Background: CCL19 ligand controls signaling events in T cells.
Results: CCL19 promotes up-regulation of ERK5 to regulate KLF-2, which leads to expression of EDG-1.
Conclusion: CCL19 stimulation of naïve T cells regulates expression of ERK5, KLF-2, and EDG-1.
Significance: CCL19 links the control of expression of EDG-1 in naïve T cells that mediate T cell entry to, and exit from, lymph nodes.
Keywords: Cell Migration, Cell Surface Receptor, Chemokines, Chemotaxis, G Protein-coupled Receptors (GPCR), Signal Transduction
Abstract
T lymphocytes circulate between the blood, tissues, and lymph. These T cells carry out immune functions, using the C-C chemokine receptor 7 (CCR7) and its cognate ligands, CCL19 and CCL21, to enter and travel through the lymph nodes. Distinct roles for each ligand in regulating T lymphocyte trafficking have remained elusive. We report that in the human T cell line HuT78 and in primary murine T lymphocytes, signaling from CCR7/CCL19 leads to increased expression and phosphorylation of extracellular signal-regulated kinase 5 (ERK5) within eight hours of stimulation. Within 48–72 h we observed peak levels of endothelial differentiation gene 1 (EDG-1), which mediates the egress of T lymphocytes from lymph nodes. The increased expression of EDG-1 was preceded by up-regulation of its transcription factor, Krüppel-like factor 2 (KLF-2). To determine the cellular effect of disrupting ERK5 signaling from CCR7, we examined the migration of ERK5flox/flox/Lck-Cre murine T cells to EDG-1 ligands. While CCL19-stimulated ERK5flox/flox naïve T cells showed increased migration to EDG-1 ligands at 48 h, the migration of ERK5flox/flox/Lck-Cre T cells remained at a basal level. Accordingly, we define a novel signaling pathway that controls EDG-1 up-regulation following stimulation of T cells by CCR7/CCL19. This is the first report to link the two signaling events that control migration through the lymph nodes: CCR7 mediates entry into the lymph nodes and EDG-1 signaling controls their subsequent exit.
Introduction
During homeostasis, naïve T lymphocytes travel through the circulation and enter the lymph nodes via high endothelial venules (HEV)3 (1). C-C chemokine receptor 7 (CCR7)-expressing naïve T lymphocytes respond to two different ligands, CCL19 and CCL21. CCL21 is expressed along the HEV, and promotes migration of naïve T cells into the lymph nodes from peripheral tissues (2, 3). Stromal cells within the T zone express both CCL19 and CCL21, while CCL19 is also expressed on mature dendritic cells (4–6). Although both ligands are found in the lymph nodes, physiologically distinct roles for each of the ligands remain mostly undefined. Recent studies by our laboratory and others, however, have revealed that T lymphocytes respond differentially to CCL19 and CCL21 (7–10).
Studies to define physiological roles for CCL19 and CCL21 have been carried out in paucity of lymph node T lymphocyte (plt) mice, a spontaneous mutant that lacks functional CCL19 and CCL21 (3, 11, 12). Because plt mice lack expression of both CCR7 ligands, the specific, individual contribution of CCL19 or CCL21 are unknown. As expected, the phenotype of the plt mouse is similar to that of the CCR7−/− mouse (13). Further efforts to define individual roles for CCR7 ligands have used ectopically expressed CCL19 or CCL21 and have revealed that CCL21 promotes lympho-neogenesis more efficiently than CCL19 (2, 8). More recently it was observed that the CCL19−/− mice, in which the CCL19 locus has been homozygously deleted, had no distinct phenotype. However adoptive transfer of wild type CD4+ T cells into the CCL19−/− strain showed a delayed clearance from peripheral lymph nodes when compared with the behavior of the same cells in the corresponding wild type strain (14). Similarly, mice treated with the CCL19-specific antagonist ELC8–83 showed a significant increase in the number of T cells present in the lymph nodes, compared with vehicle treated controls (15). Both of these studies associate a loss of CCR7 signaling through CCL19 to T lymphocyte retention in the lymph nodes. However, because CCR7 is thought to promote lymph node entry, it remains unclear how CCR7 contributes to the length of time it takes for a T cell to travel through the lymph nodes.
T lymphocytes exit the lymph nodes via the endothelial differentiation gene 1 (EDG-1, also known as sphingosine-1-phosphate receptor 1 [S1P1]), a receptor for sphingosine 1-phosphate (S1P) (16). During this process, the T lymphocytes down-regulate CCR7, as evidenced by their reduced ability to migrate to CCL21 (16, 17). We, along with other groups, have shown that CCR7 shows a more efficient internalization in response to CCL19 found on the surface of activated dendritic cells, than to CCL21, which lines lymph nodes and mediates attraction of T cells to the lymph nodes (7, 10). Interestingly, the mean level of EDG-1 expressed on the surface is lower in the CCR7−/− T cells than on wild type, which may implicate CCR7 in the up-regulation of EDG-1 (17). In T lymphocytes, Krüppel-like factor 2 (KLF-2) is required for the expression of EDG-1 (18). Yet, what role CCR7 stimulation may have in regulating the levels of KLF-2 expressed is unknown. It remains unclear, then, whether CCR7 could be involved in regulating the egress of cells from the lymph nodes by controlling the expression of EDG-1.
In this study, we use the HuT78 human T lymphocyte cell line, and primary murine T lymphocytes, to examine the contributions of CCL19 in the regulation of EDG-1 expression. In the HuT78 line, we found that CCL19-activated CCR7 led to increased expression of KLF-2 and an increase in cell migration to EDG-1. Because extracellular signal-regulated kinase 5 (ERK5) has been implicated in the regulation of KLF-2, we examined the migration of ERK5flox/flox/Lck-Cre cells following stimulation with CCL19, and found that these cells no longer responded to sphingosine 1-phosphate.
Activated dendritic cells are a likely source for CCL19 in the lymph nodes (8). In our studies, we used small hairpin RNA (shRNA) to selectively knock down the expression of CCL19 in dendritic cells, which allowed us to determine the extent to which CCL19 mediated the recruitment of naïve T lymphocytes. We found that, in the absence of CCL19, mature ovalbumin-primed dendritic cells failed to form conjugates with ovalbumin-specific T lymphocytes in vitro. Taken together, our studies define a novel pathway in which CCL19 controls T cell regulation of EDG-1 expression.
EXPERIMENTAL PROCEDURES
Cell Lines and Mice
The HuT78 human T lymphocyte line was purchased from ATCC and maintained in complete media (RPMI 1640 (Sigma)/10% heat-inactivated fetal bovine serum (FBS) (Hyclone)/2 mm l-glutamine (Invitrogen)) in a humidified atmosphere at 37 °C and 5% CO2. B6.129P2(C)-CCR7tm1Rfor/mice and C57BL/6 mice were purchased from Jackson Labs. ERK5flox/flox/Lck-Cre mice were generated by crossing ERK5flox/flox mice (a generous gift from Dr. Cathy Tournier (19)) with Lck-Cre (Jackson labs 003802 (20)). Genotypes were verified by PCR. Isolated T splenocytes were maintained for up to 1 week in splenocyte media (RPMI 1640 (Sigma), 10% heat-inactivated FBS, 2 mm l-glutamine (Invitrogen), 50 μm β-mercaptoethanol (Fisher), 20 units/ml IL-2, 100 units/ml penicillin/100 μg/ml streptomycin).
Chemotaxis Assays
Chemotaxis assays were carried out as described (7). Briefly, HuT78 were grown in the presence of 40 nm CCL19 (R&D), CCL21 (R&D), or an equal volume of vehicle (phosphate-buffered saline (PBS)) for 0, 24, 48, 72, and 96 h. Following the preincubation, for the EDG-1 migration assays, 50,000 cells were placed in the top well of a Neuro Probe migration chamber and allowed to migrate to 10 nm sphingosine 1-phosphate across a 5 μm membrane (Neuro Probe, Inc.) at 37 °C in a 5% CO2 humidified environment for 2 h (Sigma) in serum-free RPMI 1640 medium in a 48-well chemotaxis chamber. Cells in the bottom chamber were counted by hemacytometer. Assays were performed in duplicate and replicated three times.
Dendritic Cell/T Lymphocyte Conjugates
Bone marrow-derived dendritic cells were generated from C57BL/6 wild type mice as described (21). Briefly, bone marrow was isolated from C57BL/6 wild type mice. 2 × 106 bone marrow cells were plated on 100 mm bacterial Petri dishes and cultured in 10 ml of DC media (RPMI 1640, 10% heat-inactivated FBS, 100 μg/ml penicillin-streptomycin, 50 μm β-mercaptoethanol, 20 ng/ml murine granulocyte macrophage colony stimulating factor (GM-CSF) (R&D), and 2 nm l-glutamine) (Day 1). At 48 h (Day 3), 10 ml of fresh DC media was added to each dish. At 3–4 day intervals, 10 ml of supernatant/cells was removed, cells isolated by centrifugation (90 × g) and resuspended with fresh DC medium. On day 9, non-adherent dendritic cells were collected and nucleofected using the Amaxa DC Nucleofection kit, Nuclefector II, and program Y-001 (Amaxa) with murine CCL19 shRNA (Origene). 106 nucleofected cells were plated into each well of a 12-well dish and allowed to proliferate for 48 h in DC media. On day 11, immature dendritic cells were labeled with (5 μm) 5(and 6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE) for 10 min, or 10 μm 5 (and 6)-(((4-chloromethyl)benzoyl)amino) tetramethyl-rhodamine (CMTMR) for 30 min at 37 °C, washed 3× in PBS/2%FBS, primed with 50 μg/ml ovalbumin, and matured with 100 ng/ml TNFα. T lymphocytes were isolated from OT-I/RAG1 (C57BL/6-Tg[OT-I]-RAG1tm1Mom) spleens and purified by negative selection (EasySep). Purified T lymphocytes were labeled with 10 μm CMTMR (when DCs were labeled with CSFE) for 10 min at 37 °C, or 5 μm CSFE (when DCs were labeled with CMTMR) for 30 min at 37 °C, rinsed 3× in PBS/2%FBS and plated in the well with ovalubumin-primed, labeled mature dendritic cells. Cells were allowed to form conjugates for 2 h, fixed, unbound cells removed by a PBS rinse and conjugates counted per 100 dendritic cells.
Western Blots
As described (22). Briefly, cells were lysed in radio immunoprecipitation assay buffer (RIPA) (100 mm Tris pH 8.0, 300 mm NaCl, 2% Nonidet P40, 1% sodium deoxycholate, 0.2% SDS) supplemented with protease inhibitor mixture (Sigma), 1 mm sodium orthovanadate, 1 mm sodium pyrophosphate, 10 mm sodium fluoride (Sigma) and 1 mm β-glycerophosphate (Sigma). Lysates were centrifuged for 20 min following homogenization. 5 × 105 cell equivalents were solubilized in sample buffer with 10 mm dithiothreitol, fractionated by SDS-PAGE and transferred to PVDF membranes. Membranes were incubated in 5% milk/Tris buffered saline (50 mm Tris-HCl pH→7.5, 150 mm NaCl) 0.1% Tween-20 (TBST) and probed with anti-ERK5 (Cell Signaling Technologies or Upstate), anti-α-tubulin (Cell Signaling Technologies), anti-β-actin (Cell Signaling Technologies), anti-KLF-2 (CeMines) or anti-MIP3β (AbCam). To detect phospho-ERK5, membranes were incubated in 3% bovine serum albumin (Sigma A3058) and probed with anti-phosphoERK5 (Upstate). Secondary antibodies, used at a dilution of 1:10,000, included horseradish peroxidase (HRP) conjugated rabbit anti-goat IgG (Jackson ImmunoResearch) and HRP conjugated goat anti-rabbit IgG (Pierce). Immune complexes were developed with chemilluminescence Supersignal West Femto (Pierce) on X-MAT film and quantified with a Fuji LAS-4000. Membranes were stripped using Blotfresh (SignaGen) stripping buffer for 15 min, rinsed a minimum of three times in TBST (until no residual smell remained), blocked, and re-probed.
Reverse Transcriptase-PCR
Total RNA was extracted from Hut78 cells or splenocytes using TRIzol (Invitrogen). cDNA was prepared as follows: 50 ng of random primers (Invitrogen), 5 μg of RNA, 0.25 mm dNTP (TakaRa) and ddH2O, and were heated to 65 °C for 5 min, then chilled on ice. 5X First Strand Buffer (Invitrogen) was added to the mixture and adjusted to 1× in the presence of 5 mm DTT (Invitrogen), and 40 units of RNasin (Promega). The reaction was incubated at 25 °C for 2 min, and 200 units of SuperScript IITM RT (Invitrogen) was added. The cDNA was primed at 25 °C for 10 min, and then extended by incubating at 42 °C for 50 min. Super Script IITM RT was inactivated by heating to 70 °C for 15 min. To assay for expression, PCR was carried out by addition of Green Go Taq flexi buffer (Promega), 1.5 mm MgCl2 (Invitrogen), 0.25 mm dNTP (TakaRa), 1Unit TaqDNA polymerase (Invitrogen), cDNA, H2O, and mRNA specific primers. Primers used: murine EDG-1 and murine β-actin forward: 5′-ATGACGATATCGCTGCGCTG-3′ reverse: 5′-AGTAACAGTCCGCCTAGAAG-3′ (16). PCR products were resolved on a 2% agarose gel containing ethidium bromide. Products were amplified 30 cycles using a thermocycler. EDG-1 and β-actin yielded products of the predicted size.
Immunofluorescent Imaging
HuT78 or wild type T cells were incubated ± 10 nm CCL19 for 72 h at 37C in a humidified incubator/5% CO2. At the end of the incubation cells were fixed in 2% PFA(EM Sciences)/1× PBS (Cellgro) for 15 min at room temperature, and permeabilized (0.1% Nonidet P-40 in 1× PBS (Cellgro)) for 15 min at room temperature. Cells were diluted in 1× PBS, pelleted at 300 × g, then washed two more times in 1× PBS. Cells were stained with goat anti-CCR7 (Santa Cruz sc-9700) and mouse anti-human EDG-1 (Santa Cruz sc-48356) for 1 h at room temperature. Cells were rinsed twice in 0.1% Nonidet P-40/1× PBS with a final rinse in 1× PBS. Cells were plated on glass slides in a drop of Prolong Gold with DAPI and allowed to cure overnight before imaging. Cells were imaged using Metamorph imaging program.
FACS Assays
HuT78 cells were incubated ± 10 nm CCL19 for 96 h. During this period, CCL19 was added to individual dishes at timed intervals as follows: 96 h was added on day 0, 72 h was added on day 1, 48 h was added on day 2, and 24 h was added on day 3. Cells were collected on day 4, pelleted by centrifugation at 90 × g for 10 min, rinsed in 1× PBS/2% BSA, and incubated in APC conjugated anti-human EDG-1 (R&D) on ice for 1 h per manufacturer's instructions. Cells were rinsed 2× in 1× PBS/2% BSA, resuspended in PBS and assayed by FACS. Mean channel fluorescence intensities were measured.
RESULTS
CCL19 Expression by Activated Dendritic Cells Mediates Recruitment of Naïve T Lymphocytes
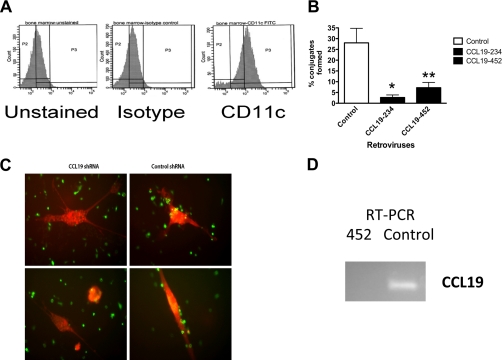
During their residence within the lymph nodes, T cells form contacts with activated dendritic cells. We hypothesized that T cells would migrate to, and form adhesions with, dendritic cells, in response to the high levels of CCL19 expressed by the activated dendritic cells (Fig. 1). To examine the contribution of activated dendritic cell CCL19 to the recruitment of naïve T lymphocytes we used an in vitro conjugate assay. CD11c+ bone marrow-derived dendritic cells (Fig. 1A), loaded with ovalbumin and matured in the presence of tumor necrosis factor α (TNFα), were incubated with purified OTI cells for 2 h. The OTI CD8+T cells express a transgenic T lymphocyte receptor (TCR), which is specific for the ovalbumin receptor. Under these conditions, we found that ∼20% of the T cells formed conjugates with antigen primed, activated dendritic cells transfected with control shRNA (Fig. 1, B and C). In contrast, in the presence of two different CCL19 shRNAs, levels of CCL19 were knocked down when compared with controls (Fig. 1D). Using different plasmid combinations, we found that the number of conjugates was significantly reduced for combinations 452 (e.g. plasmids 64, 65, and 62 (0.0125/cell)) and 234 (0.0313/cell) (Fig. 1). We concluded that CCL19 expressed by dendritic cells promotes conjugates between naïve T lymphocytes and activated dendritic cells.
FIGURE 1.
Loss of CCL19 expression in dendritic cells blocks T cells from forming conjugates with dendritic cells in vitro. A, bone marrow-derived dendritic cells (DC) generated from C57BL/6 mice, stained with an isotype control or CD11c antibodies were analyzed by flow cytometry. B, DC that lack CCL19 expression fails to form conjugates with OTI T cells. DC were transfected with control, 234 (CCL19), or 452(CCL19) shRNA, and 72 h later loaded with ovalbumin during maturation. Cells were incubated with OTI T cells for 2 h. Cell were rinsed, fixed in paraformaldehyde, and the number of conjugates formed per 100 cells counted. Error bars are mean ± S.E. *, p = 0.0313 for 234, and **, p = 0125 for 452 Student's t test versus control. C, representative image of CMTMR-labeled OVA loaded, TNF-α matured DCs forming conjugates with CSFE-labeled OTI T cells. D, RT-PCR of 5 μg of mRNA isolated from DC transfected with control, or 452(CCL19) shRNA plasmids. Data are representative of three independent experiments.
Sustained CCL19 Activation of T Cells Leads to EDG-1 Expression
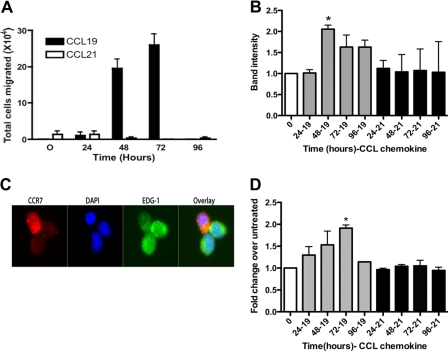
During an adaptive immune response, naïve T cells bind to cognate antigen on activated dendritic cells for 48–72 h before detaching and beginning to divide (23, 24). Following activation, the T cells exit the lymph nodes via up-regulation of the EDG-1 receptor (16). Therefore, to determine if CCR7 adhesion to dendritic cells in response to CCL19 for 48 and 72 h led to up-regulation of EDG-1, we co-incubated the HuT78 cells in the presence of CCL19 or CCL21 for up to 72 h. To determine if the EDG-1 expressed by the T cells could promote migration to sphingosine 1-phosphate, we used a migration assay (Fig. 2). Following incubation with ligand, HuT78 cells were induced to migrate in response to 10 nm sphingosine 1-phosphate. We found that CCL19, but not CCL21, promoted increased migration to EDG-1. Cells stimulated with PBS showed no increase in migration compared with untreated controls (data not shown). Because multiple G-protein coupled receptors can bind to sphingosine 1-phosphate, we confirmed that the EDG-1 receptor was expressed on the CCL19-activated HuT78 through the use of RT-PCR, immunofluorescence microscopy and FACS (Fig. 2). We found that following prolonged stimulation with CCL19, T cells lost surface expression of CCR7, which correlated with a significant up-regulation in the levels of EDG-1 expressed on the surface. From these studies we concluded that CCR7 stimulation by CCL19 for 48 to 72 h led to up-regulation of EDG-1.
FIGURE 2.
CCL19 stimulation of HuT78 T cells induces expression of EDG-1 and migration to 10 nm sphingosine 1-phosphate. A, HuT78 cells migrate to 10 nm sphingosine 1-phosphate after 48 and 72 h of stimulation with 40 nm CCL19 but not CCL21. B, histogram of mRNA levels isolated from cells treated with 40 nm CCL19 or CCL21. mRNA was reverse transcribed and amplifed for EDG-1 and actin. EDG-1 levels were normalized to actin. C, HuT78 cells were incubated in the presence of 10 nm CCL19 for 72 h, stained for EDG-1 and counterstained with DyeLight 488-anti mouse and stained for CCR7 and counterstained with Texas Red anti-goat, and imaged. D, HuT78 cells were incubated in the presence of 10 nm CCL19 or CCL21 for the indicated times, stained with APC-anti-human EDG-1 and assayed by FACS. Data are representative of at least three independent experiments. Error bars are means ± S.E. *, p = 0.0011 for 0 h versus CCL19 for 72 h (72–19) Student's paired t test for EDG-1 protein levels.
KLF-2 Is Up-regulated in Response to CCL19 Stimulation of CCR7
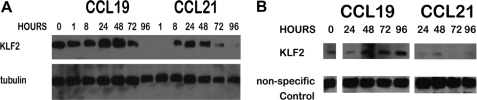
Expression of EDG-1 in T lymphocytes is regulated by the transcription factor KLF-2 (18). Therefore, we used Western blots to determine if KLF-2 was up-regulated in HuT78 cells in the presence of CCR7/CCL19 (Fig. 3A). We found that following stimulation with CCL19, KLF-2 levels increased during the first 48 h and started to decrease at 72 h. This increased level of KLF-2 expression preceded the increased expression of EDG-1. In contrast, signaling through CCL21 mediated only a rapid, but transient, increase in KLF-2. To confirm that this signaling pathway was active in primary T lymphocytes, we stimulated splenic T lymphocytes with CCL19 or CCL21 (Fig. 3B) in culture for 24, 48, 72, or 96 h. As determined by Western blot, expression of KLF-2 increased in primary T lymphocytes following exposure to CCL19, but not in response to CCL21. Because in T lymphocytes KLF-2 is a transcription factor that is required for the expression of EDG-1 (18), we concluded that CCL19 mediated CCR7 signaling mediates increased expression of KLF-2.
FIGURE 3.
Stimulation of HuT78 T cells and murine primary T cells with CCL19 mediates up-regulation of KLF-2. A, HuT78 and B, primary T cells were stimulated with 40 nm CCL19 or CCL21 for the indicated times. Cell lysates were fractionated by SDS-PAGE, transferred to PVDF, and probed for KLF-2. A, membranes were stripped and reprobed for tubulin or B, a nonspecific band was used as a loading reference. Blots are representative of at least three independent experiments.
Activation of CCR7 via CCL19 Leads to Phosphorylation of ERK5
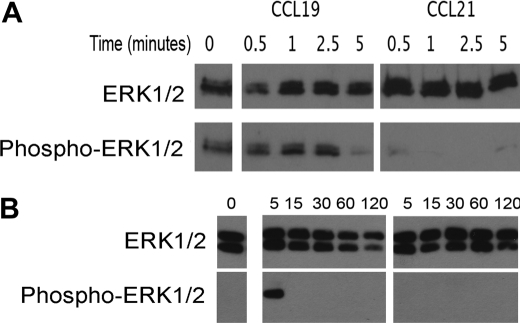
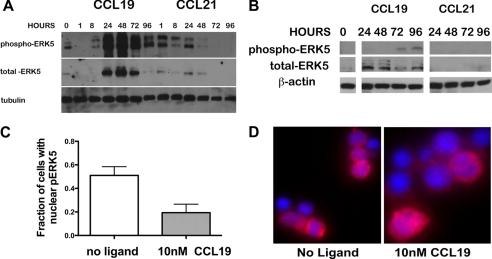
In the Erk5−/− embryonic lethal mouse it was determined that ERK5 regulated the expression of KLF-2; however, no effect has been observed on the regulation of T lymphocytes (19, 25). Activation of ERK5 can parallel activation of ERK1/2 (26, 27). In addition, in heterologous systems, CCL19 induced a 4-fold increase in activation of ERK1/2 when compared with ERK1/2 phosphorylation levels induced by CCL21 (28). To determine if CCL19 preferentially activated ERK1/2 in T lymphocytes, we compared activation of HuT78 by CCL19 to activation by CCL21 (Fig. 4A). We observed a transient increase in the phosphorylation of ERK1/2 in the presence of CCL19. In the presence of CCL21 we observed a reduction of ERK1/2 phosphorylation when compared with unstimulated cells. We used mouse splenic T lymphocytes to confirm the signaling event in primary cells (Fig. 4B). We found that, while CCL21 failed to mediate sustained ERK1/2 phosphorylation in primary murine T lymphocytes or in the HuT78 cell line, CCL19 mediated activation of ERK1/2 within 5 min of stimulation. Because in both HuT78 and primary T cells the phosphorylation of ERK1/2 was reduced to levels below our detection after 5 min, we concluded that ERK1/2 did not contribute to signaling over the extended times of 48 and 72 h, which were required to induce EDG-1 expression. In addition, although at early time points ERK1/2 was phosphorylated, we were unable to detect phosphorylation of ERK5, a transcription factor required for expression of KLF-2, during this interval (data not shown). To determine if ERK5 was involved in upregulating EDG-1 following activation of T lymphocytes by CCL19, we stimulated T lymphocytes with CCL19 and evaluated the expression and phosphorylation of ERK5. Up-regulation of KLF-2 and EDG-1 takes place between 2 and 4 days. Therefore, we examined the phosphorylation of ERK5 in the presence of CCL19 over 96 h in both primary T lymphocytes and the HuT78 cells. After 48 h of stimulation with CCL19, we observed an increase in ERK5 phosphorylation, which was maintained throughout the 96 h of stimulation (Fig. 5A). ERK5 was also phosphorylated in cells treated with CCL21, but to a lesser extent. In both HuT78 and primary T lymphocytes we also observed that ERK5 protein levels increased in parallel with the phosphorylation. We concluded that in T lymphocytes, CCL19 differentially mediates increased expression and phosphorylation of ERK5.
FIGURE 4.
Stimulation of HuT78 human T cells and murine primary T cells with CCL19, but not CCL21 leads to phosphorylation of ERK1/2. A, HuT78 or B, C57BL/6 primary T cells were stimulated with 40 nm CCL19 or 40 nm CCL21 for A, 0.5, 1, 2.5, and 5 min or B, 0, 5, 15, 30, 60, and 120 min. Cell lysates were fractionated by SDS-PAGE, transferred to PVDF and probed for phospho-ERK1/2. The membranes were stripped and reprobed for total ERK1/2. Blots are representative of at least three independent experiments.
FIGURE 5.
The HuT78 cells and primary T cells induce increased levels of ERK5 expression and phosphorylation following activation with CCL19. A, HuT78 and B, C57BL/6 primary T cells were stimulated in vitro for the indicated time courses with 40 nm CCL19 or CCL21. Cell lysates were fractionated by SDS-PAGE, transferred to PVDF and probed for phospho-ERK5. The membranes were stripped and reprobed for total-ERK5 and for A, tubulin or B, actin as a loading control. Blots are representative of at least three independent experiments. C, histogram of cells scored for pERK5 in the nucleus or cytoplasm. At least 30 cells were counted per group. Histograms are means ± S.E. Significantly more T cells stained for pERK5 in the nucleus, in the absence of CCL19 when compared with cells stimulated for 72 h (p = 0.0044). D, metamorph images of wild type primary T cells ± 10 nm CCL19 at 72 h stained for phospho-ERK5 (Texas Red) and DNA (DAPI).
CCL19 Promotes Redistribution of ERK5
ERK5 can be found in both the nucleus and the cytoplasm (29), however the functional significance of the movement into the nucleus is unclear. To determine if CCR7 stimulation of primary T cells promoted translocation of phosphorylated ERK5 (pERK5), we used immunofluorescence microscopy. At 0 h pERK5 was found predominantly in the nucleus. At 72 h following stimulation with CCL19, we found that pERK5 was present in the cytoplasm in significantly more cells, when compared with cells that had not been treated with CCL19 (p = 0.0044) (Fig. 5C). From this result we concluded that stimulation of T cells with CCL19 promotes movement of pERK5 from the nucleus to the cytoplasm.
Migration to Sphingosine 1-Phosphate Is Lost in ERK5f/f/Lck-Cre Cells
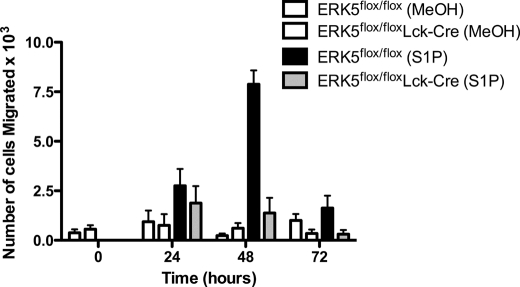
To determine a physiological significance for ERK5 in regulating chemotaxis of T cells to sphingosine 1-phosphate we used migration assays. To this end we compared migration of ERK5flox/flox T cells to ERK5flox/flox/Lck-Cre T cells. We found that ERK5flox/flox T cells migrated to sphingosine 1-phosphate at levels similar to the wild type cells, while the ERK5flox/flox/Lck-Cre T cells failed to migrate. These results suggested that T cells are unable to up-regulate EDG-1 in the absence of ERK5.
DISCUSSION
Circulating T cells express high levels of EDG-1 prior to entering lymph nodes (16). Twenty-four hours after entering the lymph node and encountering cognate ligands, the T cells lose their ability to migrate toward sphingosine 1-phosphate; the ability to migrate to sphingosine 1-phosphate is restored within 72 h of lymph node entry. In this study, we have found that expression levels of KLF-2 are up-regulated in response to stimulation of CCR7/CCL19 but not CCR7/CCL21. In addition, CCR7/CCL19 activation leads to changes in the expression levels and phosphorylation state of ERK5, and the levels of KLF-2 expressed are increased (Figs. 3 and 5).
FIGURE 6.
T cells isolated from Erk5flox/flox/Lck-Cre mice fail to migrate to S1P following exposure to CCL19. Primary T cells were isolated from Erk5flox/flox/Lck-Cre, or Erk5flox/flox mouse spleens and stimulated in vitro with 40 nm CCL19 for 0–72 h. Cells were allowed to migrate to 10 nm sphingosine 1-phosphate or vehicle control (methanol) for 90 min and counted. The graph is representative of three independent experiments. Histograms are means of the number of cells migrated ± S.E.
CCR7 is involved in regulating egress of T cells from lymph nodes. In one study using CCR7−/− cells in an adoptive transfer assay, it was shown that CCR7−/− T cells leave lymph nodes more rapidly than wild type CCR7+/+ cells that have been co-injected into the same mouse (17). Given our observation that T cells use CCR7 to mediate conjugates with activated dendritic cells, this is may not be surprising. In these mice the adoptively transferred cells were found near the high endothelial venules but not deep within the T zone. In this case, the cells would not bind to activated dendritic cells and it is likely that the T cells would exit the lymph nodes more rapidly than their wild type cohorts. In contrast, transgenic overexpression of CCR7 in T cells resulted in retention within the lymph nodes compared with control cells (13, 17), which could be due to prolonged adhesion to dendritic cells. We have observed that, in the presence of CCL19, CCR7 is lost from the surface and EDG-1 is up-regulated. Furthermore, it is not surprising that cells, which overexpress CCR7 fail to properly internalize CCR7, and remove it from the surface, which keeps the T cell from leaving the lymph nodes. Therefore, we examined the signaling events that are mediated by CCR7, following activation by its ligands, that could regulate the timing of lymph node egress.
Our results are further supported by reports in which T lymphocytes, adoptively transferred to a CCL19−/− mouse, failed to exit the lymph nodes at the same rate as T lymphocytes injected into a wild-type mouse at the same time (14). In this model, cells are able to enter the lymph nodes, via high endothelial venules, which are a source of CCL21. In this study, however, the authors did not mention a connection between CCL19 signaling and the egress of T lymphocytes from the lymph nodes. Based on our observations, we propose that in these studies, loss of signaling through CCR7/CCL19 blocked T lymphocyte exit from the lymph nodes by preventing signaling to up-regulate EDG-1.
Our observations are not unexpected given the distribution of CCL21 and CCL19 and the extent of internalization of CCR7 following engagement of these two ligands (7, 10). We and others (7) have shown that only ∼20% of CCR7 is internalized when it is bound to CCL21, allowing for CCR7 to remain on the surface of the cell and continue to sense CCL19 or CCL21 in the lymph nodes. We speculate that if T lymphocytes fail to make a conjugate with a dendritic cell that lasts for more than an hour, the signaling pathway is not activated, allowing T lymphocytes to maintain their level of EDG-1 expression and exit the lymph nodes. In contrast, if the T lymphocytes form a conjugate with activated dendritic cells that expresses a cognate ligand that T lymphocytes recognize, the T cell maintains the close contact required to internalize over 80% of CCR7 and to activate ERK5. Studies are ongoing to determine the cytoplasmic adaptors that are required for activation of ERK5 in the presence of continued internalization of CCR7/CCL19.
As mentioned, CCL19 induces internalization of 80% of the CCR7 on the cell surface. In contrast, CCL21 induces internalization of ∼20% of the cell surface CCR7. Therefore, it is possible that the differential signaling that we observed with respect to ERK5, KLF-2, and EDG-1 is due to differences in levels of CCR7 on the surface. Such a hypothesis would be difficult to test without generating numerous T lymphocyte lines that express different levels of CCR7 on the cell surface. An alternative explanation is that internalization of CCR7 via CCL19 versus CCL21 mediates recruitment of different adaptor proteins that remain associated with the cytoplasmic C terminus and intracellular loops following recycling of the receptor. We have observed that internalization of CCL19-bound CCR7 mediates the recruitment of arrestin 3, while CCL21 does not (7). Thus, it is possible that the activated form of CCR7 recruits ERK5 following engagement of CCL19 via arrestins. The recruitment of arrestins takes place within minutes of CCR7 activation by CCL19, and while ERK1/2 is activated within this timeframe, activation of ERK5 requires several hours of CCL19 exposure. From this observation, we speculate that other adaptors may be required, that associate only after CCR7/CCL19 has trafficked within the cell to sites that allow for recruitment of these adaptors. The adaptors would then remain associated when the receptor recycles. In contrast, signaling through CCR7/CCL21 fails to activate this pathway since it takes a different route when it is internalized, although this ligand/receptor pair has been linked to other distinct cellular events (7, 9, 28, 31, 32). If the receptor is re-internalized rapidly, due to the presence of ligand the subsequent internalizations may recruit novel adaptors to the already modified cytoplasmic tail. Subsequently, ERK5 associates with the activated receptor and is internalized. CCR7 has been observed in the nucleus in some cells (33); its function in the nucleus, however, has not yet been defined, though CCR7 has been found to be associated with topoisomerase I (32). In future studies, it will be important to determine the mechanisms that mediate increased expression and increased phosphorylation of ERK5 after prolonged stimulation of T lymphocyte CCR7, to be able to regulate T lymphocyte egress during immune responses.
Because the Erk5−/− mouse is embryonic lethal, we were unable to use the T lymphocytes from the Erk5−/− mouse to study the role of ERK5 in T lymphocyte egress. Therefore, we used ERK5flox/flox/Lck-Cre mouse T cells. We found that, instead of a reduction in the level of ERK5 expressed, the T cells isolated from these mice showed a significant increase in the levels of ERK5 (data not shown). Nonetheless, we found that with this perturbed level of ERK5 expression, we were no longer able to stimulate expression of EDG-1 following ligation of CCR7/CCL19. From our studies, we concluded that ERK5 is indeed required for regulation of EDG-1.
KLF-2 expression is tightly regulated in T lymphocytes during differentiation and maturation (34). Overexpression of activated ERK5 leads to up-regulation of KLF-2 in a reconstituted fetal thymic organ culture, while dominant negative ERK5 blocks KLF-2 (35). Recently it was reported that Foxo1, one of four members of the Foxo subfamily of transcription factors that control cell cycle progression and (36, 37) apoptosis, mediates the expression of CCR7 and KLF-2 (37). In these studies, Foxo1 was homozygously deleted, producing a reduction in the expression levels of both CCR7 and KLF-2. In other studies, which used a microarray of RNAs enriched for immune function, KLF-2 mRNA levels were found to be up-regulated during positive selection at the same time that CCR7 is first expressed in T lymphocytes (38) in the thymus and lymph nodes. This was not surprising, given the role that CCR7 has in the up-regulation of KLF-2. Furthermore, KLF-2 levels increase following in vitro stimulation of naïve T lymphocytes, with IL-2 or IL-17 (30). KLF-2 expression correlated with long-term survival, and we therefore speculate that KLF-2 contributes to the long-term viability of T lymphocytes, that have had sustained interactions with CCL19-expressing dendritic cells, for future immune responses.
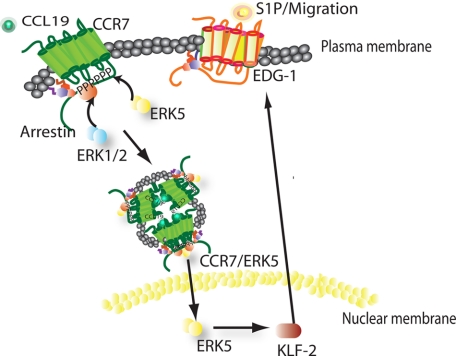
In conclusion, our data define differential signaling events that take place within naïve T lymphocytes following activation by CCL19 or CCL21. After binding to CCL19, CCR7 signaling leads to increased expression and phosphorylation of ERK5 and up-regulation of KLF-2, which results in EDG-1 expression and a migration of T lymphocytes (Fig. 7). This is the first report to show that these signaling events, which are required to control the migration of T lymphocytes through the lymph node, are in response to CCR7/CCL19.
FIGURE 7.
Model of proposed CCR7/CCL19 signaling pathway in T cells.
Acknowledgments
We thank Colin A. Bill, Brian Kaiser, Steve Benedict, and Hattie Gresham for critical discussions and reading of the manuscript. We greatly appreciate the generous gift of the ERK5flox/flox mice from Dr. Cathy Tournier, University of Manchester.
This work was supported, in whole or in part, by National Institutes of Health Grants P20RR016443, K12HD052027, and K22AI060815 and a grant from the KUMC Institute for Advancing Medical Innovation, a KUMC Bridging Award, and the KUMC Biomedical Research Training Program.
- HEV
- high endothelial venules
- CCR7
- C-C chemokine receptor 7
- HuT78
- human T cell line
- CCL19
- chemokine (C-C) ligand 19
- CCL21
- chemokine (C-C) ligand 21
- KLF-2
- Kruppel-like factor 2
- EDG-1
- endothelial differentiation gene-1
- PLT
- paucity of lymph node T lymphocyte.
REFERENCES
- 1. Dailey M. O., Gallatin W. M., Weissman I. L. (1985) The in vivo behavior of T cell clones: altered migration due to loss of the lymphocyte surface homing receptor. J. Mol. Cell. Immunol. 2, 27–36 [PubMed] [Google Scholar]
- 2. Fan L., Reilly C. R., Luo Y., Dorf M. E., Lo D. (2000) Cutting edge: ectopic expression of the chemokine TCA4/SLC is sufficient to trigger lymphoid neogenesis. J. Immunol. 164, 3955–3959 [DOI] [PubMed] [Google Scholar]
- 3. Gunn M. D., Kyuwa S., Tam C., Kakiuchi T., Matsuzawa A., Williams L. T., Nakano H. (1999) Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 189, 451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luther S. A., Tang H. L., Hyman P. L., Farr A. G., Cyster J. G. (2000) Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc. Natl. Acad. Sci. U.S.A. 97, 12694–12699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ngo V. N., Korner H., Gunn M. D., Schmidt K. N., Riminton D. S., Cooper M. D., Browning J. L., Sedgwick J. D., Cyster J. G. (1999) Lymphotoxin α/β and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 189, 403–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Campbell J. J., Bowman E. P., Murphy K., Youngman K. R., Siani M. A., Thompson D. A., Wu L., Zlotnik A., Butcher E. C. (1998) 6-C-kine (SLC), a lymphocyte adhesion-triggering chemokine expressed by high endothelium, is an agonist for the MIP-3β receptor CCR7. J. Cell Biol. 141, 1053–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Byers M. A., Calloway P. A., Shannon L., Cunningham H. D., Smith S., Li F., Fassold B. C., Vines C. M. (2008) Arrestin 3 mediates endocytosis of CCR7 following ligation of CCL19 but not CCL21. J. Immunol. 181, 4723–4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luther S. A., Bidgol A., Hargreaves D. C., Schmidt A., Xu Y., Paniyadi J., Matloubian M., Cyster J. G. (2002) Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J. Immunol. 169, 424–433 [DOI] [PubMed] [Google Scholar]
- 9. Otero C., Groettrup M., Legler D. F. (2006) Opposite fate of endocytosed CCR7 and its ligands: recycling versus degradation. J. Immunol. 177, 2314–2323 [DOI] [PubMed] [Google Scholar]
- 10. Bardi G., Lipp M., Baggiolini M., Loetscher P. (2001) The T cell chemokine receptor CCR7 is internalized on stimulation with ELC, but not with SLC. Eur. J. Immunol. 31, 3291–3297 [DOI] [PubMed] [Google Scholar]
- 11. Nakano H., Mori S., Yonekawa H., Nariuchi H., Matsuzawa A., Kakiuchi T. (1998) A novel mutant gene involved in T-lymphocyte-specific homing into peripheral lymphoid organs on mouse chromosome 4. Blood 91, 2886–2895 [PubMed] [Google Scholar]
- 12. Nakano H., Tamura T., Yoshimoto T., Yagita H., Miyasaka M., Butcher E. C., Nariuchi H., Kakiuchi T., Matsuzawa A. (1997) Genetic defect in T lymphocyte-specific homing into peripheral lymph nodes. Eur. J. Immunol. 27, 215–221 [DOI] [PubMed] [Google Scholar]
- 13. Förster R., Schubel A., Breitfeld D., Kremmer E., Renner-Müller I., Wolf E., Lipp M. (1999) CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33 [DOI] [PubMed] [Google Scholar]
- 14. Link A., Vogt T. K., Favre S., Britschgi M. R., Acha-Orbea H., Hinz B., Cyster J. G., Luther S. A. (2007) Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat. Immunol. 8, 1255–1265 [DOI] [PubMed] [Google Scholar]
- 15. Pilkington K. R., Clark-Lewis I., McColl S. R. (2004) Inhibition of generation of cytotoxic T lymphocyte activity by a CCL19/macrophage inflammatory protein (MIP)-3β antagonist. J. Biol. Chem. 279, 40276–40282 [DOI] [PubMed] [Google Scholar]
- 16. Matloubian M., Lo C. G., Cinamon G., Lesneski M. J., Xu Y., Brinkmann V., Allende M. L., Proia R. L., Cyster J. G. (2004) Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 [DOI] [PubMed] [Google Scholar]
- 17. Pham T. H., Okada T., Matloubian M., Lo C. G., Cyster J. G. (2008) S1P1 receptor signaling overrides retention mediated by Gαi-coupled receptors to promote T cell egress. Immunity 28, 122–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carlson C. M., Endrizzi B. T., Wu J., Ding X., Weinreich M. A., Walsh E. R., Wani M. A., Lingrel J. B., Hogquist K. A., Jameson S. C. (2006) Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature 442, 299–302 [DOI] [PubMed] [Google Scholar]
- 19. Ananieva O., Macdonald A., Wang X., McCoy C. E., McIlrath J., Tournier C., Arthur J. S. (2008) ERK5 regulation in naïve T-cell activation and survival. Eur. J. Immunol. 38, 2534–2547 [DOI] [PubMed] [Google Scholar]
- 20. Hennet T., Hagen F. K., Tabak L. A., Marth J. D. (1995) T-cell-specific deletion of a polypeptide N-acetylgalactosaminyl-transferase gene by site-directed recombination. Proc. Natl. Acad. Sci. U.S.A. 92, 12070–12074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lutz M. B., Kukutsch N., Ogilvie A. L., Rössner S., Koch F., Romani N., Schuler G. (1999) An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 [DOI] [PubMed] [Google Scholar]
- 22. Vines C. M., Potter J. W., Xu Y., Geahlen R. L., Costello P. S., Tybulewicz V. L., Lowell C. A., Chang P. W., Gresham H. D., Willman C. L. (2001) Inhibition of β2 integrin receptor and Syk kinase signaling in monocytes by the Src family kinase Fgr. Immunity 15, 507–519 [DOI] [PubMed] [Google Scholar]
- 23. Stoll S., Delon J., Brotz T. M., Germain R. N. (2002) Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science 296, 1873–1876 [DOI] [PubMed] [Google Scholar]
- 24. Mempel T. R., Henrickson S. E., Von Andrian U. H. (2004) T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 [DOI] [PubMed] [Google Scholar]
- 25. Sohn S. J., Li D., Lee L. K., Winoto A. (2005) Transcriptional regulation of tissue-specific genes by the ERK5 mitogen-activated protein kinase. Mol. Cell. Biol. 25, 8553–8566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rovida E., Spinelli E., Sdelci S., Barbetti V., Morandi A., Giuntoli S., Dello Sbarba P. (2008) ERK5/BMK1 is indispensable for optimal colony-stimulating factor 1 (CSF-1)-induced proliferation in macrophages in a Src-dependent fashion. J. Immunol. 180, 4166–4172 [DOI] [PubMed] [Google Scholar]
- 27. Nishimoto S., Nishida E. (2006) MAPK signaling: ERK5 versus ERK1/2. EMBO Rep. 7, 782–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kohout T. A., Nicholas S. L., Perry S. J., Reinhart G., Junger S., Struthers R. S. (2004) Differential desensitization, receptor phosphorylation, β-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor 7. J. Biol. Chem. 279, 23214–23222 [DOI] [PubMed] [Google Scholar]
- 29. Buschbeck M., Ullrich A. (2005) The unique C-terminal tail of the mitogen-activated protein kinase ERK5 regulates its activation and nuclear shuttling. J. Biol. Chem. 280, 2659–2667 [DOI] [PubMed] [Google Scholar]
- 30. Schober S. L., Kuo C. T., Schluns K. S., Lefrancois L., Leiden J. M., Jameson S. C. (1999) Expression of the transcription factor lung Krüppel-like factor is regulated by cytokines and correlates with survival of memory T cells in vitro and in vivo. J. Immunol. 163, 3662–3667 [PubMed] [Google Scholar]
- 31. Otero C., Eisele P. S., Schaeuble K., Groettrup M., Legler D. F. (2008) Distinct motifs in the chemokine receptor CCR7 regulate signal transduction, receptor trafficking, and chemotaxis. J. Cell Sci. 121, 2759–2767 [DOI] [PubMed] [Google Scholar]
- 32. Arcand J., Robitaille G., Koenig M., Senecal J. L., Raymond Y. (2012) Arthritis Rheum. 64, 826–834 [DOI] [PubMed] [Google Scholar]
- 33. Cabioglu N., Yazici M. S., Arun B., Broglio K. R., Hortobagyi G. N., Price J. E., Sahin A. (2005) CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin. Cancer Res. 11, 5686–5693 [DOI] [PubMed] [Google Scholar]
- 34. Kuo C. T., Veselits M. L., Leiden J. M. (1997) LKLF: A transcriptional regulator of single-positive T cell quiescence and survival. Science 277, 1986–1990 [DOI] [PubMed] [Google Scholar]
- 35. Sohn S. J., Lewis G. M., Winoto A. (2008) Non-redundant function of the MEK5-ERK5 pathway in thymocyte apoptosis. EMBO J. 27, 1896–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Greer E. L., Brunet A. (2005) FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 24, 7410–7425 [DOI] [PubMed] [Google Scholar]
- 37. Kerdiles Y. M., Beisner D. R., Tinoco R., Dejean A. S., Castrillon D. H., DePinho R. A., Hedrick S. M. (2009) Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat. Immunol. 10, 176–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huang Y. H., Li D., Winoto A., Robey E. A. (2004) Distinct transcriptional programs in thymocytes responding to T cell receptor, Notch, and positive selection signals. Proc. Natl. Acad. Sci. U.S.A. 101, 4936–4941 [DOI] [PMC free article] [PubMed] [Google Scholar]