Summary
Objectives
To test the hypothesis that surfactant, when given prophylactically during one lung ventilation, improves physiological stability and reduces inflammation.
Methods
Prospective controlled animal study. After 30 minutes of mechanical ventilation, surfactant was administered to the left lung of the treatment group. Right lung mechanical ventilation continued for 3 hours, after which the left lung was unblocked. Bilateral mechanical ventilation was continued for 30 minutes thereafter. Physiological parameters and biomarkers of inflammation in plasma, lung tissue homogenates, and bronchoalveolar lavage were measured.
Measurements and Main Results
Oxygenation improved in the surfactant group, reaching statistical significance at 3 hours of one lung ventilation and again after 30 minutes of bilateral mechanical ventilation following the one lung ventilation. Plasma levels of interleukin-1 β, interleukin-6, and tumor necrosis factor-α showed a trend for reduction. The lung homogenates from the ventilated lungs had significantly lower levels of interleukin-1 β (P < 0.01) and interleukin-6 (P < 0.01). The bronchoalveolar lavage specimen showed an overall reduction in the cytokine levels; interleukin-1 β was significantly lower in the ventilated lungs (P < 0.01).
Conclusions
Surfactant administration improves oxygenation and decreases inflammation, as evidenced by a decrease in several inflammatory cytokines both in the plasma and lungs of a piglet model of one lung ventilation.
Keywords: acute lung injury, alleviate, inflammation
Introduction
Surfactant administration is beneficial in human and animal models of acute lung injury/acute respiratory distress syndrome (ALI/ARDS). One lung ventilation (OLV) induces endothelial and alveolar injury; however, to date, no prospective, randomized study has been conducted to determine the effects of surfactant administration prior to OLV on inflammatory and physiological markers that determine lung injury.
One lung ventilation is a procedure usually performed to facilitate surgery, and it is mandatory when a thoracoscopic approach is used for surgery. During OLV, the lung on the operative side of the thoracic cavity is collapsed. The collapsed lung is known to sustain pan endothelial and alveolar epithelial injury as well as activation of the inflammatory cascade (1,2). In animal models, vascular congestion and edema have been noted due to changes in the endothelial permeability that allow exudation of proteinaceous fluid into the alveolar space (3). Damage to the endothelium has significant implications in the modulation of the hypoxic pulmonary vasoconstriction, which is the primary mechanism by which significant hypoxemia is avoided during OLV (4). Other feared complications of OLV are ischemia-reperfusion injury and reexpansion pulmonary edema (5). Thus, a cascade of events follows the collapse and re-expansion of a lung, eventually resulting in leukocyte sequestration, further leading to inflammation and microvascular injury (5,6).
Injury resulting from OLV shares many histopathological features with ALI/ARDS, one of which is surfactant dysfunction. Loss or inactivation of surfactant biophysical activity and damage to the type II alveolar cells results in an exacerbation of the pulmonary injury. Clinical studies involving surfactant therapy have shown improvement in oxygenation and ventilation in children with ALI/ARDS. In a recent multicenter trial, surfactant was shown to improve oxygenation, decrease mortality, and decrease ventilator days in children (7). In the present study, we hypothesize that calfactant (Infasurf; ONY, Amherst, New York, USA), a natural, animal-derived surfactant, will not only improve lung functional-mechanics, but will also reduce inflammatory injury sustained from OLV in our piglet model (8).
We chose calfactant (Infasurf), a natural calf lung surfactant extract that contains the highest concentration of surfactant protein B of the various available surfactants. One ml of Infasurf contains 35 mg of phospholipids and 0.65 mg of protein, which includes 0.26 mg of surfactant protein B. The mean protein weight relative to phospholipids is closest to the natural lung surfactant. It is the most active surfactant protein in increasing overall adsorption and dynamic surface activity. Surfactant protein B is required for lung function, host defense, and recovery following lung injury. The deficiency or absence of surfactant protein B has been shown to be associated with decreased lung compliance and increased hyperoxic lung injury (9-12). Surfactant protein B also has been shown to decrease inflammation by enhancing surfactant function and making it more resistant to inhibition by blood proteins (13). Given this background, a conceptual rationale exists for administering surfactant prior to OLV.
Materials and Methods
Twenty juvenile pigs (8-10 kg; 3-4 wk postnatal age) were included in this trial. The animals were chosen due to the similarity between human and swine bronchial anatomy. The Institutional Animal Care and Use Committee, Department of Biomedical Research, Nemours, approved this study in accordance with the National Institutes of Health guidelines.
Animals were randomly assigned to two groups of 10 (control group [4 males, 6 females] and surfactant group [5males, 5 females]). The animals were anesthetised by injections (two 1-ml/kg) of an anaesthetic mixture (ketamine: 23 mg/ml, azepromazine: 0.1 mg/ml, and xylazine: 0.05 mg/ml). Prior to instrumentation, animals were placed on a radiant warmer bed (Resuscitaire; Hill-Rom Air-Shields, Hatboro, Pennsylvania, USA) to maintain a rectal temperature of 37°C to 39°C. Following initial anesthesia, the trachea was intubated with a 6.0-mm uncuffed endotracheal tube. All piglets had a small leak around the tube that was minimized by inserting tonsillar packs made of soft gauze around the tube, deep into the oropharynx around the laryngeal opening. An uncuffed endotracheal tube was chosen due to the anatomy of the pig trachea. The pigs have a tracheal bronchus and very short main stem bronchi relative to humans, thus inflated cuffs could have caused partial or complete occlusion of the apical bronchi (14).
Lungs were ventilated with an anesthesia machine (Modulus II Plus; Ohmeda, Hartford, Connecticut, USA) using a tidal volume of 10 ml/kg and no positive end expiratory pressure. The tidal volume was measured at the Y piece using a standard pediatric anesthesia monitoring system (Model M1175A; Hewlett Packard, Palo Alto, California, USA). The ventilator delivered breaths in a volume-control mode. The end-tidal carbon dioxide was kept between 35 and 45 mmHg by adjusting the ventilatory rate. Customarily, during one lung ventilation, an initial tidal volume of 10 mL.kg-1 is used with a peak inspiratory pressure of 30 cm H2O (15), and this normally provides adequate ventilation (1). Eight-Fr umbilical catheters were inserted into the internal jugular vein and carotid artery through incisions in the neck for venous and arterial access, respectively.
Vital parameters, including arterial and central venous blood pressure, electrocardiogram, and rectal temperature, were monitored for a stabilization period of 30 min and throughout the duration of the protocol using the anesthesia monitoring system. Pulmonary mechanics variables were recorded from a pediatric patient monitoring system (CO2SMO Plus; Novametrix Medical Systems, Willingford, Connecticut, USA). Pulmonary mechanics analyses involved pneumotachography and airway manometry to determine integrated tidal volumes and calculate respiratory compliance. Arterial blood chemistry (arterial blood gas [ABG], Stat Profile; Nova Biomedical, Waltham, Massachusetts, USA) was measured at each assessment point and monitored every half-hour throughout the protocol.
After completion of catheter placement and stabilization, maintenance anesthesia was initiated as follows: sufentanil infusion at 0.2-0.3 μg/hr and isoflurane at 1% inspired concentration in 100% oxygen. Pancuronium was administered intermittently at a dosage of 0.2 mg/kg intravenously. Depth of anesthesia was assessed using changes in vital signs as primary criteria, and when appropriate, the infusion rate of sufentanil was titrated as needed. Following the initiation of maintenance anesthesia, baseline vital parameters and arterial blood-gas measurements were obtained. Blood samples were drawn at blood to sodium citrate ratio of 9:1 for subsequent analysis of inflammatory mediators associated with ALI.
After the initial 30 minutes of stabilization, an Arndt endobronchial blocker (Cook Medical, Bloomington, Indiana, USA) was inserted into the left main stem bronchus (under bronchoscopic visualisation), and the balloon was inflated. Surfactant was then administered via the endobronchial blocker lumen in a dosage of 1 ml/kg in four divided aliquots. The dose varied from 8-10 ml, dependent upon the weight of the pig. The surfactant was administered in four different positions: head-down, head-up, right-lateral, and left-lateral positions. Administration of each aliquot was followed by instillation of 5 cc of air for distribution in the surfactant group. Air was not instilled in the control group. The different positions were chosen to facilitate equal distribution of surfactant material within the left lung. During this manipulation, the endobronchial blocker was inflated to prevent surfactant spillage into the right lung; the trachea was examined bronchoscopically for spillage and none was noted.
One Lung Ventilation Protocol
The left primary bronchus was blocked using a fiberoptic bronchoscope and an Arndt endobronchial blocker. An airway adaptor was used during the blocking procedure so ventilation was minimally affected. The piglet was turned to the right-lateral position to simulate surgical positioning during OLV. To simulate thoracoscopic instrumentation, following local anesthesia with 1% lidocaine, a 5-mm trocar was placed through the nondependent thoracic wall between the seventh and eighth rib. Placement of the trocar provided additional information regarding the collapsed status of the left lung by allowing observation of the collapsed lung through the trocar using the bronchoscope. Following initiation of OLV, measurements of outcome parameters were repeated immediately and then every half-hour throughout the experiment for comparison with bilateral lung ventilation. Breath sounds were periodically checked by auscultation, and the position of the bronchial blocker was rechecked using the fiberoptic bronchoscope in 30-min intervals throughout the duration of the OLV period. Following 3 hours of OLV, measurements were repeated. The bronchial blocker was then retrieved after deflating the balloon to restore bilateral ventilation. Three deep breaths (twice the peak inflating pressure generated for the initial tidal volume) were given manually to recruit alveoli, the animal was turned back to the supine position, and ventilation was continued for another 30 min before a final set of measurements was performed for comparison with pre-OLV data. The anesthesia was then deepened by intravenous administration of 20 mg/kg ketamine and 2-4 μg/kg of sufentanil in preparation for tissue harvest.
A sternotomy was performed, and catheters were placed in the pulmonary artery and left ventricle. The catheters allowed the lungs to be perfused with Millonig’s buffer to flush blood from the vasculature. The lungs were removed from the body after flushing with Millonig’s solution. A 5-Fr x 16-in tube was attached to a 5-ml syringe containing 5 ml of cold citrate phosphate-buffered saline (PBS-citrate) solution (PBS-citrate solution for lavage is a 9:1 mixture of PBS, pH 7.4, and 3.8% sodium. The final 9:1 solution is 0.38% sodium citrate in PBS). The tube was inserted into the main stem bronchus and fed down to the base portion of the lung. Five milliliters of PBS-citrate was slowly flushed into the lung tissue and then drawn back up in the same syringe, recovering as much of the solution as possible. The recovered lavage fluid was then transferred to 1.5-ml microcentrifuge tubes and centrifuged at 2000 rpm for 20 minutes. The supernatant was removed from the pellet and transferred to a clean tube for storage. Both the supernatant and the pellet were frozen at -70°C until enzyme-linked immunosorbent assays (ELISAs) were run on the supernatant. Lung tissue from both right and left lungs was harvested and snap-frozen in liquid nitrogen for measurements of inflammatory markers. Lung tissue obtained from the base nondependent section of each lung, in an area unaffected by the lavage step above, was fixed in 10% formalin for 24 to 48 hours. The tissue was gently washed in PBS before storage in 70% ethanol. Trained histologists then processed the tissue, including paraffin-embedding, slide preparation (5-um-thick sections), and haematoxylin and eosin staining.
Measurement of Inflammatory Mediators
The levels of interleukin (IL)-1 β, IL-6, and tumor necrosis factor (TNF)-α in plasma samples were measured with quantitative ELISA using porcine-specific Quantikine ELISA kits (R&D Systems, Minneapolis, Minnesota, USA). Plasma and lung homogenates and bronchoalveolar lavage (BAL) were appropriately diluted to fall within the detection range of each assay, and all standards and samples were assayed in duplicate. The test sensitivities for respective immunoassays were as follows: IL-1 β (10 pg/ml), IL-6 (10 pg/ml), and TNF-α (3.7 pg/ml). Interassay and intra-assay coefficients of variance were < 10%.
Statistical Analysis
Continuous study variables were summarized using mean and standard deviation, and categorical variables were described using frequencies and percentages. A two-sample t-test was used to examine the distribution of continuous covariates between the two groups, and a repeated measures analysis of variance was used to test the differences of means of the two groups of outcome variables measured during the experiment over time. Each model was adjusted for significant covariates such as age and weight. The sphericity assumption of the model was checked using Mauchley’s test, and an appropriate corrected method was used in case of violation of this model assumption. A log 10 transformation was performed for all cytokine measures to linearize and reduce the volatility of data. Lung tissue cytokine measurements of two treatment groups were compared using a nonparametric Mann–Whitney U-test. All analyses were two-tailed at 5% level of significance and were performed using the statistical package SPSS version 17.0 (SPSS Inc, Chicago, Illinois, USA).
Results
The mean weight of the two groups was 9.4 kg ± 0.54 (control) and 9.2 kg ± 0.77 (surfactant), and the mean age was 35.2 ± 8.7 days (control) and 44.7 ± 4.4 days (surfactant). The mean weight of the two groups was not different (P = 0.48); however, there was a small but significant difference (P < 0.01) in age between the two groups. Peak inspiratory pressure, mean airway pressures, partial pressure of carbon dioxide, tidal volume, and respiratory rates for different time-points are given in Table 1.
Table 1.
Mean and standard deviations of confounding variables measured over time at different time points during the experiment are represented. P value was obtained by analysis using repeated measures ANOVA.
| Control Group (Mean and Standard deviation) |
Surfactant Group (Mean and Standard deviation) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T-BL | T-0 | T-0.5 | T-1 | T-1.5 | T-2 | T-2.5 | T-3 | End | T-BL | T-0 | T-0.5 | T-1 | T-1.5 | T-2 | T-2.5 | T-3 | End | P value | |
| Temp (°C) | 36.8 (0.6) | 36.7 (0.4) | 36.6 (0.6) | 36.6 (0.6) | 36.7 (0.6) | 36.7 (0.7) | 36.7 (0.8) | 36.8 (0.8) | 36.9 (0.8) | 36.6 (0.7) | 36.5 (0.7) | 36.6 (0.7) | 36.7 (0.7) | 35.8 (2.6) | 36.8 (0.8) | 36.8 (0.6) | 36.6 (0.5) | 36.4 (0.5) | 0.46 |
| MAP (mm Hg) | 73 (7.9) | 77 (11.2) | 80 (8.2) | 80 (9.6) | 79 (9.5) | 74 (8.4) | 74 (5.6) | 72 (6.7) | 68 (4.7) | 75 (10) | 86 (13.7) | 81 (13.5) | 79 (12.5) | 79 (12.4) | 78 (11.7) | 77 (11.4) | 75 (8.6) | 71 (7.8) | 0.47 |
| HR (b/min) | 99 (9.5) | 100 (17) | 101 (17) | 99 (13) | 95 (14) | 99 (16) | 95 (13) | 97 (15) | 98 (18) | 100 (23) | 116 (12) | 118 (19) | 116 (18) | 114 (16) | 116 (18) | 114 (17) | 114 (16) | 125 (19) | 0.01 |
| PIP (cm H2O) | 13 (4.6) | 21 (3.4) | 21 (3.2) | 22 (3.3) | 22 (3.7) | 22 (3.7) | 22 (3.9) | 22 (4.0) | 20 (3.0) | 14 (2.7) | 25 (6.0) | 25 (3.1) | 24 (3.6) | 23 (3.4) | 23 (3.6) | 23 (3.3) | 23 (3.8) | 20 (2.8) | 0.21 |
| MAWP (cm H2O) | 4 (.97) | 5.9 (1.2) | 5.7 (.48) | 5.7 (.82) | 5.6 (.69) | 5.7 (1) | 5.9 (.99) | 5.9 (.99) | 5.5 (.85) | 4.5 (.97) | 6.7 (1.5) | 6.4 (1.4) | 6 (.81) | 5.8 (.78) | 6 (.81) | 6 (.66) | 5.9 (.87) | 5.6 (1) | 0.4 |
| PaCO2 (mm Hg) | 39 (4.0) | 46 (8.2) | 44 (3.8) | 45 (3.5) | 46 (3.9) | 46 (4.0) | 45 (2.5) | 47 (3.2) | 48 (5.7) | 37 (5.9) | 51 (8.7) | 44 (6.6) | 44 (3.7) | 46 (5.5) | 44 (4.6) | 44 (4.1) | 43 (3.7) | 46 (6.3) | 0.62 |
| TV (ml) | 97 (7.6) | 94 (7.4) | 93 (9.5) | 93 (6.4) | 92 (7.4) | 95 (6.8) | 96 (6.5) | 96 (6.2) | 95 (6.5) | 90 (9.3) | 87 (13.1) | 91 (7.4) | 90 (8.5) | 88 (8.1) | 89 (5.2) | 89 (6.1) | 91 (6.3) | 89 (10.2) | 0.1 |
| RR (/min) | 18 (4.5) | 20 (5.6) | 17 (5.0) | 17 (5.0) | 17 (4.1) | 18 (4.8) | 17 (4.3) | 17 (4.4) | 18 (3.9) | 17 (3.4) | 19 (3.3) | 19 (2.6) | 19 (4.8) | 21 (5.7) | 20 (4.4) | 19 (4.5) | 20 (5.1) | 20 (4.4) | 0.12 |
ANOVA = analysis of variance, BL = baseline, HR = heart rate, MAP = mean arterial pressure, PIP = positive inspiratory pressure, MAWP = mean airway pressure, TV = tidal volume, RR = respiratory rate
Physiological Parameters
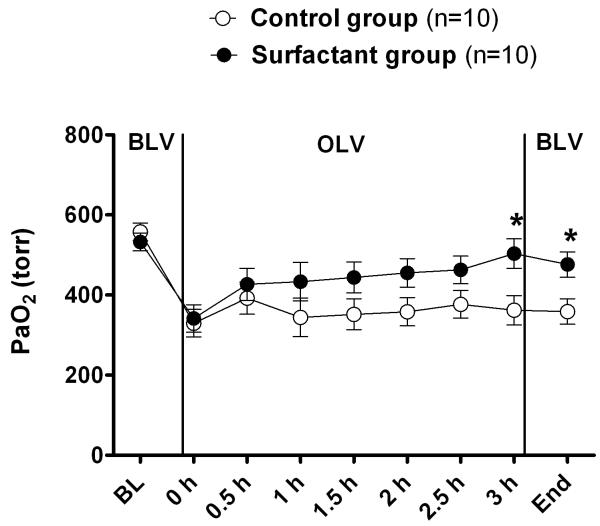
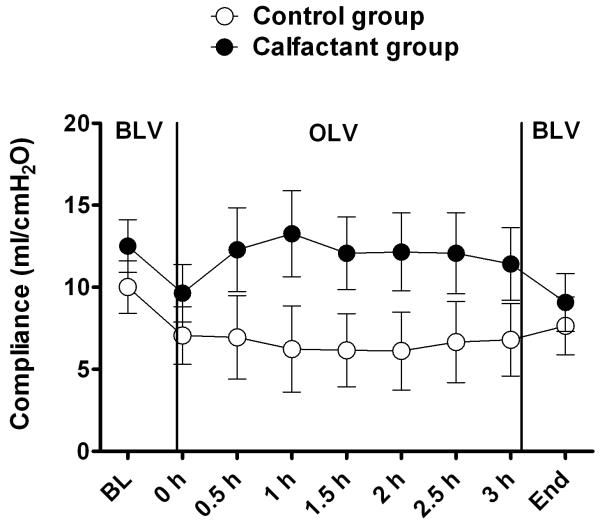
The surfactant group showed overall greater values for PaO2 compared with the control group at all time-points (Figure 1). This difference achieved statistical significance at measurements taken after three hours of OLV and again at the end of the experiment (following re-expansion and 30 min of bilateral ventilation). Respiratory compliance (Figure 2), measured throughout the entire experiment, trended towards an improvement at all measurement points favoring the surfactant group, whereas the “group by time” (interaction) was not different. Respiratory compliance decreased significantly (P < 0.001) with onset of OLV in both groups; however, the surfactant group trended with a better compliance compared to the control group throughout the remainder of the experiment.
Figure 1.
Surfactant (SF)-group and control-group PaO2 over time. There was an improvement in oxygenation as a function of time in the SF group, which reached significance at three hours of one lung ventilation (OLV) and again at the end of the experiment when compared with the control group. Overall, the P value for comparing means of two groups after age and weight adjustment was 0.15.
Figure 2.
Compliance over time for surfactant (SF) and control group. The P value for comparing means of two groups after age and weight adjustment was 0.16. Although this P value is not significant, the trend favored the SF group.
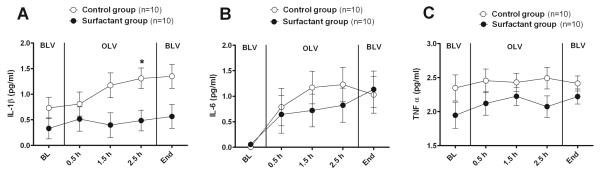
Plasma Cytokines
Figure 3 shows plasma levels of IL-1 β, IL-6, and TNF-α as a function of group and time interaction after log 10 transformation. These cytokines show a trend towards reduction of mediators in the surfactant group compared with the control group over time.
Figure 3.
Plasma levels of (A) interleukin (IL)-1 β, (B) IL-6, and (C) tumor necrosis factor (TNF)-α shown as a function of group and time after log 10 transformation. As shown, there was a trend for a reduction of mediators in the surfactant compared with the control group over time. The P values for comparing means of two groups after age and weight adjustment are shown in text. There is no significant difference between baseline means of two groups for log 10 transformed values of IL-1 β and TNF-α (P > 0.80), and there is no difference between the baseline means of the two groups for IL-6. All of baseline values for IL-6 were 0. *Represents the points at which mean cytokine levels were significantly different.
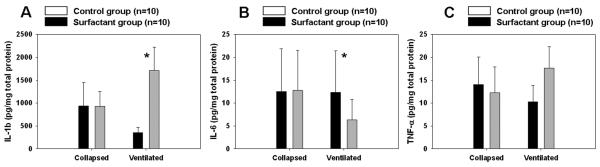
Lung Homogenates
Figure 4 shows the cytokine levels from the lung tissue homogenates. The lung homogenates from the ventilated lungs in the surfactant group had significantly lower levels of IL-1 β (P < 0.01); and IL 6 (P < 0.01). However, TNF-α was not significantly different between groups.
Figure 4.
The lung homogenates, (A) interleukin (IL)-1 β, (B) IL-6, and (C) tumor necrosis factor (TNF)-α, from the ventilated lungs in the surfactant (SF) group had significantly lower levels of IL-6 (P < 0.01) and IL-1 β (P < 0.01); TNF-α was not significantly different between groups. Analysis by Mann–Whitney U-test. Median, range, and P values are represented.
Bronchoalveolar Lavage Specimens
IL-1 β from the ventilated lungs was significantly lower in the surfactant group compared with the control group (P < 0.01) (Figure 5). Similarly, TNF-α was lower in the BAL of ventilated lungs in the surfactant group, although this difference did not reach statistical significance (P = 0.07). The IL-6 levels in the BAL were not different between the two groups.
Figure 5.
Bronchioalveolar lavage specimens obtained from both lungs are illustrated; (A) interleukin (IL)-1 β, (B) IL-6, and (C) tumor necrosis factor (TNF)-α. Interleukin-1β from the ventilated lungs was significantly lower in the surfactant group compared with the control group (P < 0.01). Analysis by Mann–Whitney U-test. Median, range, and P values are represented.
Histopathology Specimens
Lung tissue from the right and left lungs was examined for histological appearance after staining with haematoxylin and eosin (in each group, N = 5). There was less infiltration of alveolar space with polymorphonuclear leukocytes and better preservation of alveolar architecture in the ventilated lungs of the surfactant group (Figure 6). Although it is hard to quantify, without actually measuring the BAL for inflammatory cells, it appears to us that there is lesser exudative process and fewer inflammatory cells in the alveoli and interstitium of right and left lung of the surfactant group, which is consistent with the observations in the measured cytokines and is further suggestive of attenuation of the inflammatory process.
Figure 6.

Haematoxylin and eosin staining of lung tissue harvested at completion of the OLV experiment. Lung histology specimens from the left lung of the control and surfactant (SF) group are shown alongside right lungs of the control and SF group (the specimens were obtained from the base sections of both sides).
Discussion
We investigated the effect of surfactant therapy on the physiological parameters measured during OLV and simultaneously examined the markers of inflammation in plasma and lung. To our knowledge, this is the first study to examine the efficacy of surfactant to prevent injury induced by OLV. In this animal model of OLV, we have shown that surfactant treatment improved the overall physiological parameters of the lungs and improved oxygenation while decreasing pro-inflammatory biomarkers. Surfactant dysfunction is a well recognized component of ALI and ARDS; OLV leads to a similar surfactant dysfunction (16-18) but is a necessary technique to facilitate a thoracoscopic approach to the thoracic cavity. Ashbaugh et al. initially reported abnormalities in surfactant function while describing ALI/ARDS (18). In recent years, further research has focused on understanding this injury, which causes surfactant dysfunction due to inactivation or inhibition of one of the components of a complex process much like the injury sustained from OLV.
During OLV, inflammatory injury is prevalent and has been described in animal models of OLV (1-3,8). Surfactant protein B has been shown to reduce inflammation by making the surfactant more resistant to inhibition by blood proteins and exudative material. In this regard, Calfactant, among the available surfactants, has the highest concentration of surfactant protein B, which is required for lung function, host defense, and recovery following lung injury. A deficiency of surfactant protein B also is associated with decreased lung compliance and increased hyperoxic lung injury (9-12).
One lung ventilation, which is commonly used to assist in thorocacoscopic surgery poses special challenges. Hypoxemia is a commonly encountered problem during OLV. The major cause of hypoxemia during OLV is the shunting of de-oxygenated blood through the non-ventilated lung. Factors that influence this shunt are hypoxic pulmonary vasoconstriction (HPV), gravity, the pressure differential between the thoraces, and physical lung collapse. Moreover, unlike in situations where longer term respiratory support is provided, respiratory mechanics during OLV procedures are dynamic and fluctuating based on the size of the surgical instruments placed in the chest and based on the compression of not only lung tissue but also other structures in the thoracic cavity. An FiO2 of 1.0 is commonly used during OLV (19,20) because it a) provides a margin of safety and b) dilates the pulmonary vessels (especially in the ventilated, dependent lung).
Improvement in oxygenation is likely the first physiological change that occurs with surfactant instillation (21). The improvement in oxygenation in the surfactant treatment group in our study is consistent with trials conducted in the past in both animal (22-28) and human (15,29-31) models. The mechanism of induced injury and surfactant used were different in the various studies; however, most studies reported an improvement in oxygenation (32).
Although there have been multiple reports of improvement in oxygenation after surfactant administration, there has been a renewed interest in investigating the use of surfactant in ALI/ARDS. In a recent multicenter trial conducted by the Pediatric Acute Lung Injury and Sepsis investigators, Willson et al. demonstrated a decrease in mortality and a significantly lower oxygenation index in the surfactant-treated group (7). More patients in the placebo group failed to respond to conventional mechanical ventilation. A post hoc analysis of the same trial studied the effects of surfactant in immuno-compromised children and suggested a potential benefit in the surfactant-treated group (33). A randomized controlled trial by the PALISI group is currently investigating the role of surfactant in oncology patients suffering from ALI.
Oxygenation is affected by multiple factors during OLV. Improvement in oxygenation has been seen in some patients during OLV. Although the mechanism of this improvement is not clearly understood, the postulated mechanism includes re-direction of blood flow to the dependent lung by physical compression and compression of the blood vessels (34). Cardiac output has been shown to effect oxygenation during OLV (35,36). Although the cardiovascular and hemodynamic monitoring in this study were limited to heart rate and invasive arterial pressure monitoring, interestingly enough, the heart rate was significantly different between the two groups (P < 0.01).
The beneficial effects in the current study may also be explained by the decrease in inflammation in the non-collapsed, ventilated lung. Padley et al. (37) have demonstrated greater injury in the non-collapsed, ventilated lungs following OLV. A decrease in systemic and pulmonary inflammation, which was also clearly demonstrated in our study, may have a role to play in the observed improvement in oxygenation.
The endobronchial blocker was inflated prior to surfactant administration to prevent spillage. The trachea, carina, and right main stem bronchus were continuously visualised with a bronchoscope. Although microscopic spillage is a possibility, we did not observe any gross surfactant spillage. Furthermore, after removal of lungs, and while obtaining BAL specimens and harvesting lung tissue, we did not recover any surfactant material from the non-collapsed lung; residuals were seen only in the surfactant-instilled lungs.
Respiratory compliance was measured throughout the entire experiment and trended towards an improvement after OLV at all measurement points favoring the surfactant-treated group. Since surfactant was only administered to the left lung, this finding may be explained by the fact that the respiratory compliance is a combination of the chest-wall compliance and lung compliance of both lungs. In our study, the improvement in the compliance observed was small and may be explained by the following: first, we only measured the respiratory compliance of one lung (the other lung was blocked during OLV), and second, it was the blocked lung that was treated with surfactant, while the chest wall compliance was unaffected.
We selected specific cytokines based on prior literature and experimental time frame. Studies have attempted to predict the onset, outcome, and prognosis of ALI and ARDS using specific cytokine measurements in the BAL fluid. In our pilot study, we demonstrated an increase in the plasma cytokine level during the OLV period with TNF-α showing a second surge after the reexpansion of the collapsed lung (8). The most extensively studied cytokines in the setting of ALI are TNF-α (38-41), IL-1 β (40,41), and IL-6 (39-41). In the ARDSNet lower tidal volume ventilation trial, Parsons et al. showed a higher baseline level of IL-6 and IL-8 in the non-survivors (42). Meduri et al. showed that baseline levels of IL-1 β, IL-6, IL-8, and TNF-α are higher in non-survivors, and that persistent elevation of these markers predict mortality (39). Meduri et al. also showed that an unfavorable outcome in ALI is associated with an exaggerated initial pulmonary inflammatory response. In their study, plasma cytokine levels paralleled changes in BAL cytokine levels, which suggests a pulmonary origin of the cytokine production. In survivors in their study, a reduction over time in BAL cytokine levels was associated with a decline in the BAL protein and albumin level, which suggests a decrease in the exudative component of the BAL. Which particular cytokines can be used as prognostic indicators remains controversial because of the complex nature of the inflammatory cascade. Also, although measurement of BAL cytokines has provided deeper insights into the pathophysiology of ALI/ARDS, it may be misleading to indict single cytokines as prediction tools.
This experiment is a part of series of studies to test interventions that may reduce inflammation and attenuate lung injury sustained during OLV. Our group has studied the effects of protective ventilation with 5 ml/kg of tidal volume as a strategy to alleviate this injury (43,44) Tidal volume in this study was used as a variable that we controlled. To target a normal end-tidal CO2 and PaCO2, we maintained a tidal volume of approximately 10 ml/kg (Table 1). Due to the design of this study, the FiO2 was kept at 1.0 in both the surfactant and control groups. While this could have led to a finite amount of oxidative injury, due to similar treatment of the study and control groups, the overall influence of this should be minimal.
There are a few limitations of this study. One limitation of our study is that we only collected data for a half-hour period following re-expansion of the left lung and therefore obtained only one time-point for measurement of outcome parameters. Perhaps a longer period for data collection following re-expansion may have given a greater insight into the trends. Although the sample size may not have been enough to detect the difference with adequate power for some parameters to demonstrate a statistically significant difference, the overall trends consistently favored surfactant. It would also be interesting to compare calfactant with other commercially available surfactants with a low concentration of surfactant protein B. Although this was considered, we did not perform this intervention.
In conclusion, the present study shows that surfactant administration in association with OLV procedures improves oxygenation and decreases inflammation, as evidenced by a decrease in several inflammatory cytokines both in the plasma and lungs.
Acknowledgements
This study was supported in part by NIH COBRE Grant 1 P20 RR020173-06 (recipient Thomas H. Shaffer) and by the Nemours Foundation. Calfactant was provided by ONY, Inc.
Financial support: This study was supported in part by NIH COBRE Grant 1 P20 RR020173-06 (recipient Thomas H. Shaffer) and by the Nemours Foundation. None of the sponsors were involved with the study design; collection, analysis, and interpretation of data; writing of the manuscript; or decision to submit for publication.
Footnotes
No conflict of interest, real or perceived, exists for any of the authors.
References
- 1.Jordan S, Mitchell JA, Quinlan GJ, Goldstraw P, Evans TW. The pathogenesis of lung injury following pulmonary resection. Eur Respir J. 2000;15:790–799. doi: 10.1034/j.1399-3003.2000.15d26.x. [DOI] [PubMed] [Google Scholar]
- 2.Williams EA, Evans TW, Goldstraw P. Acute lung injury following lung resection: is one-lung anaesthesia to blame? Thorax. 1996;51:114–116. doi: 10.1136/thx.51.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin K, Gribbin E, Emanuel S, Orndorff R, Walker J, Weese J, Fallahnejad M. Histochemical alterations in one lung ventilation. J Surg Res. 2007;137:16–20. doi: 10.1016/j.jss.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 4.Vanhoutte PM. The endothelium--modulator of vascular smooth-muscle tone. N Engl J Med. 1988;319:512–513. doi: 10.1056/NEJM198808253190809. [DOI] [PubMed] [Google Scholar]
- 5.Her C, Mandy S. Acute respiratory distress syndrome of the contralateral lung after reexpansion pulmonary edema of a collapsed lung. J Clin Anesth. 2004;16:244–250. doi: 10.1016/j.jclinane.2003.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Jackson RM, Veal CF, Alexander CB, Brannen AL, Fulmer JD. Neutrophils in reexpansion pulmonary edema. J Appl Physiol. 1988;65:228–234. doi: 10.1152/jappl.1988.65.1.228. [DOI] [PubMed] [Google Scholar]
- 7.Willson DF, Thomas NJ, Markovitz BP, Bauman LA, DiCarlo JV, Pon S, Jacobs BR, Jefferson LS, Conaway MR, Egan EA, Pediatric Acute Lung Injury and Sepsis Investigators Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA. 2005;293:470–476. doi: 10.1001/jama.293.4.470. [DOI] [PubMed] [Google Scholar]
- 8.Miller TL, Costarino AT, Olivant A, Lim D, Shaffer TH, Theroux MC. An animal model for the study of lung protective therapies during one lung ventilation (OLV) in children. Open Anesthesiol J. 2008;2:58–62. [Google Scholar]
- 9.Tokieda K, Whitsett JA, Clark JC, Weaver TE, Ikeda K, McConnell KB, Jobe AH, Ikegami M, Iwamoto HS. Pulmonary dysfunction in neonatal SP-B-deficient mice. Am J Physiol. 1997;273:L875–L882. doi: 10.1152/ajplung.1997.273.4.L875. [DOI] [PubMed] [Google Scholar]
- 10.Clark JC, Wert SE, Bachurski CJ, Stahlman MT, Stripp BR, Weaver TE, Whitsett JA. Targeted disruption of the surfactant protein B gene disrupts surfactant homeostasis, causing respiratory failure in newborn mice. Proc Natl Acad Sci U S A. 1995;92:7794–7798. doi: 10.1073/pnas.92.17.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tokieda K, Iwamoto HS, Bachurski C, Wert SE, Hull WM, Ikeda K, Whitsett JA. Surfactant protein-B-deficient mice are susceptible to hyperoxic lung injury. Am J Respir Cell Mol Biol. 1999;21:463–472. doi: 10.1165/ajrcmb.21.4.3436. [DOI] [PubMed] [Google Scholar]
- 12.Tokieda K, Ikegami M, Wert SE, Baatz JE, Zou Y, Whitsett JA. Surfactant protein B corrects oxygen-induced pulmonary dysfunction in heterozygous surfactant protein B-deficient mice. Pediatr Res. 1999;46:708–714. doi: 10.1203/00006450-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Epaud R, Ikegami M, Whitsett JA, Jobe AH, Weaver TE, Akinbi HT. Surfactant protein B inhibits endotoxin-induced lung inflammation. Am J Respir Cell Mol Biol. 2003;28:373–378. doi: 10.1165/rcmb.2002-0071OC. [DOI] [PubMed] [Google Scholar]
- 14.Mouton WG, Pfitzner J, Bessell JR, Maddern GJ. Bronchial anatomy and single-lung ventilation in the pig. Can J Anaesth. 1999;46:701–703. doi: 10.1007/BF03013963. [DOI] [PubMed] [Google Scholar]
- 15.Willson DF, Zaritsky A, Bauman LA, Dockery K, James RL, Conrad D, Craft H, Novotny WE, Egan EA, Dalton H. Instillation of calf lung surfactant extract (calfactant) is beneficial in pediatric acute hypoxemic respiratory failure. Members of the Mid-Atlantic Pediatric Critical Care Network. Crit Care Med. 1999;27:188–195. doi: 10.1097/00003246-199901000-00050. [DOI] [PubMed] [Google Scholar]
- 16.Petty TL, Ashbaugh DG. The adult respiratory distress syndrome. Clinical features, factors influencing prognosis and principles of management. Chest. 1971;60:233–239. doi: 10.1378/chest.60.3.233. [DOI] [PubMed] [Google Scholar]
- 17.Bigelow DB, Petty TL, Ashbaugh DG, Levine BE, Nett LM, Tyler SW. Acute respiratory failure. Experiences of a respiratory care unit. Med Clin North Am. 1967;51:323–340. doi: 10.1016/s0025-7125(16)33059-0. [DOI] [PubMed] [Google Scholar]
- 18.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2:319–323. [Google Scholar]
- 19.Schwarzkopf K, Klein U, Schreiber T, Preussetaler NP, Bloos F, Helfritsch H, Sauer F, Karzai W. Oxygenation during one-lung ventilation: the effects of inhaled nitric oxide and increasing levels of inspired fraction of oxygen. Anesth Analg. 2001;92:842–847. doi: 10.1097/00000539-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Karzai W, Schwarzkopf K. Hypoxemia during one-lung ventilation: prediction, prevention, and treatment. Anesthesiology. 2009;110:1402–1411. doi: 10.1097/ALN.0b013e31819fb15d. [DOI] [PubMed] [Google Scholar]
- 21.Goldsmith LS, Greenspan JS, Rubenstein SD, Wolfson MR, Shaffer TH. Immediate improvement in lung volume after exogenous surfactant: alveolar recruitment versus increased distention. J Pediatr. 1991;119:424–428. doi: 10.1016/s0022-3476(05)82057-8. [DOI] [PubMed] [Google Scholar]
- 22.Nieman GF, Gatto LA, Paskanik AM, Yang B, Fluck R, Picone A. Surfactant replacement in the treatment of sepsis-induced adult respiratory distress syndrome in pigs. Crit Care Med. 1996;24:1025–1033. doi: 10.1097/00003246-199606000-00024. [DOI] [PubMed] [Google Scholar]
- 23.Lutz CJ, Picone A, Gatto LA, Paskanik A, Landas S, Nieman GF. Exogenous surfactant and positive end-expiratory pressure in the treatment of endotoxin-induced lung injury. Crit Care Med. 1998;26:1379–1389. doi: 10.1097/00003246-199808000-00025. [DOI] [PubMed] [Google Scholar]
- 24.Tashiro K, Li WZ, Yamada K, Matsumoto Y, Kobayashi T. Surfactant replacement reverse respiratory failure induced by intratracheal endotoxin in rats. Crit Care Med. 1995;23:149–156. doi: 10.1097/00003246-199501000-00024. [DOI] [PubMed] [Google Scholar]
- 25.Sun B, Curstedt T, Song GW, Robertson B. Surfactant improves lung function and morphology in newborn rabbits with meconium aspiration. Biol Neonate. 1993;63:96–104. doi: 10.1159/000243917. [DOI] [PubMed] [Google Scholar]
- 26.Harris JD, Jackson F, Jr, Moxley MA, Longmore WJ. Effect of exogenous surfactant instillation on experimental acute lung injury. J Appl Physiol. 1989;66:1846–1851. doi: 10.1152/jappl.1989.66.4.1846. [DOI] [PubMed] [Google Scholar]
- 27.van Daal GJ, So KL, Gommers D, Eijking EP, Fiévez RB, Sprenger MJ, van Dam DW, Lachmann B. Intratracheal surfactant administration restores gas exchange in experimental adult respiratory distress syndrome associated with viral pneumonia. Anesth Analg. 1991;72:589–595. [PubMed] [Google Scholar]
- 28.Matalon S, Holm BA, Notter RH. Mitigation of pulmonary hyperoxic injury by administration of exogenous surfactant. J Appl Physiol. 1987;62:756–761. doi: 10.1152/jappl.1987.62.2.756. [DOI] [PubMed] [Google Scholar]
- 29.Herting E, Möller O, Schiffmann JH, Robertson B. Surfactant improves oxygenation in infants and children with pneumonia and acute respiratory distress syndrome. Acta Paediatr. 2002;91:1174–1178. doi: 10.1080/080352502320777397. [DOI] [PubMed] [Google Scholar]
- 30.Willson DF, Jiao JH, Bauman LA, Zaritsky A, Craft H, Dockery K, Conrad D, Dalton H. Calf’s lung surfactant extract in acute hypoxemic respiratory failure in children. Crit Care Med. 1996;24:1316–1322. doi: 10.1097/00003246-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Lotze A, Knight GR, Martin GR, Bulas DI, Hull WM, O’Donnell RM, Whitsett JA, Short BL. Improved pulmonary outcome after exogenous surfactant therapy for respiratory failure in term infants requiring extracorporeal membrane oxygenation. J Pediatr. 1993;122:261–268. doi: 10.1016/s0022-3476(06)80131-9. [DOI] [PubMed] [Google Scholar]
- 32.Bhatia R, Cox T, Hertzog J. Surfactant Replacement Therapy in Pediatric Acute Lung Injury/Acute Respiratory Distress Syndrome. Curr Respir Med Rev. 2009;5:160–167. [Google Scholar]
- 33.Tamburro RF, Thomas NJ, Pon S, Jacobs BR, Dicarlo JV, Markovitz BP, Jefferson LS, Willson DF, Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network Post hoc analysis of calfactant use in immunocompromised children with acute lung injury: Impact and feasibility of further clinical trials. Pediatr Crit Care Med. 2008;9:459–464. doi: 10.1097/PCC.0b013e3181849bec. [DOI] [PubMed] [Google Scholar]
- 34.Ishikawa S, Nakazawa K, Makita K. Progressive changes in arterial oxygenation during one-lung anaesthesia are related to compression of the non-dependent lung. Br J Anaesth. 2003;90:21–26. [PubMed] [Google Scholar]
- 35.Nomoto Y, Kawamura M. Pulmonary gas exchange effects by nitroglycerine, dopamine, and dobutamine during one-lung ventilation in man. Can J Anaesth. 1998;36:273–277. doi: 10.1007/BF03010764. [DOI] [PubMed] [Google Scholar]
- 36.Abe K, Yoshija I. The effects of propofol, isoflurane, and sevoflurane on oxygenation and shunt fraction during one-lung ventilation. Anesth Analg. 1998;87:1164–1169. doi: 10.1097/00000539-199811000-00035. [DOI] [PubMed] [Google Scholar]
- 37.Padley SP, Jordan SJ, Goldstraw P, Wells AU, Hansell DM. Asymmetric ARDS following pulmonary resection: CT findings initial observations. Radiology. 2002;223:468–473. doi: 10.1148/radiol.2232010721. [DOI] [PubMed] [Google Scholar]
- 38.Millar AB, Foley NM, Singer M, Johnson NM, Meager A, Rook GA. Tumour necrosis factor in bronchopulmonary secretions of patients with adult respiratory distress syndrome. Lancet. 1989;2:712–714. doi: 10.1016/s0140-6736(89)90772-1. [DOI] [PubMed] [Google Scholar]
- 39.Meduri GU, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107:1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 40.Levitt JE, Gould MK, Ware LB, Matthay MA. The pathogenetic and prognostic value of biologic markers in acute lung injury. J Intensive Care Med. 2009;24:151–167. doi: 10.1177/0885066609332603. [DOI] [PubMed] [Google Scholar]
- 41.Levitt JE, Bedi H, Calfee CS, Gould MK, Matthay MA. Identification of early acute lung injury at initial evaluation in an acute care setting prior to the onset of respiratory failure. Chest. 2009;135:936–943. doi: 10.1378/chest.08-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, Wheeler AP, NHLBI Acute Respiratory Distress Syndrome Clinical Trials Network Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. discussion 230–232. [DOI] [PubMed] [Google Scholar]
- 43.Theroux MC, Fisher AO, Horner LM, Rodriguez ME, Costarino AT, Miller TL, Shaffer TH. Protective ventilation to reduce inflammatory injury from one lung ventilation in a piglet model. Paediatr Anaesth. 2010;20:356–364. doi: 10.1111/j.1460-9592.2009.03195.x. [DOI] [PubMed] [Google Scholar]
- 44.Theroux MC, Olivant A, Lim D, Bernardi JP, Costarino AT, Shaffer TH, Miller TL. Low dose methylprednisolone prophylaxis to reduce inflammation during one-lung ventilation. Paediatr Anaesth. 2008;18:857–864. doi: 10.1111/j.1460-9592.2008.02667.x. [DOI] [PubMed] [Google Scholar]