Abstract
Background
Although altered metabolism has long been known to affect human breath, generating clinically usable metabolic tests from exhaled compounds has proven challenging. If developed, a breath-based lipid test would greatly simplify management of diabetes and serious pathological conditions (e.g., obesity, familial hyperlipidemia, and coronary artery disease), in which systemic lipid levels are a critical risk factor for onset and development of future cardiovascular events.
Methods
We, therefore, induced controlled fluctuations of plasma lipids (insulin-induced lipid suppression or intravenous infusion of Intralipid) during 4-h in vivo experiments on 23 healthy volunteers (12 males/11 females, 28.0 ± 0.3 years) to find correlations between exhaled volatile organic compounds and plasma lipids. In each subject, plasma triglycerides (TG) and free fatty acids (FFA) concentrations were both directly measured and calculated via individualized prediction equations based on the multiple linear regression analysis of a cluster of 4 gases. In the lipid infusion protocol, we also generated common prediction equations using a maximum of 10 gases.
Results
This analysis yielded strong correlations between measured and predicted values during both lipid suppression (r = 0.97 for TG; r = 0.90 for FFA) and lipid infusion (r = 0.97 for TG; r = 0.94 for FFA) studies. In our most accurate common prediction model, measured and predicted TG and FFA values also displayed very strong statistical agreement (r = 0.86 and r = 0.81, respectively).
Conclusions
Our results demonstrate the feasibility of measuring plasma lipids through breath analysis. Optimization of this technology may ultimately lead to the development of portable breath analyzers for plasma lipids, replacing blood-based bioassays.
Keywords: breath tests, diabetes mellitus, diagnostic techniques and procedures, gases, lipid metabolism, volatile organic compounds
Introduction
Human breath contains several hundred different compounds, of which many are direct or indirect products of the metabolism of carbohydrates, lipids, and other energy substrates.1 Some are spontaneously present in gas form [volatile organic compounds (VOCs)], while others enter the exhaled gas mixture as aerosolized particles.2,3 As breath sampling is a painless, noninvasive procedure easily accepted by patients, these components of exhaled breath, therefore, represent potentially ideal biomarkers.4,5 In fact, attempts to correlate specific breath VOCs with endogenous metabolic processes indeed date back more than a century.6 Yet despite an exponential increase since the 1990s of published associations between exhaled VOCs and various physiological events or pathologies,7–13 translating these findings into clinically useful applications has still proven challenging. However, one of the fields in which rapid progress appears possible is in the development of breath-based testing devices for diabetes-related variables. Among these, plasma glucose and insulin are obvious candidates and are currently explored by several research groups. Significant potential benefits also exist for breath testing of plasma lipids, as evidenced by the number of research projects in the area that are producing excellent papers on the subject. This technology may be especially relevant to diabetes patients because prevention of cardiovascular disease, through the control of key risk factors such as elevated plasma lipid concentrations, is a crucial component to their long-term survival and quality of life. Facilitating plasma lipid measurement through a breath-based test, possibly performed simultaneously with a breath test for plasma glucose, could therefore substantially improve prevention and management of these conditions.
While some previous studies have addressed multiple aspects of the interaction between endogenous lipid metabolism and composition of exhaled breath,14–22 very few studies have attempted to specifically quantify systemic levels of triglycerides (TG), free fatty acids (FFA), or other lipids. In this article, we propose a noninvasive methodology to estimate lipidemia indirectly through the analysis of breath VOCs. Previously, our group reported the possibility of deriving accurate estimates of plasma glucose and insulin concentrations by integrating the simultaneous kinetic profiles of several exhaled VOCs in carefully controlled metabolic conditions.23–26 Because of the close biochemical ties found between these VOCs and systemic metabolism, plasma lipid concentrations also appeared amenable to estimation via parallel VOC analyses. Because our prior work also indicated that exhaled VOC profiles constantly change in response to the extreme variability of the endogenous plasma milieu, we decided to focus on exhaled VOC patterns observed during sizable and prolonged metabolic perturbations rather than at individual time points so that we could better capture the “breath equivalent” of simultaneous systemic metabolic processes. This consideration is especially important for defining and monitoring the time course of evolving, complex metabolic conditions, including diabetes and dyslipidemia.
Our overall hypothesis was that by integrating measurements of multiple exhaled VOCs at several consecutive time points, it is possible to estimate plasma concentrations of a given variable through multivariate regression analysis. In this study, we have designed a repeated-measure approach to VOC analysis, which, in the present study, was applied to the prediction of plasma TG and FFA. Fluctuations of plasma lipid concentrations were induced in a group of healthy young adults via intravenous (IV) insulin-mediated suppression of lipolysis or lipid infusion, and simultaneous plasma and exhaled breath samples were collected at multiple time points over 4 h.
Methods
All procedures were approved by the University of California, Irvine (UCI), Institutional Review Board and conducted by specialized personnel at the UCI Institute for Clinical and Translational Science (ICTS). Volatile organic compound analysis was conducted in the Rowland–Blake Atmospheric Chemistry Laboratory.
Subjects
Twenty-three healthy volunteers [12 males (M) and 11 females (F), 28.0 ± 0.3 years] were enrolled in our study. Of these, 17 (8 M/9 F) participated in study 1, as described later, and 15 (9 M/6 F) in study 2 [with 9 (5 M/4 F) participating in both]. All volunteers signed informed consent forms prior to participation, did not smoke, had no evidence or record of recent or chronic illness, were not taking medications, and had no known allergies in general or in particular to soy products (contained in some of the study infusates).
Study Procedures
For both studies, subjects reported to the ICTS at 7:30 a.m. after an overnight fast. Intravenous catheters were placed in the antecubital veins of both arms for subsequent blood drawing and IV glucose/insulin/lipid infusions. (We chose to induce acute hyperglycemia and hyperlipidemia in our subjects by IV infusion to avoid confounding effects from metabolism and absorption in the gastrointestinal tract.) Matched breath, room air, and blood samples were collected at 12 time points: baseline (8 a.m., t = 0 min) and then at t = 60, 90, 110, 130, 140, 150, 180, 200, 220, 230, 240 min). For breath collection, after two tidal volume ventilations and a deep inspiration, subjects slowly exhaled for ∼10 s through a three-way valve mouthpiece into custom-made 1.9 liter stainless steel canisters that had been sterilized before use at 150 °C, pumped to 10−5 atm, flushed with purified helium, and repumped to 10−5 atm. The first 3 s (∼500 ml) of exhaled breath was vented to the room to clear anatomic dead space. As subjects had practiced the maneuver several times, the full canister volume was collected in all instances. A room air sample was simultaneously collected in an identical canister. Blood was collected in 10 ml samples drawn in Vacutainer ethylene diamine tetraacetic acid-treated tubes (BD Biosciences, Franklin Lakes, NJ); additional 0.5 ml blood aliquots were collected every 5 min throughout the study for the monitoring of plasma glucose.
Study 1 (Glucose Infusion)
After a baseline euglycemic period (t = 0–60 min), plasma glucose was gradually increased over 30 min (t = 60–90 min) via IV administration of 20% dextrose to a target level of ∼220 mg/dl. For each subject, the infusion rate of glucose was adjusted every 5 min based on a negative feedback principle. If the measured blood glucose concentration was lower than desired (i.e., because of the glucose-lowering effects of spontaneous insulin), the dextrose infusion rate would be raised. On the other hand, if the blood glucose concentration was too high, the infusion rate would be reduced. While this procedure can be difficult if subjects are exceptionally insulin-sensitive or if the target glycemia is ≥300 mg/dl, requiring amounts of IV dextrose so high that it may cause complications at the IV site, it was performed successfully in all studies with our experimental protocol. Hyperglycemia was then maintained for 1 h (t = 90–150 min), allowing for natural hyperinsulinemia to occur. At t = 150 min, the glucose infusion was reduced and a 1.5 mU/kg/min IV infusion of fast-acting insulin (Novolin R, Novo Nordisk, Princeton, NJ) was started so that plasma glucose was back to basal levels by t = 180 min. A stable hyperinsulinemia of ∼10-fold basal levels was then established and maintained for the last hour of the study (t = 180–240 min).
Study 2 (Lipid Infusion)
Baseline lipidemia was maintained for 1 h (t = 0–60 min), and then IV administration of a 20% fat emulsion (Intralipid, Baxter, Deerfield, IL) was started. The major component fatty acids of the emulsion are linoleic (44–62%), oleic (19–30%), palmitic (7–14%), linolenic (4–11%), and stearic (1.4–5.5%) acids. To test for possible allergic hypersensitivity to the emulsion, the infusion was performed at the reduced rate of 10 ml/h for the first 10 min. In the absence of signs of an allergic reaction (none was ever detected in any of the participants), the infusion rate was increased to 1.1 ml/kg/h, for induction of hyperlipidemia over the following 170 min (t = 70–240 min), which allowed plasma FFA and TG concentrations to increase ∼2.5-fold over basal levels. During these studies, plasma glucose concentrations never significantly changed as compared to baseline values.
Blood Analysis
Blood samples were centrifuged immediately following each draw, and plasma glucose concentrations were determined with a Beckman Glucose Analyzer II (Beckman Ltd., Fullerton, CA); remaining plasma was stored at −80 °C until assays were performed. Triglycerides concentrations were measured with Triglyceride-SL Reagent System Kit (Equal Diagnostics, Exton, PA). Free fatty acid concentrations were measured with a NEFA-ACS-ACOD Reagent System Kit (Equal Diagnostics, Exton, PA).
Analysis of Breath and Room Air
The canisters containing study breath and room samples were taken to the Rowland–Blake Atmospheric Chemistry Laboratory, stored at room temperature, and analyzed within 1 week. Stability of VOC concentrations within the canisters has been tested in dozens of prior studies; specific VOC mixtures were transferred from large high-pressure cylinders into our collection canisters and compared at multiple time points up to over 1 year. By this technique, VOCs identified as having changing concentrations over time are systematically excluded from data analysis.
On assay day, a 275 cm3 sample aliquot (at standard temperature and pressure) was introduced in the system manifold and passed over glass beads chilled by liquid nitrogen (−196 °C) with flow kept below 500 cm3/min to ensure complete trapping of the relevant components. This procedure preconcentrated the relatively less-volatile sample components (halocarbons, hydrocarbons) while allowing volatile components (nitrogen, oxygen, and argon) to be pumped away. The less volatile compounds were then revolatilized by immersing the loop containing the beads in hot water (80 °C) and flushed into a helium carrier flow (head pressure 48 psi). The sample flow was split into five streams at an eight-port union (Valco Instruments, 1/16ʺ manifold, 1–8 ports, 0.75-mm inlet bore, 0.25-mm outlet bore, with three outlet port capped off). Each stream was chromatographically separated on different column/detector combinations.
Three HP 6890 gas chromatographs (GCs, Hewlett-Packard, Sunnyvale, CA) form the core of the analytical system, utilizing various combinations of electron-capture detectors (ECD, sensitive to halocarbons and alkyl nitrates), flame-ionization detectors (FID, sensitive to hydrocarbons), sulfur chemiluminiscence detector (SCD, sensitive to sulfur-containing compounds), and quadrupole mass spectrometer detector (MSD, for unambiguous compound identification and selected ion monitoring). The oven parameters for the three instruments are given in Table 1. The first HP 6890 (GC 1) contains two columns: one is a J&W DB-5 (30 m; i.d., 0.25 mm; film, 1 µm) connected in series to a RESTEK 1701 (5 m; i.d., 0.25 mm; film, 0.5 µm), which is then output to an ECD. The J&W DB-5/RESTEK 1701 union helps resolve halocarbon and organic nitrate species that have similar polarity through higher retention of the nitrate species. The second column is a J&W DB-5ms (60 m; i.d., 0.25 mm; film, 0.5 µm), which is output to a MSD detector (HP 5973). The J&W DB-5/RESTEK 1701 received 6.84% and the J&W DB-5ms/MSD received 10.1% of the total carrier flow, respectively. The second HP 6890 (GC 2) contains a J&W DB-1 column (60 m; i.d., 0.32 mm; film, 1 µm) output to a FID and SCD in series. This column received 15.1% of the flow. The third HP 6890 (GC 3) contains a J&W GS-Alumina PLOT column (30 m; i.d., 0.53 mm) connected in series to a J&W DB-1 column (5 m; i.d., 0.53 mm; film, 1 µm), which is output to a FID, and a RESTEK 1701 (60 m; i.d., 0.25 mm; film, 0.50 µm), which is output to a ECD. The PLOT/DB-1 union helps to reduce signal spikes from PLOT column bleed and tightens up the carbon dioxide (CO2) peak width. The GS-Alumina PLOT column received 60.8% of the flow, and the RESTEK 1701 received the remaining 7.16% of the flow. The signal from each FID, ECD, and SCD was recorded digitally using Chromeleon software (Dionex Corporation, San Jose, CA). The output of each MSD was digitally recorded using Chemstation software (Hewlett-Packard). Representative chromatograms are shown in Figures 1 and 2. All VOCs are individually quantified through integration of the area under each peak on the chromatogram. Area limits are initially identified by our analytical software, and correct placement is confirmed by at least two team members. The area under each peak is then compared to whole air standards containing the same compound at a known concentration. During this process, any coelution is detected by comparing measurements for the same compound from different column/detector combinations. Only when clear agreement across quantifications is obtained, a compound is included in subsequent data analysis. This built-in redundancy ensures that reported VOCs we report are not affected by coelution.
Table 1.
Gas Chromatograph Oven Temperature Parameters
| Starting temperature (°C) | −60 | −60 | −20 |
| Time at starting temperature (min) | 1.5 | 1.5 | 1.5 |
| Temperature ramp 1 (°C/min) | 15 | 10 | 30 |
| Temperature 1 (°C) | 110 | 0 | 60 |
| Time at temperature 1 (min) | 0 | 0 | 0 |
| GC 1 | GC 2 | GC 3 | |
|---|---|---|---|
| Temperature ramp 2 (°C/min) | 29 | 17 | 14 |
| Temperature 2 (°C) | 220 | 145 | 200 |
| Time at temperature 2 (min) | 1.88 | 0 | 4.7 |
| Temperature ramp 3 (°C/min) | – | 65 | – |
| Temperature 3 (°C) | – | 220 | – |
| Time at temperature 3 (min) | – | 1.3 | – |
Figure 1.
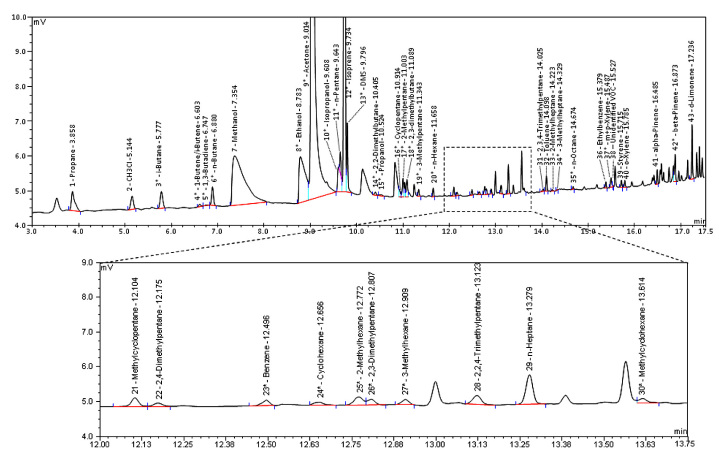
Representative J&W DB-1/FID chromatogram of a breath sample. This representative chromatogram was obtained from a HP-6890 chromatograph containing a J&W DB-1 column (60 m; i.d., 0.32 mm; film, 1 μm) with output to a FID. Minutes 12.00–13.75 have been enlarged to illustrate the resolution of our instruments.
Figure 2.
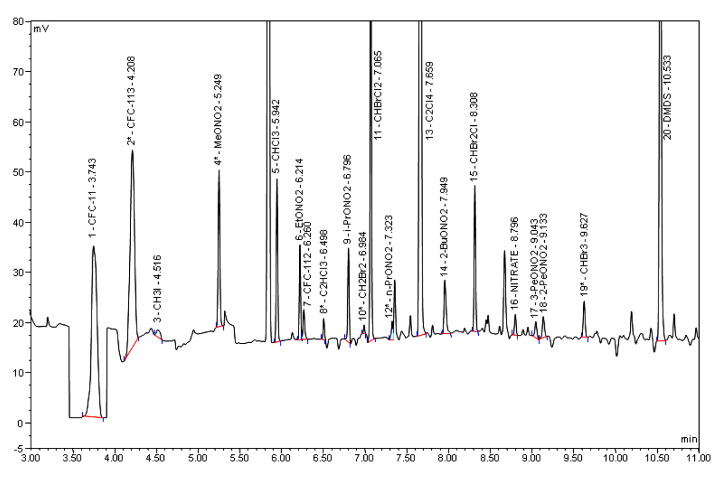
Representative RESTEK 1701/ECD chromatogram of a breath sample. This chromatogram was obtained from a HP 6890 chromatograph utilizing a RESTEK 1701 column (60 m; i.d., 0.25 mm; film, 0.50 μm), which was output to an ECD.
Because our analytical protocols were originally designed for atmospheric air measurements, as detailed in previous studies,27,28 we incorporated some minor procedural changes to take into account the higher CO2 concentrations present in breath as compared to ambient air. This process included running whole air standards enriched with 5% CO2 (to mimic breath concentrations) and trapping/injecting only about 15% (<300 ml) of the volume used for remote ambient air analysis (2000 ml) because greater volumes can be negatively affected by higher CO2 concentrations. Our breath analysis technique has now been successfully used in numerous studies.10,23–26,29
In total, concentrations of ∼100 VOCs were quantified and utilized for analyses pertinent to this study. As our system, originally developed for atmospheric gas analysis, is progressively optimized for samples of human origin as our studies advance, a number of additional VOCs that had not been available for study 1 could be included in study 2's calculations.
Degassing of Substrates
To avoid including exhaled VOCs that had potentially been introduced in the body via study infusates in our data analysis, 18 ml aliquots of dextrose and lipid infusates were introduced into a custom-designed sealed bioreactor and exposed to a constant flow of helium micro-bubbles, capturing all VOCs suspended in the fluid sample. Extracted compounds were then collected and analyzed similarly to other VOC samples. By this technique, we have identified a number of VOCs (heptane, hexane, methyl- and cycloheptane/hexane, butanal, heptanal) that had clearly been introduced into the body through the infusate during the study; these VOCs were excluded from our analysis.
Data Analysis and Statistics
Matched exhaled breath, room air, and peripheral blood samples were collected at 12 time points for each subject enrolled in our clamp protocols. Changes in VOC values (differences between room air and breath concentrations) from each collection point were compared to their corresponding plasma TG and FFA concentrations, and prediction models for each lipid variables were generated using multivariate regression analysis. Agreement between measured and predicted TG and FFA concentrations was quantified with Pearson's product-moment correlation coefficients.
Individualized Predictions
For study 1, we first performed a best subset regression analysis with SAS software, version 9.2 (SAS Institute, Cary, NC), to calculate the set of 4 VOCs out of ∼100 VOCs that could estimate plasma TG and FFA concentrations with the highest accuracy. We then used multivariate regression analysis on these 4 VOCs to generate prediction models for TG and FFA that were individualized to each subject via JMP software, version 8 (SAS Institute). Every model included the same set of 4 VOCs as covariates, but each VOC was weighted differently for each subject. We arbitrarily defined the set of four compounds included in each prediction model as a 4-VOC “cluster” and will refer to each as such throughout the article. In some instances, individual VOC measurements were technically unavailable; in these cases, the whole data point was dropped from the analysis. Of the 204 possible total data points in study 1, only 198 were used for TG analysis and 186 for FFA analysis.
To identify additional 4-VOC clusters usable in alternative prediction models, we repeated the aforementioned process by alternatively eliminating each of the 4 VOCs included in the “best subset.” In this way, we identified several clusters allowing predictions with slightly lower, but still strong, correlations with measured values (r > 0.90 for TGs, and r > 0.85 for FFAs).
In study 2, the original set of 4 VOCs obtained from study 1 was used again to generate individualized prediction models of TG and FFA in this new data set. Again, of 180 possible total data points, 174 were usable for TG and 161 for FFA predictions due to occasional missing VOC readings.
Common Predictions
In the attempt to derive a common prediction model applicable to the whole group of subjects, we then performed best subset regression analysis using SAS software on ∼100 VOCs. Given the much greater complexity of this predictive approach, a maximum of 10 VOCs per model was allowed to be incorporated in the analysis. Each common prediction model included a set of VOCs that were weighted the same for all subjects. To check their validity of each model, 10% of all data points were randomly withheld from the model-building set for cross-validation.
Results
Plasma Concentrations
In study 1 (glucose infusion), 204 matched plasma and VOC samples were collected from each of our 17 subjects at 12 time points during 4 h of glucose/insulin fluctuations that induced an average drop of 6 mg/dl (or 7% below basal level) in TGs and of 289 mmol/liter (or 72% below basal levels) in FFAs. In study 2 (lipid infusion), we induced an average increase of 119 mg/dl (or 167% above basal level) for TGs and 831 mmol/liter (or 168% above basal levels) for FFAs in 15 subjects (9 of whom also participated in the first study). Mean plasma concentrations across all subjects are listed in Table 2.
Table 2.
Mean Plasma Concentrations with Individualized Predictions
| Study time (min) | TG (mg/dl) | Predicted TG (mg/dl) | FFA (mM) | Predicted FFA (mM) | Glucose (mg/dl) | Insulin (mU/ml) |
|---|---|---|---|---|---|---|
| Study 1: glucose infusion (n = 17) | ||||||
| 30–60 | 89.9 ± 13.6 | 83.8 ± 10.9 | 406 ± 26 | 360 ± 20 | 92.0 ± 1.2 | 3.7 ± 0.5 |
| 130–150 | 65.0 ± 6.6 | 70.1 ± 6.7 | 133 ± 9 | 163 ± 10 | 199.1 ± 2.9 | 60.6 ± 7.4 |
| 220–240 | 65.7 ± 7.7 | 62.9 ± 7.5 | 94 ± 8 | 80 ± 9 | 89.4 ± 1.4 | 86.0 ± 5.3 |
| Study 2: lipid infusion (n = 15) | ||||||
|---|---|---|---|---|---|---|
| 30–60 | 76.1 ± 10.4 | 88.7 ± 13.1 | 477 ± 31 | 678 ± 55 | 92.4 ± 1.4 | 4.5 ± 0.5 |
| 130–150 | 179.4 ± 14.4 | 174.7 ± 14.2 | 1045 ± 82 | 975 ± 69 | 88.4 ± 1.0 | 4.7 ± 0.3 |
| 220–240 | 204.1 ± 19.4 | 205.3 ± 17.2 | 1295 ± 113 | 1252 ± 104 | 89.6 ± 1.1 | 4.6 ± 0.4 |
VOC Concentrations
All VOC were measured in parts per trillion by volume (pptv) concentrations and varied across compounds. The measured concentration range of each VOC included in our prediction models can be found in the Appendix. Mean concentration deltas for the VOCs selected for the individualized prediction models are listed in Table 3. Some VOCs displayed considerable stability, in terms of both quantity and direction of observed changes, while others showed greater variability. For each subject in study 2, the net change in VOC concentrations (difference between study beginning and end) for all compounds selected for our individualized prediction models is listed in Table 4.
Table 3.
Mean VOC Concentrations (Deltas)
| Study time (min) | CO2 (%) | CH3ONO2 (pptv) | Toluene (pptv) | 2-PeONO2 (pptv) | Butanone (pptv) | 2-Pentanone (pptv) | MTBE (pptv) |
|---|---|---|---|---|---|---|---|
| Study 1: glucose infusion (n = 17) | |||||||
| 30–60 | 4.81 ± 0.08 | 13.9 ± 3.1 | −99±272 | −2.09 ± 0.20 | 7573 ± 3274 | 6234 ± 1457 | 5562 ± 1302 |
| 130–150 | 4.62 ± 0.09 | 10.0 ± 1.8 | −101 ± 168 | −3.40 ± 0.32 | 8608 ± 3083 | 3993 ± 846 | 1975 ± 414 |
| 220–240 | 4.78 ± 0.08 | 7.7 ± 1.5 | 96 ± 191 | −4.39 ± 0.38 | 13,762 ± 3525 | 2584 ± 869 | 2341 ± 561 |
| Study 2: lipid infusion (n = 15) | |||||||
|---|---|---|---|---|---|---|---|
| 30–60 | 4.45 ± 0.12 | 9.0 ± 1.9 | −257 ± 64 | −1.95 ± 0.39 | 13,871 ± 5903 | 6769 ± 1214 | 1855 ± 363 |
| 130–150 | 4.62 ± 0.11 | 7.3 ± 1.3 | −123 ± 73 | −2.78 ± 0.44 | 18,916 ± 2966 | 10,882 ± 1443 | 1083 ± 175 |
| 220–240 | 4.41 ± 0.11 | 5.7 ± 1.1 | 20 ± 49 | −2.58 ± 0.54 | 32,391 ± 8103 | 16,964 ± 2272 | 877 ± 135 |
Table 4.
Change in Reported VOCs from Baseline to Study End for All Subjects in Study 2
| Subject | 2-PeONO2 | 2-Pentanone | CO2 (%) | Butanone | CH3ONO2 | MTBE | Toluene |
|---|---|---|---|---|---|---|---|
| 1 | −1.18 | 11,655 | 0.09 | 9630 | −3.98 | −610 | 170 |
| 2 | 2.23 | 2841 | 0.60 | −7969 | −11.98 | −3276 | 428 |
| 3 | −1.55 | 21,956 | 0.35 | 90,632 | −9.78 | −4157 | 146 |
| 4 | −3.71 | 1659 | 0.09 | −7707 | −2.66 | −6864 | 205 |
| 5 | −3.03 | 2036 | −0.71 | −2122 | −3.82 | −208 | 422 |
| 6 | 0.39 | 10,230 | −0.18 | 10,541 | −1.30 | −391 | 161 |
| 7 | −0.87 | 661 | 1.09 | 4431 | −9.00 | −251 | −7 |
| 8 | −0.75 | 1962 | 0.78 | 3139 | −1.74 | −804 | 302 |
| 9 | −0.59 | 29,536 | −1.07 | 32,094 | −1.02 | −891 | 147 |
| 10 | −2.07 | 29,232 | −0.35 | −30,709 | −11.32 | −2982 | 713 |
| 11 | 4.99 | 6859 | −0.05 | 20,366 | −1.82 | −1657 | 210 |
| 12 | −0.69 | 14,125 | 0.47 | 21,484 | −2.26 | −682 | 266 |
| 13 | −1.19 | −285 | −0.39 | 16,673 | −1.12 | −964 | 188 |
| 14 | 0.05 | 4520 | 0.18 | 5644 | −2.27 | 400 | 612 |
| 15 | 0.20 | 12,611 | 0.30 | 14,541 | −1.81 | −3525 | 657 |
| Mean | −0.52 | 9973 | 0.08 | 12,045 | −4.39 | −1791 | 308 |
| SE | 0.54 | 2585 | 0.15 | 6821 | 1.02 | 510 | 55 |
| Mean % change | 24% | 277% | 2% | 417% | −55% | −62% | 22% |
| # Increased | 5 (33%) | 14 (93%) | 9 (60%) | 11 (73%) | 0 (0%) | 1 (7%) | 14 (93%) |
Individualized Predictions
Several individualized lipid prediction models were generated in this standard format: TG or FFA = X0 + X1 [VOC 1] + X2 [VOC 2] + X3 [VOC 3] + X4 [VOC 4], where X0, X1, X2, X 3, and X4 represent the expected difference in TG or FFA when the concentration of each corresponding VOC is increased by one unit while other VOCs are kept constant.
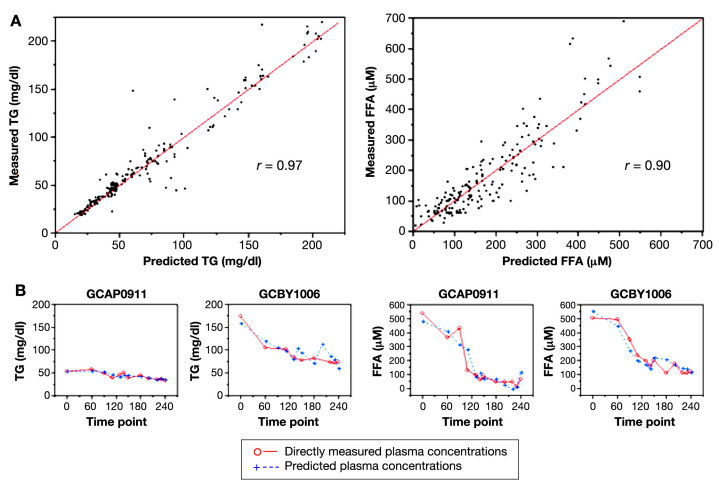
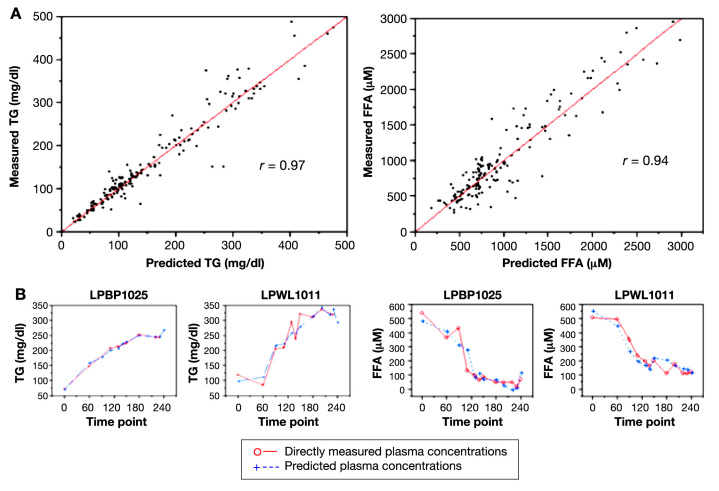
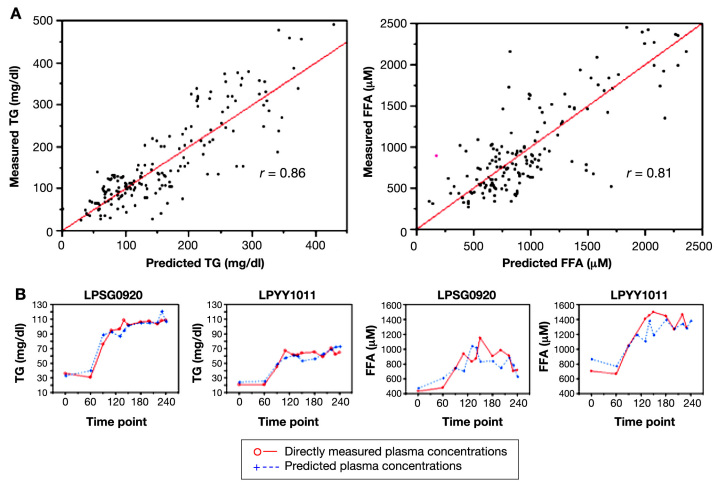
For study 1, the 4-VOC cluster that yielded the highest overall correlation between our breath-based estimates of plasma TGs and measured plasma values was 2-pentyl nitrate (2-PeONO2), CO2, methyl nitrate (CH3ONO2), and toluene; the overall correlation coefficient was 0.97 (Figure 3, left top). Similarly for FFAs, the set of 4 VOCs yielding the highest overall correlation of predicted and measured values was 2-pentanone, 2-PeONO2, butanone, and methyl tert-butyl ether (MTBE) with an overall correlation coefficient of 0.90 (Figure 3, right top). For study 2, estimates of plasma TGs and FFA using the same 4-VOC cluster also resulted in high concordance. The strength of the correlations between measured and predicted values was very similar to those observed in study 1 (0.97 for TGs and 0.94 for FFA; Figure 4). As an example of the flexibility of our methodology, a summary of the overall correlation between measured and predicted TG using five alternative clusters is also reported in Table 5.
Figure 3.
Individualized prediction models of TG and FFAs during glucose/insulin infusion. (A) Plots of directly measured vs predicted plasma TG and FFA concentrations in 17 healthy young adults. A total of 12 breath and room air samples were taken for each subject during a 4 h clamp study with broad fluctuations of plasma glucose and insulin. Individualized prediction models based on multilinear regression analyses of 4-VOC clusters (2-PeONO2, CO2, CH3ONO2, and toluene for TGs, left; 2-pentanone, 2-PeONO2, butanone, MTBE for FFAs, right) demonstrated the highest overall correlation with directly measured lipid concentrations. (B) Time course of measured and predicted lipid values in two representative subjects.
Figure 4.
Individualized prediction models of TG and FFAs during lipid infusion. (A) Plots of directly measured vs predicted plasma TG and FFA concentrations in 15 healthy young adults. A total of 12 breath and room air samples were taken for each subject during a 4-h lipid infusion study, which resulted in a ∼2.5-fold increase of plasma lipids above basal levels. Individualized prediction models based on multilinear regression analyses of 4-VOC clusters (2-PeONO2, CO2, CH3ONO2, and toluene for TGs, left; 2-pentanone, 2-PeONO2, butanone, MTBE for FFAs, right) demonstrated the highest overall correlation with directly measured lipid concentrations. (B) Time course of measured and predicted lipid values in two representative subjects.
Table 5.
Comparison of Alternative VOC Clusters for Individualized TG Predictions
| All subjects:(studies 1 and 2; n = 32) | 2-PeONO2, CH3ONO2, CO2, toluene | 2-PeONO2, CH3ONO2, isoprene, toluene | 2-PeONO2, CO2, isoprene, toluene | CH3ONO2, CO2, isoprene, toluene | 2-PeONO2, 2-BuONO2, CH3ONO2, CO2 |
|---|---|---|---|---|---|
| Overall correlation coefficient | 0.97 | 0.97 | 0.97 | 0.97 | 0.96 |
| Mean correlation coefficient | 0.83 | 0.82 | 0.80 | 0.80 | 0.81 |
| # Subjects that selected cluster is stronger | – | 18 (56%) | 23 (72%) | 18 (56%) | 23 (72%) |
The strong correlation between measured and predicted values was maintained when data was compared separately for each subject, by overlaying the individual time courses of measured and predicted lipid concentrations during the 4 h of the study (Figures 3 and 4, bottom panels). Of course, providing the best overall correlation does not automatically translate into the best prediction model for each subject. For example, at least some of the tested subjects displayed a better correlation when using some of the four alternative clusters than using our best overall model. However, our reported model always yielded a higher correlation in the majority of the subjects as well as the highest mean correlation (Table 5). As noted earlier, while the profiles of the same four VOCs were used in all subjects to predict TG and FFA, the actual prediction models were unique to each subject.
Common Predictions
In study 1, attempts to generate a common prediction model for TG and FFA, applicable to the whole set of subjects, using combinations of up to 10 VOCs, were relatively unsuccessful. Correlations between measured and predicted lipid values, initially weak with only a few VOCs in the model, grew somewhat stronger as more VOC covariates were added. As expected, these increases were larger with addition of the first few covariates, but as the model neared 10 covariates, only negligible albeit measurable improvements with additional covariates were noted; the overall predictive ability of the model remained weak.
In study 2, on the other hand, we successfully developed several common prediction models to predict lipidemia. We believe this improved ability was due to both the availability of a greater number of usable exhaled VOCs in study 2 as well as the much broader range of TG and FFA values induced by study procedures.
Our most accurate prediction model for TG utilized 10 VOCs and resulted in a correlation coefficient of 0.86 from 174 observations across all the lipid infusion subjects (Figure 5, left top):
Figure 5.
Common prediction model for TG and FFAs during lipid infusion. (A) Plots of directly measured vs predicted plasma TG and FFA concentrations in 15 healthy young adults. A total of 12 breath and room air samples were taken for each subject during a 4 h lipid infusion study, which resulted in a ∼2.5-fold increase of plasma lipids above basal levels. Common prediction models for TG and FFA were derived from the multilinear regression analyses of 10-VOC clusters. The models that demonstrated the highest correlation with directly measured lipid concentrations are shown (TG, left; FFA, right). (B) Time course of measured and predicted lipid values in two representative subjects.
TG (mg/dl) = 241.4 + 0.012 [β-pinene] + 1.06 [bromomethane (CH3Br)] − 5.44 [CH3ONO2] − 0.0034 [CO2 (in ppmv)] − 0.00049 [d Limonene] + 0.0024 [dimethyl disulfide] + 0.042 [ethane] + 0.0016 [methacrolein] + 3.16 [methane (CH4) (in ppmv)] + 0.12 [tetrachloroethylene (C2Cl4)].
A separate combination of 10 VOCs was used to construct a common prediction model for FFA with a correlation coefficient of 0.81 from 161 observations on the same cohort (Figure 5, right top):
FFA (μM) = 404.9 + 15.46 [2-butyl nitrate (2-BuONO2)] − 46.87 [bromoform (CHBr3)] + 0.76 [C2Cl4] + 15.65 [CH3Br] + 0.16 [ethane] − 203.56 [ethyl nitrate (EtONO2)] − 1.08 [hydrocholorofluorocarbon-22] − 251.89 [methyl iodide (CH3I)] + 0.66 [toluene] − 4.50 [trichloroethylene (C2HCl3)].
The p values for two-tailed tests of the significance of each regression coefficient ranged from <0.0001 to 0.0279 (Table 6).
Table 6.
Parameter Estimates for the Common Prediction Models
| Term | Estimate | Standard error | t ratio | Prob. > |t| |
|---|---|---|---|---|
| Common prediction model for triglyerides (mg/dl) | ||||
| Intercept | 241.4117 | 37.98443 | 6.36 | <.0001 |
| β-Pinene | 0.011504 | 0.002906 | 3.96 | .0001 |
| CH3Br | 1.0607384 | 0.164033 | 6.47 | <.0001 |
| CH3ONO2 | −5.440771 | 0.633954 | −8.58 | <.0001 |
| CO2 (in ppmv) | −0.003369 | 0.000732 | −4.6 | <.0001 |
| d-Limonene | −0.00049 | 0.000132 | −3.71 | .0003 |
| DMDS | 0.002399 | 0.000311 | 7.72 | <.0001 |
| Ethane | 0.0420142 | 0.005481 | 7.66 | <.0001 |
| Methacrolein | 0.001608 | 0.00032 | 5.03 | <.0001 |
| CH4 (in ppmv) | 3.1552289 | 0.435703 | 7.24 | <.0001 |
| C2Cl4 | 0.1151845 | 0.015501 | 7.43 | <.0001 |
| Common prediction model for free fatty acids (μM) | ||||
|---|---|---|---|---|
| Intercept | 404.94404 | 57.28307 | 7.07 | <.0001 |
| 2-BuONO2 | 15.458042 | 3.525271 | 4.38 | <.0001 |
| CHBr3 | −46.86606 | 16.02472 | −2.92 | .0040 |
| C2Cl4 | 0.7575156 | 0.083584 | 9.06 | <.0001 |
| CH3Br | 15.65419 | 1.357382 | 11.53 | <.0001 |
| Ethane | 0.1576478 | 0.04068 | 3.88 | .0002 |
| EtONO2 | −203.5591 | 28.63184 | −7.11 | <.0001 |
| Hydrochloro-fluorocarbon-22 | −1.078886 | 0.415386 | −2.6 | .0103 |
| CH3I | −251.8913 | 40.53543 | −6.21 | <.0001 |
| Toluene | 0.6567578 | 0.127792 | 5.14 | <.0001 |
| C2HCl3 | −4.500372 | 2.026429 | −2.22 | .0279 |
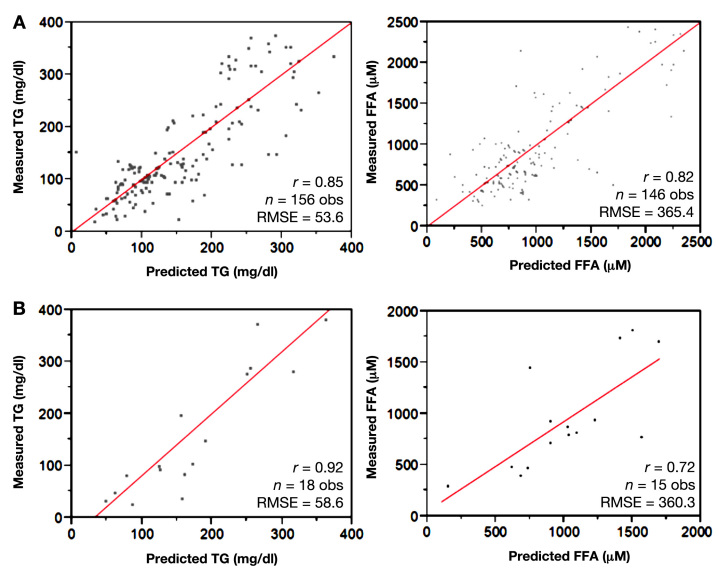
Strong predictive accuracy was maintained in a cross-validation step, in which predictive equations were developed on a randomly selected 90% subset of the data and tested on the remaining 10% of the data. Correlation coefficients and root mean square errors (RMSE) for the model-building set and validation set were comparable (TG: r = 0.85 and RMSE = 53.6 mg/dl for the training set, r = 0.92 and RMSE = 58.6 mg/dl for the validation set; FFA: r = 0.82 and RMSE = 365 µM for the training set, r = 0.72 and RMSE = 360 µM for the validation set; Figure 6).
Figure 6.
Cross-validation of common prediction model for TG and FFAs during lipid infusion. For cross-validation of TG and FFA common prediction models, data were randomly separated into a training set (90% of data points, top panels), on which predictive algorithms were built, and a validation set (remaining 10% of data points, bottom panels), on which algorithms were applied. Correlation coefficients and root mean square errors (RMSE) were comparable between the training sets (A) and validation sets (B).
Discussion
Our main finding is that plasma concentrations of TGs and FFAs were estimated accurately via integrated analysis of exhaled VOCs in a group of healthy young adults. These estimates for plasma lipid concentrations were calculated for each subject using the same 4-VOC cluster, albeit with individualized calibrations of the coefficients in each prediction model. Our results were achieved first during relatively small fluctuations of plasma lipids (∼50% drop below basal levels during insulin infusion) and then confirmed during much greater lipid fluctu-ations (>150% increase over baseline via lipid infusion). For study 2, a common prediction model was also derived from the collective data of all subjects and utilized to estimate TG and FFA for each individual subject. This model, which integrates 10 exhaled VOCs into a single equation, retained considerable accuracy when compared to the individualized prediction models. As some of the VOCs examined in study 2 were technically not available at the time of study 1, the common prediction equation could not be extended to our earlier data set.
To facilitate the transition from proof-of-concept of this methodology to future clinical applications, it is important that the number of VOCs used for plasma lipid prediction is kept as low as possible and that the selected VOCs are relatively uncomplicated to quantify. In this regard, our methodology offers considerable flexibility. In fact, we initially restricted the number of covariates in our model on the notion that 4 VOCs is an acceptably small number to be included in a portable device from an engineering perspective. In our reported individualized lipid prediction model, we, therefore, selected the 4-VOC clusters with the best predictive ability for TGs and FFAs across both protocols. Several alternative VOC clusters yielded lipid estimates with a level of accuracy often only marginally inferior to those selected (Table 5). If, at the stage of product development, some of the VOCs included in our reported models were to prove unpractical to measure, numerous viable 4-VOC clusters retaining clinically relevant predictive accuracy would be available with VOCs that are easier to measure or exist at higher concentrations. (Even with our sophisticated equipment, in fact, many VOCs are more challenging than others to quantify accurately.)
It is important to note that we were only able to effectively use 4-VOC models with individualized prediction models. This means that while the same 4 VOCs were used in all participants, a separate equation was generated for each subject. Accordingly, the coefficients for each VOC were adjusted separately for each subject so that the subject's plasma lipid values could be calculated from the exhaled profiles of those VOCs. We also attempted to develop an “exploratory” common prediction model (i.e., testing the overall feasibility of this concept) that could be used for all our subjects in study 2. Of course, we are aware that, in its present form, this model was less than ideal for at least two reasons. First, compared to the individualized model, there was a loss of correlation (dropping to 0.86 and 0.81 for TGs and FFAs, respectively, which is still relatively strong but undeniably weaker); second, this model required integration of the profiles of 10 different VOCs, a number probably too high to be reasonably included in a portable breath analyzer, especially a device monitoring multiple plasma metabolites.
Nonetheless, the fact that we were able to generate similarly strong individualized prediction models when using the same 4-VOC cluster as study 1 (even though the earlier protocol included much narrower lipid fluctuations) highlights the flexibility of our methodology and bodes well for its potential future applicability to clinical settings. We are very much aware that the practical usefulness of a model for monitoring plasma lipids is closely linked to the model's accuracy in the context of different and rapidly changing metabolic conditions. In real life, the same lipid concentrations or their changes may occur in the presence of very different attending metabolic milieus. Acutely, this can be caused by ingestion of large meals with different percentage of carbohydrate and lipid composition and, chronically, by the presence of specific conditions. For example, hyperlipidemia can be accompanied by both hyperglycemia and hyperinsulinemia in the obese patient with early-stage type 2 diabetes; however, those with familial hyperlipidemic syndromes can have normal plasma glucose and insulin. We, therefore, incorporated some of this variability in the different arms of our experimental design, fully expecting that some (possibly many) of the measured exhaled compounds would display markedly different exhaled profiles, while others would maintain a consistent relationship with lipidemia (leading to stronger predictions) across the two conditions.
Several general observations are probably in order regarding the origins, pathways, and selection of VOCs reported in our models. As the changes in VOC concentrations were often many orders of magnitude smaller than the changes in lipids they appear to reflect, it seems logical that complex intermediate biochemical events are involved in these changes. Given the number and variety of VOCs involved, addressing these issues in detail for each compound would clearly be beyond the scope of this study. Additionally, as the dynamics of gas exchange across the lungs are so complex, estimated partition coefficients between fluid and gas phase of many VOCs often defy estimates by Henry's Law. Complete explanation of these discrepancies will require considerable future experimental efforts. However, initial experiments by our group have found that the VOCs described in this article have stable levels across subjects, both in blood and breath, i.e., with very stable blood/breath ratios. A schematic summary of chemical characteristics of our selected VOCs, possibly relevant to endogenous lipid metabolism, as well as related literature, can be found in the Appendix.
It is also indeed possible that some of the reported VOCs were simply inhaled from ambient sources. While at first sight, this may appear to provide strong grounds to remove that compound from analysis, we believe that correlation with systemic levels of lipids (or other variables) may not be just coincidental in many cases. Some inhaled VOCs, while not part of human origin, can be absorbed and partly degraded via various enzymatic activities, resulting in exhaled levels lower than inhaled. Intervening metabolic changes can modify the ratio between inhaled and exhaled concentrations, linking the exhaled levels to specific metabolic events. We identified a similar situation during an earlier experiment on plasma glucose prediction. Some aromatic VOCs normally present in the atmosphere (ethylbenzene, o-xylene, mp-xylene) are metabolized by the liver30 and were among the strongest covariates for glucose prediction. These VOCs were exhaled at ∼20% of inhaled concentrations initially but increased two- to three-fold during hyperglycemia.24 A likely explanation is hyperglycemia alters glucose load and blood flow to the liver, which in turn shifts hepatic metabolism away from the VOCs and results in their increased exhalation. The VOCs thereby are indirect markers of glucose metabolism. Similar pathways may be in place for VOCs involved in lipid prediction analysis. Only VOCs present in ambient air at relatively constant and reproducible concentrations, of course, can be used in this context. All compounds used for our calculations with uncertain biological origin have demonstrably stable and measurable concentrations throughout the earth atmosphere (including several man-made substances whose number, incidentally, is unfortunately increasing). Further, several of our selected VOCs (e.g., trichloroethene, toluene, tetrachloroethene) can be systematically extracted at measurable levels from blood samples of nonoccupationally exposed adult U.S. populations.31
The variability of the VOCs selected across our models may seem contrary to the expectation that TGs and FFAs should have similar metabolism and kinetics. In fact, most of the VOCs selected as covariates for the individualized prediction models from our study were also not utilized as covariates for the common prediction models. While one might initially expect a direct one-to-one relationship between plasma metabolites and breath components and therefore the same VOCs in both models, it is important to realize that we are only reporting the combinations of VOCs with the highest correlation for each proposed condition; an additional 10–20 VOCs were also showing some level of correlation with the lipid variables. Each of these VOCs can possibly account for a different component of a variable's plasma concentrations, i.e., in addition to absolute value, an upward or downward shift, a time lag, reaching a certain threshold, and having remained elevated or suppressed for a certain period. Furthermore, multiple VOCs were likely generated during intracellular intermediate metabolism of FFAs or TGs, and therefore, combinations of VOCs in the common predictions models may jointly account for different steps of the same biological pathways as some of the VOCs from the individualized models. Possible partial clarification of their sources may derive from ongoing studies in our group; we have developed a technique to capture VOC emissions from human cells cultured in custom-made glass bioreactors during both resting culture conditions and exposure to metabolic stimuli. We have applied this technique to isolated immune cells32 and plan to extend these studies to a number of other tissues.
Yet, several VOCs did appear in multiple models: CH3Br, ethane, and C2Cl4 were found to be significant covariates for both the TG and FFA common equations, and two VOCs in the common TG predictions (CH3ONO2, CO2) were also important covariates in the 4-VOC individual TG models. On the other hand, no VOCs were used in both the common and individualized FFA prediction models. It should be noted, however, that to be able to compare the predictive accuracy of individualized models from studies 1 and 2, we used sets of VOCs that were available for both studies. It is likely that if the technology to quantify all the VOCs used in study 2 had been available in study 1, other VOC combinations may have been chosen for individualized models, possibly utilizing several of the VOCs from the common 10-VOC prediction models.
In its current incomplete phase of development, we openly acknowledge that our methodology presents several areas of concern. Lag times between changes in plasma variables and their corresponding changes in breath VOC concentrations are also potential confounders. Lag times might vary across different VOCs, and mathematically correcting this problem is paramount for the breath-based measurement of rapidly changing variables that require frequent testing, such as plasma glucose in patients with diabetes. This should, however, be less of a problem for lipid measurements because they are typically measured only every few months in clinical settings. Additionally, these tests are primarily performed in fasting, metabolically stable conditions, effectively rendering such lag times irrelevant. Lastly, other limitations of our current work is the lack of a full independent cohort to validate our common predictions and the ratio between number of samples collected per subject in comparison with the terms in our individualized models. There is no question that the optimal way to definitively validate our models would be to repeat at least part of the study on a separate cohort and to increase the sampling frequency; we are indeed planning to incorporate such changes in future studies. Still, cross-validation via splitting our data set demonstrated comparable results between training and prediction sets.
Conclusions
In conclusion, our data shows that prediction of plasma TG and FFA from the exhaled breath is feasible in controlled experimental conditions. Although the project is still at a relatively early phase, results appear very encouraging; further, marked improvements in predictive ability (ideally above r > 0.90) and a reduced number of VOCs in our common prediction models are possible after more extensive studies. Validation with additional subjects under a broader range of experimental conditions (e.g., subjects with type 1 and type 2 diabetes, history of smoking, acute illness) will also be necessary before the translation of this technology into practical clinical applications for diabetes mellitus and other related conditions, such as dyslipidemia, obesity, and cardio-vascular disease.
Acknowledgments
The authors would like to acknowledge Tu-Anh Nguyen, Brian Tran, Nicole Tse, and Nancy Wong for their help in preparing the manuscript and the anonymous reviewers for their helpful suggestions. Our work would not have been possible without the excellent technical support of the UC Irvine ICTS research nurses (Barbara Bodenhoefer, A. Diane Capobianco, and Connie Parido) and staff.
Glossary
Abbreviations
- (2-BuONO2)
2-butyl nitrate
- (2-PeONO2)
2-pentyl nitrate
- (C2Cl4)
tetrachloroethylene
- (C2HCl3)
trichloroethylene
- (CHBr3)
bromoform
- (CH3Br)
bromomethane
- (CH3I)
methyl iodide
- (CH3ONO2)
methyl nitrate
- (CH4)
methane
- (CO2)
carbon dioxide
- (DMDS)
dimethyl disulfide
- (ECD)
electron-capture detectors
- (EtONO2)
ethyl nitrate
- (F)
female
- (FFA)
free fatty acids
- (FID)
flame-ionization detectors
- (GC)
gas chromatograph
- (ICTS)
Institute for Clinical and Translational Science
- (IV)
intravenous
- (M)
male
- (MSD)
mass spectrometer detector
- (MTBE)
methyl tert-butyl ether
- (RMSE)
root mean square errors
- (SCD)
sulfur chemiluminiscence detector
- (TG)
triglycerides
- (UCI)
University of California, Irvine
- (VOC)
volatile organic compounds
Funding
This work was supported by grants from the Juvenile Diabetes Research Foundation (#1-2006-76), National Institutes of Health (NIH, M01-RR00827-28), and American Diabetes Association (7-08-CR-22). Timothy Minh was supported by the NIH Medical Scientist Training Program at UC Irvine (T32-GM008620) and the NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Kirschstein Predoctoral MD/PhD Fellowship (F30-DK088401). Pietro R. Galassetti was supported by the NIH/NIDDK Mid-Career Development Grant (K24-DK085223).
References
- 1.Miekisch W, Schubert JK, Noeldge-Schomburg GFE. Diagnostic potential of breath analysis—focus on volatile organic compounds. Clin Chim Acta. 2004;347(1–2):25–39. doi: 10.1016/j.cccn.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 2.Phillips M, Herrera J, Krishnan S, Zain M, Greenberg J, Cataneo RN. Variation in volatile organic compounds in the breath of normal humans. J Chromatogr B Biomed Sci Appl. 1999;729(1–2):75–88. doi: 10.1016/s0378-4347(99)00127-9. [DOI] [PubMed] [Google Scholar]
- 3.Horváth I, Hunt J, Barnes PJ, Alving K, Antczak A, Baraldi E, Becher G, van Beurden WJ, Corradi M, Dekhuijzen R, Dweik RA, Dwyer T, Effros R, Erzurum S, Gaston B, Gessner C, Greening A, Ho LP, Hohlfeld J, Jöbsis Q, Laskowski D, Loukides S, Marlin D, Montuschi P, Olin AC, Redington AE, Reinhold P, van Rensen EL, Rubinstein I, Silkoff P, Toren K, Vass G, Vogelberg C, Wirtz H ATS/ERS Task Force on Exhaled Breath Condensate. Exhaled breath condensate: methodo-logical recommendations and unresolved questions. Eur Respir J. 2005;26(3):523–548. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 4.Thomas KW, Pellizzari ED, Cooper SD. A canister-based method for collection and GC/MS analysis of volatile organic compounds in human breath. J Anal Toxicol. 1991;15(2):54–59. doi: 10.1093/jat/15.2.54. [DOI] [PubMed] [Google Scholar]
- 5.Moser B, Bodrogi F, Eibl G, Lechner M, Rieder J, Lirk P. Mass spectrometric profile of exhaled breath—field study by PTR-MS. Respir Physiol Neurobiol. 2005;145(2–3):295–300. doi: 10.1016/j.resp.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Anstie FE. London and Cambridge: Macmillan; 1864. Stimulants and narcotics, their mutual relations: with special researches on the action of alcohol, ether and chloroform, on the vital organism. [Google Scholar]
- 7.Enderby B, Smith D, Carroll W, Lenney W. Hydrogen cyanide as a biomarker for Pseudomonas aeruginosa in the breath of children with cystic fibrosis. Pediatr Pulmonol. 2009;44(2):142–147. doi: 10.1002/ppul.20963. [DOI] [PubMed] [Google Scholar]
- 8.Olopade CO, Zakkar M, Swedler WI, Rubinstein I. Exhaled pentane levels in acute asthma. Chest. 1997;111(4):862–865. doi: 10.1378/chest.111.4.862. [DOI] [PubMed] [Google Scholar]
- 9.Phillips M, Cataneo RN, Condos R, Ring Erickson GA, Greenberg J, La Bombardi V, Munawar MI, Tietje O. Volatile biomarkers of pulmonary tuberculosis in the breath. Tuberculosis. 2007;87(1):44–52. doi: 10.1016/j.tube.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Kamboures MA, Blake DR, Cooper DM, Newcomb RL, Barker M, Larson JK, Meinardi S, Nussbaum E, Rowland FS. Breath sulfides and pulmonary function in cystic fibrosis. Proc Natl Acad Sci USA. 2005;102(44):15762–15767. doi: 10.1073/pnas.0507263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips M, Gleeson K, Hughes JM, Greenberg J, Cataneo RN, Baker L, McVay WP. Volatile organic compounds in breath as markers of lung cancer: a cross-sectional study. Lancet. 1999;353(9168):1930–1933. doi: 10.1016/S0140-6736(98)07552-7. [DOI] [PubMed] [Google Scholar]
- 12.Weitz ZW, Birnbaum AJ, Sobotka PA, Zarling EJ, Skosey JL. High breath pentane concentrations during acute myocardial infarction. Lancet. 1991;337(8747):933–935. doi: 10.1016/0140-6736(91)91569-g. [DOI] [PubMed] [Google Scholar]
- 13.Kneepkens CM, Ferreira C, Lepage G, Roy CC. The hydrocarbon breath test in the study of lipid peroxidation: principles and practice. Clin Invest Med. 1992;15(2):163–186. [PubMed] [Google Scholar]
- 14.McCloy U, Ryan MA, Pencharz PB, Ross RJ, Cunnane SC. A comparison of the metabolism of eighteen-carbon 13C-unsaturated fatty acids in healthy women. J Lipid Res. 2004;45(3):474–485. doi: 10.1194/jlr.M300304-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Wang CJ, Yang NH, Liou SH, Lee HL. Fast quantification of the exhaled breath condensate of oxidative stress 8-iso-prostaglandin F2[alpha] using on-line solid-phase extraction coupled with liquid chromatography/electrospray ionization mass spectrometry. Talanta. 2010;82(4):1434–1438. doi: 10.1016/j.talanta.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Janicka M, Kot-Wasik A, Kot J, Namiesnik J. Isoprostanes-biomarkers of lipid peroxidation: their utility in evaluating oxidative stress and analysis. Int J Mol Sci. 2010;11(11):4631–4659. doi: 10.3390/ijms11114631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montuschi P. LC/MS/MS analysis of leukotriene B4 and other eicosanoids in exhaled breath condensate for assessing lung inflammation. J Chromatogr B Anal Technol Biomed Life Sci. 2009;877(13):1272–1280. doi: 10.1016/j.jchromb.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 18.Votruba SB, Zeddun SM, Schoeller DA. Validation of deuterium-labeled fatty acids for the measurement of dietary fat oxidation: a method for measuring fat-oxidation in free-living subjects. Int J Obesity. 2001;25(8):1240–1245. doi: 10.1038/sj.ijo.0801672. [DOI] [PubMed] [Google Scholar]
- 19.Martínez-Lozano P, Fernández de la Mora J. Direct analysis of fatty acid vapors in breath by electrospray ionization and atmospheric pressure ionization-mass spectrometry. Anal Chem. 2008;80(21):8210–8215. doi: 10.1021/ac801185e. [DOI] [PubMed] [Google Scholar]
- 20.Martínez-Lozano P, Zingaro L, Finiguerra A, Cristoni S. Secondary electrospray ionization-mass spectrometry: breath study on a control group. J Breath Res. 2011;5 doi: 10.1088/1752-7155/5/1/016002. 016002. [DOI] [PubMed] [Google Scholar]
- 21.Arterbery VE, Pryor WA, Jiang L, Sehnert SS, Foster WM, Abrams RA, Williams JR, Wharam MD, Jr., Risby TH. Breath ethane generation during clinical total body irradiation as a marker of oxygen-free-radical-mediated lipid peroxidation: a case study. Free Radical Biol Med. 1994;17(6):569–576. doi: 10.1016/0891-5849(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 22.Musa-Veloso K, Likhodii SS, Cunnane SC. Breath acetone is a reliable indicator of ketosis in adults consuming ketogenic meals. Am J Clin Nutr. 2002;76(1):65–70. doi: 10.1093/ajcn/76.1.65. [DOI] [PubMed] [Google Scholar]
- 23.Novak BJ, Blake DR, Meinardi S, Rowland FS, Pontello A, Cooper DM, Galassetti PR. Exhaled methyl nitrate as a noninvasive marker of hyperglycemia in type 1 diabetes. Proc Natl Acad Sci USA. 2007;104(40):15613–15618. doi: 10.1073/pnas.0706533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Ngo J, Blake D, Meinardi S, Pontello AM, Newcomb R, Galassetti PR. Improved predictive models for plasma glucose estimation from multi-linear regression analysis of exhaled volatile organic compounds. J Appl Physiol. 2009;107(1):155–160. doi: 10.1152/japplphysiol.91657.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galassetti PR, Novak B, Nemet D, Rose-Gottron C, Cooper DM, Meinardi S, Newcomb R, Zaldivar F, Blake DR. Breath ethanol and acetone as indicators of serum glucose levels: an initial report. Diabetes Tech Therap. 2005;7(1):115–123. doi: 10.1089/dia.2005.7.115. [DOI] [PubMed] [Google Scholar]
- 26.Minh TD, Oliver SR, Ngo J, Flores RL, Midyett J, Meinardi S, Carlson MK, Rowland FS, Blake DR, Galassetti PR. Non-invasive measurement of plasma glucose from exhaled breath in healthy and type 1 diabetic mellitus subjects. Am J Physiol Endocrinol Metab. 2011;300(6):E1166–E1175. doi: 10.1152/ajpendo.00634.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colman JJ, Swanson AL, Meinardi S, Sive BC, Blake DR, Rowland FS. Description of the analysis of a wide range of volatile organic compounds in whole air samples collected during PEM-tropics A and B. Anal Chem. 2001;73(15):3723–3731. doi: 10.1021/ac010027g. [DOI] [PubMed] [Google Scholar]
- 28.Simpson I, Colman J, Swanson A, Bandy A, Thornton D, Blake D, Rowland F. Aircraft measurements of dimethyl sulfide (DMS) using a whole air sampling technique. J Atmos Chem. 2001;39(2):191–213. [Google Scholar]
- 29.Gorham KA, Sulbaek Andersen MP, Meinardi S, Delfino RJ, Staimer N, Tjoa T, Rowland FS, Blake DR. Ethane and n-pentane in exhaled breath are biomarkers of exposure not effect. Biomarkers. 2009;14(1):17–25. doi: 10.1080/13547500902730680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Âstrand I, Engström J, Övrum P. Exposure to xylene and ethyl-benzene. I. Uptake, distribution and elimination in man. Scand J Work Environ Health. 1978;4(3):185–194. doi: 10.5271/sjweh.2707. [DOI] [PubMed] [Google Scholar]
- 31.Ashley DL, Bonin MA, Cardinali FL, McCraw JM, Wooten JV. Blood concentrations of volatile organic compounds in a non-occupationally exposed US population and in groups with suspected exposure. Clin Chem. 1994;40(7 Pt 2):1401–1404. [PubMed] [Google Scholar]
- 32.Shin HW, Umber BJ, Meinardi S, Leu SY, Zaldivar F, Blake DR, Cooper DM. Acetaldehyde and hexanaldehyde from cultured white cells. J Transl Med. 2009;7:31. doi: 10.1186/1479-5876-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]