Abstract
Antiphospholipid (aPL)/anti-β2 glycoprotein I (anti-β2GPI) antibodies stimulates tissue factor (TF) expression within vasculature and in blood cells, thereby leading to increased thrombosis. Several cellular receptors have been proposed to mediate these effects, but no convincing evidence for the involvement of a specific one has been provided. We investigated the role of Apolipoprotein E receptor 2 (ApoER2′) on the pathogenic effects of a patient-derived polyclonal aPL IgG preparation (IgG-APS), a murine anti-β2GPI monoclonal antibody (E7) and of a constructed dimeric β2GPI I (dimer), which in vitro mimics β2GPI-antibody immune complexes, using an animal model of thrombosis, and ApoER2-deficient (−/−) mice. In wild type mice, IgG-APS, E7 and the dimer increased thrombus formation, carotid artery TF activity as well as peritoneal macrophage TF activity/expression. Those pathogenic effects were significantly reduced in ApoER2 (−/−) mice. In addition, those effects induced by the IgG-APS, by E7 and by the dimer were inhibited by treatment of wild-type mice with soluble binding domain 1 of ApoER2 (sBD1). Altogether these data show that ApoER2 is involved in pathogenesis of antiphospholipids antibodies.
Introduction
The association between persistently present antiphospholipid (aPL) antibodies and the clinical manifestations of thrombosis or pregnancy morbidity is known as the antiphospholipid syndrome (APS).1 aPL antibodies are heterogeneous and recognize a wide variety of plasma proteins with phospholipid-binding properties, such as prothrombin2 and β2 glycoprotein I (β2GPI).3,4 aPL antibodies directed against β2GPI, a plasma protein without known physiologic function, are considered the most pathologically relevant antibodies.
There is strong experimental evidence that anti-β2GPI antibodies have thrombogenic properties. In studies on endothelial cell activation,5–8 authors have shown the induction of a prothrombotic and proinflammatory phenotype upon exposure to anti-β2GPI antibodies, indicated by expression of tissue factor (TF) and increased surface expression of adhesion molecules, such as intercellular adhesion molecule-1, vascular-cell adhesion molecule-1, and E-selectin. Activation of monocytes by anti-β2GPI antibodies leads to TF expression as well.9 Furthermore, anti-β2GPI antibodies, or recombinant dimers of β2GPI that mimic β2GPI-antibody immune complexes, increase platelet deposition to extracellular matrix components in in vitro flow models.10 Injection of anti-β2GPI antibodies in murine11 or hamster12 thrombosis models leads to increased thrombus formation.
Several receptors were postulated to mediate the prothrombotic cellular effects of anti-β2GPI antibodies. The interaction between annexin A2 and β2GPI-antibody immune complexes has been reported to lead to endothelial cell activation.13 It seems unlikely, however, that annexin A2 is able to convey activation signals across the cell membrane because this phospholipid-binding protein lacks a transmembrane domain. Toll-like receptor-4 (TLR-4) is another candidate receptor for aPL antibodies because TLR-4–like signaling was reported in endothelial cells upon incubation with aPL antibodies.14 Furthermore, a mutation in murine TLR4, known to disrupt lipopolysaccharide binding, attenuated the increased prothrombotic state observed in wild-type mice injected with aPL antibodies.15 A direct interaction between TLR-4 and β2GPI-antibody immune complexes, however, remains to be confirmed to this date.
Members of the low-density lipoprotein (LDL) receptor family do bind β2GPI-antibody immune complexes.16 The interaction between β2GPI-antibody immune complexes and both apolipoprotein E receptor 2′ (ApoER2′), the only LDL receptor family member present on platelets,17 and the platelet adhesive receptor glycoprotein Ibα was shown to lead to increased thrombus formation in vitro.18,19 Platelet activation could be attenuated by inhibition of the interaction between either receptor and β2GPI.20
We investigated whether ApoER2, which is present on endothelial cells21 and monocytes,22 mediates the prothrombotic effects of aPL antibodies in a murine thrombosis model. Here we present evidence that aPL antibodies and dimeric β2GPI enhance in vivo thrombus formation through ApoER2 expressed on endothelial cells and monocytes.
Methods
Reagents
Recombinant apple4-C321S-β2GPI (dimer), a construct of the apple4 dimerization domain of coagulation factor XI fused to human β2GPI, and apple2-β2GPI (monomer), which contains the nondimerizing apple2 domain of coagulation factor XI, were expressed and purified as described previously.23 The soluble first LDL-binding domain of ApoER2 (sBD1) was expressed and purified as described previously.24 Purity of all recombinant proteins was assessed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
A total IgG fraction (IgG-APS, immunoglobulin from a patient with APS) was affinity-purified with protein G Sepharose chromatography from the serum of a 53-year-old patient with primary APS (without systemic lupus erythematosus) who had a history of 1 transient ischemic attack, 2 myocardial infarctions, 3 episodes of deep vein thrombosis, and 1 pulmonary embolism. His anticardiolipin antibody (aCL) titer was 456 G phospholipids units (GPL) mL−1, his anti-β2GPI antibodies (Abs) titer was 256 standard G units (SGU) mL−1, and he was positive for lupus anticoagulant. The APS patient's serum also contained antibodies that bound to domain I of β2GPI (50 DI Units/mL) by enzyme-linked immunosorbent assay (prototype kit kindly provided by INOVA Diagnostics, Inc). Human total immunoglobulin G (IgG) from a healthy patient (IgG-NHS, ie, IgG from a patient with normal human serum) was purified by an identical method. The patients who donated serum for this study signed a consent form approved by the Institutional Review Board Committee at University of Texas Medical Branch in accordance with the Declaration of Helsinki.
A murine monoclonal IgG (named E7) with specificity for human and murine β2GPI was prepared as described elsewhere.25 A murine monoclonal antibody of irrelevant specificity (MuMoAbC) was used as a control (mouse IgG 1k MPOC-21; Sigma-Aldrich). Absence of endotoxin was confirmed with the limulus amoebocyte lysate assay (E-Toxate; Sigma-Aldrich; assay sensitivity, < 0.06 IU · mL−1) for all preparations that were injected in the mice.
Levels of aCL and anti-β2GPI antibodies were measured by enzyme-linked immunosorbent assay in the human and murine Ig preparations, as previously described.26,27 Lupus anticoagulant activity was determined in the IgG fractions by a modified Kaolin clotting time on spiked normal plasma.28 The IgG-APS fraction injected in the mice had aCL (135 GPL/mL), anti-β2GPI (145 SGU/mL), and lupus anticoauglant activity (ratio: 1.6). IgG-NHS was negative in the 3 tests.
Animals
C57BL/6 (ApoER2+/+) and B6;129S6-Lrp8tm1Her/J (ApoER2−/−) mice were obtained from The Jackson Laboratory. During this study the (ApoER2−/−) mice were bred and then genotyped with the following primers: (1) GAT TGG GAA GAC AAT AGC AGG CAT GC, (2) GCT TGT TGG AAT TCA GCC AGT TAC C, (3) ACG ATG ACC CCA ATG ACA GCA GCG, and (4) CCA CAG TGT CAC ACA GGT AAT GTG. Animals were housed in the Animal Care facilities of the University of Texas Medical Branch at Galveston (an Association for Assessment and Accreditation of Laboratory Animal Care-approved facility). Animals were handled by trained personnel according to Institutional Animal Care and Use Committee guidelines.
Analysis of thrombus dynamics
ApoER2+/+ and ApoER2−/− mice (7-9 animals per group) were injected intraperitoneally with either IgG-APS (500 μg), IgG-NHS (500 μg), E7 (100 μg), MuMoAbC (100 μg), dimer (200 μg), or monomer (200 μg) twice, with an interval of 48 hours between injections. In addition, other groups of ApoER2+/+ mice received an intraperitoneal injection of sBD1 (50 μg) 30 minutes before each injection when they were injected with either IgG-APS (500 μg), IgG-NHS (500 μg), dimer (200 μg), or monomer (200 μg). In all cases, surgical procedures were performed to study thrombus dynamics at 72 hours after the first reagent injections, as described previously.7 In brief, the femoral vein was pinched with a standard pressure to introduce an injury and induce a clot. Clot formation and dissolution in the transilluminated vein were visualized with a microscope equipped with a closed-circuit video system. When a thrombus reached its maximum size, it was measured (in μm2) by digitizing the image and tracing the outer margin of the thrombus. A total of 2 to 5 thrombi were induced in each animal, and the mean thrombus area was computed for each group of animals. Mice were subsequently used to determine TF activity in carotid arteries and peritoneal macrophages.
TF activity in murine carotid artery homogenates
Pieces of approximately 5 mm of uninjured carotid arteries were dissected from both sides in each animal and were collected in Tris-buffered saline (50mM Tris, 150mM NaCl, pH 7.4) with 0.1% Triton X-100 containing heparin as anticoagulant. Samples were subsequently homogenized, as described elsewhere.29 TF activity in carotid artery homogenates was determined with a commercial chromogenic assay (Actichrome TF; American Diagnostica). TF activity was expressed in pM · mg−1 · mL−1. Each pooled sample was assayed in duplicate, and experiments were repeated 3 times.
TF activity in murine peritoneal macrophages
After the animals were killed, peritoneal macrophages were collected immediately after by flushing the peritoneal cavity of each mouse with 5 mL of phosphate-buffered saline (PBS). TF activity was determined as described previously.5 TF activity was expressed in pM · mg−1 · mL−1.
Ex vivo immunolabeling of TF in harvested murine peritoneal macrophages
Peritoneal macrophages were collected immediately after euthanasia by flushing the peritoneal cavity of each mouse with 5 mL of ice-cold Dulbecco minimum essential medium (GIBCO, Invitrogen) supplemented with polymyxin B (20 μg mL−1) as previously described.30 Resident cells were harvested, pelleted at 4°C, and suspended in 50 μL of ice-cold Dulbecco minimum essential medium supplemented with polymyxin B (20 μg mL−1). The cellular suspension was dropped on a sterile glass slide and incubated for 60 minutes at 37°C to allow adherence of macrophages. Nonadherent cells were removed by washing 5 times in 500 μL of warm PBS with the use of a gentle swirling action. When this method was used, more than 90% of adherent cells consisted in macrophages.30
Quantum-dot immunolabeling of TF on macrophages was performed according to the manufacturer's instructions (Invitrogen). In brief, macrophages were with 10% formalin for 10 minutes. Nonspecific binding was blocked by incubation with PBS containing 2% bovine serum albumin. Cells were subsequently labeled with rabbit antimouse TF IgG (American Diagnostica), followed by incubation with goat antirabbit IgG conjugated with Qdot 655 (1:50; Molecular Probes). Finally, nuclear counterstaining was performed with Hoechst dye. Images were captured on a custom built 2-photon microscope consisting of a Zeiss 410 LSM 2-photon excitation laser scanning microscope, equipped with a near-infrared titanium-sapphire femtosecond laser (Spectra Physics) that was tuned and mode-locked at 750 nm. Quantitation of the Qdot fluorescence was performed with Metamorph software (Molecular Devices).
Statistical analysis
Results are presented as means ± SEM or ± SD, as detailed in each experiment. The statistically significant differences of the means of thrombus sizes and TF activity were determined by the use of nonparametric Mann-Whitney U tests and an unpaired t test, respectively. Quantitative fluorescence microscopy of TF expression ex vivo was analyzed with the one-way analysis of variance test, followed by a Tukey multiple comparison test. P values ≤ .05 denote a statistical difference between groups.
Results
Thrombogenic properties of IgG-APS and an anti- β2GPI monoclonal Ab (E7)–injected mice are ameliorated in ApoER2−/− mice
At the time of vascular injury, serum titers of aCL and anti-β2GPI Abs were highly positive in both ApoER2−/− and ApoER2+/+ mice injected with IgG-APS (75.7 ± 12.9 GPL units and 79.6 ±11.8 GPL units, respectively). Anti-β2GPI titers were positive in both ApoER2−/− (63.2 ± 19.1 SGU) and ApoER2+/+ mice (52.1 ± 21.3 SGU) injected with IgG-APS. Similarly, all mice injected with E7 monoclonal Abs were positive for anti-β2GPI Abs (O.D. = 2.0 ± 0.1 [ApoER2−/−] or 1.9 ± 0.7 [ApoER2+/+]) and negative for aCL (O.D. = 0.4 ± 0.2 [ApoER2−/−] or 0.2 ±0.1 [ApoER2+/+]). Mice injected with IgG-NHS or MuMoAbC were negative for aCL and for anti- β2GPI Abs.
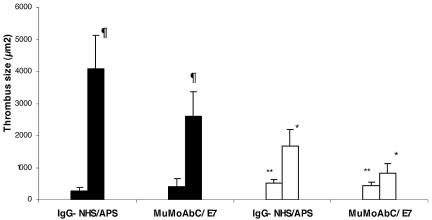
Injection with IgG-APS resulted in significantly larger thrombi (4094.4 ± 1036.8 μm2) in ApoER2+/+ mice compared with mice injected with control IgG-NHS (287.4 ± 110.0 μm2; P < .0001, Figure 1). After IgG-APS injection, mean thrombus size was 59% smaller in ApoER2−/− mice than in ApoER2+/+ mice. In ApoER2−/− mice, however, thrombus size was still larger upon injection with IgG-APS versus IgG-NHS (P < .0001, Figure 1), indicating a partial but significant abrogation of the thrombogenic effect in ApoER2−/− mice induced by IgG-APS.
Figure 1.
aPL antibodies and a murine monoclonal anti-β2GPI antibody (E7) increase thrombus size in a murine thrombosis model through an interaction with ApoER2. ApoER2+/+ (filled bars) or ApoER2−/− (open bars) mice were injected with either IgG-NHS, IgG-APS, MuMoAbC, or E7, as indicated in the section “Analysis of thrombus dynamics.” Thrombi were induced in the animals by the use of a standardized injury, and thrombus size was measured in square microns (μm2). The data are expressed as means ± SD (5-10 animals were used per group). The Mann-Whitney 2-tailed test was used to assess statistical significance. ¶Statistically different from apoER2+/+ treated with control (P < .0001). *Statistically different from apoER2+/+ treated with IgG-APS or E7(P < .0001). **Statistically different from apoER2−/− treated with IgG-APS or E7(P < .0001).
To determine whether these in vivo effects obtained with a polyclonal IgG–APS were specifically related to the anti-β2GPI Abs, we performed experiments by using a murine E7 MoAb that displayed specificity for β2GPI (human and murine). Mean thrombus size was 6-7 times larger in ApoER2+/+ mice injected with E7 compared with same type of mice injected with MuMoAbC (P < .0001). The thrombogenic effects of E7 were significantly diminished in ApoER2−/− mice. In ApoER2−/− mice, however, thrombus size was still significantly larger after injection with E7 compared with MuMoAbC (P < .0001), indicating that a partial but significant abrogation of thrombogenic effect in ApoER2−/− mice induced by E7 took place.
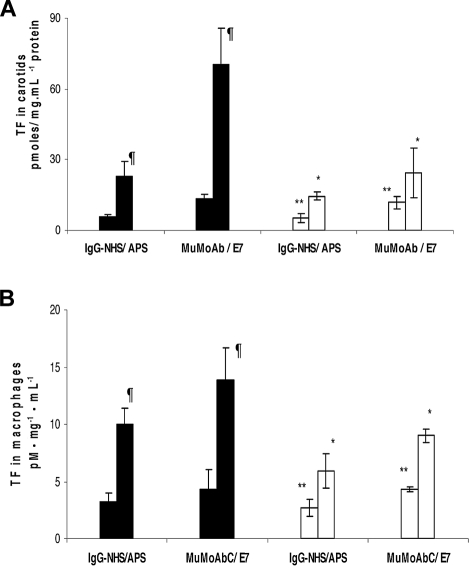
TF activity in carotid artery homogenates was increased 3- to 4-fold in ApoER2+/+ mice treated with IgG-APS versus IgG-NHS (P = .014, Figure 2A). Importantly, upon treatment with IgG-APS, TF activity was diminished by 32% in ApoER2−/− mice compared with their ApoER2+/+ counterparts. The absence of ApoER2, however, did not completely eliminate the increase in TF in carotid arteries of mice treated with IgG-APS compared with those treated with IgG-NHS (P < .0001). Similarly, TF activity in carotids of ApoER2+/+ mice injected with E7 was significantly increased compared with ApoER2+/+ mice injected with MuMoAbC (P = .003). This TF activity was diminished significantly in ApoER2−/− mice treated with E7. Again in this case the up-regulation of TF in carotid arteries in ApoER2−/− mice was significantly elevated (P = .030) compared with ApoER2−/− mice treated with MuMoAbC, indicating an incomplete abrogation of the effect of E7 on TF in carotids when ApoER2 was absent.
Figure 2.
aPL antibodies and a murine monoclonal anti-β2GPI antibody (E7) increase carotid artery and peritoneal macrophages TF activity in mice through an interaction with ApoER2. ApoER2+/+ (filled bars) or ApoER2−/− (open bars) mice were injected with either IgG-NHS, IgG-APS, IgG MoAbC, or E7. Carotid arteries (A) or peritoneal macrophages (B) were harvested from ApoER2+/+ or ApoER2−/− mice, and TF activity in homogenates was determined with a commercial chromogenic assay for Xa formation. At least 3 animals were used per condition. Results are expressed as pM · mg−1 · mL−1 ± SEM. An unpaired t test was applied to examine statistical significance. ¶Statistically different from apoER2+/+ treated with control; (A) NHS (P = .014) or MuMoAb (P = .003) and (B) NHS (P = .004) or MuMoAb (P = .003). *Statistically different from apoER2+/+ treated with IgG-APS (A, P = .009 and B, P < .0001) or E7 (A, P < .0001 and B, P = .018). **Statistically different from apoER2−/− treated with IgG-APS (A, P = < .0001 and B, P = .040) or E7 (A, P = .030 and B, P = .0004).
We performed complementary studies to examine prothrombotic effects of IgG-APS or E7 MoAb by measuring TF activity in peritoneal macrophages (Figure 2B). There was significantly greater TF activity in peritoneal macrophage homogenates of ApoER2+/+ mice injected with IgG-APS or E7 than those given in their controls (10.0 ± 1.4 vs 3.2 ± 0.8 pmoles/ mg.mL−1 protein; P = .004) and (13.9 ± 2.8 vs 4.3 ± 1.7 pmoles/ mg.mL−1 protein; P = .003), respectively. This TF activity was significantly diminished by 41% in ApoER2−/− mice treated with IgG-APS and by 35% in ApoER2−/− mice treated with E7. Again in this case the up-regulation of TF in peritoneal macrophages by IgG-APS or E7 in ApoER2−/− mice was significantly elevated compared with ApoER2−/− mice treated with IgG-NHS or MuMoAbC (5.9 ± 1.5 pM·mg−1·mL−1 protein vs 2.7 ± 0.8 pM·mg−1·mL−1 protein, P = .040, and 9.0 ± 0.6 pM·mg−1·mL−1 protein vs 4.3 ± 0.2 pM·mg.mL−1 protein, P = .004, respectively), indicating an incomplete but significant abrogation of the effect of IgG-APS or E7 on TF in macrophages when ApoER2 was absent.
Dimerized β2GPI induces a prothrombotic state similar to aPL antibodies, and this effect is ameliorated in ApoER2−/− mice
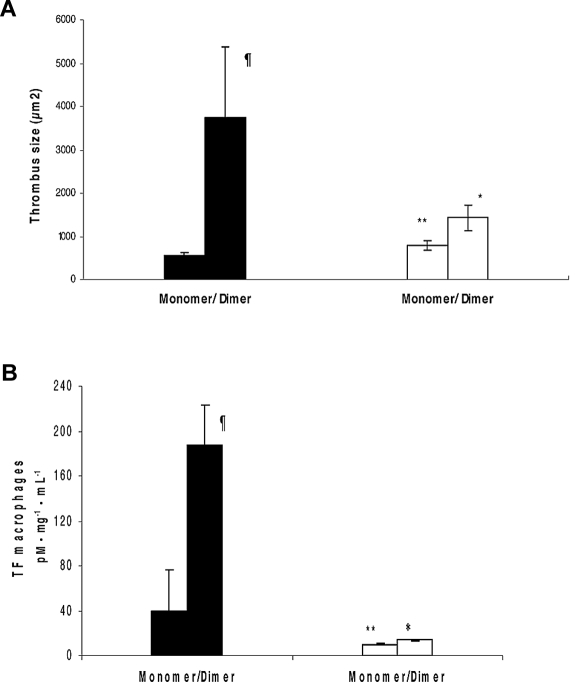
We investigated the thrombogenic properties of a dimer of β2GPI in vivo. To determine whether this dimer is able to induce a prothrombotic state, we studied thrombus formation and up-regulation of TF in peritoneal macrophages of ApoER2+/+ and ApoER2−/− mice. Dimerized β2GPI showed significantly larger thrombi compared with the monomer control in ApoER2+/+ mice (3757.2 ± 1624.5 μm2 vs 552 ± 79.6 μm2; P = .011; Figure 3A). Treatment of ApoER2+/+ mice with dimerized β2GPI caused a significant increase in peritoneal macrophages TF activity compared with mice treated with monomer (187 ± 36 pmoles/mg.mL−1 protein vs 40 ± 5 pmoles/mg.mL−1 protein; P = 0.023, Figure 3B).
Figure 3.
Dimerized β2GPI increases thrombus size, carotid artery, and peritoneal macrophages TF activity in mice through an interaction with ApoER2. ApoER2+/+ (filled bars) or ApoER2−/− (open bars) mice were injected with dimeric β2GPI or monomer β2GPI as control. (A) Thrombi were induced in the animals, and thrombus size was measured in square microns (μm2). The data are expressed as mean ± SD (5-10 animals were used per group). (B) In peritoneal macrophages as described in “Statistical analysis,” TF activity was determined with a commercial chromogenic assay for Xa formation. Results are expressed as means ± SEM in pM · mg−1 · mL−1. Experiments were assayed in duplicate and repeat thrice. ¶Statistically different from apoER2+/+ treated with control; (A, P = .011 and B, P = .023). *Statistically different from apoER2+/+ treated with dimer (A, P = .007 and B, P = .013). **Statistically different from apoER2−/− treated with dimmer (A, P < .0001 and B, P < .0001).
ApoER2−/−mice injected with dimerized β2GPI showed a 63% decrease in thrombus size compared with wild-type mice. This effect, although statistically significant was partial. Furthermore, TF activity in peritoneal macrophages was diminished by 92% in ApoER2−/− mice injected with dimerized β2GPI, indicating a strong involvement of ApoER2 in TF up-regulation in macrophages.
TF expression is up-regulated in ApoER2+/+ mice treated with IgG-APS or dimer through an interaction with ApoER2
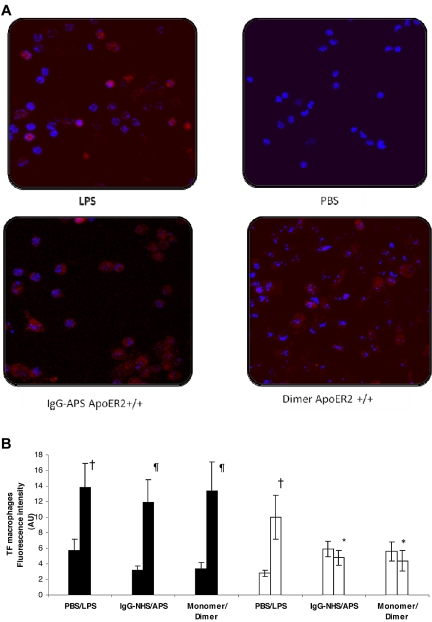
The up-regulation of TF in response to IgG-APS or dimer on the surface of intact murine macrophages ex vivo was also mapped and quantified by the use of quantum-dot nanocrystal immunolabeling, as shown in Figure 4A-B. This technique, performed independently of the chromogenic assay experiments assessing TF activity from cells lysates, confirmed the significant induction of TF expression within macrophages by IgG-APS or dimer comparable with the induction in lipopolysaccharide (LPS)-treated mice (used as positive control) in ApoER2+/+ mice (11.9 ± 2.8 arbitrary units (AU) vs 3.2 ± 0.5 AU or 13.4 ± 3.7 AU vs 3.4 ± 0.8 AU or 13.8 ± 3.1 AU vs 5.7 ± 1.5 AU, respectively). Significantly reduction (by 60% for IgG-APS and by 67% for dimer) was observed in TF expression in ApoER2−/− mice, indicating an involvement of ApoER2 in pathogenic properties of aPL Abs or a dimer that have shown to mimic the effects of β2GPI /anti-β2GPI complexes in vitro.
Figure 4.
TF expression was up-regulated in apoER2+/+ mice treated with IgG-APS or dimer through an interaction with ApoER2. ApoER2+/+ (filled bars) or ApoER2−/− (open bars) mice were injected either with IgG-NHS, IgG-APS, monomer, dimer, PBS, or LPS as described in the section “Ex vivo immunolabeling of TF in harvested murine peritoneal macrophages.” TF expression was determined by immunostaining with specific Qdot bioconjugates and quantitated after examination with a dual-photon laser confocal microscope. Mice treated with LPS or PBS were used as controls. (A) Representative images of TF expression of the different treatment groups. The red fluorescent staining (655-nm Qdot bioconjugate) indicates surface TF immunoreactivity, whereas the blue color represents cell nuclei stained with Hoechst dye. (B) Quantitative TF expression are assessed as fluorescence intensity in AU (mean ± SD; n = 10 images per mouse and 2 mice per group). †Statistically different from PBS-treated mice control (P < .0001). ¶Statistically different from apoER2+/+ treated with control, NHS (P < .0001), or monomer (P < .0001). *Statistically different from apoER2+/+ treated with agonist, IgG-APS (P < .0001), or dimer (P < .0001).
Soluble binding domain of ApoER2 (sBD1-ApoER2) ameliorates thrombogenic properties of IgG-APS or dimer in ApoER2+/+ mice
To confirm the involvement of apoER2′ on aPL thrombogenic effects, we investigated the influence of sBD1, which has been shown to inhibit the binding of dimerized β2GPI to ApoER2 in vitro, in IgG-APS, and dimer-injected ApoER2+/+ mice. Treatment with sBD1 reduced significantly thrombus size in ApoER2+/+ mice injected with IgG-APS (P < .0001) or with dimerized β2GPI (P = .004) compared with those animals injected only with control (Table 1). Similarly, TF expression assessed in peritoneal macrophages as fluorescence intensity (AU) was significantly diminished in ApoER2+/+ mice treated with sBD1 and with IgG-APS (P < .0001) or dimerized β2GPI (P < .0001). This inhibition in thrombogenic properties of IgG-APS and dimerized β2GPI in mice indicates a possible binding/interaction between sBD1 (of apoER2) and anti-β2GPI/β2GPI complexes or dimeric β2GPI that production an abrogation of the previously demonstrated thrombogenic properties in the animal model studied.
Table 1.
Effects of sBD1 on thrombus size and TF expression in murine macrophages induced by IgG-APS
| Treatments | Thrombus size, μm2 | TF expression in macrophages, AU |
|---|---|---|
| IgG-APS | 4094.4 ± 1036.8* | 10.1 ± 1.4† |
| IgG-NHS | 287.4 ± 110.0 | 3.2 ± 0.5 |
| IgG-APS + sBD1 | 665.0 ± 173.0 | 4.4 ± 0.6 |
| IgG-NHS + sBD1 | 486.0 ± 164.0 | 3.2 ± 0.7 |
| Dimer | 3757 ± 162‡ | 13.4 ± 3.7§ |
| Monomer | 552.0 ± 79.6 | 3.4 ± 0.8 |
| Dimer + sBD1 | 533 ± 148 | 6.9 ± 1.5 |
| Monomer + sBD1 | 552.0 ± 133.0 | 4.9 ± 1.2 |
AU indicates arbitrary unit; IgG-APS, immunoglobulin G from a patient with antiphospholipid syndrome; IgG-NHS, immunoglobulin G from a patient with normal human serum; sBD1, soluble binding domain 1 of ApoER2; and TF, tissue factor.
Statistically significant from their controls and from sBD1-treated mice; IgG-APS (*P < .0001) and †(P < .0001) or dimer (‡P = .0044) and (§P = .0001).
Discussion
We have shown that injection of both a polyclonal IgG from a patient with primary APS and a murine monoclonal anti-β2GPI antibody caused a significant increase in thrombus formation as well as increased vascular TF activity and monocyte activation in a murine thrombosis model. The importance of ApoER2 in the induction of a prothrombotic state by aPL antibodies in vivo was confirmed in ApoER2−/− mice, in which injection of aPL antibodies (both polyclonal and monoclonal) did not result in enhanced thrombus formation, vessel wall, or monocyte TF activity. Interestingly, a constructed dimeric β2GPI that in vitro mimics β2GPI-antibody immune complexes induced also in vivo a potent prothrombotic effect, as evidenced by increased in thrombus formation and TF function/expression in peritoneal macrophages. Similarly, the thrombogenic properties of the dimerized β2GPI were diminished in ApoER2−/− mice. In addition, these thrombogenic effects could be inhibited by addition of the soluble first LDL-binding domain of ApoER2, indicative of the importance of ApoER2 in endothelial cell activation by aPL antibodies. Importantly, our studies confirmed the findings from Prof de Groot's laboratory, who demonstrated a role of ApoER2′ on effects aPL antibodies derived from several different APS patients on platelets in vitro.19,20,23,24
ApoER2 is a member of the LDL receptor family, a multiligand receptor family with a wide tissue distribution.31 Originally identified in neurons, in which it mediates neuronal plasticity,32,33 and it also is involved in neuronal migration during brain development,32 ApoER2 is expressed on platelets, endothelial cells, and monocytes. Studies on the role of ApoER2 in platelets showed ApoER2 mediates both ApoE-dependent platelet inhibition17 and LDL-dependent platelet activation.34 The platelet activating properties of ApoER2-derived signaling were supported by a genome-wide linkage-scan that linked the onset of premature cardiovascular disease to a polymorphism in the ApoER2 gene.35 This polymorphism results in increased platelet sensitivity for ligands such as LDL.35 These observations point to ApoER2 as a key regulator of thrombus formation, not only under pathologic conditions such as in the APS, but also under physiologic conditions.
Our data indicate a relevant and new in vivo role for ApoER2 in thrombus formation in the APS. We cannot, however, exclude a potential role for additional receptors, as demonstrated by the partial inhibition of thrombogenic properties of aPL antibodies or dimer observed in apoER2−/− mice. Furthermore, we cannot exclude the possibility that the residual thrombus formation observed in apoER (−/−) mice may be mediated by other receptors. To that extent, TLR-4 seems a likely candidate because it is already implicated in aPL antibody-induced signaling in endothelial cells14 and because there are structural similarities with glycoprotein Ibα; both glycoprotein Ibα and TLR-4 contain Leucine-rich repeats.36 Moreover, dysfunctional TLR-4 was shown to abrogate the prothrombotic effects of aPL antibodies in a murine thrombosis model.15 Annexin A2, a receptor for plasminogen and tissue plasminogen activator, might play a role in cellular activation by aPL antibodies as well,37 although it most likely functions as a docking site. It is difficult to envision intracellular signaling upon binding to a protein without a transmembrane domain. Other studies have shown the involvement of TLR2 in aPL-mediated effects in fibroblasts and in endothelial cells in vitro.38,39 Interestingly, the lack of aPOER2 in mice appeared to have a more pronounced effect on expression of TF in macrophages of mice treated with IgG-APS or with dimer compared with the partial, albeit significant, decrease on thrombus size, possibly indicating that other mechanisms related to thrombosis may have been responsible for this apparently paradoxical effect.
Antibody binding to a cellular surface has been shown to lead to cellular activation via Fc receptors. Nevertheless, the role of Fc receptor–mediated cellular activation in the thrombotic complications of APS is debatable. F(ab′)2 fragments of aPL antibodies were shown to have the same in vitro40 and in vivo12 effects in models of APS as whole IgG. Our data do not support involvement of Fc receptors either, because dimeric β2GPI does not contain the Fc domains of IgG.
Involvement of the complement system in the prothrombotic effects of dimeric β2GPI seems questionable. Complement components C3 and C5a are reported to mediate aPL antibody-induced fetal loss41,42 and are suggested to mediate the thrombotic complications of the syndrome as well.43,44 Dimeric β2GPI, however, does not support antibody-mediated complement activation. The classical complement activation pathway is therefore unlikely to play a major role in our system. We cannot exclude that dimeric β2GPI, which causes increased vessel wall TF expression and therefore thrombin formation, causes activation of complement components indirectly.45
Thrombotic events occur only occasionally in patients with the APS, despite the continuous presence of circulating aPL antibodies. A possible explanation is that aPL antibodies can only exert their prothrombotic influence when the local environment is primed for further activation. This second-hit mechanism was shown in an animal model of the APS, in which injection of aPL antibodies in rats only resulted in increased thrombus formation when rats were pretreated with lipopolysaccharide, but not when rats were injected with buffer.46 Our results are in line with these data because in our model of thrombosis, the effects of the antibodies were seen after the thrombus formation was initiated by a standardized injury in the vessel wall. We propose the following sequence of events that leads to the thrombotic manifestations of the APS: β2GPI binds to anionic phospholipids exposed on the surface of cells that become activated as a result of a small injury. Subsequently, β2GPI undergoes a conformational change. This conformational change leads to exposure of the binding site for “pathogenic aPL” antibodies, that may include antibodies with lupus anticoagulant, anticardiolipin, and/or anti-β2GPI activities. β2GPI is dimerized upon antibody binding, which stabilizes the interaction with cells and allows interaction with ApoER2.47 Interaction with ApoER2 results in cell activation and the induction of a prothrombotic cellular phenotype. The newly identified role of ApoER2 in this thrombotic mechanism may be a potential therapeutic target for treatment of the thrombotic manifestations of the APS.
Acknowledgments
This work was partially supported with funds from the Antiphospholipid Standardization Laboratory (University of Texas Medical Branch, Galveston, TX), a grant from the American Heart Association (#0855272F) a grant from the National Institutes of Health (1ROI), and a grant from the Netherlands Heart Foundation (2003B074).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Z.R.P., R.A.-V., R.T.U., M.T.T.P, E.P., and Y.H. performed research experiments; R.T.U. contributed to vital new reagents or analytical tools; Z.R.P, T.S., and G.V. performed the microscopy studies and analysis of the corresponding data; R.H.W.M.D., P.G.d.G., and S.S.P. contributed with design of the studies, critical analysis of the data, and writing the manuscript; Z.R.P. and R.TU. also contributed with writing of the manuscript; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Silvia S. Pierangeli, PhD, Division of Rheumatology, Department of Internal Medicine, 301 University Blvd, Galveston, TX 77555-0883; e-mail: sspieran@utmb.edu.
References
- 1.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 2.Bevers EM, Galli M, Barbui T, Comfurius P, Zwaal RF. Lupus anticoagulant IgG's (LA) are not directed to phospholipids only, but to a complex of lipid-bound human prothrombin. Thromb Haemost. 1991;66(6):629–632. [PubMed] [Google Scholar]
- 3.McNeil HP, Simpson RJ, Chesterman CN, Krilis SA. Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: beta 2-glycoprotein I (apolipoprotein H). Proc Natl Acad Sci U S A. 1990;87(11):4120–4124. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galli M, Comfurius P, Maassen C, et al. Anticardiolipin antibodies (ACA) directed not to cardiolipin but to a plasma protein cofactor. Lancet. 1990;335(8705):1544–1547. doi: 10.1016/0140-6736(90)91374-j. [DOI] [PubMed] [Google Scholar]
- 5.Vega-Ostertag M, Casper K, Swerlick R, Ferrara D, Harris EN, Pierangeli SS. Involvement of p38 MAPK in the up-regulation of tissue factor on endothelial cells by antiphospholipid antibodies. Arthritis Rheum. 2005;52(5):1545–1554. doi: 10.1002/art.21009. [DOI] [PubMed] [Google Scholar]
- 6.Simantov R, LaSala JM, Lo SK, et al. Activation of cultured vascular endothelial cells by antiphospholipid antibodies. J Clin Invest. 1995;96(5):2211–2219. doi: 10.1172/JCI118276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierangeli SS, Colden-Stanfield M, Liu X, Barker JH, Anderson GL, Harris EN. Antiphospholipid antibodies from antiphospholipid syndrome patients activate endothelial cells in vitro and in vivo. Circulation. 1999;99(15):1997–2002. doi: 10.1161/01.cir.99.15.1997. [DOI] [PubMed] [Google Scholar]
- 8.Del Papa N, Guidali L, Sala A, et al. Endothelial cells as target for antiphospholipid antibodies. Human polyclonal and monoclonal anti-beta 2-glycoprotein I antibodies react in vitro with endothelial cells through adherent beta 2-glycoprotein I and induce endothelial activation. Arthritis Rheum. 1997;40(3):551–561. doi: 10.1002/art.1780400322. [DOI] [PubMed] [Google Scholar]
- 9.Sorice M, Longo A, Capozzi A, et al. Anti-beta2-glycoprotein I antibodies induce monocyte release of tumor necrosis factor alpha and tissue factor by signal transduction pathways involving lipid rafts. Arthritis Rheum. 2007;56(8):2687–2697. doi: 10.1002/art.22802. [DOI] [PubMed] [Google Scholar]
- 10.Lutters BC, Derksen RH, Tekelenburg WL, Lenting PJ, Arnout J, de Groot PG. Dimers of beta 2-glycoprotein I increase platelet deposition to collagen via interaction with phospholipids and the apolipoprotein E receptor 2′. J Biol Chem. 2003;278(36):33831–33838. doi: 10.1074/jbc.M212655200. [DOI] [PubMed] [Google Scholar]
- 11.Gharavi AE, Pierangeli SS, Colden-Stanfield M, Liu XW, Espinola RG, Harris EN. GDKV-induced antiphospholipid antibodies enhance thrombosis and activate endothelial cells in vivo and in vitro. J Immunol. 1999;163(5):2922–2927. [PubMed] [Google Scholar]
- 12.Jankowski M, Vreys I, Wittevrongel C, et al. Thrombogenicity of beta 2-glycoprotein I-dependent antiphospholipid antibodies in a photochemically induced thrombosis model in the hamster. Blood. 2003;101(1):157–162. doi: 10.1182/blood-2002-05-1310. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, McCrae KR. Annexin A2 mediates endothelial cell activation by antiphospholipid/anti-beta2 glycoprotein I antibodies. Blood. 2005;105(5):1964–1969. doi: 10.1182/blood-2004-05-1708. [DOI] [PubMed] [Google Scholar]
- 14.Raschi E, Testoni C, Bosisio D, et al. Role of the MyD88 transduction signaling pathway in endothelial activation by antiphospholipid antibodies. Blood. 2003;101(9):3495–3500. doi: 10.1182/blood-2002-08-2349. [DOI] [PubMed] [Google Scholar]
- 15.Pierangeli SS, Vega-Ostertag ME, Raschi E, et al. Toll-like receptor and antiphospholipid mediated thrombosis: in vivo studies. Ann Rheum Dis. 2007;66(10):1327–1333. doi: 10.1136/ard.2006.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pennings MT, van Lummel M, Derksen RH, et al. Interaction of beta2-glycoprotein I with members of the low density lipoprotein receptor family. J Thromb Haemost. 2006;4(8):1680–1690. doi: 10.1111/j.1538-7836.2006.02036.x. [DOI] [PubMed] [Google Scholar]
- 17.Riddell DR, Vinogradov DV, Stannar d AK, Chadwick N, Owen JS. Identification and characterization of LRP8 (apoER2) in human blood platelets. J Lipid Res. 1999;40(10):1925–1930. [PubMed] [Google Scholar]
- 18.Shi T, Giannakopoulos B, Yan X, et al. Anti-beta2-glycoprotein I antibodies in complex with beta2-glycoprotein I can activate platelets in a dysregulated manner via glycoprotein Ib-IX-V. Arthritis Rheum. 2006;54(8):2558–2567. doi: 10.1002/art.21968. [DOI] [PubMed] [Google Scholar]
- 19.Pennings MT, Derksen RH, van Lummel M, et al. Platelet adhesion to dimeric beta-glycoprotein I under conditions of flow is mediated by at least two receptors: glycoprotein Ibalpha and apolipoprotein E receptor 2′. J Thromb Haemost. 2007;5(2):369–377. doi: 10.1111/j.1538-7836.2007.02310.x. [DOI] [PubMed] [Google Scholar]
- 20.Urbanus RT, Pennings MT, Derksen RH, de Groot PG. Platelet activation by dimeric beta2-glycoprotein I requires signaling via both glycoprotein Ibalpha and apolipoprotein E receptor 2′. J Thromb Haemost. 2008;6(8):1405–1412. doi: 10.1111/j.1538-7836.2008.03021.x. [DOI] [PubMed] [Google Scholar]
- 21.Sacre SM, Stannard AK, Owen JS. Apolipoprotein E (apoE) isoforms differentially induce nitric oxide production in endothelial cells. FEBS Lett. 2003;540(1–3):181–187. doi: 10.1016/s0014-5793(03)00261-8. [DOI] [PubMed] [Google Scholar]
- 22.Yang XV, Banerjee Y, Fernandez JA, et al. Activated protein C ligation of ApoER2 (LRP8) causes Dab1-dependent signaling in U937 cells. Proc Natl Acad Sci U S A. 2009;106(1):274–279. doi: 10.1073/pnas.0807594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lutters BC, Meijers JC, Derksen RH, Arnout J, de Groot PG. Dimers of beta 2-glycoprotein I mimic the in vitro effects of beta 2-glycoprotein I-anti-beta 2-glycoprotein I antibody complexes. J Biol Chem. 2001;276(5):3060–3067. doi: 10.1074/jbc.M008224200. [DOI] [PubMed] [Google Scholar]
- 24.Pennings MT, Derksen RH, Urbanus RT, Tekelenburg WL, Hemrika W, de Groot PG. Platelets express three different splice variants of ApoER2 that are all involved in signaling. J Thromb Haemost. 2007;5(7):1538–1544. doi: 10.1111/j.1538-7836.2007.02605.x. [DOI] [PubMed] [Google Scholar]
- 25.Gharavi AE, Pierangeli SS, Gharavi EE, et al. Thrombogenic properties of antiphospholipid antibodies do not depend on their binding to beta2 glycoprotein 1 (beta2GP1) alone. Lupus. 1998;7(5):341–346. doi: 10.1191/096120398678920190. [DOI] [PubMed] [Google Scholar]
- 26.Harris EN. Antiphospholipid antibodies. Br J Haematol. 1990;74(1):1–9. doi: 10.1111/j.1365-2141.1990.tb02530.x. [DOI] [PubMed] [Google Scholar]
- 27.Palomo I, Alarcon M, Sepulveda C, Pereira J, Espinola R, Pierangeli S. Prevalence of antiphospholipid and antiplatelet antibodies in human immunodeficiency virus (HIV)-infected Chilean patients. J Clin Lab Anal. 2003;17(6):209–215. doi: 10.1002/jcla.10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Exner T, Rickard KA, Kronenberg H. A sensitive test demonstrating lupus anticoagulant and its behavioural patterns. Br J Haematol. 1978;40(1):143–151. doi: 10.1111/j.1365-2141.1978.tb03648.x. [DOI] [PubMed] [Google Scholar]
- 29.Day SM, Reeve JL, Pedersen B, et al. Macrovascular thrombosis is driven by tissue factor derived primarily from the blood vessel wall. Blood. 2005;105(1):192–198. doi: 10.1182/blood-2004-06-2225. [DOI] [PubMed] [Google Scholar]
- 30.Davies JQ, Gordon S. Isolation and culture of murine macrophages. Methods Mol Biol. 2005;290:91–103. doi: 10.1385/1-59259-838-2:091. [DOI] [PubMed] [Google Scholar]
- 31.Herz J, Gotthardt M, Willnow TE. Cellular signalling by lipoprotein receptors. Curr Opin Lipidol. 2000;11(2):161–166. doi: 10.1097/00041433-200004000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Beffert U, Durudas A, Weeber EJ, et al. Functional dissection of Reelin signaling by site-directed disruption of Disabled-1 adaptor binding to apolipoprotein E receptor 2: distinct roles in development and synaptic plasticity. J Neurosci. 2006;26(7):2041–2052. doi: 10.1523/JNEUROSCI.4566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weeber EJ, Beffert U, Jones C, et al. Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J Biol Chem. 2002;277(42):39944–39952. doi: 10.1074/jbc.M205147200. [DOI] [PubMed] [Google Scholar]
- 34.Korporaal SJ, Relou IA, van Eck M, et al. Binding of low density lipoprotein to platelet apolipoprotein E receptor 2′ results in phosphorylation of p38MAPK. J Biol Chem. 2004;279(50):52526–52534. doi: 10.1074/jbc.M407407200. [DOI] [PubMed] [Google Scholar]
- 35.Shen GQ, Li L, Girelli D, et al. An LRP8 variant is associated with familial and premature coronary artery disease and myocardial infarction. Am J Hum Genet. 2007;81(4):780–791. doi: 10.1086/521581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bell JK, Mullen GE, Leifer CA, Mazzoni A, Davies DR, Segal DM. Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol. 2003;24(10):528–533. doi: 10.1016/s1471-4906(03)00242-4. [DOI] [PubMed] [Google Scholar]
- 37.Romay-Penabad Z, Montiel-Manzano MG, Shilagard T, et al. Annexin A2 is involved in antiphospholipid antibody-mediated pathogenic effects in vitro and in vivo. Blood. 2009;114(14):3074–3083. doi: 10.1182/blood-2008-11-188698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satta N, Dunoyer-Geindre S, Reber G, et al. The role of TLR2 in the inflammatory activation of mouse fibroblasts by human antiphospholipid antibodies. Blood. 2007;109(4):1507–1514. doi: 10.1182/blood-2005-03-024463. [DOI] [PubMed] [Google Scholar]
- 39.Alard JE, Gaillard F, Daridon C, Shoenfeld Y, Jamin C, Youinou P. TLR2 is one of the endothelial receptors for beta 2-glycoprotein I. J Immunol. 2010;185(3):1550–1557. doi: 10.4049/jimmunol.1000526. [DOI] [PubMed] [Google Scholar]
- 40.Vega-Ostertag M, Harris EN, Pierangeli SS. Intracellular events in platelet activation induced by antiphospholipid antibodies in the presence of low doses of thrombin. Arthritis Rheum. 2004;50(9):2911–2919. doi: 10.1002/art.20434. [DOI] [PubMed] [Google Scholar]
- 41.Holers VM, Girardi G, Mo L, et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J Exp Med. 2002;195(2):211–220. doi: 10.1084/jem.200116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Girardi G, Berman J, Redecha P, et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest. 2003;112(11):1644–1654. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Redecha P, Tilley R, Tencati M, et al. Tissue factor: a link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood. 2007;110(7):2423–2431. doi: 10.1182/blood-2007-01-070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pierangeli SS, Girardi G, Vega-Ostertag M, Liu X, Espinola RG, Salmon J. Requirement of activation of complement C3 and C5 for antiphospholipid antibody-mediated thrombophilia. A rthritis Rheum. 2005;52(7):2120–2124. doi: 10.1002/art.21157. [DOI] [PubMed] [Google Scholar]
- 45.Huber-Lang M, Sarma JV, Zetoune FS, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12(6):682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 46.Fischetti F, Durigutto P, Pellis V, et al. Thrombus formation induced by antibodies to beta2-glycoprotein I is complement dependent and requires a priming factor. Blood. 2005;106(7):2340–2346. doi: 10.1182/blood-2005-03-1319. [DOI] [PubMed] [Google Scholar]
- 47.de Laat B, Derksen RH, van Lummel M, Pennings MT, de Groot PG. Pathogenic anti-beta2-glycoprotein I antibodies recognize domain I of beta2-glycoprotein I only after a conformational change. Blood. 2006;107(5):1916–1924. doi: 10.1182/blood-2005-05-1943. [DOI] [PubMed] [Google Scholar]