Abstract
Clinical trials using mesenchymal stem cells (MSCs) have been initiated worldwide. An improved understanding of the mechanisms by which allogeneic MSCs evade host immune responses is paramount to regulating their survival after administration. This study has focused on the novel role of serine protease inhibitor (SPI) in the escape of MSCs from host immunosurveillance through the inhibition of granzyme B (GrB). Our data indicate bone marrow–derived murine MSCs express SPI6 constitutively. MSCs from mice deficient for SPI6 (SPI6−/−) exhibited a 4-fold higher death rate by primed allogeneic cytotoxic T cells than did wild-type MSCs. A GrB inhibitor rescued SPI6−/− MSCs from cytotoxic T-cell killing. Transduction of wild-type MSCs with MigR1-SPI6 also protected MSCs from cytotoxic T cell–mediated death in vitro. In addition, SPI6−/− MSCs displayed a shorter lifespan than wild-type MSCs when injected into an allogeneic host. We conclude that SPI6 protects MSCs from GrB-mediated killing and plays a pivotal role in their survival in vivo. Our data could serve as a basis for future SPI-based strategies to regulate the survival and function of MSCs after administration and to enhance the efficacy of MSC-based therapy for diseases.
Introduction
Mesenchymal stem cell (MSC) developmental plasticity has generated remarkable interest in their potential use in cell-based tissue engineering and regeneration.1 MSCs were also observed to have profound immunomodulatory effects.2 As a result, researchers are evaluating their clinical use in an expanding array of common immune-mediated diseases, including type 1 diabetes mellitus, graft-versus-host disease, collagen-induced arthritis, and multiple sclerosis.3–5 To date, more than 120 clinical trials have been initiated using MSCs, at a pace faster than any other cell therapy (www.clinicaltrials.org).
Given the potential for expansion of MSCs from a single donor followed by subsequent use in patients, there has been growing interest in the use of allogeneic MSCs.2 An understanding of MSC survival, as well as of the mechanisms by which these cells evade cytotoxic immune responses, is paramount for tailoring such MSC-based strategies. MSCs lack expression of major histocompatibility complex (MHC) class II and classic positive costimulatory molecules. As a result, MSCs historically were regarded as hypoimmunogenic cells.6 Indeed, MSCs cultured with T cells do not cause T-cell proliferation, fail to induce interferon-γ and tumor necrosis factor-α production in human CD8+ cytotoxic T-cell (CTL) clones, and trigger weaker up-regulation of CD25 on CTLs.7,8 MSCs were also shown to be capable of down-regulating CTL-mediated lysis.9 Indeed, after exposure of MSCs to primed CTLs in a mixed lymphocyte reaction, donor T cells but not donor MSCs were lysed by the CTLs in cocultures.9 Rasmusson et al7 have shown that MSCs are also resistant to lysis by fully differentiated effector CTLs.
However, recent studies suggest that MSCs are not as immunoprivileged as once thought.10 MSCs were found to up-regulate the expression of MHC class II and costimulatory molecules in an inflammatory milieu, and they are indeed recognized by the host immune system, which results in their rejection, albeit with delay.11 The partial immunogenicity of MSCs suggests that these cells possess tools by which they, in part, evade host immune responses.
The therapeutic success of using MSCs across MHC barriers is not only dependent on their failure to induce activation of CD4 and CD8 T-cell responses but also on their escape from the granzyme B (GrB) lytic activity of CTLs.12,13 GrB is a major constituent of CTL/natural killer cell granules, binds mannose-6-phosphate receptor, and induces killing of target cells via activation of caspases and the promotion of mitochondrial permeabilization.14–17 GrB activity is, in turn, tightly regulated through its interaction with peptidase inhibitors that belong to the serine protease inhibitor (serpin) superfamily.18,19 Endogenous serpins have been characterized in both mice and humans that specifically inactivate GrB in an irreversible manner and, when overexpressed, allow cells to evade GrB-mediated cytotoxicity.18,20
Serpins that inhibit GrB are expressed in the cytoplasm and nuclei of CTLs and in immunoprivileged sites, such as the placenta, testis, ovaries, and brain.21 Serine protease inhibitor 6 (SPI6) is required to protect CTLs from GrB-mediated death and facilitates the survival of virally infected cells and tumors.22,23 No data are yet available on the presence and role of serpins in mouse MSCs. The work we describe herein substantially enhances our knowledge of MSC immune evasion and sets forth a potential SPI-based strategy to regulate the survival of MSCs and to prove the efficacy of MSC-based therapy.
Methods
Mice
Wild-type (WT), SPI6−/−, and BALB/c mice (6-8 weeks old) were obtained from The Jackson Laboratory. NOD-SCID–IL-2rγ−/− (nonobese diabetic/severe combined immunodeficiency, interleukin-2–deficient) mice were generously provided by Lenny Shultz (The Jackson Laboratory). Mice were kept in a standard animal housing setting at the Harvard Medical School. Protocols were approved by the Institutional Animal Care and Use Committee.
MSC characterization and differentiation
MSCs were harvested from femurs and tibia bone marrow of WT and SPI6−/− mice. Cells were cultured at a concentration of 15 × 106/25 cm2 flasks in Dulbecco modified Eagle medium (Lonza) that contained 10% fetal bovine serum (Gemini Bio-Products), 1% penicillin-streptomycin, and 1% glutamine (Lonza) supplemented with 6 ng/mL basic fibroblastic growth factor (PeproTech). Cells were incubated at 37°C and 5% CO2 until confluence reached > 80%, after which cells were trypsinized (Gibco 0.25% trypsin-EDTA [ethylenediaminetetraacetic acid] 1×) to a new passage. All experiments were performed at passages 5 or 6. Anti-mouse Sca1, CD44, CD73, CD45, CD29, CD90.2, CD105, CD80, and CD86 fluorescence-activated cell sorting (FACS) antibodies were purchased from eBioscience. Intracellular anti-SPI6 was purchased from Hycult Biotech. For the immunostaining of SPI6, MSCs were trypsinized, and cells (50 000/well) were incubated for 20 minutes in Cytofix/Cytoperm solution (BD Biosciences) at 4°C for permeabilization. Cells were washed, and secondary antibody was added for 20 minutes. MSCs were differentiated into chondrocytes, osteocytes, and adipocytes as reported previously.3 Chondrogenic differentiation was induced by 50 μg/mL ascorbic acid and 1 ng/mL transforming growth factor-β1 (PeproTech); osteogenic differentiation was induced by 50 μg/mL ascorbic acid, 10mM sodium α-glycerophosphate, and 10−8M dexamethasone; and adipogenic differentiation was induced by addition of 10−7M dexamethasone and 6 ng/mL insulin.
T-cell proliferation assays
WT and SPI6−/− MSCs were incubated at increasing concentrations in an anti-CD3/CD28 proliferation assay. BALB/c lymphocytes were stimulated to proliferate with pure-grade anti-CD3/CD28 antibodies (2 μg per well; eBioscience) for 72 hours in RPMI containing 10% fetal bovine serum (Gemini Bio-Products), 1% penicillin-streptomycin, and 1% glutamine (Lonza) in the presence of 6 different concentrations of irradiated MSCs (3000 rad). Proliferation was assessed by tritiated thymidine (3H-TdR), as described previously.4
Cell killing assay
Isolated CTLs from BALB/c spleen were cocultured for 4 hours with murine MSCs stained with 15μM calcein AM (Molecular Probes, Invitrogen) according to the manufacturer's recommendation. MSCs were subjected to killing in a V-shaped 96-well plate and signified as “experimental wells.” As negative and positive controls, “spontaneous release” cells (only target cells in complete medium) and “maximum release” cells (target cells in complete medium supplemented with 2% Triton X-100) were added for each MSC group in triplicates. After incubation at 37°C in 5% CO2 for 4 hours, the supernatant was harvested and transferred into new 96-well flat-bottom plates (Costar 3596; Corning Inc). Samples were then measured with a dual-scanning microplate spectrofluorometer (Versamax microplate reader; excitation filter 485 ± 9 nm, band-pass filter 530 ± 9 nm). Percentage of lysis was calculated with the same formula used for the 51Cr-release assay and presented as follows: % cell lysis = (experimental wells − spontaneous release)/(maximum release − spontaneous release).24
GranToxiLux assay
A GranToxiLux kit (OncoImmune) was used to determine early MSC GrB activity for cell-mediated cytotoxicity when challenged with CTLs in our killing assay according to the manufacturer's recommendation. MSCs were fluorescently labeled (red) before coincubation with CTLs in the presence of a fluorogenic GrB substrate. Cleavage of the substrate results in increased green fluorescence in MSCs with GrB activity. MSCs with GrB activity revealed a green fluorescence assessed by FACS compared with MSCs alone and analyzed on FlowJo Version 6.3.3 (TreeStar).
Caspase-3 colorimetric activity
Caspase-3, a GrB intracellular second messenger in MSCs, was assessed with the caspase-3 colorimetric assay (BF3100; R&D Systems) per the manufacturer's recommendations. Briefly, lysed MSCs were tested for protease activity by the addition of a caspase-specific peptide conjugated to the color reporter molecule p-nitroanaline. Cleavage of the peptide by caspase releases the chromophore p-nitroanaline, quantified spectrophotometrically at a wavelength of 405 nm. The level of caspase enzymatic activity in the cell lysate is directly proportional to the color reaction.
Transduction of MSCs with hGH
A human growth hormone (hGH) lentivirus-based plasmid construct (pHRST-hGH) was designed. A vesicular stomatitis virus glycoprotein pseudotyped lentiviral vector was generated by transient transfection of 5 plasmids (pHDM-Hgpm2, pMD-Tat, pRC/CMV-rev, pHDM-G, and pHRST-hGH) into human embryonic kidney 293T cells with TransIT-293 transfection reagent (Mirus Bio LLC). The viral vector was assessed by Southern blot analysis on genomic DNA isolated from infected U2OS cells. After transfection, we transduced the lentivirus hosting the pHRST-hGH into the MSCs used in the present study at a multiplicity of infection of 5 in Dulbecco modified Eagle medium for 24 hours with 8 μg/mL Polybrene (hexadimethrine bromide; Sigma-Aldrich). Transduced MSCs were washed twice after 24 hours, and sample medium was assessed for hGH secretion at 72 hours. Sample medium was assessed for hGH by enzyme-linked immunosorbent assay (Roche Diagnostics).
Overexpression of SPI6 in MSCs
DNA was diluted in TE buffer to a final concentration of 1 μg (MigR1; MigR1-proteinase inhibitor-9). Lipofectamine transduction of cells was performed according to the manufacturer's protocol (Invitrogen). Transduction of samples at a final concentration of 1 μg was made in 50 μL of Dulbecco modified Eagle medium (without serum) and incubated at room temperature for 5 minutes. Lipofectamine reagent was diluted to 2 μL per 50 μL of media. Diluted DNA was then added to the lipofectamine reagent, mixed gently, and incubated for 20 minutes at room temperature to allow for the DNA-lipofectamine complexes to develop. After incubation, DNA-lipofectamine mixture was added to the 60%-70% confluent Phoenix amphotrophic cells (ATCC Corp) cultured in T150 flasks. Transduced Phoenix cells were left to incubate at 37°C. After 24 hours, the medium was changed, and cells were incubated at 35°C to induce production of viral packaging proteins that contained the plasmid. Conditioned medium was collected every 8 hours from the Phoenix cell cultures and transferred to human MSCs seeded in T75 flasks (at 70%-80% confluence) and replaced with fresh media. Conditioned medium was removed every 8 hours over a period of 3 days.
Results
Mouse MSC characterization and differentiation
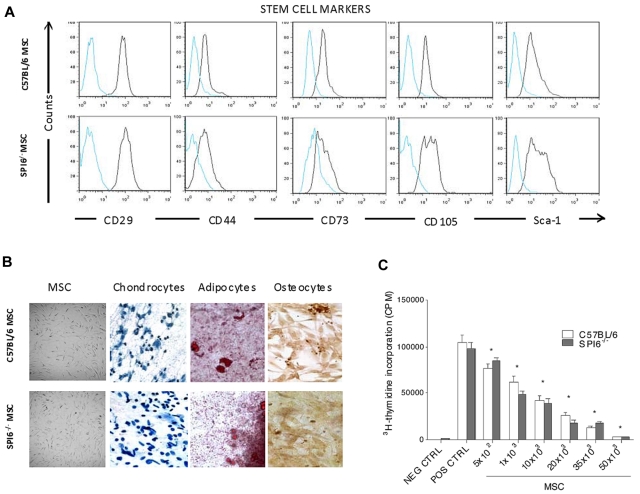
Mouse MSCs were harvested from the bone marrow of 6- to 8-week-old C57BL/6J (WT) and C57BL/6J-serpin9tm1Arp/J (SPI6−/−) mice. Using FACS, we assessed the expression of the acknowledged MSC surface markers. MSCs revealed positive expression for the surface stem cell markers CD29, CD44, CD73, CD105, and Sca-1 (Figure 1A), whereas no expression for the hematopoietic lineage markers CD45 and CD90.2 was observed, as described previously.25,26 FACS results also showed negative expression for the costimulatory molecules CD80 and CD86 as reported previously (data not shown).27,28 Both MSC types shared similar characteristic morphology, with large, clear nuclei (Figure 1B). Cultured MSCs at passages 5 and 6 were stimulated to differentiate, respectively, either into chondrocytes, osteocytes, or adipocytes (Figure 1B), as described previously.4 To also characterize WT and SPI6−/− MSCs with regard to their immunosuppressive capabilities, lymphocytes from BALB/c mice were stimulated with anti-CD3/CD28 in the presence of increasing MSC concentrations. WT and SPI6−/− MSCs showed significant inhibition of T-cell proliferation compared with the positive control (P < .01; Figure 1C). There was no significant difference in inhibition between SPI6−/− and WT MSCs.
Figure 1.
Characterization of WT and SPI6−/− MSCs. MSCs were harvested from femurs and tibia bone marrow of WT and SPI6−/− mice. Stem cell markers were assessed at passage 5 by FACS analysis (n = 4). (A) CD29, CD44, CD73, CD105, and Sca-1 were positive in WT and SPI6−/− MSCs. (B) MSCs from WT and SPI6−/− mice showed similar fibroblastic morphology. Both WT and SPI6−/− MSCs differentiated into chondrocytes, osteocytes, and adipocytes when stimulated with differentiation medium. Nikon E-1000 epifluorescence microscope with 200× magnification was used to capture images. (C) Lymphocyte proliferation was assessed in the presence of an increasing concentration of MSCs from WT (open bars) and SPI6−/− (shaded bars) mice. MSCs from WT and SPI6−/− mice significantly and comparably inhibited lymphocyte proliferation (P < .01). This is the average of 3 experiments, with every parameter performed in 6 replicates. NEG CTRL indicates negative control (lymphocytes in medium); POS CTRL, positive control (lymphocytes cocultured with anti-CD3/CD28); and CPM, counts per minute.
Constitutive expression of SPI6 in MSCs and its role in protecting MSCs from allogeneic CTLs
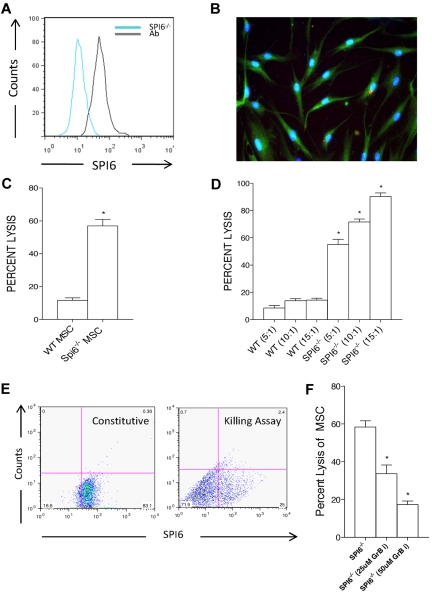
We examined the intracellular expression of SPI6 in WT MSCs by FACS analysis and immunostaining. SPI6 expression in WT MSCs was compared with that of SPI6−/− MSCs. As shown in Figure 2A, WT MSCs revealed constitutive intracellular expression of SPI6 in culture. Immunostaining with SPI6/fluorescein isothiocyanate revealed free SPI6 within the cytoplasm, accompanied by clusters of granules (Figure 2B). To examine the role of SPI6 in protecting MSCs against CTLs, we subjected SPI6−/− and WT MSCs to primed CTLs and assessed their percentage of lysis using the fluorescent dye calcein as reported previously.29 Primed CTLs were obtained from the spleen of sensitized allogeneic BALB/c mice. Sensitization was achieved by skin transplantation, during which BALB/c mice received a skin allograft from WT donor mice. Lymphocytes recovered from the spleen of recipient mice showed a distinct activated CTL population of CD8+ T cells. SPI6−/− and WT MSCs stained for calcein were cocultured with activated CTLs at an effector/target cell (E/T) ratio of 5:1 for 4 hours. Compared with WT MSCs, SPI6−/− MSCs experienced a 4 times higher rate of cell death expressed as percentage of lysis (11.66% ± 1.56% vs 57.00% ± 3.91%, respectively; P < .01; Figure 2C). Both WT and SPI6−/− MSCs were subjected to an increasing E/T ratio by the addition of 5, 10, and 15 times higher numbers of activated CTLs to the number of MSCs. There was a significant and proportional increase in the percentage of lysis of SPI6−/− MSCs to the number of CTLs, as opposed to WT MSCs (P < .01; Figure 2D). We also examined the SPI6 expression of WT MSCs after exposure to primed CTLs at an E/T ratio of 5:1 for 4 hours in our killing assay. MSCs at time zero and at 4 hours in the killing assay system showed a significant decrease in SPI6 expression compared with its constitutive standing (83% ± 6% vs 25% ± 3%, respectively, P < .01; Figure 2E). These data show the dynamic changes in the expression of SPI6 on exposure to CTLs.
Figure 2.
Constitutive expression of SPI6 on MSCs and its role in protecting MSCs from allogeneic CTLs. (A) MSCs revealed constitutive expression of SPI6 as assessed by FACS. SPI6−/− MSC staining was used as a negative control (blue line) versus the positive stain (black line) in WT MSCs. Ab indicates antibody. (B) Immunostaining was performed on WT MSCs, with intracellular SPI6 shown in green versus blue for DAPI (4′,6-diamidino-2-phenylindole). Immunostained images were taken with a Nikon E-1000 epifluorescence microscope at 200× magnification. (C) MSCs from WT and SPI6−/− mice were incubated with primed allogeneic CTLs in a killing assay (n = 5 experiments). SPI6−/− MSCs had 4 times higher cell death rates (expressed as percentage of cell lysis) than WT MSCs (P < .01). (D) MSCs were incubated with increasing E/T ratios, and SPI6−/− MSCs showed a dose-dependent significant increase in cell death with increasing E/T ratios (P < .01). (E) MSCs were stained for SPI6 before (constitutive) and after (killing assay) incubation with CTLs, and SPI6 expression was analyzed by a dot plot on FACS. Data showed a significant decrease in the expression of SPI6 after exposure to CTLs (n = 3 experiments; P < .01). (F) GrB-i resulted in a significant reduction in the percentage of lysis of SPI6−/− MSCs at the 2 concentrations used (n = 3 experiments; P < .02 and P < .01, respectively).
To further explore the functional role of GrB in MSC-mediated killing, we used compound 19, a potent and specific GrB inhibitor (GrB-i), in our killing assay system to examine whether GrB-i can rescue SPI6−/− MSCs from killing by CTLs.30 We added 2 concentrations (25 and 50μM) of the GrB-i to primed CTLs for 45 minutes before the killing assay. SPI6−/− MSC percentage of lysis decreased significantly from 58.33% ± 3.33% to 33.67% ± 4.66% with 25μM of GrB-i, while decreasing further to 17.33% ± 1.85% with the use of 50μM (P < .02 and P < .01, respectively; Figure 2F).
Detection of early GrB activity in MSCs after exposure to CTLs
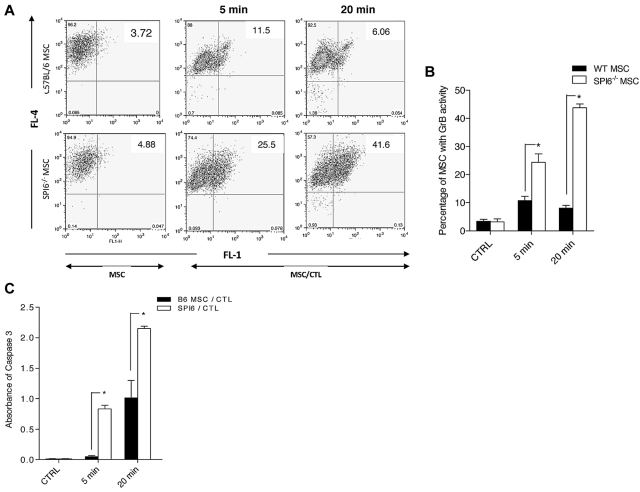
GrB enzymatic activity in MSCs challenged with CTLs was detected in a GranToxiLux assay, as reported previously.30 MSCs were labeled fluorescently (red) and then coincubated with CTLs in the presence of a fluorogenic GrB substrate. In this assay, cleavage of the fluorogenic substrate by GrB results in an increase of green fluorescence in the target cells that experience GrB activity in them. MSCs were analyzed for GrB activity by flow cytometry after 5 and 20 minutes of incubation time with CTLs. GrB activity was identified by an increase in the percentage of MSCs with green fluorescence (top right quadrant) compared with MSCs alone (Figure 3A). At 5 and 20 minutes, a much higher percentage of SPI6−/− MSCs showed GrB activity than did WT MSCs (6.06% ± 2.56% vs 11.5% ± 3.54% and 41.6% ± 4.32% vs 25.5% ± 2.89%, respectively; P < .02; Figure 3B). These data indicate that SPI6 suppresses GrB activity in MSCs after incubation with CTLs. Because GrB induced activation of caspase-3 in the target cells, we also measured the activity of caspase-3 in MSCs using the caspase-3 colorimetric assay. Compared with WT MSCs, significantly higher caspase-3 activity was evident in SPI6−/− MSCs at 5 and 20 minutes after incubation (P < .02; Figure 3C). These data indicate that SPI6 protects MSCs from CTL-mediated apoptosis by suppressing GrB activity.
Figure 3.
Early GrB and caspase-3 activity showed early killing of SPI6−/− MSCs. (A) Early GrB activity in WT and SPI6−/− MSCs subjected to CTLs was detected by a GranToxiLux assay and characterized by a population shift from the top left quadrant to the top right quadrant. MSCs were fluorescently labeled (red; FL-4) before coincubation with CTLs in the presence of a fluorogenic GrB substrate. Cleavage of the substrate results in increased green fluorescence (FL-1) in MSCs with GrB activity. (B) The percentage of MSCs with GrB activity (top right quadrant) is shown in the bar graph. SPI6−/− MSCs showed significantly higher GrB activity at 5 and 20 minutes (6.06% ± 2.56% vs 11.5% ± 3.54% and 41.6% ± 4.32% vs 25.5% ± 2.89%, respectively; P < .02; n = 3 experiments). (C) Caspase-3 was assessed in WT and SPI6−/− MSCs subjected to CTLs at the same time points as for GrB. Results showed significantly higher caspase-3 activity in SPI6−/− MSCs than in WT MSCs (B6 MSC/CTL) at 5 and 20 minutes when challenged with CTLs (1.01 ± 0.36 vs 2.14 ± 0.042 and 0.051 ± 0.009 vs 0.831 ± 0.067, respectively; n = 3 experiments; P < .02).
In vivo longevity of SPI6−/− MSCs is diminished
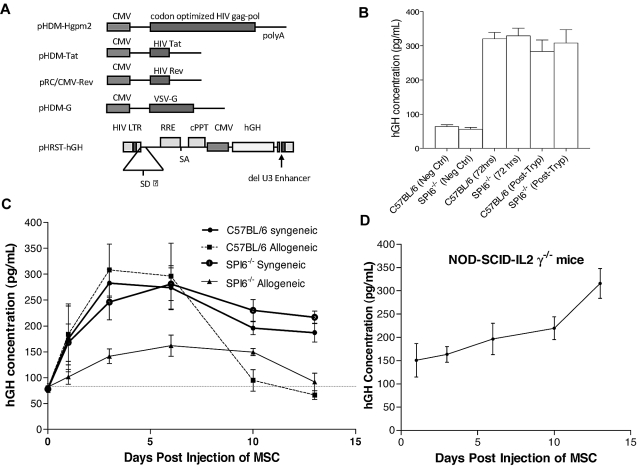
To assess the role of SPI6 in the survival of MSCs in vivo, we generated a lentivirus-based plasmid construct (pHRST-hGH) to express hGH. HRST-hGH resulted from transfection of 5 plasmids into human 293T cells. Schematic representation of the constructs is shown in Figure 4A. At a multiplicity of infection of 5, WT and SPI6−/− MSCs were transduced with the lentivirus, and MSCs stably expressed hGH at various time points after trypsinization in the culture medium by enzyme-linked immunosorbent assay (Figure 4B).
Figure 4.
Longevity assessment of MSCs from WT and SPI6−/− mice. (A) Schematic showing the vesicular stomatitis virus glycoprotein (VSV-G) pseudotyped lentiviral vector (HRST-hGH) generated by transient transfection of 5 plasmids (pHDM-Hgpm2, pMD-Tat, pRC/CMV-rev, pHDM-G, and pHRST-hGH) into human 293T cells. CMV indicates cytomegalovirus. (B) Before injection, lentiviral transduction was assessed in vitro, at several time points, for the detection of hGH in MSC culture medium. Neg Ctrl indicates negative control; and Post-Tryp, after trypsinization. (C) Transduced MSCs were injected into allogeneic or syngeneic recipients, and serum was collected at 6 time points to assess hGH secretion (n = 5 mice with serum hGH assessed in duplicates). (C) Syngeneic MSC injections showed a stable hGH concentration in the serum at all time points assessed. Allogeneic MSCs showed a decrease in hGH at day 14, which indicates their rejection by the host immune response. The hGH secretion was significantly lower for SPI6−/− MSCs than for WT MSCs (P < .02). (D) Transduced WT MSCs were injected into NOD-SCID-IL-2rγ−/− mice, and serum concentration of hGH was stable for the same time points assessed.
We injected the 2 transduced MSC types into 2 groups of syngeneic and allogeneic mice and assessed serum hGH as an indicator for MSC survival after injection. The syngeneic groups consisted of WT MSCs injected into C57BL/6 recipients and SPI6−/− MSCs injected into SPI6−/− recipients, whereas the allogeneic groups consisted of WT and SPI6−/− MSCs each injected into BALB/c mice. MSCs (1 × 106 cells/animal) injected in an allogeneic setting revealed a significantly lower serum hGH concentration in SPI6−/− than in WT MSCs at all time points (P < .02; Figure 4C). A surge in hGH level was detected at day 3 in all groups except the SPI6−/− allogeneic group, which revealed a significantly lower serum hGH. At day 14, the serum hGH secreted from allogeneic cells reached nadir levels, which indicated their rejection by the host immune response (Figure 4C). To take into account the possibility of hGH in the MSCs triggering an immune response from the recipient and thus influencing MSC survival, we injected both transduced MSCs in a syngeneic setting. WT and SPI6−/− MSCs injected in a syngeneic setting revealed serum hGH at a concentration of approximately 200 pg/mL (Figure 4C). To rule out any potential effect of transduction on the viability of MSCs in vivo, we injected 1 × 106 WT MSCs into humanized NOD-SCID IL-2rγnull mice, which represent completely immunodeficient mice that lack natural killer cell activity and thus are devoid of alloimmunity or innate immunity.31,32 WT MSCs were injected and serum hGH was assessed at the same time points as for the allogeneic and syngeneic injections. The results revealed a stable hGH serum concentration at all time points analyzed (Figure 4D).
Overexpression of SPI6 protects mouse MSCs from GrB-mediated killing
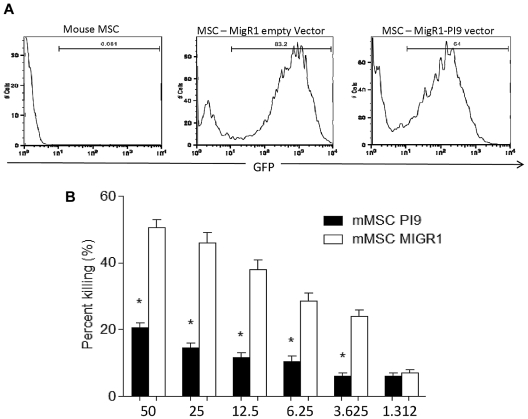
To investigate the specific significance of SPI6 in protecting MSCs from primed CTLs, we transduced WT MSCs with a retrovirus that contained MigR1-PI9 to overexpress SPI6. Transduction results revealed 64% efficiency in SPI6 expression for the MigR1-PI9, as measured by FACS (Figure 5A). Transduced MSCs were then incubated with primed allotype CTLs at increasing E/T ratios. Results revealed that MigR1-PI9–transduced MSCs were better protected than WT MSCs, because a significant reduction in percentage of lysis was observed vs the control MSCs at an E/T ratio of 50 (50.5% ± 2.5% for control and 20.0% ± 1.5% for MigR1-PI9–transduced MSCs; P < .01; Figure 5B). These data highlight the immunoprotective characteristics of SPI6 in MSCs when challenged with CTLs.
Figure 5.
Overexpression of SPI6 protects mouse MSCs from CTL killing. (A) FACS showing the transduction of MigR1-PI9 into WT MSCs for overexpression of SPI6 compared with MigR1 (empty vector) as control. Transduced MSCs were incubated with increasing E/T ratios of primed CTLs (n = 3 experiments). (B) MSCs with MigR1-PI9 displayed significant protection against cytotoxic killing at the E/T ratios examined (P < .01).
Discussion
The extensive proliferative properties of MSCs, their abundant availability, and their immunomodulatory effect have made them an exciting therapeutic modality for a growing list of immune-mediated diseases and regenerative medicine.33–35 Although a large number of these studies rely on the therapeutic potential of allogeneic MSCs, there is a need to explore the life span of MSCs after injection and the mechanisms by which they survive rejection.
Historically, MSCs were considered to be hypoimmunogenic.36 MSCs exhibit low levels of expression of MHC-I, no expression of MHC class II markers, and no expression of costimulatory molecules, which allows them to avoid immunosurveillance.11,36 Nevertheless, MSCs up-regulate the expression of these molecules in an inflammatory milieu.11 In addition to their hypoimmunogenicity, it is important to understand the mechanisms by which MSCs counteract the cytotoxicity of CTLs and evade host immune responses. CTLs are known to play an important role in the rejection of allogeneic grafts and cells.37 CTLs lyse allogeneic cells by the release of cytotoxic effectors, such as GrB. GrB is instrumental in the rapid induction of target cell death by apoptosis. Although it has been reported that MSCs inhibit the formation of CTLs and down-regulate their activity, the mechanisms by which MSCs exert such actions remain to be explored.38 These data are pivotal to regulate the survival and function of allogeneic MSCs after in vivo administration for therapeutic purposes.
The present data indicated the constitutive expression of SPI6 and revealed a dynamic change of SPI6 expression in WT MSCs when challenged with CTLs. To generate efficient CTL machinery, we sensitized BALB/c mice by transplanting a skin allograft from WT donors onto them. This model mimics a scenario in which allogeneic WT MSCs become targets of CTLs after administration. MSC killing is dependent on the experimental model in which the effector population has been raised.39,40 With CTLs raised in this model, SPI6−/− MSCs showed 4-fold higher death rates than WT MSCs. The specificity of GrB-mediated killing was further confirmed when we impeded the lytic capacity of the CTLs with a GrB-i. The significant decrease in percentage of lysis of SPI6−/− MSCs with GrB-i underscores the central role of the GrB-mediated process in the killing of MSCs and emphasizes the fundamental importance of SPI6 in the survival of MSCs. To examine the level of early GrB activity, we also performed a GranToxiLux assay, which showed much higher GrB activity in the SPI6−/− MSCs than in the WT MCSs as early as 5 minutes after exposure to CTLs. The higher GrB activity in SPI6−/− MSCs was also associated with higher caspase-3 activity than in WT MSCs. These data show that in the absence of the protective effect of SPI6, SPI6−/− MSCs are rendered susceptible to GrB-induced apoptosis.
To further examine the role of SPI6 in allogeneic MSC survival in vivo, we injected hGH-transduced WT and SPI6−/− MSCs into BALB/c mice. The steady hGH serum concentration from syngeneic MSCs and NOD-SCID-IL-2rγ−/− mice ensured that antibody formation against hGH and actual transduction did not affect their survival. Compared with the syngeneic MSCs, allogeneic MSCs were rejected approximately 2 weeks after administration. Our strategy with the hGH could be used as a very sensitive methodology to assess the survival of MSCs. The present study results are in agreement with more recent reports that indicate that MSCs do not completely evade the host immune response.10 To demonstrate strategies based on the protective role of SPI6 in MSCs against GrB-mediated CTL killing, we transduced MSCs with MigR1-PI9 vector as a means to overexpress SPI6. The killing of the transduced MSCs was inversely proportional to the increasing E/T ratio, which implies an undeviating role for SPI6 in the protection of MSCs subjected to CTLs.
The present study shows, for the first time, the presence of SPI6 in MSCs and examines its role in protecting MSCs from the GrB machinery of CTLs. Our data highlighting the life span of allogeneic MSCs and the role of SPI6 in their survival after administration could be crucial in designing preclinical or clinical trials with MSC-based therapy.
Acknowledgments
This work was supported by Juvenile Diabetes Research Foundation grant 4-2007-1065 and research and development grant 1-2007-713, and by NAID R01 AI45108 (P.G.A.-R.). R.A. is the recipient of a regular grant from the Juvenile Diabetes Research Foundation.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: N.E.H., M.H.S., P.G.A.-R., and R.A. proposed and were principally responsible for the design of the research, and all coauthors contributed to the writing of the manuscript and analysis of the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Reza Abdi, MD, 221 Longwood Ave, LMRC Bldg, Rm 310, Boston, MA 02115; e-mail: rabdi@rics.bwh.harvard.edu.
References
- 1.Granero-Molto F, Weis JA, Longobardi L, Spagnoli A. Role of mesenchymal stem cells in regenerative medicine: application to bone and cartilage repair. Expert Opin Biol Ther. 2008;8(3):255–268. doi: 10.1517/14712598.8.3.255. [DOI] [PubMed] [Google Scholar]
- 2.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 3.Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56(4):1175–1186. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- 4.Fiorina P, Jurewicz M, Augello A, et al. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol. 2009;183(2):993–1004. doi: 10.4049/jimmunol.0900803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyndall A, Houssiau FA. Mesenchymal stem cells in the treatment of autoimmune diseases. Ann Rheum Dis. 2010;69(8):1413–1414. doi: 10.1136/ard.2010.132639. [DOI] [PubMed] [Google Scholar]
- 6.Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmusson I, Uhlin M, Le Blanc K, Levitsky V. Mesenchymal stem cells fail to trigger effector functions of cytotoxic T lymphocytes. J Leukoc Biol. 2007;82(4):887–893. doi: 10.1189/jlb.0307140. [DOI] [PubMed] [Google Scholar]
- 8.Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57(7):1759–1767. doi: 10.2337/db08-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76(8):1208–1213. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 10.Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108(6):2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Blanc K, Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy. 2005;7(1):36–45. doi: 10.1080/14653240510018118. [DOI] [PubMed] [Google Scholar]
- 12.Rasmusson I. Immune modulation by mesenchymal stem cells. Exp Cell Res. 2006;312(12):2169–2179. doi: 10.1016/j.yexcr.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shresta S, Heusel JW, Macivor DM, Wesselschmidt RL, Russell JH, Ley TJ. Granzyme B plays a critical role in cytotoxic lymphocyte-induced apoptosis. Immunol Rev. 1995;146:211–221. doi: 10.1111/j.1600-065x.1995.tb00690.x. [DOI] [PubMed] [Google Scholar]
- 15.Cullen SP, Martin SJ. Mechanisms of granule-dependent killing. Cell Death Differ. 2008;15(2):251–262. doi: 10.1038/sj.cdd.4402244. [DOI] [PubMed] [Google Scholar]
- 16.Adrain C, Murphy BM, Martin SJ. Molecular ordering of the caspase activation cascade initiated by the cytotoxic T lymphocyte/natural killer (CTL/NK) protease granzyme B. J Biol Chem. 2005;280(6):4663–4673. doi: 10.1074/jbc.M410915200. [DOI] [PubMed] [Google Scholar]
- 17.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94(4):481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 18.Hirst CE, Buzza MS, Bird CH, et al. The intracellular granzyme B inhibitor, proteinase inhibitor 9, is up-regulated during accessory cell maturation and effector cell degranulation, and its overexpression enhances CTL potency. J Immunol. 2003;170(2):805–815. doi: 10.4049/jimmunol.170.2.805. [DOI] [PubMed] [Google Scholar]
- 19.Stout-Delgado HW, Getachew Y, Rogers TE, Miller BC, Thiele DL. The role of serpinb9/serine protease inhibitor 6 in preventing granzyme B-dependent hepatotoxicity. Hepatology. 2007;46(5):1530–1540. doi: 10.1002/hep.21820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun J, Bird CH, Sutton V, et al. A cytosolic granzyme B inhibitor related to the viral apoptotic regulator cytokine response modifier A is present in cytotoxic lymphocytes. J Biol Chem. 1996;271(44):27802–27809. doi: 10.1074/jbc.271.44.27802. [DOI] [PubMed] [Google Scholar]
- 21.Bladergroen BA, Strik MC, Bovenschen N, et al. The granzyme B inhibitor, protease inhibitor 9, is mainly expressed by dendritic cells and at immune-privileged sites. J Immunol. 2001;166(5):3218–3225. doi: 10.4049/jimmunol.166.5.3218. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Park SM, Wang Y, et al. Serine protease inhibitor 6 protects cytotoxic T cells from self-inflicted injury by ensuring the integrity of cytotoxic granules. Immunity. 2006;24(4):451–461. doi: 10.1016/j.immuni.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Medema JP, de Jong J, Peltenburg LT, et al. Blockade of the granzyme B/perforin pathway through overexpression of the serine protease inhibitor PI-9/SPI-6 constitutes a mechanism for immune escape by tumors. Proc Natl Acad Sci U S A. 2001;98(20):11515–11520. doi: 10.1073/pnas.201398198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang XM, Terasaki PI, Rankin GW, Jr, Chia D, Zhong HP, Hardy S. A new microcellular cytotoxicity test based on calcein AM release. Hum Immunol. 1993;37(4):264–270. doi: 10.1016/0198-8859(93)90510-8. [DOI] [PubMed] [Google Scholar]
- 25.Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann N Y Acad Sci. 1176:101–117. doi: 10.1111/j.1749-6632.2009.04607.x. [DOI] [PubMed] [Google Scholar]
- 26.Charbord P. Bone marrow mesenchymal stem cells: historical overview and concepts. Hum Gene Ther. 2010;21(9):1045–1056. doi: 10.1089/hum.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun S, Guo Z, Xiao X, et al. Isolation of mouse marrow mesenchymal progenitors by a novel and reliable method. Stem Cells. 2003;21(5):527–535. doi: 10.1634/stemcells.21-5-527. [DOI] [PubMed] [Google Scholar]
- 28.Ohishi M, Schipani E. Bone marrow mesenchymal cells. J Cell Biochem. 2010;109(2):277–282. doi: 10.1002/jcb.22399. [DOI] [PubMed] [Google Scholar]
- 29.Papadopoulos NG, Dedoussis GV, Spanakos G, Gritzapis AD, Baxevanis CN, Papamichail M. An improved fluorescence assay for the determination of lymphocyte-mediated cytotoxicity using flow cytometry. J Immunol Methods. 1994;177(1-2):101–111. doi: 10.1016/0022-1759(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 30.Phillips T, Opferman JT, Shah R, Liu N, Froelich CJ, Ashton-Rickardt PG. A role for the granzyme B inhibitor serine protease inhibitor 6 in CD8+ memory cell homeostasis. J Immunol. 2004;173(6):3801–3809. doi: 10.4049/jimmunol.173.6.3801. [DOI] [PubMed] [Google Scholar]
- 31.Greiner DL, Hesselton RA, Shultz LD. SCID mouse models of human stem cell engraftment. Stem Cells. 1998;16(3):166–177. doi: 10.1002/stem.160166. [DOI] [PubMed] [Google Scholar]
- 32.Christianson GJ, Brooks W, Vekasi S, et al. Beta 2-microglobulin-deficient mice are protected from hypergammaglobulinemia and have defective antibody responses because of increased IgG catabolism. J Immunol. 1997;159(10):4781–4792. [PubMed] [Google Scholar]
- 33.Sadan O, Melamed E, Offen D. Bone-marrow-derived mesenchymal stem cell therapy for neurodegenerative diseases. Expert Opin Biol Ther. 2009;9(12):1487–1497. doi: 10.1517/14712590903321439. [DOI] [PubMed] [Google Scholar]
- 34.Paczesny S, Choi SW, Ferrara JL. Acute graft-versus-host disease: new treatment strategies. Curr Opin Hematol. 2009;16(6):427–436. doi: 10.1097/MOH.0b013e3283319a6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Bari C, Dell'accio F. Mesenchymal stem cells in rheumatology: a regenerative approach to joint repair. Clin Sci (Lond) 2007;113(8):339–348. doi: 10.1042/CS20070126. [DOI] [PubMed] [Google Scholar]
- 36.Jones BJ, McTaggart SJ. Immunosuppression by mesenchymal stromal cells: from culture to clinic. Exp Hematol. 2008;36(6):733–741. doi: 10.1016/j.exphem.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Sayegh MH, Turka LA. The role of T-cell costimulatory activation pathways in transplant rejection. N Engl J Med. 1998;338(25):1813–1821. doi: 10.1056/NEJM199806183382506. [DOI] [PubMed] [Google Scholar]
- 38.Pistoia V, Raffaghello L. Potential of mesenchymal stem cells for the therapy of autoimmune diseases. Expert Rev Clin Immunol. 2010;6(2):211–218. doi: 10.1586/eci.09.86. [DOI] [PubMed] [Google Scholar]
- 39.Sun J, Ooms L, Bird CH, Sutton VR, Trapani JA, Bird PI. A new family of 10 murine ovalbumin serpins includes two homologs of proteinase inhibitor 8 and two homologs of the granzyme B inhibitor (proteinase inhibitor 9). J Biol Chem. 1997;272(24):15434–15441. doi: 10.1074/jbc.272.24.15434. [DOI] [PubMed] [Google Scholar]
- 40.Tseng HC, Arasteh A, Paranjpe A, et al. Increased lysis of stem cells but not their differentiated cells by natural killer cells: de-differentiation or reprogramming activates NK cells. PLoS One. 2010;5(7):e11590. doi: 10.1371/journal.pone.0011590. [DOI] [PMC free article] [PubMed] [Google Scholar]