Background: p38MAPK plays an essential role in myoblast differentiation.
Results: TAK1 and ASK1 interact with Cdo and JLP to promote myogenesis.
Conclusion: TAK1 and ASK1 act as MAP3Ks to activate p38MAPK in Cdo-mediated myogenesis.
Significance: This might be the first report to identify MAP3Ks in Cdo-mediated myogenesis.
Keywords: Cell Signaling, MAP Kinases (MAPKs), Myogenesis, p38, p38MAPK, ASK1, Cdo, TAK1
Abstract
p38MAPK plays an essential role in the transition of myoblasts to differentiated myotubes through the activation of MyoD family transcription factors. A promyogenic cell surface molecule, Cdo, promotes myogenic differentiation mainly through activation of the p38MAPK pathway. Two MAP3Ks, TAK1 and ASK1, can activate p38MAPK via MKK6 in various cell systems. Moreover TAK1 has been shown to promote myogenic differentiation via p38MAPK activation. In this study, we hypothesized that TAK1 and ASK1 might function as MAP3Ks in Cdo-mediated p38MAPK activation during myoblast differentiation. Both ASK1 and TAK1 were expressed in myoblasts and interacted with the cytoplasmic tail of Cdo and a scaffold protein, JLP. The depletion of TAK1 or ASK1 in C2C12 cells decreased myoblast differentiation, whereas overexpression of TAK1 or ASK1 in C2C12 cells enhanced myotube formation. In agreement with this, overexpression of ASK1 or TAK1 resulted in enhanced p38MAPK activation, and their knockdown inhibited p38MAPK in C2C12 cells. Overexpression of TAK1 or ASK1 in Cdo−/− myoblasts and Cdo-depleted C2C12 cells restored p38MAPK activation as well as myotube formation. Furthermore, ASK1 and TAK1 compensated for each other in p38MAPK activation and myoblast differentiation. Taken together, these findings suggest that ASK1 and TAK1 function as MAP3Ks in Cdo-mediated p38MAPK activation to promote myogenic differentiation.
Introduction
Skeletal muscle differentiation is a well orchestrated process that includes cell cycle withdrawal, expression of muscle-specific genes, and morphological changes that include cell elongation, alignment, and fusion to form multinucleated myofibers (1). The processes of myogenic specification and differentiation are coordinated by a MyoD family of transcription factors, which function in concert with the MEF2 family of transcription factors (2). The activities of these transcription factors need to be tightly regulated to ensure efficient muscle development, and several signaling pathways are involved in posttranslational regulation of these transcription factors (3, 4). The mitogen-activated protein kinases (MAPKs) belong to a family of serine/threonine kinases that transduce signals from extracellular cues to regulate a variety of biological processes, including cell growth, migration, survival, and differentiation. MAPKs function as a part of three-tiered cascades of kinases consisting of a MAPK kinase kinase (MAP3K), MAPK kinase (MAP2K), and MAPK. Three major groups of MAPKs have been characterized in mammals, including extracellular signal-regulated kinase (ERK), c-Jun N-terminal-activating kinase (JNK), and p38MAPK (5). It is well established that p38MAPK plays a prominent role in myogenesis for the biochemical as well as morphological differentiation of myoblasts (3, 4, 6). p38MAPKs consist of four isoforms: p38α, -β, -γ, and -δ in mammalian cells (7, 8). Among these, p38α appears to be essential for myoblast differentiation, whereas the other three are dispensable for the differentiation of primary myoblasts and muscle regeneration (6, 9). p38α positively regulates myogenic differentiation through modulation of MyoD function (8) via phosphorylation of several proteins in muscle-specific gene expression, including MEF2 isoforms, the MyoD heterodimeric partner E47, and the chromatin-modifying enzyme SWI/SNF subunit BAF60 (10–12).
TGF-β-activated kinase 1 (TAK1)3 and apoptosis signal-regulating kinase 1 (ASK1) are MAP3Ks that function upstream of the p38MAPK pathway in response to various stimuli including cytokines and cellular stresses (13–15). TAK1 was originally identified as a key regulator of MAPK in response to TGF-β and can phosphorylate MAPK kinase 6 (MKK6), the upstream activator of p38MAPK (16, 17). Mice lacking TAK1 display embryonic lethality, suggesting its essential role in mouse development. Studies with tissue-specific knock-out mice suggest that TAK1 is involved in multiple developmental processes including vasculature development, differentiation of keratinocytes, survival of hematopoietic cells and hepatocytes, and development of cartilage and immune responses (17–22). Recently it has been reported that TAK1 plays a key role in proliferation and differentiation of myoblasts via activation of p38MAPK (23). Inactivation of TAK1 by expression of RNAi or a dominant-negative form of TAK1 decreases the expression of myogenic differentiation markers in C2C12 cells. TAK1-deficient mouse embryonic fibroblasts (MEFs) show a defect in myoblast differentiation induced by ectopically transfected MyoD, a defect that is rescued by expression of a constitutively active form of MKK6, suggesting that TAK1 may function as a MAP3K for p38MAPK in myoblast differentiation. On the other hand, ASK1, originally identified as a mediator of tumor necrosis factor α-induced apoptosis by activation of p38MAPK and JNK (24), can promote cell differentiation and survival rather than apoptosis in PC12 cells and keratinocytes (25, 26), suggesting that ASK1 may play distinct roles depending on the cellular context. ASK1 has also been shown to promote neuronal differentiation of adult hippocampal neural progenitors via activation of p38MAPK (27). However, whether ASK1 can activate p38MAPK in myoblast differentiation, thereby regulating myogenesis, is unknown.
Cdo is an immunoglobulin (Ig)/fibronectin type III (FNIII) superfamily of cell surface protein that positively regulates myogenesis in vivo and in vitro. Cdo-deficient mice exhibit delayed skeletal muscle development, and Cdo-deficient myoblasts differentiate defectively with the reduction in levels of muscle-specific proteins (28). Similarly, Cdo depletion in C2C12 cells by RNAi causes inefficient differentiation, whereas overexpression of Cdo enhances differentiation. Cdo is implicated in the promotion of myoblast differentiation mediated by cell to cell adhesion as well as cell to substratum adhesion signal mediated by integrin β1/FAK signaling (29). Cdo functions as a component of multiprotein complexes that include the closely related protein Boc, the Ig superfamily receptor neogenin and its ligand netrin-3, and the adhesion molecules N-cadherin and Gas1 (30–33). The promyogenic function of Cdo is exerted largely through activation of the p38MAPK pathway (34, 35) to stimulate MyoD activity through phosphorylation of E47, leading to the enhancement of heterodimerization (28). N-cadherin-mediated cell contact enhances myoblast differentiation via p38MAPK activation in a Cdo-dependent manner, suggesting that the N-cadherin/Cdo/p38MAPK pathway mediates cell contact-dependent differentiation of myoblasts (32, 36). The Cdo intracellular region binds to JLP, a scaffold protein for the p38MAPK pathway, and Bnip-2, a scaffold-like protein for the small GTPase Cdc42, during myoblast differentiation. This interaction leads to the Cdc42-dependent activation of JLP-bound p38MAPK, which promotes myoblast differentiation (35). In agreement with this notion, Cdo−/− primary myoblasts are unable to induce the differentiation-specific p38MAPK, and their defective differentiation is rescued by the expression of an activated form of MKK6, an immediate upstream kinase of p38MAPK. In addition, Cdo-mediated p38MAPK activation is required for the neuronal differentiation of C17.2 neuronal progenitor cells and P19 embryonal carcinoma cells (37). It is anticipated that p38MAPK activation by the Cdo-JLP complex will require specific MAP3Ks. The fact that p38MAPK acts downstream of Cdo and TAK1 in myoblast differentiation and of Cdo and ASK1 in neuronal differentiation led us to hypothesize that ASK1 and TAK1 might function as MAP3Ks in Cdo-mediated p38MAPK activation and myoblast differentiation. In this study, we demonstrate that the knockdown or overexpression of TAK1 or ASK1 in C2C12 cells decreases or enhances myoblast differentiation, respectively. Cdo and JLP interacted with ASK1 or TAK1 in 293T cells and C2C12 myoblasts. As expected, overexpression of ASK1 and TAK1 in C2C12 cells resulted in enhanced p38MAPK activation, whereas knockdown reduced the level of pp38. Overexpression of TAK1 or ASK1 in Cdo-depleted C2C12 cells and Cdo−/− myoblasts rescued the deficient p38MAPK activation and the defective myotube formation. Furthermore, ASK1 and TAK1 can compensate for each other in p38MAPK activation and myoblast differentiation. Taken together, these findings suggest that ASK1 and TAK1 function as MAP3Ks in Cdo-mediated p38MAPK activation and promotion of myoblast differentiation.
EXPERIMENTAL PROCEDURES
Cell Culture and Expression Vectors
C2C12 and 293T cells were cultured as described previously (38). C2C12 cells were cultured in Dulbecco modified Eagle's medium (DMEM) containing 15% fetal bovine serum (growth medium) and induced to differentiate at near confluence in DMEM, 2% horse serum (differentiation medium (DM)). Myotube formation in stable and transient transfection assays was quantified as described previously (38). Primary myoblasts were obtained from the hind limbs of wild type and Cdo−/− mice as described previously (34). Briefly, cells were grown in F10 medium containing 20% fetal bovine serum and bFGF (100 ng/ml). For the rescue experiment, cells were cotransfected with TAK1 or ASK1 expression vectors plus an RFP or GFP vectors to mark transfectant using Lipofectamine 2000, and 24 h later, cells were induced to differentiate by removing bFGF for 1 day followed by immunostaining with antibodies to pp38 or myosin heavy chain (MHC). Tak1−/− mouse embryonic fibroblasts (kindly provided by Dr. Takeuchi, Osaka University) were cultured in 10% FBS containing DMEM and transfected with various constructs by the reverse transfection method (39) with Lipofectamine 2000 (Invitrogen). Because the transfection efficiency generally was 70–90%, the transiently transfected cells were used to analyze for pp38 detection and BrdU incorporation. The statistical analysis of myotube formation was performed using Student's t test. For overexpression studies, pRK5/HA-TAK1 (40), pRK5/HA-TAK1(KN) (41), pcDNA/FLAG-ASK1, or pcDNA/FLAG-ASK1(KN) (24) and pBabePuro control vectors were cotransfected into C2C12 cells using FuGENE 6 reagent (Roche Applied Science). To generate stable C2C12 cell lines, cultures were selected in puromycin-containing medium. Drug-resistant cells were pooled and analyzed for Western blotting or MHC staining. The rescue ability of ASK1 and TAK1 for differentiation of Cdo-depleted C2C12 cells was assessed by a transient coexpression approach as described previously (38). Briefly, those cells were cotransfected with ASK1 or TAK1 expression vector plus a GFP expression vector with a ratio of 10:1, respectively. Forty-eight hours after transfection, the cells were transferred into DM for 2 days followed by immunostaining for both MHC and GFP expression. To generate C2C12 cell lines that stably expressed small hairpin RNAs (shRNAs) against ASK1 or TAK1, three different sequences for each gene were chosen and inserted into pSuper-puro vector. From among them, the following sequences were chosen based on reproducibility: shAsk1-1, 5′- CCGGCCAGGTCAGAATTGCTATTAACTCGAGTTAATAGCAATAGCAATTCTGAC- CTTGTTTTT-3′; shAsk1-2, CCGGCCTGTGCTAATGACTTGCTTACTCGAGTAAGCAAGTCATTAGCACAGGTTTTT; shTak1-1, 5′-CCGGCGCCCTTCAATGGAGGAAATTCTCGAGAATTTCCTCCATTGAAGGGCGTTTTT-3′; and shTak1-2, 5′-CCGGCAGCCCTAGTGTCAGAATGATCTCGAGATCATTCTGACACTAGGGCGGTTTTT-3′. pSuper-shCdo vectors were reported previously (42).
Western Blot Analyses and Immunoprecipitation
Western blot analyses were performed as described previously (38). Briefly, cells were lysed in extraction buffer (50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 10% glycerol, 1.5 mm MgCl2, 1 mm EGTA, 1% Triton X-100, 10 mm NaF, 1 mm Na3VO4, and complete protease inhibitor mixture (Roche Applied Science)), and SDS-PAGE was performed. The primary antibodies used were anti-ASK1, anti-MyoD, anti-myogenin, anti-S probe, anti-TAK1 (Santa Cruz Biotechnology), anti-pp38 (Cell Signaling Technology), anti-Cdo (Zymed Laboratories Inc.), anti-JLP (Abcam), anti-pan-cadherin, anti-troponin T, anti-p38, anti-FLAG (Sigma), anti-MHC (MF-20; Developmental Studies Hybridoma Bank), anti-β-tubulin (Zymed Laboratories Inc.), and anti-HA (Roche Applied Science) antibodies. For immunoprecipitation experiments, 293T cells were transfected with a combination of S-tagged JLP and either FLAG-tagged ASK1 or HA-tagged TAK1. Forty-eight hours after transfection, whole-cell extracts were incubated with 20 μl of 50% slurry S-agarose beads for 2 h at 4 °C. Beads were washed three times with extraction buffer and resuspended in extraction buffer, and samples were analyzed by Western blotting. To study the formation of ASK1-Cdo and TAK1-Cdo complexes, coimmunoprecipitation was performed as described previously (38).
Immunocytochemistry and Microscopy
Immunostaining for MHC expression was performed as described previously (38), and images were captured and processed with a Nikon Eclipse Ti-U and NIS-Elements F software. For the results shown in Fig. 5, C2C12 cells or primary myoblasts on coverslips in 12-well plates were cotransfected with 100 ng of enhanced GFP expression vector and 900 ng of the indicated DNA construct for 2 days, fixed with 4% paraformaldehyde for 20 min, permeabilized with 1% Triton X-100 in phosphate-buffered saline (PBS), blocked, and stained with anti-pp38MAPK or anti-MHC followed by an Alexa Fluor 568-conjugated secondary antibody (Invitrogen). An image was obtained on a Zeiss LSM-510 Meta confocal microscope. Quantification of the fluorescent signal for pp38 was performed with Image Gauge software (Fujifilm, Tokyo).
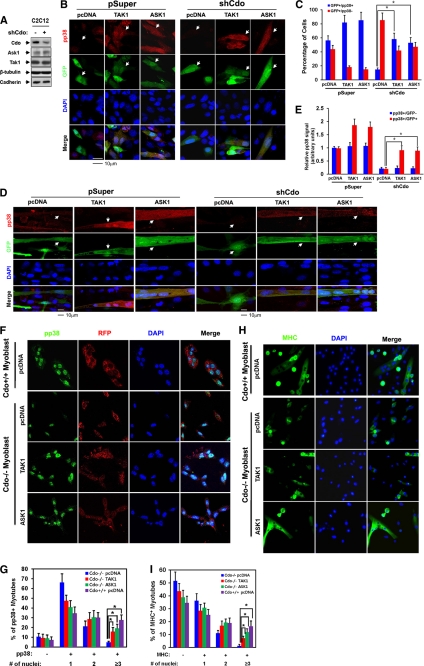
FIGURE 5.
TAK1 and ASK1 rescue defective p38MAPK activation and myotube formation of Cdo-depleted myoblasts and Cdo−/− myoblasts. A, lysates of C2C12/pSuper and C2C12/shCdo cells were immunoblotted with antibodies to Cdo, ASK1, and TAK1 and to β-tubulin and cadherin as loading controls. B, C2C12/pSuper and C2C12/shCdo cells were transiently transfected with ASK1(FLAG), TAK1(HA), or control expression vector plus a GFP vector to mark the transfectants, and 2 days later cells were immunostained with antibodies to pp38 (red) and visualized for GFP expression (green). Cell nuclei were visualized by staining with DAPI (blue). The white arrowheads indicate transfected cells. C, quantification of cultures shown in B. GFP+ cells were scored as positive or negative for pp38 staining. Values represent the means of triplicate determinations ± 1 S.D. The experiment was repeated three times with similar results. Asterisks indicate difference from the control at p < 0.01. D, C2C12/pSuper and C2C12/shCdo cells were transiently transfected with ASK1(FLAG), TAK1(HA), or the control expression vector and a GFP vector to mark the transfectants. Cells at differentiation day 2 were fixed and immunostained with antibodies to pp38 (red) and visualized for GFP expression (green). White arrowheads indicate transfected cells. E, quantification of cultures shown in D. The intensity of the immunofluorescent pp38 signals in GFP+ and GFP− cells was quantified with the values obtained from control vector-transfected cultures set to 1.0. Values represent the means of triplicate determinations ± 1 S.D. The experiment was repeated three times with similar results. Asterisks indicate difference from the control at p < 0.01. F, Cdo−/− and Cdo+/+ myoblasts were transiently transfected with ASK1(FLAG), TAK1(HA), or control expression vector and an RFP vector to mark the transfectants. Cells were induced to differentiate for 1 day by removing bFGF, immunostained with antibodies to pp38 (green), and visualized for RFP expression (red). Cell nuclei were visualized by staining with DAPI (blue). G, quantification of pp38-positive multinucleated myotubes shown in F. Values represent the means of triplicate determinations ± 1 S.D. The experiment was repeated three times with similar results. Asterisks indicate difference from the control at p < 0.01. H, Cdo−/− and Cdo+/+ myoblasts were transfected with expression vectors for ASK1(FLAG), TAK1(HA), or the control. Cells were induced to differentiate for 1 day in DM, fixed, and stained with antibodies to MHC (green). I, quantification of MHC-positive multinucleate myotubes shown in H. Values represent the means of triplicate determinations ± 1 S.D. The experiment was repeated three times with similar results. Asterisks indicate difference from the control at p < 0.01.
For reactivation of p38 in Cdo-depleted cells by an ASK1 or TAK1 overexpression experiment, C2C12 cells in 12-well plates were cotransfected with 100 ng of a GFP expression vector and 900 ng of the indicated DNA construct for 2 days and then fixed with 4% paraformaldehyde for 20 min. Cultures were then permeabilized with 1% Triton X-100 in PBS, blocked, and incubated with anti-pp38 or anti-MHC antibodies followed by incubation with an Alexa Fluor 568-conjugated secondary antibody (Invitrogen). An image was obtained on a Zeiss LSM-510 Meta confocal microscope. Quantification of the fluorescent signal for pp38 was performed with Image Gauge software (Fujifilm).
In Vitro Kinase Assay
the kinase reaction buffer comprised 20 mm HEPES (pH 7.5), 20 mm MgCl2, 1 mm EDTA, 2 mm NaF, 10 mm pNPP, 1 mm DTT, and 10 μm ATP. The purified kinase proteins used for this study were as follows: active ASK1 (R&D Systems), MKK6 (Millipore), and p38α (BIOSOURCE). The mixtures were incubated for 15 min at 30 °C followed by SDS-PAGE and Western blotting. Primary antibodies used were anti-pp38 (Cell Signaling Technology) and anti-Mkk6 (Santa Cruz Biotechnology).
BrdU Incorporation Assay
C2C12 cells stably expressing ASK1, TAK1, shAsk1-1, shAsk1-2, shTak1-1, or shTak1-2 were incubated with BrdU for 10 min in growth medium and analyzed as described previously (43). Briefly, cells were rinsed with PBS twice and then fixed for 10 min at room temperature with 4% paraformaldehyde. Cells were then washed with 1% Triton X-100/PBS twice for 5 min, incubated in 2 n HCl for 30 min, and neutralized with 0.1 m sodium borate buffer for 12 min at room temperature. After three washings with 1% Triton X-100/PBS for 5 min, cells were blocked with 5% FBS in PBS for 1 h at room temperature followed by incubation with anti-BrdU antibody (Chemicon), Alexa Fluor 488-conjugated secondary antibody (Invitrogen), and DAPI, sequentially. Signals were obtained and quantified with a Nikon Eclipse Ti-U and NIS-Elements F software.
Annexin V-FITC/Propidium Iodide Assay
The annexin V-FITC apoptosis detection kit, ApopscanTM (Biobud Inc., Seoul, Korea) was used according to the manufacturer's protocol to detect phosphatidylserine translocation from the inner to the outer plasma membrane. Briefly, for each assay, cells were washed with PBS, diluted in annexin V binding buffer containing annexin V and propidium iodide, and incubated for 15 min in the dark at room temperature. Those cells were processed with fluorescence-activated cell sorting (FACSCalibur, BD Biosciences) by collecting 10,000 events/sample to ensure statistical significance. Data were analyzed with FCS Express software.
RESULTS
ASK1 and TAK1 Form a Complex with Cdo and JLP during Myoblast Differentiation
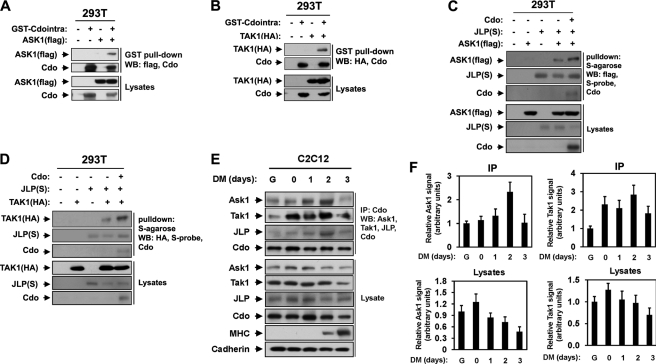
To test the possibility that TAK1 and ASK1 may function as MAP3Ks in Cdo-mediated p38MAPK activation, we analyzed whether TAK1 and ASK1 could interact with Cdo. Thus, 293T cells were transiently transfected with expression vectors for the GST-tagged cytoplasmic tail of Cdo, HA-tagged TAK1 (TAK1(HA)), or FLAG-tagged ASK1 (ASK1(FLAG)) and the control vector. Lysates were then subjected to pulldown with glutathione-Sepharose beads and immunoblotted with antibodies recognizing Cdo, HA (for TAK1), or FLAG (for ASK1). As shown in Fig. 1, A and B, the cytoplasmic region of Cdo interacted with TAK1 as well as ASK1. In our previous study, Cdo interacted directly with JLP via its cytoplasmic tail, thereby activating the p38MAPK signaling pathway in myoblast differentiation (34). Therefore we investigated whether ASK1 and TAK1 can interact with JLP and Cdo. To that end, 293T cells were transfected with ASK1(FLAG), TAK1(HA), S-tagged JLP (JLP-S), Cdo, or the control expression vectors, and lysates were subjected to pulldown with S-agarose followed by immunoblotting with antibodies recognizing FLAG, HA, Cdo, or S-probe. As shown in Fig. 1, C and D, ASK1 or TAK1 interacted with JLP, and this interaction was further increased when Cdo was coexpressed, suggesting that ASK1 and TAK1 may function as MAP3Ks in Cdo-mediated p38 activation during myogenic differentiation.
FIGURE 1.
Cdo interacts with ASK1 and TAK1. A, lysates of 293T cells transiently transfected with the GST-Cdo intracellular region (GST-Cdointra), FLAG-tagged ASK1 (ASK1(FLAG)), or control (−) expression vectors, as indicated, were pulled down with glutathione-Sepharose beads and blotted (WB, Western blot) with antibodies to FLAG or the Cdo intracellular region. Total lysates were also immunoblotted with the indicated antibodies. B, lysates of 293T cells transiently transfected with GST-Cdo intracellular region, HA-tagged TAK1 (TAK1(HA)), or control (−) expression vectors, as indicated, were pulled down with glutathione-Sepharose beads and blotted with antibodies to HA or the Cdo intracellular region. Total lysates were also immunoblotted with the indicated antibodies. C, lysates of 293T cells transiently transfected with Cdo, ASK1(FLAG), S epitope-tagged JLP (JLP(S)), or control (−) expression vectors, as indicated, were pulled down with S-agarose and immunoblotted with antibodies to Cdo, FLAG, or S-epitope. Total lysates were also immunoblotted with the indicated antibodies. D, lysates of 293T cells transiently transfected with Cdo, TAK1(HA), JLP(S), or control (−) expression vectors, as indicated, were pulled down with S-agarose and immunoblotted with antibodies to Cdo, HA, or S-epitope. Total lysates were immunoblotted with the indicated antibodies. E, lysates of C2C12 cells that were proliferating in growth medium (lane G), at near confluence (day 0) or in DM for the indicated times were immunoprecipitated with anti-Cdo antibody and immunoblotted with anti-ASK1, anti-TAK1, anti-JLP, or anti-Cdo antibodies. Total lysates were also immunoblotted with antibodies to ASK1, TAK1, JLP, Cdo, and MHC (an indicator of differentiation) and to cadherin as a loading control. F, quantification of three independent experiments shown in E. The intensity of the ASK1 and TAK1 signals was quantified, and the values from condition lane G were set to 1.0. Values represent the means of triplicate determinations ± 1 S.D.
Next we tested whether the endogenous proteins also form complexes in myoblasts during differentiation. C2C12 cells at high cell density (near confluence) were triggered to differentiate in DM. Lysates of C2C12 cells proliferating in growth medium at low density (G), at near confluence (day 0), or in DM for the indicated times were immunoprecipitated with anti-Cdo antibodies followed by Western blot analysis with antibodies recognizing ASK1, TAK1, Cdo, MHC (to monitor differentiation), and cadherin as a loading control. Both ASK1 and TAK1 were expressed in myoblasts, and the levels of ASK1 and TAK1 were modestly increased in the cells at near confluence and decreased slightly in differentiating cells, as shown in the quantification of three independent experiments (Fig. 1, E and F). Both ASK1 and TAK1 were coimmunoprecipitated with Cdo in proliferating and differentiating myoblasts. The interaction among Cdo, ASK1, TAK1, and JLP was significantly enhanced at day 2 in DM (Fig. 1, E and F) when myoblast differentiation was initiated, which is evident by MHC expression; p38MAPK activation is generally detected at this time point (34). We concluded that ASK1 and TAK1 interact with Cdo and JLP in myoblasts and that this interaction is strongly enhanced at the early stage of differentiation.
Knockdown or Overexpression of TAK1 or ASK1 Decreases or Enhances Myoblast Differentiation
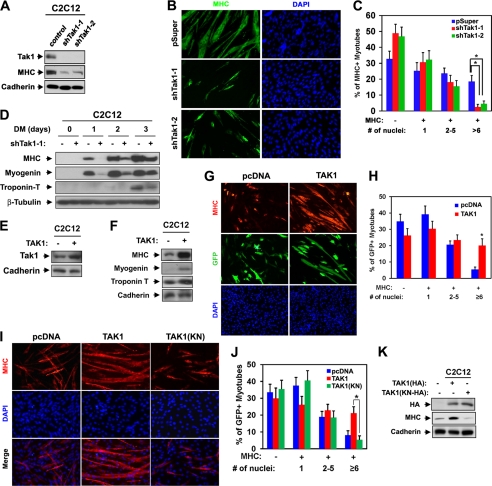
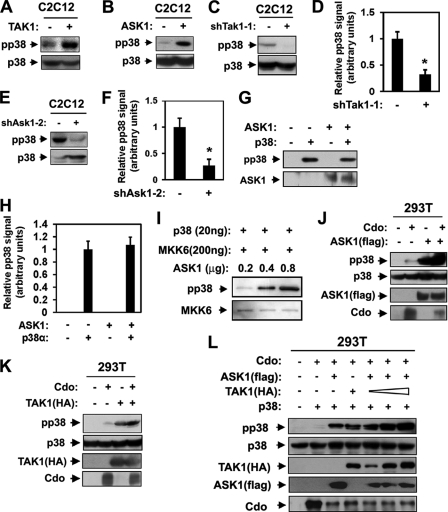
We next analyzed the role of ASK1 and TAK1 in myoblast differentiation. C2C12 cells were stably transfected with expression vectors for a control shRNA or two shRNAs targeting TAK1, and the differentiation ability of such cells was analyzed by Western blot analysis and immunostaining with anti-MHC antibody at differentiation day 3 when cells are fully differentiated. In agreement with the published report (23), TAK1 depletion by either of the shRNAs inhibited myotube formation and muscle-specific gene expression, such as MHC, myogenin, and troponin T (Fig. 2, A–D), suggesting that TAK1 is critical for efficient myoblast differentiation. To investigate whether TAK1 promotes myoblast differentiation, C2C12 cells were transiently transfected with the control, pcDNA, or TAK1(HA) expression vectors plus the GFP expression vector to mark the transfectants. Cells were then induced to differentiate for only 2 days at the early differentiation time point and subjected to immunostaining for MHC and GFP expression followed by DAPI staining to visualize cell nuclei. The extent of myotube formation was quantified by determining the percentage of nuclei present in myotubes. As shown in Fig. 2, E and F, TAK1-overexpressing C2C12 cells displayed an increase in muscle-specific gene expression, such as MHC, myogenin, and troponin T, relative to that of the control cells. In addition, C2C12/pcDNA cells were mostly single nucleus-containing myocytes and small myotubes with two to five nuclei at this time point of differentiation. However C2C12/TAK1 cells formed larger myotubes with more nuclei per myotube. Specifically, 20% of the myotubes contained more than six nuclei as compared with 5% in control myotubes (Fig. 2, G and H). To further assess the requirement of TAK1 kinase activity for myoblast differentiation, C2C12 cells were stably transfected with the expression vectors for the control, wild type TAK1(HA), or kinase-negative TAK1(KN/HA) and assessed for their differentiation ability. In contrast to TAK1(HA), the ectopic expression of TAK1(KN) failed to enhance myoblast differentiation as analyzed by myotube formation and MHC expression (Fig. 2, I–K). These data suggest that the increase in TAK1 protein levels, and its activity, caused accelerated myoblast differentiation at the morphological as well as the biochemical level.
FIGURE 2.
TAK1 promotes myoblast differentiation. A, lysates of C2C12 cells that stably express shTak1-1, shTak1-2, or control (pSuper) vectors at differentiation day 3 (DM3) were immunoblotted with antibodies to TAK1, MHC, and cadherin as a loading control. B, photomicrographs of C2C12 cells shown in A that were cultured in DM for 3 days, fixed, and stained with antibody to MHC. Nuclei were visualized by DAPI staining. C, quantification of myotube formation by the cell lines shown in B. The values represented are the means of triplicate determinations ± 1 S.D. The experiment was repeated three times with similar results. Asterisks indicate difference from the control at p < 0.01. D, lysates of C2C12/shTak1-1 cells from various differentiation times were immunoblotted with antibodies to MHC, myogenin, or troponin T and to β-tubulin as a loading control. E, lysates of C2C12/control or C2C12/TAK1 cells were immunoblotted with antibodies to TAK1 and to cadherin as a loading control. F, lysates of C2C12/control or C2C12/TAK1 cells that were cultured in DM for 2 days were immunoblotted with antibodies to MHC, myogenin, or troponin T and to cadherin as a loading control. G, C2C12 cells were transiently transfected with control or TAK1 expression vector and a GFP expression vector to mark transfectants. Cells at differentiation day 2 were immunostained for MHC (red) and visualized for GFP expression (green). Nuclei were visualized by staining with DAPI (blue). H, quantification of myotube formation shown in G. Values represent the means of triplicate determinations ± 1 S.D. The experiment was repeated three times with similar results. The asterisk indicates difference from the control at p < 0.01. I, C2C12 cells were stably transfected with TAK1, a kinase-negative form of TAK1 (TAK1(KN)), or a control expression vector and differentiated for 2 days followed by immunostaining for MHC (red). Nuclei were visualized by DAPI staining (blue). J, quantification of myotube formation by the cell lines shown in I. Values represent the means of triplicate determinations ± 1 S.D. The experiment was repeated three times with similar results. The asterisk indicates difference from the control at p < 0.01. K, lysates of C2C12 cells that stably express TAK1, TAK1(KN), or control vectors in I were immunoblotted with antibodies to HA and MHC and to cadherin as a loading control.
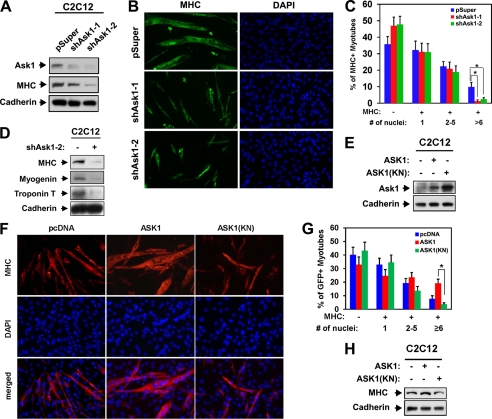
Next, we analyzed the role of ASK1 in myoblast differentiation. C2C12 cells stably transfected with either the control pSuper or two different ASK1 shRNA expression vectors were induced to differentiate for 3 days followed by Western blot analysis or immunostaining with anti-MHC antibodies. Expression of ASK1 protein was nearly abrogated with both ASK1 shRNAs in expressing C2C12 cells, which resulted in a decrease in expression of MHC relative to the control cells (Fig. 3A). Moreover these cells formed smaller myotubes with fewer nuclei per myotube compared with the control vector-transfected cells (Fig. 3, B and C). Roughly 64% of the control cells were MHC-positive, and these cells were scored as mononucleate (∼32%), containing two to five nuclei (∼23%), or containing six or more nuclei (∼11%). Expression of either ASK1 shRNA decreased the percentage of MHC-positive cells to ∼51–53%, with a dramatic decrease in larger myotube formation (those with more than six nuclei), which dropped from 10% to ∼1–2%. To analyze the effect of ASK1 overexpression on myoblast differentiation, stable transfectants of C2C12 cells with control, wild type ASK1, or the kinase-negative ASK1(KN) expression vectors were induced to differentiate for 2 days and then immunostained with anti-MHC antibodies followed by DAPI staining to visualize cell nuclei. As shown in Fig. 3E, overexpression of ASK1 and ASK1(KN) in C2C12 cells resulted in about a 3- and 5-fold increase in the ASK1 protein levels, respectively, compared with that of the control cells. ASK1 overexpression resulted in the formation of larger myotubes with more nuclei per myotube, whereas the ectopic expression of ASK1(KN) exhibited a significant decrease in myotube formation as compared with C2C12/ASK1 cells. Roughly 60% of the control MHC-positive cells were scored as mononucleate (∼33%), containing two to five nuclei (∼19%), or containing six or more nuclei (∼8%). Overexpression of ASK1 increased the percentage of MHC-positive cells to 67%: mononucleate (24%), containing two to five nuclei (∼23%), and containing six or more nuclei (∼20%). In contrast, ∼55% of the C2C12/ASK1(KN) cells were MHC-positive: mononucleate (∼36%), containing two to five nuclei (∼15%), and containing six or more nuclei (∼4%) (Fig. 3, F and G). In agreement, ASK1-overexpressing cells displayed enhanced MHC expression, whereas ASK1(KN) cells expressed a lower level of MHC relative to the control cells (Fig. 3H). These data suggest that TAK1 and ASK1 are required for efficient myoblast differentiation and the kinase activity of these kinases is critical for their promyogenic effect.
FIGURE 3.
ASK1 promotes myoblast differentiation. A, lysates of C2C12 cells that stably express shAsk1-1, shAsk1-2, or control (pSuper) vectors were immunoblotted with antibodies to ASK1 and MHC and to cadherin as a loading control. B, photomicrographs of C2C12 cells shown in A that were cultured in DM for 3 days, fixed, and stained with antibody to MHC. C, quantification of myotube formation by the cell lines shown in B. Values represent the means of triplicate determinations ± 1 S.D. The experiment was repeated three times with similar results. Asterisks indicate difference from the control at p < 0.01. D, lysates of cell lines shown in B were immunoblotted with antibodies to MHC, myogenin, or troponin T and to cadherin as a loading control. E, lysates of C2C12/control, C2C12/ASK1, or C2C12/ASK1(KN) cells from proliferating high density cultures were immunoblotted with antibodies to ASK1 and to cadherin as a loading control F, C2C12 cells were stably transfected with expression vectors for ASK1, a kinase-negative form of ASK1 (ASK1(KN)), or the control. Cells at differentiation day 2 were immunostained for MHC (red), and nuclei were visualized by DAPI staining (blue). G, quantification of myotube formation shown in F. Values represent the means of triplicate determinations ± 1 S.D. The experiment was repeated three times with similar results. The asterisk indicates difference from the control at p < 0.01. H, lysates of C2C12/pcDNA, C2C12/ASK1, or C2C12/ASK1(KN) cells cultured in DM for 2 days were immunoblotted with antibodies to MHC and to cadherin as a loading control.
Knockdown of ASK1 or TAK1 in C2C12 Cells Reduces Proliferation without Altering Cell Death
As ASK1 and TAK1 are also implicated in cell proliferation and death in various cell types, we analyzed their role in proliferation and apoptosis of myoblasts. C2C12 cells were stably transfected with the expression vectors for TAK1 or ASK1 shRNAs or with the control pSuper, and their proliferation in differentiating culture for 1 proliferation at differentiation day 1 (DM1) was analyzed by BrdU incorporation and DAPI staining to visualize cell nuclei. In agreement with the previous report, TAK1-depleted C2C12 cells exhibited a decrease in the level of BrdU incorporation relative to the control cells (supplemental Fig. 1, A and B). Furthermore, C2C12 cells with ASK1 knockdown also showed a reduction in BrdU incorporation compared with the control cells. However, we did not observe any significant decrease in BrdU incorporation when cells were cultured in growth medium with 15% FBS (data not shown). These data suggest that TAK1 and ASK1 are involved in the proliferation of myoblasts prior to differentiation. Next we analyzed the effect of overexpression or knockdown of ASK1 or TAK1 on cell death in C2C12 cells from proliferating cultures or cultures at differentiation day 1 by flow cytometry. C2C12 cells with altered ASK1 or TAK1 expression levels did not exhibit any discernable changes in cell death under either proliferative or differentiation-induced conditions (supplemental Fig. 1, C and D). These data suggest that ASK1 and TAK1 are involved in the regulation, but not in apoptosis during myoblast differentiation.
TAK1 and ASK1 Are Critical for p38MAPK Activation in C2C12 Myoblasts
Next we investigated the effect of overexpression or knockdown of TAK1 and ASK1 on p38MAPK activation in C2C12 cells during differentiation. Control and C2C12 cells overexpressing TAK1 or ASK1 were induced to differentiate for 2 days, the point at which robust p38MAPK activation is generally observed (34), and p38MAPK activation was analyzed by Western blotting with antibodies to the active phosphorylated form of p38MAPK (pp38) or p38MAPK. As shown in Fig. 4, A and B, overexpression of TAK1 and ASK1 led to a substantial increase in pp38 levels without alteration in total p38 levels relative to that of control cells. These data suggest that TAK1 and ASK1 enhance p38MAPK activation in differentiating myoblasts. Next we investigated the effect of TAK1 or ASK1 depletion on p38MAPK activation in C2C12 cells. C2C12/shTak1-1 and C2C12/shAsk1-2 cells were induced to differentiate for 2 days followed by Western blot analysis. The depletion of TAK1 or ASK1 in C2C12 cells caused a significant reduction in pp38 levels relative to that of the control cells (Fig. 4, C–F), suggesting that TAK1 and ASK1 are required for p38MAPK activation during myoblast differentiation. Taken together, these data suggest that both TAK1 and ASK1 are critical for both biochemical and morphological differentiation of myoblasts, most likely via p38MAPK activation.
FIGURE 4.
TAK1 and ASK1 are required for p38MAPK activation in C2C12 myoblasts. A and B, lysates of C2C12 cells stably transfected with TAK1, ASK1, or the respective control expression vectors at differentiation day 2 were immunoblotted with antibodies to pp38 or p38. C and E, lysates of C2C12 cells stably transfected with shTak1-1, shAsk1-2, or the respective control expression vectors at differentiation day 2 were immunoblotted with antibodies to pp38 or p38. D and F, quantification of immunoblots shown in C and E. The intensity of the pp38 and p38 signals was quantified, with values obtained from control vector-transfected cultures set to 1.0. Values represent the means of triplicate determinations ± 1 S.D. The experiment was repeated three times with similar results. Asterisks indicate difference from the control at p < 0.01. G, in vitro kinase assay of p38MAPK by using purified p38α and ASK1 proteins. The p38α protein was used as a substrate, and its phosphorylation was measured by immunoblot analysis with antibodies to pp38 and ASK1. H, quantification of three blots similar immunoblots to those shown in G. The intensity of the pp38 was quantified, with the values obtained from the p38α alone set to 1.0. Values represent the means of triplicate determinations ± 1 S.D. I, the result from in vitro kinase assay using purified p38α, ΜKK6, and ASK1 with the indicated amounts followed by immunoblotting with antibodies to pp38 and MKK6. The experiment was repeated three times with similar results. J, lysates of 293T cells transiently transfected with indicated expression vectors were subjected to Western blot analysis with antibodies to pp38, p38, FLAG (for ASK1), or Cdo. K, lysates of 293T cells transiently transfected with indicated expression vectors were immunoblotted with pp38, p38, HA (for TAK1), or Cdo antibodies. L, 293T cells were transiently transfected with expression vectors for Cdo, ASK1 (FLAG), or p38 plus varying amount of TAK1(HA) vectors as indicated. The lysates were immunoblotted with antibodies to pp38, p38, FLAG, HA, and Cdo.
Next we investigated whether ASK1 could directly activate p38MAPK by in vitro kinase assays with purified p38α and activated ASK1 proteins followed by Western blot analysis with antibodies to pp38, p38 and ASK1. As shown in Fig. 4G, p38α had an autophosphorylation activity, and pp38 levels did not increase when 0.8 μg of ASK1 was added. Fig. 4H shows the quantification of three independent experiments, setting the pp38 signal from p38α alone to 1. These data suggest that ASK1 does not directly phosphorylate p38α. Therefore we then analyzed whether ASK1 activates p38MAPK through MKK6. To this end, kinase assays were carried out by using purified p38α, MKK6, and increasing amount of ASK1 followed by Western blotting with antibodies to pp38. As shown in Fig. 4I, pp38 levels were increased substantially in the response of increased ASK1 proteins. These data suggest that ASK1 enhances p38 activation in the presence of MKK6.
To investigate the role of ASK1 and TAK1 in Cdo-mediated myoblast differentiation, 293T cells were transfected with expression vectors for Cdo and ASK1(FLAG) as indicated, and the lysates were analyzed by Western blotting with antibodies to FLAG, Cdo, pp38, or p38. Consistent with published reports (24, 34), expression of Cdo or ASK1(FLAG) in 293T cells enhanced the level of pp38. However the degree of p38 activation was much greater in ASK1-overexpressing cells than in Cdo-overexpressing cells. This was further increased by cotransfection of ASK1(FLAG) with Cdo, suggesting that Cdo and ASK1 function cooperatively to activate p38 (Fig. 4J). Similarly, Cdo-overexpressing cells showed a slightly increase in pp38 levels, whereas TAK1 overexpression greatly induced p38 activation as compared with the control vector-transfected cells. This increase in pp38 levels was further enhanced when both Cdo and TAK1 were coexpressed in 293T cells (Fig. 4K). Taken together, these data suggest that Cdo cooperates with ASK1 or TAK1 to activate p38MAPK.
We then asked whether ASK1 and TAK1 function cooperatively in p38MAPK activation in the presence of Cdo. To this end, 293T cells were transfected with 2 μg of ASK1(FLAG) or TAK1(HA) singly or cotransfected with a gradually increasing amount of TAK1 (1, 2, and 3 μg), 2 μg of ASK1, and 5 μg of Cdo, and cell lysates were analyzed by Western blotting with antibodies to pp38, p38, HA, FLAG, or Cdo. As shown in Fig. 4L, 293T cells expressing Cdo and ASK1 or TAK1 displayed a significant increase in pp38 levels. ASK1 expression levels were substantially decreased in 293T cells cotransfected with TAK1. This effect was seen also with Cdo in cells cotransfected with either ASK1 or TAK1. Currently the reason for this down-regulation is unknown. Coexpression of ASK1 with TAK1 in combination resulted in stronger activation of p38MAPK compared with cells expressing ASK1 or TAK1 singly. Taken together, these data suggest ASK1 and TAK1 can cooperate with Cdo to enhance p38MAPK activation in 293T cells.
Overexpression of ASK1 or TAK1 Restores p38MAPK Activation and Myotube Formation of Cdo-depleted C2C12 Cells
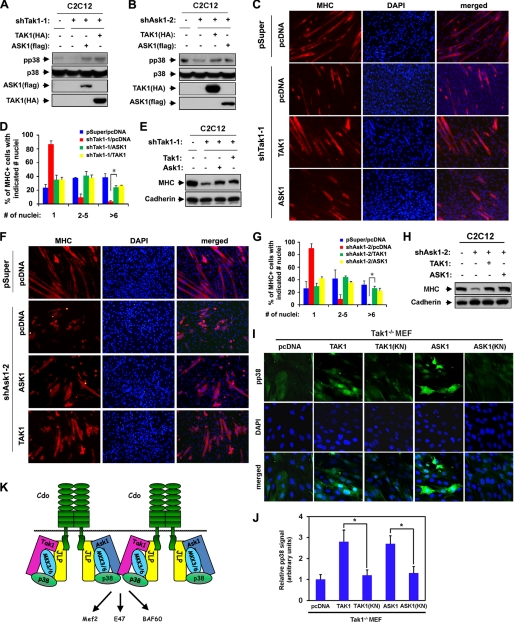
We reported previously that Cdo depletion causes defects in p38MAPK activation and myotube formation, which can be rescued by expression of an activated form of MKK6 (34). Hence, we asked whether the defective p38MAPK activation in Cdo-depleted C2C12 cells could be rescued by overexpression of ASK1 or TAK1. C2C12 cells were stably transfected with Cdo shRNA (shCdo) or pSuper expression vectors. The Cdo depletion in C2C12 cells did not alter the levels of ASK1 and TAK1 proteins (Fig. 5A). C2C12/pSuper and C2C12/shCdo cells were transiently transfected with ASK1, TAK1, or the control expression vectors plus a GFP expression vector to label the transfectants. Two different culture conditions resulting in p38MAPK activation were tested: 1) cultures that were at high density without freshly added serum for 48 h (Fig. 5, B and C); and 2) cultures that had been in DM for 2 days, a midpoint in the differentiation time course (Fig. 5, D and E). Cells were immunostained with antibodies to pp38 and GFP to mark the transfected cells followed by DAPI staining to visualize nuclei. Under culture condition 1, the control vector-transfected C2C12/pSuper cells were mainly mononucleate, whereas ASK1- or TAK1-overexpressing C2C12/pSuper cells had some cells with two nuclei (Fig. 5B, white arrowheads). 56% of the control vector-transfected C2C12/pSuper cells were positive for pp38, whereas 81% of TAK1-expressing cells and 85% of ASK1-expressing cells were positive for pp38 (Fig. 5, B and C (white arrowheads)). The signal intensity was also much enhanced in TAK1- and ASK1-expressing control cells. On the other hand, only 15% of the C2C12/shCdo cells were weakly positive for pp38, whereas TAK1- or ASK1-transfected C2C12/shCdo cells displayed a restoration of pp38 levels to the level in the control C2C12/pSuper/pcDNA cells. 58% of TAK1-expressing cells and 53% of ASK1-expressing C2C12/shCdo cells were positive for pp38 (Fig. 5, B and C (white arrowheads)). Under culture condition 2, whereas C2C12/pSuper cells transfected with control pcDNA, TAK1, or ASK1 expression vectors all formed multinucleate myotubes and were positive for pp38 signal, C2C12/pSuper/TAK1 or C2C12/pSuper/ASK1 cells displayed more intense pp38 staining. In agreement with our previous study, C2C12/shCdo/pcDNA cells showed greatly reduced myotube formation, with only a few cells immunoreactive to anti-pp38 antibodies. In contrast, C2C12/shCdo cells expressing either TAK1 or ASK1 formed multinucleate myotubes that were strongly positive for pp38 signal (Fig. 5D, white arrowheads). To quantify these results, we scored the intensity of the immunofluorescent pp38 signal in untransfected (GFP−) versus transfected (GFP+) cells on the same coverslips, with the average pp38 signal in untransfected control cells set to 1.0. Control vector-transfected cells had a pp38 signal of ∼1, whereas the signal for C2C12/pSuper/TAK1 or C2C12/pSuper/ASK1 cells was ∼1.9 or ∼1.8, respectively. The untransfected or the control vector-transfected C2C12/shCdo cells had a pp38 signal of ∼0.2, whereas the signal for both C2C12/shCdo/TAK1 and C2C12/shCdo/ASK1 cells was restored to ∼0.9 relative to that of the control cells (Fig. 5E). In these cells we did not observe any alteration in total p38 signal (data not shown). These data suggest that TAK1 and ASK1 function downstream of Cdo to activate p38MAPK and promote myoblast differentiation. To verify this data, Cdo+/+ and Cdo−/− primary myoblasts were cotransfected with the control pcDNA, ASK1, or TAK1 and RFP expression vectors to mark transfectants; these cells were induced to differentiate for 1 day by the removal of bFGF followed by immunostaining with antibodies to pp38 and DAPI staining to visualize nuclei. ASK1- or TAK1-overexpressing Cdo−/− myoblast cultures contained more pp38-positive multinucleated myotubes compared with the control transfected Cdo−/− myoblasts. Among pp38-positive cells, 17 or 19% of Cdo−/− myoblasts expressing ASK1 and TAK1 had more than three nuclei, whereas ∼29% of the control-transfected Cdo+/+ myotubes had more than three nuclei, and only ∼4% of the control-transfected Cdo−/− myotubes were in the category of more than three nuclei/myotube (Fig. 5, F and G). This result was further confirmed by immunostaining with MHC antibodies (Fig. 5, H and I). These data suggest that overexpression of ASK1 and TAK1 restores the myogenic differentiation of Cdo−/− myoblasts.
Overexpression of ASK1 or TAK1 in ASK1- or TAK1-depleted Cells Reactivates p38MAPK and Restores Myoblast Differentiation
To further investigate whether these kinases may play qualitatively different roles in p38MAPK activation during myoblast differentiation, C2C12/pSuper and C2C12/shTak1–1 cells were transfected with the control, TAK1(HA), or ASK1(FLAG) expression vectors and induced to differentiate for 2 days followed by immunoblotting with antibodies to pp38, p38, FLAG, or HA. As shown in Fig. 6A, overexpression of either ASK1 or TAK1 in TAK1-depleted C2C12 cells enhanced pp38 levels even higher than the pp38 levels observed in the control cells. Conversely, overexpression of either ASK1 or TAK1 in ASK1-depleted cells restored pp38 levels to the control levels (Fig. 6B). These data demonstrate that ASK1 and TAK1 play a compensatory role in p38MAPK activation. We next analyzed whether this reactivation of p38MAPK would result in the rescue of myoblast differentiation. C2C12/pSuper and C2C12/shTak1-1 were transfected with expression vectors for TAK1, ASK1, or pcDNA and induced to differentiate for 2 days followed by immunostaining with anti-MHC antibodies and DAPI staining. As shown in Fig. 6, C and D, the defective myotube formation of C2C12/shTak1-1 cells was rescued by the reintroduction of TAK1 or the ectopic expression of ASK1. In addition, cell lysates from similar cultures (shown in Fig. 6C) were analyzed by immunoblotting with MHC and cadherin antibodies as a loading control. Consistently, C2C12/shTak1-1 cells expressing either TAK1 or ASK1 displayed restored MHC expression in comparable levels with the control cells (Fig. 6E). In addition, C2C12/pSuper and C2C12/shAsk1-2 cells were also analyzed using methods similar to those described above. The expression of either TAK1 or ASK1 in these cells restored myotube formation as well as MHC expression (Fig. 6, F–H). To verify the effect on p38 activation, Tak1−/− MEF cells were stably cotransfected with the control pcDNA, ASK1, TAK1, ASK1(KN), or TAK1(KN) plus MyoD expression vectors, and p38 activation was analyzed in cells at differentiation day 2 by immunostaining with pp38 antibodies. As shown in Fig. 6I, overexpression of TAK1 and ASK1 resulted in enhanced immunoreactivity for pp38 by ∼2.6–2.7-fold relative to the control-transfected cells. In contrast, kinase-negative TAK1 and ASK1 failed to enhance pp38 signals (Fig. 6, I and J). These data demonstrate that ASK1 and TAK1 can compensate for each other in p38MAPK activation and myogenic differentiation. Taken together, these data demonstrate that ASK1 and TAK1 function as MAP3Ks in Cdo-mediated p38MAPK activation to promote myoblast differentiation.
FIGURE 6.
Overexpression of ASK1 or TAK1 can reactivate p38MAPK in TAK1- or ASK1-depleted cells and restore myogenic differentiation. A, C2C12/pSuper and C2C12/shTak1-1 cells were transfected with TAK1(HA), ASK1(FLAG), or control (−) expression vectors as indicated, and the lysates were subjected to Western blotting with antibodies to pp38, p38, FLAG (for ASK1), or HA (for TAK1). B, C2C12/pSuper and C2C12/shAsk2-1 cells were transfected with TAK1(HA), ASK1(FLAG), or control (−) expression vectors as indicated, and the lysates were subjected to Western blotting with antibodies to pp38, p38, FLAG, or HA. C, photomicrographs of C2C12/pSuper and C2C12/shTak1-1 cells transfected with TAK1(HA), ASK1(FLAG), or pcDNA expression vectors cultured in DM for 2 days, fixed, and immunostained with an antibody to MHC (red). Nuclei were visualized by staining with DAPI (blue). D, quantification of myotube formation by the cell lines shown in C. Values represent the means of triplicate determinations ± 1 S.D. The experiments were repeated three times with similar results. The asterisk indicates difference from the control at p < 0.01. E, lysates of cell lines shown in C were analyzed for myoblast differentiation by immunoblotting with antibodies to MHC and to cadherin as the loading control. F, photomicrographs of C2C12/pSuper and C2C12/shAsk2-1 cells transfected with TAK1(HA), ASK1(FLAG), or pcDNA expression vectors cultured in DM for 2 days, fixed, and immunostained with an antibody to MHC (red). Nuclei were visualized by staining with DAPI (blue). G, quantification of myotube formation by the cell lines shown in F. Values represent the means of triplicate determinations ± 1 S.D. The experiments were repeated three times with similar results. The asterisk indicates difference from the control at p < 0.01. H, lysates of cell lines shown in F were analyzed for myoblast differentiation by immunoblotting with antibodies to MHC and to cadherin as a loading control. I, Tak1−/− MEF cells were transfected sequentially with MyoD expression vectors and expression vectors for ASK1, ASK1(KN), TAK1, TAK1(KN), or the control. Cells were induced to differentiate for 48 h in DM, fixed, and stained with antibodies to pp38MAPK (green). Cell nuclei were visualized by staining with DAPI (blue). J, quantification of cultures shown in I. The intensity of the immunofluorescent pp38 signals was quantified, with the values obtained from control vector-transfected cultures set to 1.0. Values represent the means of triplicate determinations ± 1 S.D. The experiment was repeated three times with similar results. Asterisks indicate difference from the control at p < 0.01. K, schematic diagram summarizing the role of ASK1 and TAK1 as MAP3Ks in Cdo-initiated p38MAPK activation.
DISCUSSION
TAK1 has been proposed to function as a MAP3K to activate p38 and promotes myoblast differentiation (23), whereas the role of ASK1 in myogenesis and whether it activates p38MAPK in myogenesis is unknown. ASK1 has been shown to promote neuronal differentiation of PC12 cells via activation of p38MAPK (25). In our previous study, we showed Cdo promotes neuronal differentiation via activation of p38MAPK (37). This correlation led us to hypothesize that TAK1 and ASK1 may function as MAP3Ks to link Cdo to the p38MAPK signaling pathway. In agreement with this hypothesis, ASK1 or TAK1 forms a complex with Cdo and JLP in 293T cells when overexpressed, or endogenously in C2C12 cells, with a robust interaction at the early differentiation stage. Our preliminary analysis to identify the interacting domain of JLP with ASK1 and TAK1 showed that JLP interacts with p38MAPK and ASK1 or TAK1 via distinct regions. Two separated regions of JLP, amino acids 1–110 and 160–209 interact with p38MAPK (34), whereas JLP binds ASK1 and TAK1 through amino acids 465–1008 (data not shown). This region overlaps with the Cdo-interacting domain, which lies within amino acids 465–647 (34). Further study is required to determine the exact domain of JLP responsible for the interaction with ASK1 and TAK1. The expression levels of ASK1 and TAK1 increased only slightly in myoblasts at high cell density prior to differentiation and decreased modestly in differentiated cells. The association of ASK1 and TAK1 with Cdo has been detected in growing and differentiating myoblasts until differentiation day 2. The highest level of association was detected transitionally in cells at differentiation day 2, which coincides with the activation of p38MAPK (34), and in myogenic bHLH transcription factors, which is evidenced by expression of MHC at differentiation day 2. Our results suggest that both ASK1 and TAK1 are essential for myoblast differentiation, as the depletion of either ASK1 or TAK1 caused a reduction in p38MAPK activation and defective myotube formation. Conversely, overexpression of TAK1 or ASK1 enhanced the expression of myogenic markers and myotube formation. This promyogenic role of TAK1 and ASK1 may also involve their role in cell proliferation, because both TAK1 and ASK1 knockdown impaired the proliferative capacity of C2C12 cells at differentiation day 1 without increasing cell death. These data are consistent with a previous study reporting that TAK1 depletes C2C12 cells and Tak1−/− fibroblasts proliferate more slowly than control cells (23).
ASK1 and TAK1 may function independently in a temporal regulation of p38MAPK activation during myoblast differentiation. It appears that both ASK1 and TAK1 are required for p38MAPK activation and efficient myoblast differentiation. Because cotransfection of Cdo, ASK1, and TAK1 affected the expression levels of ASK1 and Cdo, we were unable to address whether ASK1 and TAK1 interact with Cdo cooperatively or independently. However, it appears that ASK1 and TAK1 play an interchangeable role in p38MAPK activation, because overexpression of either ASK1 or TAK1 in ASK1- or TAK1-depleted cells or in Tak−/− MEFs restored p38MAPK activation and myoblast differentiation. These data suggest that the threshold level of p38MAPK activation by either ASK1 or TAK1 is required for myoblast differentiation rather than implying any independent role for ASK1 or TAK1 in myoblast differentiation triggered by high cell density and serum removal. Similar to the ability of MKK6 to rescue defective p38MAPK activation and myoblast differentiation caused by Cdo deficiency, TAK1 or ASK1 overexpression restored myotube formation and p38MAPK activation in Cdo-depleted myoblasts and Cdo−/− myoblasts. These data indicate that ASK1 and TAK1 function downstream of Cdo-mediated promyogenic signaling. ASK1 and TAK1 have been shown to activate both JNK and p38 in response to various stimuli including cellular stress (16, 44). In contrast to the promyogenic role of p38MAPK, activation of JNK is associated with inhibition of myogenesis (45). ASK1 and TAK1 appear to activate p38MAPK specifically without modulating JNK activation under the differentiation conditions used in this study (data not shown). The interaction of ASK1 and TAK1 with the scaffold protein JLP may be one way to activate p38MAPK specifically in response to cell adhesion signaling triggered by N-cadherin/Cdo during myoblast differentiation. Because Cdo lacks an intrinsic enzyme activity (e.g. receptor tyrosine kinase), the molecular mechanism of MAPK activation by Cdo may differ from other known receptor-mediated signaling mechanisms. It is conceivable that the formation of multiple kinase complexes through JLP may cause the activation of kinases via conformational changes in these kinases (Fig. 6K). In our recent report (43), the non-receptor tyrosine kinase Abl interacts with the cytoplasmic region of Cdo and JLP and cooperates with JLP and Cdo to activate p38MAPK and promote myoblast differentiation. It is possible that Abl may be responsible for activating ASK1 and TAK1 to initiate the downstream signaling pathway. However Abl may not act solely as a kinase in this context, as a kinase-deficient mutant form of Abl retains partial activity in stimulating p38MAPK activity (43). Based on these data, we propose that N-cadherin-mediated cell contact may induce a complex formation of Cdo with JLP, ASK1/TAK1, and p38MAPK, thereby enhancing the activation of p38MAPK.
Supplementary Material
Acknowledgments
We thank Drs. Ruth Simon and Kyung Lee for critical reading of the manuscript.
This work was supported by National Research Foundation (NRF) Bio and Medical Technology Development Program Grant 2011-0030154 and NRF Grant 2011-0017315 (to J. S. K.) funded by the Korean government (MEST).

This article contains supplemental Fig. 1.
- TAK1
- TGF-β-activated kinase 1
- ASK1
- apoptosis signal-regulating kinase 1
- DM
- differentiation medium
- bFGF
- basic fibroblast growth factor
- RFP
- red fluorescent protein
- MEF
- mouse embryonic fibroblast
- JLP
- JNK-associated leucine zipper protein.
REFERENCES
- 1. Molkentin J. D., Olson E. N. (1996) Defining the regulatory networks for muscle development. Curr. Opin. Genet. Dev. 6, 445–453 [DOI] [PubMed] [Google Scholar]
- 2. Tapscott S. J. (2005) The circuitry of a master switch: MyoD and the regulation of skeletal muscle gene transcription. Development 132, 2685–2695 [DOI] [PubMed] [Google Scholar]
- 3. Cuenda A., Cohen P. (1999) Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J. Biol. Chem. 274, 4341–4346 [DOI] [PubMed] [Google Scholar]
- 4. Wu Z., Woodring P. J., Bhakta K. S., Tamura K., Wen F., Feramisco J. R., Karin M., Wang J. Y., Puri P. L. (2000) p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol. Cell. Biol. 20, 3951–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson G. L., Lapadat R. (2002) Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298, 1911–1912 [DOI] [PubMed] [Google Scholar]
- 6. Perdiguero E., Ruiz-Bonilla V., Gresh L., Hui L., Ballestar E., Sousa-Victor P., Baeza-Raja B., Jardí M., Bosch-Comas A., Esteller M., Caelles C., Serrano A. L., Wagner E. F., Muñoz-Cánoves P. (2007) Genetic analysis of p38MAP kinases in myogenesis: fundamental role of p38α in abrogating myoblast proliferation. EMBO J. 26, 1245–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keren A., Tamir Y., Bengal E. (2006) The p38 MAPK signaling pathway: a major regulator of skeletal muscle development. Mol. Cell. Endocrinol. 252, 224–230 [DOI] [PubMed] [Google Scholar]
- 8. Lluís F., Ballestar E., Suelves M., Esteller M., Muñoz-Cánoves P. (2005) E47 phosphorylation by p38 MAPK promotes MyoD/E47 association and muscle-specific gene transcription. EMBO J. 24, 974–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruiz-Bonilla V., Perdiguero E., Gresh L., Serrano A. L., Zamora M., Sousa-Victor P., Jardí M., Wagner E. F., Muñoz-Cánoves P. (2008) Efficient adult skeletal muscle regeneration in mice deficient in p38β, p38γ, and p38δ MAP kinases. Cell Cycle 7, 2208–2214 [DOI] [PubMed] [Google Scholar]
- 10. de la Serna I. L., Carlson K. A., Imbalzano A. N. (2001) Mammalian SWI/SNF complexes promote MyoD-mediated muscle differentiation. Nat. Genet. 27, 187–190 [DOI] [PubMed] [Google Scholar]
- 11. Simone C., Forcales S. V., Hill D. A., Imbalzano A. N., Latella L., Puri P. L. (2004) p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat. Genet. 36, 738–743 [DOI] [PubMed] [Google Scholar]
- 12. Serra C., Palacios D., Mozzetta C., Forcales S. V., Morantte I., Ripani M., Jones D. R., Du K., Jhala U. S., Simone C., Puri P. L. (2007) Functional interdependence at the chromatin level between the MKK6/p38 and IGF1/PI3K/AKT pathways during muscle differentiation. Mol. Cell 28, 200–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shim J. H., Xiao C., Paschal A. E., Bailey S. T., Rao P., Hayden M. S., Lee K. Y., Bussey C., Steckel M., Tanaka N., Yamada G., Akira S., Matsumoto K., Ghosh S. (2005) TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 19, 2668–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huangfu W. C., Omori E., Akira S., Matsumoto K., Ninomiya-Tsuji J. (2006) Osmotic stress activates the TAK1-JNK pathway while blocking TAK1-mediated NF-κB activation. TAO2 regulates TAK1 pathways. J. Biol. Chem. 281, 28802–28810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takeda K., Noguchi T., Naguro I., Ichijo H. (2008) Apoptosis signal-regulating kinase 1 in stress and immune response. Annu. Rev. Pharmacol. Toxicol. 48, 199–225 [DOI] [PubMed] [Google Scholar]
- 16. Wang C., Deng L., Hong M., Akkaraju G. R., Inoue J., Chen Z. J. (2001) TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412, 346–351 [DOI] [PubMed] [Google Scholar]
- 17. Sato S., Sanjo H., Takeda K., Ninomiya-Tsuji J., Yamamoto M., Kawai T., Matsumoto K., Takeuchi O., Akira S. (2005) Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 6, 1087–1095 [DOI] [PubMed] [Google Scholar]
- 18. Omori E., Matsumoto K., Sanjo H., Sato S., Akira S., Smart R. C., Ninomiya-Tsuji J. (2006) TAK1 is a master regulator of epidermal homeostasis involving skin inflammation and apoptosis. J. Biol. Chem. 281, 19610–19617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu H. H., Xie M., Schneider M. D., Chen Z. J. (2006) Essential role of TAK1 in thymocyte development and activation. Proc. Natl. Acad. Sci. U.S.A. 103, 11677–11682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sayama K., Hanakawa Y., Nagai H., Shirakata Y., Dai X., Hirakawa S., Tokumaru S., Tohyama M., Yang L., Sato S., Shizuo A., Hashimoto K. (2006) Transforming growth factor-β-activated kinase 1 is essential for differentiation and the prevention of apoptosis in epidermis. J. Biol. Chem. 281, 22013–22020 [DOI] [PubMed] [Google Scholar]
- 21. Tang M., Wei X., Guo Y., Breslin P., Zhang S., Zhang S., Wei W., Xia Z., Diaz M., Akira S., Zhang J. (2008) TAK1 is required for the survival of hematopoietic cells and hepatocytes in mice. J. Exp. Med. 205, 1611–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shim J. H., Greenblatt M. B., Xie M., Schneider M. D., Zou W., Zhai B., Gygi S., Glimcher L. H. (2009) TAK1 is an essential regulator of BMP signaling in cartilage. EMBO J. 28, 2028–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhatnagar S., Kumar A., Makonchuk D. Y., Li H., Kumar A. (2010) Transforming growth factor-β-activating kinase 1 is an essential regulator of myogenic differentiation. J. Biol. Chem. 285, 6401–6411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ichijo H., Nishida E., Irie K., ten Dijke P., Saitoh M., Moriguchi T., Takagi M., Matsumoto K., Miyazono K., Gotoh Y. (1997) Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275, 90–94 [DOI] [PubMed] [Google Scholar]
- 25. Takeda K., Hatai T., Hamazaki T. S., Nishitoh H., Saitoh M., Ichijo H. (2000) Apoptosis signal-regulating kinase 1 (ASK1) induces neuronal differentiation and survival of PC12 cells. J. Biol. Chem. 275, 9805–9813 [DOI] [PubMed] [Google Scholar]
- 26. Sayama K., Hanakawa Y., Shirakata Y., Yamasaki K., Sawada Y., Sun L., Yamanishi K., Ichijo H., Hashimoto K. (2001) Apoptosis signal-regulating kinase 1 (ASK1) is an intracellular inducer of keratinocyte differentiation. J. Biol. Chem. 276, 999–1004 [DOI] [PubMed] [Google Scholar]
- 27. Faigle R., Brederlau A., Elmi M., Arvidsson Y., Hamazaki T. S., Uramoto H., Funa K. (2004) ASK1 inhibits astrogial development via p38 mitogen-activated protein kinase and promotes neuronal differentiation in adult hippocampus-derived progenitor cells. Mol. Cell. Biol. 24, 280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cole F., Zhang W., Geyra A., Kang J. S., Krauss R. S. (2004) Positive regulation of myogenic bHLH factors and skeletal muscle development by the cell surface CDO. Dev. Cell 7, 843–854 [DOI] [PubMed] [Google Scholar]
- 29. Han J. W., Lee H. J., Bae G. U., Kang J. S. (2011) Promyogenic function of integrin/FAK signaling is mediated by Cdo, Cdc42, and MyoD. Cell. Signal. 23, 1162–1169 [DOI] [PubMed] [Google Scholar]
- 30. Kang J. S., Mulieri P. J., Hu Y., Taliana L., Krauss R. S. (2002) BOC, an Ig superfamily member, associates with CDO to positively regulate myogenic differentiation. EMBO J. 21, 114–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kang J. S., Feinleib J. L., Knox S., Ketteringham M. A., Krauss R. S. (2003) Promyogenic members of the Ig and cadherin families associate to positively regulate differentiation. Proc. Natl. Acad. Sci. U.S.A. 100, 3989–3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kang J. S., Yi M. J., Zhang W., Feinleib J. L., Cole F., Krauss R. S. (2004) Netrins and neogenin promote myotube formation. J. Cell Biol. 167, 493–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leem Y. E., Han J. W., Lee H. J., Ha H. L., Kwon Y. L., Ho S. M., Kim B. G., Tran P., Bae G. U., Kang J. S. (2011) Gas1 cooperates with Cdo and promotes myogenic differentiation via activation of p38MAPK. Cell. Signal. 12, 2021–2029 [DOI] [PubMed] [Google Scholar]
- 34. Takaesu G., Kang J. S., Bae G. U., Yi M. J., Lee C. M., Reddy E. P., Krauss R. S. (2006) Activation of p38α/β MAPK in myogenesis via binding of the scaffold protein JLP to the cell surface protein Cdo. J. Cell Biol. 175, 383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kang J. S., Bae G. U., Yi M. J., Yang Y. J., Oh J. E., Takaesu G., Zhou Y. T., Low B. C., Krauss R. S. (2008) A Cdo-Bnip2-Cdc42 signaling pathway regulates p38α/β MAPK activity and myogenic differentiation. J. Cell Biol. 182, 497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lu M., Krauss R. S. (2010) N-cadherin ligation, but not Sonic hedgehog binding, initiates Cdo-dependent p38α/β MAPK signaling in skeletal myoblasts. Proc. Natl. Acad. Sci. U.S.A. 107, 4212–4217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oh J. E., Bae G. U., Yang Y. J., Yi M. J., Lee H. J., Kim B. G., Krauss R. S., Kang J. S. (2009) Cdo promotes neuronal differentiation via activation of the p38 mitogen-activated protein kinase pathway. FASEB J. 23, 2088–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bae G. U., Lee J. R., Kim B. G., Han J. W., Leem Y. E., Lee H. J., Ho S. M., Hahn M. J., Kang J. S. (2010) Cdo interacts with APPL1 and activates Akt in myoblast differentiation. Mol. Biol. Cell 21, 2399–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee H. J., Bae G. U., Leem Y. E., Choi H. K., Kang T. M., Cho H., Kim S. T., Kang J. S. (2012) Phosphorylation of Stim1 at serine-575 via Netrin-2/Cdo-activated ERK1/2 is critical for the promyogenic function of Stim. Mol. Biol. Cell, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamaguchi K., Shirakabe K., Shibuya H., Irie K., Oishi I., Ueno N., Taniguchi T., Nishida E., Matsumoto K. (1995) Identification of a member of the MAPKKK family as a potential mediator of TGF-β signal transduction. Science 270, 2008–2011 [DOI] [PubMed] [Google Scholar]
- 41. Shibuya H., Yamaguchi K., Shirakabe K., Tonegawa A., Gotoh Y., Ueno N., Irie K., Nishida E., Matsumoto K. (1996) TAB1, an activator of the TAK1 MAPKKK in TGF-β signal transduction. Science 272, 1179–1182 [DOI] [PubMed] [Google Scholar]
- 42. Zhang W., Kang J. S., Cole F., Yi M. J., Krauss R. S. (2006) Cdo functions at multiple points in the Sonic Hedgehog pathway, and Cdo-deficient mice accurately model human holoprosencephaly. Dev. Cell 10, 657–665 [DOI] [PubMed] [Google Scholar]
- 43. Bae G. U., Kim B. G., Lee H. J., Oh J. E., Lee S. J., Zhang W., Krauss R. S., Kang J. S. (2009) Cdo binds Abl to promote p38α/β mitogen-activated protein kinase activity and myogenic differentiation. Mol. Cell. Biol. 29, 4130–4143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tobiume K., Matsuzawa A., Takahashi T., Nishitoh H., Morita K., Takeda K., Minowa O., Miyazono K., Noda T., Ichijo H. (2001) ASK1 is required for sustained activations of JNK/p38MAP kinases and apoptosis. EMBO Rep. 2, 222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meriane M., Roux P., Primig M., Fort P., Gauthier-Rouvière C. (2000) Critical activities of Rac1 and Cdc42Hs in skeletal myogenesis: antagonistic effects of JNK and p38 pathways. Mol. Biol. Cell 11, 2513–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.