Background: The interplay of lipid signaling with macrophage phenotype is critical for vascular disease progression.
Results: ER stress links scavenger receptor signaling to macrophage phenotype and foam cell formation through a JNK- and PPARγ-dependent pathway.
Conclusion: ER stress is a functional switch controlling macrophage phenotype and cellular cholesterol content.
Significance: Suppression of ER stress is a potential therapeutic target to reduce atherosclerosis progression.
Keywords: Atherosclerosis, Diabetes, ER Stress, Jun N-terminal Kinase (JNK), Peroxisome Proliferator-activated Receptor (PPAR), Foam Cell, Macrophage Phenotype
Abstract
Macrophages are essential in atherosclerosis progression, but regulation of the M1 versus M2 phenotype and their role in cholesterol deposition are unclear. We demonstrate that endoplasmic reticulum (ER) stress is a key regulator of macrophage differentiation and cholesterol deposition. Macrophages from diabetic patients were classically or alternatively stimulated and then exposed to oxidized LDL. Alternative stimulation into M2 macrophages lead to increased foam cell formation by inducing scavenger receptor CD36 and SR-A1 expression. ER stress induced by alternative stimulation was necessary to generate the M2 phenotype through JNK activation and increased PPARγ expression. The absence of CD36 or SR-A1 signaling independently of modified cholesterol uptake decreased ER stress and prevented the M2 differentiation typically induced by alternative stimulation. Moreover, suppression of ER stress shifted differentiated M2 macrophages toward an M1 phenotype and subsequently suppressed foam cell formation by increasing HDL- and apoA-1-induced cholesterol efflux indicating suppression of macrophage ER stress as a potential therapy for atherosclerosis.
Introduction
Macrophages play a critical role in the development of atherosclerosis. Monocytes recruited to the subendothelial space at sites of endothelial cell activation respond to environmental signals and differentiate into macrophages with diverse functional programs. In atherosclerotic plaque, IFNγ, a T-helper 1 cytokine, induces classically activated macrophages (M1) known to express membrane receptors C-C chemokine receptor type 7 (CCR7)4 and CD86 (cluster of differentiation 86) and characterized by the generation of proinflammatory cytokines and reactive oxygen and nitrogen intermediates, accelerating additional immune cell recruitment, and subendothelial cell remodeling (1–3). Recent evidence suggests that alternatively activated macrophages (M2) known to be induced by IL-4 or -10 or immunocomplex also are abundantly expressed in advanced human atherosclerotic lesions (4). These cells are characterized by the expression of CD163 and mannose receptor (MR), as well as increased IL-10 secretion and suppressed IL-12 expression, attenuating excessive inflammation and facilitating collagen production and fibrosis (5, 6). Previous studies have demonstrated that macrophages are plastic cells that can shift their differentiated phenotype back and forth from M1 to M2 under various environmental conditions (7). In chow-fed apolipoprotein E knock-out (ApoE−/−) mice, lesion-infiltrated macrophages of young mice exhibit predominantly the M2 phenotype, whereas M1 macrophages are dominant in more advanced lesions of aged mice (8). Moreover, phenotypic conversion of plaque-infiltrated macrophages from M1 to M2 was implicated in plaque regression in atherosclerotic mouse models (9, 10); therefore, understanding environmental conditions that promote anti-inflammatory macrophage properties and identifying macrophage signaling pathways that control phenotype plasticity are critical to the development of novel therapeutic strategies for the treatment of atherosclerosis.
The endoplasmic reticulum (ER) is a dynamic membranous organelle that facilitates correct protein modification, folding, and maturation of transmembrane, secretory, and ER-resident proteins. Unfolded protein response (UPR) is an adaptive intracellular signaling pathway that responds to ER stress by attenuating global protein translation and degrading unfolded proteins. During monocyte differentiation by macrophage colony stimulating factor (M-CSF), the ER undergoes structural as well as functional reorganization to perform the new cell functions, leading to ER stress and up-regulated UPR (11–14). In mouse models of diet-induced insulin resistance and atherosclerosis, up-regulated UPR markers are detected in intimal macrophages at early stages of vascular inflammation, even before the formation of fatty streaks or atherosclerotic plaques (15), and as the plaque evolves into late stages, adverse environmental changes, including increased macrophage-free cholesterol, accelerate macrophage apoptosis through an ER stress-dependent mechanism (16). Moreover, persistent macrophage ER stress accelerates modified cholesterol uptake by these cells through peroxisome proliferator-activated receptor γ (PPARγ)-signaling activation (17). Stimulation of PPARγ and -δ also facilitates transformation of undifferentiated macrophages to the alternatively activated M2 phenotype (18–20). Therefore, the ER stress response in the vessel wall could integrate macrophage cholesterol metabolism and the mechanisms controlling macrophage phenotype differentiation, both key factors in atherosclerosis progression.
Insulin resistance is a potent inducer of prolonged ER stress in macrophages. Patients with type 2 diabetes mellitus (T2DM) have accelerated plaque progression and larger, more necrotic plaques than their non-diabetic counterparts (21). Macrophages with defective insulin signaling are more susceptible to ER stress and the associated apoptosis and plaque necrosis in a mouse model of diet-induced insulin resistance and atherosclerosis (22). Conversely, ER stress promotes insulin resistance, generating a vicious cycle that leads to plaque instability. In mouse models of diet-induced insulin resistance and atherosclerosis, suppression of ER stress decreases vascular inflammation and prevents the development of atherosclerosis (23, 24). In T2DM patients, a predominance of the M1 over M2 phenotype in peripheral blood monocytes is linked to arterial wall stiffness (25). This makes T2DM an ideal model to study whether regulation of ER stress in insulin-resistant macrophages could influence macrophage phenotype and alter the increased foam cell formation seen in this disease.
We thus evaluated whether ER homeostasis regulates the selection of the macrophage phenotype in T2DM patients and clarified whether macrophage phenotype influences foam cell formation in vitro and in vivo. In addition, we explored whether mediators of ER stress could shift macrophage phenotype.
EXPERIMENTAL PROCEDURES
Population
Our study population included 50 predominantly female (62%), African-American (56%), obese adult subjects with T2DM. Subjects were recruited voluntarily from the outpatient clinic at Barnes-Jewish Hospital (St. Louis, MO). Each subject was provided with written informed consent, approved by the Human Research Protection Office of Washington University School of Medicine. The population had a mean age of 54 ± 1.3 years, body mass index of 36 ± 1.3 kg/m2, blood pressure of 133/80 ± 2.1/1.8 mm Hg, and hemoglobin A1c level of 7.8 ± 0.3%. Approximately half were on treatment for hypertension (45%) and/or dyslipidemia (55%), and most (>70%) had T2DM for >5 years with 20% on insulin therapy. All parametric variables tested were distributed normally.
Isolation and Preparation of Primary Human Monocytes
Peripheral monocytes were isolated by standard Ficoll isolation techniques and selected by CD14 marker positivity (Miltenyi Biotec, Auburn, CA). CD14+/CD11b+ cell purity reached 97% as assessed by flow cytometry (FACStar Plus, BD Biosciences). Monocytes were cultured with 100 ng/ml of M-CSF for 5 days in DMEM plus 10% FBS. Macrophages were cultured for an additional 24 h in DMEM plus 10% FBS and IFNγ (20 ng/ml) plus LPS (100 ng/ml) (classical stimulation for M1 differentiation) or with either IL-4 (20 ng/ml), immunocomplex (IC; 10 μg/ml ovalbumin with 100 μg/ml anti-ovalbumin) plus LPS (100 ng/ml), or IL-10 (10 ng/ml) (alternative stimulation for M2a, M2b, and M2c differentiation, respectively)., Reduction of ER stress was obtained by adding a chemical chaperone, phenylbutyric acid (PBA; 20 mm, Calbiochem, San Diego, CA) for the 24 h during or for 24 h following M1 or M2 differentiation. Induction of ER stress was obtained by adding thapsigargin (0.25 nm, Sigma) to cultured macrophages for 24 h. Inhibition of phospho-JNK (p-JNK) was obtained by adding SP600125 (100 nmol/liter, SA Bioscience Corp., Frederick, MD) for the 24 h during or for 24 h following M1 or M2 differentiation.
Isolation of Murine Peritoneal Macrophages
Peritoneal macrophages from mice lacking either CD36 (provided by Roy L. Silverstein at Cleveland Clinic), scavenger receptor (SR)-A1, JNK2, or CCAAT/Enhancer binding protein homologous protein (CHOP) (The Jackson Laboratory), from M lysozyme (LysM)-Cre PPARγ mice (provided by F. J. Gonzalez, National Institutes of Health, Bethesda, MD), and from WT mice were isolated 3 days after intraperitoneal injection of 4% thioglycollate solution, as described previously (26). Cells were selected by fluorescence-activated cell sorting for F4/80 (e-Biosciences, San Diego, CA) and CD11b (BD Biosciences) antigen expression. Macrophages were evaluated subsequently for membrane receptor expression, cholesterol metabolism, and harvested to isolate RNA or protein according to standard methods.
Macrophage Cholesterol Homeostasis
Foam cell formation (Oil Red O stain), cholesteryl ester formation, total cholesterol, and cholesterol uptake, binding, and efflux were assessed as we described previously (17) in differentiated human or murine peritoneal macrophages. Images for qualitative macrophage cholesterol uptake were assessed by confocal microscopy. Detailed description is presented in supplemental data.
Mouse Atherosclerotic Lesions
To detect the colocalization of differentiated macrophages with cholesterol deposition in the atherosclerotic plaque in vivo, we stained formalin-fixed 10-μm serial cryosections of the proximal aorta from ApoE−/− mice fed a Western diet for 8 weeks with antibodies specific for CCR7 (M1 marker) or MR (M2 marker) (Santa Cruz Biotechnology) and adipocyte differentiation-related protein (American Research Product, Belmont, MA) following the manufacturer's recommendations. Plaque M1 or M2 macrophages were measured by the percentage of total plaque area with staining for the membrane receptor for each phenotype. Lipid colocalization with M1 or M2 macrophages was measured by the percentage of total adipocyte differentiation-related protein staining area colocalizing with staining for the membrane receptor for each phenotype.
Flow Cytometry
Macrophage protein analysis was performed using FACStar Plus with PE-Conjugated anti-CCR7 and anti-CD86 (E-Bioscience) for M1 macrophage membrane protein expression and FITC-conjugated anti-CD 163 and anti-MR (E-Bioscience) for M2 macrophage membrane protein expression.
Gene Expression, Western Blot Analysis
Quantitative RT-PCR analyses were performed by Sybrgreen methodologies. Results were normalized to the housekeeping gene L32. Western blot protein expression analysis from macrophages was normalized to β-actin expression; that of phosphoproteins was normalized to the respective total protein expression. Detailed description is included in the supplemental data.
Statistical Analysis
Experiments were carried out with duplicate or triplicate samples. All data are expressed as means ± S.E. of the mean for continuous variables and as a ratio for categorical data. Statistical significance of differences was calculated using t tests for parametric data involving two groups and ANOVA for parametric data with Tukey's test for multiple groups. Differences were considered statistically significant if p ≤ 0.05.
RESULTS
M2 Macrophage Differentiation Increases Foam Cell Formation
To investigate the interplay between macrophage cholesterol metabolism and phenotype, we collected monocytes from 50 predominantly obese type 2 diabetics. We stimulated macrophages previously induced by M-CSF with either IFNγ plus LPS to promote formation of M1 macrophages (classical stimulation) or IL-4, IC plus LPS, or IL-10 to promote formation of M2 macrophage subtypes (alternative stimulation; M2a, M2b, and M2c, respectively) (supplemental Fig. S1). In contrast, the expression of DC-SIGN (dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin) and CD1a was almost undetectable in M1 and M2 macrophages, showing that dendritic cell differentiation is not induced in our model (data not shown). We then exposed M1 and M2 macrophages to oxidized low density lipoprotein (oxLDL) to determine the effects of macrophage differentiation on foam cell formation. M2 macrophages exhibited a significant increase in foam cell formation compared with M1 macrophages (Fig. 1A, arrows). M2 macrophages exposed to oxLDL had 2–3 times higher cholesterol deposition (total cholesterol and cholesteryl ester formation), compared with M1 and unstimulated macrophages (Fig. 1, B and C, p < 0.001 for all).
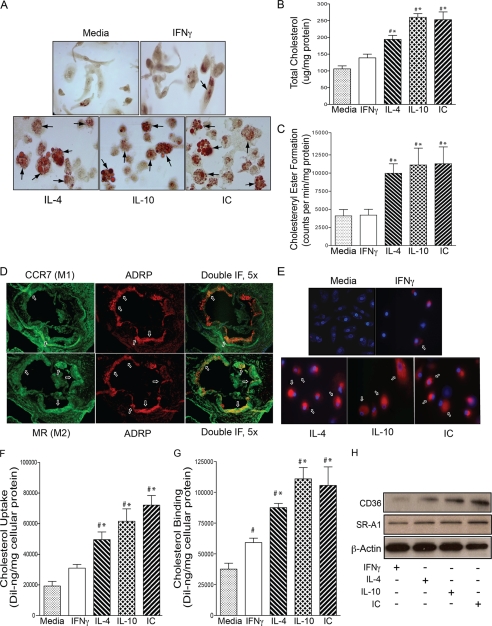
FIGURE 1.
M2 macrophage differentiation induces foam cell formation. For A–C and E–H, macrophages from diabetic patients were differentiated with IFNγ to promote M1 differentiation or with IL-4, IL-10, or IC to promote M2 differentiation and then stimulated with oxLDL. A, staining with Oil Red O. Top panel, media, IFNγ-treated cells; Bottom panel, IL-4, IL-10, and IC-treated cells. Arrowheads indicate foam cells. Shown are total cholesterol (B) and cholesteryl ester (C) formation in macrophages incubated with media (gray), IFNγ (white), IL-4 (back-slashed), IL-10 (hatched), and IC (front-slashed) (n = 8 per group). *, p < 0.0001 versus media; #, p < 0.001 versus IFNγ by ANOVA. D, immunofluorescent staining of antibodies to M1 or M2 macrophages in the aortic sinus atherosclerotic lesions from ApoE−/− mice after 8 weeks on Western diet (n = 5 per group). Top panel, arrows indicate stain for M1 marker CCR7 (green), ARDP (red), and co-staining for ARDP and CCR7 (yellow). Bottom panel, arrows indicate stain for M2 marker MR (green), ARDP (red), and costaining for ARDP and MR (yellow). Scale bars, 100 μm. E, macrophage cholesterol uptake assessed by confocal microscopy. Red represents labeled cholesterol uptake after 6 h of stimulation with 1,1′-dioctadecyl-3,3, 3′3′-tetramethylindocarbocyanine perchlorate oxLDL; blue represents nuclear counterstain. Shown are quantification of macrophage cholesterol uptake (F) and cholesterol binding (G) by mean fluorescence absorbance after DiI-oxLDL stimulation (n = 8 per group). *, p < 0.0001 versus media; #, p < 0.01 versus IFNγ by ANOVA for both. H, Western blot for CD36 and SR-A1 expression. IF, immunofluorescence.
To confirm that M2 macrophages differentiate into foam cells in atherosclerotic plaques in vivo, we examined frozen sections of the aortic root from ApoE−/− mice after 8 weeks of high fat diet. ApoE−/− mice are a model of diet-induced insulin resistance and atherosclerosis. M2 macrophages (MR in green, bottom panel) were localized more centrally within the plaque compared with M1 macrophages (CCR7 in green, upper panel), which localized to the periphery of the plaque. M2 macrophages were more prevalent as a percentage of the atherosclerotic plaque and had a higher proportion of adipocyte differentiation-related protein expression (a marker of lipid deposition; red with colocalization in yellow) compared with M1 macrophages, suggesting that M2 cells accumulate more cholesteryl ester (Fig. 1D and supplemental Fig. S2).
M2 Macrophage Differentiation Accelerates Cholesterol Uptake
To investigate the mechanism underlying increased foam cell formation in M2 macrophages, we assessed cholesterol uptake in M1 versus M2 macrophages from diabetic patients. Confocal microscopy after fluorescence-labeled 1,1′-dioctadecyl-3,3, 3′3′-tetramethylindocarbocyanine perchlorate oxLDL stimulation showed that M2 macrophages had increased oxLDL cholesterol uptake both qualitatively and quantitatively by 40 to 70% when compared with M1 and unstimulated macrophages (Fig. 1, E and F, p < 0.01 for all). In addition, M2 macrophages also had 20–42% more macrophage cholesterol binding compared with M1 and unstimulated macrophages (Fig. 1G, p < 0.002 for all). In this work, we found that M2 macrophages had higher CD36 and SR-A1 (essential macrophage scavenger receptors for internalization of modified LDL) protein expression when compared with M1 macrophages (Fig. 1H and supplemental Fig. S3), suggesting that during M2 differentiation, macrophages up-regulate a common pathway that facilitates modified LDL cholesterol uptake.
We also evaluated cholesterol efflux in M1 versus M2 macrophages. M2 macrophages had no differences in apolipoprotein A-I (apoA-1) or HDL-stimulated cholesterol efflux compared with M1 macrophages (supplemental Fig. S4). These findings indicate clear differences between M2 and M1 macrophages in the regulation of cholesterol handling, resulting in a net increase in cholesterol deposition due to increased cholesterol uptake.
ER Stress Controls M2 Macrophage Differentiation and OxLDL Cholesterol Uptake
Excess cellular cholesterol trafficking to the ER triggers the unfolded protein response (27). ER stress and UPR are known to occur during early atherosclerotic plaque formation and induce SR-A1 and CD36 expression (15, 16, 23). In our patients, alternatively stimulated (with IL-4, IL-10, or IC) macrophages had significantly increased activation of UPR markers phosphopancreatic ER kinase, CHOP, and phosphoinositol requiring transmembrane kinase/endonuclease 1α when compared with classically stimulated (with IFNγ) macrophages (Fig. 2A and supplemental Fig. S5A).
FIGURE 2.
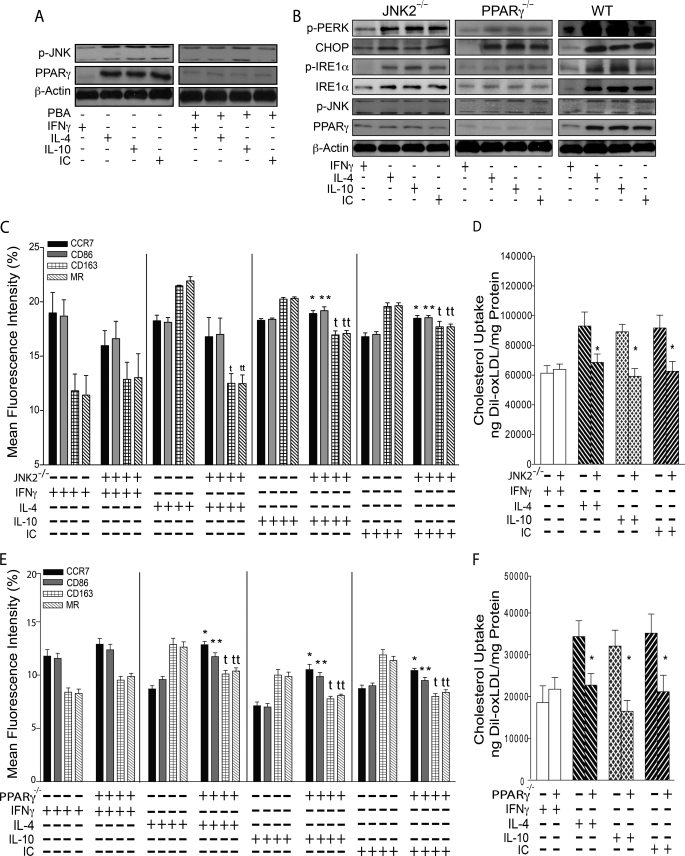
M2 macrophage differentiation and cholesterol uptake are ER stress-dependent in diabetics. For A–C, macrophages from diabetic patients were differentiated with INFγ to promote M1 differentiation or with IL-4, IL-10, or IC to promote M2 differentiation and co-incubated with or without PBA (ER stress inhibitor). A, ER stress protein activation/expression. B, flow cytometry quantification of membrane receptors. M1 receptors, CCR7 (black), CD86 (gray); M2 receptors, CD163 (grid), MR (back-slashed) (n = 9 per group). t and tt, p < 0.001 versus same receptor in non-PBA-treated. C, quantification of cholesterol uptake after differentiation with INFγ (white), IL-4 (back-slashed), IL-10 (hatched), or IC (front-slashed) and stimulation with oxLDL (n = 9 per group). *, p < 0.01 versus same stimulation in non-PBA-treated. For D–F, cultured macrophages from diabetic patients were treated with or without ER stress inducer thapsigargin. D, ER stress protein activation/expression. E, flow cytometry quantification of membrane receptors (n = 8 per group). *, p < 0.04; **, p < 0.02; t and tt, p < 0.01 versus same receptor in non-thapsigargin-treated. F, quantification of cholesterol uptake after stimulation with oxLDL with (black) or without (white) thapsigargin (n = 6 per group). *, p < 0.02 versus non-thapsigargin-treated. For G and H, peritoneal macrophages from CHOP−/− or WT mice were differentiated to M1 or M2 macrophages as described above for A–C. G, flow cytometry quantification of membrane receptors (n = 8 per group). * and **, p < 0.04; t and tt, p < 0.01 versus same receptor in WT. H, quantification of cholesterol uptake after stimulation with oxLDL (n = 8 per group). *, p < 0.04 versus same stimulation in WT.
To clarify whether ER stress activation is responsible for M2 differentiation and increased cholesterol uptake seen in alternatively stimulated macrophages, we treated macrophages with or without PBA (chemical chaperone, known to reduce ER stress), concurrently with classical or alternative stimuli of differentiation. PBA decreased ER stress, demonstrated by reduced UPR protein activation compared with non-PBA-treated cells (Fig. 2A and supplemental Fig. S5B). In classically stimulated cells, PBA did not alter the M1 pattern of membrane receptor expression (high CCR7 and CD86 and low CD163 and MR). In alternatively stimulated macrophages, PBA prevented the M2 expression pattern (by suppressing CD163 and MR expression) when compared with non-PBA-treated cells (Fig. 2B, p < 0.001 for both receptors). These macrophages also had low IL-10 and high IL-12 mRNA expression, similar to M1 macrophages (supplemental Fig. S6, A and B), suggesting ER stress inhibition prevents M2 macrophage differentiation. In addition, PBA decreased cholesterol uptake induced by oxLDL by ∼30% in alternatively stimulated macrophages, and cholesterol uptake remained low (as expected) in classically stimulated macrophages when compared with non-PBA-treated cells (Fig. 2C, p < 0.01 for all).
To confirm ER stress as a critical switch for macrophage differentiation and foam cell formation, we induced ER stress with thapsigargin in macrophages differentiated with M-CSF. Thapsigargin-treated macrophages had increased activation of ER stress proteins phosphopancreatic ER kinase, phosphoinositol requiring transmembrane kinase/endonuclease 1α, and CHOP expression compared with non-thapsigargin-treated cells (Fig. 2D). Thapsigargin-treated macrophages had increased CD163 and MR and suppressed CCR7 and CD86 membrane expression (Fig. 2E, p < 0.04 for all receptors), as well as increased IL-10 and decreased IL-12 mRNA expression compared with non-thapsigargin-treated macrophages (supplemental Fig. S6, C and D), confirming induction of M2 differentiation by ER stress activation. Finally, thapsigargin-treated macrophages also had 30% increased cholesterol uptake when compared with non-thapsigargin-treated cells (Fig. 2F, p < 0.02). These data suggest that ER stress regulates the macrophage differentiation phenotype.
Because PBA is a nonspecific ER stress inhibitor, we studied whether CHOP (a downstream protein of a branch of the UPR known to be induced in advanced murine and human coronary artery plaques) is essential for the regulation of macrophage cholesterol uptake and membrane receptor phenotype by ER stress (28, 29). We obtained peritoneal macrophages from CHOP−/− and WT mice and cultured them with IFNγ (classical stimulation) or IL-4, IL-10, or IC (alternative stimulation). After classical stimulation, CHOP−/− macrophages showed an M1 pattern of membrane receptor expression (high CCR7 and CD86 and low CD163 and MR), similar to WT macrophages. After alternative stimulation, CHOP−/− macrophages maintained an M1 expression pattern when compared with WT cells (increased expression of CCR7 and CD86 and suppressed expression of CD163 and MR; Fig. 2G, p < 0.04 for all receptors), with high IL-12 and low IL-10 mRNA expression (supplemental Fig. S6, E and F). Also, CHOP−/− macrophages had 28–43% lower oxLDL-induced cholesterol uptake after alternative stimulation compared with WT macrophages (Fig. 2H, p < 0.04 for all). Cholesterol uptake remained low (as expected) in classically stimulated CHOP−/− macrophages when compared with WT macrophages. Therefore, the downstream ER stress signal CHOP is critical for M2 macrophage differentiation and oxLDL-induced cholesterol uptake in response to alternative activation.
ER Stress Controls M2 Macrophage Differentiation by JNK-PPARγ-dependent Pathway
In diabetics, the suppression of macrophage ER stress prevents CD36 and SR-A1-dependent cholesterol deposition by decreasing JNK activation and PPARγ expression (4, 17). We found up-regulation of p-JNK and PPARγ by alternative stimulation in macrophages from diabetics, which was suppressed by PBA (Fig. 3A). To determine whether increased cholesterol uptake and M2 differentiation induced by ER stress are JNK- and/or PPARγ-dependent, we obtained peritoneal macrophages from JNK2−/−, LysM-Cre PPARγ−/−, and WT mice and cultured them with classical or alternative stimulation. We confirmed up-regulation of p-JNK by alternative stimulation in LysM-Cre PPARγ−/− murine macrophages by Western blot (Fig. 3B); conversely, PPARγ expression was suppressed in JNK2−/− macrophages, suggesting, as we have reported previously, that PPARγ is downstream of JNK during ER stress activation (17). In the absence of JNK2 or PPARγ expression, alternative stimulation induced macrophage ER stress compared with IFNγ-treated cells (Fig. 3B). After classical stimulation with INFγ, JNK2−/− macrophages showed an M1 pattern of membrane receptors, similar to WT macrophages. However, alternatively stimulated (with IL-4, IL-10, or IC) JNK2−/− macrophages behaved similar to CHOP−/− macrophages, maintaining an M1 pattern of membrane receptors with suppressed CD163 and MR (Fig. 3C, p < 0.02 for all receptors), as well as high IL-12 and low IL-10 mRNA expression, compared with WT (supplemental Fig. S7, A and B). JNK2−/− macrophages also had 26–34% lower oxLDL-induced cholesterol uptake after alternative stimulation compared with WT macrophages (Fig. 3D, p < 0.04 for all). Cholesterol uptake remained low (as expected) in classically stimulated JNK2−/− macrophages when compared with WT macrophages. In human macrophages from diabetics, we confirmed these results by treating with or without a JNK inhibitor (SP600125) during classical or alternative simulation (supplemental Fig. S7, C–E) suggesting that induction of p-JNK by ER stress facilitates the M2 phenotype. Finally, the absence of PPARγ had the same effects as CHOP−/− and JNK2−/− macrophages, preventing M2 differentiation and decreasing macrophage cholesterol uptake in alternatively activated macrophages compared with WT cells (Fig. 3E, p < 0.03 for all receptors; Fig. 3F, p < 0.01 for all; supplemental Fig. S7, F and G), suggesting that ER stress facilitates M2 macrophage differentiation and accelerated cholesterol uptake via a JNK-PPARγ-dependent pathway.
FIGURE 3.
Effects of ER stress on M2 macrophage differentiation and cholesterol uptake are JNK- and PPARγ-dependent. Cultured macrophages from diabetic patients were differentiated with INFγ to promote M1 differentiation or with IL-4, IL-10, or IC to promote M2 differentiation and co-incubated with or without PBA (ER stress inhibitor). A, p-JNK and PPARγ expression. For B–F, peritoneal macrophages from JNK2−/−, LysM-Cre PPARγ−/−, and WT mice were differentiated with IFNγ to promote M1 or IL-4, IL-10, or IC to promote M2 macrophages (n = 8 per group). B, ER stress protein activation/expression. C, flow cytometry quantification of membrane receptors from JNK2−/− macrophages. M1 receptors, CCR7 (black), CD86 (gray); M2 receptors, CD163 (grid), MR (back-slashed). * and **, p < 0.001; t, p < 0.02; tt, p < 0.001 versus same receptor in WT. D, quantification of cholesterol uptake after differentiation with IFNγ (white), IL-4 (back-slashed), IL-10 (hatched), or IC (front-slashed) and stimulation with oxLDL. *, p < 0.04 versus same stimulation in WT. E, flow cytometry quantification of membrane receptors from LyzM-Cre PPARγ−/− macrophages. * and **, p < 0.01; t and tt, p < 0.03 versus same receptor in WT. F, quantification of cholesterol uptake after stimulation with oxLDL. *, p < 0.01 versus same stimulation in WT.
Absence of Scavenger Receptors Prevents ER Stress-induced M2 Macrophage Differentiation
Because cholesterol uptake was increased in M2 macrophages, we clarified whether scavenger receptor signaling is required for ER stress-induced M2 macrophage differentiation by obtaining peritoneal macrophages from mice lacking scavenger receptor CD36 or SR-A1, stimulating with oxLDL and AcLDL, respectively, and comparing to WT mice stimulated with both types of modified LDL. The absence of CD36 or SR-A1 expression prevented ER stress activation after alternative stimulation (Fig. 4A and supplemental Fig. S8). First, to ensure that we could achieve adequate macrophage cholesterol uptake in our knock-out mouse macrophages, we incubated CD36−/− and SR-A1−/− macrophages with AcLDL and oxLDL, respectively, and WT macrophages with both types of modified cholesterol after classical and alternative stimulation. In all cases, we were able to significantly increase cholesterol uptake with the addition of modified LDL (supplemental Fig. S9). Then, we assessed whether increased cholesterol load or scavenger receptor signaling is critical to macrophage differentiation. Classically stimulated macrophages from CD36−/− or SR-A1−/− mice had an M1 membrane expression pattern similar to WT (Fig. 4B). The suppression of scavenger receptor signaling in CD36−/− or SR-A1−/− mice prevented M2 macrophage differentiation by alternative stimulation (decreased CD163 and MR expression compared with WT; p < 0.001 for both receptors). Surprisingly, this suppression of M2 differentiation was persistent despite cholesterol loading, suggesting that CD36- and SR-A1-mediated signaling, rather than cholesterol uptake, are required for M2 differentiation induced by alternative stimulation (Fig. 4, C–E, p < 0.001 for all). Finally, the addition of thapsigargin to macrophages cultured from SR-A1−/−, CD36−/−, or WT mice induced M2 differentiation, with increased CD163 and MR expression compared with cells cultured without thapsigargin (Fig. 4F, p < 0.01 for all). This suggests that ER stress is necessary to induce M2 macrophage differentiation.
FIGURE 4.
Scavenger receptor signaling induces ER stress and regulates M2 macrophage differentiation. Peritoneal macrophages from CD36−/−, SR-A1−/−, and WT mice were cultured with IFNγ to promote M1 differentiation or with IL-4, IL-10, or IC to promote M2 differentiation and stimulated with modified cholesterol (n = 6 per group). A, ER stress protein activation/expression. Flow cytometry quantification of macrophage membrane receptors CCR7 (black), CD86 (gray), CD163 (grid), MR (back-slashed) after differentiation with IFNγ (B), IL-4 (C), IL-10 (D), or IC (E) and stimulation with oxLDL, AcLDL, or neither. t and tt, p < 0.001 versus same receptor/LDL stimulation by ANOVA. F, flow cytometry quantification of membrane receptors from peritoneal macrophages treated with or without ER stress inducer thapsigargin from CD36−/−, SR-A1−/−, and WT mice (n = 6 per group). t and tt, p < 0.01 versus same receptor in thapsigargin-treated.
Suppression of ER Stress Shifts M2 toward M1 Macrophages and Reverses Foam Cells
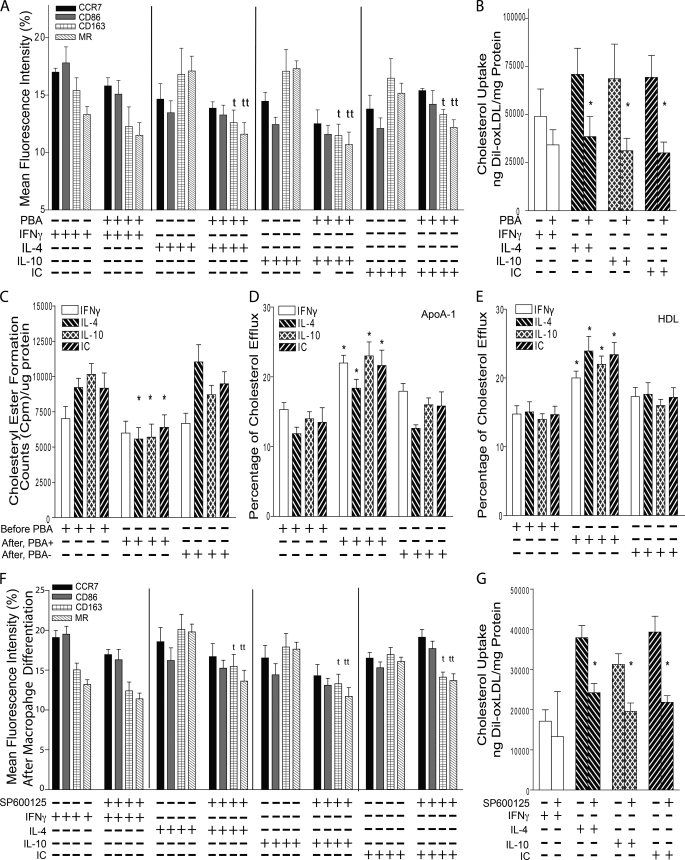
To determine whether ER stress-induced M2 differentiation and cholesterol deposition are reversible, we treated macrophages from diabetic patients with or without PBA following differentiation into an M1 or M2 phenotype. In M2 macrophages, PBA shifted the phenotype to that of M1 macrophages, significantly suppressing the expression of CD163 and MR compared with that of non-PBA-treated, M2-differentiated macrophages (Fig. 5A, p < 0.02 for both receptors). Additionally, PBA decreased cholesterol uptake induced by oxLDL by ∼50% (p < 0.02 for all) and decreased net cholesteryl ester formation (p < 0.04 for all) by increasing apoA-I- and HDL-induced cholesterol efflux compared with non-PBA-treated cells (p < 0.05 for all) (Fig. 5, B–E). These findings demonstrate the plasticity of macrophage phenotype and suggest ER stress as a key regulator of macrophage differentiation and cholesterol deposition.
FIGURE 5.
Suppression of ER stress and JNK reverses M2 macrophage differentiation and cholesterol deposition. For A–E, macrophages from diabetic patients were differentiated into M1 or M2 and then incubated with or without PBA (ER stress inhibitor). A, flow cytometry quantification of membrane receptors. M1 receptors, CCR7 (black), CD86 (gray); M2 receptors, CD163 (grid), MR (back-slashed) (n = 8 per group). t, p < 0.02; tt, p < 0.01 versus same receptor in non-PBA-treated. B, quantification of cholesterol uptake after IFNγ (white), IL-4 (back-slashed), IL-10 (hatched), or IC (front-slashed) and stimulation with oxLDL (n = 12 per group). *, p < 0.02 versus same stimulation in non-PBA-treated. C, cholesteryl ester formation (n = 6 per group). *, p < 0.04 versus same stimulation by ANOVA. Shown are apoA-I-induced (D) and HDL-induced (E) cholesterol efflux (n = 6 per group). *, p < 0.05 versus same stimulation by ANOVA. For F and G, macrophages from diabetic patients were differentiated into M1 or M2 and then incubated with or without SP600125 (JNK inhibitor). F, flow cytometry quantification of membrane receptors (n = 6 per group). t, p < 0.05; tt, p < 0.04 versus same receptor in non-SP600125-treated. G, quantification of cholesterol uptake after stimulation with oxLDL (n = 4 per group). *, p < 0.02 versus same stimulation in non-SP600125-treated.
To determine whether ER stress regulation of macrophage plasticity or cholesterol uptake is JNK-dependent, we treated macrophages with or without JNK inhibitor, SP600125, after M1 or M2 differentiation. In M2 macrophages, SP600125 shifted the phenotype to that of M1 macrophages, significantly suppressing the expression of CD163 and MR (Fig. 5F, p < 0.05 for both receptors) and decreasing cholesterol uptake induced by oxLDL by ∼40% when compared with non-SP600125-treated cells (Fig. 5G, p < 0.02 for all). These findings suggest that ER stress modulates macrophage plasticity and cholesterol uptake through a JNK-dependent pathway (Fig. 6).
FIGURE 6.
Influence of ER stress pathways in macrophage differentiation. ER stress pathways include the following: c-Jun N-terminal kinase (JNK), PPARγ, scavenger receptor CD36, and SR-A1. Macrophage M1 receptors include CCR7 and CD86; M2 receptors include MR and CD163.
DISCUSSION
Despite our knowledge that differentiated macrophages are essential in the development of atherosclerosis, the mechanism(s) controlling their phenotype, the interplay between phenotype and scavenger receptor signaling, and the contribution of phenotype to vascular cholesterol deposition in vivo is unknown. In addition, it is unclear which signaling pathways control shifting between phenotypes. In this study of macrophages from diabetic patients, we demonstrated that alternative stimulation into M2 macrophages lead to increased foam cell formation by inducing scavenger receptor CD36 and SR-A1 expression. Induction of ER stress by alternative stimulation was necessary and sufficient to generate the M2 phenotype. Suppression of scavenger receptor signaling during alternative stimulation prevented M2 formation and foam cell formation by an ER stress-p-JNK-PPARγ-dependent mechanism. Moreover, the suppression of ER stress-p-JNK activation shifted M2 toward M1 cells and decreased foam cell formation by reducing cholesterol uptake and increasing cholesterol efflux, indicating suppression of macrophage ER stress as a potential therapy for atherosclerosis.
Although the proinflammatory effect of M1 macrophages in atherosclerosis progression is well established, the role of M2 macrophages in plaque progression is more complex (30, 31). The absence of IL-5 or IL-10 in mice, known factors that facilitate M2 macrophage differentiation, decreases atherosclerosis, whereas the absence of IL-4, another cytokine which induces M2 macrophage differentiation and foam cell formation in murine macrophages, has no effect or a proatherogenic effect in mice (32–36). In human atherosclerotic plaques, alternative MR+ macrophages were found to have smaller lipid droplets and less foam cell formation when compared with MR− macrophages. In addition, IL-4-stimulated macrophages from healthy donors showed less foam cell formation compared with resting macrophages (37). However, macrophages derived from healthy donors and alternatively stimulated with M-CSF show increased cholesterol uptake, cholesteryl ester formation, and a proinflammatory phenotype upon oxLDL stimulation, (38), suggesting that M2 macrophages activated by different stimuli may have different cholesterol handling behavior. In this study, in contrast to previous studies in chow-fed ApoE−/− mice, we found a higher presence of M2 macrophages with higher cholesterol deposition compared with M1 macrophages in the atherosclerotic plaque from ApoE−/− mice fed a high fat diet. Also, alternative stimulation with IL-4, IL-10, or IC transformed human diabetic macrophages into foam cells by increasing CD36 and SR-A1 expression to increase total cholesterol through accelerated oxLDL and AcLDL cholesterol uptake. Therefore, the promotion of M2 macrophage differentiation as a possible atheroprotective therapy promoted by others to decrease inflammation and slow atherosclerotic plaque progression may not be an ideal therapeutic target.
Intracellular accumulation of lipoprotein-derived free cholesterol increases UPR and drives increased macrophage apoptosis and plaque instability in late atherosclerotic plaques (24). Up-regulated UPR markers are detected in intimal macrophages as the plaque evolves into late stages in murine and human plaques (15, 29). In mouse models of diet-induced insulin resistance and atherosclerosis, multiple mechanisms decreasing ER stress, including knock-out of ER stress protein CHOP or suppression with PBA, prevent the development of atherosclerosis (23, 39), suggesting that macrophage ER stress signaling links the effects of proatherogenic lipoproteins to macrophage behavior and plaque progression. In this study, we found that in diabetic patients, ER stress is an essential regulator of macrophage plasticity and cholesterol metabolism. During macrophage differentiation, inhibition of ER stress with PBA or the absence of CHOP suppressed macrophage cholesterol uptake and prevented M2 differentiation in alternatively stimulated macrophages. Moreover, the induction of ER stress with thapsigargin induced M2 macrophage differentiation and accelerated cholesterol uptake. Therefore, macrophage ER stress is required and sufficient for M2 differentiation, and its suppression prevents foam cell formation induced by alternative activation.
A central question in atherogenesis is whether macrophage phenotype and behavior are altered by modified cholesterol loading. Macrophage scavenger receptors account for the vast majority of modified LDL cholesterol uptake and foam cell formation during atherogenesis (40). The activation of SR-A1 and CD36 increases free cholesterol deposition in the ER, inducing ER stress, accelerating macrophage apoptosis, and causing atherosclerotic plaque necrosis and instability (16, 24). However, multiple studies indicate that scavenger receptor signaling activation is engaged independently of cholesterol in ER stress-induced macrophage inflammatory responses and apoptosis. SR-A1 functions as a pattern recognition receptor in the innate immune system, binding diverse, non-cholesterol ligands and facilitating Toll-like receptor 4 mediation of proapoptotic events in ER-stressed macrophages (41). In addition, CD36 signaling activation contributes to the generation of proinflammatory eicosanoids in response to macrophage ER stress by regulation of membrane calcium influx and release of arachidonic acid from cellular membranes (42, 43), suggesting that a complex interaction of scavenger receptor signaling activation and changes in ER stress alter macrophage behavior and plaque progression. Of note, in ApoE−/− mice, targeted deletion of SR-A1 and CD36 reduces expression of inflammatory genes in atherosclerotic lesions, macrophage apoptosis, and aortic root plaque necrosis but does not reduce atherosclerotic lesion area (44). In this study, we found that M2 macrophages from patients with diabetes had increased CD36 and SR-A1 expression (2). Moreover, we found that the absence of CD36 or SR-A1 protein expression prevented induction of the M2 macrophage phenotype by alternative stimulation despite increased modified cholesterol deposition, suggesting that scavenger receptor signaling is required to induce ER stress and M2 macrophage differentiation by alternative simulation. M2 macrophages perpetuate a vicious cycle, inducing more ER stress through increased scavenger receptor expression, cholesterol uptake, and cholesterol deposition, all of which lead to foam cell formation, potentially inducing plaque progression.
In advanced atherosclerotic lesions, multiple studies in mice suggest that aggressive lipid lowering leads to decreased plaque macrophage and foam cell content with a predominance of the M2 phenotype within the atherosclerotic plaque (9, 10, 45). Reduction in plaque macrophages is associated with the suppression of monocyte recruitment (45) and/or egression from the plaque by induction of the M1 migration marker CCR7 in foam cells (46, 47). Injection of wild-type recipient animals with antibodies against CCR7 ligands inhibited the majority of foam cell egression from aortic plaques, establishing a functional role for CCR7 and macrophage phenotype in plaque regression (46), suggesting that understanding the mechanism by which macrophages shift their phenotype could be key to understanding atherosclerotic plaque behavior. In humans, M2 macrophage markers are present in human carotid atherosclerotic lesions and correlate positively with PPARγ expression levels. Studies with PPARδ−/−, PPARγ−/−, and PPARδ/γ−/− macrophages reveal that both receptors are required for expression of M2 markers (6, 48, 49). Additionally, transgenic mice with overexpression of PGC-1β (peroxisome proliferator-activated receptor γ coactivator-1β) in macrophages primes them for alternative activation (50). However, PPARγ activation did not shift resting or M1 macrophages into an M2 phenotype in vitro (4). A recent study in mouse peritoneal macrophages and human adipose tissue macrophages suggests that differential expression of Krüppel-like factors, a subfamily of the zinc finger class of DNA-binding transcriptional regulators, is also critical in regulating M1/M2 polarization during macrophage differentiation, but its role shifting macrophage phenotype is unknown (51). In our study from diabetic patients, suppression of ER stress-JNK activation shifted M2 macrophages induced by alternative stimuli toward M1 macrophages with increased CCR7 expression. Moreover, improvement of ER stress reversed foam cells and cholesteryl ester deposition in macrophages by suppressing cholesterol uptake and increasing cholesterol efflux. These data suggest that these cells are functionally plastic and that regulation of ER stress is a critical switch that links macrophage cholesterol metabolism to functional phenotype. Also, shifting differentiated macrophages from M2 toward M1 cells by suppression of ER stress represents a novel therapeutic target to not only prevent plaque formation but also to potentially facilitate plaque regression.
This study establishes macrophage ER stress as a potential link between macrophage cholesterol deposition and macrophage differentiation. ER stress is a critical mechanism of regulation in macrophage phenotype differentiation. With alternative stimulation, scavenger receptor signaling facilitates the induction of ER stress, JNK2, and PPARγ activation, resulting in the M2 macrophage phenotype. Shifting M2 toward CCR7+ M1 macrophages by suppression of ER stress facilitates cholesterol efflux and potentially plaque regression. Human interventional trials with ER suppressers are needed to determine the effects on atherosclerosis.
Supplementary Material
Acknowledgments
We thank Drs. Adriana Dusso, Daniel S. Ory, Mark S. Sands, and Clay F. Semenkovich for helpful discussions and review of the manuscript.
This work was supported in part by National Institutes of Health Grants R01HL094818-0, P30 DK079333, and P60 20579. This work was also supported by the American Diabetes Association (7-08-CR-08).

This article contains supplemental “Methods,” Figs. S1–S9, and additional references.
- CCR7
- C-C chemokine receptor type 7
- ER
- endoplasmic reticulum
- CD
- cluster of differentiation
- MR
- mannose receptor
- ApoE
- apolipoprotein E
- UPR
- unfolded protein response
- M-CSF
- macrophage colony-stimulating factor
- PPAR
- peroxisome proliferator-activated receptor
- T2DM
- type 2 diabetes mellitus
- IC
- immunocomplex
- PBA
- 4-phenylbutyric acid
- SR
- scavenger receptor
- CHOP
- CCAAT/Enhancer binding protein homologous protein
- LysM
- M lysozyme
- oxLDL
- oxidized LDL
- apoA-I
- apolipoprotein A-I
- ANOVA
- analysis of variance.
REFERENCES
- 1. Mantovani A., Garlanda C., Locati M. (2009) Macrophage diversity and polarization in atherosclerosis: A question of balance. Arterioscler. Thromb. Vasc. Biol. 29, 1419–1423 [DOI] [PubMed] [Google Scholar]
- 2. Martinez F. O., Gordon S., Locati M., Mantovani A. (2006) Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J. Immunol. 177, 7303–7311 [DOI] [PubMed] [Google Scholar]
- 3. Mills C. D., Kincaid K., Alt J. M., Heilman M. J., Hill A. M. (2000) M1/M2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164, 6166–617310843666 [Google Scholar]
- 4. Bouhlel M. A., Derudas B., Rigamonti E., Dièvart R., Brozek J., Haulon S., Zawadzki C., Jude B., Torpier G., Marx N., Staels B., Chinetti-Gbaguidi G. (2007) PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 6, 137–143 [DOI] [PubMed] [Google Scholar]
- 5. Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 [DOI] [PubMed] [Google Scholar]
- 6. Martinez F. O., Helming L., Gordon S. (2009) Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 27, 451–483 [DOI] [PubMed] [Google Scholar]
- 7. Porcheray F., Viaud S., Rimaniol A. C., Leone C., Samah B., Dereuddre-Bosquet N., Dormont D., Gras G. (2005) Macrophage activation switching: An asset for the resolution of inflammation. Clin. Exp. Immunol. 142, 481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khallou-Laschet J., Varthaman A., Fornasa G., Compain C., Gaston A. T., Clement M., Dussiot M., Levillain O., Graff-Dubois S., Nicoletti A., Caligiuri G. (2010) Macrophage plasticity in experimental atherosclerosis. PLoS One 5, e8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feig J. E., Parathath S., Rong J. X., Mick S. L., Vengrenyuk Y., Grauer L., Young S. G., Fisher E. A. (2011) Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation 123, 989–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feig J. E., Rong J. X., Shamir R., Sanson M., Vengrenyuk Y., Liu J., Rayner K., Moore K., Garabedian M., Fisher E. A. (2011) HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc. Natl. Acad. Sci. U.S.A. 108, 7166–7171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schaefer A., Magócsi M., Stöcker U., Kósa F., Marquardt H. (1994) Early transient suppression of c-myb mRNA levels and induction of differentiation in Friend erythroleukemia cells by the [Ca2+]i-increasing agents cyclopiazonic acid and thapsigargin. J. Biol. Chem. 269, 8786–8791 [PubMed] [Google Scholar]
- 12. Dickout J. F., Lhotak S., Hilditch B. A., Basseri S., Colgan S. M., Lynn E. F., Carlisle R. E., Zhou J., Sood S. K., Ingram A. J., Austin R. C. (2011) Induction of the unfolded protein response after monocyte to macrophage differentiation augments cell survival in early atherosclerotic lesions. FASEB J. 25, 576–589 [DOI] [PubMed] [Google Scholar]
- 13. Launay S., Giannì M., Kovàcs T., Bredoux R., Bruel A., Gélébart P., Zassadowski F., Chomienne C., Enouf J., Papp B. (1999) Lineage-specific modulation of calcium pump expression during myeloid differentiation. Blood 93, 4395–4405 [PubMed] [Google Scholar]
- 14. Berridge M. J. (1995) Calcium signaling and cell proliferation. Bioessays 17, 491–500 [DOI] [PubMed] [Google Scholar]
- 15. Zhou J., Lhoták S., Hilditch B. A., Austin R. C. (2005) Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation 111, 1814–1821 [DOI] [PubMed] [Google Scholar]
- 16. Devries-Seimon T., Li Y., Yao P. M., Stone E., Wang Y., Davis R. J., Flavell R., Tabas I. (2005) Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J. Cell Biol. 171, 61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oh J., Weng S., Felton S. K., Bhandare S., Riek A., Butler B., Proctor B. M., Petty M., Chen Z., Schechtman K. B., Bernal-Mizrachi L., Bernal-Mizrachi C. (2009) 1,25(OH)2 vitamin D inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation 120, 687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chawla A., Barak Y., Nagy L., Liao D., Tontonoz P., Evans R. M. (2001) PPARγ-dependent and -independent effects on macrophage gene expression in lipid metabolism and inflammation. Nat. Med. 7, 48–52 [DOI] [PubMed] [Google Scholar]
- 19. Odegaard J. I., Ricardo-Gonzalez R. R., Goforth M. H., Morel C. R., Subramanian V., Mukundan L., Red Eagle A., Vats D., Brombacher F., Ferrante A. W., Chawla A. (2007) Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tabas I. (2010) Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 10, 36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tabas I., Tall A., Accili D. (2010) The impact of macrophage insulin resistance on advanced atherosclerotic plaque progression. Circulation Res. 106, 58–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Han S., Liang C. P., DeVries-Seimon T., Ranalletta M., Welch C. L., Collins-Fletcher K., Accili D., Tabas I., Tall A. R. (2006) Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 3, 257–266 [DOI] [PubMed] [Google Scholar]
- 23. Erbay E., Babaev V. R., Mayers J. R., Makowski L., Charles K. N., Snitow M. E., Fazio S., Wiest M. M., Watkins S. M., Linton M. F., Hotamisligil G. S. (2009) Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 15, 1383–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tabas I. (2010) The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ. Res. 107, 839–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Satoh N., Shimatsu A., Himeno A., Sasaki Y., Yamakage H., Yamada K., Suganami T., Ogawa Y. (2010) Unbalanced M1/M2 phenotype of peripheral blood monocytes in obese diabetic patients: Effect of pioglitazone. Diabetes Care 33, e7. [DOI] [PubMed] [Google Scholar]
- 26. Schneider J. G., Finck B. N., Ren J., Standley K. N., Takagi M., Maclean K. H., Bernal-Mizrachi C., Muslin A. J., Kastan M. B., Semenkovich C. F. (2006) ATM-dependent suppression of stress signaling reduces vascular disease in metabolic syndrome. Cell Metab. 4, 377–389 [DOI] [PubMed] [Google Scholar]
- 27. Feng B., Yao P. M., Li Y., Devlin C. M., Zhang D., Harding H. P., Sweeney M., Rong J. X., Kuriakose G., Fisher E. A., Marks A. R., Ron D., Tabas I. (2003) The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat. Cell Biol. 5, 781–792 [DOI] [PubMed] [Google Scholar]
- 28. Ozcan U., Cao Q., Yilmaz E., Lee A. H., Iwakoshi N. N., Ozdelen E., Tuncman G., Görgün C., Glimcher L. H., Hotamisligil G. S. (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 [DOI] [PubMed] [Google Scholar]
- 29. Myoishi M., Hao H., Minamino T., Watanabe K., Nishihira K., Hatakeyama K., Asada Y., Okada K., Ishibashi-Ueda H., Gabbiani G., Bochaton-Piallat M. L., Mochizuki N., Kitakaze M. (2007) Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation 116, 1226–1233 [DOI] [PubMed] [Google Scholar]
- 30. Buono C., Come C. E., Stavrakis G., Maguire G. F., Connelly P. W., Lichtman A. H. (2003) Influence of interferon-γ on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 23, 454–460 [DOI] [PubMed] [Google Scholar]
- 31. Gupta S., Pablo A. M., Jiang X., Wang N., Tall A. R., Schindler C. (1997) IFN-γ potentiates atherosclerosis in ApoE knock-out mice. The Journal of clinical investigation 99, 2752–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huo Y., Zhao L., Hyman M. C., Shashkin P., Harry B. L., Burcin T., Forlow S. B., Stark M. A., Smith D. F., Clarke S., Srinivasan S., Hedrick C. C., Praticò D., Witztum J. L., Nadler J. L., Funk C. D., Ley K. (2004) Critical role of macrophage 12/15-lipoxygenase for atherosclerosis in apolipoprotein E-deficient mice. Circulation 110, 2024–2031 [DOI] [PubMed] [Google Scholar]
- 33. Binder C. J., Hartvigsen K., Chang M. K., Miller M., Broide D., Palinski W., Curtiss L. K., Corr M., Witztum J. L. (2004) IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J. Clin. Invest. 114, 427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pinderski Oslund L. J., Hedrick C. C., Olvera T., Hagenbaugh A., Territo M., Berliner J. A., Fyfe A. I. (1999) Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 19, 2847–2853 [DOI] [PubMed] [Google Scholar]
- 35. King V. L., Szilvassy S. J., Daugherty A. (2002) Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler. Thromb. Vasc. Biol. 22, 456–461 [DOI] [PubMed] [Google Scholar]
- 36. King V. L., Cassis L. A., Daugherty A. (2007) Interleukin-4 does not influence development of hypercholesterolemia or angiotensin II-induced atherosclerotic lesions in mice. Am. J. Pathol. 171, 2040–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chinetti-Gbaguidi G., Baron M., Bouhlel M. A., Vanhoutte J., Copin C., Sebti Y., Derudas B., Mayi T., Bories G., Tailleux A., Haulon S., Zawadzki C., Jude B., Staels B. (2011) Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARγ and LXRα pathways. Circ. Res. 108, 985–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Tits L. J., Stienstra R., van Lent P. L., Netea M. G., Joosten L. A., Stalenhoef A. F. (2011) Oxidized LDL enhances proinflammatory responses of alternatively activated M2 macrophages: A crucial role for Krüppel-like factor 2. Atherosclerosis 214, 345–349 [DOI] [PubMed] [Google Scholar]
- 39. Tsukano H., Gotoh T., Endo M., Miyata K., Tazume H., Kadomatsu T., Yano M., Iwawaki T., Kohno K., Araki K., Mizuta H., Oike Y. (2010) The endoplasmic reticulum stress-C/EBP homologous protein pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 30, 1925–1932 [DOI] [PubMed] [Google Scholar]
- 40. Rader D. J., Puré E. (2005) Lipoproteins, macrophage function, and atherosclerosis: Beyond the foam cell? Cell Metab. 1, 223–230 [DOI] [PubMed] [Google Scholar]
- 41. Lim W. S., Timmins J. M., Seimon T. A., Sadler A., Kolodgie F. D., Virmani R., Tabas I. (2008) Signal transducer and activator of transcription-1 is critical for apoptosis in macrophages subjected to endoplasmic reticulum stress in vitro and in advanced atherosclerotic lesions in vivo. Circulation 117, 940–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kuda O., Jenkins C. M., Skinner J. R., Moon S. H., Su X., Gross R. W., Abumrad N. A. (2011) CD36 protein is involved in store-operated calcium flux, phospholipase A2 activation, and production of prostaglandin E2. J. Biol. Chem. 286, 17785–17795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Seimon T. A., Nadolski M. J., Liao X., Magallon J., Nguyen M., Feric N. T., Koschinsky M. L., Harkewicz R., Witztum J. L., Tsimikas S., Golenbock D., Moore K. J., Tabas I. (2010) Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 12, 467–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Manning-Tobin J. J., Moore K. J., Seimon T. A., Bell S. A., Sharuk M., Alvarez-Leite J. I., de Winther M. P., Tabas I., Freeman M. W. (2009) Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler. Thromb. Vasc. Biol. 29, 19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Potteaux S., Gautier E. L., Hutchison S. B., van Rooijen N., Rader D. J., Thomas M. J., Sorci-Thomas M. G., Randolph G. J. (2011) Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of ApoE−/− mice during disease regression. J. Clin. Invest. 121, 2025–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Trogan E., Feig J. E., Dogan S., Rothblat G. H., Angeli V., Tacke F., Randolph G. J., Fisher E. A. (2006) Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 103, 3781–3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Feig J. E., Pineda-Torra I., Sanson M., Bradley M. N., Vengrenyuk Y., Bogunovic D., Gautier E. L., Rubinstein D., Hong C., Liu J., Wu C., van Rooijen N., Bhardwaj N., Garabedian M., Tontonoz P., Fisher E. A. (2010) LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J. Clin. Invest. 120, 4415–4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Desvergne B. (2008) PPARδ/β: The lobbyist switching macrophage allegiance in favor of metabolism. Cell Metab. 7, 467–469 [DOI] [PubMed] [Google Scholar]
- 49. Chawla A. (2010) Control of macrophage activation and function by PPARs. Circ. Res. 106, 1559–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vats D., Mukundan L., Odegaard J. I., Zhang L., Smith K. L., Morel C. R., Wagner R. A., Greaves D. R., Murray P. J., Chawla A. (2006) Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab. 4, 13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liao X., Sharma N., Kapadia F., Zhou G., Lu Y., Hong H., Paruchuri K., Mahabeleshwar G. H., Dalmas E., Venteclef N., Flask C. A., Kim J., Doreian B. W., Lu K. Q., Kaestner K. H., Hamik A., Clément K., Jain M. K. (2011) Krüppel-like factor 4 regulates macrophage polarization. J. Clin. Invest. 121, 2736–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.