Abstract
Objective
To present the specific public health indicators recently developed by EUROCAT that aim to summarise important aspects of the public health impact of congenital anomalies (CA) in a few quantitative measures.
Methods
The six indicators are: 1) CA Perinatal Mortality, 2) CA Prenatal Diagnosis Prevalence, 3) CA Termination of pregnancy, 4) Down Syndrome livebirth prevalence, 5) CA Paediatric Surgery and 6) Neural Tube Defect total prevalence. Data presented for this report pertained to all cases (live births, foetal deaths or stillbirths after 20 weeks of gestation and terminations of pregnancy for foetal anomaly) of CA from 27 full member registries of EUROCAT that could provide data for at least three years during the period 2004–2008. Prevalence of anomalies, prenatal diagnosis, terminations of pregnancy for foetal anomaly (TOPFA), paediatric surgery and perinatal mortality were calculated per 1,000 births.
Results
The overall perinatal mortality was approximately 1.0 per 1,000 births for EUROCAT registries with almost half due to foetal and the other half due to first week deaths. There were wide variations in perinatal mortality across the registries with the highest rates observed in Dublin and Malta, registries in countries where TOPFA are illegal, and in Ukraine. The overall perinatal mortality across EUROCAT registries slightly decreased between 2004 and 2008 due to a decrease in first week deaths. The prevalence of TOPFA was fairly stable at about 4 per 1,000 births. There were variations in live birth prevalence of cases typically requiring surgery across the registries; however for most registries this prevalence was between 3 and 5 per 1,000 births. Prevalence of NTD decreased by about 10% from 1.05 in 2004 to 0.94 per 1,000 in 2008.
Conclusion
It is hoped that by publishing the data on EUROCAT indicators, the public health importance of congenital anomalies can be clearly summarised to policy makers, the need for accurate data from registries emphasized, the need for primary prevention and treatment services highlighted, and the impact of current services measured.
Keywords: Abortion, Eugenic; Congenital Abnormalities; epidemiology; surgery; Down Syndrome; epidemiology; Europe; epidemiology; Female; Fetal Death; epidemiology; Health Status Indicators; Humans; Malta; epidemiology; Neural Tube Defects; epidemiology; Perinatal Mortality; Pregnancy; Prenatal Diagnosis; methods; Public Health; Registries; statistics & numerical data; Stillbirth; epidemiology; Ukraine; epidemiology
Introduction
Public health indicators are increasingly proposed for different diseases or disease groups, as well as, various risk factors and health outcomes1–4. The terminology “public health indicator” is not precisely or consistently defined, but generally refers to a quantitative summary to guide public health policy and to measure in gross terms the effect of policy interventions. According to the European Commission “an indicator is a quantitative or qualitative measure of how close we are to achieving a set goal (policy outcome). They help us analyse and compare performance across population groups or geographic areas, and can be useful for determining policy priorities. Health indicators based on reliable, comparable data are essential for designing strategies and policies to improve the health of Europeans, and then monitoring their implementation.” (http://ec.europa.eu/health/indicators/policy/index_en.htm)
One body of indicators has aimed to measure burden of illness, as well as, the impact of health services and interventions in terms of a common “currency” such as Quality-Adjusted Life Years (QALYs) or Disability-Adjusted Life Years (DALYs)1. This allows comparisons to be made across diseases or interventions. There are also disease-specific or risk factor or intervention specific indicators, which do not aim at a common currency of expression but allow the tracking of change over time, and differences between places or population subgroups.
The European Community Health Indicators (ECHI) consist of over 40 core indicators covering demographic and socioeconomic factors, health status, health determinants, and health interventions and services. The list is limited by the availability of comparable data in different European countries but is set to grow. (http://ec.europa.eu/health/indicators/echi/index_en.htm). Specific public health indicators have also been proposed in Europe for perinatal conditions (http://www.europeristat.com/) and in particular for rare diseases.4 However, these indicators do not reflect broader issues related to overall health outcomes and interventions for congenital anomalies or those related to certain “key” anomalies such as Down syndrome.
In this paper, we present the specific public health indicators recently developed by the European network for the surveillance of congenital anomalies, EUROCAT (http://www.eurocat-network.eu/) as part of its work programme of 2007–2010. These indicators aim to summarise important aspects of the public health impact of congenital anomalies in a few quantitative measures.
Materials and Methods
Indicator definitions
Indicators were proposed by the EUROCAT Central Registry to the EUROCAT Project Management Committee for discussion and agreement. One constraint was that the information on which the indicator was based should be available within the EUROCAT standard dataset collected by registries. The following list was agreed on:
CA Perinatal Mortality due to congenital anomaly (CA): Perinatal mortality associated with a congenital anomaly (per 1,000 births), defined as first week deaths plus late foetal deaths or stillbirths from 20 weeks gestation (excluding pregnancy terminations for foetal anomaly). This serves as an indicator of mortality burden.
CA Prenatal Diagnosis Prevalence. Prevalence (per 1,000 births) of prenatally diagnosed cases of congenital anomaly. The indicator is split into chromosomal and non-chromosomal since these have different screening methods. This serves as an indicator of the degree to which prenatal screening services are detecting cases of congenital anomaly.
CA Termination of pregnancy: Prevalence (per 1,000 births) of terminations of pregnancy for foetal anomaly (TOPFA). The indicator is split into chromosomal and non-chromosomal since these have different screening methods, and a different proportion of prenatally diagnosed cases leading to TOPFA. This serves as an indicator of the degree to which terminations of pregnancy are the choice following prenatal diagnosis, and the degree to which terminations of pregnancy may be affecting perinatal mortality and livebirth prevalence.
Down Syndrome livebirth prevalence. Live born Down syndrome cases (per 1,000 births). Since specific prenatal screening programmes have been established in many European countries for this anomaly, this specific indicator gives the combined effect of delayed childbearing and prenatal screening policy and termination of pregnancy.
CA Paediatric Surgery: Prevalence of selected malformations usually requiring surgery (per 1,000 births). Since liveborn CA differ in severity and consequences for services, this indicator relates to the public health impact in terms of need for surgery. Total prevalence is given, broken down by type of birth (livebirths, foetal deaths and TOPFA) to show in particular the impact that TOPFA may have on the number of live born surgical cases.
Neural Tube Defect total prevalence. Total prevalence of neural tube defects (per 1,000 births). Since many countries have focused their primary preventive efforts on prevention of NTD by folic acid supplementation, this indicator allows the success of these preventive programmes to be measured.
Source of Data
We used data on all cases (live births, foetal deaths or stillbirths after 20 weeks of gestation and terminations of pregnancy for foetal anomaly) of congenital anomalies from 27 full member population-based registries of EUROCAT (http://www.eurocat-network.eu/) that could provide data for at least three years during the period 2004–2008. Data for the indicators presented here correspond to the entire study period except for registries from Mainz, Wielkopolska, Barcelona, Basque Country (all 2004–2007), Norway (2004–2006) and Ukraine (2005–2008), based on availability of data from different registries (see Figure 1a for complete list of the registries and the countries they represent).
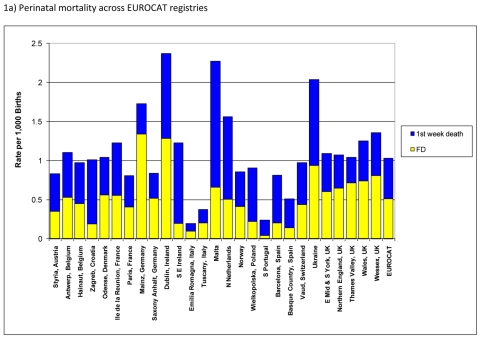
Figure 1.
Perinatal mortality (foetal deaths (FD) + 1st week deaths) per 1000 births, EUROCAT Registries, 2004 – 2008
Data analysis
A congenital anomaly was any case with one or more codes in the Q chapter of ICD10 plus a very limited set of conditions coded outside the Q chapter, namely D215, D821, D1810, P350, P352 and P371. Foetal deaths included stillbirths and any spontaneous abortion from 20 weeks gestation but excluded terminations of pregnancy for foetal anomaly. Termination of Pregnancy for Foetal Anomaly refers to cases where prenatal diagnosis of a congenital anomaly is made in a live foetus and the pregnancy is then terminated. Prenatal Diagnosis was defined as suspicion of a major congenital anomaly (excluding soft markers) in a live foetus. Cases with surgery included those who had surgery performed in the first year of life (or were expected to have surgery at the appropriate age) for one of more of the registered congenital anomalies.
Prevalence of anomalies, prenatal diagnosis, terminations of pregnancy for foetal anomaly (TOPFA), paediatric surgery and perinatal mortality were calculated per 1,000 births. Prevalence of prenatal diagnosis was calculated for all anomalies but also broken down by cases prenatally diagnosed for a chromosomal condition, those prenatally diagnosed for a non-chromosomal condition, those not prenatally diagnosed and those for which timing of diagnosis (i.e., whether the diagnosis was pre or postnatal) was not known. Prevalence of TOPFA was divided into chromosomal and non-chromosomal cases and prevalence of perinatal mortality was divided into foetal and early neonatal (within first week of life) deaths. Livebirth prevalence of Down syndrome was analysed by maternal age groups (< 35 vs. ≥ 35 years or maternal age unknown) and prevalence of neural tube defects (NTD) by type of birth (pregnancy termination/foetal death/live birth. As noted above, our main indicator for NTD is the total prevalence, which is the most relevant variable for evaluating prevention policies related to folic acid. However, we also show the NTD prevalence data by type of birth, as it may be helpful to know the specific changes in the live birth prevalence of NTD vs. those in foetal deaths or TOPFA.
The analysis of the anomalies typically requiring surgery was based on previous work that we had done on rates of surgery per subgroup. Based on data from Odense and Vaud registries for 2005–2006, we selected the anomalies that had high rates of surgery performed (or expected to be performed) and high proportions of livebirths. Hence, six EUROCAT subgroups (see EUROCAT Guide 1.3 available on the website(http://www.eurocat-network.eu/ABOUTUS/DataCollection/GuidelinesforRegistration/Guide1_3InstructionManual) : severe CHD (includes: single ventricle, hypoplastic left heart, hypoplastic right heart, Ebstein’s anomaly, tricuspid atresia, pulmonary valve atresia, common arterial truncus, atrioventricular septal defects, aortic valve atresia/stenosis, transposition of great vessels, tetralogy of Fallot, total anomalous pulmonary venous return and coarctation of aorta), omphalocele, gastroschisis, digestive system (includes: oesophageal atresia with or without tracheo-oesophageal fistula, duodenal atresia or stenosis, atresia or stenosis of other parts of small intestine, ano-rectal atresia and stenosis, Hirschsprung’s disease, atresia of bile ducts, annular pancreas and diaphragmatic hernia), craniosynostosis and oriofacial clefts (includes: cleft lip with or without cleft palate, cleft palate), were considered together as the “typically surgical” group. This group covered about 50% of cases having surgery in Odense and Vaud. Looking at this ‘typically surgical group’ of anomalies only, we calculated the prevalence of these in ALL registries.
Assessment of time trends was done by pooling data from all registries together. Below, we will present the results of part of the analyses conducted on EUROCAT indicators overall and by registry. Additional data are available on the EUROCAT website (http://www.eurocat-network.eu/) or from authors upon request.
Perinatal mortality
Figure 1a) shows the mortality burden of congenital anomalies using the indicator of perinatal mortality (foetal plus first week neonatal deaths) due to congenital anomalies per 1,000 births across the registries in Europe. The overall perinatal mortality was approximately 1.0 per 1,000 births for EUROCAT registries with almost half due to foetal and the other half due to first week deaths. There were wide variations in perinatal mortality across the registries with the highest rates observed in Dublin and Malta, registries in countries where TOPFA are illegal, and in Ukraine. These registries had more than a two-fold higher rate of perinatal mortality as compared to the EUROCAT average rate. Registries in Italy and Portugal reported much lower rates of perinatal mortality than the EUROCAT average. Differences across registries were in general due to differences in rates of both foetal and first week deaths.
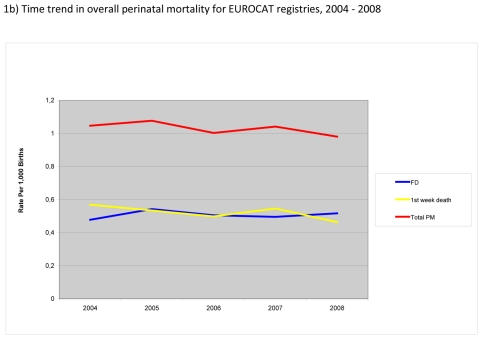
Figure 1b) shows the time trend in perinatal mortality during the study period. The overall perinatal mortality across EUROCAT registries slightly decreased between 2004 and 2008 due to a decrease in first week deaths. In contrast, there was no obvious trend in foetal deaths due to anomalies.
Prenatal diagnosis
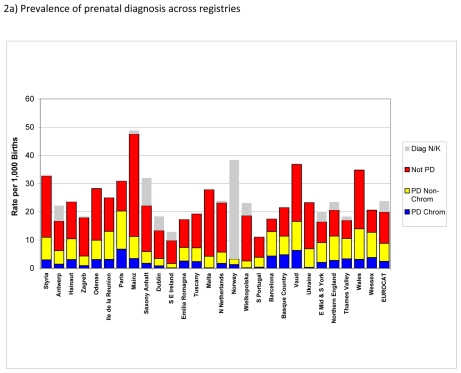
Figure 2a) shows prevalence of prenatal diagnosis for chromosomal as well as non-chromosomal anomalies per 1,000 births across EUROCAT registries. The overall prevalence of prenatal diagnosis for EUROCAT registries was about 9 per 1,000 births. Non-chromosomal anomalies accounted for about two-thirds of the cases prenatally diagnosed. By far most registries could provide information on timing of diagnosis (pre- vs. post-natal) with little missing data. There were wide variations in prevalence of prenatal diagnosis across registries, both in the prevalence of prenatal diagnosis for chromosomal and non-chromosomal anomalies. Prevalence of cases not prenatally diagnosed also differed across registries in ways that were not obviously due to differences in prevalence of prenatal diagnosis.
Figure 2.
Prevalence of prenatal diagnosis per 1,000 births, EUROCAT Registries, 2004 – 2008
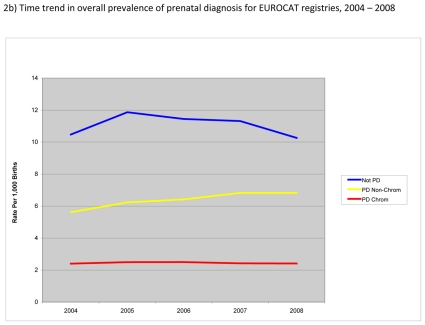
Figure 2b) shows trends in the prevalence of prenatal diagnosis during the study period. Overall, the prevalence of prenatal diagnosis was fairly stable for chromosomal anomalies (about 2.4 per 1,000 births) but increased for non-chromosomal anomalies reaching a prevalence of almost 7 per 1,000 in 2008. Prevalence of cases not prenatally diagnosed initially increased but subsequently decreased to its level in 2004 with a prevalence of about 10.3 per 1,000 births in 2008.
Termination of pregnancy for foetal anomaly (TOPFA)
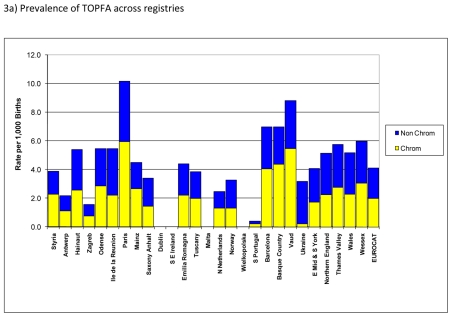
Figure 3a) shows prevalence of TOPFA for chromosomal and non-chromosomal anomalies. The overall prevalence of TOPFA for EUROCAT registries was about 4 per 1,000 with about half of the TOPFA accounted for by chromosomal and the other half by non-chromosomal anomalies. Both the overall prevalence of TOPFA and the proportion accounted for by chromosomal vs. non-chromosomal cases varied widely across registries. In particular, there were no TOPFA for registries in Ireland, Malta and Poland, where TOPFA are illegal, whereas the prevalence of TOPFA was highest, particularly for chromosomal anomalies, in registries in Paris and Vaud.
Figure 3.
Prevalence of termination of pregnancy for foetal anomaly (TOPFA) per 1,000 births, EUROCAT Registries, 2004 – 2008
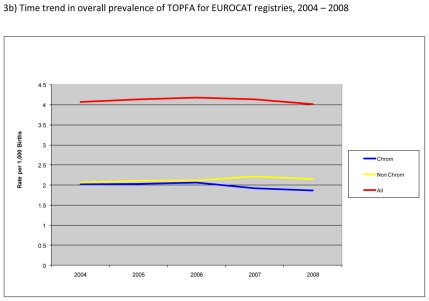
Figure 3b) shows the time trend in prevalence of TOPFA. Overall, the prevalence of TOPFA was fairly stable at about 4 per 1,000 births. There appeared to be a small decrease in the prevalence of TOPFA for chromosomal anomalies and a slight increase for non-chromosomal anomalies between 2004 and 2008.
Down syndrome live births
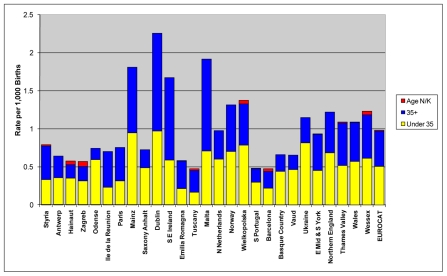
Figure 4 shows the prevalence of Down syndrome live births per 1,000 births for EUROCAT registries. The overall live birth prevalence of Down syndrome was about 1 per 1,000 with about half of the cases born to women 35 years of age and older. Registries with no or a low prevalence of TOPFA (e.g., Ireland, Poland and Malta) tended to have a higher live birth prevalence of Down syndrome.
Figure 4.
Live birth prevalence of Down syndrome per 1000 births, EUROCAT Registries, 2004 – 2008
Typical surgery case
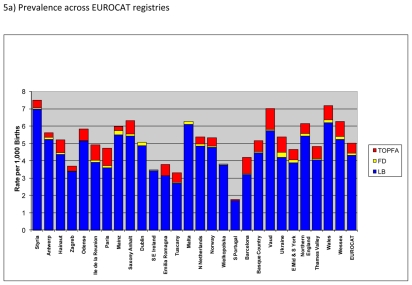
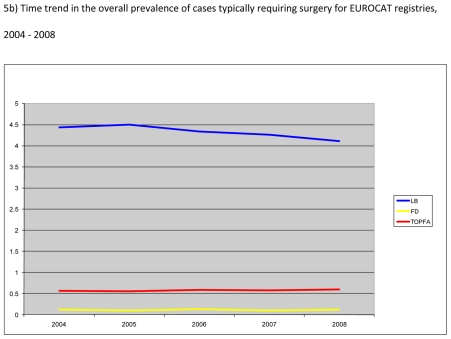
Figure 5a) shows the overall prevalence of six malformations and/or malformation groups, namely severe congenital heart defects, orofacial clefts, malformations of the digestive system, gastroschisis, omphalocele and craniocynostosis that usually require surgery. By far most of these cases were live births with an average prevalence of about 4.3 per 1,000 births for EUROCAT registries. There were variations in live birth prevalence of cases typically requiring surgery across the registries; however for most registries this prevalence was between 3 and 5 per 1,000 births.
Figure 5.
Prevalence of six malformations and/or malformation groups typically requiring surgery per 1000 births, EUROCAT Registries, 2004 – 2008
Figure 5b) shows the time trends in the prevalence of anomalies typically requiring surgery. Overall, the live birth prevalence of these cases decreased by about 10% from 4.5 in 2004 to 4.1 per 1,000 births in 2008; whereas the prevalence of TOPFA and foetal deaths for these cases remained essentially the same during the study period.
Neural tube defects (NTD)
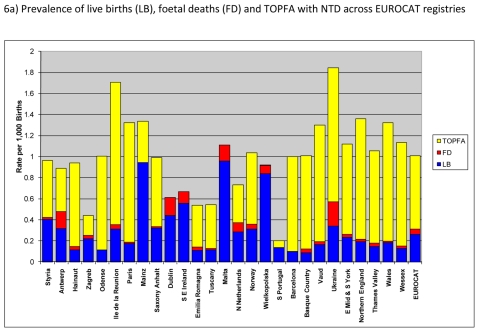
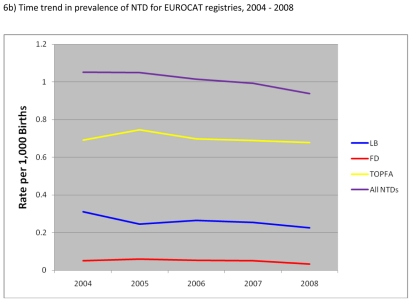
Figure 6a) shows the total and live birth prevalence of neural tube defects. Overall, total prevalence of NTD was about 1.0 per 1,000 births in EUROCAT registries with more than two-thirds of the cases accounted for by TOPFA. Prevalence of NTD was particularly high in Ukraine and Ile de la Reunion.
Figure 6.
Prevalence of neural tube defects (NTD) per 1000 births, EUROCAT Registries, 2004 – 2008
Figure 6b) shows the time trend in prevalence of NTD between 2004 and 2008. Overall, total prevalence of NTD decreased by about 10% from 1.05 in 2004 to 0.94 per 1,000 in 2008. There was in particular a decrease in prevalence of TOPFA and live births with NTD with lesser essentially no change in the prevalence of foetal deaths with NTD.
Comment
In this paper, we described the public health indicators for congenital anomalies proposed by EUROCAT and presented recent data on these indicators from 27 registries representing 10.1% of births in Europe. The EUROCAT indicators address important areas relevant to evaluation of the public health impact and assessment of interventions for congenital anomalies. These indicators address the mortality burden, prenatal diagnosis, termination of pregnancy for foetal anomaly (TOPFA), impact of maternal age distribution and prenatal screening on Down syndrome live births, need for surgical interventions for anomalies, and the impact of policies aimed at prevention of neural tube defects through periconceptional folic acid supplementation.
An important advantage of these indicators is that they are well-defined. In contrast, the prevalence of all major congenital anomalies combined, though a desirable indicator, is not well defined as it depends critically on the quite arbitrary boundary between major and minor anomalies, and the degree of diagnosis (often related to screening methods) and reporting in medical records of the less severe anomalies.
Another advantage of these indicators is that joint consideration of certain indicators can provide insights as to the reasons for differences one may observe across countries (registries) and/or over time for a given indicator. This is facilitated by defining, as we have done, the prevalence of indicators using a common denominator (per 1,000 births). For example, by comparing the prevalence of prenatal diagnosis and TOPFA, one can glean some, albeit indirect and only partial, evidence for evaluating whether differences in TOPFA may be mostly related to differences across registries in the probability of prenatal diagnosis of anomalies and/or the decision to continue the pregnancy after a prenatal diagnosis is made. Comparing differences in TOPFA and perinatal mortality can in turn generate hypotheses about the extent to which differences in early neonatal mortality and/or foetal deaths across registries or over time may be due to TOPFA for severe anomalies. However, alternative hypotheses must also be considered as the relation between TOPFA and neonatal survival is more complicated than that of reduced prevalence of severe anomalies at birth due to TOPFA alone (Dolk et al, Circulation paper submitted, in revision). In particular, since the prevalence of TOPFA is correlated with, though does not always follow, the prevalence of prenatal diagnosis and the latter is known to improve survival for certain anomalies 5;6, TOPFA can also be correlated with perintatal mortality through mechanisms other than that of a decrease in the live birth prevalence of severe anomalies.
A further advantage of these indicators is that we can place congenital anomalies more clearly on the public health agenda by having a few summary measures, than by publishing prevalence rates and other characteristics of the 96 congenital anomaly subgroups on which EUROCAT regularly published information on its website (http://www.eurocat-network.eu/). Sometimes “less is more”.
Of the indicators published here, we will be giving the highest priority to further development of the surgery indicator. Currently we have included only those anomaly subgroups where we know that surgery is usually needed. It would be preferable to have an indicator showing the number of cases of all CA needing surgery in the population. However at present only few EUROCAT registries are completing the variable regarding surgery on a case by case basis. We know from the information we have from selected registries that the total prevalence of surgical cases may be about 1% of births (Odense: 1.4% and Vaud 1.2% of births in 2005–2006). Hospital episode statistics are generally not good at producing the information required, as they tend to concern the rate of hospitalisation for congenital anomaly surgery, which includes children needing repeat surgery.
In addition to, or perhaps instead of, the proposed indicator for prenatal diagnosis (prevalence of cases prenatally diagnosed per 1,000 births), many clinicians and others may want to have data on the proportion of cases prenatally diagnosed. The reason we have not proposed this as an indicator is that the proportion of cases prenatally diagnosed would be influenced by the registered prevalence of malformed cases diagnosed after the early postnatal period, which differs between registries according to their ascertainment methods. However, we have provided the prevalence of both prenatally and non-prenatally diagnosed cases, which allow calculation of the proportion of cases prenatally diagnosed. In addition, data on the percentage of cases prenatally diagnosed are available on the EUROCAT website for selected anomalies, overall for EUROCAT and by registry. (http://www.eurocat-network.eu/PRENATALSCREENINGAndDIAGNOSIS/PrenatalDetectionRates)
Our main objective in this paper was to present the data on EUROCAT indicators across the member registries rather than analyzing the underlying reasons or mechanisms for the observed differences across the registries or for trends in the indicators over time. Many factors, including those related to registration and data quality issues, differences in population characteristics (e.g., maternal age), variations in “baseline” prevalence and distribution of congenital anomalies, differences in prenatal testing policies7;8 and factors related to medical services and cultural factors, among others, can potentially explain the differences we documented here for the EUROCAT indicators across registries or over time. Hence, future studies will be needed in order to more adequately examine the reasons for and/or the implications of differences in the indicators across registries or over time. Nevertheless, it is hoped that by publishing the data on EUROCAT indicators, the public health importance of congenital anomalies can be clearly summarised to policy makers, the need for accurate data from registries emphasized, the need for primary prevention and treatment services highlighted, and the impact of current services measured.
Reference List
- 1.Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 2.de Hollander AE, Melse JM, Lebret E, Kramers PG. An aggregate public health indicator to represent the impact of multiple environmental exposures. Epidemiology. 1999;10:606–617. [PubMed] [Google Scholar]
- 3.Ezzati M, Lopez AD, Rodgers A, Vander HS, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 4.Rare Diseases Task Force Working Group on Health Indicators. Health indicators for rare diseases: Conceptual framework and development of indicators from existing sources. 2010. http://www.eucerd.eu/upload/file/RDTFReportIndicatorsApril2010.pdf.
- 5.Bonnet D, Coltri A, Butera G, et al. Detection of transposition of the great arteries in fetuses reduces neonatal morbidity and mortality. Circulation. 1999;99:916–918. doi: 10.1161/01.cir.99.7.916. [DOI] [PubMed] [Google Scholar]
- 6.Khoshnood B, De VC, Vodovar V, et al. Trends in prenatal diagnosis, pregnancy termination, and perinatal mortality of newborns with congenital heart disease in France, 1983–2000: a population-based evaluation. Pediatrics. 2005;115:95–101. doi: 10.1542/peds.2004-0516. [DOI] [PubMed] [Google Scholar]
- 7.Boyd PA, Devigan C, Khoshnood B, Loane M, Garne E, Dolk H. Survey of prenatal screening policies in Europe for structural malformations and chromosome anomalies, and their impact on detection and termination rates for neural tube defects and Down’s syndrome. BJOG. 2008;115:689–696. doi: 10.1111/j.1471-0528.2008.01700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garne E, Khoshnood B, Loane M, Boyd P, Dolk H. Termination of pregnancy for fetal anomaly after 23 weeks of gestation: a European register-based study. BJOG. 2010;117:660–666. doi: 10.1111/j.1471-0528.2010.02531.x. [DOI] [PubMed] [Google Scholar]