Abstract
Taste buds consist of at least three principal cell types that have different functions in processing gustatory signals: glial-like (type I) cells, receptor (type II) cells, and presynaptic (type III) cells. Using a combination of Ca2+ imaging, single-cell reverse transcriptase-PCR and immunostaining, we show that GABA is an inhibitory transmitter in mouse taste buds, acting on GABAA and GABAB receptors to suppress transmitter (ATP) secretion from receptor cells during taste stimulation. Specifically, receptor cells express GABAA receptor subunits β2, δ, and π, as well as GABAB receptors. In contrast, presynaptic cells express the GABAA β3 subunit and only occasionally GABAB receptors. In keeping with the distinct expression pattern of GABA receptors in presynaptic cells, we detected no GABAergic suppression of transmitter release from presynaptic cells. We suggest that GABA may serve function(s) in taste buds in addition to synaptic inhibition. Finally, we also defined the source of GABA in taste buds: GABA is synthesized by GAD65 in type I taste cells as well as by GAD67 in presynaptic (type III) taste cells and is stored in both those two cell types. We conclude that GABA is an inhibitory transmitter released during taste stimulation and possibly also during growth and differentiation of taste buds.
Introduction
Mammalian taste buds contain three morphologically and functionally distinct cell types (for review, see Chaudhari and Roper, 2010). Type I cells appear to be supporting or glial-like cells (Bartel et al., 2006; Dvoryanchikov et al., 2009). Some of the type I cells may also play a role in salt (Na+) taste (Vandenbeuch et al., 2008; Chandrashekar et al., 2010). Type II (receptor) cells are the primary detectors of sweet, bitter, and umami compounds; they express G-protein-coupled taste receptors and effectors for these taste stimuli (Pérez et al., 2002; Zhao et al., 2003; Clapp et al., 2004; DeFazio et al., 2006). Type III (presynaptic) cells detect sour tastants. Presynaptic cells also are the only taste bud cells showing well-differentiated synapses and expressing synaptic proteins (Yee et al., 2001; DeFazio et al., 2006).
During taste stimulation and after the primary transduction response, the different types of cells in the taste bud interact and process gustatory signals via chemical signaling intrinsic to the taste bud. Taste stimulation triggers receptor cells to secrete ATP and presynaptic cells to release serotonin (5-HT) and norepinephrine (Dvoryanchikov et al., 2007; Huang et al., 2007, 2008; Romanov et al., 2007). ATP appears to be a transmitter between receptor cells and primary afferent nerve fibers (Finger et al., 2005; Huang et al., 2007; Romanov et al., 2007). Both 5-HT and ATP play critical roles in cell-to-cell signaling within the taste bud, establishing positive and negative feedback circuits that shape the afferent signal and may contribute to the coding of sensory information (Roper, 2007; Huang et al., 2009). Other transmitters such as glutamate and acetylcholine also serve in cell–cell communication within the taste bud (Ogura et al., 2007; Vandenbeuch et al., 2010). Additionally, cholecystokinin and neuropeptide Y may function in this capacity (Herness and Zhao, 2009).
In addition to the above transmitters, there is evidence that an inhibitory amino acid transmitter, GABA, figures in taste buds. Early immunocytochemical and autoradiography data revealed GABA in taste cells and gustatory nerve endings in amphibians and rodents (Jain and Roper, 1991; Obata et al., 1997; Nagai et al., 1998). Electrophysiological recordings from sensory ganglion cells that innervate taste buds showed that GABA mainly produces hyperpolarizing responses when applied to the cell body (Koga and Bradley, 2000). This was interpreted as a possible role for GABA as an afferent taste transmitter at the central and/or peripheral sensory endings of these ganglion cells. More recently, patch-clamp recordings have shown that GABA hyperpolarizes cells in rat taste buds (Cao et al., 2009). Those workers proposed that GABA is involved in cell-to-cell communication within taste buds. Responses to GABA can be produced via ionotropic (GABAA) and metabotropic (GABAB) receptors. In different cells, responses to GABA may vary depending on the intracellular concentration of Cl−, the particular receptor subunits expressed, and the signaling pathways within cells.
Our understanding of the role of GABA in taste buds is very limited. The specific taste cells that synthesize and secrete this transmitter and the cells that respond to GABA in cell-to-cell communication are presently very incompletely defined. Importantly, the influence of GABA signaling on the sensory signal itself remains unexplored. Here, we begin to address these questions, focusing on the origin and cellular targets of GABA and its functional effects on the taste-evoked signal.
Materials and Methods
Animals and tissues.
Adult mice of both sexes were used in this study, including C57BL/6J (wild-type) mice and mice from two transgenic strains. In PLCβ2–GFP mice, green fluorescent protein (GFP) is expressed in >95% of all phospholipase Cβ2 (PLCβ2)-expressing (i.e., receptor) cells (Kim et al., 2006), whereas in GAD1–GFP mice, GFP fluorescence is detected in ≈75% of presynaptic cells of taste buds (Chattopadhyaya et al., 2004; Tomchik et al., 2007). Mice were killed by CO2 asphyxiation following National Institutes of Health guidelines, with procedures approved by the University of Miami Animal Care and Use Committee. Taste buds were obtained from lingual and palatal epithelia by enzymatic digestion as outlined below.
Biosensor cells.
Chinese hamster ovary cells expressing P2X2/P2X3 or 5-HT2c receptors, or both served as ATP, 5-HT, or dual biosensors, respectively (Huang et al., 2005, 2007). Biosensors were loaded with fura-2 AM for Ca2+ imaging. We verified that Ca2+ mobilization in biosensors was neither directly elicited nor altered by bath-applied KCl (up to 140 mm), by the taste stimuli used in this study, or by any of the GABA agonists and antagonists (supplemental Fig. S1, available at www.jneurosci.org as supplemental material).
Buffers, drugs, and stimuli.
During the dissection and recording, unless otherwise specified, we bathed tissues and cells in Tyrode's solution, composed of the following (in mm): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, 10 Na-pyruvate, and 5 NaHCO3, pH 7.2 (310–320 mOsm). Muscimol, baclofen, and bicuculline were purchased from Sigma. CGP55845 [(2S)-3-[(1S)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl)(phenylmethyl)phosphinic acid] was purchased from Tocris Bioscience. The taste stimulus mix consisted of cycloheximide (10 μm), denatonium (1 mm), saccharin (2 mm), and the artificial sweetener SC45647 (0.1 mm), i.e., two “bitter” and two “sweet” compounds, respectively.
Ca2+ imaging.
We used two different preparations in this study: (1) dispersed taste buds and single cells removed from vallate epithelium and imaged with fura-2, and (2) semi-intact taste buds embedded in lingual tissue slices and imaged with Calcium Green dextran (CaGD) using confocal microscopy.
Dispersed taste buds or isolated taste cells were prepared as we described previously (Huang et al., 2007). Taste buds or taste cells were transferred to a shallow recording chamber, loaded with 5 μm fura-2 AM, and superfused with buffer. The ratio of F340/F380 was converted to approximate [Ca2+]i as described by Grynkiewicz et al. (1985) using a fura-2 calibration kit (Invitrogen). Taste buds and/or cells were stimulated by bath perfusion of taste mix, ATP, or KCl. All solutions were made in Tyrode's buffer adjusted to pH 7.2. Stimuli were bath-applied for 30 s after which the perfusion was returned to Tyrode's solution. All experiments were conducted at room temperature.
For functional imaging in the lingual slice preparation, vallate taste cells were iontophoretically loaded with CaGD in blocks of tissue containing the vallate papilla. Tissue was sliced and imaged as detailed previously (Caicedo et al., 2000; Dando and Roper, 2009). Lingual slices were perfused at a rate of 2 ml/min at room temperature with Tyrode's buffer containing elevated Ca2+ (8 mm) to improve signal/noise. Taste stimuli were focally applied to the taste pore for 2 s via a focal “puffer” micropipette; KCl and pharmacological agents were bath applied. Images were captured with a confocal microscope (Olympus Fluoview FVx) at 2 s intervals, and responses are presented as changes in relative fluorescence, ΔF/F [i.e., (F − F0)/F0].
In both of the above cell/tissue preparations, we identified receptor (type II) or presynaptic (type III) taste cells by the expression of GFP in PLCβ2–GFP mice (receptor cells) or GAD1–GFP mice (presynaptic cells) or physiologically as follows. Cells that responded to taste stimulation but not to KCl depolarization were classified as receptor cells; cells that responded to KCl depolarization but not taste stimulation were identified as presynaptic cells (DeFazio et al., 2006; Tomchik et al., 2007). The two methods for identifying cell types were mutually consistent.
Reverse transcriptase-PCR analysis.
Total RNA was isolated after DNase I digestion using the Absolutely RNA Nanoprep kit (Stratagene). First-strand cDNA synthesis was with Superscript III (Invitrogen) and PCR (Taq Polymerase; Qiagen) as detailed previously (Dvoryanchikov et al., 2009). Dispersed taste buds were washed individually in Tyrode's buffer to remove contaminating, adherent, non-taste cells. For analyzing single cells, taste buds were dissociated, and GFP-labeled or GFP-lacking individual taste cells were collected. RNA was extracted from individual cells or from pools of 10 similar cells (e.g., GFP positive). The RNA was subjected to T7 linear RNA amplification (Message BOOSTER cDNA kit for qPCR; Epicenter), and the resulting cDNA was used in PCR to assess expression as detailed previously (Dvoryanchikov et al., 2009). PCR primers were designed for each of 19 GABAA subunits currently recognized (Olsen and Sieghart, 2008). PCR primers and conditions are listed in supplemental Table 1 (available at www.jneurosci.org as supplemental material).
Quantitative reverse transcriptase-PCR (RT-PCR) was performed on a Bio-Rad iCycler using cDNA from one taste bud in each reaction (Dvoryanchikov et al., 2009). Synaptosome-associated protein of 25 kDa (SNAP25) mRNA in each tissue sample was used as a reference for normalization.
Immunostaining.
Taste tissues were dissected from mice after perfusion–fixation with 4% paraformaldehyde and were processed for immunostaining as described (Dvoryanchikov et al., 2009). Antibodies used were rabbit anti-GABA (1:1500; A2052; Sigma), rabbit anti-glutamic acid decarboxylase 65 (GAD65) (1:500; AB5082; Millipore), rabbit anti-PLCβ2 (1:1000; SC-1488; Santa Cruz Biotechnology), rabbit anti-NTPDase2 (1:1000; N-1082; J.-P. Sevigny, University of Laval, Laval, QC, Canada), and guinea pig anti-GABAB1 receptor (1:500; AB2256; Millipore). Secondary antibodies were goat anti-rabbit IgG Alexa Fluor 488 (A-11034), goat anti-rabbit IgG Alexa Fluor 568 (A-11011), goat anti-rabbit IgG Alexa Fluor 594 (A-11012), or goat anti-guinea pig IgG Alexa Fluor 594 (A-11076) (all from Invitrogen). When two primary antibodies to different antigens were both raised in rabbit, we prelabeled one of the antibodies with Alexa Fluor 568 or Alexa Fluor 647 using a Zenon IgG labeling kit (Z-25306 or Z-25308; Invitrogen). For GABAB1 immunostaining, antigen retrieval was performed in citrate buffer (Borg Decloaker RTU, BD1000 MM; Biocare Medical) at 95°C for 30 min. Negative control sections incubated without primary antibody were included in each experiment.
Fluorescent and bright-field (with Nomarski differential interference contrast optics) micrographs were captured either on a Carl Zeiss LSM510 or an Olympus Fluoview FV1000 confocal microscope. All images included in a given figure were adjusted in parallel for brightness and contrast.
Results
We examined whether and how GABA affected taste cells isolated from mouse vallate papillae. Isolated taste cells were identified as receptor (type II) or presynaptic (type III) cells based on their responses to taste stimulation and KCl depolarization and on their green fluorescence if obtained from PLCβ2–GFP or GAD1–GFP transgenic mice (see Materials and Methods). Bath-applied GABA (10 μm) did not evoke Ca2+ responses in isolated receptor cells (0 of 6) but did elicit modest changes of cytoplasmic Ca2+ in a minority of single presynaptic cells (6 of 29). We reasoned, however, that if GABA was an inhibitory neurotransmitter, as is the case elsewhere in the nervous system and described for rat taste cells by Cao et al. (2009), one might not necessarily observe significant GABA-evoked Ca2+ mobilization (as indeed the case, shown below). Thus, we turned to other measures of taste bud function to measure the action of GABA.
GABA inhibits transmitter release from taste buds
Using highly sensitive biosensors, we have shown previously that gustatory stimulation elicits 5-HT release from intact taste buds removed from lingual epithelium (Huang et al., 2005). Using that same methodology, we now asked whether GABA alters taste-evoked 5-HT secretion. Indeed, bath-applied GABA (10 μm) markedly suppressed the 5-HT release that was triggered when dispersed taste buds were stimulated with tastants (Fig. 1). To classify the GABA receptor types involved, we repeated the experiments with muscimol and baclofen, agonists at ionotropic (GABAA) or metabotropic (GABAB) receptors, respectively (Fig. 1B,C). Each agonist (at 1 μm) significantly inhibited 5-HT secretion, suggesting that both GABAA and GABAB receptors are present in taste buds (Fig. 1D). There was no significant difference between the depression produced by GABA, muscimol, and baclofen (ANOVA with post hoc Newman–Keuls multiple comparison test).
Figure 1.

GABA inhibits taste-evoked serotonin release from mouse taste buds. Taste buds were isolated from vallate papillae and recorded with biosensors to detect release of serotonin (5-HT). A, Stimulating an isolated taste bud with a bitter–sweet taste mixture (arrows) containing cycloheximide (10 μm), denatonium (1 mm), saccharin (2 mm), and SC45647 (0.1 mm) elicited 5-HT secretion, as shown by the pronounced biosensor response (5-HT-bio). 5-HT secretion was recorded as Δ[Ca2+]i in the serotonin biosensor, measured in nanomolar (see Materials and Methods). Repeating the taste stimulation on the same taste bud in the presence of 10 μm GABA (shaded region, middle trace) showed a marked reduction of 5-HT secretion that was reversed during washout of GABA [GABA did not directly affect 5-HT biosensors (data not shown)]. Similar results were obtained by applying the GABAA receptor agonist muscimol (1 μm) (B) or the GABAB receptor agonist baclofen (C). D, Summary of data from several experiments such as shown in A–C. Taste-evoked serotonin release in the presence of GABA agonists was normalized to serotonin release under control conditions for the same taste bud/biosensor pair (paired Student's t test; *p < 0.05; **p < 0.01). Bars show mean ± SEM. mus, Muscimol (1 μm); bac, baclofen (1 μm); af, after washout of drugs.
Taste stimulation also triggers ATP secretion from taste buds (Finger et al., 2005; Huang et al., 2007; Romanov et al., 2007). Thus, we tested whether GABA inhibits taste-evoked ATP release. We used ATP biosensors to monitor ATP release from dispersed taste buds and found that GABA also significantly reduced taste-evoked ATP secretion (Fig. 2A). Furthermore, both muscimol and baclofen were effective inhibitors of ATP secretion (Fig. 2B,C), reinforcing the notion that GABAA and GABAB receptors influence transduction and/or transmission pathways in mouse taste buds. The inhibitory action of muscimol was blocked by the GABAA-selective antagonist bicuculline (10 μm) (Fig. 2D). Conversely, the action of baclofen was antagonized by the GABAB selective blocker CGP55845 (10 μm) (Fig. 2E). These findings confirm that GABA, muscimol, and baclofen act specifically on GABAA and GABAB receptors in mouse taste cells and are not depressing taste cell responses nonselectively.
Figure 2.
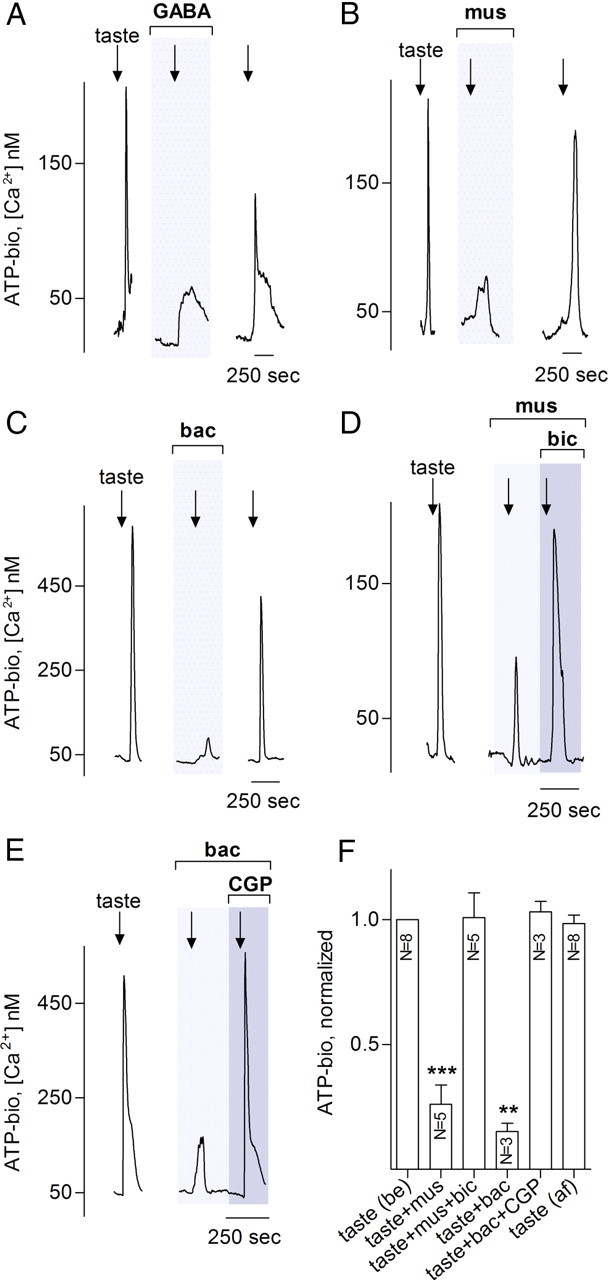
GABA inhibits taste-evoked ATP release from taste buds. Taste stimulation as in Figure 1 evokes ATP release that is reduced by 10 μm GABA (A), 1 μm muscimol (B), or 1 μm baclofen (C). Traces in A–C show responses from an ATP biosensor that was closely apposed to an isolated taste bud (3 different experiments). Arrows show application of taste mixture, and gray shaded area shows bath perfusion with agonists. D, E, Inhibition of ATP release by muscimol or baclofen is eliminated by specific antagonists to the respective GABA receptors. Thus, 10 μm bicuculline, a GABAA receptor antagonist, rescues taste-evoked ATP secretion even in the presence of muscimol (D) and 10 μm CGP55845, a GABAB receptor antagonist, restores ATP release that was blocked by baclofen. Lightly shaded areas in D and E show bath application of the GABA agonist; and darker shaded area shows perfusion of agonist plus antagonist. Data in D and E verify that the GABA agonists were indeed acting on specific GABA receptors, not generally depressing taste bud cells. F, Summary of data from several experiments as in A–E. Bars show means ± SEM of ATP biosensor responses, normalized as in Figure 1. Paired Student's t test compared each taste-evoked response in the presence of GABAergic drugs to the control evoked response in the same taste bud/biosensor pair (paired Student's t test; ns, no significant difference; **p < 0.01). N, Numbers of taste buds tested; mus, muscimol; bic, bicuculline; bac, baclofen; CGP, CGP55845; be, control before drug application; af, after washout of drugs.
Does GABA inhibit receptor cells, presynaptic cells, or both?
The above experiments were conducted on dispersed but otherwise intact taste buds. To identify which taste cells are directly inhibited by GABA, we dissociated taste buds into isolated cells and used biosensors to monitor transmitter secretion from cells identified by type. GABA, muscimol, and baclofen significantly decreased taste-evoked ATP release from receptor cells (Fig. 3A,B). ATP release was inhibited, although none of the agents (GABA, muscimol, or baclofen) altered taste-evoked Ca2+ mobilization in receptor cells. In contrast, transmitter release from presynaptic cells (i.e., 5-HT secretion) was not directly affected by either GABA or GABA agonists (Fig. 3C,D).
Figure 3.
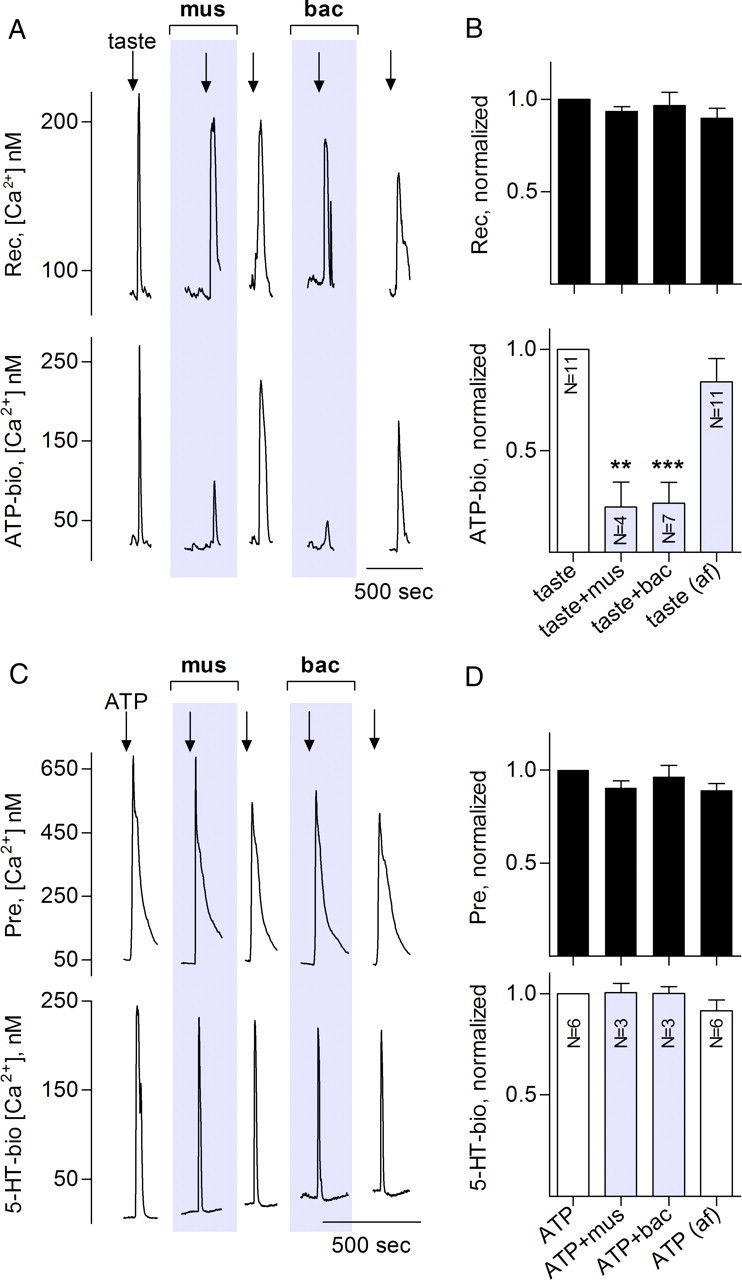
Muscimol and baclofen inhibit taste-evoked transmitter release from receptor (type II) but not presynaptic (type III) taste bud cells. Traces in A and C show recordings from an isolated receptor cell (A) or presynaptic cell (B) and its closely apposed biosensor for detecting transmitter release. A, Top trace, Recordings from a receptor cell; bottom trace, ATP biosensor. Repeated taste stimulation (arrows) produces responses in receptor cells and triggers ATP secretion. ATP secretion is significantly and reversibly reduced in presence of 1 μm muscimol or 1 μm baclofen (shaded areas). B, Summary of data from several experiments such as shown in A. Top bars (black) show mean ± SEM for receptor cell responses; bottom bars, concurrent measurements of ATP release (mean ± SEM). Normalization, analyses, and labels as in Figure 1. GABA reduced ATP release (to 34 ± 7%, n = 3; data not shown) similar to that seen for muscimol or baclofen. C, Sequential responses of a paired presynaptic cell and 5-HT biosensor to repeated stimulation with 1 μm ATP (arrows). The presynaptic cell repetitively releases 5-HT, and this release is unaffected by muscimol or baclofen (shaded areas). D, Summary of several experiments on presynaptic cells, similar to summary shown in B for receptor cells. mus, Muscimol; bic, bicuculline; bac, baclofen; be, control before drug application; af, after washout of drugs.
We infer from these results on isolated taste cells that, in intact, dispersed taste buds, taste-evoked transmitter secretion (both ATP and 5-HT) (Figs. 1, 2) is inhibited primarily by the actions of GABA on receptor cells, as follows:
 |
Consistent with this interpretation, GABA did not reduce 5-HT secretion from taste buds that were stimulated with ATP (Fig. 4A), although taste-evoked 5-HT release in the same taste bud was inhibited (Fig. 4B,C).
Figure 4.
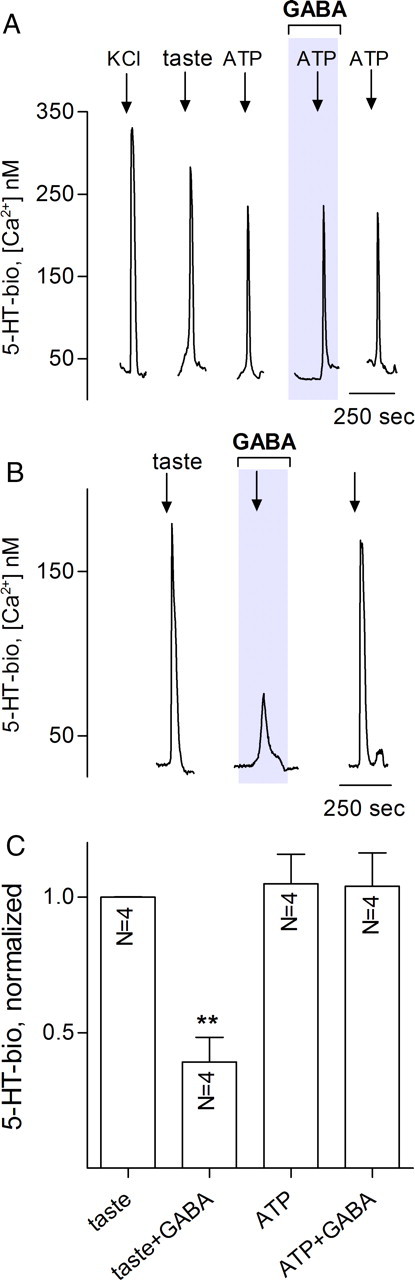
In isolated taste buds, GABA interrupts communication between receptor cells and presynaptic cells. Isolated taste buds were sequentially depolarized with KCl, stimulated with tastants and with ATP, in the absence or presence of 10 μm GABA. A, Traces show stimulus-evoked 5-HT release from presynaptic cells within the taste bud (i.e., responses from a 5-HT biosensor apposed to the taste bud). All stimuli (arrows) elicit 5-HT release as shown previously (Huang et al., 2007). GABA has no effect on ATP-evoked 5-HT release. B, Same taste bud, showing effects of GABA on taste-evoked 5-HT release. Here, presynaptic cells are indirectly triggered to release 5-HT, and GABA-mediated inhibition is consistent with GABA acting to reduce ATP release from receptor cells, thereby interfering with cell–cell excitation of presynaptic cells. C, Summary of several experiments as those in A and B. Details of bars and analyses as in Figure 1.
Taste receptor and presynaptic cells express distinct GABA receptors
As an independent confirmation of GABAergic mechanisms in mouse taste buds, we used RT-PCR on cleanly isolated taste buds to evaluate the expression of all the known subunits of GABAA and GABAB receptors. Taste buds abundantly express mRNAs for several of GABAA subunits, particularly β1, β2, β3, δ, and π, as well as for an obligate subunit of GABAB receptors, GABAB1 (Fig. 5A,D). mRNAs for the remaining GABAA subunits (α1, α2, α3, α4, α5, α6, γ1, γ2, γ3, ρ1, ρ2, ρ3, ε, and θ) were either undetectable or appeared at very low levels and inconsistently across taste bud samples (supplemental Fig. S2, available at www.jneurosci.org as supplemental material). GABA receptor subunits were not expressed to any appreciable level in the non-taste epithelium adjacent to taste buds (Fig. 5A) (supplemental Fig. S2, available at www.jneurosci.org as supplemental material).
Figure 5.

Mouse taste buds express distinct GABAA and GABAB receptors in receptor and presynaptic cells. A, RT-PCR on dissected vallate papillae (va), two preparations of cleanly isolated vallate taste buds (tb1, tb2), enzymatically peeled non-taste lingual epithelium (nt), and in parallel, brain (br), and no cDNA (−) control reactions. Expression was tested for several GABAA receptor and one GABAB receptor subunits. RT-PCR for β-actin and the taste-specific marker PLCβ2 on the same samples validated RNA quality and the presence of taste buds only in the first three samples. Data on additional GABAA subunits are presented in supplemental Figure S2 (available at www.jneurosci.org as supplemental material). B, RT-PCR on pools of isolated receptor cells (type II) and presynaptic cells (see Materials and Methods). Three pools of each class were validated by expression of either PLCβ2 or SNAP25 and then were tested for expression of the GABA receptor subunits shown in A. [Although GABAA β1 is expressed in a taste-selective manner (see A), expression was not sufficiently high to permit detection in a few isolated cells.] Control PCRs are as in A. C, Summary of data from expression profiles of receptor and presynaptic cells in B. Incidence of GABA receptor subunits is represented in a modified, overlapping Venn diagram. For example, all three pools of receptor cells but only one of three pools of presynaptic cells expressed GABAB receptors (blue). Receptor cells expressed several different GABAA subunits (warm colors), whereas presynaptic cells express only the β3 isoform (for details, see Results). D, Immunostaining with anti-GABAB1 shows that all receptor cells (visualized as immunopositive for PLCβ2; green) coexpress GABAB1 receptors (red). Scale bar, 20 μm.
To assess which taste cell types express this limited set of GABA receptors, we tested cDNAs from receptor cells (three pools, each pool containing 10 cells) and presynaptic cells (also three pools, each with 10 cells). These pools were obtained by harvesting GFP-positive taste cells after isolating and dissociating taste buds from PLCβ2–GFP mice (receptor cells) or GAD67–GFP mice (presynaptic cells). The homogeneity of each pool of cells was verified by RT-PCR for two diagnostic genes: PLCβ2 (for receptor cells) and SNAP25 (for presynaptic cells). Receptor cell pools consistently expressed GABAB1. In addition, GABAA subunits β2, δ, and π were detected in some but not all pools of receptor cells. Of the three pools of presynaptic cells, two expressed GABAA β3, and one of these also expressed GABAB1 (Fig. 5B). When expression levels for a particular gene are low, the mRNA is inconsistently detected by single-cell RT-PCR. Thus, the uneven pattern of expression of GABAA subunits in both cell types may reflect either low mRNA abundance or heterogeneity in the particular subunits expressed in each cell. GABAB subunits, conversely, were detected in all receptor cell pools, suggesting that most receptor cells express the metabotropic receptor for GABA. These RT-PCR data on the expression pattern of GABA subunits are summarized in Figure 5C.
We also performed immunofluorescence on mouse taste tissue for the one subunit most consistently detected by RT-PCR, GABAB1. Consistent with our single-cell RT-PCR data, we detected GABAB1 in all receptor cells and also at lower intensity in additional, unidentified cells (Fig. 5D, arrow).
In summary, receptor cells express GABAA and GABAB receptor subunits, consistent with the calcium imaging data. GABAB subunits are prominently expressed in receptor cells but are also seen, although less frequently, in presynaptic cells. GABAA subunits are expressed at lower levels, or in subsets of cells: receptor cells heterogeneously express β2, δ and π, whereas presynaptic cells express β3 (Fig. 5C).
GABA inhibits cell–cell communication in the lingual slice preparation
As an additional investigation of GABAergic inhibition in mouse taste buds and to verify the above findings in a more intact preparation, we tested whether GABA affects taste-evoked responses in lingual slices. For this, we recorded taste- and depolarization-evoked responses using confocal Ca2+ imaging. Receptor and presynaptic taste cells were identified by their Ca2+ responses to a tastant mix or to KCl, as above (DeFazio et al., 2006; Tomchik et al., 2007). Consistent with the data from isolated taste cells (above), taste-evoked responses in receptor cells were unaltered by bath-applied GABA (100 μm) (Fig. 6A,B). However, Ca2+ responses in presynaptic cells (i.e., “downstream” of receptor cells) were significantly reduced by GABA (Fig. 6C,D). These data strongly support the interpretation that GABA inhibits taste-triggered and ATP-mediated communication from receptor to presynaptic cells.
Figure 6.
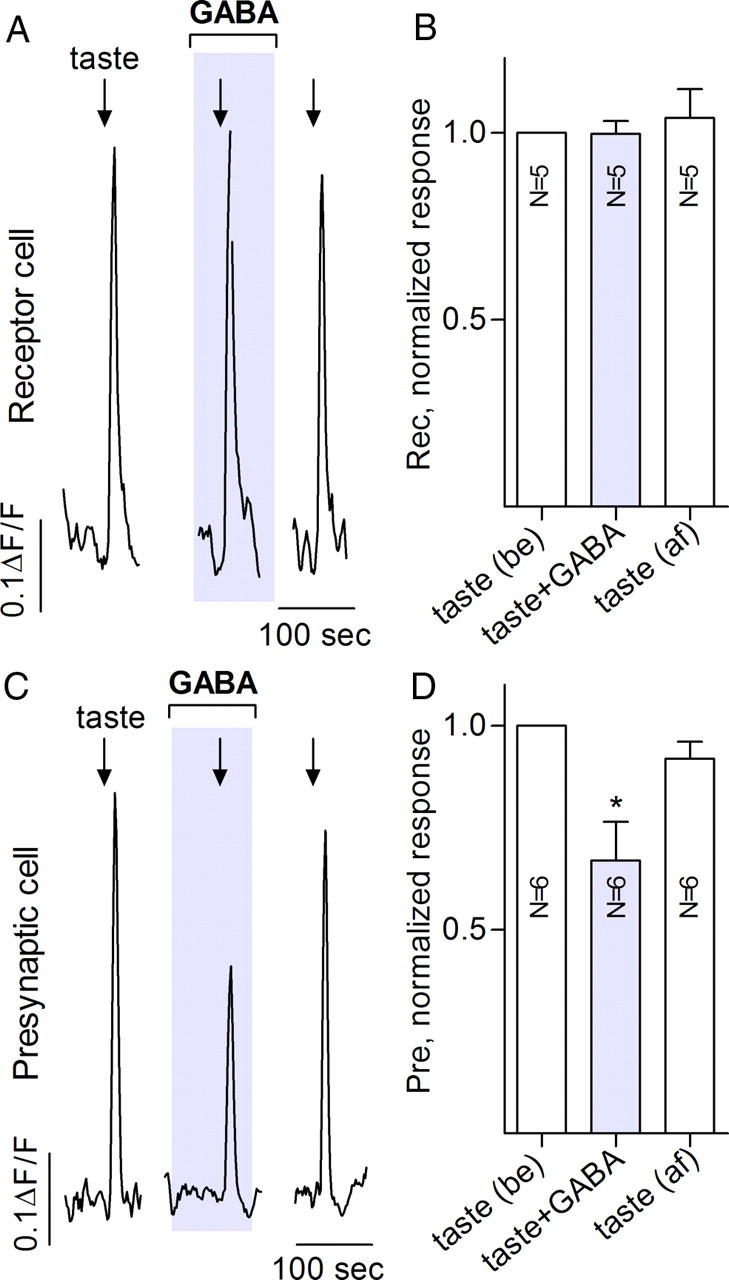
When tested in a semi-intact preparation, GABA inhibits communication between receptor and presynaptic taste bud cells. Traces show Ca imaging from vallate taste cells in a lingual slice preparation. Receptor cells (A, B) were identified as responding to taste mixture but not 50 mm KCl. Presynaptic cells (C, D) were identified as responding to both the taste mixture and KCl. A, Focally applied taste mixture (arrow) evoked responses in a receptor cell. Taste-evoked receptor cell Ca responses were not significantly affected by bath application of 100 μm GABA (shaded area). B, Summary of data from five experiments as in A. Bars are means ± SEM responses normalized to the group mean for taste responses before (be) GABA. C, In contrast, 100 μm GABA significantly reduces taste-evoked responses recorded from a presynaptic cell. D, Summary of six experiments such as shown in C. Details of bars as in B. Calibration: A, C, 100 s, 0.1 ΔF/F0. be, Control before drug application; af, after washout of drugs.
GABA is synthesized in glial-like (type I) and presynaptic (type III) taste bud cells
We next investigated potential sources of GABA in mouse taste buds. In neurons, GABA is synthesized by GAD, which exists as one of two isoforms: GAD65 (GAD2) or GAD67 (GAD1) (Soghomonian and Martin, 1998). Using RT-PCR, we found that both GAD65 and GAD67 are abundant in taste buds across the oral cavity, including vallate, foliate, fungiform, and palate. In contrast, non-taste lingual epithelium did not express GAD65 at all, whereas GAD67 was detectable in some samples at low abundance (Fig. 7A). The presence of GAD67 mRNA in non-taste samples may derive from small amounts of contaminating Von Ebner's glands, which have been reported to express GAD67 (Kosuge et al., 2009). With real-time RT-PCR, we found that, in taste buds, both the synthetic enzymes are expressed at levels similar to SNAP25, a prominent mRNA selectively expressed in taste buds (supplemental Fig. S3, available at www.jneurosci.org as supplemental material).
Figure 7.
GABA is synthesized and accumulates in both type I and presynaptic (type III) taste bud cells. A, RT-PCR for GAD subtypes in enzymatically peeled epithelium containing taste buds from four oral taste fields: vallate (va), foliate (fo), fungiform (fu), and palate (pa). Both GAD65 and GAD67 are expressed in all fields and little, if at all, in peeled non-taste lingual epithelium (nt). Control reactions include no cDNA (−) and brain cDNA (br). B, Taste bud cells were subjected to single-cell RT-PCR, first to test for expression of NTPDase2, PLCβ2, and SNAP25. Of >50 cells tested, none expressed more than one of these three cell-type markers. The expression of GAD67 and GAD65 in each cell was then tested. The gel shows three representative cells of each type. No expression of GAD65 or GAD67 was detected in receptor cells (Plcβ2+). C, Summary of single-cell profiling of 45 cells as in B shows that GAD65 is only expressed in type I (glial-like) cells, whereas GAD67 is only expressed in presynaptic cells. Receptor (type II) cells do not express either GABA-synthesizing enzyme. D–G, Double immunofluorescence for GABA or its synthetic enzymes and markers for type I, receptor, and presynaptic cells in mouse vallate taste buds. D, GAD65 immunoreactivity (red) is in cells that express NTPDase2 (green), a marker for type I cells. E, GFP fluorescence (green) in taste buds from GAD67–GFP transgenic mice is in cells distinct from cells that are immunoreactive for GAD65 (red). F, GABA immunoreactivity (red) in vallate taste buds from a GAD67–GFP mouse occurs in cells displaying GFP fluorescence (green) as well as in cells that are immunoreactive for NTPDase2 (blue). That is, GABA accumulates only in GAD67-positive type III cells and in NTPDase2-positive type I cells. G, In contrast, GFP fluorescence (green) in taste buds from PLCβ2–GFP transgenic mice does not colocalize with GABA immunostaining, confirming that receptor (PLCβ2+) cells do not synthesize or store GABA.
To examine which taste cells express GAD isoforms and to test whether both GAD65 and GAD67 are present in the same cells, we used single-cell RT-PCR and immunofluorescence microscopy. Individually isolated cells were classified according to their expression of NTPDase2 (glial-like, type I), PLCβ2 (receptor, type II), or SNAP25 (presynaptic, type III). Type I glial-like cells (10 of 17, i.e., ≈ 60%) prominently expressed GAD65, whereas only 1 of 18 (≈6%) presynaptic cells were positive for GAD65 (Fig. 7B,C). In contrast, only presynaptic cells (15 of 18, i.e., 83%) expressed GAD67. None of the receptor cells tested expressed either isoform of GAD. These expression patterns of GAD67 and GAD65 across the three cell types were significantly different from each other (χ2 test, p ≤ 0.0002).
We extended our single-cell RT-PCR results on GAD isoforms using immunofluorescence microscopy. We have shown previously (DeFazio et al., 2006; Tomchik et al., 2007) that GAD67 is expressed only in type III presynaptic cells. As shown in Figure 7D, GAD65 immunoreactivity substantially colocalized with NTPDase2, a marker for type I glial-like taste cells. We further examined GAD65 expression in taste buds from two well-characterized strains of transgenic mice, PLCβ2–GFP (in which receptor cells are labeled) and GAD67–GFP, in which most presynaptic cells are labeled. GAD65 was lacking from GFP-positive taste cells in both GAD67–GFP tissue [i.e., from presynaptic cells (Fig. 7E)] and PLCβ2–GFP tissue [i.e., receptor cells (data not shown)]. These observations are fully consistent with the non-overlapping distribution of GAD65 and GAD67 seen in our single-cell RT-PCR data (Fig. 7B).
Does GABA accumulate in the cells that express GAD65 or GAD67? Using anti-GABA antibodies, we observed GABA immunofluorescence in several cells in vallate, fungiform, and palate taste buds (Fig. 7F,G) (supplemental Fig. S4, available at www.jneurosci.org as supplemental material). Using taste buds in which receptor or presynaptic cells are marked with GFP and by immunostaining for cell-type markers, we determined that GABA-immunoreactive cells were either NTPDase2 expressing (i.e., type I) or GAD67 expressing [i.e., presynaptic cells (Fig. 7F)]. Receptor cells (PLCβ2-positive or PLCβ2–GFP) were never seen to accumulate GABA (Fig. 7G), consistent with their lack of GABA-synthesizing enzymes. We noted that occasional GAD67–GFP-positive presynaptic cells did not accumulate GABA; we did not explore this further. In vallate taste buds, there are relatively large numbers of presynaptic cells (Dvoryanchikov et al., 2007), and GABA may be secreted from either type I or presynaptic cells. Because fungiform and palatal taste buds contain few presynaptic cells (supplemental Fig. S4, available at www.jneurosci.org as supplemental material), type I glial-like cells may constitute the main stores of GABA in these taste buds.
Discussion
Results presented here document that GABA is an inhibitory transmitter in mouse vallate taste buds. A prominent action of GABA is to reduce transmitter (ATP) secretion from receptor cells by acting on GABAA and GABAB receptors expressed by those cells. GABA is synthesized by and found in type I (glial-like) and presynaptic (type III) taste cells. These findings suggest that GABA is an important taste bud transmitter, likely released from type I and presynaptic taste cells during gustatory activation of taste buds and modulating the signal output from these peripheral sensory organs (Fig. 8).
Figure 8.
Schematic diagram of GABAergic inhibition in taste buds. The three functional taste cell types are illustrated. Sweet, bitter, or umami stimuli evoke Ca2+ mobilization in receptor cells, leading to ATP secretion. This ATP stimulates both afferent fibers (data not shown) and adjacent presynaptic cells that then secrete 5-HT. GABA, released from presynaptic, type I cells, or both activates GABAA and GABAB receptors (gray and black) on receptor cells and reduces the taste-evoked secretion of ATP. The trigger(s) for GABA secretion and signaling events downstream of GABA receptor activation remain to be elucidated. For clarity, GABA receptors on non-receptor cells are not shown.
ATP release from receptor cells requires the combined actions of a membrane depolarization (mediated by TrpM5) concurrent with an increased intracellular Ca2+, mediated by inositol 1,4,5-trisphosphate-triggered release from stores (Huang and Roper, 2010). These two factors act in concert to open Px1 gap junction hemichannels and allow ATP efflux. GABA presumably inhibits taste-evoked ATP secretion by hyperpolarizing receptor cells (i.e., countering TrpM5-mediated depolarization), an action not readily measured by Ca2+ imaging. This interpretation is entirely consistent with recent patch recordings in rat taste buds that showed that activating GABA receptors in taste cells increased membrane Cl− and K+ conductances, thereby stabilizing or even hyperpolarizing receptor cells (Cao et al., 2009). Our study confirms some of the previous findings and significantly extends them. Although Cao et al. (2009) demonstrated the accumulation of GABA and the expression of GABAA and GABAB receptors in rat taste buds, these entities were not specifically localized to functionally identified cellular populations. Importantly, because Cao et al. (2009) did not discriminate GAD65 from GAD67, the two source cell populations for GABA were not apparent. The expression of GABAergic proteins in taste buds from mice and rats bear overall similarities. Importantly, our data now provide a more complete explanation for the role of GABA in taste buds, to inhibit taste-evoked transmitter secretion from receptor cells. The significance of such inhibition in the intact system remains to be addressed. It is possible that GABAergic inhibition is used in shaping responses to one or other of the taste qualities (sweet, bitter) in the mix we used. Alternatively, secreted GABA may tonically set the resting potential or responsivity of taste cells. In neurons, such tonic inhibition is mediated through extrasynaptic GABAA receptors, which typically include the δ subunit (Belelli et al., 2009). We note that we detected δ subunits in receptor cells. Finally, because GABA may be secreted from presynaptic or type I cells (or both), it remains unclear which cell type is responsible for GABA secretion during taste stimulation. Our future experiments will address these questions.
Previous studies (Cao et al., 2009; Starostik et al., 2010) have examined GABA receptor subunits in taste tissues of rodents. Our semiquantitative RT-PCR data suggest that expression levels for most GABA subunits are relatively or very low in taste cells. This may account for some of the apparent discrepancies across data in these three reports. Furthermore, our comparisons between isolated taste buds and dissected taste papillae (Fig. 5) (supplemental Fig. S2, available at www.jneurosci.org as supplemental material) suggest that many of the GABA receptor subunits may be expressed in cells underneath the taste epithelium (e.g., nerves). Nevertheless, we and Starostik et al. (2010) both find that GABAA β3 is a relatively abundant subunit in taste buds; all three reports show the presence of GABAB receptors.
We note that muscimol, baclofen, and GABA all produced similar levels of inhibition (Figs. 1D, 3B). These results may suggest that signals from GABAA and GABAB receptors in taste cells converge on a common step that is upstream of the transmitter release. Thus, a saturating dose of any one agonist would produce maximum inhibition. Furthermore, receptor cells express both GABAA and GABAB subunits and ATP secretion from receptor cells is the primary target of GABAergic inhibition (Fig. 3A,B). That is, the taste cells that express GABA receptors prominently are the ones that are obviously affected by GABA.
Although both receptor (type II) and presynaptic (type III) cells express GABAA receptors, we only detected GABAergic inhibition of transmitter secretion from receptor cells. This may suggest that GABA has additional functions in mouse taste buds. For instance, presynaptic cells express principally GABAA β3 receptors. The β3 subunit has been shown to be important in the development of the palate (Hagiwara et al., 2003). Indeed, there is by now substantial evidence that GABA acts as a trophic factor and modulates cell proliferation and synaptic formation during neuronal development (Owens and Kriegstein, 2002). One might speculate that the action of GABA on presynaptic cells is related to the development and maturation of these taste bud cells and their synapses, as is the case in the CNS (Wang and Kriegstein, 2009).
Our results in mice, combined with those of Cao et al. (2009) in rats, firmly establish GABA as an inhibitory transmitter in taste buds. However, the implication of this for how animals discriminate sweet, sour, salty, etc. and how taste behavior is affected by GABAergic mechanisms is unclear. Our data indicate that GABA is synthesized and stored in specific taste cells: GABA-biosynthetic enzymes are found in type I glial-like cells (GAD65) and presynaptic (type III) cells (GAD67). Although neurons have long been known to secrete GABA as a transmitter, recent evidence indicates that many glia also synthesize and secrete GABA (Jow et al., 2004), and, indeed, GABA is recognized as a significant “gliotransmitter” (Angulo et al., 2008). Taste preference experiments have been conducted on GAD65 knock-out mice (Shimura et al., 2004), but the findings have no straightforward interpretation. Namely, GAD65 knock-out mice did not differ from wild-type mice in taste preferences for sucrose, NaCl, HCl, or quinine when these solutions were presented alone. However, GAD65 knock-out versus wild-type mice responded differently to binary taste mixtures, notably sucrose plus quinine. The authors concluded that GABA (from GAD65) is not involved in basic taste discrimination, per se, but is instead involved in signal processing for more complex information such as taste mixtures. A major complication in interpreting these data is that knocking out GAD65 also interrupts GABAergic synapses in the CNS, not just the actions of GABA (including trophic, if any) in taste buds. This may have profound effects on all behaviors, including taste preference and discrimination ability. Taste behavioral assays on GAD67 knock-out mice have not been conducted; the genetic mutation is lethal at birth. In addition to the genetic studies, pharmacological investigations have revealed that GABAergic drugs, such as benzodiazepines, do indeed influence taste preferences (Cooper, 1989). Again, there is the caveat that these drugs are inevitably exerting powerful CNS actions. In short, given its inhibitory effects in taste buds, it is likely that GABA plays a distinct peripheral role in taste reception and signaling (in addition to the aforementioned role in development). However, pinpointing whether and how GABA is released from type I glial-like cells, presynaptic cells, or both during taste reception has not been undertaken, and it remains to be determined what overall effects this inhibitory transmitter exerts in taste reception.
Footnotes
This work was supported by National Institutes of Health/National Institute on Deafness and Other Communication Disorders Grants R01DC7630 (S.D.R.), R01DC374 (S.D.R.), and R01DC6308 (N.C.).
The authors declare no competing financial interests.
References
- Angulo MC, Le Meur K, Kozlov AS, Charpak S, Audinat E. GABA, a forgotten gliotransmitter. Prog Neurobiol. 2008;86:297–303. doi: 10.1016/j.pneurobio.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Bartel DL, Sullivan SL, Lavoie EG, Sévigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol. 2006;497:1–12. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Jafri MS, Roper SD. In situ Ca2+ imaging reveals neurotransmitter receptors for glutamate in taste receptor cells. J Neurosci. 2000;20:7978–7985. doi: 10.1523/JNEUROSCI.20-21-07978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Zhao FL, Kolli T, Hivley R, Herness S. GABA expression in the mammalian taste bud functions as a route of inhibitory cell-to-cell communication. Proc Natl Acad Sci U S A. 2009;106:4006–4011. doi: 10.1073/pnas.0808672106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190:285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. J Comp Neurol. 2004;468:311–321. doi: 10.1002/cne.10963. [DOI] [PubMed] [Google Scholar]
- Cooper SJ. Benzodiazepine receptor-mediated enhancement and inhibition of taste reactivity, food choice, and intake. Ann N Y Acad Sci. 1989;575:321–336. doi: 10.1111/j.1749-6632.1989.tb53253.x. discussion 336–337. [DOI] [PubMed] [Google Scholar]
- Dando R, Roper SD. Cell-to-cell communication in intact taste buds through ATP signalling from pannexin 1 gap junction hemichannels. J Physiol. 2009;587:5899–5906. doi: 10.1113/jphysiol.2009.180083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 2006;26:3971–3980. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoryanchikov G, Tomchik SM, Chaudhari N. Biogenic amine synthesis and uptake in rodent taste buds. J Comp Neurol. 2007;505:302–313. doi: 10.1002/cne.21494. [DOI] [PubMed] [Google Scholar]
- Dvoryanchikov G, Sinclair MS, Perea-Martinez I, Wang T, Chaudhari N. Inward rectifier channel, ROMK, is localized to the apical tips of glial-like cells in mouse taste buds. J Comp Neurol. 2009;517:1–14. doi: 10.1002/cne.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hagiwara N, Katarova Z, Siracusa LD, Brilliant MH. Nonneuronal expression of the GABA(A) beta3 subunit gene is required for normal palate development in mice. Dev Biol. 2003;254:93–101. doi: 10.1016/s0012-1606(02)00030-1. [DOI] [PubMed] [Google Scholar]
- Herness S, Zhao FL. The neuropeptides CCK and NPY and the changing view of cell-to-cell communication in the taste bud. Physiol Behav. 2009;97:581–591. doi: 10.1016/j.physbeh.2009.02.043. [DOI] [PubMed] [Google Scholar]
- Huang YA, Roper SD. Intracellular Ca2+ and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells. J Physiol. 2010;588:2343–2350. doi: 10.1113/jphysiol.2010.191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, Roper SD. Norepinephrine is coreleased with serotonin in mouse taste buds. J Neurosci. 2008;28:13088–13093. doi: 10.1523/JNEUROSCI.4187-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Dando R, Roper SD. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci. 2009;29:13909–13918. doi: 10.1523/JNEUROSCI.2351-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, Wu D, Roper SD. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci. 2005;25:843–847. doi: 10.1523/JNEUROSCI.4446-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Roper SD. Immunocytochemistry of gamma-aminobutyric acid, glutamate, serotonin, and histamine in Necturus taste buds. J Comp Neurol. 1991;307:675–682. doi: 10.1002/cne.903070412. [DOI] [PubMed] [Google Scholar]
- Jow F, Chiu D, Lim HK, Novak T, Lin S. Production of GABA by cultured hippocampal glial cells. Neurochem Int. 2004;45:273–283. doi: 10.1016/j.neuint.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Kim JW, Roberts C, Maruyama Y, Berg S, Roper S, Chaudhari N. Faithful expression of GFP from the PLCbeta2 promoter in a functional class of taste receptor cells. Chem Senses. 2006;31:213–219. doi: 10.1093/chemse/bjj021. [DOI] [PubMed] [Google Scholar]
- Koga T, Bradley RM. Biophysical properties and responses to neurotransmitters of petrosal and geniculate ganglion neurons innervating the tongue. J Neurophysiol. 2000;84:1404–1413. doi: 10.1152/jn.2000.84.3.1404. [DOI] [PubMed] [Google Scholar]
- Kosuge Y, Kawaguchi M, Sawaki K, Okubo M, Shinomiya T, Sakai T. Immunohistochemical study on GABAergic system in salivary glands. Eur J Pharmacol. 2009;610:18–22. doi: 10.1016/j.ejphar.2009.02.043. [DOI] [PubMed] [Google Scholar]
- Nagai T, Delay RJ, Welton J, Roper SD. Uptake and release of neurotransmitter candidates, [3H]serotonin, [3H]glutamate, and [3H]gamma-aminobutyric acid, in taste buds of the mudpuppy, Necturus maculosus. J Comp Neurol. 1998;392:199–208. [PubMed] [Google Scholar]
- Obata H, Shimada K, Sakai N, Saito N. GABAergic neurotransmission in rat taste buds: immunocytochemical study for GABA and GABA transporter subtypes. Brain Res Mol Brain Res. 1997;49:29–36. doi: 10.1016/s0169-328x(97)00118-6. [DOI] [PubMed] [Google Scholar]
- Ogura T, Margolskee RF, Tallini YN, Shui B, Kotlikoff MI, Lin W. Immuno-localization of vesicular acetylcholine transporter in mouse taste cells and adjacent nerve fibers: indication of acetylcholine release. Cell Tissue Res. 2007;330:17–28. doi: 10.1007/s00441-007-0470-y. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Pérez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26:657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper SD. Signal transduction and information processing in mammalian taste buds. Pflugers Arch. 2007;454:759–776. doi: 10.1007/s00424-007-0247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura T, Watanabe U, Yanagawa Y, Yamamoto T. Altered taste function in mice deficient in the 65-kDa isoform of glutamate decarboxylase. Neurosci Lett. 2004;356:171–174. doi: 10.1016/j.neulet.2003.11.041. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol Sci. 1998;19:500–505. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- Starostik MR, Rebello MR, Cotter KA, Kulik A, Medler KF. Expression of GABAergic receptors in mouse taste receptor cells. PLoS One. 2010;5:e13639. doi: 10.1371/journal.pone.0013639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 2007;27:10840–10848. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci. 2008;9:1. doi: 10.1186/1471-2202-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Tizzano M, Anderson CB, Stone LM, Goldberg D, Kinnamon SC. Evidence for a role of glutamate as an efferent transmitter in taste buds. BMC Neurosci. 2010;11:77. doi: 10.1186/1471-2202-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Kriegstein AR. Defining the role of GABA in cortical development. J Physiol. 2009;587:1873–1879. doi: 10.1113/jphysiol.2008.167635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee CL, Yang R, Böttger B, Finger TE, Kinnamon JC. “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol. 2001;440:97–108. doi: 10.1002/cne.1372. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]




