Abstract
Preventing amputations in persons with lower extremity complications of diabetes is a complex endeavor, particularly in those with concomitant ischemia and tissue loss. Fluorescence angiography (Novadaq SPY system) may provide a tool for objective evaluations of tissue viability in the diabetic foot, which is an important indicator of the ability of the diabetic ulcer to heal adequately. The SPY system uses a low-power laser coupled with a charge-coupled device camera and indocyanine green (ICG) to sequence perfusion at the surface of the skin. We present an illustrated example of the potential utility of ICG fluorescence angiography (ICGFA) before and after vascular intervention in a high-risk limb. ICGFA appeared to reveal demarcation between viable and nonviable tissue and real-time perfusion, specifically capillary fill. ICGFA clarified the extent of necessary debridement and provided an immediate indication of improvement in regional perfusion status following revascularization. Future studies involving ICGFA may include pre- and postdebridement and closure perfusion, comparison of tissue perfusion pre- and post-endovascular therapy, and lower extremity flap viability. Future works will also address the consistency of results with ICGFA by analyzing a larger cohort of patients being treated by our unit.
Keywords: diabetic foot ulcers, noninvasive imaging, peripheral vascular disease, tissue health, wound healing
Introduction
Management of diabetic foot disease presents a significant challenge for health professionals due to the many complications that lead to foot ulcers and subsequent amputations. Peripheral vascular disease (PVD) and diabetic peripheral neuropathy (DPN) are the two most common risk factors that contribute to the disease burden and are associated with an increased morbidity and mortality. The relative 5-year mortality rate for neuropathic ulcers was reported to be 45% for neuropathic ulcers, 47% for amputations, 55% for ischemic ulcers, and 64% for peripheral arterial disease.1 Surviving amputees may experience a reduced quality of life, a higher rate of chronic wounds and an increased probability of a second amputation.2–5
The goal of the physician when treating the diabetic foot is to prevent amputations, a process that begins with the prevention of foot ulcers. One of the main causes of foot ulcers is PVD, which is found in half of all patients with diabetes who develop foot ulcers.6 Peripheral vascular disease is characterized by atherosclerosis (deposition of plaque) in patients with longstanding diabetes mellitus.
In the United States, there are an estimated 12 million people with PVD, many of whom are asymptomatic. Typical symptoms of PVD include claudication and critical limb ischemia. Peripheral vascular disease leads to rheological changes in blood and can impair nutritional supply to the lower extremity thereby making the foot more susceptible to ulceration in the presence of a triggering factor such as minor trauma.7 Additionally, it is an important risk factor for postoperative complications in patients with ulceration and is indicative of poor healing and infection proliferation.8
Multiple methods are utilized to determine vascular flow including the ankle-brachial index test, Doppler measurement, duplex ultrasound scanning, arteriogram, toe systolic pressure index, and other more invasive procedures such as CT-fluoroscopy. In the following case study, the SPY Fluorescence Imaging Device (Novadaq, USA) was used to determine real-time arterial blood flow to an ulcerated and/or partially necrotic foot, capillary perfusion within the tissue in question, as well as the most current degree of necrosis. Higher rates of tissue salvage not only improve the patient's quality of life postsurgery but also positively impact the closure and healing rates of an open wound from a partial amputation or incision. Often, the viability of the tissue is determined by a clinical assessment of the skin coloration, which is in many cases highly subjective. Other evidence in the literature suggest using hyperspectral imaging for determination of tissue viability and ulcer healing by measuring oxyhemoglobin and deoxyhemoglobin.9,10 The goal of utilizing ICGFA was to determine the extent of viable tissue available to facilitate limb salvage in a patient undergoing surgical bypass. The following case study addresses the accuracy of ICGFA.
Methods
The SPY Fluorescent Imaging System consists of an imaging head equipped with a charge- coupled device camera, a laser light source, and a distance sensor. The laser operates at a power density of 40 mW/cm2, far below the hazard threshold of 200 mW/cm2. The camera head has a proximity limitation of 5 cm or 2 inches from the area of interest to prevent overexposure. The imaging head is attached to an articulating arm to a mobile cart containing the central processing unit, keyboard, monitor, and mouse. For use during surgeries, the sterile Novadrape covers the camera and articulating arm.
The SPY system is used in tandem with indocyanine green or ICG, a water soluble, nonradioactive tricarbocyanine dye with a peak spectral absorption at 800–810 nm in blood plasma or blood. ICG contains no more than 5.0% sodium iodide and can be injected after it is reconstituted with prepared sterile water. Immediately prior to each ICGFA sequence, 5 cc of the mixture or 12.5 mg of ICG was injected. Following injection, the ICG is rapidly bound to plasma proteins, primarily lipoproteins, and to a lesser more variable respect to albumin. Negligible amounts of the dye are taken up by arterial and venous blood in renal, peripheral, lung or cerebrospinal regions. ICG is taken up almost exclusively by the hepatic parenchymal cells and secreted entirely into the bile. It does not undergo significant enterohepatic recirculation. The dye is nontoxic and has a very low incidence of adverse reactions: 4 out of 240,000 doses. In 34 years, 17 adverse reactions have occurred including 2 deaths. It is strongly suggested that patients with allergic reactions to iodine and iodine products be excluded from any iodine based imaging11 and that the patient should not undergo radioactive iodine uptake studies within 1 week after using ICG. However, most patients experience only a sore throat and a slight warming feeling from ICG.
Case Report
A 43-year old male with a 40-year history of type 1 diabetes presented at the Southern Arizona Limb Salvage Alliance's (SALSA) outpatient clinics with extremely painful interdigital ulceration of the lateral aspect of the fourth digit of the right foot, which had been present for a month. The patient had a history of hypertension, loss of protective sensation (particularly in the left lower extremity), PVD, and an extensive history of endovascular intervention including a superficial femoral artery angio-plasty and stent, and a below-knee popliteal and reverse vein bypass. The patient underwent comprehensive clinical examination and noninvasive vascular tests. His medications included Plavix, Simvastatin, and Lantus and he had no known allergies. The patient smoked for 30 years. Vascular examination revealed markedly abnormal hemodynamics in the right lower extremity (posterior tibial and dorsalis pedis waveforms were barely pulsatile, the great toe waveforms were flat and the ankle brachial index was unreliable due to medial calcification) and arterial duplex findings of a complete occlusion of the right superficial femoral angioplasty stent and the right femoral popliteal bypass.
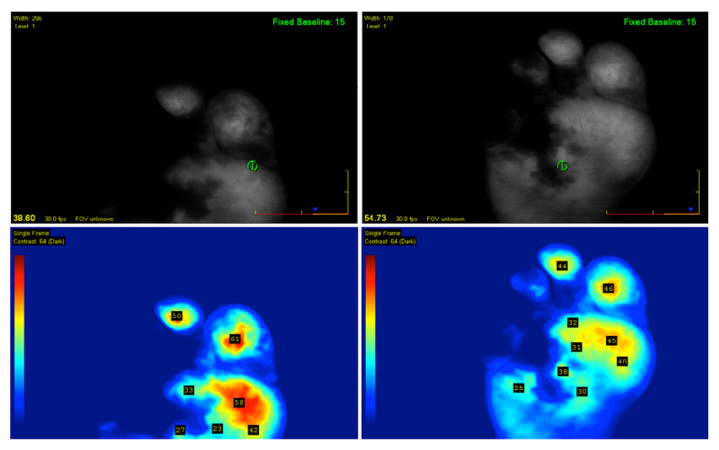
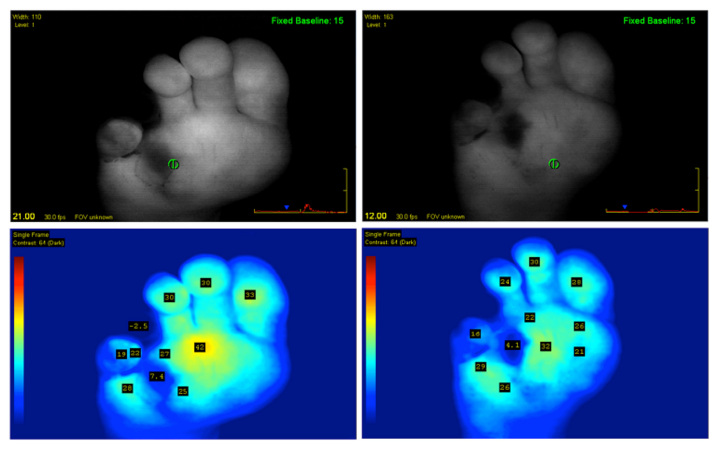
The patient presented again in 2 months, with minor trauma at the fourth digit. The fourth digit was ischemic at the time of presentation and conservative treatment failed to resolve any symptoms. Upon referral to the vascular surgeon, the patient was advised to undergo a femoral anterior tibial bypass to increase arterial flow to the lateral aspect of his right foot. During the surgery, the fourth toe on the right foot showed clinically necrotic tissue. ICGFA was used to determine the perfusion to the plantar region during occlusion as well as the efficacy of the bypass in reintroducing blood flow (Figure 1). The bypass was manually occluded during the first 40 s of the SPY imaging sequence. Only the distal lateral region of the plantar did not fluoresce including the third, fourth, and fifth digits, and the metatarsal tissue beneath, which signified inadequate supply. Clinically, the corresponding tissue appeared viable. Once the bypass was unimpeded, all digits filled in except the fourth digit and the tissue covering the fourth metatarsal on the plantar region of the foot (Figure 2). In subsequent ICGFA sequences, that area resisted illumination and ultimately appeared clinically necrotic under physical examination.
Figure 1.
First scans during surgery show reduced perfusion with manual graft compression at the medial portion covering three digits. Note the fourth digit and metatarsal head region, which showed clinical signs of necrosis.
Figure 2.
Two follow-up SPY sequences following bypass procedure. Note that the fourth digit continues to demonstrate signs of necrosis and was eventually amputated.
Following the bypass, while the patient did experience markedly improved waveform patterns and graft patency, the fourth toe did not show signs of improvement during physical examination or ICGFA and a fourth-toe amputation was performed. Tissue directly beneath the amputation site on the plantar bed was irrigated and debrided, removing the necrotic and infected tissue. The wound was left open and was scheduled for a closure procedure. Finally, ICGFA determined adequate perfusion was available to close the open wound, but the wound was closed some time later due to widespread cellulitis. The wound remained healed at 12 months.
Discussion
ICGFA was able to determine the direct effects of the arterial occlusion, confirm the extent of viable tissue post-bypass and corroborate the necrosis of the fourth digit, substantiating the necessity for amputation of that digit (Figure 2).
Fluorescence angiography using SPY technology can provide data about viability from arterial flow and capillary fill in the lower extremities to a degree that is not found by other noninvasive vascular methods. Subsequently, it can also be used as a means of determining efficacy of a particular vascular technique/procedure allowing for a greater confidence in the outcome as well as providing some foreknowledge of the need for additional intervention. It should not be used as a sole indicator of vascular health, but used in tandem with standard imaging procedures of vascular imaging: angiography, computerized tomography, X-ray fluoroscopy, and magnetic resonance angiogram. ICGFA can provide powerful knowledge to improve the patency of revasculari-zations. SPY technology is limited by perfusion information at a depth of 5 mm, therefore providing information solely on superficial capillary ingress and egress, and is incapable of assessing deep tissue perfusion. Additionally, patients allergic to iodine or sulfa and penicillin should be excluded from any procedures involving ICG. It is not known whether ICGFA provides the same or higher level of sensitivity and specificity demonstrated by other nonvascular measurements, but it may prove valuable as a minimally invasive tool to determine tissue viability in patients who lack toe pulses because of ulceration or amputation of the toes and in patients with abnormal ankle brachial indices due to medial calcification. A larger cohort of over 60 patients corresponding to over 100 ICGFA sequences will be analyzed in order to answer questions of consistency and continued efficacy.
Conclusion
Foot wounds can be healed only with an appropriate level of blood supply and limb salvage efforts rely heavily on perfusion to the lower extremities. With fluorescence angiography using SPY technology, surface tissue viability can be determined. As improvements in the SPY system continue to develop, it will continue to be an important tool in preventing amputations from vascular deficiencies. Future study may include pre- and postdebridement and post closure site tissue viability, pre- and post-vascular intervention levels of arterial flow, lower extremity flap viability and comparisons to the transcutaneous partial pressure of oxygen test as well as other noninvasive hemodynamic measurements. All of these concepts may be further understood by utilizing SPY Fluorescence Angiography System in future research.
Video Link
Please follow the video link to learn about the device capabilities and clinical summary of the cases discussed in this article. http://dl.dropbox.com/u/13393681/SpyVideo.mov.
Acknowledgments
The authors wish to thank Novadaq for their support regarding image analysis and equipment.
Glossary
Abbreviations
- (DPN)
diabetic peripheral neuropathy
- (ICG)
indocyanine green
- (ICGFA)
ICG fluorescence angiography
- (PVD)
peripheral vascular disease
References
- 1.Armstrong DG, Wrobel J, Robbins JM. Guest Editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J. 2007;4(4):286–287. doi: 10.1111/j.1742-481X.2007.00392.x. [DOI] [PubMed] [Google Scholar]
- 2.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation: basis for prevention. Diabetes Care. 1990;13:513–521. doi: 10.2337/diacare.13.5.513. [DOI] [PubMed] [Google Scholar]
- 3.Reiber G, Boyko E, Smith DG. Lower extremity ulcers and amputations in individuals with diabetes. In: Harris M, editor. Diabetes in America. Second ed. Bethesda, MD: National Institutes of Health; 1995. pp. 209–227. Vol 95-1468. [Google Scholar]
- 4.Reiber GE. Diabetic foot care. financial implications and practice guidelines. Diabetes Care. 1992;15(Suppl1):29–31. doi: 10.2337/diacare.15.1.s29. [DOI] [PubMed] [Google Scholar]
- 5.Johannesson A, Larsson GU, Ramstrand N, Turkiewicz A, Wiréhn AB, Atroshi I. Incidence of lower-limb amputation in the diabetic and nondiabetic general population: a 10-year population-based cohort study of initial unilateral and contralateral amputations and reamputations. Diabetes Care. 2009;32(2):275–280. doi: 10.2337/dc08-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prompers L, Schaper N, Apelqvist J, Edmonds M, Jude E, Mauricio D, Uccioli L, Urbancic V, Bakker K, Holstein P, Jirkovska A, Piaggesi A, Ragnarson-Tennvall G, Reike H, Spraul M, Van Acker K, Van Baal J, Van Merode F, Ferreira I, Huijberts M. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia. 2008;51(5):747–755. doi: 10.1007/s00125-008-0940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cobb J, Claremont D. Noninvasive measurement techniques for monitoring of microvascular function in the diabetic foot. Int J Low Extrem Wounds. 2002;1(3):161–169. doi: 10.1177/153473460200100303. [DOI] [PubMed] [Google Scholar]
- 8.Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, Edmonds M, Holstein P, Jirkovska A, Mauricio D, Ragnarson Tennvall G, Reike H, Spraul M, Uccioli L, Urbancic V, Van Acker K, Van Baal J, Van Merode F, Schaper N. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007;50(1):18–25. doi: 10.1007/s00125-006-0491-1. [DOI] [PubMed] [Google Scholar]
- 9.Nouvong A, Hoogwerf B, Mohler E, Davis B, Tajaddini A, Medenilla E. Evaluation of diabetic foot ulcer healing with hyperspectral imaging of oxyhemoglobin and deoxyhemoglobin. Diabetes Care. 2009;32(11):2056–2061. doi: 10.2337/dc08-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen M, Kerechanin C, Greenspan D, Criss T, Franckowiak SC, Vincent JA, Pattay RS. Development of a thermal and hyperspectral imaging system for wound characterization and metabolic correlation. Johns Hopkins Apl Technical Digest. 2005;26(1):67–74. [Google Scholar]
- 11.Marshall M, Rasmussen J, Tan I, Aldrich MB, Adams KE, Wang X, Fife CE, Maus EA, Smith LA, Sevick-Muraca EM. Near-infrared fluorescence imaging in humans with indocyanine green: a review and update. Open Surgical Oncology Journal. 2010;2:12–25. doi: 10.2174/1876504101002010012. [DOI] [PMC free article] [PubMed] [Google Scholar]