Abstract
Influenza is an important contributor to morbidity and mortality worldwide. Accumulation of genetic mutations termed antigenic drift, allows influenza viruses to inflict yearly epidemics that may result in 250,000 to 500,000 deaths annually. Over 90% of influenza-related deaths occur in the older adult population. This is at least in part a result of increasing dysregulation of the immune system with age, termed immunosenescence. This dysregulation results in reduced capacity to cope with infections and decreased responsiveness to vaccination. The older adult population is in dire need of improved vaccines capable of eliciting protective responses in the face of a waning immune system. This review focuses on the status of immunity, responses to influenza vaccination, and strategies that are currently being explored to elicit enhanced immune responses in this high risk population.
Keywords: Aging, Influenza, Vaccination, Immune Response, Immunosenescence
Annual influenza epidemics are the leading cause of severe virus-induced respiratory disease in the older adult population [1,2]. Influenza epidemics cause three to five million cases of severe illness, and may lead to 250,000 to 500,000 deaths annually [3]. Influenza-associated morbidity and mortality disproportionately affects older adults; defined here as adults age 65 and older. Even in a population with a high rate of influenza vaccination, influenza infects 2–5% of older adults, resulting in 10- to 30-times more hospitalizations annually compared to younger individuals [1,2,4,5]. The average length of a hospital stay due to pneumonia and influenza increases from 5.8 days for those between the ages of 5 and 49, to over 8 days for those older than 65 [4]. The influenza-associated mortality rate for individuals 5–49 years of age is 0.2 deaths per 100,000 person-years, compared with 1.3 deaths for those aged 50–64 years [1]. The influenza-associated mortality rate for individuals 65 years and older is 22.1 deaths per 100,000 [1]. Influenza impacts the frail older adult population even more severely. Individuals older than 85 years of age are 16-times more likely to die of influenza-related illness and 32-times more likely to die of influenza-associated pneumonia than those between 65 and 69 years of age [1].
Effectiveness of Influenza Vaccination in Older Adults
Vaccine Efficacy in Older Adults
Vaccination remains the most cost-effective method currently available for reducing the morbidity and mortality associated with influenza infection. Several studies have been conducted which demonstrated the ability of influenza vaccines to stimulate significant immune responses in older adults compared to placebo [6,7] However, these studies only evaluated immune parameters, while protection from disease was not evaluated. During the 1991–1992 influenza season, Govaert et al. conducted the only large, published, randomized, placebo-controlled trial of influenza vaccines in older adults, examining vaccine effects on clinical outcomes [8]. This study estimated influenza vaccine efficacy to be 58% for the prevention of clinical influenza with serologic evidence of infection in relatively healthy, adults aged 60 years and older. This study also suggested that subjects who had received the influenza vaccine in previous years were better protected than first-time vaccinees [9]. However, placebo-controlled influenza vaccine trials in the aged population are no longer considered ethical, because the United States and other countries have recommended that all persons aged 65 years and older receive annual influenza vaccination. These recommendations have limited current evaluations of influenza vaccine effectiveness in older adults to observational studies that compare the relative reduction in influenza disease outcomes in those who elect to be vaccinated compared to those who are not vaccinated. This reliance on observational studies, all of which are subject to various forms of bias, has led to considerable controversy over the effectiveness of influenza vaccination in older adults. Differences in the analysis methodology, a limited number of subjects, and variability in influenza attack rates from season to season have resulted in variable and often conflicting conclusions [10–12]. While some studies claim a definite benefit from influenza vaccination of older adults, others question the benefit of vaccination, and point to a ‘healthy vaccine bias’ that may confound the results of many large analyses [13–15].
In an effort to overcome these potential problems, several studies have been performed with large numbers of subjects across multiple influenza seasons, aimed at assessing the effectiveness of influenza vaccination in older adults. Nichol et al. evaluated data from subjects aged 65 or over, across 10 influenza seasons from 1990 to 2000 [16]. This study pooled data from community-dwelling individuals from 18 cohorts across the United States. Influenza vaccination, in this study, was associated with a 27% reduction in the risk of hospitalization due to influenza, and a 48% reduction in the risk of death [16]. A similar study by the same group examined three large managed care organizations across two influenza seasons (1998–2000) resulting in similar estimates [17]. However, concern has been raised about the likelihood of a healthy vaccinee bias resulting in an overestimation of influenza vaccine effectiveness in older adults in large cohort studies of this type [13,14]. Recent analyses designed to control for unmeasured health factors potentially associated with both vaccination status and risk of mortality have recently been developed [18,19]. More studies of this type are needed to better differentiate vaccine effects for confounding, especially the healthy vaccinee bias, particularly in studies with a mortality outcome.
While considerable controversy exists over the benefit to influenza vaccination in older adults compared to unvaccinated, it is generally agreed that vaccine responses in older adults are significantly reduced compared to young, healthy adults. In young, healthy adults, influenza vaccine effectiveness have ranged from 47% to as high as 86% effective in reducing laboratory-confirmed illness, depending upon antigenic similarity between the vaccine and the circulating influenza strains [20–23]. In older adults, influenza vaccines have been estimated to be 17% to 53% effective at reducing pneumonia and influenza hospitalizations; however, these observational studies did not use laboratory-confirmed influenza illness as a criterion [16,24,25].
A quantitative review of studies by Goodwin et al. evaluated the antibody responses to influenza vaccination in older adults [24]. Of 4,492 vaccinated subjects, 42%, 51% and 35% of subjects seroconverted (defined as a 4-fold increase in antibody titers) to H1N1, H3N2 and influenza B, respectively, compared with 60%, 62% and 58%, of younger subjects (913 subjects), respectively. Responses in subjects ≥75 years of age were especially impaired with seroconversion (a 4-fold or greater increase in serum HI antibody titer) rates of 32%, 46% and 29%, respectively. Seroprotection (defined as hemagglutination inhibition (HI) titers ≥ 40) in older adults (4,643 subjects) occurred in 69%, 74%, and 67% to H1N1, H3N2 and influenza B, respectively, compared to 83%, 84%, and 78% of younger subjects (1,151 subjects). Seroprotection in subjects age 75 or older was demonstrated in 65%, 68%, and 71% of subjects to H1N1, H3N2 and influenza B, respectively [24]. It should be noted; however, that while the so-called seroprotective titer (HI titer ≥ 40) is a widely accepted immune correlate of protection, this standard was established in younger adults [26–29]. While both the HI titer of ≥ 40 and seroconversion parameters are commonly applied to influenza studies performed in older adults to evaluate immunogenicity, there is little evidence to suggest that achievements of HI titers ≥ 40 correlate with protection in this age group. On the contrary, there is evidence to suggest that antibody titers are poor correlates of protection in the aged; T cell responses appear to correlate better with immune protection to influenza in older adults [30,31]. Further studies examining immune correlates in the older population are greatly needed for influenza research in this age group.
Immunity in Aging
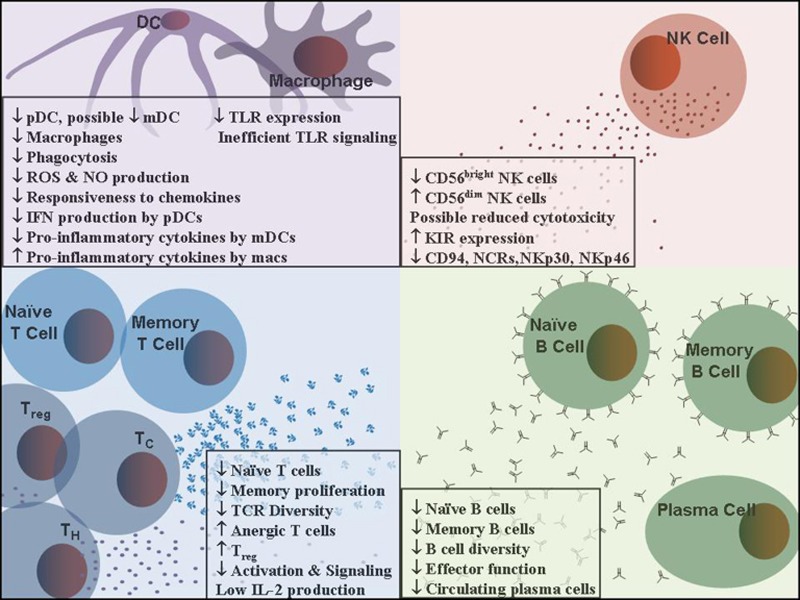
The impaired ability of older adults to develop effective immunity in response to influenza vaccines has been attributed to immunosenescence, defined as a decline in the body’s ability to fight infection, mount adequate protective immune responses, and develop immunological memory for future protection [32,33]. This age-related immunological dysfunction is summarized in Figure 1, and discussed in detail below.
Figure 1.
Immunosenesence of the aging immune system. The adult immune system becomes less responsive to vaccination, and less able to cope with infectious disease with age. Multiple arms of the immune system have been shown to develop age-related defects resulting in the loss or decreased effectiveness of key protective functions. Development of improved influenza vaccines for older adults must overcome these age related defects to improve protection of this high risk population.
Immunosenescence in Innate Immunity
The innate immune system, the first line of defense against microbial pathogens, is mediated by a diverse group of cell types composed primarily of neutrophils, natural killer (NK), natural killer T cells (NKT), monocytes/macrophages and dendritic cells (DCs). DCs are the most potent, professional antigen presenting cells and play a central, unique role in bridging innate and adaptive immunity in response to pathogen invasion [34,35]. DC progenitors in the bone marrow give rise to circulating precursors that home to the periphery where they reside as immature DCs. Upon stimulation with microbial products, DCs mature and migrate to the draining lymph nodes to stimulate antigen-specific T cells [35,36].
Defects in Dendritic Cell Populations
DCs in humans are heterogeneous and are subdivided into two major categories, conventional or myeloid DCs (mDC) and plasmacytoid DCs (pDC). mDCs play a crucial role in antigen presentation but show rather limited capacities in type I interferon (IFN) production. pDCs can produce large amounts of type I IFNs and are thought to be the major source of these cytokines in vivo [37,38]. Although the number and phenotype of DCs in aged mice seems preserved [39,40], studies in humans are not conclusive. DC number & phenotype were shown to be comparable between the young and the aged in some studies [41,42]. However, Della Bella et al. and Panda et al. demonstrated that the number of mDCs in human blood progressively declines with age, and mDCs appeared to have a more mature phenotype and impaired ability to produce pro-inflammatory cytokines (TNF-α, IL-6 and IL-12) upon Toll-like receptor (TLR) engagement [43,44]. In addition, mDCs were severely depleted in frail older subjects [41]. Most studies showed that overall pDC numbers in peripheral blood were lower in healthy older adults and the amount of IFN generated by pDCs was decreased in response to virus infection [41,45].Because of the importance of pDCs for antiviral responses, the age-related changes in pDCs likely contribute to the impaired immune response to viral infections in older persons, especially when combined with the mDC dysfunction occurring in those with compromised health.
In terms of antigen uptake and migration, DCs from older individuals were shown to display significantly reduced capacity to phagocytose antigens via macropinocytosis and endocytosis in contrast to DCs from the young. In the same study, impaired capacity to migrate in vitro in response to chemokines was observed in DCs from older subjects [42]. DCs differ from other APCs in that they have the unique capacity to prime naïve T cells, which is critical for mounting an effective response against novel antigens. Earlier studies in aged mice demonstrated a decreased ability to prime a robust T cell response against an infectious agent [39,46]. In humans, DCs generated from peripheral blood of older adults were equally effective as those from younger adults in inducing the proliferation of T cell clones after antigenic stimulation [47,48]. However, the DCs were generated in vitro with cytokines that may have overcome age-related defects that are observed in vivo in older adults. Although the role of DCs in response to foreign antigens seems controversial, DCs from aged subjects display impaired capacity to phagocytose self antigens in the form of apoptotic cells [49,50]. This would result in the accumulation of apoptotic cells leading to secondary necrosis and release of self-antigens such as DNA. DCs from older adults were shown to display increased reactivity to intracellular human DNA. These DNA-primed DCs from older adults enhanced T cell proliferation compared to younger adults [51,52]. An impaired uptake of apoptotic cells and increased reactivity to intracellular human DNA by DCs from older adults may result in both increased inflammation and autoimmunity commonly associated with aging.
Defects in Macrophages
Macrophages function as ‘pathogen sensors’ and play an important role in the phagocytosis of antigen, microorganisms and cellular debris, and elimination of invading microorganisms and tumors [53]. A significant decrease in the number of macrophages in older adults has been described [54]. While macrophage precursors, monocytes, are found in the peripheral blood. Macrophages are found primarily in tissues. This has made studies of human macrophages difficult, and restricted studies primarily to human alveolar macrophages which can be isolated more readily than those from other sites. As a result, many of the studies examining the effect of aging on macrophage function have been done in animal models, such as mice.
Phagocytosis constitutes the first line of immune defense against pathogenic bacteria that have penetrated the epithelial barrier. An age-related decline in particle internalization, reactive oxygen species (ROS) and nitric oxide (NO) production were observed in murine macrophages from aged animals which weaken defense mechanisms to clear infectious agents [55–59].
Activated macrophages secrete pro-inflammatory cytokines and chemokines such as TNF-α, IL-1, IL-6 and metalloproteinases to initiate inflammatory responses that recruit neutrophils and natural killer cells. In vitro, macrophages from the alveoli and spleen of older mice generally produced more cytokines compared to younger counterparts. Conversely, macrophages from the peritonea of older mice generally produced less cytokine in vitro compared to younger counterparts [60,61]. Macrophages play an important role during the inflammatory phase of wound healing. They keep the wound free from infection and promote angiogenesis [62]. The alterations in chemokine secretion, and a concurrent decline in wound macrophage phagocytic function may contribute to the delayed repair response of aging [63]. In addition, defects in secretion of vascular endothelial growth factor and expression of cell adhesion molecules are also thought to contribute to the delay in wound healing in the aged [64]. However, not all inflammatory mediators are produced in reduced amounts with aging, some of which may actually increase with age. Macrophages from old mice have significantly higher levels of PGE2 production compared with those from young mice [65,66]. PGE2 plays a critical role in the age-associated dysregulation of the immune and inflammatory responses. In particular, several studies have shown that increased PGE2 production in macrophages from old mice contributes to the suppression of T cell function with aging [66]. In summary, the effects of advancing age have been reported on the broad range of functional capabilities of macrophages. However, the reported impact of aging on macrophage function varies from study to study due to differences in experimental conditions assessing their function and the underlying medical conditions of the population evaluated.
Defects in Pattern Recognition Receptors with Age
As main cellular components of innate immunity, DCs and macrophages recognize molecules shared by groups of related microbes that are essential for the survival of those organisms and are not found associated with mammalian cells. These unique microbial molecules are called pathogen-associated molecular patterns or PAMPs. These PAMPs are sensed by the host’s germline encoded pattern recognition receptors (PRRs) expressed on DCs and macrophages. Toll-Like Receptors are the most widely studied PRRs and are considered to be the primary sensors of pathogens. Eleven human TLRs and 13 murine TLRs have been described to recognize specific molecular patterns present on the surface of pathogens [67]. Toll-Like Receptors are evolutionarily conserved molecules and the expression of TLRs varies in different cell subsets: the expression of TLR3 on primary human macrophages from older individuals was lower than macrophages from young individuals [68]. Decreased expression of TLR3 and TLR8 in mDC and decreased expression of TLR7 in pDC was observed [44].
After recognition of microbial pathogens, TLRs trigger intracellular signaling pathways that result in the induction of inflammatory cytokines, type I IFN and chemokines. Panda et al. found substantial decreases in older compared with young individuals in TNF-α, IL-6, and/or IL-12 (p40) production in mDCs, and in TNF-α and IFN-α production in pDCs in response to TLR engagement [44,69]. These results support the concept that increased susceptibility to infections and poor adaptive immune responses in aging may be due to the decline in TLR expression and function [70–72]. Upon ligation, TLR signaling activates a cascade of intermediates including myeloid differentiation factor-88 (MyD88), IL-1 receptor-associated kinase (IRAK) and tumor necrosis factor receptor-associated factor 6 (TRAF6) leading to activation of nuclear factor (NF)-κ B and activating protein-1 (AP-1) [73–75]. Both basal and downstream signaling components, such as the adaptor molecule MyD88, TRAF6 and several members of the NF-κB pathway, such as c-Rel, p65, NF-κB p50 and p52 were reduced in the aged suggesting that the TLR-dependent pathway is working at a significantly reduced efficiency [76]. Qian et al. observed no significant age-related difference in expression or nuclear translocation of signaling molecules in initial antiviral responses. But they showed that DCs from older donors have diminished induction of late-phase responses (eg, STAT1, IRF7, and IRF1), suggesting defective regulation of type I IFN [77].
In specific TLR 4 signaling, LPS stimulation results in endothelial activation through a receptor complex consisting of TLR4, CD14 and MD2. Serum levels of LPS Binding Protein are unaltered in aged mice or humans. However, the expression of CD14 as well as MD2 on macrophages is reduced in mice [78]. Excessive immune responses are detrimental to the host and negative feedback regulation is crucial for the maintenance of immune-system integrity. TLR signaling during subsequent or continuous exposure induces the expression of IL-1 receptor-associated kinase-M and suppressor of cytokine signaling as negative regulators. Ageing increases SOCS-3 expression in rat hypothalamus and human muscle tissue, and SOCS1 and SOCS3 in human neutrophils [79–81]. In addition, increased levels of IRAK-M mRNA as well as protein have been observed with increased age in mice after LPS stimulation [82]. Therefore, in older adults, dysfunction of TLR signaling could arise from either decreased activation of TLR signaling adaptor molecules and/or increased activity of negative regulators of TLR function.
Defects in NK Cells
NK cells play an essential role in the innate immune defense against tumors and viral infections. They mediate MHC-independent cytotoxicity through perforin and granzyme B, and regulate adaptive immune responses by the production of chemokines and cytokines for recruitment and activation of T and B cells. Two distinct populations of NK cells can be identified by the density of surface CD56 molecules. While CD56dim NK cells mediate cytotoxicity of virus-infected cells, the low density CD56bright subpopulation appears to be the primary source of NK cell-derived immunoregulatory cytokines such as IFN-γ, TNF-α, GM-CSF, IL-10 and IL-13 [83,84]. Although there is a reduction in CD56bright NK cells with aging [85,86], an expansion of CD56dim NK cells results in an increase in the absolute number [85–87].
Age-related changes in NK cell functionality are controversial. Several studies have demonstrated diminished, unaffected or even enhanced activity of NK cells from older adults [86–90]. Reduced cytotoxicity of NK cells based on diminished perforin expression was observed in older adults [87,88], although a more recent study revealed a preserved cytotoxic ability of NK cells from childhood through old age [86]. Interferon-γ production upon NK cell activation was previously considered to be reduced in older adults [90], but was recently shown to be significantly higher in the aged, particularly in subjects older than 85 years of age compared to younger older adults [89]. Altered chemokine (RANTES, MIP-1a, IL-8) production [87] and suppressed trafficking of matured NK cells into draining lymph nodes [91] have also been associated with ageing.
Several potential mechanisms have been observed to account for the functional decline of NK cells in older adults. Reduced cytotoxicity may be a consequence of impaired zinc [87,92] or calcium [93] homeostasis in NK cells from older adults. An increased expression of Ly49-receptors downregulates NK activation [94], and diminishes sensitivity of aged NK cells to stimulatory effects of IL-2 [90] and IFN-α [95]. Moreover, several studies [86,96,97] demonstrated significant age-related changes in expression of NK cell receptors that inhibit or activate NK cells to lyse target cells, including killer cell immunoglobulin-like receptors (KIRs), natural cytotoxicity receptors (NCRs) and C-type lectins. The increased expression of KIR [96] particularly on the CD56bright subset [86], along with the decreased expression of C-type lectin CD94 [97] and activating NCRs, NKp30 and NKp46 in both CD56 subsets [86] were observed in older adults compared to younger adults.
Defects in T cells with Aging
Aging affects all stages of T cell responses including generation, maturation, differentiation, activation and functionality of both CD4 and CD8 T cells. Progressive accumulation of these defects leads to several key changes in T cell immunity: decreased production of naïve T cells, increased proportion of memory cells, decreased activation and T cell signaling, and decreased proliferation and antigen-specific response. Beginning with the most crucial event – depletion of naïve T cells due to thymic involution – all subsequent changes are strongly interconnected revealing imbalanced T cell responses with altered survival and activity of immature and mature T cells.
Thymic Involution and T cell Repertoire
The size of the naïve T cell pool is significantly decreased in aging. Naïve T cells are generated from bone marrow precursors and develop into mature T cells upon migration to the thymus. The age-associated involution of the thymus begins in the initial years of life and continues into almost complete adipose tissue replacement by age 70 [98,99]. Thymic atrophy involves considerable contraction of thymic epithelial space and substantial increase in perivascular space that contains low levels of recruited peripheral T and B cells and is not directly involved in thymopoiesis [100]. Another factor contributing to naïve T cell pool depletion in older adults is a lack of IL-7 production. IL-7 plays a key role in survival of both preselected and matured T cells, including memory cells that express the IL-7 receptor. Treatment with IL-7 reversed thymic atrophy and increased thymic output in aged mice [101,102], and increased the number of naïve CD4 and CD8 T cells in aged rhesus macaques [103]. In a small human study involving volunteers under 60 years old, recombinant IL-7 slightly enhanced CD45RA+ and diminished CD45RO+ levels in CD4 and CD8 T cells, indicating expansion of the naïve T cell pool [104]. Decreased production of IL-7 was associated with observed thymic involution both in animal studies [105], and aged humans [106].
In addition to limited development and survival, naïve T cells in older adults appear to be more susceptible to apoptosis. Decreased expression of bcl-2 molecules in naïve CD4 and CD8 T cells along with increased activation of caspase 8 and 3 was observed in aged humans indicating that aged naive T cells maintain both apoptotic pathways [107–109]. The age-related decrease in the ability of naïve T cells to survive and renew, results in a significant loss in the ability to mount primary responses to novel antigens [110,111].
The age-associated reduction in naïve T cells in older adults is partially compensated by an increased memory T cell pool that provides cross reactive responses to new antigens. This age associated increased memory T cell pool is not simply a proportional increase as the naïve T cell pool wanes due to reduced thymic output. Several animal studies reported an increased memory phenotype even in newly developed CD4 T cells [112,113]. Bone marrow cells from young mice were transferred to both young and aged animals. Peripheral CD4 T cells reconstituted in aged mice revealed phenotype and lymphokine profiles similar to those from the control aged mice. However, these are distinctly different from those in the young mice. These data suggest that the aged microenvironment is defective and influences the maturation of newly produced CD4 T cells. Besides antigen-independent accumulation of memory T cells, clinical data indicate that chronic viral stimulation in the older adults leads to expansion of CD4 and CD8 T cells with a typical memory phenotype [114,115]. However, memory T cells from older adults have decreased differentiation and proliferative ability [116] and demonstrate low CD28 and CD27 expression reflecting their poor activation [116].
Poor adaptive immune response in aging is also related to narrowing of the T cell repertoire by decreased TCR diversity and increased levels of anergic CD28- cells. Reduced TCR diversity reveals the inability of naïve T cells to differentiate efficiently into new effector T cells with high specificity for antigen. Animal studies and in vitro analysis of isolated human CD4 and CD8 T cells indicate significantly decreased TCR beta-chain diversity [117–119]. In individuals over 70 years old, CD4 T cell repertoire decreased 100 times compared with young adult controls [117]. Another factor that narrows T cell repertoire is progressive accumulation of cells lacking the costimulatory molecule, CD28, which occupy available immunological space. CD28-negative T cells are anergic to antigen stimulation [120] and resistant to apoptosis [121], allowing them to expand considerably in vivo [120,122,123]. Significant expansion of CD28null T cells was observed upon chronic stimulation with viral [115,122–124], auto- [125] and tumor-associated antigens [126,127]. In addition CD8+CD28- cells seem to retain cytokine production that disrupt APC function, suppress T cell responses, and disturb the Th1:Th2 balance [128,129].
Regulatory T Cells
Regulatory T cells (Treg) expressing a CD4+CD25+ phenotype along with effector/memory markers are believed to suppress redundant T cell immune responses, preventing autoimmune diseases and immune pathology following excessive immune reactivity [130]. Although the data for Treg functional activity in older adults is controversial [131–133], a significant increase in the number of Treg cells with age is well established [131,133,134]. Resistant to apoptosis, and independent of IL-7 signaling [135,136], Tregs seem to suppress CD4 and CD8 T cell functionality with aging [136,137] by impairment of costimulatory CD40 and CD86 molecule expression on DCs, thereby diminishing the ability of APCs to form stable contacts with responding T cells [138]. Several studies show a correlation between increased levels of Tregs in aged mice and older adults, and low levels of effector/memory T cells to viral antigens [139–142], suggesting that age-associated defects in the regulatory T cell compartment affects the immune control of acute and persistent viral infections.
Decreased T Cell Activation and Signaling
Although the T cell compartment appears to accumulate effector/memory CD4 and CD8 T cells with age, the ability of those cells to respond to antigenic stimulation, achieve activation and eventually respond is significantly decreased with aging. Several factors are considered to be involved in this process: (i) the lack of costimulatory molecules; (ii) low efficiency of receptor synapse formation between the T cell receptor and antigen-MHC complex on the surface of antigen-presenting cells; (iii) weak activation of intracellular signaling; and (iv) low expression of surface activation/differentiation markers. T cells from older individuals lose the costimulatory molecule CD28, becoming anergic to antigen stimulation and thus resulting in a weak immune response. A significant proportion of CD4 and CD8 T cells expressing a CD28null phenotype was observed in subjects over 65 years old vaccinated with influenza trivalent vaccine, and only 17% of vaccine recipients demonstrated increased antibody titers to all three vaccine components, while almost half of the vaccinees failed to respond at all [143]. The whole synaptic complex of TCR and antigen-MHC presented by APCs becomes less efficient with aging [144–146]. Subsequent intracellular signal transduction is weak due to defects in signaling pathway gene expression [147], calcium and tyrosine kinase metabolism and alterations in plasma membrane lipids [144,145,148,149]. All these factors together provide poor T cell activation that is reflected in low expression of activation/differentiation markers.
Decreased T Cell Functionality
Defects in the functionality of aged naïve and memory T cells reveal suboptimal effector responses. The decreased proliferation of peripheral CD4 and CD8 T cells isolated from older adults to mitogens and antigens is well established [150,151]. Altered functionality of the effector/memory compartment has been associated with low IL-2 production [148,151]. While there has been an overall cytokine imbalance demonstrated in mice with polarization towards Th2, it was not very clear in humans [129,152–159]. Furthermore, antigen-experienced T cells from aged mice exhibit decreased trafficking into sites of inflammation [160,161], and reduced helper or cytolytic activity [118,162–164]. Furthermore, young mice that received naïve CD4 cells from aged donors have dramatically decreased numbers of antigen-specific B cells after immunization, suggesting reduced help from CD4 T cells from the older animals [162]. Clinical trials of influenza vaccines show substantially lower Granzyme B expression by effector CD8 T cells in vaccine recipients over 65 years old [163,164]. Besides altered functionality, memory T cells in older adults also seem to be more susceptible to apoptosis, presenting decreased expression of bcl-2 and increased Fas-mediated activation of caspase 8 and 3 [165,166]. In addition, diminished T cell responses with aging are partially a result of age-associated changes in antigen presenting cells. Aged mice demonstrated limited expansion of influenza-specific CD8 T cells transferred from young mice [167], but the ability of CD8 cells to proliferate and produce IFN-γ significantly increased after co-transfer of DCs from young donors [168].
Immunosenescence in NK T Cells
NK T cells represent a heterogeneous population expressing T cell markers such as CD3, CD4, CD8 and CD1d restricted TCR (Vα24/Vβ11 in humans) as well as NK-cell markers such as CD56, CD94 and KIR [169]. While an age-associated decrease in NK T cell numbers was observed in mice [170,171], ageing in humans does not seem to affect NK T cell levels [86,172], although a significant decrease in expression of CD94 in aged human NKT cells was noticed [86,96]. In contrast to T lymphocytes maturating in the thymus, NK T cells may migrate directly from the bone marrow to the liver. The ability to maintain extrathymic development is considered to be a compensatory mechanism for thymus involution during ageing [173]. However, studies with viral infections in aged mice identified the NK T cells as producers of high levels of IL-17 which was associated with increased inflammation and hepatic injury [174,175] suggesting that NK T cells have the potential to account for adverse infection-related outcomes in aging.
Aging in Humoral Immunity
The effect of aging on humoral immunity is characterized by quantitative and qualitative alterations in antibody responses. These render older adults increasingly susceptible to infectious diseases and prone to suboptimal responses to vaccination. Since most current vaccines are designed to generate a neutralizing antibody response against infectious pathogens such as influenza viruses, the senescence of humoral immunity directly influences vaccine immunogenicity and efficacy in the aged. While several players of the immune system intricately affect each other’s function, defects in antigen presenting cell function, T cell number and function, namely diminished CD4 T cell help and increased T cell-mediated suppression play significant roles in reduced humoral immunity [99,160]. However, a number of studies point to intrinsic changes in B cells associated with aging, indicating senescence of humoral immunity itself. These changes include reductions in cellular composition, in B cell diversity, and in B cell effector function. In this section, we discuss findings of intrinsic alterations of B cells associated with aging. Although much of our knowledge on immunosenescence of humoral immunity derives from animal studies, there are some discrepancies between animal vs. human data [176]. Therefore, in this review, we focus on findings from human B cells.
Cellular Composition
Both the total number and percentage of mature B cells and antigen-specific B cells in the periphery alter with aging [177]. A number of studies have found that the absolute number and percentage of mature B cells including naïve and memory B cells in the periphery decrease compared to younger counterparts [178]. Especially, the reduction in overall human memory B cells as well as compromised recall memory responses is noted. Human memory B cells are composed of IgM memory and class-switched memory B cells, which include IgG, IgA and IgE memory B cells. IgM memory B cells play an important role in bacterial infection, such as pneumococcal infection [179]. In older adults, the frequency of antigen-specific IgM memory B cells was shown to be dramatically reduced, i.e. the pneumococcal bacteria-specific IgM response was reduced following vaccination [180]. A reduction in IgG responses and alteration in IgG subclass composition in response to influenza vaccination has also been reported [181]. In contrast to a dramatic reduction in mature B cells in the periphery, B lymphopoiesis in bone marrow stays relatively active and the percentage and numbers of B cell precursors decline only moderately with aging [182,183]. This pattern may reflect age-associated changes in the microenvironment that supports the survival of mature B cells in the periphery. B cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL) are TNF ligand superfamily proteins that are critical survival factors for mature B cells in the periphery [184,185]. It has been reported that in healthy older adults, the blood plasma levels of BAFF and APRIL are reduced [186]. Primary sources of BAFF and APRIL are monocytes, macrophages and dendritic cells [187,188], and aging is associated with the decline in the number and function of dendritic cells [41] as outlined above. Thus, these studies point out a global alteration in innate and adaptive immunity associated with aging.
B Cell Diversity
Changes in the B cell repertoire associated with aging involve an increase in the reactivity against autologous antigens and impaired responses against foreign antigens, expansion of oligoclonal populations, reduced size and diversity of the B cell repertoire, and reduced antibody specificity and affinity [189–191]. A shift in the B cell repertoire from foreign to self-antigens accompanies selectively decreased activity of conventional B2 (CD5−) B cell subsets compared to autoreactive B1 (CD5+) B cell subsets, resulting in production of autoantibodies [192]. Reduced B cell diversity could be in part due to impaired B cell lymphopoiesis in the bone marrow and due to diminished diversification in the germinal center [191]. The diversity of the B cell repertoire can be measured by the diversity of mRNA coding for the 3rd complementarity-determining region (CDR3) of the immunoglobulin molecule [193]. This region is highly diverse both in sequence and length of the sequence and important for antigen binding. The diversity of the Ig heavy chain CDR3 region is due to the addition and deletion of nucleotides when variable (VH), diversity (D), and joining (JH) genes rearrange [194,195]. Mutations in this region are reduced in older adults compared to young adults [196] and the preference for certain VH family genes in older individuals has been reported [197]. In healthy, young individuals, the mRNA CDR3 size diversity is represented by a bell-shaped profile of peaks. However, frail older individuals exhibit a distorted profile in their CDR3 spectratype from the normal distribution, indicating a collapse in B cell diversity [189]. Interestingly, the collapse in B cell diversity is associated with poor health status (frail vs. healthy) in this study cohort. Reduced B cell diversity also means oligoclonal expansions in the B cell repertoire [189]. Most of these oligoclonal B cells are derived from the CD5+ subset (B1) of B cells, which accounts for increased autoreactive antibodies in the aged [190,198].
B Cell Activation and Effector Function
Immunosenescence is also evident at the individual cell level in biochemical and signaling pathways. In older adults, the activity of protein tyrosine kinase (PTK) and the expression of protein kinase C (PKC) in response to B cell receptor engagement is reduced, thus perturbing downstream signaling events, such as antigen-induced proliferation [199]. Detailed molecular mechanisms behind the reduced antibody response associated with immunosenescence is mostly derived from murine studies [176]. In aged mice, the number and duration of germinal center formation is reduced [200]. Within the germinal center, molecular events including class switch recombination (CSR) and somatic hypermutation (SHM) in the IgH and IgV genes are critical in generating high affinity antibodies against antigen. In aged mice, reduced germinal center function accompanies reduced activity of activation-induced cytidine deaminase (AID), an enzyme necessary for CSR and SHM events, and reduced expression of E47 protein, a transcription factor regulating the gene encoding AID, and reduced stability of E47 mRNA [201–204]. However, corresponding, direct evidence concerning human germinal centers has been limited for obvious reasons. Recently, studies employing human peripheral blood B cells (from subjects 18–86 years of age) showed that expression of E47, AID and Igγ1 circle transcripts progressively decrease with age [205]. This, together with the reduction in the magnitude and duration of antibody responses to specific antigen, such as tetanus toxin, encephalitis viruses, Salmonella, or pneumonococcus indirectly indicates the diminished germinal center reaction of aged human B cells in lymphoid organs [206].
B Cell Differentiation
Progressive defects in mounting high-affinity antibody responses in older people is in part due to a decrease in the number and percentage of circulating plasma cells in the peripheral blood [207]. This decrease could be due to reduced survival niches that support plasma cells in the bone marrow, due to progressive replacement of hematopoietic bone marrow with fat cells, or reduced differentiation processes from mature B cells to plasma cells [208]. These changes could lead to a reduction in the number of antigen-specific plasma cells in peripheral blood as well as in bone marrow. The reduction in antigen-specific plasma cells, together with the oligoclonal expansion of plasma cells with cross-reactivity and low affinity to specific antigen, may account for reduction in the antibody responses to influenza vaccination in older adults [24].
Mechanisms for Improving Influenza Vaccine Efficacy in Older Adults
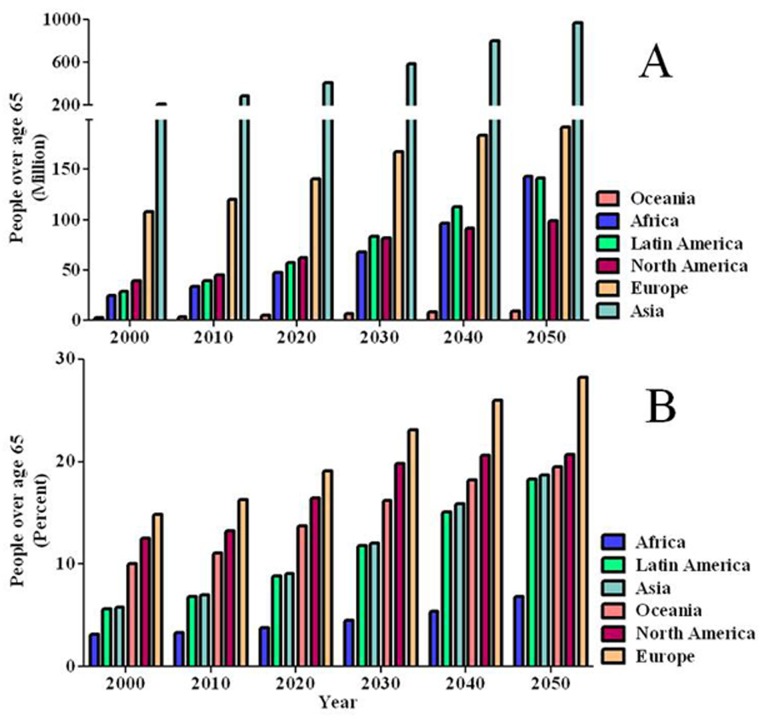
Older adults are at high risk for severe complications resulting from influenza infection, and are increasing rapidly as a proportion of the world population [209]. Adults over the age of 65 currently compose 8% of the world’s population, but projections by the U.S. Census Bureau predict that proportion will more than double by 2050 [209]. In some regions of the world, the older adults are expected to grow to nearly 30% of the population. Growth of this high-risk population, as projected by the U.S. Census Bureau, is depicted in Figure 2. In the near future, there will be an increasing need for protection of this age group from influenza.
Figure 2.
Projected increase in the aged population. Adults over the age of 65 are projected to increase worldwide over the next 40 years. The summary above, generated from population projections by the U.S. Census Bureau [209], predict that the older population within every region of the world will drastically increase in (A) number, and (B) will make up a larger proportion of the overall population. This could have a significant impact on influenza spread and control as the proportion of high risk individuals dramatically increases.
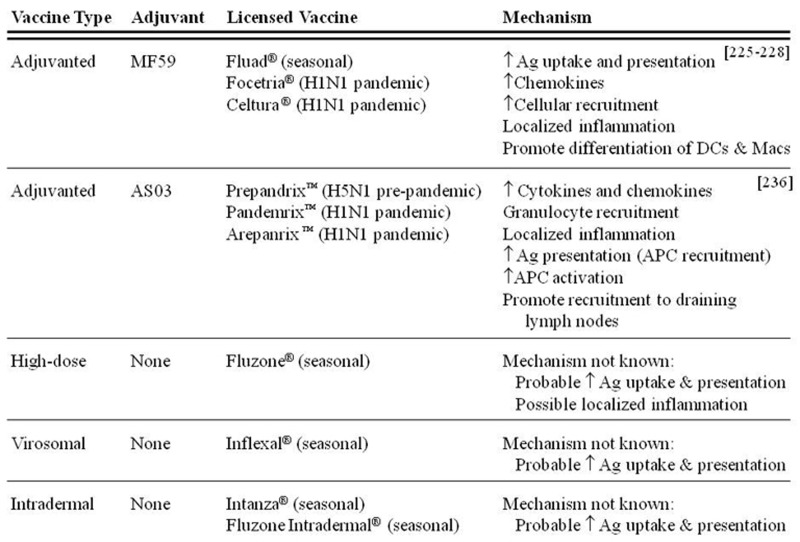
Despite controversies that exist about the effectiveness of influenza vaccines in older adults, it is clear that improved vaccines need to be developed that enhance the immunogenicity of influenza vaccines for this age group [210,211]. These improved vaccines need to overcome the age-related decline in immune function and stimulate both humoral and cellular immunity. Several strategies in various stages of development have been proposed and tested, including the use of adjuvants, cytokines, immunostimulatory complexes, increased antigen doses, intradermal vaccine delivery, live-attenuated vaccines, and virosomal vaccines [210,212–216]. However, very few of these proposed strategies have demonstrated the level of safety and efficacy required for licensure (Fig. 3). Two adjuvanted influenza vaccines containing MF59 and AS03 marketed by Novartis Vaccines and GlaxoSmithKline (GSK), respectively, are licensed for use primarily in Europe for older adults [217,218]. Canada recently granted licensure for the Arepanrix™ pandemic H1N1 vaccine and Fluad® seasonal influenza vaccine containing AS03 and MF59, respectively [219,220]. These adjuvants are based on an oil-in-water formulation utilizing the biodegradable oil, squalene [217,218].
Figure 3.
Licensed influenza vaccines eliciting improved immune responses in older adults. Conventional vaccines have been shown to elicit poor immune responses in older adults. Several novel mechanisms have resulted in influenza vaccines which elicit improved immune responses in this population, yet are considered safe enough by regulatory agencies to warrant licensure.
MF59, licensed in 1997, is the first adjuvant approved for the use in humans since alum [221]. Although the MF59-adjuvanted TIV vaccine has been marketed in Europe for older adults for over 13 years and has been shown to be safe and immunogenic [221–223], it is not yet approved for use in the US [224]. MF59 has been shown to significantly enhance the immunogenicity of influenza vaccines in older adults, resulting in higher antibody titers than conventional unadjuvanted vaccines [225–227]. Additionally, the antibody responses induced by MF59-adjuvanted vaccine demonstrate broader cross-reactivity with influenza strains not included in the vaccine, potentially offering improved protection in the event of vaccine mismatch with currently circulating strains [228,229]. In studies performed in mice and humans, MF59 has been shown to enhance the number of influenza specific CD4 T cells and memory B cells [230–232]. In clinical trials evaluating MF59 as an adjuvant for a H5N1 pre-pandemic vaccine, MF59 induced significant broadly reactive, H5-specific, CD4 T cells with a Th1 prone effector/memory phenotype after 1 injection [230]. A second vaccine dose stimulated H5N1-specific, IgG+ memory B cells, and microneutralization titers of ≥ 80. It should be noted that MF59’s enhancement of cellular immunity has only been studied in subjects aged 18 to 64, and has yet to be evaluated in older subjects [233]. Fluad®, the MF59 adjuvanted seasonal influenza vaccine, as well as the H5N1 pre-pandemic mentioned above are subunit vaccines containing influenza hemagglutinin (HA) and neuraminidase (NA), but lacking influenza nucleoproteins, matrix proteins, and polymerases. As it has been shown that the majority of influenza T cell epitopes are derived from the nucleoproteins, matrix proteins, and polymerases, with minimal epitopes derived from HA and NA [234,235], the capacity of these vaccines to expand a broader repertoire of cross-reactive T cells may be narrower than that of a comparable split-virion vaccine. This may be especially true from the perspective of older adults, whose T cell repertoire has been shown to be narrower than that of younger adults due to immunosenescence [117–119]. An evaluation of this vaccine’s ability to expand cross-reactive T cells in older adults would give a more complete understanding of the breadth of this vaccine’s effectiveness from the perspective of cellular as well as humoral immunity in the older population.
From studies primarily performed in mice, MF59 has been shown to enhance vaccine responses by increasing antigen uptake at the site of injection, stimulating the release of chemokines to attract additional immune cells, and stimulating localized inflammation at the injection site, enhancing responses [236–239]. MF59 has also been shown to induce the differentiation of monocytes into dendritic cells [237].
More recently, GSK Biologicals has developed its proprietary adjuvant system, AS03, to enhance responses to pre-pandemic vaccines. AS03 was initially used with an H5N1 split-virion pre-pandemic vaccine called Prepandrix™ [218]. In test subjects aged 18 to 60, subjects received 2 doses of a split virion vaccine, 21 days apart [240]. Subjects received 1 of 4 concentrations of antigen. All adjuvanted vaccines in this trial were significantly more immunogenic than their unadjuvanted counterparts as determined by HI and microneutralization titers, including the lowest antigen concentration of 3.8μg (25% the amount commonly used in conventional seasonal vaccines). Furthermore, the AS03 adjuvant was shown to stimulate cross-neutralizing antibody to related drift strains, as well as cross-reactive B and T cells [240–242]. Studies in subjects over the age of 61 showed significantly higher geometric mean HI titers than unadjuvanted vaccine, and antigen specific CD4 T cells [243]. Prepandrix™ is currently licensed to market in all members of the European Union [218]. With the emergence of H1N1 pandemic influenza in 2009, the monovalent H1N1 pandemic vaccine, Pandemrix™ was adjuvanted with AS03. In clinical trials evaluating the immunogenicity of the H1N1 pandemic vaccine, 85.7% of subjects older than 60 years of age demonstrated seroprotection (defined as an HI titer ≥ 1:40) after the first dose, and 87.8%, after a booster vaccination at 21 days [244]. An AS03-adjuvanted seasonal influenza vaccine for older adults is currently in development [224].
In studies performed in mice and humans, the AS03 adjuvant system has been shown to enhance immune responses by inducing localized inflammation at the injection site, increasing production of cytokines and chemokines, and recruiting granulocytes, macrophages and dendritic cells to the injection site and draining lymph nodes [245]. Antigen presenting cells displayed increased activation and increased antigen presentation [245].
Several studies have shown that increasing the vaccine dose from the standard 15 μg of HA per virus strain can enhance the level of protective antibodies [246–249]. In studies in older adults, vaccines were generated matching that year’s seasonal vaccine strains (2001–2002, and 2004–2005 seasons), but containing 60 μg of HA for each viral strain rather than the conventional 15 μg. These high-dose vaccines generated significantly higher HI and neutralizing antibody titers than the conventional-dose vaccines [244,247]. While each of the vaccine doses was well tolerated, a dose related increase in injection site reactions was observed in the studies. A recent study by Chen et al., demonstrated similar findings using a 60 μg dose influenza vaccine [249]. While the high-dose vaccine did not increase responses to the level observed in younger volunteers, the number of complete non-responders in the older adult group was significantly reduced.
In December of 2009, the US Food and Drug Administration (FDA) granted a license to Sanofi Pasteur for Fluzone® High-Dose, a trivalent, inactivated influenza vaccine containing 60 μg of HA for each of the viral strains [250,251]. Approval for this vaccine was granted under an accelerated process based on favorable safety and efficacy data [250]. Under this accelerated process, the manufacturer is required to provide additional data evaluating vaccine effectiveness in preventing seasonal influenza [250]. The Advisory Committee on Immunization Practices (ACIP) reviewed data generated during the first year after licensure, acknowledging the vaccine’s favorable immunogenicity and safety data. However, the committee has expressed no preference for Fluzone® High-Dose, nor for any other influenza vaccine, for preferred use in older adults [250,252].
The use of virus-like particles, called virosomes, as an alternative delivery vehicle has been proposed for some time. Virosomes are liposomes with the viral surface antigens on their surface, but with relevant T cell antigens inside rather than the viral genetic material. This creates a delivery system which mimics viral infection, without the pathology associated with live virus. Virosomes are able to present intact surface antigen to B cells, and are readily taken up by APCs for presentation to T cells. Upon uptake by APCs, the virosomes fuse with the endosomal membrane [253,254]. This fusion releases the internal antigens into the cytosol where they can be loaded onto MHC class I for presentation to T cytotoxic cells, while the surface antigens are processed within the endosome for presentation on MHC class II to T helper cells [253,255]. The first virosomal influenza vaccine, Inflexal® V marketed by Crucell N.V., was introduced in Switzerland in 1997, in Italy in 1998, and throughout the rest of Europe in 2001 [256]. Safety studies have demonstrated that Inflexal® V is safe and well tolerated by all age groups, including children, older adults, and immunocompromised patients [256–260]. However, studies in older adults examining the immunogenicity of virosomal vaccines have varied widely in their conclusions, ranging from greater responses to inferior responses compared with conventional vaccines [257,261–265].
The route of delivery has long been known to affect the immune response elicited by a vaccine. The most common route of influenza vaccine delivery is by intramuscular injection. This method does not require significant skill for delivery thereby making mass vaccination during influenza season possible. However, the muscle environment is not considered to be an efficient site for vaccination due to the low numbers of APCs [266]. In contrast, intradermal vaccination is considered a significantly more efficient route of delivery due to the high number of resident macrophages and dendritic cells [266–269]. Intradermal vaccine delivery; however, requires more skill, making intradermal vaccines less popular in the past. Recent advances in intradermal vaccine delivery systems, including patches, microneedle injection systems, and needle free systems have made this route of vaccine delivery more attractive and feasible for influenza vaccines [270–273]. Clinical trials in the elderly evaluating intradermal delivery of influenza vaccines using these new delivery systems compared to conventional intramuscular vaccines has shown equivalent or enhanced immunogenicity by the intradermal route [270,274–276]. The intradermal influenza vaccine, Intanza® produced by Sanofi Pasteur, was granted licensure by the European Medicines Agency for marketing throughout the European Union in February 2009, and was approved for marketing in Australia by the Therapeutic Goods Agency in March of 2009 [277]. Intanza® became available in Canada in 2010, and was recently licensed in May 2011 by the FDA for marketing in the U.S. under the name Fluzone Intradermal® [278,279]. However, this vaccine is only approved for ages 18 to 59 years in Canada and Australia, and ages 18 to 64 years in the U.S. The European Union has approved a 15μg Intanza® vaccine for use in adults over age 60 years (compared to the 9μg dose approved for ages 18 to 59 years). In a recent phase III, multi-center, randomized, controlled study performed in adults aged 65 years or more, Intanza® was shown to have immunogenicity and safety comparable to the MF59 adjuvanted Fluad® vaccine [280].
Many strategies to enhance the immunogenicity of influenza vaccines for older adults are still in the early stages of development and these include the use of toll-like receptor (TLR) ligands as adjuvants to enhance immune responses to vaccines. These ligands act by binding TLRs on the surface of APCs resulting in APC activation, release of pro-inflammatory cytokines and chemokines, thus enhancing adaptive immune responses. However, as discussed earlier, older adults demonstrate a reduced capacity to respond to many TLR ligands which may reduce the effectiveness of this mechanism. A recent study performed in an aging mouse model has shown that Poly I:C, a TLR-3 ligand, retains its ability to stimulate APCs resulting in release of pro-inflammatory cytokines, leading to enhanced T helper function [281]. In a brief report, McElhaney suggested that a TLR4 ligand may also be effective in improving the IFN-γ:IL-10 ratio and Granzyme B activity in aged mice [282]; functions that she has previously demonstrated correlate with protection in the elderly [30,31].
Conclusions
Influenza causes considerable morbidity and mortality worldwide, but disproportionately affects older adults. Conventional TIV vaccines provide adequate protection in young, healthy adults against seasonal epidemics. However, older adults do not develop effective protective immunity in response to these vaccines due to immunosenescence which impacts the cellular components of both innate and adaptive immune systems. While the dysregulation of the immune system with aging is becoming better understood, strategies for overcoming these deficiencies are being explored, which include high-dose vaccines, adjuvanted-vaccines, and alternate routes of immunization.
References
- [1].Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- [2].Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- [3].World Health Organization (WHO) Fact sheet no. No211. April 2009. [Available at: http://www.who.int/mediacentre/factsheets/fs211/en/index.html. Accessed May 25, 2011].
- [4].Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- [5].Mullooly JP, Bridges CB, Thompson WW, Chen J, Weintraub E, Jackson LA, Black S, Shay DK. Influenza- and RSV-associated hospitalizations among adults. Vaccine. 2007;25:846–855. doi: 10.1016/j.vaccine.2006.09.041. [DOI] [PubMed] [Google Scholar]
- [6].Powers DC, Fries LF, Murphy BR, Thumar B, Clements ML. In elderly persons live attenuated influenza A virus vaccines do not offer an advantage over inactivated virus vaccine in inducing serum or secretory antibodies or local immunologic memory. J Clin Microbiol. 1991;29:498–505. doi: 10.1128/jcm.29.3.498-505.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Treanor J, Dumyati G, O’Brien D, Riley MA, Riley G, Erb S, Betts R. Evaluation of cold-adapted, reassortant influenza B virus vaccines in elderly and chronically ill adults. J Infect Dis. 1994;169:402–407. doi: 10.1093/infdis/169.2.402. [DOI] [PubMed] [Google Scholar]
- [8].Govaert TM, Sprenger MJ, Dinant GJ, Aretz K, Masurel N, Knottnerus JA. Immune response to influenza vaccination of elderly people. A randomized double-blind placebo-controlled trial. Vaccine. 1994;12:1185–1189. doi: 10.1016/0264-410x(94)90241-0. [DOI] [PubMed] [Google Scholar]
- [9].Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA. 1994;272:1661–1665. [PubMed] [Google Scholar]
- [10].Nichol KL. Influenza vaccination in the elderly: impact on hospitalisation and mortality. Drugs Aging. 2005;22:495–515. doi: 10.2165/00002512-200522060-00004. [DOI] [PubMed] [Google Scholar]
- [11].Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366:1165–1174. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- [12].Jacobson RM, Targonski PV, Poland GA. Meta-analyses in vaccinology. Vaccine. 2007;25:3153–3159. doi: 10.1016/j.vaccine.2007.01.047. [DOI] [PubMed] [Google Scholar]
- [13].Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis. 2007;7:658–666. doi: 10.1016/S1473-3099(07)70236-0. [DOI] [PubMed] [Google Scholar]
- [14].Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. Lancet. 2008;372:398–405. doi: 10.1016/S0140-6736(08)61160-5. [DOI] [PubMed] [Google Scholar]
- [15].Jansen AG, Sanders EA, Nichol KL, van Loon AM, Hoes AW, Hak E. Decline in influenza-associated mortality among Dutch elderly following the introduction of a nationwide vaccination program. Vaccine. 2008;26:5567–5574. doi: 10.1016/j.vaccine.2008.08.003. [DOI] [PubMed] [Google Scholar]
- [16].Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357:1373–1381. doi: 10.1056/NEJMoa070844. [DOI] [PubMed] [Google Scholar]
- [17].Nichol KL, Nordin J, Mullooly J, Lask R, Fillbrandt K, Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med. 2003;348:1322–1332. doi: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]
- [18].Fireman B, Lee J, Lewis N, Bembom O, van der Laan M, Baxter R. Influenza vaccination and mortality: differentiating vaccine effects from bias. Am J Epidemiol. 2009;170:650–656. doi: 10.1093/aje/kwp173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Baxter R, Ray GT, Fireman BH. Effect of influenza vaccination on hospitalizations in persons aged 50 years and older. Vaccine. 2010;28:7267–7272. doi: 10.1016/j.vaccine.2010.08.088. [DOI] [PubMed] [Google Scholar]
- [20].Gross PA. Review: inactivated vaccines provide the greatest protection against influenza in healthy persons. ACP J Club. 2002;136:103. doi: 10.7326/acpjc-2002-136-3-103. [DOI] [PubMed] [Google Scholar]
- [21].Edwards KM, Dupont WD, Westrich MK, Plummer WD, Jr, Palmer PS, Wright PF. A randomized controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza A disease. J Infect Dis. 1994;169:68–76. doi: 10.1093/infdis/169.1.68. [DOI] [PubMed] [Google Scholar]
- [22].Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine. 1997;15:1114–1122. doi: 10.1016/s0264-410x(97)00003-0. [DOI] [PubMed] [Google Scholar]
- [23].Bridges CB, Thompson WW, Meltzer MI, Reeve GR, Talamonti WJ, Cox NJ, Lilac HA, Hall H, Klimov A, Fukuda K. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: A randomized controlled trial. JAMA. 2000;284:1655–1663. doi: 10.1001/jama.284.13.1655. [DOI] [PubMed] [Google Scholar]
- [24].Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- [25].Nordin J, Mullooly J, Poblete S, Strikas R, Petrucci R, Wei F, Rush B, Safirstein B, Wheeler D, Nichol KL. Influenza vaccine effectiveness in preventing hospitalizations and deaths in persons 65 years or older in Minnesota, New York, and Oregon: data from 3 health plans. J Infect Dis. 2001;184:665–670. doi: 10.1086/323085. [DOI] [PubMed] [Google Scholar]
- [26].Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972;70:767–777. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull. 1979;35:69–75. doi: 10.1093/oxfordjournals.bmb.a071545. [DOI] [PubMed] [Google Scholar]
- [28].Beare AS, Hobson D, Reed SE, Tyrrell DA. A comparison of live and killed influenza-virus vaccines. Report to the Medical Research Council’s Committee on Influenza and other Respiratory Virus Vaccines. Lancet. 1968;2:418–422. doi: 10.1016/s0140-6736(68)90463-7. [DOI] [PubMed] [Google Scholar]
- [29].Beare AS, Tyrrell DA, Hobson D, Howells CH, Pereira MS, Pollock TM, Tyler LE. Live influenza B vaccine in volunteers. A report to the Medical Research Council by their Committee on Influenza and Other Respiratory Virus Vaccines. J Hyg (Lond) 1969;67:1–11. doi: 10.1017/s002217240004136x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, Ewen C, Kane KP, Bleackley RC. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176:6333–6339. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- [31].McElhaney JE, Ewen C, Zhou X, Kane KP, Xie D, Hager WD, Barry MB, Kleppinger A, Wang Y, Bleackley RC. Granzyme B: Correlates with protection and enhanced CTL response to influenza vaccination in older adults. Vaccine. 2009;27:2418–2425. doi: 10.1016/j.vaccine.2009.01.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Targonski PV, Jacobson RM, Poland GA. Immunosenescence: role and measurement in influenza vaccine response among the elderly. Vaccine. 2007;25:3066–3069. doi: 10.1016/j.vaccine.2007.01.025. [DOI] [PubMed] [Google Scholar]
- [33].Aspinall R, Del Giudice G, Effros RB, Grubeck-Loebenstein B, Sambhara S. Challenges for vaccination in the elderly. Immun Ageing. 2007;4:9. doi: 10.1186/1742-4933-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- [35].Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- [36].Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- [37].Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- [38].Colonna M, Krug A, Cella M. Interferon-producing cells: on the front line in immune responses against pathogens. Curr Opin Immunol. 2002;14:373–379. doi: 10.1016/s0952-7915(02)00349-7. [DOI] [PubMed] [Google Scholar]
- [39].Grolleau-Julius A, Garg MR, Mo R, Stoolman LL, Yung RL. Effect of aging on bone marrow-derived murine CD11c+CD4-CD8alpha- dendritic cell function. J Gerontol A Biol Sci Med Sci. 2006;61:1039–1047. doi: 10.1093/gerona/61.10.1039. [DOI] [PubMed] [Google Scholar]
- [40].Tesar BM, Walker WE, Unternaehrer J, Joshi NS, Chandele A, Haynes L, Kaech S, Goldstein DR. Murine [corrected] myeloid dendritic cell-dependent toll-like receptor immunity is preserved with aging. Aging Cell. 2006;5:473–486. doi: 10.1111/j.1474-9726.2006.00245.x. [DOI] [PubMed] [Google Scholar]
- [41].Jing Y, Shaheen E, Drake RR, Chen N, Gravenstein S, Deng Y. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum Immunol. 2009;70:777–784. doi: 10.1016/j.humimm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol. 2007;178:6912–6922. doi: 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- [43].Della Bella S, Bierti L, Presicce P, Arienti R, Valenti M, Saresella M, Vergani C, Villa ML. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin Immunol. 2007;122:220–228. doi: 10.1016/j.clim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- [44].Panda A, Qian F, Mohanty S, van Duin D, Newman FK, Zhang L, Chen S, Towle V, Belshe RB, Fikrig E, Allore HG, Montgomery RR, Shaw AC. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010;184:2518–2527. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Shodell M, Siegal FP. Circulating, interferon-producing plasmacytoid dendritic cells decline during human ageing. Scand J Immunol. 2002;56:518–521. doi: 10.1046/j.1365-3083.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- [46].Moretto MM, Lawlor EM, Khan IA. Aging mice exhibit a functional defect in mucosal dendritic cell response against an intracellular pathogen. J Immunol. 2008;181:7977–7984. doi: 10.4049/jimmunol.181.11.7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Steger MM, Maczek C, Grubeck-Loebenstein B. Morphologically and functionally intact dendritic cells can be derived from the peripheral blood of aged individuals. Clin Exp Immunol. 1996;105:544–550. doi: 10.1046/j.1365-2249.1996.d01-790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Grewe M. Chronological ageing and photoageing of dendritic cells. Clin Exp Dermatol. 2001;26:608–612. doi: 10.1046/j.1365-2230.2001.00898.x. [DOI] [PubMed] [Google Scholar]
- [49].Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Aprahamian T, Takemura Y, Goukassian D, Walsh K. Ageing is associated with diminished apoptotic cell clearance in vivo. Clin Exp Immunol. 2008;152:448–455. doi: 10.1111/j.1365-2249.2008.03658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Agrawal A, Tay J, Yang GE, Agrawal S, Gupta S. Age-associated epigenetic modifications in human DNA increase its immunogenicity. Aging (Albany NY) 2010;2:93–100. doi: 10.18632/aging.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Agrawal A, Tay J, Ton S, Agrawal S, Gupta S. Increased reactivity of dendritic cells from aged subjects to self-antigen, the human DNA. J Immunol. 2009;182:1138–1145. doi: 10.4049/jimmunol.182.2.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- [54].Zissel G, Schlaak M, Muller-Quernheim J. Age-related decrease in accessory cell function of human alveolar macrophages. J Investig Med. 1999;47:51–56. [PubMed] [Google Scholar]
- [55].Tasat DR, Mancuso R, O’Connor S, Molinari B. Age-dependent change in reactive oxygen species and nitric oxide generation by rat alveolar macrophages. Aging Cell. 2003;2:159–164. doi: 10.1046/j.1474-9728.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- [56].Antonini JM, Roberts JR, Clarke RW, Yang HM, Barger MW, Ma JY, Weissman DN. Effect of age on respiratory defense mechanisms: pulmonary bacterial clearance in Fischer 344 rats after intratracheal instillation of Listeria monocytogenes. Chest. 2001;120:240–249. doi: 10.1378/chest.120.1.240. [DOI] [PubMed] [Google Scholar]
- [57].Khare V, Sodhi A, Singh SM. Effect of aging on the tumoricidal functions of murine peritoneal macrophages. Nat Immun. 1996;15:285–294. [PubMed] [Google Scholar]
- [58].Videla LA, Tapia G, Fernandez V. Influence of aging on Kupffer cell respiratory activity in relation to particle phagocytosis and oxidative stress parameters in mouse liver. Redox Rep. 2001;6:155–159. doi: 10.1179/135100001101536265. [DOI] [PubMed] [Google Scholar]
- [59].Martin G, Sewell RB, Yeomans ND, Morgan DJ, Smallwood RA. Hepatic Kupffer cell function: the efficiency of uptake and intracellular degradation of 14C-labelled mitochondria is reduced in aged rats. Mech Ageing Dev. 1994;73:157–168. doi: 10.1016/0047-6374(94)90048-5. [DOI] [PubMed] [Google Scholar]
- [60].Kohut ML, Senchina DS, Madden KS, Martin AE, Felten DL, Moynihan JA. Age effects on macrophage function vary by tissue site, nature of stimulant, and exercise behavior. Exp Gerontol. 2004;39:1347–1360. doi: 10.1016/j.exger.2004.07.001. [DOI] [PubMed] [Google Scholar]
- [61].Murciano C, Yanez A, O’Connor JE, Gozalbo D, Gil ML. Influence of aging on murine neutrophil and macrophage function against Candida albicans. FEMS Immunol Med Microbiol. 2008;53:214–221. doi: 10.1111/j.1574-695X.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- [62].Barbul A, Regan MC. Immune involvement in wound healing. Otolaryngol Clin North Am. 1995;28:955–968. [PubMed] [Google Scholar]
- [63].Swift ME, Burns AL, Gray KL, DiPietro LA. Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol. 2001;117:1027–1035. doi: 10.1046/j.0022-202x.2001.01539.x. [DOI] [PubMed] [Google Scholar]
- [64].Swift ME, Kleinman HK, DiPietro LA. Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest. 1999;79:1479–1487. [PubMed] [Google Scholar]
- [65].Meydani SN, Meydani M, Verdon CP, Shapiro AA, Blumberg JB, Hayes KC. Vitamin E supplementation suppresses prostaglandin E1(2) synthesis and enhances the immune response of aged mice. Mech Ageing Dev. 1986;34:191–201. doi: 10.1016/0047-6374(86)90034-5. [DOI] [PubMed] [Google Scholar]
- [66].Wu D, Meydani SN. Mechanism of age-associated up-regulation in macrophage PGE2 synthesis. Brain Behav Immun. 2004;18:487–494. doi: 10.1016/j.bbi.2004.05.003. [DOI] [PubMed] [Google Scholar]
- [67].Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- [68].Kong KF, Delroux K, Wang X, Qian F, Arjona A, Malawista SE, Fikrig E, Montgomery RR. Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. J Virol. 2008;82:7613–7623. doi: 10.1128/JVI.00618-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Agius E, Lacy KE, Vukmanovic-Stejic M, Jagger AL, Papageorgiou AP, Hall S, Reed JR, Curnow SJ, Fuentes-Duculan J, Buckley CD, Salmon M, Taams LS, Krueger J, Greenwood J, Klein N, Rustin MH, Akbar AN. Decreased TNF-alpha synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging. J Exp Med. 2009;206:1929–1940. doi: 10.1084/jem.20090896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- [71].Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ. Aging negatively skews macrophage TLR2- and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mech Ageing Dev. 2005;126:1305–1313. doi: 10.1016/j.mad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- [72].Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3:161–167. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- [73].Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- [74].Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- [75].Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- [76].Chelvarajan RL, Liu Y, Popa D, Getchell ML, Getchell TV, Stromberg AJ, Bondada S. Molecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophages. J Leukoc Biol. 2006;79:1314–1327. doi: 10.1189/jlb.0106024. [DOI] [PubMed] [Google Scholar]
- [77].Qian F, Wang X, Zhang L, Lin A, Zhao H, Fikrig E, Montgomery RR. Impaired interferon signaling in dendritic cells from older donors infected in vitro with West Nile virus. J Infect Dis. 2011;203:1415–1424. doi: 10.1093/infdis/jir048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Dunston CR, Griffiths HR. The effect of ageing on macrophage Toll-like receptor-mediated responses in the fight against pathogens. Clin Exp Immunol. 2010;161:407–416. doi: 10.1111/j.1365-2249.2010.04213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Tortorella C, Simone O, Piazzolla G, Stella I, Cappiello V, Antonaci S. Role of phosphoinositide 3-kinase and extracellular signal-regulated kinase pathways in granulocyte macrophage-colony-stimulating factor failure to delay fas-induced neutrophil apoptosis in elderly humans. J Gerontol A Biol Sci Med Sci. 2006;61:1111–1118. doi: 10.1093/gerona/61.11.1111. [DOI] [PubMed] [Google Scholar]
- [80].Leger B, Derave W, De Bock K, Hespel P, Russell AP. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res. 2008;11:163–175B. doi: 10.1089/rej.2007.0588. [DOI] [PubMed] [Google Scholar]
- [81].Peralta S, Carrascosa JM, Gallardo N, Ros M, Arribas C. Ageing increases SOCS-3 expression in rat hypothalamus: effects of food restriction. Biochem Biophys Res Commun. 2002;296:425–428. doi: 10.1016/s0006-291x(02)00906-3. [DOI] [PubMed] [Google Scholar]
- [82].Li Y, Howell EA, Lagoo AS, Kuchibhatla M, Pan H, Cohen HJ, Lagoo SA. Differential gene expression of interleukin-1 receptor associated kinase-1 and interleukin-1 receptor associated kinase-M in peripheral blood mononuclear cells of young and aged rats following preconditioning with endotoxin. Shock. 2009;31:55–63. doi: 10.1097/SHK.0b013e3181778ab2. [DOI] [PubMed] [Google Scholar]
- [83].Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. [Google Scholar]
- [84].Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- [85].Chidrawar SM, Khan N, Chan YL, Nayak L, Moss PA. Ageing is associated with a decline in peripheral blood CD56bright NK cells. Immun Ageing. 2006;3:10. doi: 10.1186/1742-4933-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Almeida-Oliveira A, Smith-Carvalho M, Porto LC, Cardoso-Oliveira J, Ribeiro Ados S, Falcao RR, Abdelhay E, Bouzas LF, Thuler LC, Ornellas MH, Diamond HR. Age-related changes in natural killer cell receptors from childhood through old age. Hum Immunol. 2011;72:319–329. doi: 10.1016/j.humimm.2011.01.009. [DOI] [PubMed] [Google Scholar]
- [87].Mocchegiani E, Giacconi R, Cipriano C, Malavolta M. NK and NKT cells in aging and longevity: role of zinc and metallothioneins. J Clin Immunol. 2009;29:416–425. doi: 10.1007/s10875-009-9298-4. [DOI] [PubMed] [Google Scholar]
- [88].Mariani E, Sgobbi S, Meneghetti A, Tadolini M, Tarozzi A, Sinoppi M, Cattini L, Facchini A. Perforins in human cytolytic cells: the effect of age. Mech Ageing Dev. 1996;92:195–209. doi: 10.1016/s0047-6374(96)01829-5. [DOI] [PubMed] [Google Scholar]
- [89].Kaszubowska L, Kaczor JJ, Hak L, Dettlaff-Pokora A, Szarynska M, Kmiec Z. Sensitivity of natural killer cells to activation in the process of ageing is related to the oxidative and inflammatory status of the elderly. J Physiol Pharmacol. 2011;62:101–109. [PubMed] [Google Scholar]
- [90].Krishnaraj R, Bhooma T. Cytokine sensitivity of human NK cells during immunosenescence. 2 IL2-induced interferon gamma secretion. Immunol Lett. 1996;50:59–63. doi: 10.1016/0165-2478(96)02519-9. [DOI] [PubMed] [Google Scholar]
- [91].Fang M, Roscoe F, Sigal LJ. Age-dependent susceptibility to a viral disease due to decreased natural killer cell numbers and trafficking. J Exp Med. 2010;207:2369–2381. doi: 10.1084/jem.20100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Mariani E, Neri S, Cattini L, Mocchegiani E, Malavolta M, Dedoussis GV, Kanoni S, Rink L, Jajte J, Facchini A. Effect of zinc supplementation on plasma IL-6 and MCP-1 production and NK cell function in healthy elderly: interactive influence of +647 MT1a and -174 IL-6 polymorphic alleles. Exp Gerontol. 2008;43:462–471. doi: 10.1016/j.exger.2007.12.003. [DOI] [PubMed] [Google Scholar]
- [93].Borrego F, Alonso MC, Galiani MD, Carracedo J, Ramirez R, Ostos B, Pena J, Solana R. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol. 1999;34:253–265. doi: 10.1016/s0531-5565(98)00076-x. [DOI] [PubMed] [Google Scholar]
- [94].Dorfman JR, Raulet DH. Acquisition of Ly49 receptor expression by developing natural killer cells. J Exp Med. 1998;187:609–618. doi: 10.1084/jem.187.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kutza J, Murasko DM. Effects of aging on natural killer cell activity and activation by interleukin-2 and IFN-alpha. Cell Immunol. 1994;155:195–204. doi: 10.1006/cimm.1994.1112. [DOI] [PubMed] [Google Scholar]
- [96].Lutz CT, Moore MB, Bradley S, Shelton BJ, Lutgendorf SK. Reciprocal age related change in natural killer cell receptors for MHC class I. Mech Ageing Dev. 2005;126:722–731. doi: 10.1016/j.mad.2005.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Hayhoe RP, Henson SM, Akbar AN, Palmer DB. Variation of human natural killer cell phenotypes with age: identification of a unique KLRG1-negative subset. Hum Immunol. 2010;71:676–681. doi: 10.1016/j.humimm.2010.03.014. [DOI] [PubMed] [Google Scholar]
- [98].Aspinall R, Andrew D. Thymic involution in aging. J Clin Immunol. 2000;20:250–256. doi: 10.1023/a:1006611518223. [DOI] [PubMed] [Google Scholar]
- [99].Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- [100].Flores KG, Li J, Sempowski GD, Haynes BF, Hale LP. Analysis of the human thymic perivascular space during aging. J Clin Invest. 1999;104:1031–1039. doi: 10.1172/JCI7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Andrew D, Aspinall R. Il-7 and not stem cell factor reverses both the increase in apoptosis and the decline in thymopoiesis seen in aged mice. J Immunol. 2001;166:1524–1530. doi: 10.4049/jimmunol.166.3.1524. [DOI] [PubMed] [Google Scholar]
- [102].Henson SM, Snelgrove R, Hussell T, Wells DJ, Aspinall R. An IL-7 fusion protein that shows increased thymopoietic ability. J Immunol. 2005;175:4112–4118. doi: 10.4049/jimmunol.175.6.4112. [DOI] [PubMed] [Google Scholar]
- [103].Aspinall R, Pido-Lopez J, Imami N, Henson SM, Ngom PT, Morre M, Niphuis H, Remarque E, Rosenwirth B, Heeney JL. Old rhesus macaques treated with interleukin-7 show increased TREC levels and respond well to influenza vaccination. Rejuvenation Res. 2007;10:5–17. doi: 10.1089/rej.2006.9098. [DOI] [PubMed] [Google Scholar]
- [104].Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, Stetler-Stevenson M, Morton KE, Mavroukakis SA, Morre M, Buffet R, Mackall CL, Gress RE. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006;29:313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ortman CL, Dittmar KA, Witte PL, Le PT. Molecular characterization of the mouse involuted thymus: aberrations in expression of transcription regulators in thymocyte and epithelial compartments. Int Immunol. 2002;14:813–822. doi: 10.1093/intimm/dxf042. [DOI] [PubMed] [Google Scholar]
- [106].Andrew D, Aspinall R. Age-associated thymic atrophy is linked to a decline in IL-7 production. Exp Gerontol. 2002;37:455–463. doi: 10.1016/s0531-5565(01)00213-3. [DOI] [PubMed] [Google Scholar]
- [107].Aggarwal S, Gupta S. Increased apoptosis of T cell subsets in aging humans: altered expression of Fas (CD95), Fas ligand, Bcl-2, and Bax. J Immunol. 1998;160:1627–1637. [PubMed] [Google Scholar]
- [108].Aggarwal S, Gupta S. Increased activity of caspase 3 and caspase 8 in anti-Fas-induced apoptosis in lymphocytes from ageing humans. Clin Exp Immunol. 1999;117:285–290. doi: 10.1046/j.1365-2249.1999.00957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Tsukamoto H, Huston GE, Dibble J, Duso DK, Swain SL. Bim dictates naive CD4 T cell lifespan and the development of age-associated functional defects. J Immunol. 2010;185:4535–4544. doi: 10.4049/jimmunol.1001668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Plowden J, Renshaw-Hoelscher M, Gangappa S, Engleman C, Katz JM, Sambhara S. Impaired antigen-induced CD8+ T cell clonal expansion in aging is due to defects in antigen presenting cell function. Cell Immunol. 2004;229:86–92. doi: 10.1016/j.cellimm.2004.07.001. [DOI] [PubMed] [Google Scholar]
- [111].Jiang J, Gross D, Elbaum P, Murasko DM. Aging affects initiation and continuation of T cell proliferation. Mech Ageing Dev. 2007;128:332–339. doi: 10.1016/j.mad.2007.02.002. [DOI] [PubMed] [Google Scholar]
- [112].Timm JA, Thoman ML. Maturation of CD4+ lymphocytes in the aged microenvironment results in a memory-enriched population. J Immunol. 1999;162:711–717. [PubMed] [Google Scholar]
- [113].Clise-Dwyer K, Huston GE, Buck AL, Duso DK, Swain SL. Environmental and intrinsic factors lead to antigen unresponsiveness in CD4(+) recent thymic emigrants from aged mice. J Immunol. 2007;178:1321–1331. doi: 10.4049/jimmunol.178.3.1321. [DOI] [PubMed] [Google Scholar]
- [114].Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, Nayak L, Moss PA. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169:1984–1992. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- [115].Pourgheysari B, Khan N, Best D, Bruton R, Nayak L, Moss PA. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J Virol. 2007;81:7759–7765. doi: 10.1128/JVI.01262-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Decman V, Laidlaw BJ, Dimenna LJ, Abdulla S, Mozdzanowska K, Erikson J, Ertl HC, Wherry EJ. Cell-intrinsic defects in the proliferative response of antiviral memory CD8 T cells in aged mice upon secondary infection. J Immunol. 2010;184:5151–5159. doi: 10.4049/jimmunol.0902063. [DOI] [PubMed] [Google Scholar]
- [117].Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- [118].Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;205:711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Rudd BD, Venturi V, Davenport MP, Nikolich-Zugich J. Evolution of the antigen-specific CD8+ TCR repertoire across the life span: evidence for clonal homogenization of the old TCR repertoire. J Immunol. 2011;186:2056–2064. doi: 10.4049/jimmunol.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Boucher N, Dufeu-Duchesne T, Vicaut E, Farge D, Effros RB, Schachter F. CD28 expression in T cell aging and human longevity. Exp Gerontol. 1998;33:267–282. doi: 10.1016/s0531-5565(97)00132-0. [DOI] [PubMed] [Google Scholar]
- [121].Spaulding C, Guo W, Effros RB. Resistance to apoptosis in human CD8+ T cells that reach replicative senescence after multiple rounds of antigen-specific proliferation. Exp Gerontol. 1999;34:633–644. doi: 10.1016/s0531-5565(99)00033-9. [DOI] [PubMed] [Google Scholar]
- [122].Effros RB, Allsopp R, Chiu CP, Hausner MA, Hirji K, Wang L, Harley CB, Villeponteau B, West MD, Giorgi JV. Shortened telomeres in the expanded CD28-CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 1996;10:F17–22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- [123].Sansoni P, Vescovini R, Fagnoni F, Biasini C, Zanni F, Zanlari L, Telera A, Lucchini G, Passeri G, Monti D, Franceschi C, Passeri M. The immune system in extreme longevity. Exp Gerontol. 2008;43:61–65. doi: 10.1016/j.exger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- [124].Ouyang Q, Wagner WM, Wikby A, Walter S, Aubert G, Dodi AI, Travers P, Pawelec G. Large numbers of dysfunctional CD8+ T lymphocytes bearing receptors for a single dominant CMV epitope in the very old. J Clin Immunol. 2003;23:247–257. doi: 10.1023/a:1024580531705. [DOI] [PubMed] [Google Scholar]
- [125].Schirmer M, Goldberger C, Wurzner R, Duftner C, Pfeiffer KP, Clausen J, Neumayr G, Falkenbach A. Circulating cytotoxic CD8(+) CD28(−) T cells in ankylosing spondylitis. Arthritis Res. 2002;4:71–76. doi: 10.1186/ar386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Tsukishiro T, Donnenberg AD, Whiteside TL. Rapid turnover of the CD8(+)CD28(−) T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol Immunother. 2003;52:599–607. doi: 10.1007/s00262-003-0395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Pilch H, Hoehn H, Schmidt M, Steiner E, Tanner B, Seufert R, Maeurer M. CD8+CD45RA+CD27-CD28-T-cell subset in PBL of cervical cancer patients representing CD8+T-cells being able to recognize cervical cancer associated antigens provided by HPV 16 E7. Zentralbl Gynakol. 2002;124:406–412. doi: 10.1055/s-2002-38130. [DOI] [PubMed] [Google Scholar]
- [128].Cortesini R, LeMaoult J, Ciubotariu R, Cortesini NS. CD8+CD28- T suppressor cells and the induction of antigen-specific, antigen-presenting cell-mediated suppression of Th reactivity. Immunol Rev. 2001;182:201–206. doi: 10.1034/j.1600-065x.2001.1820116.x. [DOI] [PubMed] [Google Scholar]
- [129].Saurwein-Teissl M, Lung TL, Marx F, Gschosser C, Asch E, Blasko I, Parson W, Bock G, Schonitzer D, Trannoy E, Grubeck-Loebenstein B. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- [130].Wing K, Ekmark A, Karlsson H, Rudin A, Suri-Payer E. Characterization of human CD25+ CD4+ T cells in thymus, cord and adult blood. Immunology. 2002;106:190–199. doi: 10.1046/j.1365-2567.2002.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Zhao L, Sun L, Wang H, Ma H, Liu G, Zhao Y. Changes of CD4+CD25+Foxp3+ regulatory T cells in aged Balb/c mice. J Leukoc Biol. 2007;81:1386–1394. doi: 10.1189/jlb.0506364. [DOI] [PubMed] [Google Scholar]
- [132].Tsaknaridis L, Spencer L, Culbertson N, Hicks K, LaTocha D, Chou YK, Whitham RH, Bakke A, Jones RE, Offner H, Bourdette DN, Vandenbark AA. Functional assay for human CD4+CD25+ Treg cells reveals an age-dependent loss of suppressive activity. J Neurosci Res. 2003;74:296–308. doi: 10.1002/jnr.10766. [DOI] [PubMed] [Google Scholar]
- [133].Rosenkranz D, Weyer S, Tolosa E, Gaenslen A, Berg D, Leyhe T, Gasser T, Stoltze L. Higher frequency of regulatory T cells in the elderly and increased suppressive activity in neurodegeneration. J Neuroimmunol. 2007;188:117–127. doi: 10.1016/j.jneuroim.2007.05.011. [DOI] [PubMed] [Google Scholar]
- [134].Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, Nayak L, Moss PA. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin Exp Immunol. 2005;140:540–546. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Chougnet CA, Tripathi P, Lages CS, Raynor J, Sholl A, Fink P, Plas DR, Hildeman DA. A major role for Bim in regulatory T cell homeostasis. J Immunol. 2011;186:156–163. doi: 10.4049/jimmunol.1001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Trzonkowski P, Szmit E, Mysliwska J, Mysliwski A. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of CTL and NK cells in humans-impact of immunosenescence. Clin Immunol. 2006;119:307–316. doi: 10.1016/j.clim.2006.02.002. [DOI] [PubMed] [Google Scholar]
- [137].Bryl E, Witkowski JM. Decreased proliferative capability of CD4(+) cells of elderly people is associated with faster loss of activation-related antigens and accumulation of regulatory T cells. Exp Gerontol. 2004;39:587–595. doi: 10.1016/j.exger.2003.10.029. [DOI] [PubMed] [Google Scholar]
- [138].Chiu BC, Stolberg VR, Zhang H, Chensue SW. Increased Foxp3(+) Treg cell activity reduces dendritic cell co-stimulatory molecule expression in aged mice. Mech Ageing Dev. 2007;128:618–627. doi: 10.1016/j.mad.2007.09.002. [DOI] [PubMed] [Google Scholar]
- [139].Williams-Bey Y, Jiang J, Murasko DM. Expansion of regulatory T cells in aged mice following influenza infection. Mech Ageing Dev. 2011;132:163–170. doi: 10.1016/j.mad.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Cusi MG, Martorelli B, Di Genova G, Terrosi C, Campoccia G, Correale P. Age related changes in T cell mediated immune response and effector memory to Respiratory Syncytial Virus (RSV) in healthy subjects. Immun Ageing. 2010;7:14. doi: 10.1186/1742-4933-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Tenorio AR, Spritzler J, Martinson J, Gichinga CN, Pollard RB, Lederman MM, Kalayjian RC, Landay AL. The effect of aging on T-regulatory cell frequency in HIV infection. Clin Immunol. 2009;130:298–303. doi: 10.1016/j.clim.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, Belkaid Y, Chougnet C. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181:1835–1848. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O’Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75:12182–12187. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Garcia GG, Berger SB, Sadighi Akha AA, Miller RA. Age-associated changes in glycosylation of CD43 and CD45 on mouse CD4 T cells. Eur J Immunol. 2005;35:622–631. doi: 10.1002/eji.200425538. [DOI] [PubMed] [Google Scholar]
- [145].Sadighi Akha AA, Miller RA. Signal transduction in the aging immune system. Curr Opin Immunol. 2005;17:486–491. doi: 10.1016/j.coi.2005.07.004. [DOI] [PubMed] [Google Scholar]
- [146].Pereira LF, de Souza AP, Borges TJ, Bonorino C. Impaired in vivo CD4+ T cell expansion and differentiation in aged mice is not solely due to T cell defects: decreased stimulation by aged dendritic cells. Mech Ageing Dev. 2011;132:187–194. doi: 10.1016/j.mad.2011.03.005. [DOI] [PubMed] [Google Scholar]
- [147].Cao JN, Gollapudi S, Sharman EH, Jia Z, Gupta S. Age-related alterations of gene expression patterns in human CD8+ T cells. Aging Cell. 2010;9:19–31. doi: 10.1111/j.1474-9726.2009.00534.x. [DOI] [PubMed] [Google Scholar]
- [148].Fulop T, Larbi A, Wikby A, Mocchegiani E, Hirokawa K, Pawelec G. Dysregulation of T-cell function in the elderly : scientific basis and clinical implications. Drugs Aging. 2005;22:589–603. doi: 10.2165/00002512-200522070-00005. [DOI] [PubMed] [Google Scholar]
- [149].Larbi A, Douziech N, Dupuis G, Khalil A, Pelletier H, Guerard KP, Fulop T., Jr Age-associated alterations in the recruitment of signal-transduction proteins to lipid rafts in human T lymphocytes. J Leukoc Biol. 2004;75:373–381. doi: 10.1189/jlb.0703319. [DOI] [PubMed] [Google Scholar]
- [150].Hallgren HM, Buckley CE, 3rd, Gilbertsen VA, Yunis EJ. Lymphocyte phytohemagglutinin responsiveness, immunoglobulins and autoantibodies in aging humans. J Immunol. 1973;111:1101–1107. [PubMed] [Google Scholar]
- [151].Haynes L, Linton PJ, Eaton SM, Tonkonogy SL, Swain SL. Interleukin 2, but not other common gamma chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999;190:1013–1024. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Ouyang Q, Wagner WM, Wikby A, Remarque E, Pawelec G. Compromised interferon gamma (IFN-gamma) production in the elderly to both acute and latent viral antigen stimulation: contribution to the immune risk phenotype? Eur Cytokine Netw. 2002;13:392–394. [PubMed] [Google Scholar]
- [153].Rink L, Cakman I, Kirchner H. Altered cytokine production in the elderly. Mech Ageing Dev. 1998;102:199–209. doi: 10.1016/s0047-6374(97)00153-x. [DOI] [PubMed] [Google Scholar]
- [154].Haynes L, Eaton SM, Burns EM, Rincon M, Swain SL. Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo. J Immunol. 2004;172:5194–5199. doi: 10.4049/jimmunol.172.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Linton PJ, Haynes L, Klinman NR, Swain SL. Antigen-independent changes in naive CD4 T cells with aging. J Exp Med. 1996;184:1891–1900. doi: 10.1084/jem.184.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Candore G, Di Lorenzo G, Melluso M, Cigna D, Colucci AT, Modica MA, Caruso C. gamma-Interferon, interleukin-4 and interleukin-6 in vitro production in old subjects. Autoimmunity. 1993;16:275–280. doi: 10.3109/08916939309014646. [DOI] [PubMed] [Google Scholar]
- [157].al-Rayes H, Pachas W, Mirza N, Ahern DJ, Geha RS, Vercelli D. IgE regulation and lymphokine patterns in aging humans. J Allergy Clin Immunol. 1992;90:630–636. doi: 10.1016/0091-6749(92)90136-p. [DOI] [PubMed] [Google Scholar]
- [158].Yen CJ, Lin SL, Huang KT, Lin RH. Age-associated changes in interferon-gamma and interleukin-4 secretion by purified human CD4+ and CD8+ T cells. J Biomed Sci. 2000;7:317–321. doi: 10.1007/BF02253251. [DOI] [PubMed] [Google Scholar]
- [159].Gardner EM, Murasko DM. Age-related changes in Type 1 and Type 2 cytokine production in humans. Biogerontology. 2002;3:271–290. doi: 10.1023/a:1020151401826. [DOI] [PubMed] [Google Scholar]
- [160].Ely KH, Roberts AD, Kohlmeier JE, Blackman MA, Woodland DL. Aging and CD8+ T cell immunity to respiratory virus infections. Exp Gerontol. 2007;42:427–431. doi: 10.1016/j.exger.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Toapanta FR, Ross TM. Impaired immune responses in the lungs of aged mice following influenza infection. Respir Res. 2009;10:112. doi: 10.1186/1465-9921-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200:1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].McElhaney JE, Upshaw CM, Hooton JW, Lechelt KE, Meneilly GS. Responses to influenza vaccination in different T-cell subsets: a comparison of healthy young and older adults. Vaccine. 1998;16:1742–1747. doi: 10.1016/s0264-410x(98)00133-9. [DOI] [PubMed] [Google Scholar]
- [164].Xie D, McElhaney JE. Lower GrB+ CD62Lhigh CD8 TCM effector lymphocyte response to influenza virus in older adults is associated with increased CD28null CD8 T lymphocytes. Mech Ageing Dev. 2007;128:392–400. doi: 10.1016/j.mad.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Gupta S, Gollapudi S. CD95-mediated apoptosis in naive, central and effector memory subsets of CD4+ and CD8+ T cells in aged humans. Exp Gerontol. 2008;43:266–274. doi: 10.1016/j.exger.2007.12.006. [DOI] [PubMed] [Google Scholar]
- [166].Smithey MJ, Renkema KR, Rudd BD, Nikolich-Zugich J. Increased apoptosis, curtailed expansion and incomplete differentiation of CD8+ T cells combine to decrease clearance of L. monocytogenes in old mice. Eur J Immunol. 2011;41:1352–1364. doi: 10.1002/eji.201041141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Jiang J, Bennett AJ, Fisher E, Williams-Bey Y, Shen H, Murasko DM. Limited expansion of virus-specific CD8 T cells in the aged environment. Mech Ageing Dev. 2009;130:713–721. doi: 10.1016/j.mad.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Jiang J, Fisher E, Bennett AJ, Murasko DM. Enhancement of virus-specific expansion of transgenic CD8 T cells in aged mice by dendritic cells. Mech Ageing Dev. 2010;131:580–583. doi: 10.1016/j.mad.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- [170].Faunce DE, Palmer JL, Paskowicz KK, Witte PL, Kovacs EJ. CD1d-restricted NKT cells contribute to the age-associated decline of T cell immunity. J Immunol. 2005;175:3102–3109. doi: 10.4049/jimmunol.175.5.3102. [DOI] [PubMed] [Google Scholar]
- [171].Kawabata T, Kinoshita M, Inatsu A, Habu Y, Nakashima H, Shinomiya N, Seki S. Functional alterations of liver innate immunity of mice with aging in response to CpG-oligodeoxynucleotide. Hepatology. 2008;48:1586–1597. doi: 10.1002/hep.22489. [DOI] [PubMed] [Google Scholar]
- [172].Zeng W, Maciejewski JP, Chen G, Risitano AM, Kirby M, Kajigaya S, Young NS. Selective reduction of natural killer T cells in the bone marrow of aplastic anaemia. Br J Haematol. 2002;119:803–809. doi: 10.1046/j.1365-2141.2002.03875.x. [DOI] [PubMed] [Google Scholar]
- [173].Mocchegiani E, Malavolta M. NK and NKT cell functions in immunosenescence. Aging Cell. 2004;3:177–184. doi: 10.1111/j.1474-9728.2004.00107.x. [DOI] [PubMed] [Google Scholar]
- [174].Stout-Delgado HW, Du W, Shirali AC, Booth CJ, Goldstein DR. Aging promotes neutrophil-induced mortality by augmenting IL-17 production during viral infection. Cell Host Microbe. 2009;6:446–456. doi: 10.1016/j.chom.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Tesar BM, Du W, Shirali AC, Walker WE, Shen H, Goldstein DR. Aging augments IL-17 T-cell alloimmune responses. Am J Transplant. 2009;9:54–63. doi: 10.1111/j.1600-6143.2008.02458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Frasca D, Landin AM, Riley RL, Blomberg BB. Mechanisms for decreased function of B cells in aged mice and humans. J Immunol. 2008;180:2741–2746. doi: 10.4049/jimmunol.180.5.2741. [DOI] [PubMed] [Google Scholar]
- [177].Ademokun A, Wu YC, Dunn-Walters D. The ageing B cell population: composition and function. Biogerontology. 2010;11:125–137. doi: 10.1007/s10522-009-9256-9. [DOI] [PubMed] [Google Scholar]
- [178].Chong Y, Ikematsu H, Yamaji K, Nishimura M, Nabeshima S, Kashiwagi S, Hayashi J. CD27(+) (memory) B cell decrease and apoptosis-resistant CD27(−) (naive) B cell increase in aged humans: implications for age-related peripheral B cell developmental disturbances. Int Immunol. 2005;17:383–390. doi: 10.1093/intimm/dxh218. [DOI] [PubMed] [Google Scholar]
- [179].Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter HH, Berner R, Peters A, Boehm T, Plebani A, Quinti I, Carsetti R. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197:939–945. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Shi Y, Yamazaki T, Okubo Y, Uehara Y, Sugane K, Agematsu K. Regulation of aged humoral immune defense against pneumococcal bacteria by IgM memory B cell. J Immunol. 2005;175:3262–3267. doi: 10.4049/jimmunol.175.5.3262. [DOI] [PubMed] [Google Scholar]
- [181].Powers DC. Effect of age on serum immunoglobulin G subclass antibody responses to inactivated influenza virus vaccine. J Med Virol. 1994;43:57–61. doi: 10.1002/jmv.1890430111. [DOI] [PubMed] [Google Scholar]
- [182].Rossi MI, Yokota T, Medina KL, Garrett KP, Comp PC, Schipul AH, Jr, Kincade PW. B lymphopoiesis is active throughout human life, but there are developmental age-related changes. Blood. 2003;101:576–584. doi: 10.1182/blood-2002-03-0896. [DOI] [PubMed] [Google Scholar]
- [183].McKenna RW, Washington LT, Aquino DB, Picker LJ, Kroft SH. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) in 662 consecutive bone marrow specimens by 4-color flow cytometry. Blood. 2001;98:2498–2507. doi: 10.1182/blood.v98.8.2498. [DOI] [PubMed] [Google Scholar]
- [184].Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- [185].Mackay F, Ambrose C. The TNF family members BAFF and APRIL: the growing complexity. Cytokine Growth Factor Rev. 2003;14:311–324. doi: 10.1016/s1359-6101(03)00023-6. [DOI] [PubMed] [Google Scholar]
- [186].Jin R, Kaneko H, Suzuki H, Arai T, Teramoto T, Fukao T, Kondo N. Age-related changes in BAFF and APRIL profiles and upregulation of BAFF and APRIL expression in patients with primary antibody deficiency. Int J Mol Med. 2008;21:233–238. [PubMed] [Google Scholar]
- [187].MacLennan I, Vinuesa C. Dendritic cells, BAFF, and APRIL: innate players in adaptive antibody responses. Immunity. 2002;17:235–238. doi: 10.1016/s1074-7613(02)00398-9. [DOI] [PubMed] [Google Scholar]
- [188].Tezuka H, Abe Y, Asano J, Sato T, Liu J, Iwata M, Ohteki T. Prominent role for plasmacytoid dendritic cells in mucosal T cell-independent IgA induction. Immunity. 2011;34:247–257. doi: 10.1016/j.immuni.2011.02.002. [DOI] [PubMed] [Google Scholar]
- [189].Gibson KL, Wu YC, Barnett Y, Duggan O, Vaughan R, Kondeatis E, Nilsson BO, Wikby A, Kipling D, Dunn-Walters DK. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009;8:18–25. doi: 10.1111/j.1474-9726.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Weksler ME. Changes in the B-cell repertoire with age. Vaccine. 2000;18:1624–1628. doi: 10.1016/s0264-410x(99)00497-1. [DOI] [PubMed] [Google Scholar]
- [191].Weksler ME, Szabo P. The effect of age on the B-cell repertoire. J Clin Immunol. 2000;20:240–249. doi: 10.1023/a:1006659401385. [DOI] [PubMed] [Google Scholar]
- [192].Hu A, Ehleiter D, Ben-Yehuda A, Schwab R, Russo C, Szabo P, Weksler ME. Effect of age on the expressed B cell repertoire: role of B cell subsets. Int Immunol. 1993;5:1035–1039. doi: 10.1093/intimm/5.9.1035. [DOI] [PubMed] [Google Scholar]
- [193].Delassus S, Gey A, Darche S, Cumano A, Roth C, Kourilsky P. PCR-based analysis of the murine immunoglobulin heavy-chain repertoire. J Immunol Methods. 1995;184:219–229. doi: 10.1016/0022-1759(95)00091-n. [DOI] [PubMed] [Google Scholar]
- [194].Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- [195].VanDyk L, Meek K. Assembly of IgH CDR3: mechanism, regulation, and influence on antibody diversity. Int Rev Immunol. 1992;8:123–133. doi: 10.3109/08830189209055568. [DOI] [PubMed] [Google Scholar]
- [196].van Dijk-Hard I, Soderstrom I, Feld S, Holmberg D, Lundkvist I. Age-related impaired affinity maturation and differential D-JH gene usage in human VH6-expressing B lymphocytes from healthy individuals. Eur J Immunol. 1997;27:1381–1386. doi: 10.1002/eji.1830270613. [DOI] [PubMed] [Google Scholar]
- [197].Wang X, Stollar BD. Immunoglobulin VH gene expression in human aging. Clin Immunol. 1999;93:132–142. doi: 10.1006/clim.1999.4781. [DOI] [PubMed] [Google Scholar]
- [198].Hijmans W, Radl J, Bottazzo GF, Doniach D. Autoantibodies in highly aged humans. Mech Ageing Dev. 1984;26:83–89. doi: 10.1016/0047-6374(84)90167-2. [DOI] [PubMed] [Google Scholar]
- [199].Whisler RL, Grants IS. Age-related alterations in the activation and expression of phosphotyrosine kinases and protein kinase C (PKC) among human B cells. Mech Ageing Dev. 1993;71:31–46. doi: 10.1016/0047-6374(93)90033-n. [DOI] [PubMed] [Google Scholar]
- [200].Zheng B, Han S, Takahashi Y, Kelsoe G. Immunosenescence and germinal center reaction. Immunol Rev. 1997;160:63–77. doi: 10.1111/j.1600-065x.1997.tb01028.x. [DOI] [PubMed] [Google Scholar]
- [201].Sayegh CE, Quong MW, Agata Y, Murre C. E-proteins directly regulate expression of activation-induced deaminase in mature B cells. Nat Immunol. 2003;4:586–593. doi: 10.1038/ni923. [DOI] [PubMed] [Google Scholar]
- [202].Frasca D, Van der Put E, Riley RL, Blomberg BB. Reduced Ig class switch in aged mice correlates with decreased E47 and activation-induced cytidine deaminase. J Immunol. 2004;172:2155–2162. doi: 10.4049/jimmunol.172.4.2155. [DOI] [PubMed] [Google Scholar]
- [203].Nussenzweig MC, Alt FW. Antibody diversity: one enzyme to rule them all. Nat Med. 2004;10:1304–1305. doi: 10.1038/nm1204-1304. [DOI] [PubMed] [Google Scholar]
- [204].Frasca D, Van der Put E, Landin AM, Gong D, Riley RL, Blomberg BB. RNA stability of the E2A-encoded transcription factor E47 is lower in splenic activated B cells from aged mice. J Immunol. 2005;175:6633–6644. doi: 10.4049/jimmunol.175.10.6633. [DOI] [PubMed] [Google Scholar]
- [205].Frasca D, Landin AM, Lechner SC, Ryan JG, Schwartz R, Riley RL, Blomberg BB. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J Immunol. 2008;180:5283–5290. doi: 10.4049/jimmunol.180.8.5283. [DOI] [PubMed] [Google Scholar]
- [206].LeMaoult J, Szabo P, Weksler ME. Effect of age on humoral immunity, selection of the B-cell repertoire and B-cell development. Immunol Rev. 1997;160:115–126. doi: 10.1111/j.1600-065x.1997.tb01032.x. [DOI] [PubMed] [Google Scholar]
- [207].Caraux A, Klein B, Paiva B, Bret C, Schmitz A, Fuhler GM, Bos NA, Johnsen HE, Orfao A, Perez-Andres M. Circulating human B and plasma cells. Age-associated changes in counts and detailed characterization of circulating normal CD138- and CD138+ plasma cells. Haematologica. 2010;95:1016–1020. doi: 10.3324/haematol.2009.018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- [209].U.S. Census Bureau International Data Base (IDB). [Available at: http://www.census.gov/ipc/www/idb/informationGateway.php. Accessed May 20, 2011].
- [210].Hoelscher M, Gangappa S, Zhong W, Jayashankar L, Sambhara S. Vaccines against epidemic and pandemic influenza. Expert Opin Drug Deliv. 2008;5:1139–1157. doi: 10.1517/17425247.5.10.1139. [DOI] [PubMed] [Google Scholar]
- [211].Katz JM, Plowden J, Renshaw-Hoelscher M, Lu X, Tumpey TM, Sambhara S. Immunity to influenza: the challenges of protecting an aging population. Immunol Res. 2004;29:113–124. doi: 10.1385/IR:29:1-3:113. [DOI] [PubMed] [Google Scholar]
- [212].Sambhara S, McElhaney JE. Immunosenescence and influenza vaccine efficacy. Curr Top Microbiol Immunol. 2009;333:413–429. doi: 10.1007/978-3-540-92165-3_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Sambhara S, Kurichh A, Miranda R, James O, Underdown B, Klein M, Tartaglia J, Burt D. Severe impairment of primary but not memory responses to influenza viral antigens in aged mice: costimulation in vivo partially reverses impaired primary immune responses. Cell Immunol. 2001;210:1–4. doi: 10.1006/cimm.2001.1799. [DOI] [PubMed] [Google Scholar]
- [214].Ferko B, Kittel C, Romanova J, Sereinig S, Katinger H, Egorov A. Live attenuated influenza virus expressing human interleukin-2 reveals increased immunogenic potential in young and aged hosts. J Virol. 2006;80:11621–11627. doi: 10.1128/JVI.01645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Sambhara S, Kurichh A, Miranda R, Tamane A, Arpino R, James O, McGuinness U, Kandil A, Underdown B, Klein M, Burt D. Enhanced immune responses and resistance against infection in aged mice conferred by Flu-ISCOMs vaccine correlate with up-regulation of costimulatory molecule CD86. Vaccine. 1998;16:1698–1704. doi: 10.1016/s0264-410x(98)00130-3. [DOI] [PubMed] [Google Scholar]
- [216].Sambhara S, Woods S, Arpino R, Kurichh A, Tamane A, Bengtsson KL, Morein B, Underdown B, Klein M, Burt D. Influenza (H1N1)-ISCOMs enhance immune responses and protection in aged mice. Mech Ageing Dev. 1997;96:157–169. doi: 10.1016/s0047-6374(97)01889-7. [DOI] [PubMed] [Google Scholar]
- [217].O’Hagan DT, Wack A, Podda A. MF59 is a safe and potent vaccine adjuvant for flu vaccines in humans: what did we learn during its development? Clin Pharmacol Ther. 2007;82:740–744. doi: 10.1038/sj.clpt.6100402. [DOI] [PubMed] [Google Scholar]
- [218].Baras B, Bouveret N, Devaster JM, Fries L, Gillard P, Sanger R, Hanon E. A vaccine manufacturer’s approach to address medical needs related to seasonal and pandemic influenza viruses. Influenza Other Respi Viruses. 2008;2:251–260. doi: 10.1111/j.1750-2659.2008.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Novartis Pharmaceuticals Canada, Inc. Health Canada approves Fluad, new influenza vaccine for seniors [Available at: http://www.novartis.ca/downloads/en/News/FLUAD_Release_Feb2010_eng.pdf. Accessed July 19, 2011].
- [220].Health Canada Health Canada has authorized the sale of Arepanrix H1N1 based on limited clinical testing in humans under the provision of an Interim Order (IO) issued on October 13, 2009. [Available at: http://hc-sc.gc.ca/dhp-mps/prodpharma/legislation/interimorders-arretesurgence/prodinfo-vaccin-eng.php. Accessed July 19, 2011].
- [221].Schultze V, D’Agosto V, Wack A, Novicki D, Zorn J, Hennig R. Safety of MF59 adjuvant. Vaccine. 2008;26:3209–3222. doi: 10.1016/j.vaccine.2008.03.093. [DOI] [PubMed] [Google Scholar]
- [222].Podda A. The adjuvanted influenza vaccines with novel adjuvants: experience with the MF59-adjuvanted vaccine. Vaccine. 2001;19:2673–2680. doi: 10.1016/s0264-410x(00)00499-0. [DOI] [PubMed] [Google Scholar]
- [223].O’Hagan DT, Rappuoli R, De Gregorio E, Tsai T, Del Giudice G. MF59 adjuvant: the best insurance against influenza strain diversity. Expert Rev Vaccines. 2011;10:447–462. doi: 10.1586/erv.11.23. [DOI] [PubMed] [Google Scholar]
- [224].Parodi V, de Florentiis D, Martini M, Ansaldi F. Inactivated influenza vaccines: recent progress and implications for the elderly. Drugs Aging. 2011;28:93–106. doi: 10.2165/11586770-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [225].De Donato S, Granoff D, Minutello M, Lecchi G, Faccini M, Agnello M, Senatore F, Verweij P, Fritzell B, Podda A. Safety and immunogenicity of MF59-adjuvanted influenza vaccine in the elderly. Vaccine. 1999;17:3094–3101. doi: 10.1016/s0264-410x(99)00138-3. [DOI] [PubMed] [Google Scholar]
- [226].Minutello M, Senatore F, Cecchinelli G, Bianchi M, Andreani T, Podda A, Crovari P. Safety and immunogenicity of an inactivated subunit influenza virus vaccine combined with MF59 adjuvant emulsion in elderly subjects, immunized for three consecutive influenza seasons. Vaccine. 1999;17:99–104. doi: 10.1016/s0264-410x(98)00185-6. [DOI] [PubMed] [Google Scholar]
- [227].Gasparini R, Pozzi T, Montomoli E, Fragapane E, Senatore F, Minutello M, Podda A. Increased immunogenicity of the MF59-adjuvanted influenza vaccine compared to a conventional subunit vaccine in elderly subjects. Eur J Epidemiol. 2001;17:135–140. doi: 10.1023/a:1017919305501. [DOI] [PubMed] [Google Scholar]
- [228].Galli G, Hancock K, Hoschler K, DeVos J, Praus M, Bardelli M, Malzone C, Castellino F, Gentile C, McNally T, Del Giudice G, Banzhoff A, Brauer V, Montomoli E, Zambon M, Katz J, Nicholson K, Stephenson I. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc Natl Acad Sci U S A. 2009;106:7962–7967. doi: 10.1073/pnas.0903181106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Fragapane E, Gasparini R, Schioppa F, Laghi-Pasini F, Montomoli E, Banzhoff A. A heterologous MF59-adjuvanted H5N1 prepandemic influenza booster vaccine induces a robust, cross-reactive immune response in adults and the elderly. Clin Vaccine Immunol. 2010;17:1817–1819. doi: 10.1128/CVI.00461-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Galli G, Medini D, Borgogni E, Zedda L, Bardelli M, Malzone C, Nuti S, Tavarini S, Sammicheli C, Hilbert AK, Brauer V, Banzhoff A, Rappuoli R, Del Giudice G, Castellino F. Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proc Natl Acad Sci U S A. 2009;106:3877–3882. doi: 10.1073/pnas.0813390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Wack A, Baudner BC, Hilbert AK, Manini I, Nuti S, Tavarini S, Scheffczik H, Ugozzoli M, Singh M, Kazzaz J, Montomoli E, Del Giudice G, Rappuoli R, O’Hagan DT. Combination adjuvants for the induction of potent, long-lasting antibody and T-cell responses to influenza vaccine in mice. Vaccine. 2008;26:552–561. doi: 10.1016/j.vaccine.2007.11.054. [DOI] [PubMed] [Google Scholar]
- [232].Higgins DA, Carlson JR, Van Nest G. MF59 adjuvant enhances the immunogenicity of influenza vaccine in both young and old mice. Vaccine. 1996;14:478–484. doi: 10.1016/0264-410x(95)00240-2. [DOI] [PubMed] [Google Scholar]
- [233].Clinical Trials.gov, trial registered as NCT 00382187. [Available at: http://clinicaltrials.gov. Accessed May 18, 2011].
- [234].Wang M, Lamberth K, Harndahl M, Roder G, Stryhn A, Larsen MV, Nielsen M, Lundegaard C, Tang ST, Dziegiel MH, Rosenkvist J, Pedersen AE, Buus S, Claesson MH, Lund O. CTL epitopes for influenza A including the H5N1 bird flu; genome-, pathogen-, and HLA-wide screening. Vaccine. 2007;25:2823–2831. doi: 10.1016/j.vaccine.2006.12.038. [DOI] [PubMed] [Google Scholar]
- [235].Jameson J, Cruz J, Terajima M, Ennis FA. Human CD8+ and CD4+ T lymphocyte memory to influenza A viruses of swine and avian species. J Immunol. 1999;162:7578–7583. [PubMed] [Google Scholar]
- [236].Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F, Iavarone C, O’Hagan D, Rappuoli R, De Gregorio E. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci U S A. 2008;105:10501–10506. doi: 10.1073/pnas.0804699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Seubert A, Monaci E, Pizza M, O’Hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol. 2008;180:5402–5412. doi: 10.4049/jimmunol.180.8.5402. [DOI] [PubMed] [Google Scholar]
- [238].Dupuis M, Denis-Mize K, LaBarbara A, Peters W, Charo IF, McDonald DM, Ott G. Immunization with the adjuvant MF59 induces macrophage trafficking and apoptosis. Eur J Immunol. 2001;31:2910–2918. doi: 10.1002/1521-4141(2001010)31:10<2910::aid-immu2910>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [239].Dupuis M, Murphy TJ, Higgins D, Ugozzoli M, van Nest G, Ott G, McDonald DM. Dendritic cells internalize vaccine adjuvant after intramuscular injection. Cell Immunol. 1998;186:18–27. doi: 10.1006/cimm.1998.1283. [DOI] [PubMed] [Google Scholar]
- [240].Leroux-Roels I, Borkowski A, Vanwolleghem T, Drame M, Clement F, Hons E, Devaster JM, Leroux-Roels G. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet. 2007;370:580–589. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- [241].Moris P, van der Most R, Leroux-Roels I, Clement F, Drame M, Hanon E, Leroux-Roels GG, Van Mechelen M. H5N1 Influenza Vaccine Formulated with AS03(A) Induces Strong Cross-Reactive and Polyfunctional CD4 T-Cell Responses. J Clin Immunol. 2011;31:443–454. doi: 10.1007/s10875-010-9490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Leroux-Roels I, Roman F, Forgus S, Maes C, De Boever F, Drame M, Gillard P, van der Most R, Van Mechelen M, Hanon E, Leroux-Roels G. Priming with AS03 A-adjuvanted H5N1 influenza vaccine improves the kinetics, magnitude and durability of the immune response after a heterologous booster vaccination: an open non-randomised extension of a double-blind randomised primary study. Vaccine. 2010;28:849–857. doi: 10.1016/j.vaccine.2009.10.017. [DOI] [PubMed] [Google Scholar]
- [243].Heijmans S, De Meulemeester M, Reynders P, Giet D, Demanet E, Devresse PY, Icardi G, Drame M, Roman F, Gillard P. Immunogenicity profile of a 3.75-mug hemagglutinin pandemic rH5N1 split virion AS03A-adjuvanted vaccine in elderly persons: a randomized trial. J Infect Dis. 2011;203:1054–1062. doi: 10.1093/infdis/jiq174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Roman F, Vaman T, Kafeja F, Hanon E, Van Damme P. AS03(A)-Adjuvanted influenza A (H1N1) 2009 vaccine for adults up to 85 years of age. Clin Infect Dis. 2010;51:668–677. doi: 10.1086/655830. [DOI] [PubMed] [Google Scholar]
- [245].Morel S, Didierlaurent A, Bourguignon P, Delhaye S, Baras B, Jacob V, Planty C, Elouahabi A, Harvengt P, Carlsen H, Kielland A, Chomez P, Garcon N, Van Mechelen M. Adjuvant System AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29:2461–2473. doi: 10.1016/j.vaccine.2011.01.011. [DOI] [PubMed] [Google Scholar]
- [246].Couch RB, Winokur P, Brady R, Belshe R, Chen WH, Cate TR, Sigurdardottir B, Hoeper A, Graham IL, Edelman R, He F, Nino D, Capellan J, Ruben FL. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine. 2007;25:7656–7663. doi: 10.1016/j.vaccine.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Keitel WA, Atmar RL, Cate TR, Petersen NJ, Greenberg SB, Ruben F, Couch RB. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med. 2006;166:1121–1127. doi: 10.1001/archinte.166.10.1121. [DOI] [PubMed] [Google Scholar]
- [248].Palache AM, Beyer WE, Sprenger MJ, Masurel N, de Jonge S, Vardy A, Charpentier B, Noury J, van Beek WC, Borst RJ, et al. Antibody response after influenza immunization with various vaccine doses: a double-blind, placebo-controlled, multi-centre, dose-response study in elderly nursing-home residents and young volunteers. Vaccine. 1993;11:3–9. doi: 10.1016/0264-410x(93)90333-s. [DOI] [PubMed] [Google Scholar]
- [249].Chen WH, Cross AS, Edelman R, Sztein MB, Blackwelder WC, Pasetti MF. Antibody and Th1-type cell-mediated immune responses in elderly and young adults immunized with the standard or a high dose influenza vaccine. Vaccine. 2011;29:2865–2873. doi: 10.1016/j.vaccine.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Sullivan SJ, Jacobson R, Poland GA. Advances in the vaccination of the elderly against influenza: role of a high-dose vaccine. Expert Rev Vaccines. 2010;9:1127–1133. doi: 10.1586/erv.10.117. [DOI] [PubMed] [Google Scholar]
- [251].U.S. Food and Drug Administration (FDA) News and Events. December 23, 2009. [Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm195483.htm. Accessed May 10, 2011].
- [252].Centers for Disease Control and Prevention Licensure of a high-dose inactivated influenza vaccine for persons aged > or = 65 years (Fluzone High-Dose) and guidance for use – United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:485–486. [PubMed] [Google Scholar]
- [253].Huckriede A, Bungener L, Stegmann T, Daemen T, Medema J, Palache AM, Wilschut J. The virosome concept for influenza vaccines. Vaccine. 2005;23(Suppl 1):S26–38. doi: 10.1016/j.vaccine.2005.04.026. [DOI] [PubMed] [Google Scholar]
- [254].Stegmann T, Morselt HW, Booy FP, van Breemen JF, Scherph of G, Wilschut J. Functional reconstitution of influenza virus envelopes. EMBO J. 1987;6:2651–2659. doi: 10.1002/j.1460-2075.1987.tb02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Bungener L, Huckriede A, de Mare A, de Vries-Idema J, Wilschut J, Daemen T. Virosome-mediated delivery of protein antigens in vivo: efficient induction of class I MHC-restricted cytotoxic T lymphocyte activity. Vaccine. 2005;23:1232–1241. doi: 10.1016/j.vaccine.2004.09.002. [DOI] [PubMed] [Google Scholar]
- [256].Calcagnile S, Zuccotti GV. The virosomal adjuvanted influenza vaccine. Expert Opin Biol Ther. 2010;10:191–200. doi: 10.1517/14712590903431014. [DOI] [PubMed] [Google Scholar]
- [257].de Bruijn IA, Nauta J, Gerez L, Palache AM. Virosomal influenza vaccine: a safe and effective influenza vaccine with high efficacy in elderly and subjects with low pre-vaccination antibody titers. Virus Res. 2004;103:139–145. doi: 10.1016/j.virusres.2004.02.026. [DOI] [PubMed] [Google Scholar]
- [258].Holm KJ, Goa KL. Liposomal influenza vaccine. BioDrugs. 1999;11:137–144. doi: 10.2165/00063030-199911020-00007. [DOI] [PubMed] [Google Scholar]
- [259].Kunzi V, Dornseiff M, Horwath J, Hartmann K. Safe vaccination of children with a virosomal adjuvanted influenza vaccine. Vaccine. 2009;27:1261–1265. doi: 10.1016/j.vaccine.2008.12.008. [DOI] [PubMed] [Google Scholar]
- [260].Amendola A, Boschini A, Colzani D, Anselmi G, Oltolina A, Zucconi R, Begnini M, Besana S, Tanzi E, Zanetti AR. Influenza vaccination of HIV-1-positive and HIV-1-negative former intravenous drug users. J Med Virol. 2001;65:644–648. doi: 10.1002/jmv.2085. [DOI] [PubMed] [Google Scholar]
- [261].de Bruijn I, Meyer I, Gerez L, Nauta J, Giezeman K, Palache B. Antibody induction by virosomal, MF59-adjuvanted, or conventional influenza vaccines in the elderly. Vaccine. 2007;26:119–127. doi: 10.1016/j.vaccine.2007.10.051. [DOI] [PubMed] [Google Scholar]
- [262].Conne P, Gauthey L, Vernet P, Althaus B, Que JU, Finkel B, Gluck R, Cryz SJ., Jr Immunogenicity of trivalent subunit versus virosome-formulated influenza vaccines in geriatric patients. Vaccine. 1997;15:1675–1679. doi: 10.1016/s0264-410x(97)00087-x. [DOI] [PubMed] [Google Scholar]
- [263].Pregliasco F, Mensi C, Serpilli W, Speccher L, Masella P, Belloni A. Immunogenicity and safety of three commercial influenza vaccines in institutionalized elderly. Aging (Milano) 2001;13:38–43. doi: 10.1007/BF03351492. [DOI] [PubMed] [Google Scholar]
- [264].Baldo V, Baldovin T, Pellegrini M, Angiolelli G, Majori S, Floreani A, Busana MC, Bertoncello C, Trivello R. Immunogenicity of three different influenza vaccines against homologous and heterologous strains in nursing home elderly residents. Clin Dev Immunol. 2010;2010:517198. doi: 10.1155/2010/517198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Ruf BR, Colberg K, Frick M, Preusche A. Open, randomized study to compare the immunogenicity and reactogenicity of an influenza split vaccine with an MF59-adjuvanted subunit vaccine and a virosome-based subunit vaccine in elderly. Infection. 2004;32:191–198. doi: 10.1007/s15010-004-3204-z. [DOI] [PubMed] [Google Scholar]
- [266].Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351:2295–2301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- [267].Ueno H, Klechevsky E, Schmitt N, Ni L, Flamar AL, Zurawski S, Zurawski G, Palucka K, Banchereau J, Oh S. Targeting human dendritic cell subsets for improved vaccines. Semin Immunol. 2011;23:21–27. doi: 10.1016/j.smim.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity. 2010;33:464–478. doi: 10.1016/j.immuni.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14:75–78. doi: 10.1016/0167-5699(93)90062-P. [DOI] [PubMed] [Google Scholar]
- [270].Frech SA, Kenney RT, Spyr CA, Lazar H, Viret JF, Herzog C, Gluck R, Glenn GM. Improved immune responses to influenza vaccination in the elderly using an immunostimulant patch. Vaccine. 2005;23:946–950. doi: 10.1016/j.vaccine.2004.06.036. [DOI] [PubMed] [Google Scholar]
- [271].Beran J, Ambrozaitis A, Laiskonis A, Mickuviene N, Bacart P, Calozet Y, Demanet E, Heijmans S, Van Belle P, Weber F, Salamand C. Intradermal influenza vaccination of healthy adults using a new microinjection system: a 3-year randomised controlled safety and immunogenicity trial. BMC Med. 2009;7:13. doi: 10.1186/1741-7015-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Amorij JP, Hinrichs W, Frijlink HW, Wilschut JC, Huckriede A. Needle-free influenza vaccination. Lancet Infect Dis. 2010;10:699–711. doi: 10.1016/S1473-3099(10)70157-2. [DOI] [PubMed] [Google Scholar]
- [273].Ansaldi F, Durando P, Icardi G. Intradermal influenza vaccine and new devices: a promising chance for vaccine improvement. Expert Opin Biol Ther. 2011;11:415–427. doi: 10.1517/14712598.2011.557658. [DOI] [PubMed] [Google Scholar]
- [274].Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, McKeirnan M, Salem H, Mills G, Reid J, Weber F, Saville M. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis. 2008;198:650–658. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- [275].Arnou R, Icardi G, De Decker M, Ambrozaitis A, Kazek MP, Weber F, Van Damme P. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine. 2009;27:7304–7312. doi: 10.1016/j.vaccine.2009.10.033. [DOI] [PubMed] [Google Scholar]
- [276].Chi RC, Rock MT, Neuzil KM. Immunogenicity and safety of intradermal influenza vaccination in healthy older adults. Clin Infect Dis. 2010;50:1331–1338. doi: 10.1086/652144. [DOI] [PubMed] [Google Scholar]
- [277].European Medicines Agency Intanza influenza vaccine (split virion, inactivated). [Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000957/human_med_000842.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d124. Accessed.
- [278].Sanofi Pasteur. Sanofi Pasteur introduces new option for flu vaccination - tiny needle, same effectiveness as traditional flu shots. [Available at: http://www.sanofipasteur.com/sanofi-pasteur2/front/index.jsp?siteCode=SP_CORP&codeRubrique=34&codePage=PAG_34_PR11. Accessed July 21, 2011].
- [279].U.S. Food and Drug Administration May 9, 2011. Approval Letter - Fluzone Intradermal. [Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm255160.htm. Accessed July 21, 2011].
- [280].Van Damme P, Arnou R, Kafeja F, Fiquet A, Richard P, Thomas S, Meghlaoui G, Samson SI, Ledesma E. Evaluation of non-inferiority of intradermal versus adjuvanted seasonal influenza vaccine using two serological techniques: a randomised comparative study. BMC Infect Dis. 2010;10:134. doi: 10.1186/1471-2334-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Maue AC, Eaton SM, Lanthier PA, Sweet KB, Blumerman SL, Haynes L. Proinflammatory adjuvants enhance the cognate helper activity of aged CD4 T cells. J Immunol. 2009;182:6129–6135. doi: 10.4049/jimmunol.0804226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].McElhaney JE. Influenza vaccine responses in older adults. Ageing Res Rev. 2011;10:379–388. doi: 10.1016/j.arr.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]