Abstract
Stress hyperglycemia (SH) commonly occurs during critical illness in children. The historical view that SH is beneficial has been questioned in light of evidence that demonstrates the association of SH with worse outcomes. In addition to intrinsic changes in glucose metabolism and development of insulin resistance, specific intensive care unit (ICU) practices may influence the development of SH during critical illness. Mechanical ventilation, vasoactive infusions, renal replacement therapies, cardiopulmonary bypass and extracorporeal life support, therapeutic hypothermia, prolonged immobility, nutrition support practices, and the use of medications are all known to mediate development of SH in critical illness. Tight glucose control (TGC) to manage SH has emerged as a promising therapy to improve outcomes in critically ill adults, but results have been inconclusive. Large variations in ICU practices across studies likely resulted in inconsistent results. Future studies of TGC need to take into account the impact of commonly used ICU practices and, ideally, standardize protocols in an attempt to improve the accuracy of conclusions from such studies.
Keywords: blood glucose, children, critical illness, stress hyperglycemia, tight glucose control
Introduction
Stress hyperglycemia (SH) commonly occurs during critical illness in children, even in those with previously normal glucose homeostasis.1–7 Historically, SH during pediatric critical illness was considered to be, at best, an adaptive response that improved survival or, at worst, inconsequential.8,9 However, studies in children have challenged this assertion by observing that SH during critical illness is associated with poor outcomes.1–7,10–16 Based on the premise that SH during critical illness is possibly harmful, tight glucose control (TGC) to normalize blood glucose (BG) concentrations has emerged as a rational but unproven therapy to improve outcomes in critically ill children. Studies of TGC in critically ill adults have had mixed results, with some observing worse outcomes from TGC.17–21 Notably, all studies of TGC in critically ill adults observed significant increases in hypoglycemia.17–21 Consequently, the initial rush to embrace this therapy has justifiably given way to a more cautious approach in the adult critical care community.22 Various reasons have been put forth to explain the observed differences in results of these trials. These include disparities in patient populations, differences in glucose control targets, variability in attaining these targets, differences in glucose control protocols and nutrition delivery, variable sampling and measurement techniques, and variable expertise in protocol implementation.23
The pediatric critical care community faces an even greater dilemma due to the lack of large-scale clinical trials of TGC in critically ill children. A single-center study of TGC in critically ill children predominantly recovering from cardiac surgery observed reductions in inflammation and length of intensive care unit (ICU) stay, but at the cost of a substantial increase in hypoglycemia.24 While most practitioners agree that SH is likely harmful and should be avoided in critically ill children, they worry about iatrogenic hypoglycemia and few use a standardized approach to TGC.25,26 This review examines the mechanisms for development of SH and discusses the impact of factors specific to the environment of the ICU on the development of SH and resulting implications for TGC in critically ill children.
Stress Hyperglycemia in Pediatric Critical Illness
Stress hyperglycemia is common in pediatric critical illness, with an estimated 49–72% of children experiencing BG concentrations >150 mg/dl (>8.3 mmol/liter).1–7 Additionally, it is estimated that BG concentrations >200 mg/dl (>11 mmol/liter) occur in as many as 20–35% of critically ill children.1–7 In comparison, 3.8–5% of children presented to the emergency room experience BG levels >150 mg/dl (8.3 mmol/liter).27,28 Peak BG concentrations in critically ill children can often range as high as 172 + 78 mg/dl (9.6 + 4.3 mmol/liter) to 283 + 115 mg/dl (15.7 + 6.4 mmol/liter).1,2,6,29 Stress hyperglycemia can also remain sustained over a prolonged period of ICU admission (ranging from 42 + 14% to 44 + 28% of duration of ICU stay).1,29
Several studies have demonstrated the association of SH in critically ill children with mortality.1–5,10–16 Specifically, peak and duration of SH appear to be associated with mortality. Peak BG concentrations tend to be much higher in nonsurvivors compared with survivors.1–5 Similarly, non-survivors tend to have exposure to longer duration of SH compared with survivors.1,29 This association of SH with mortality appears across different pediatric disease states, including septic shock, burns, traumatic brain injury, post cardiac surgery, and trauma.10–16 Additionally, SH is associated with longer periods of ICU and hospital stay and more frequent nosocomial infections, including surgical site infections in critically ill children.2–6,29,30 While all these studies demonstrate strong associations between SH and poor clinical outcomes, they do not necessarily demonstrate a cause and effect relationship, because SH tends to be more marked in patients with greater illness severity. Table 1 summarizes key pediatric studies that have examined the association between SH and mortality in critically ill children.
Table 1.
Key Studies of Association of Stress Hyperglycemia and Mortality in Critically Ill Children
| Study (number denotes study reference) | Setting/patient population | Sample size | Definition of SH | Mortality associated with SH | |
|---|---|---|---|---|---|
| OR/RR | 95% CI | ||||
| Srinivasan1 | ICU | 152 | BG ≥150 mg/dl (≥8.3 mmol/liter) | OR 3.4 | 1.4–8.6 |
| Faustino2 | ICU | 942 | BG ≥150 mg/dl (≥8.3 mmol/liter) | RR 2.5 | 1.3–4.9 |
| Wintergerst3 | ICU | 1094 | BG >150 mg/dl (>8.3 mmol/liter) | RR 4.8 | 1.2–19.5 |
| Yung4 | ICU | 409 | BG >126 mg/dl (>7.0 mmol/liter) | OR 3.1 | 1.3–7.7 |
| Hirshberg5 | ICU | 863 | BG ≥150 mg/dl (≥8.3 mmol/liter) | OR 11.1 | 1.5–85.6 |
| Gore10 | Burns | 58 | BG ≥140 mg/dl (≥7.8 mmol/liter) | RR 5.1 | 2.1–12.7 |
| Michaud12 | Traumatic brain injury | 54 | BG ≥250 mg/dl (≥13.9 mmol/liter) | OR 8.3 | 1.3–53.6 |
| Branco13 | Septic shock | 57 | BG >178 mg/dl (>9.9 mmol/liter) | RR 2.6 | 1.4–4.9 |
| Yates14 | Cardiac surgery | 184 | BG ≥126 mg/dl (≥7.0 mmol/liter) | OR 1.5 | Not specified |
RR, relative risk; OR, odds ratio; CI, confidence interval
Pathophysiology of Stress Hyperglycemia
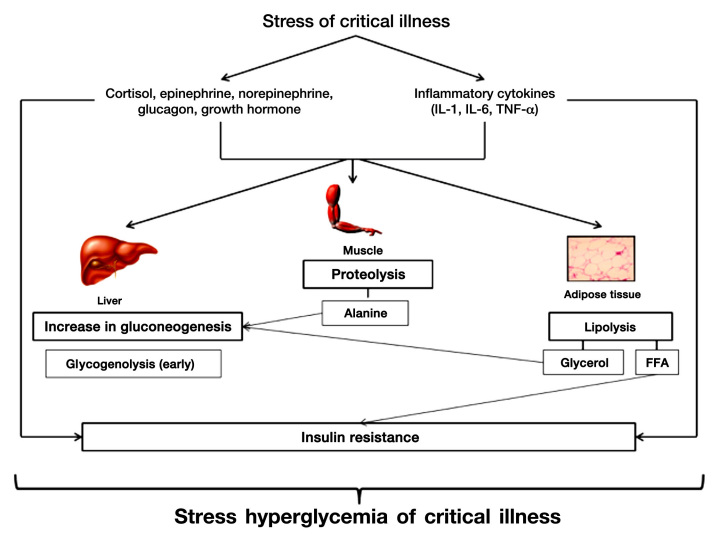
Critical illness is characterized by injury to the cellular environment from a variety of factors such as hypoxia, oxidative stress, systemic inflammation, and reduced or redistributed blood flow. In the setting of critical illness, SH develops principally through a combination of (1) increased gluconeogenesis relative to glucose clearance and (2) development of insulin resistance affecting cellular uptake of glucose31 (Figure 1). Both of these mechanisms appear to be mediated via increases in counterregulatory hormones (i.e., epinephrine, norepinephrine, glucagon, cortisol, growth hormone) and proinflammatory cytokines [tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), interleukin-6 (IL-6)].32,33 Additionally, proinflammatory cytokines may directly inhibit insulin secretion by pancreatic β cells through stimulation of a adrenergic receptors.34 The overall effect of SH in critical illness is to increase BG concentrations and provide a ready source of fuel for vital organs in the body at a time of increased metabolic demand. While initially SH may represent an adaptive response by the body during the acute phase of illness to improve the likelihood of survival, persistence of SH during chronic illness may be harmful.
Figure 1.
Pathophysiology of stress hyperglycemia in critical illness.
Alterations in Glucose Metabolism
During health, a balanced combination of glycogenolysis and gluconeogenesis maintains adequate BG concentrations between meals. After a meal, elevated BG concentrations result in insulin release with suppression of gluconeogenesis and increased formation of glycogen. During conditions of stress, increased concentrations of counter-regulatory hormones and proinflammatory cytokines initially mediate rapid glycogenolysis and gluconeogenesis, resulting in elevated BG concentrations.31 Glycogen stores are rapidly depleted in the unfed state, with glycogenolysis contributing to limited glucose production.32 However, hepatic gluconeogenesis persists, resulting in increased glucose production and development of SH.35,36 The surge in catecholamines during critical illness results in increased levels of glucagon so that gluconeogenesis is maintained even in the presence of elevated levels of insulin.37 The kidney is also an important source of gluconeogenesis in critical illness and may account for up to 40% of glucose production in response to catecholamines.38 Other hormonal changes, such as increase in growth hormone (GH) and reduction in insulin-like growth factor-1 (IGF-1), facilitate the breakdown of muscle to release alanine to the liver to support continued gluconeogenesis.39
Alterations in Insulin Sensitivity and Secretion
Critical illness is characterized by development of both central and peripheral insulin resistance. Central insulin resistance at the level of the liver is mediated by glucagon, epinephrine, and cortisol, resulting in sustained hepatic gluconeogenesis even in the face of elevated levels of insulin.37 Hepatic insulin resistance is also associated with increase in GH and reduction in IGF-1 levels.39 Peripheral insulin resistance occurs in muscle and adipose tissue due to alterations in the insulin-signaling pathway mediated largely by counter regulatory hormones and inflammatory cytokines. Increased cortisol, GH, and epinephrine levels in critical illness impair the translocation of the insulin-dependent glucose transporter protein 4 (GLUT-4) from internal membrane stores and reduce insulin binding.40,41 Inflammatory cytokines affect serine phosphorylation of insulin receptor substrate 1 and inhibit insulin receptor tyrosine kinase, thereby reducing cellular utilization of glucose via GLUT-4.42,43 Increased free fatty acid (FFA) concentrations due to lipolysis mediated by catecholamines and GH also increase insulin resistance.42,43 Such acquired peripheral insulin resistance may persist for an extended period of time following recovery from critical illness in children.44 In addition to the development of insulin resistance, studies have also demonstrated abnormalities in pancreatic β-cell function and reduced insulin secretion in critically ill children.45,46
Stress Hyperglycemia and Harm
Acute SH has more adverse consequences in critically ill patients than in healthy individuals or even patients with diabetes.47 Under normal conditions, elevated BG concentrations stimulate secretion of insulin by the pancreas, which in turn blocks hepatic glucose production and stimulates glucose uptake by the liver, muscle, and adipose tissue. Peripheral glucose uptake is regulated by the GLUT family of proteins via facilitated diffusion. Elevated BG concentrations downregulate insulin-independent GLUT-1, GLUT-2, and GLUT-3 to prevent cellular glucose overload. In contrast, critical illness causes an overexpression of these transporters, leading to glucose overload and toxicity in organ systems that express these transporters. Upregulation of these insulin-independent GLUTs is seen in the central and peripheral nervous systems, as well as in endothelial, hepatic and immune cells, renal tubules, and gastrointestinal mucosa.47 Glucose overload results in excessive glycolysis and oxidative phosphorylation, with increased production of reactive oxygen species (ROS), such as peroxynitrite and superoxide in these cells. These highly reactive species cause mitochondrial dysfunction and altered energy metabolism, leading to increased apoptosis, and consequently, cellular and organ system failure in critically ill patients.47,48 Insufficient cellular autophagy exacerbated by SH may worsen cellular damage and delay recovery from critical illness.49
Additionally, SH impairs macrophage and neutrophil activity (via reduced phagocytosis) and alters complement fixation (via glycosylation of immunoglobulins).50,51 Stress hyperglycemia can also exacerbate the inflammatory state by increasing binding of nuclear factor kappa B, leading to increased transcription of proinflammatory cytokines.52,53 Additionally, SH is implicated in other abnormalities commonly seen during critical illness, such as endothelial dysfunction, alterations in vascular smooth muscle tone, and abnormalities in coagulation pathways.54–57
Stress Hyperglycemia and the Intensive Care Unit
Specific ICU interventions, such as mechanical ventilation, vasoactive infusions, renal replacement therapies (RRT), cardiopulmonary bypass (CPB), extracorporeal life support (ECLS), therapeutic hypothermia, prolonged immobility, nutrition support practices, and the use of medications can mediate development of SH during critical illness.
Mechanical Ventilation and Stress Hyperglycemia
Mechanical ventilation is commonly associated with SH in pediatric critical illness, ranging from 60–89% of patients.1,4,6,15 Mechanical ventilation is known to induce both pulmonary and systemic cytokine responses in conjunction with shear stress and barotrauma.58 These changes may result in development of SH, especially in the context of multiorgan dysfunction. In turn, SH likely prolongs the duration of mechanical ventilation directly via lung damage and indirectly through the development of critical illness myopathy.59,60 Poor control of hyperglycemia in children with type 1 diabetes mellitus and cystic fibrosis is associated with worsening of pulmonary function.61,62 It is conceivable that similar mechanisms may play a role during critical illness in children.
Vasoactive Infusions and Stress Hyperglycemia
Stress hyperglycemia can develop with the use of vasoactive infusions such as epinephrine, norepinephrine, and dopamine in the pediatric ICU setting.63 In a 2009 study, 90% of critically ill children requiring vasoactive infusions were noted to have BG >140 mg/dl (>7.8 mmol/liter).6 Mechanisms for development of SH due to epinephrine administration include changes in glucose metabolism, characterized by rapid glycogenolysis and sustained gluconeogenesis (via stimulation of b2 receptors), as well as development of insulin resistance (mediated by release of glucagon and cortisol) along with reduction in insulin secretion (via stimulation of a2 receptors).63 Norepinephrine and dopamine have less potent activity at the b2 receptor and are associated with correspondingly lower degrees of SH.63
Renal Replacement Therapies and Stress Hyperglycemia
Renal replacement therapies are commonly used in the pediatric ICU to manage acute kidney injury that develops during critical illness, especially in the setting of multiorgan dysfunction.64 Peritoneal dialysis is more commonly associated with the occurrence of SH, compared with hemodialysis. Traditional dialysate solutions used in peritoneal dialysis pose a substantial glucose load, and plasma insulin levels rise with glucose loading in a dosage-dependent manner.65 These changes induced by the hypertonic dialysate solutions may also result in adverse short-term hemodynamic changes.65 In turn, SH predisposes patients to acute kidney injury during critical illness via glucose toxicity, resulting in mitochondrial dysfunction, inflammation, apoptosis, endothelial dysfunction, and lipid abnormalities.66 It is unclear at this time, though, if TGC reduces the need for RRT in critical illness.24,67
Cardiopulmonary Bypass/Extracorporeal Life Support and Stress Hyperglycemia
Cardiopulmonary bypass is commonly associated with the development of SH in children undergoing cardiac surgery. The incidence of hyperglycemia varies from 52–97%, depending on the definition used.68,69 Cardiopulmonary bypass is thought to result in SH through a combination of inflammatory cytokine release, vasoactive infusion use, pancreatic β-cell dysfunction, hypothermia with insulin resistance, and steroid use.68 While some studies have demonstrated the association of SH with poor outcomes in children undergoing cardiac surgery14,29,30,69,70, other studies have not shown any impact of SH on long-term neurodevelopmental outcomes following cardiac surgery involving CPB.71–73
Similar to CPB, ECLS is also associated with development of SH in critically ill children. In the study by Pressig and Rigby, 100% of children on ECLS were observed to develop SH.6 The mechanisms for development of SH in the context of ECLS are likely to be similar to those of CPB. However, it is unclear if SH is associated with worse outcomes in the setting of pediatric ECLS.
Therapeutic Hypothermia and Stress Hyperglycemia
Application of mild therapeutic hypothermia has emerged as a promising approach to improve outcomes from cardiac arrest due to presumed cardiac origin in adults.74 However, implementation of such hypothermia protocols has been associated with development of SH, likely due to a combination of reduction in insulin secretion and development of insulin resistance.75 Limited studies in adults suggest that SH associated with application of therapeutic hypothermia may be associated with worse outcomes.76 The application of therapeutic hypothermia to improve outcomes from pediatric cardiac arrest and traumatic brain injury continues to be studied and debated.
Prolonged Immobility and Stress Hyperglycemia
Prolonged bed rest is a well-known factor in the occurrence of SH in adults.77 The mechanisms likely involve development of insulin resistance in the skeletal muscle as a direct consequence of inactivity.78 In turn, SH may directly influence development of critical illness-related neuro-muscular dysfunction via mechanisms involving apoptosis and mitochondrial oxidative damage to worsen immobility.60,79 Critical illness-related neuromuscular dysfunction is poorly characterized in children, likely due to under-diagnosis.80 Evidence that SH may be involved in critical illness-related neuromuscular dysfunction also emerges from the beneficial effects of TGC in adults and children through reductions in mechanical ventilation dependency and ICU lengths of stay.17,24,79
Nutrition Support and Stress Hyperglycemia
Nutrition support practices can strongly influence the development of SH during critical illness. Critically ill children are often prescribed parenteral nutrition (PN) for various reasons, such as inability to tolerate enteral nutrition (EN) and ICU practitioner concerns. Provision of excess carbohydrate calories in PN can result in the development of SH. While normal infants and children may have substantially higher glucose turnover rates than adults,81 limited data from critically ill children suggest that glucose infusion rates (GIR) less than 5 mg/kg/min may be optimal for glucose utilization from PN.82,83 Further, BG concentrations may not accurately reflect glucose turnover and utilization.82 The practice of cycling PN may also be associated with development of SH, most likely due to impaired insulin secretion.84 On the other hand, provision of a high carbohydrate diet in critically ill children with severe burns was associated with reduced skeletal muscle protein breakdown with increase in endogenous insulin secretion.85 Regardless of whether PN or EN is employed as the preferred mode of nutrition, overfeeding is common in critically ill children, especially during periods of acute metabolic stress, and may also contribute to SH.86 Studies have demonstrated that commonly used predictive equations to calculate energy expenditure needs are inferior to the practice of targeted indirect calorimetry and often result in overprescription of calories.87–89 In contrast, nutrition strategies, such as supplementation of PN with glutamine and the administration of low calorie PN, may reduce development of SH during critical illness.90,91
In turn, SH can affect delivery of nutrition during critical illness in a variety of ways. Stress hyperglycemia may influence the ability to provide consistent or adequate EN during critical illness. Stress hyperglycemia can result in delayed gastric emptying and slowing down of gut motility, even in the absence of diabetes mellitus.92 Stress hyperglycemia can also impair the prokinetic action of erythromycin on gastric emptying.93 Altered gut motility and insensitivity to prokinetic agents may result in intolerance to EN. Studies in critically ill adults have demonstrated the association of intolerance to EN with SH and BG variability.94 Stress hyperglycemia also results in altered nutrient utilization during critical illness. Stress hyperglycemia exacerbates protein catabolism in skeletal muscle in critically ill adults with severe burns.95 Stress hyperglycemia may also reduce the activity of lipoprotein lipase, contributing to the development of hypertriglyceridemia through reduced clearance of circulating triglycerides.96
Medications and Stress Hyperglycemia
In addition to vasoactive infusions, several medications that are commonly used in the ICU setting result in development of SH. Glucocorticoids can increase the risk of SH in critically ill children, especially when administered in pulse doses.97 The mechanisms of action by which glucocorticoids result in SH include increase in gluconeogenesis, increase in insulin resistance, and impaired insulin secretion by the pancreas.98 Thiazide diuretics are associated with the occurrence of SH, largely due to a decrease in whole body potassium, with corresponding reduction in insulin secretion.99 Stress hyperglycemia is observed with the use of beta blocking agents, due to reduction in insulin secretion from pancreatic β cells.99 Chronic administration of pentamidine can result in SH, due to impaired insulin release and pancreatic β-cell destruction.98 Calcineurin inhibitors, such as tacrolimus and cyclosporine, can result in SH and post-transplant diabetes, due to decreases in insulin biosynthesis and release.98 Newer atypical antipsychotics (clozapine and olanzapine) are associated with SH, diabetes mellitus, and even life-threatening diabetic ketoacidosis. The mechanisms are likely due to development of insulin resistance and inhibition of insulin secretion.98,99 Other causes of SH in critical illness include administration of antibiotic and antifungal medications in large volumes of dextrose-containing solutions.
Tight Glucose Control in Critical Illness
Single-center studies of TGC in critically ill adults demonstrated improved outcomes in mortality and morbidity, especially in long-stay patients.17 However, other multicenter studies were unable to replicate the same observations, with some even observing worse outcomes from TGC.18–21 Notably, all studies of TGC in critically ill adults observed significant increases in hypoglycemia.17–21 Consequently, adult ICU practitioners now approach TGC with a great deal more caution.22 Various reasons have been put forth to explain the observed differences in results in these trials. These include disparities in patient populations, differences in glucose control targets, variability in attaining these targets, differences in ICU specific protocols, glucose control protocols and nutrition support practices, variable sampling and measurement techniques, and variable expertise in protocol implementation.23 Table 2 summarizes major differences in study design and methodology between key studies of TGC in adults that might explain differences in observed outcomes.
Table 2.
Major Differences in Study Design and Methodology between Key Studies of TGC in Critically Ill Adults
| Study (number denotes study reference) | ICU Type, No. of centers, sample size (n) | TGC range vs. control range (mg/dl) | Nutrition support | Primary outcome in TGC vs control range | Hypoglycemia (BG ≤40 mg/dl) in TGC vs control range | Other comments |
|---|---|---|---|---|---|---|
| Van den Berghe17 | Surgical, 1 center, n = 1548 | 80–110 vs. 180–200 | PN » EN, standard protocol, goal calories reached by day 1–2 | ICU all cause mortality: 4.6% vs 8% (p < .04) | 5.1% vs 0.8% | Steroids given as infusions, dedicated study team |
| Van den Berghe18 | Medical, 1 center, n = 1200 | 80–110 vs 180–200 | PN » EN, standard protocol, goal calories reached by day 3–4 | Hospital all cause mortality: 37.3% vs 40% (p = .33) | 18.7% vs 3.1% | Steroids given as boluses, benefit in long stay (> 3 days) patients |
| Brunkhorst19 | Mixed, 18 centers, n = 537 | 80–110 vs 180–200 | PN > EN, standard protocol, goal calories reached by day 5-6 | 28-day all cause mortality: 24.7% vs 26% (p = .74) SOFA score: 7.8 vs 7.7 (p = .88) | 17% vs 4.1% | Stopped early for safety reasons, based on Leuven protocol |
| Preiser20 | Mixed, 21 centers n = 1101 | 80–110 vs 140–180 | EN > PN, no standard protocol, no nutrition support for > 50% of ICU days | ICU all cause mortality: 17.2% vs 15.3% (p = .41) | 8.7% vs 2.7% | Stopped early due to multiple protocol violations |
| NICE-SUGAR21 | Mixed, 42 centers n = 6104 | 81–108 vs 144–180 | EN » PN, no standard protocol, goal calories reached by day 9–10 | 90-day all cause mortality: 27.5% vs 24.9% (p = .02) | 6.8% vs 0.5% | POCT, multiple sites of sampling |
NICE SUGAR, normoglycemia in intensive care evaluation and survival using glucose algorithm regulation; SOFA, sequential organ failure assessment; POCT, point-of-care testing.
The only pediatric study of TGC in critically ill children predominantly recovering from cardiac surgery has observed reductions in mortality, length of ICU stay, and inflammation, but at the cost of a substantial increase in hypoglycemia.24 A multicenter trial of insulin infusion in very low birth weight neonates ended prematurely because of concerns about futility and potential harm from hypoglycemia.100 Pediatric ICU practitioners have great concerns about the risk of iatrogenic hypoglycemia, but most practitioners agree that SH is likely harmful and should be avoided in critically ill children.25,26 Several multicenter studies of TGC in critically ill children are underway to examine whether this strategy can improve outcomes and do so safely without increasing hypoglycemia. The Control of Hyperglycemia in Pediatric Intensive Care trial in the United Kingdom aims to study the impact of TGC on numbers of days alive and freed of ventilator support at 30 days in 1500 critically ill children who are mechanically ventilated and on vasoactive infusions.101 The Safe Pediatric Euglycemia in Cardiac Surgery study is examining the impact of TGC in reducing nosocomial infections and improving cardiac index at 24 hours following cardiac surgery using a continuous glucose monitoring system in 980 children.102 The Pediatric ICUs at Emory-Children's Center Glycemic Control trial aims to study the impact of TGC on recovery of organ function by measuring pediatric logistic organ dysfunction scores in 1004 critically ill children with persistent hyperglycemia.103 Finally, the Heart and Lung Failure – Pediatric Insulin Titration trial is underway to examine the impact of TGC to improve 28-day hospital mortality-adjusted ICU length of stay (equivalent to ICU-free days) in 1880 critically ill children using a continuous glucose monitoring system.104 Standardization of ICU-specific practices across the many centers involved in these trials will be crucial to the validity and reproducibility of the observed results. The results from these large pediatric trials will hopefully answer important questions for the pediatric ICU practitioner, including, but not limited to the: timing of initiation of TGC, optimum target BG range for TGC, target population(s) of interest, and optimum protocol for safely attaining this BG target range without increasing hypoglycemia in critically ill children.
Glossary
Abbreviations
- (BG)
blood glucose
- (CBP)
cardiopulmonary bypass
- (EN)
enteral nutrition
- (ECLS)
extracorporeal life support
- (FFA)
free fatty acids
- (GIR)
glucose infusion rate
- (GLUT)
glucose transporter protein
- (GH)
growth hormone
- (IGF-1)
insulin-like growth factor-1
- (ICU)
intensive care unit
- (IL-1)
interleukin-1
- (IL-6)
interleukin-6
- (PN)
parenteral nutrition
- (ROS)
reactive oxygen species
- (RRT)
renal replacement therapy
- (SH)
stress hyperglycemia
- (TGC)
tight glucose control
- (TNF-α)
tumor necrosis factor-α
References
- 1.Srinivasan V, Spinella PC, Drott HR, Roth CL, Helfaer MA, Nadkarni V. Association of timing, duration, and intensity of hyperglycemia with intensive care unit mortality in critically ill children. Pediatr Crit Care Med. 2004;5(4):329–336. doi: 10.1097/01.pcc.0000128607.68261.7c. [DOI] [PubMed] [Google Scholar]
- 2.Faustino EV, Apkon M. Persistent hyperglycemia in critically ill children. J Pediatr. 2005;146(1):30–34. doi: 10.1016/j.jpeds.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 3.Wintergerst KA, Buckingham B, Gandrud L, Wong BJ, Kache S, Wilson DM. Association of hypoglycemia, hyperglycemia, and glucose variability with morbidity and death in the pediatric intensive care unit. Pediatrics. 2006;118(1):173–179. doi: 10.1542/peds.2005-1819. [DOI] [PubMed] [Google Scholar]
- 4.Yung M, Wilkins B, Norton L, Slater A, Paediatric Study Group; Australian New Zealand Intensive Care Society Glucose control, organ failure, and mortality in pediatric intensive care. Pediatr Crit Care Med. 2008;9(2):147–152. doi: 10.1097/PCC.0b013e3181668c22. [DOI] [PubMed] [Google Scholar]
- 5.Hirshberg E, Larsen G, Van Duker H. Alterations in glucose homeostasis in the pediatric intensive care unit: Hyperglycemia and glucose variability are associated with increased mortality and morbidity. Pediatr Crit Care Med. 2008;9(4):361–366. doi: 10.1097/PCC.0b013e318172d401. [DOI] [PubMed] [Google Scholar]
- 6.Preissig CM, Rigby MR. Pediatric critical illness hyperglycemia: risk factors associated with development and severity of hyperglycemia in critically ill children. J Pediatr. 2009;155(5):734–739. doi: 10.1016/j.jpeds.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Ognibene KL, Vawdrey DK, Biagas KV. The association of age, illness severity, and glycemic status in a pediatric intensive care unit. Pediatr Crit Care Med. 2011;12(6):e386–e390. doi: 10.1097/PCC.0b013e3182192c53. [DOI] [PubMed] [Google Scholar]
- 8.Weise K, Zaritsky A. Endocrine manifestations of critical illness in the child. Pediatr Clin North Am. 1987;34(1):119–130. doi: 10.1016/s0031-3955(16)36185-5. [DOI] [PubMed] [Google Scholar]
- 9.Gupta P, Natarajan G, Agarwal KN. Transient hyperglycemia in acute childhood illnesses: to attend or ignore? Indian J Pediatr. 1997;64(2):205–210. doi: 10.1007/BF02752447. [DOI] [PubMed] [Google Scholar]
- 10.Gore DC, Chinkes D, Heggers J, Herndon DN, Wolf SE, Desai M. Association of hyperglycemia with increased mortality after severe burn injury. J Trauma. 2001;51(3):540–544. doi: 10.1097/00005373-200109000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Cochran A, Scaife ER, Hansen KW, Downey EC. Hyperglycemia and outcomes from pediatric traumatic brain injury. J Trauma. 2003;55(6):1035–1038. doi: 10.1097/01.TA.0000031175.96507.48. [DOI] [PubMed] [Google Scholar]
- 12.Michaud LJ, Rivara FP, Longstreth WT, Jr, Grady MS. Elevated initial blood glucose levels and poor outcome following severe brain injuries in children. J Trauma. 1991;31(10):1356–1362. doi: 10.1097/00005373-199110000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Branco RG, Garcia PC, Piva JP, Casartelli CH, Seibel V, Tasker RC. Glucose level and risk of mortality in pediatric septic shock. Pediatr Crit Care Med. 2005;6(4):470–472. doi: 10.1097/01.PCC.0000161284.96739.3A. [DOI] [PubMed] [Google Scholar]
- 14.Yates AR, Dyke PC, 2nd, Taeed R, Hoffman TM, Hayes J, Feltes TF, Cua CL. Hyperglycemia is a marker for poor outcome in the postoperative pediatric cardiac patient. Pediatr Crit Care Med. 2006;7(4):351–355. doi: 10.1097/01.PCC.0000227755.96700.98. [DOI] [PubMed] [Google Scholar]
- 15.Day KM, Haub N, Betts H, Inwald DP. Hyperglycemia is associated with morbidity in critically ill children with meningococcal sepsis. Pediatr Crit Care Med. 2008;9(6):636–640. doi: 10.1097/PCC.0b013e31818d350b. [DOI] [PubMed] [Google Scholar]
- 16.Tuggle DW, Kuhn MA, Jones SK, Garza JJ, Skinner S. Hyperglycemia and infections in pediatric trauma patients. Am Surg. 2008;74(3):195–198. doi: 10.1177/000313480807400302. [DOI] [PubMed] [Google Scholar]
- 17.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 18.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 19.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhard K. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 20.Preiser JC, Devos P, Ruiz-Santana S, Mélot C, Annane D, Groeneveld J, Iapichino G, Leverve X, Nitenberg G, Wernerman J, Joannidis M, Stecher A, Chioléro R. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35(10):1738–1748. doi: 10.1007/s00134-009-1585-2. [DOI] [PubMed] [Google Scholar]
- 21.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 22.Preiser JC. NICE-SUGAR: the end of a sweet dream? Crit Care. 2009;13(3):143. doi: 10.1186/cc7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scurlock C, Raikhelkar J, Mechanick JI. Critique of normoglycemia in intensive care evaluation: survival using glucose algorithm regulation (NICE-SUGAR)–a review of recent literature. Curr Opin Clin Nutr Metab Care. 2010;13(2):211–214. doi: 10.1097/MCO.0b013e32833571f4. [DOI] [PubMed] [Google Scholar]
- 24.Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, Mesotten D, Casaer MP, Meyfroidt G, Ingels C, Muller J, Van Cromphaut S, Schetz M, Van den Berghe G. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009;373(9663):547–556. doi: 10.1016/S0140-6736(09)60044-1. [DOI] [PubMed] [Google Scholar]
- 25.Hirshberg E, Lacroix J, Sward K, Willson D, Morris AH. Blood glucose control in critically ill adults and children: a survey on stated practice. Chest. 2008;133(6):1328–1335. doi: 10.1378/chest.07-2702. [DOI] [PubMed] [Google Scholar]
- 26.Preissig CM, Rigby MR. A disparity between physician attitudes and practice regarding hyperglycemia in pediatric intensive care units in the United States: a survey on actual practice habits. Crit Care. 2010;14(1):R11. doi: 10.1186/cc8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhisitkul DM, Morrow AL, Vinik AI, Shults J, Layland JC, Rohn R. Prevalence of stress hyperglycemia among patients attending a pediatric emergency department. J Pediatr. 1994;124(4):547–551. doi: 10.1016/s0022-3476(05)83132-4. [DOI] [PubMed] [Google Scholar]
- 28.Valerio G, Franzese A, Carlin E, Pecile P, Perini R, Tenore A. High prevalence of stress hyperglycaemia in children with febrile seizures and traumatic injuries. Acta Paediatr. 2001;90(6):618–622. [PubMed] [Google Scholar]
- 29.Ulate KP, Lima Falcao GC, Bielefeld MR, Morales JM, Rotta AT. Strict glycemic targets need not be so strict: a more permissive glycemic range for critically ill children. Pediatrics. 2008;122(4):e898–e904. doi: 10.1542/peds.2008-0871. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien JE, Jr, Marshall JA, Tarrants ML, Stroup RE, Lofland GK. Intraoperative hyperglycemia and postoperative bacteremia in the pediatric cardiac surgery patient. Ann Thorac Surg. 2010;89(2):578–583. doi: 10.1016/j.athoracsur.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 31.Mechanick JI. Metabolic mechanisms of stress hyperglycemia. JPEN J Parenter Enteral Nutr. 2006;30(2):157–163. doi: 10.1177/0148607106030002157. [DOI] [PubMed] [Google Scholar]
- 32.Dufour S, Lebon V, Shulman GI, Petersen KF. Regulation of net hepatic glycogenolysis and gluconeogenesis by epinephrine in humans. Am J Physiol Endocrinol Metab. 2009;297(1):E231–E235. doi: 10.1152/ajpendo.00222.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulp GA, Herndon DN, Lee JO, Suman OE, Jeschke MG. Extent and magnitude of catecholamine surge in pediatric burned patients. Shock. 2010;33(4):369–374. doi: 10.1097/SHK.0b013e3181b92340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizock BA. Alterations in fuel metabolism in critical illness: hyperglycaemia. Best Pract Res Clin Endocrinol Metab. 2001;15(4):533–551. doi: 10.1053/beem.2001.0168. [DOI] [PubMed] [Google Scholar]
- 35.Jeevanandam M, Young DH, Schiller WR. Glucose turnover, oxidation, and indices of recycling in severely traumatized patients. J Trauma. 1990;30(5):582–589. doi: 10.1097/00005373-199005000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Gebara BM, Gelmini M, Sarnaik A. Oxygen consumption, energy expenditure, and substrate utilization after cardiac surgery in children. Crit Care Med. 1992;20(11):1550–1554. doi: 10.1097/00003246-199211000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Shamoon H, Hendler R, Sherwin RS. Synergistic interactions among antiinsulin hormones in the pathogenesis of stress hyperglycemia in humans. J Clin Endocrinol Metab. 1981;52(6):1235–1241. doi: 10.1210/jcem-52-6-1235. [DOI] [PubMed] [Google Scholar]
- 38.Stumvoll M, Chintalapudi U, Perriello G, Welle S, Gutierrez O, Gerich J. Uptake and release of glucose by the human kidney. Postabsorptive rates and responses to epinephrine. J Clin Invest. 1995;96(5):2528–2533. doi: 10.1172/JCI118314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gardelis JG, Hatzis TD, Stamogiannou LN, Dona AA, Fotinou AD, Brestas PS, Constantopoulos AG. Activity of the growth hormone/insulin-like growth factor-I axis in critically ill children. J Pediatr Endocrinol Metab. 2005;18(4):363–372. doi: 10.1515/jpem.2005.18.4.363. [DOI] [PubMed] [Google Scholar]
- 40.Hunt DG, Ivy JL. Epinephrine inhibits insulin-stimulated muscle glucose transport. J Appl Physiol. 2002;93(5):1638–1643. doi: 10.1152/japplphysiol.00445.2002. [DOI] [PubMed] [Google Scholar]
- 41.Dimitriadis G, Leighton B, Parry-Billings M, Sasson S, Young M, Krause U, Bevan S, Piva T, Wegener G, Newsholme EA. Effects of glucocorticoid excess on the sensitivity of glucose transport and metabolism to insulin in rat skeletal muscle. Biochem J. 1997;321(Pt 3):707–712. doi: 10.1042/bj3210707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L, Thompson LH, Zhao L, Messina JL. Tissue-specific difference in the molecular mechanisms for the development of acute insulin resistance after injury. Endocrinology. 2009;150(1):24–32. doi: 10.1210/en.2008-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118(9):2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gauglitz GG, Herndon DN, Kulp GA, Meyer WJ, 3rd, Jeschke MG. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. J Clin Endocrinol Metab. 2009;94(5):1656–1664. doi: 10.1210/jc.2008-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Preissig CM, Rigby MR. Hyperglycaemia results from beta-cell dysfunction in critically ill children with respiratory and cardiovascular failure: a prospective observational study. Crit Care. 2009;13(1):R27. doi: 10.1186/cc7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Waardenburg DA, Jansen TC, Vos GD, Buurman WA. Hyperglycemia in children with meningococcal sepsis and septic shock: the relation between plasma levels of insulin and inflammatory mediators. J Clin Endocrinol Metab. 2006;91(10):3916–3921. doi: 10.1210/jc.2006-0525. [DOI] [PubMed] [Google Scholar]
- 47.Van den Berghe G. How does blood glucose control with insulin save lives in intensive care? J Clin Invest. 2004;114(9):1187–1195. doi: 10.1172/JCI23506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vanhorebeek I, De Vos R, Mesotten D, Wouters PJ, De Wolf-Peeters C, Van den Berghe G. Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. Lancet. 2005;365(9453):53–59. doi: 10.1016/S0140-6736(04)17665-4. [DOI] [PubMed] [Google Scholar]
- 49.Vanhorebeek I, Gunst J, Derde S, Derese I, Boussemaere M, Güiza F, Martinet W, Timmermans JP, D'Hoore A, Wouters PJ, Van den Berghe G. Insufficient activation of autophagy allows cellular damage to accumulate in critically ill patients. J Clin Endocrinol Metab. 2011;96(4):E633–E645. doi: 10.1210/jc.2010-2563. [DOI] [PubMed] [Google Scholar]
- 50.Turina M, Fry DE, Polk HC., Jr Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit Care Med. 2005;33(7):1624–1633. doi: 10.1097/01.ccm.0000170106.61978.d8. [DOI] [PubMed] [Google Scholar]
- 51.Hennessey PJ, Black CT, Andrassy RJ. Nonenzymatic glycosylation of immunoglobulin G impairs complement fixation. JPEN J Parenter Enteral Nutr. 1991;15(1):60–64. doi: 10.1177/014860719101500160. [DOI] [PubMed] [Google Scholar]
- 52.Nareika A, Im YB, Game BA, Slate EH, Sanders JJ, London SD, Lopes-Virella MF, Huang Y. High glucose enhances lipopolysaccharide-stimulated CD14 expression in U937 mononuclear cells by increasing nuclear factor kappaB and AP-1 activities. J Endocrinol. 2008;196(1):45–55. doi: 10.1677/JOE-07-0145. [DOI] [PubMed] [Google Scholar]
- 53.Aljada A, Friedman J, Ghanim H, Mohanty P, Hofmeyer D, Chaudhuri A, Dandona P. Glucose ingestion induces an increase in intranuclear nuclear factor kappaB, a fall in cellular inhibitor kappaB, and an increase in tumor necrosis factor alpha messenger RNA by mononuclear cells in healthy human subjects. Metabolism. 2006;55(9):1177–1185. doi: 10.1016/j.metabol.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 54.Langouche L, Vanhorebeek I, Vlasselaers D, Vander Perre S, Wouters PJ, Skogstrand K, Hansen TK, Van den Berghe G. Intensive insulin therapy protects the endothelium of critically ill patients. J Clin Invest. 2005;115(8):2277–2286. doi: 10.1172/JCI25385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yerneni KK, Bai W, Khan BV, Medford RM, Natarajan R. Hyperglycemia-induced activation of nuclear transcription factor kappaB in vascular smooth muscle cells. Diabetes. 1999;48(4):855–864. doi: 10.2337/diabetes.48.4.855. [DOI] [PubMed] [Google Scholar]
- 56.Ellger B, Langouche L, Richir M, Debaveye Y, Vanhorebeek I, Teerlink T, Van Leeuwen PA, Van den Berghe G. Modulation of regional nitric oxide metabolism: blood glucose control or insulin? Intensive Care Med. 2008;34(8):1525–1533. doi: 10.1007/s00134-008-1118-4. [DOI] [PubMed] [Google Scholar]
- 57.Rao AK, Chouhan V, Chen X, Sun L, Boden G. Activation of the tissue factor pathway of blood coagulation during prolonged hyperglycemia in young healthy men. Diabetes. 1999;48(5):1156–1161. doi: 10.2337/diabetes.48.5.1156. [DOI] [PubMed] [Google Scholar]
- 58.Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282(1):54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 59.Baker EH, Wood DM, Brennan AL, Clark N, Baines DL, Philips BJ. Hyperglycaemia and pulmonary infection. Proc Nutr Soc. 2006;65(3):227–235. doi: 10.1079/pns2006499. [DOI] [PubMed] [Google Scholar]
- 60.Stevens RD, Dowdy DW, Michaels RK, Mendez-Tellez PA, Pronovost PJ, Needham DM. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med. 2007;33(11):1876–1891. doi: 10.1007/s00134-007-0772-2. [DOI] [PubMed] [Google Scholar]
- 61.Cazzato S, Bernardi F, Salardi S, Tassinari D, Corsini I, Ragni L, Cicognani A, Cacciari E. Lung function in children with diabetes mellitus. Pediatr Pulmonol. 2004;37(1):17–23. doi: 10.1002/ppul.10399. [DOI] [PubMed] [Google Scholar]
- 62.Milla CE, Warwick WJ, Moran A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med. 2000;162(3 Pt 1):891–895. doi: 10.1164/ajrccm.162.3.9904075. [DOI] [PubMed] [Google Scholar]
- 63.Barth E, Albuszies G, Baumgart K, Matejovic M, Wachter U, Vogt J, Radermacher P, Calzia E. Glucose metabolism and catecholamines. Crit Care Med. 2007;35(9 Suppl):S508–S518. doi: 10.1097/01.CCM.0000278047.06965.20. [DOI] [PubMed] [Google Scholar]
- 64.Goldstein SL. Advances in pediatric renal replacement therapy for acute kidney injury. Semin Dial. 2011;24(2):187–191. doi: 10.1111/j.1525-139X.2011.00834.x. [DOI] [PubMed] [Google Scholar]
- 65.Selby NM, Fialova J, Burton JO, McIntyre CW. The haemodynamic and metabolic effects of hypertonic-glucose and amino-acid-based peritoneal dialysis fluids. Nephrol Dial Transplant. 2007;22(3):870–879. doi: 10.1093/ndt/gfl654. [DOI] [PubMed] [Google Scholar]
- 66.Gunst J, Schetz M. Clinical benefits of tight glycaemic control: effect on the kidney. Best Pract Res Clin Anaesthesiol. 2009;23(4):431–439. doi: 10.1016/j.bpa.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 67.Kansagara D, Fu R, Freeman M, Wolf F, Helfand M. Intensive insulin therapy in hospitalized patients: a systematic review. Ann Intern Med. 2011;154(4):268–282. doi: 10.7326/0003-4819-154-4-201102150-00008. [DOI] [PubMed] [Google Scholar]
- 68.Verhoeven JJ, Hokken-Koelega AC, den Brinker M, Hop WC, van Thiel RJ, Bogers AJ, Helbing WA, Joosten KF. Disturbance of glucose homeostasis after pediatric cardiac surgery. Pediatr Cardiol. 2011;32(2):131–138. doi: 10.1007/s00246-010-9829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Falcao G, Ulate K, Kouzekanani K, Bielefeld MR, Morales JM, Rotta AT. Impact of postoperative hyperglycemia following surgical repair of congenital cardiac defects. Pediatr Cardiol. 2008;29(3):628–636. doi: 10.1007/s00246-007-9178-8. [DOI] [PubMed] [Google Scholar]
- 70.Polito A, Thiagarajan RR, Laussen PC, Gauvreau K, Agus MS, Scheurer MA, Pigula FA, Costello JM. Association between intraoperative and early postoperative glucose levels and adverse outcomes after complex congenital heart surgery. Circulation. 2008;118(22):2235–2242. doi: 10.1161/CIRCULATIONAHA.108.804286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ballweg JA, Wernovsky G, Ittenbach RF, Bernbaum J, Gerdes M, Gallagher PR, Dominguez TE, Zackai E, Clancy RR, Nicolson SC, Spray TL, Gaynor JW. Hyperglycemia after infant cardiac surgery does not adversely impact neurodevelopmental outcome. Ann Thorac Surg. 2007;84(6):2052–2058. doi: 10.1016/j.athoracsur.2007.06.099. [DOI] [PubMed] [Google Scholar]
- 72.Ballweg JA, Ittenbach RF, Bernbaum J, Gerdes M, Dominguez TE, Zackai EH, Clancy RR, Gaynor JW. Hyperglycaemia after Stage I palliation does not adversely affect neurodevelopmental outcome at 1 year of age in patients with single-ventricle physiology. Eur J Cardiothorac Surg. 2009;36(4):688–693. doi: 10.1016/j.ejcts.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Ferranti S, Gauvreau K, Hickey PR, Jonas RA, Wypij D, du Plessis A, Bellinger DC, Kuban K, Newburger JW, Laussen PC. Intraoperative hyperglycemia during infant cardiac surgery is not associated with adverse neurodevelopmental outcomes at 1, 4, and 8 years. Anesthesiology. 2004;100(6):1345–1352. doi: 10.1097/00000542-200406000-00005. [DOI] [PubMed] [Google Scholar]
- 74.Arrich J, Holzer M, Herkner H, Müllner M. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2009;4 doi: 10.1002/14651858.CD004128.pub2. CD004128. [DOI] [PubMed] [Google Scholar]
- 75.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37(7 Suppl):S186–S202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- 76.Nielsen N, Sunde K, Hovdenes J, Riker RR, Rubertsson S, Stammet P, Nilsson F, Friberg H, Hypothermia Network Adverse events and their relation to mortality in out-of-hospital cardiac arrest patients treated with therapeutic hypothermia. Crit Care Med. 2011;39(1):57–64. doi: 10.1097/CCM.0b013e3181fa4301. [DOI] [PubMed] [Google Scholar]
- 77.Brower RG. Consequences of bed rest. Crit Care Med. 2009;37(10 Suppl):S422–S428. doi: 10.1097/CCM.0b013e3181b6e30a. [DOI] [PubMed] [Google Scholar]
- 78.Stuart CA, Shangraw RE, Prince MJ, Peters EJ, Wolfe RR. Bed-rest-induced insulin resistance occurs primarily in muscle. Metabolism. 1988;37(8):802–806. doi: 10.1016/0026-0495(88)90018-2. [DOI] [PubMed] [Google Scholar]
- 79.Van den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005;64(8):1348–1353. doi: 10.1212/01.WNL.0000158442.08857.FC. [DOI] [PubMed] [Google Scholar]
- 80.Williams S, Horrocks IA, Ouvrier RA, Gillis J, Ryan MM. Critical illness polyneuropathy and myopathy in pediatric intensive care: A review. Pediatr Crit Care Med. 2007;8(1):18–22. doi: 10.1097/01.pcc.0000256623.01254.40. [DOI] [PubMed] [Google Scholar]
- 81.Bier DM, Leake RD, Haymond MW, Arnold KJ, Gruenke LD, Sperling MA, Kipnis DM. Measurement of “true” glucose production rates in infancy and childhood with 6,6-dideuteroglucose. Diabetes. 1977;26(11):1016–1023. doi: 10.2337/diab.26.11.1016. [DOI] [PubMed] [Google Scholar]
- 82.Sheridan RL, Yu YM, Prelack K, Young VR, Burke JF, Tompkins RG. Maximal parenteral glucose oxidation in hyper-metabolic young children: a stable isotope study. JPEN J Parenter Enteral Nutr. 1998;22(4):212–216. doi: 10.1177/0148607198022004212. [DOI] [PubMed] [Google Scholar]
- 83.Verbruggen SC, de Betue CT, Schierbeek H, Chacko S, van Adrichem LN, Verhoeven J, van Goudoever JB, Joosten KF. Reducing glucose infusion safely prevents hyperglycemia in post-surgical children. Clin Nutr. 2011;30(6):786–792. doi: 10.1016/j.clnu.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 84.Lienhardt A, Rakotoambinina B, Colomb V, Souissi S, Sadoun E, Goulet O, Robert JJ, Ricour C. Insulin secretion and sensitivity in children on cyclic total parenteral nutrition. JPEN J Parenter Enteral Nutr. 1998;22(6):382–386. doi: 10.1177/0148607198022006382. [DOI] [PubMed] [Google Scholar]
- 85.Hart DW, Wolf SE, Zhang XJ, Chinkes DL, Buffalo MC, Matin SI, DebRoy MA, Wolfe RR, Herndon DN. Efficacy of a high-carbohydrate diet in catabolic illness. Crit Care Med. 2001;29(7):1318–1324. doi: 10.1097/00003246-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 86.Chwals WJ. Overfeeding the critically ill child: fact or fantasy? New Horiz. 1994;2(2):147–155. [PubMed] [Google Scholar]
- 87.Coss-Bu JA, Jefferson LS, Walding D, David Y, Smith EO, Klish WJ. Resting energy expenditure in children in a pediatric intensive care unit: comparison of Harris-Benedict and Talbot predictions with indirect calorimetry values. Am J Clin Nutr. 1998;67(1):74–80. doi: 10.1093/ajcn/67.1.74. [DOI] [PubMed] [Google Scholar]
- 88.Framson CM, LeLeiko NS, Dallal GE, Roubenoff R, Snelling LK, Dwyer JT. Energy expenditure in critically ill children. Pediatr Crit Care Med. 2007;8(3):264–267. doi: 10.1097/01.PCC.0000262802.81164.03. [DOI] [PubMed] [Google Scholar]
- 89.Mehta NM, Bechard LJ, Dolan M, Ariagno K, Jiang H, Duggan C. Energy imbalance and the risk of overfeeding in critically ill children. Pediatr Crit Care Med. 2011;12(4):398–405. doi: 10.1097/PCC.0b013e3181fe279c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grau T, Bonet A, Miñambres E, Piñeiro L, Irles JA, Robles A, Acosta J, Herrero I, Palacios V, Lopez J, Blesa A, Martínez P, Metabolism, Nutrition Working Group, SEMICYUC, Spain The effect of L-alanyl-L-glutamine dipeptide supplemented total parenteral nutrition on infectious morbidity and insulin sensitivity in critically ill patients. Crit Care Med. 2011;39(6):1263–1268. doi: 10.1097/CCM.0b013e31820eb774. [DOI] [PubMed] [Google Scholar]
- 91.Ahrens CL, Barletta JF, Kanji S, Tyburski JG, Wilson RF, Janisse JJ, Devlin JW. Effect of low-calorie parenteral nutrition on the incidence and severity of hyperglycemia in surgical patients: a randomized, controlled trial. Crit Care Med. 2005;33(11):2507–2512. doi: 10.1097/01.ccm.0000186746.64572.8a. [DOI] [PubMed] [Google Scholar]
- 92.Hebbard GS, Sun WM, Dent J, Horowitz M. Hyperglycaemia affects proximal gastric motor and sensory function in normal subjects. Eur J Gastroenterol Hepatol. 1996;8(3):211–217. doi: 10.1097/00042737-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 93.Petrakis IE, Kogerakis N, Prokopakis G, Zacharioudakis G, Antonakakis S, Vrachassotakis N, Chalkiadakis G. Hyperglycemia attenuates erythromycin-induced acceleration of liquid-phase gastric emptying of hypertonic liquids in healthy subjects. Dig Dis Sci. 2002;47(1):67–72. doi: 10.1023/a:1013211419605. [DOI] [PubMed] [Google Scholar]
- 94.Nguyen N, Ching K, Fraser R, Chapman M, Holloway R. The relationship between blood glucose control and intolerance to enteral feeding during critical illness. Intensive Care Med. 2007;33(12):2085–2092. doi: 10.1007/s00134-007-0869-7. [DOI] [PubMed] [Google Scholar]
- 95.Gore DC, Chinkes DL, Hart DW, Wolf SE, Herndon DN, Sanford AP. Hyperglycemia exacerbates muscle protein catabolism in burn-injured patients. Crit Care Med. 2002;30(11):2438–2442. doi: 10.1097/00003246-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 96.Kovár J, Fejfarová V, Pelikánová T, Poledne R. Hyperglycemia downregulates total lipoprotein lipase activity in humans. Physiol Res. 2004;53(1):61–68. [PubMed] [Google Scholar]
- 97.Zimmerman JJ. A history of adjunctive glucocorticoid treatment for pediatric sepsis: moving beyond steroid pulp fiction toward evidence-based medicine. Pediatr Crit Care Med. 2007;8(6):530–539. doi: 10.1097/01.PCC.0000288710.11834.E6. [DOI] [PubMed] [Google Scholar]
- 98.Thomas Z, Bandali F, McCowen K, Malhotra A. Drug-induced endocrine disorders in the intensive care unit. Crit Care Med. 2010;38(6 Suppl):S219–S230. doi: 10.1097/CCM.0b013e3181dda0f2. [DOI] [PubMed] [Google Scholar]
- 99.Luna B, Feinglos MN. Drug-induced hyperglycemia. JAMA. 2001;286(16):1945–1948. doi: 10.1001/jama.286.16.1945. [DOI] [PubMed] [Google Scholar]
- 100.Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, Vanhole C, Palmer CR, van Weissenbruch M, Midgley P, Thompson M, Thio M, Cornette L, Ossuetta I, Iglesias I, Theyskens C, de Jong M, Ahluwalia JS, de Zegher F, Dunger DB. Early insulin therapy in very-low-birth-weight infants. N Engl J Med. 2008;359(18):1873–1884. doi: 10.1056/NEJMoa0803725. [DOI] [PubMed] [Google Scholar]
- 101.Macrae D, Pappachan J, Grieve R, Parslow R, Nadel S, Schindler M, Baines P, Forne PM, Slavik Z, Goldman A, Truesdale A, Betts H, Allen E, Snowdon C, Percy D, Broadhead M, Quick T, Peters M, Morris K, Tasker R, Elbourne D. Control of hyperglycaemia in paediatric intensive care (CHiP): study protocol. BMC Pediatr. 2010;10:5. doi: 10.1186/1471-2431-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.National Heart, Lung, and Blood Institute (NHLBI) SPECS: Safe Pediatric Euglycemia in Cardiac Surgery. ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US) 2011. Available from: http://clinicaltrials.gov/ct2/show/NCT00443599. Accessed on December 5.
- 103.Emory University. Pediatric Intensive Care Units (ICUs) at Emory-Children's Center Glycemic Control: The PedETrol Trial. ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US) 2011. Available from: http://clinicaltrials.gov/ct2/show/NCT01116752?term=NCT01116752&rank=1. Accessed on December 5.
- 104.Srinivasan, Vijay (Department of Anesthesiology and Critical Care Medicine, Children's Hospital of Philadelphia, Philadelphia, PA) 2011. Conversation with: Michael Agus (Children's Hospital Boston, Boston, MA.