling adults using advanced neuroimaging methods.
Acknowledgments
The authors wish to thank Amy J. Markowitz, JD.
References
- [1].Depp CA, Jeste DV. Definitions and predictors of successful aging: a comprehensive review of larger quantitative studies. Am J Geriatr Psychiatry. 2006;14:6–20. doi: 10.1097/01.JGP.0000192501.03069.bc. [DOI] [PubMed] [Google Scholar]
- [2].Rosano C, Newman AB, Katz R, Hirsch CH, Kuller LH. Association between lower digit symbol substitution test score and slower gait and greater risk of mortality and of developing incident disability in well-functioning older adults. J Am Geriatr Soc. 2008;56:1618–1625. doi: 10.1111/j.1532-5415.2008.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gorelick PB. Role of inflammation in cognitive impairment: results of observational epidemiological studies and clinical trials. Ann N Y Acad Sci. 2010;1207:155–162. doi: 10.1111/j.1749-6632.2010.05726.x. [DOI] [PubMed] [Google Scholar]
- [4].Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- [6].Rao KM, Pieper CS, Currie MS, Cohen HJ. Variability of plasma IL-6 and crosslinked fibrin dimers over time in community dwelling elderly subjects. Am J Clin Pathol. 1994;102:802–805. doi: 10.1093/ajcp/102.6.802. [DOI] [PubMed] [Google Scholar]
- [7].Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- [8].Luc G, Bard JM, Juhan-Vague I, Ferrieres J, Evans A, Amouyel P, Arveiler D, Fruchart JC, Ducimetiere P. C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: the PRIME Study. Arterioscler Thromb Vasc Biol. 2003;23:1255–1261. doi: 10.1161/01.ATV.0000079512.66448.1D. [DOI] [PubMed] [Google Scholar]
- [9].Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- [10].Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- [11].Buchanan JB, Sparkman NL, Chen J, Johnson RW. Cognitive and neuroinflammatory consequences of mild repeated stress are exacerbated in aged mice. Psychoneuroendocrinology. 2008;33:755–765. doi: 10.1016/j.psyneuen.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- [14].Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- [15].Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004;18:407–413. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- [16].Perry VH. Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathol. 2010;120:277–286. doi: 10.1007/s00401-010-0722-x. [DOI] [PubMed] [Google Scholar]
- [17].Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15:323–330. doi: 10.1159/000156474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Spooren A, Kolmus K, Laureys G, Clinckers R, De Keyser J, Haegeman G, Gerlo S. Interleukin-6, a mental cytokine. Brain Res Rev. 2011 doi: 10.1016/j.brainresrev.2011.01.002. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [19].Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- [20].Carty M, Bowie AG. Evaluating the role of Toll-like receptors in diseases of the central nervous system. Biochem Pharmacol. 2011;81:825–837. doi: 10.1016/j.bcp.2011.01.003. [DOI] [PubMed] [Google Scholar]
- [21].Godbout JP, Johnson RW. Interleukin-6 in the aging brain. J Neuroimmunol. 2004;147:141–144. doi: 10.1016/j.jneuroim.2003.10.031. [DOI] [PubMed] [Google Scholar]
- [22].Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006;236:13–23. doi: 10.1016/j.canlet.2005.04.009. [DOI] [PubMed] [Google Scholar]
- [23].McGeer PL, McGeer EG. Inflammation and the degenerative diseases of aging. Ann N Y Acad Sci. 2004;1035:104–116. doi: 10.1196/annals.1332.007. [DOI] [PubMed] [Google Scholar]
- [24].Marsland AL. Inflammation. In: Waldstein Shari R., editor; Elias Merrill F., editor. Neuropsychology of Cardiovascular Disease. 2nd Edition. 2011. in press. [Google Scholar]
- [25].Salthouse TA. What and when of cognitive aging. Current Directions in Psychological Science. 2004:13. doi: 10.1177/0963721414535212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004:44. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- [27].Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nature Reviews Neuroscience. 2004:5. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- [28].Davis HP, Small SA, Stern Y, Mayeux R, Feldstein SN, Keller FR. Acquisition, recall, and forgetting of verbal information in long-term memory by young, middle-aged, and elderly individuals. Cortex. 2003:39. doi: 10.1016/s0010-9452(08)70878-5. [DOI] [PubMed] [Google Scholar]
- [29].Huh TJ, Kramer JH, Gazzaley A, Delis DC. Response bias and aging on a recognition memory task Journal of the International Neuropsychological Society. 2006;12:1–7. doi: 10.1017/S1355617706060024. [DOI] [PubMed] [Google Scholar]
- [30].Wecker NS, Kramer JH, Hallam BJ, Delis DC. Mental flexibility: age effects on switching. Neuropsychology. 2005:19. doi: 10.1037/0894-4105.19.3.345. [DOI] [PubMed] [Google Scholar]
- [31].Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010;21:187–221. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- [32].O’Sullivan M. Imaging small vessel disease: lesion topography, networks, and cognitive deficits investigated with MRI. Stroke. 2010;41:S154–158. doi: 10.1161/STROKEAHA.110.595314. [DOI] [PubMed] [Google Scholar]
- [33].Laakso MP, Partanen K, Riekkinen P, Lehtovirta M, Helkala EL, Hallikainen M, Hanninen T, Vainio P, Soininen H. Hippocampal volumes in Alzheimer’s disease, Parkinson’s disease with and without dementia, and in vascular dementia: An MRI study. Neurology. 1996;46:678–681. doi: 10.1212/wnl.46.3.678. [DOI] [PubMed] [Google Scholar]
- [34].De Leon MJ, Convit A, de Santi S, Bobinski M. Structural neuroimaging: Eearly diagnosis and staging of Alzheimer’s disease. Alzheimer’s Disease and Related Disorders. 1999;14:105–126. [Google Scholar]
- [35].Schelten P, Fox N, Barkhof F, De Carli C. Structural magnetic resonance imaging in the practical assessment of dementia: beyond exclusion. Lancet Neurol. 2002;1:13–21. doi: 10.1016/s1474-4422(02)00002-9. [DOI] [PubMed] [Google Scholar]
- [36].van Dijk EJ, Prins ND, Vermeer SE, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. C-reactive protein and cerebral small-vessel disease: the Rotterdam Scan Study. Circulation. 2005;112:900–905. doi: 10.1161/CIRCULATIONAHA.104.506337. [DOI] [PubMed] [Google Scholar]
- [37].Jefferson AL, Massaro JM, Wolf PA, Seshadri S, Au R, Vasan RS, Larson MG, Meigs JB, Keaney JF, Jr, Lipinska I, Kathiresan S, Benjamin EJ, DeCarli C. Inflammatory biomarkers are associated with total brain volume: the Framingham Heart Study. Neurology. 2007;68:1032–1038. doi: 10.1212/01.wnl.0000257815.20548.df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hoshi T, Kitagawa K, Yamagami H, Furukado S, Hougaku H, Hori M. Relations of serum high-sensitivity C-reactive protein and interleukin-6 levels with silent brain infarction. Stroke. 2005;36:768–772. doi: 10.1161/01.STR.0000158915.28329.51. [DOI] [PubMed] [Google Scholar]
- [39].Wright CB, Moon Y, Paik MC, Brown TR, Rabbani L, Yoshita M, DeCarli C, Sacco R, Elkind MS. Inflammatory biomarkers of vascular risk as correlates of leukoariosis. Stroke. 2009;40:3466–3471. doi: 10.1161/STROKEAHA.109.559567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wada M, Nagasawa H, Kurita K, Koyama S, Arawaka S, Kawanami T, Tajima K, Daimon M, Kato T. Cerebral small vessel disease and C-reactive protein: results of a cross-sectional study in community-based Japanese elderly. J Neurol Sci. 2008;264:43–49. doi: 10.1016/j.jns.2007.06.053. [DOI] [PubMed] [Google Scholar]
- [41].Fornage M, Chiang YA, O’Meara ES, Psaty BM, Reiner AP, Siscovick DS, Tracy RP, Longstreth WT., Jr Biomarkers of Inflammation and MRI-Defined Small Vessel Disease of the Brain: The Cardiovascular Health Study. Stroke. 2008;39:1952–1959. doi: 10.1161/STROKEAHA.107.508135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yoshida M, Higashi K, Kobayashi E, Saeki N, Wakui K, Kusaka T, Takizawa H, Kashiwado K, Suzuki N, Fukuda K, Nakamura T, Watanabe S, Tada K, Machi Y, Mizoi M, Toida T, Kanzaki T, Tomitori H, Kashiwagi K, Igarashi K. Correlation between images of silent brain infarction, carotid atherosclerosis and white matter hyperintensity, and plasma levels of acrolein, IL-6 and CRP. Atherosclerosis. 2010;211:475–479. doi: 10.1016/j.atherosclerosis.2010.03.031. [DOI] [PubMed] [Google Scholar]
- [43].Baune BT, Ponath G, Rothermundt M, Roesler A, Berger K. Association between cytokines and cerebral MRI changes in the aging brain. J Geriatr Psychiatry Neurol. 2009;22:23–34. doi: 10.1177/0891988708328216. [DOI] [PubMed] [Google Scholar]
- [44].Kuo HK, Lipsitz LA. Cerebral white matter changes and geriatric syndromes: is there a link? J Gerontol A Biol Sci Med Sci. 2004;59:818–826. doi: 10.1093/gerona/59.8.m818. [DOI] [PubMed] [Google Scholar]
- [45].Awad IA, Johnson PC, Spetzler RF, Hodak JA. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. II. Postmortem pathological correlations. Stroke. 1986;17:1090–1097. doi: 10.1161/01.str.17.6.1090. [DOI] [PubMed] [Google Scholar]
- [46].Fazekas F, Schmidt R, Scheltens P. Pathophysiologic mechanisms in the development of age-related white matter changes of the brain. Dement Geriatr Cogn Disord. 1998;(Suppl 1):2–5. doi: 10.1159/000051182. [DOI] [PubMed] [Google Scholar]
- [47].Marshall VG, Bradley WG, Jr, Marshall CE, Bhoopat T, Rhodes RH. Deep white matter infarction: correlation of MR imaging and histopathologic findings. Radiology. 1988;167:517–522. doi: 10.1148/radiology.167.2.3357964. [DOI] [PubMed] [Google Scholar]
- [48].Pantoni LaJHG. Pathogenesis of leukoaraiosis: a review Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- [49].Peters A. The effects of normal aging on myelin and nerve fibers: A review. J Neurocytology. 2002;31:581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- [50].Raz NHoAaC. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. Handbook of Aging and Cognition. Erlbaum; Mahwah, NJ: 2000. pp. 1–90. [Google Scholar]
- [51].Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, Putcha D, Fischl B, Greve DN, Marshall GA, Salloway S, Marks D, Buckner RL, Sperling RA, Johnson KA. Amyloid-β associated cortical thinning in clinically normal elderly. Ann Neurol. 2010 doi: 10.1002/ana.22333. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Oh H, Mormino EC, Madison C, Hayenga A, Smiljic A, Jagust WJ. β-Amyloid affects frontal and posterior brain networks in normal aging. Neuroimage. 2011;54:1887–1895. doi: 10.1016/j.neuroimage.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kotur-Stevuljevic J, Memon L, Stefanovic A, Spasic S, Spasojevic-Kalimanovska V, Bogavac-Stanojevic N, Kalimanovska-Ostric D, Jelic-Ivanovic Z, Zunic G. Correlation of oxidative stress parameters and inflammatory markers in coronary artery disease patients. Clin Biochem. 2007;40:181–187. doi: 10.1016/j.clinbiochem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- [54].Rovaris M, Iannucci G, Cercignani M, Sormani MP, De Stefano N, Gerevini S, Comi G, Filippi M. Age-related changes in conventional, magnetization transfer, and diffusion-tensor MR imaging findings: study with whole-brain tissue histogram analysis. Radiology. 2003;227:731–738. doi: 10.1148/radiol.2273020721. [DOI] [PubMed] [Google Scholar]
- [55].Benedetti B, Charil A, Rovaris M, Judica E, Valsasina P, Sormani MP, Filippi M. Influence of aging on brain gray and white matter changes assessed by conventional, MT, and DT MRI. Neurology. 2006;66:535–539. doi: 10.1212/01.wnl.0000198510.73363.c6. [DOI] [PubMed] [Google Scholar]
- [56].Venkatraman V, Aizenstein HJ, Newman AB, Yaffe K, Harris T, Kritchevski S, Hayonayon HN, Rosano C. Maintaining cognition in old age is associated with cingulate cortex microstructure. Frontiers in Neuroscience. 2011 In Press. [Google Scholar]
- [57].Kenne E, Lindbom L. Imaging inflammatory plasma leakage in vivo. Thromb Haemost. 2010;105:783–789. doi: 10.1160/TH10-10-0635. [DOI] [PubMed] [Google Scholar]
- [58].Sibson NR, Anthony DC, van Kasteren S, Dickens A, Perez-Balderas F, McAteer MA, Choudhury RP, Davis BG. Molecular MRI approaches to the detection of CNS inflammation. Methods Mol Biol. 2011;711:379–396. doi: 10.1007/978-1-61737-992-5_19. [DOI] [PubMed] [Google Scholar]
- [59].Sibson NR, Blamire AM, Bernades-Silva M, Laurent S, Boutry S, Muller RN, Styles P, Anthony DC. MRI detection of early endothelial activation in brain inflammation. Magn Reson Med. 2004;51:248–252. doi: 10.1002/mrm.10723. [DOI] [PubMed] [Google Scholar]
- [60].McAteer MA, von Zur Muhlen C, Anthony DC, Sibson NR, Choudhury RP. Magnetic resonance imaging of brain inflammation using microparticles of iron oxide. Methods Mol Biol. 2011;680:103–115. doi: 10.1007/978-1-60761-901-7_7. [DOI] [PubMed] [Google Scholar]
- [61].Jin A, Tuor UI, Rushforth D, Filfil R, Kaur J, Ni F, Tomanek B, Barber PA. Magnetic resonance molecular imaging of post-stroke neuroinflammation with a P-selectin targeted iron oxide nanoparticle. Contrast Media Mol Imaging. 2009;4:305–311. doi: 10.1002/cmmi.292. [DOI] [PubMed] [Google Scholar]
- [62].Butler T, Ichise M, Teich AF, Gerard E, Osborne J, French J, Devinsky O, Kuzniecky R, Gilliam F, Pervez F, Provenzano F, Goldsmith S, Vallabhajosula S, Stern E, Silbersweig D. Imaging Inflammation in a Patient with Epilepsy Due to Focal Cortical Dysplasia. J Neuroimaging. 2011 doi: 10.1111/j.1552-6569.2010.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, Jones T, Banati RB. In-vivo measurement of activated microglia in dementia. Lancet. 2001;358:461–467. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- [64].Christoforidis GA, Yang M, Kontzialis MS, Larson DG, Abduljalil A, Basso M, Yang W, Ray-Chaudhury A, Heverhagen J, Knopp MV, Barth RF. High resolution ultra high field magnetic resonance imaging of glioma microvascularity and hypoxia using ultra-small particles of iron oxide. Invest Radiol. 2009;44:375–383. doi: 10.1097/RLI.0b013e3181a8afea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Rohani R, de Chickera SN, Willert C, Chen Y, Dekaban GA, Foster PJ. In Vivo Cellular MRI of Dendritic Cell Migration Using Micrometer-Sized Iron Oxide (MPIO) Particles. Mol Imaging Biol. 2010 doi: 10.1007/s11307-010-0403-0. [DOI] [PubMed] [Google Scholar]
- [66].Tysiak E, Asbach P, Aktas O, Waiczies H, Smyth M, Schnorr J, Taupitz M, Wuerfel J. Beyond blood brain barrier breakdown - in vivo detection of occult neuroinflammatory foci by magnetic nanoparticles in high field MRI. J Neuroinflammation. 2009;6:20. doi: 10.1186/1742-2094-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Versijpt J, Debruyne JC, Van Laere KJ, De Vos F, Keppens J, Strijckmans K, Achten E, Slegers G, Dierckx RA, Korf J, De Reuck JL. Microglial imaging with positron emission tomography and atrophy measurements with magnetic resonance imaging in multiple sclerosis: a correlative study. Mult Scler. 2005;11:127–134. doi: 10.1191/1352458505ms1140oa. [DOI] [PubMed] [Google Scholar]
- [68].Saleh A, Schroeter M, Jonkmanns C, Hartung HP, Modder U, Jander S. In vivo MRI of brain inflammation in human ischaemic stroke. Brain. 2004;127:1670–1677. doi: 10.1093/brain/awh191. [DOI] [PubMed] [Google Scholar]
- [69].Tang TY, Howarth SP, Miller SR, Graves MJ, Patterson AJ, JM UK-I, Li ZY, Walsh SR, Brown AP, Kirkpatrick PJ, Warburton EA, Hayes PD, Varty K, Boyle JR, Gaunt ME, Zalewski A, Gillard JH. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol. 2009;53:2039–2050. doi: 10.1016/j.jacc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- [70].Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- [71].de la Torre JC. Impaired cerebromicrovascular perfusion. Summary of evidence in support of its causality in Alzheimer’s disease. Ann N Y Acad Sci. 2000;924:136–152. doi: 10.1111/j.1749-6632.2000.tb05572.x. [DOI] [PubMed] [Google Scholar]
- [72].Wu M, Rosano C, Butters M, Whyte E, Nable M, Crooks R, Meltzer CC, Reynolds CF, 3rd, Aizenstein HJ. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006;148:133–142. doi: 10.1016/j.pscychresns.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bhat NR. Linking cardiometabolic disorders to sporadic Alzheimer’s disease: a perspective on potential mechanisms and mediators. J Neurochem. 2010;115:551–562. doi: 10.1111/j.1471-4159.2010.06978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].den Heijer T, Launer LJ, Prins ND, van Dijk EJ, Vermeer SE, Hofman A, Koudstaal PJ, Breteler MM. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology. 2005;64:263–267. doi: 10.1212/01.WNL.0000149641.55751.2E. [DOI] [PubMed] [Google Scholar]
- [75].Dufouil C, Chalmers J, Coskun O, Besançon V, Bousser M, Guillon P, MacMahon S, Mazoyer B, Neal B, Woodward M, Tzourio-Mazoyer N, Tzourio C. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation. 2005;112:1644–1650. doi: 10.1161/CIRCULATIONAHA.104.501163. [DOI] [PubMed] [Google Scholar]
- [76].Dufouil C, de Kersaint-Gilly A, Besancon V, Levy C, Auffray E, Brunnereau L, Alperovitch A, Tzourio C. Longitudinal study of blood pressure and white matter hyperintensities: the EVA MRI Cohort. Neurology. 2001;56:921–926. doi: 10.1212/wnl.56.7.921. [DOI] [PubMed] [Google Scholar]
- [77].Wiseman RM, Saxby BK, Burton EJ, Barber R, Ford GA, O’Brien JT. Hippocampal atrophy, whole brain volume, and white matter lesions in older hypertensive subjects. Neurology. 2004;63:1892–1897. doi: 10.1212/01.wnl.0000144280.59178.78. [DOI] [PubMed] [Google Scholar]
- [78].Momijan-mayor IaJCB. The pathophysiology of watershed infarction in internal carotid artery disease: review of cerebral perfusion studies. Stroke. 2005;36:567–577. doi: 10.1161/01.STR.0000155727.82242.e1. [DOI] [PubMed] [Google Scholar]
- [79].Vasilevko V, Passos GF, Quiring D, Head E, Kim RC, Fisher M, Cribbs DH. Aging and cerebrovascular dysfunction: contribution of hypertension, cerebral amyloid angiopathy, and immunotherapy. Ann N Y Acad Sci. 2010;1207:58–70. doi: 10.1111/j.1749-6632.2010.05786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lee AY, Jeong SH, Choi BH, Sohn EH, Chui H. Pulse pressure correlates with leukoaraiosis in Alzheimer disease. Arch Gerontol Geriatr. 2006;42:157–166. doi: 10.1016/j.archger.2005.06.009. [DOI] [PubMed] [Google Scholar]
- [81].Jongen C, Biessels GJ. Structural brain imaging in diabetes: a methodological perspective. European journal of pharmacology. 2008;585:208–218. doi: 10.1016/j.ejphar.2007.11.085. [DOI] [PubMed] [Google Scholar]
- [82].van Harten B, Oosterman JM, Potter van Loon BJ, Scheltens P, Weinstein HC. Brain lesions on MRI in elderly patients with type 2 diabetes mellitus. European neurology. 2007;57:70–74. doi: 10.1159/000098054. [DOI] [PubMed] [Google Scholar]
- [83].Brands AM, Biessels GJ, Kappelle LJ, de Haan EH, de Valk HW, Algra A, Kessels RP. Cognitive functioning and brain MRI in patients with type 1 and type 2 diabetes mellitus: a comparative study. Dementia and geriatric cognitive disorders. 2007;23:343–350. doi: 10.1159/000100980. [DOI] [PubMed] [Google Scholar]
- [84].McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment Effects on Adult Cognitive Development: Can the Functional Capacity of Older Adults Be Preserved and Enhanced? Psychological Science in the Public Interest. 2009;9:1–65. doi: 10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- [86].Bermudez EA, Rifai N, Buring J, Manson JE, Ridker PM. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arteriosclerosis, Thrombosis & Vascular Biology. 2002;22:1668–1673. doi: 10.1161/01.atv.0000029781.31325.66. [DOI] [PubMed] [Google Scholar]
- [87].Cournot M, Marquié JC, Ansiau D, Martinaud C, Fonds H, Ferrières J, Ruidavets JB.2006Relation between body mass index and cognitive function in healthy middle-aged men and women Neurology67 1208–1214. [DOI] [PubMed] [Google Scholar]
- [88].Gunstad J, Paul RH, Cohen RA, Tate DF, Gordon E. Obesity is associated with memory deficits in young and middle-aged adults. Eating & Weight Disorders. 2006;11:e15–19. doi: 10.1007/BF03327747. [DOI] [PubMed] [Google Scholar]
- [89].Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease Archives of Internal Medicine. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- [90].Gustafson D, Lissner L, Bengtsson C, Bjorkelund C, Skoog I.2004A 24-year follow-up of body mass index and cerebral atrophy Neurology63 1876–1881. [DOI] [PubMed] [Google Scholar]
- [91].Deary IJ, Bastin ME, Pattie A, Clayden JD, Whalley LJ, Starr JM, Wardlaw JM. White matter integrity and cognition in childhood and old age. Neurology. 2006;66:505–512. doi: 10.1212/01.wnl.0000199954.81900.e2. [DOI] [PubMed] [Google Scholar]
- [92].Oberheiden T, Blahak C, Nguyen XD, Fatar M, Elmas E, Morper N, Dempfle CE, Bazner H, Hennerici M, Borggrefe M, Kalsch T. Activation of platelets and cellular coagulation in cerebral small-vessel disease. Blood Coagul Fibrinolysis. 21:729–735. doi: 10.1097/MBC.0b013e328340147c. [DOI] [PubMed] [Google Scholar]
- [93].Gerges NZ, Aleisa AM, Alkadhi KA. Impaired long-term potentiation in obese zucker rats: possible involvement of presynaptic mechanism. Neuroscience. 2003:120. doi: 10.1016/s0306-4522(03)00297-5. [DOI] [PubMed] [Google Scholar]
- [94].Khaodhiar L, Ling PR, Blackburn GL, Bistrian BR. Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. Journal of Parenteral & Enteral Nutrition. 2004;28:410–415. doi: 10.1177/0148607104028006410. [DOI] [PubMed] [Google Scholar]
- [95].Marsland AL, Petersen KL, Sathanoori R, Muldoon MF, Neumann SA, Ryan C, et al. Interleukin-6 covaries inversely with cognitive performance among middle-aged community volunteers Psychosom Med. 2006;68:895–903. doi: 10.1097/01.psy.0000238451.22174.92. [DOI] [PubMed] [Google Scholar]
- [96].Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 2008;64:484–490. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Drake C, Boutin H, Jones MS, Denes A, McColl BW, Selvarajah JR, Hulme S, Georgiou RF, Hinz R, Gerhard A, Vail A, Prenant C, Julyan P, Maroy R, Brown G, Smigova A, Herholz K, Kassiou M, Crossman D, Francis S, Proctor SD, Russell JC, Hopkins SJ, Tyrrell PJ, Rothwell NJ, Allan SM. Brain inflammation is induced by co-morbidities and risk factors for stroke. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Bassuk SS, Glass TA, Berkman LF. Social disengagement and incident cognitive decline in community-dwelling elderly persons. Ann Intern Med. 1999;131:165–173. doi: 10.7326/0003-4819-131-3-199908030-00002. [DOI] [PubMed] [Google Scholar]
- [99].Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63:2322–2326. doi: 10.1212/01.wnl.0000147473.04043.b3. [DOI] [PubMed] [Google Scholar]
- [100].Lovden M, Ghisletta P, Lindenberger U. Social participation attenuates decline in perceptual speed in old and very old age Psychol Aging. 2005;20:423–434. doi: 10.1037/0882-7974.20.3.423. [DOI] [PubMed] [Google Scholar]
- [101].Zunzunegui MV, Alvarado BE, Del Ser T, Otero A. Social networks social integration and social engagement determine cognitive decline in community-dwelling Spanish older adults. J Gerontol B Psychol Sci Soc Sci. 2003;58:S93–S100. doi: 10.1093/geronb/58.2.s93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ertel KA, Glymour MM, Berkman LF. Effects of social integration on preserving memory function in a nationally representative US elderly population. Am J Public Health. 2008;98:1215–1220. doi: 10.2105/AJPH.2007.113654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol. 2006;101:1237–1242. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- [104].Kramer AF, Hahn S, Cohen NJ, Banich MTM, E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau R, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- [105].Weiss N, Miller F, Cazaubon S, Couraud PO. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta. 2009;1788:842–857. doi: 10.1016/j.bbamem.2008.10.022. [DOI] [PubMed] [Google Scholar]
- [106].Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear relations of blood pressure to cognitive function: the Baltimore Longitudinal Study of Aging. Hypertension. 2005;45:374–379. doi: 10.1161/01.HYP.0000156744.44218.74. [DOI] [PubMed] [Google Scholar]
- [107].Forette F, Seux ML, Staessen JA, Thijs L, Birkenhager WH, Babarskiene MR, Babeanu S, Bossini A, et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;52:1347–1351. doi: 10.1016/s0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- [108].Applegate WB, Pressel S, Wittes J, Luhr J, Shekelle RB, Camel GH, Greenlick MR, Hadley E, Moye L, Perry HM., Jr Impact of the treatment of isolated systolic hypertension on behavioral variables. Arch. Intern. Med. 1994;154:2154–2160. [PubMed] [Google Scholar]
- [109].Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annual review of psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Rosano C, Venkatraman VK, Guralnik J, Newman AB, Glynn NW, Launer L, Taylor CA, Williamson J, Studenski S, Pahor M, Aizenstein HJ. Psychomotor speed and functional brain MRI 2 years after completing a physical activity treatment. J Gerontol A Biol Sci Med Sci. 2010;65:639–647. doi: 10.1093/gerona/glq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wojcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].De Leeuw FE, De Groot JC, Oudkerk M, Witteman JC, Hofman A, Van Gijn J, Breteler MM. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125:765–772. doi: 10.1093/brain/awf077. [DOI] [PubMed] [Google Scholar]
- [113].Heinecke J. Sources of vascular axidative stress. Kluwer Academic Publishers; Boston: 1999. [Google Scholar]
- [114].Chung HY, Sung B, Jung KJ, Zou Y, Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8:572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- [115].Kotur-Stevuljevic J, Memon L, Stefanovic A, Spasic S, Spasojevic-Kalimanovska V, Bogavac-Stanojevic N, Kalimanovska-Ostric D, Jelic-Ivanovic Z, Zunic G. Correlation of oxidative stress parameters and inflammatory markers in coronary artery disease patients. Clinical Biochemistry. 2007;40:181–187. doi: 10.1016/j.clinbiochem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- [116].Dohi Y, Takase H, Sato K, Ueda R. Association among C-reactive protein, oxidative stress, and traditional risk factors in healthy Japanese subjects. Int J Cardiol. 2007;115:63–66. doi: 10.1016/j.ijcard.2006.04.006. [DOI] [PubMed] [Google Scholar]
- [117].Abramson JL, Hooper WC, Jones DP, Ashfaq S, Rhodes SD, Weintraub WS, Harrison DG, Quyyumi AA, Vaccarino V. Association between novel oxidative stress markers and C-reactive protein among adults without clinical coronary heart disease. Atherosclerosis. 2005;178:115–121. doi: 10.1016/j.atherosclerosis.2004.08.007. [DOI] [PubMed] [Google Scholar]
- [118].Meda L, Cassatella MA, Szendrei GI, Otvos L, Jr, Baron P, Villalba M, Ferrari D, Rossi F. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- [119].Rogers J, Strohmeyer R, Kovelowski CJ, Li R. Microglia and inflammatory mechanisms in the clearance of amyloid beta peptide. Glia. 2002;40:260–269. doi: 10.1002/glia.10153. [DOI] [PubMed] [Google Scholar]
- [120].Barrientos RM, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, et al. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience. 2003;121:847–853. doi: 10.1016/s0306-4522(03)00564-5. [DOI] [PubMed] [Google Scholar]
- [121].Minghetti L. Role of inflammation in neurodegenerative diseases. Curr Opin Neurol. 2005;18:315–321. doi: 10.1097/01.wco.0000169752.54191.97. [DOI] [PubMed] [Google Scholar]
- [122].Banks WA. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des. 2005;11:973–984. doi: 10.2174/1381612053381684. [DOI] [PubMed] [Google Scholar]
- [123].Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- [124].Banks WA, Farr SA, Morley JE. Entry of blood-borne cytokines into the central nervous system: effects on cognitive processes. Neuroimmuno-modulation. 2002;10:319–327. doi: 10.1159/000071472. [DOI] [PubMed] [Google Scholar]
- [125].Stamatovic SM, Keep RF, Andjelkovic AV. Brain endothelial cell-cell junctions: how to “open” the blood brain barrier. Curr Neuropharmacol. 2008;6:179–192. doi: 10.2174/157015908785777210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Black PH, Garbutt LD. Stress, inflammation, and cardiovascular disease. J Psychosom Res. 2002;52:1–23. doi: 10.1016/s0022-3999(01)00302-6. [DOI] [PubMed] [Google Scholar]
- [128].Gadient RA, Otten U.1994Expression of interleukin-6 (IL-6) and interleukin-6 receptor (IL-6R) mRNAs in rat brain during postnatal development Brain Research637 10–14. [DOI] [PubMed] [Google Scholar]
- [129].Schobitz B, De Kloet ER, Holsboer F. Gene expression and function of interleukin 1, interleukin 6 and tumor necrosis factor in the brain. Progress in Neurobiology. 1994;44:397–432. doi: 10.1016/0301-0082(94)90034-5. [DOI] [PubMed] [Google Scholar]
- [130].Vitkovic L, Konsman JP, Bockaert J, Dantzer R, Homburger V, Jacque C. Cytokine signals propagate through the brain. Molecular Psychiatry. 2000;5:604–615. doi: 10.1038/sj.mp.4000813. [DOI] [PubMed] [Google Scholar]
- [131].Van Wagoner NJ, Benveniste EN. Interleukin-6 expression and regulation in astrocytes. J Neuroimmunol. 1999;100:124–139. doi: 10.1016/s0165-5728(99)00187-3. [DOI] [PubMed] [Google Scholar]
- [132].Gradient R, Otten U. Expression of interleukin-6 (IL-6) and interleukin-6 receptor (IL-6R) mRNAs in rat brain during postnatal development. Brain Research. 1994:637. doi: 10.1016/0006-8993(94)91211-4. [DOI] [PubMed] [Google Scholar]
- [133].McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- [134].Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22:486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Montaner J, Rovira A, Molina CA, Arenillas JF, Ribo M, Chacon P, Monasterio J, Alvarez-Sabin J. Plasmatic level of neuroinflammatory markers predict the extent of diffusion-weighted image lesions in hyperacute stroke. J Cereb Blood Flow Metab. 2003;23:1403–1407. doi: 10.1097/01.WCB.0000100044.07481.97. [DOI] [PubMed] [Google Scholar]
- [136].Takano T, Ohno M, Takeuchi Y, Mandai R, Yoshida S. Cytotoxic edema and interleukin-6 in hypertensive encephalopathy. Pediatr Neurol. 2002;26:71–73. doi: 10.1016/s0887-8994(01)00345-9. [DOI] [PubMed] [Google Scholar]
- [137].Tong Y, Wang Z, Geng Y, Liu J, Zhang R, Lin Q, Li X, Huang D, Gao S, Hu D, Li Y, Cheng J, Lu Z. The association of functional polymorphisms of IL-6 gene promoter with ischemic stroke: analysis in two Chinese populations. Biochem Biophys Res Commun. 2010;391:481–485. doi: 10.1016/j.bbrc.2009.11.084. [DOI] [PubMed] [Google Scholar]
- [138].Ishikawa J, Tamura Y, Hoshide S, Eguchi K, Ishikawa S, Shimada K, Kario K. Low-grade inflammation is a risk factor for clinical stroke events in addition to silent cerebral infarcts in Japanese older hypertensives: the Jichi Medical School ABPM Study, wave 1. Stroke. 2007;38:911–917. doi: 10.1161/01.STR.0000258115.46765.f1. [DOI] [PubMed] [Google Scholar]
- [139].Nakase T, Yamazaki T, Ogura N, Suzuki A, Nagata K. The impact of inflammation on the pathogenesis and prognosis of ischemic stroke. J Neurol Sci. 2008;271:104–109. doi: 10.1016/j.jns.2008.03.020. [DOI] [PubMed] [Google Scholar]
- [140].Waje-Andreassen U, Krakenes J, Ulvestad E, Thomassen L, Myhr KM, Aarseth J, Vedeler CA. IL-6: an early marker for outcome in acute ischemic stroke. Acta Neurol Scand. 2005;111:360–365. doi: 10.1111/j.1600-0404.2005.00416.x. [DOI] [PubMed] [Google Scholar]
- [141].Kuriyama N, Mizuno T, Kita M, Nagakane Y, Hosomi A, Harada S, Takeda K, Ozasa K, Yamada K, Tokuda T, Watanabe Y, Nakagawa M. Predictive markers of blood cytokine and chemokine in recurrent brain infarction. J Interferon Cytokine Res. 2009;29:729–734. doi: 10.1089/jir.2009.0012. [DOI] [PubMed] [Google Scholar]
- [142].Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur Studies of Successful Aging. Neurology. 2002;59:371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- [143].Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, Culliford D, Perry VH. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73:768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Yasojima K, Schwab C, McGeer EG, McGeer PL. Human neurons generate C-reactive protein and amyloid P: upregulation in Alzheimer’s disease. Brain Res. 2000;887:80–89. doi: 10.1016/s0006-8993(00)02970-x. [DOI] [PubMed] [Google Scholar]
- [145].Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T.2003Inflammatory markers and cognition in well-functioning African-American and white elders Neurology61 76–80. [DOI] [PubMed] [Google Scholar]
- [146].D’Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factor α signaling during peripheral organ inflammation. J Neurosci Res. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Emmer BJ, van der Bijl AE, Huizinga TW, Breedveld FC, Steens SC, Th Bosma GP, van Buchem MA, van der Grond J. Brain involvement in rheumatoid arthritis: a magnetic resonance spectroscopy study. Arthritis Rheum. 2009;60:3190–3195. doi: 10.1002/art.24932. [DOI] [PubMed] [Google Scholar]
- [148].Mangiafico RA, Sarnataro F, Mangiafico M, Fiore CE. Impaired cognitive performance in asymptomatic peripheral arterial disease: relation to C-reactive protein and D-dimer levels. Age Ageing. 2006:35. doi: 10.1093/ageing/afi219. [DOI] [PubMed] [Google Scholar]
- [149].Baysal T, Ozisik HI, Karlidag R, Sarac K, Baysal O, Dusak A, et al. 2003Proton MRS in Behçet’s disease with and without neurological findings Neuroradiology45 860–864. [DOI] [PubMed] [Google Scholar]
- [150].Axford JS, Howe FA, Heron C, Griffiths JR. Sensitivity of quantitative 1H magnetic resonance spectroscopy of the brain in detecting early neuronal damage in systemic lupus erythematosus. Ann Rheum Dis. 2001;60:106–111. doi: 10.1136/ard.60.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Castellino G, Govoni M, Padovan M, Colamussi P, Borrelli M, Trotta F. Proton magnetic resonance spectroscopy may predict future brain lesions in SLE patients: a functional multi-imaging approach and follow up. Ann Rheum Dis. 2005;64:1022–1027. doi: 10.1136/ard.2004.026773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Skevington SM. Psychological aspects of pain in rheumatoid arthritis: a review. Soc Sci Med. 1986;23:567–575. doi: 10.1016/0277-9536(86)90150-4. [DOI] [PubMed] [Google Scholar]
- [153].Van der Bijl AE, Emmer BJ, Breedveld FC, Middelkoop HA, Jurgens CK, van Buchem MA, et al. Advanced magnetic resonance imaging of the brain in patients treated with TNF-α blocking agents. Clin Exp Rheumatol. 2007;25:301–304. [PubMed] [Google Scholar]
- [154].Mcgeer PL, Schulzer M, Mcgeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studies. Neurology. 1996;47:425–432. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- [155].Lleo A, Galea E, Sastre M. Molecular targets of non-steroidal anti-inflammatory drugs in neurodegenerative diseases. Cell Mol Life Sci. 2007;64:1403–1418. doi: 10.1007/s00018-007-6516-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Klegeris A, McGeer PL. Non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory agents in the treatment of neurodegenerative disease. Curr Alzheimer Res. 2005;2:355–365. doi: 10.2174/1567205054367883. [DOI] [PubMed] [Google Scholar]
- [157].Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 2008;63:1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav Immun. 2005;19:345–350. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- [160].Belanger M, Allaman I, Magistretti PJ. Differential effects of pro- and anti-inflammatory cytokines alone or in combinations on the metabolic profile of astrocytes. J Neurochem. 2011;116:564–576. doi: 10.1111/j.1471-4159.2010.07135.x. [DOI] [PubMed] [Google Scholar]
- [161].Hess A, Axmann R, Rech J, Finzel S, Heindl C, Kreitz S, Sergeeva M, Saake M, Garcia M, Kollias G, Straub RH, Sporns O, Doerfler A, Brune K, Schett G. Blockade of TNF-alpha rapidly inhibits pain responses in the central nervous system. Proc Natl Acad Sci U S A. 2011;108:3731–3736. doi: 10.1073/pnas.1011774108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Eijsbouts AM, Kempers MJ, van den Hoogen FH, Laan RF, Hermus AR, Ross HA, Sweep FC, van de Putte LB. Effects of naproxen and sulphasalazine or methotrexate on hypothalamic-pituitary-adrenal axis activity in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2011;29:35–42. [PubMed] [Google Scholar]
- [163].Licastro F, Pedrini S, Caputo L, Annoni G, Davis LJ, Ferri C, Casadei V, Grimaldi LM.2000Increased plasma levels of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin in patients with Alzheimer’s disease: peripheral inflammation or signals from the brain? Journal of Neuroimmunology103 97–102. [DOI] [PubMed] [Google Scholar]
- [164].O’Connor MF, Irwin MR. Links between behavioral factors and inflammation. Clin Pharmacol Ther. 2010;87:479–482. doi: 10.1038/clpt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clin Chim Acta. 2010;411:785–793. doi: 10.1016/j.cca.2010.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Warnberg J, Cunningham K, Romeo J, Marcos A. Physical activity, exercise and low-grade systemic inflammation. Proc Nutr Soc. 2010;69:400–406. doi: 10.1017/S0029665110001928. [DOI] [PubMed] [Google Scholar]
- [167].Teixeira-Lemos E, Nunes S, Teixeira F, Reis F. Regular physical exercise training assists in preventing type 2 diabetes development: focus on its antioxidant and anti-inflammatory properties. Cardiovasc Diabetol. 2011;10:12. doi: 10.1186/1475-2840-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Walsh NP, Gleeson M, Shephard RJ, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P. Position statement. Part one: Immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- [169].Packer N, Pervaiz N, Hoffman-Goetz L. Does exercise protect from cognitive decline by altering brain cytokine and apoptotic protein levels? A systematic review of the literature. Exerc Immunol Rev. 2010;16:138–162. [PubMed] [Google Scholar]
- [170].Corsonello A, Garasto S, Abbatecola AM, Rose G, Passarino G, Mazzei B, Pranno L, Guffanti EE, Bustacchini S, Lattanzio F. Targeting inflammation to slow or delay functional decline: where are we? Biogerontology. 2010;11:603–614. doi: 10.1007/s10522-010-9289-0. [DOI] [PubMed] [Google Scholar]
- [171].Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, Alves H, Heo S, Szabo AN, White SM, Wojcicki TR, Mailey EL, Gothe N, Olson EA, McAuley E, Kramer AF. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010:2. doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11:342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- [173].Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, Gach HM, Thompson PM, Ho AJ, Kuller LH. Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology. 2010;75:1415–1422. doi: 10.1212/WNL.0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Gates NJ, Valenzuela M, Sachdev PS, Singh NA, Baune BT, Brodaty H, Suo C, Jain N, Wilson GC, Wang Y, Baker MK, Williamson D, Foroughi N, Fiatarone Singh MA. Study of Mental Activity and Regular Training (SMART) in at risk individuals: A randomised double blind, sham controlled, longitudinal trial. BMC Geriatr. 2011:11–19. doi: 10.1186/1471-2318-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Espeland MA, Gill TM, Guralnik J, Miller ME, Fielding R, Newman AB, Pahor M, Lifestyle Interventions and Independence for Elders Study Group Designing clinical trials of interventions for mobility disability: results from the lifestyle interventions and independence for elders pilot (LIFE-P) trial. J Gerontol A Biol Sci Med Sci. 2007;62:1237–1243. doi: 10.1093/gerona/62.11.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Soreca I, Rosano C, Jennings JR, Sheu LK, Kuller LH, Matthews KA, Aizenstein HJ, Gianaros PJ. Gain in adiposity across 15 years is associated with reduced gray matter volume in healthy women. Psychosom Med. 2009;71:485–490. doi: 10.1097/PSY.0b013e3181a5429d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].van de Sande-Lee S, Pereira FR, Cintra DE, Fernandes PT, Cardoso AR, Garlipp CR, Chaim EA, Pareja JC, Geloneze B, Li LM, Cendes F, Velloso LA. Partial Reversibility of Hypothalamic Dysfunction and Changes in Brain Activity After Body Mass Reduction in Obese Subjects. Diabetes. 2011 doi: 10.2337/db10-1614. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Jang S, Johnson RW. Can consuming flavonoids restore old microglia to their youthful state? Nutr Rev. 2010;68:719–728. doi: 10.1111/j.1753-4887.2010.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- [181].O’Brien JS, Fillerup DL, Mead JF. Quantification and fatty acid and fatty aldehyde composition of ethanolamine, choline, and serine glycerophosphatides in human cerebral grey and white matter. J Lipid Res. 1964;5:329–338. [PubMed] [Google Scholar]
- [182].Jia Z, Pei Z, Maiguel D, Toomer CJ, Watkins PA. The fatty acid transport protein (FATP) family: very long chain acyl-CoA synthetases or solute carriers? J Mol Neurosci. 2007;33:25–31. doi: 10.1007/s12031-007-0038-z. [DOI] [PubMed] [Google Scholar]
- [183].Rapoport SI, Purdon D, Shetty HU, Grange E, Smith Q, Jones C, Chang MC. In vivo imaging of fatty acid incorporation into brain to examine signal transduction and neuroplasticity involving phospholipids. Ann N Y Acad Sci. 1997;820:56–73. doi: 10.1111/j.1749-6632.1997.tb46189.x. discussion 73–54. [DOI] [PubMed] [Google Scholar]
- [184].Tassoni D, Kaur G, Weisinger RS, Sinclair AJ. The role of eicosanoids in the brain. Asia Pac J Clin Nutr. 2008;17(Suppl 1):220–228. [PubMed] [Google Scholar]
- [185].Kiecolt-Glaser JK. Stress, food, and inflammation: psychoneuroimmunology and nutrition at the cutting edge. Psychosom Med. 2010;72:365–369. doi: 10.1097/PSY.0b013e3181dbf489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Ahmad A, Moriguchi T, Salem N. Decrease in neuron size in docosahexaenoic acid-deficient brain. Pediatr Neurol. 2002;26:210–218. doi: 10.1016/s0887-8994(01)00383-6. [DOI] [PubMed] [Google Scholar]
- [187].Wainwright PE, Bulman-Fleming MB, Levesque S, Mustsaers L, Mucheon D. A saturated fat diet during development alters dendritic growth in mouse brain. Nutr Neurosci. 1998;1:49–58. doi: 10.1080/1028415X.1998.11747212. [DOI] [PubMed] [Google Scholar]
- [188].Sakamoto T, Cansev M, Wurtman RJ. Oral supplementation with docosahexaenoic acid and uridine-5′-monophosphate increases dendritic spine density in adult gerbil hippocampus. Brain Res. 2007;1182:50–59. doi: 10.1016/j.brainres.2007.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Wurtman RJ, Ulus IH, Cansev M, Watkins CJ, Wang L, Marzloff G. Synaptic proteins and phospholipids are increased in gerbil brain by administering uridine plus docosahexaenoic acid orally. Brain Res. 2006;1088:83–92. doi: 10.1016/j.brainres.2006.03.019. [DOI] [PubMed] [Google Scholar]
- [190].Boudrault C, Bazinet RP, Ma DW. Experimental models and mechanisms underlying the protective effects of n-3 polyunsaturated fatty acids in Alzheimer’s disease. J Nutr Biochem. 2009;20:1–10. doi: 10.1016/j.jnutbio.2008.05.016. [DOI] [PubMed] [Google Scholar]
- [191].Moriguchi T, Greiner RS, Salem N., Jr Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J Neurochem. 2000;75:2563–2573. doi: 10.1046/j.1471-4159.2000.0752563.x. [DOI] [PubMed] [Google Scholar]
- [192].Rao JS, Ertley RN, Lee HJ, DeMar JC, Jr, Arnold JT, Rapoport SI, Bazinet RP. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry. 2007;12:36–46. doi: 10.1038/sj.mp.4001888. [DOI] [PubMed] [Google Scholar]
- [193].Conklin SM, Gianaros PJ, Brown SM, Yao JK, Hariri AR, Manuck SB, Muldoon MF. Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neurosci Lett. 2007;421:209–212. doi: 10.1016/j.neulet.2007.04.086. [DOI] [PubMed] [Google Scholar]
- [194].Freund-Levi Y, Hjorth E, Lindberg C, Cederholm T, Faxen-Irving G, Vedin I, Palmblad J, Wahlund LO, Schultzberg M, Basun H, Eriksdotter Jonhagen M. Effects of omega-3 fatty acids on inflammatory markers in cerebrospinal fluid and plasma in Alzheimer’s disease: the OmegAD study. Dement Geriatr Cogn Disord. 2009;27:481–490. doi: 10.1159/000218081. [DOI] [PubMed] [Google Scholar]
- [195].Harris W. Omega-3 Fatty Acids: The “Japanese” Factor? J Am Coll Cardiol. 2008;52:425–427. doi: 10.1016/j.jacc.2008.04.018. [DOI] [PubMed] [Google Scholar]
- [196].Csuka E, Morganti-Kossmann MC, Lenzlinger PM, Joller H, Trentz O, Kossmann T. IL-10 levels in cerebrospinal fluid and serum of patients with severe traumatic brain injury: relationship to IL-6, TNF-alpha, TGF-beta1 and blood-brain barrier function. J Neuroimmunol. 1999;101:211–221. doi: 10.1016/s0165-5728(99)00148-4. [DOI] [PubMed] [Google Scholar]
- [197].Sun YX, Minthon L, Wallmark A, Warkentin S, Blennow K, Janciauskiene S. Inflammatory markers in matched plasma and cerebrospinal fluid from patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2003;16:136–144. doi: 10.1159/000071001. [DOI] [PubMed] [Google Scholar]
- [198].Ayala A, Wang P, Ba ZF, Perrin MM, Ertel W, Chaudry IH. Differential alterations in plasma IL-6 and TNF levels after trauma and hemorrhage. Am J Physiol. 1991;260:R167–171. doi: 10.1152/ajpregu.1991.260.1.R167. [DOI] [PubMed] [Google Scholar]
- [199].Maier B, Laurer HL, Rose S, Buurman WA, Marzi I. Physiological levels of pro- and anti-inflammatory mediators in cerebrospinal fluid and plasma: a normative study. J Neurotrauma. 2005;22:822–835. doi: 10.1089/neu.2005.22.822. [DOI] [PubMed] [Google Scholar]


