Background: Autophagy is a process that cells use to degrade and recycle cellular proteins, however, the role of autophagy in kidney fibrosis remains largely unknown.
Results: Autophagy is responsible for the intracellular degradation of type I collagen.
Conclusion: Autophagy negatively regulates and prevents excess collagen accumulation in the kidney.
Significance: Our findings implicate a novel role of autophagy as a cytoprotective mechanism against renal fibrosis.
Keywords: Autophagy, Cell Signaling, Collagen, Fibrosis, Kidney, Transforming Growth Factor β (TGFβ), Collagen Degradation, Mesangial Cells
Abstract
Autophagy is a highly conserved cellular process regulating turnover of cytoplasmic proteins via a lysosome-dependent pathway. Here we show that kidneys from mice deficient in autophagic protein Beclin 1 exhibited profibrotic phenotype, with increased collagen deposition. Reduced Beclin 1 expression, through genetic disruption of beclin 1 or knockdown by specific siRNA in primary mouse mesangial cells (MMC), resulted in increased protein levels of type I collagen (Col-I). Inhibition of autolysosomal protein degradation by bafilomycin A1 also increased Col-I protein levels and colocalization of Col-I with LC3, an autophagy marker, or LAMP-1, a lysosome marker, whereas treatment with TFP, an inducer of autophagy, resulted in decreased Col-I protein levels induced by TGF-β1, without alterations in Col-I α1 mRNA. Heterozygous deletion of beclin 1 increased accumulation of aggregated Col-I under nonstimulated conditions, and stimulation with TGF-β1 further increased aggregated Col-I. These data indicate that Col-I and aggregated, insoluble procollagen I undergo intracellular degradation via autophagy. A cytoprotective role of autophagy is implicated in kidney injury, and we demonstrate that low-dose carbon monoxide, shown to exert cytoprotection against renal fibrosis, induces autophagy to suppress accumulation of Col-I induced by TGF-β1. We also show that TGF-β1 induces autophagy in MMC via TAK1-MKK3-p38 signaling pathway. The dual functions of TGF-β1, as both an inducer of Col-I synthesis and an inducer of autophagy and Col-I degradation, underscore the multifunctional nature of TGF-β1. Our findings suggest a novel role of autophagy as a cytoprotective mechanism to negatively regulate and prevent excess collagen accumulation in the kidney.
Introduction
The balance between synthesis and degradation of extracellular matrix (ECM)3 proteins is crucial for maintaining tissue homeostasis. Its relentless production and progressive accumulation are hallmarks of renal fibrosis in progressive kidney disease. Transforming growth factor-β1 (TGF-β1) is well known as a potent inducer of kidney fibrosis that leads to end stage renal disease (ESRD) (1, 2). TGF-β1 induces production of ECM proteins including fibronectin and collagens and inhibits degradation of ECM proteins mainly by matrix metalloproteinases (MMPs) (3–5). Collagens are the main components of the ECM in the kidney and type I collagen (Col-I) produced by mesangial cells is the major type associated with disease states (6–8). Besides the MMPs, recently emerging evidence suggests another mechanism that may promote collagen degradation. In heat shock protein 47 (Hsp47)-null cells, the deletion of collagen-specific molecular chaperone Hsp47 results in misfolded type I procollagens that are subjected to intracellular degradation via autophagy (9). Furthermore, enhanced degradation of Col-I correlated with increase in autophagy when the β2-adrenergic receptor is activated in cardiac fibroblasts (10). These data strongly suggest that autophagy plays a role in the turnover of ECM through intracellular degradation of Col-I.
Macroautophagy, commonly referred to as autophagy is a fundamental cellular homeostatic process that cells use to degrade and recycle cellular proteins and remove damaged organelles. The process of autophagy involves the formation of double membrane bound vesicles called autophagosomes that envelope and sequester cytoplasmic components, including macromolecular aggregates and cellular organelles for bulk degradation by a lysosomal degradative pathway (11). Autophagy can be up-regulated in response to both external and intracellular factors, including amino acid starvation (12), growth factor withdrawal (13), low cellular energy levels (14), endoplasmic reticulum (ER) stress (15), hypoxia (16), oxidative stress (17), pathogen infection (18), and organelle damage (19). To date, more than 30 genes whose products are involved in autophagy have been identified in yeast, and these have been termed autophagy-related genes (Atg) (20). To initiate autophagy in mammalian cells, two different protein complexes, namely Ulk1/mAtg13/FIP200 complex and the orthologues of yeast vesicular protein-sorting protein (Vps34)/Beclin 1 complex, are required. However, it is still unclear if these complexes act independently in parallel or act together in tandem. Pro-autophagy signals such as amino acid withdrawal lead to phosphatidylinositol 3-phosphate (PI3P) synthesis by Vps34, which is phosphatidylinositol 3-kinase (PI3K). The PI3P-rich membrane structures called omegasomes are formed at subdomains of the ER membrane (21). Autophagosomes are formed within the omegasomes, and eventually exit omegasomes and fuse with lysosomes to form autolysosomes, in which the sequestered contents are degraded by proteases and acidification of lysosomal lumen (22, 23).
Beclin 1, encoded by beclin 1 gene, is the mammalian orthologue of yeast Atg6 that is required for the initiation of autophagy through its interaction with Vps34 (24). Homozygous deletion of beclin 1 (beclin 1−/−) exhibits early embryonic lethality (25), while heterozygous deletion (beclin 1+/−) results in increased incidence of spontaneous tumorigenesis (26), abnormal proliferation of mammary epithelial cells and germinal center B lymphocytes (27), and increased susceptibility to neurodegeneration (28). A recent study has shown that heterozygous inactivation of beclin 1 resulted in a 3-fold increase in interstitial fibrosis in a model of severe form of desmin-related cardiomyopathy induced by a missense mutation in the αB-crystallin (CryAB) gene (29). In contrast to Beclin 1, the mammalian orthologue of Atg8 comprised by a family of proteins known as microtubule-associated protein 1 light chain 3 (LC3) functions, at least in part, as a structural component in the formation of autophagosomes (30). LC3B (herein referred to as LC3) is the best characterized form and the most widely used as an autophagic marker.
An increasing body of evidence supports the notion that autophagy can promote cell survival and the accumulation of autophagosomes may signify a survival response intended to get rid of misfolded proteins or damaged organelles in cells (9). Indeed, autophagy is implicated in the protective mechanism against the progression of certain human diseases, including cancer, muscular disorders, and neurodegeneration, such as Huntington, Alzheimer, and Parkinson diseases (18). Autophagy also plays an important role in cellular defense mechanism to certain pathogenic bacteria and viruses. In the kidney, a recent report has shown that autophagy decreases renal tubular cell apoptosis induced by cisplatin (31) or cyclosporine (32) suggesting the potential protective role of autophagy in cytotoxic injury. However, the role of autophagy in kidney fibrosis, which ultimately leads to ESRD, remains largely unknown.
We have previously reported that TGF-β1 induces autophagy in primary mouse mesangial cells (MMC) through activation of TGF-β-activated kinase 1 (TAK1) and protects cells from serum deprivation-induced apoptosis (33). In the present study, we examined the functional role of autophagy in the regulation of Col-I induced by TGF-β1. We also explored the role of autophagy in reducing Col-I expression by treatment with low-dose carbon monoxide (CO) which we have previously shown to exert anti-fibrotic effects in a model of kidney fibrosis induced by unilateral ureteral obstruction (UUO) (34). We hypothesized that the induction of autophagy promotes intracellular degradation of Col-I and that the autophagic process may serve as a cytoprotective mechanism to maintain cellular homeostasis.
MATERIALS AND METHODS
Reagents
Recombinant human TGF-β1 was purchased from R & D Systems (Minneapolis, MN). Carbon monoxide-releasing molecule 2 (CORM-2), trifluoperazine (TFP), SB203580, bafilomycin A1, and MG132 were from Sigma-Aldrich. Anti-type I collagen antibody was from Calbiochem and Abcam. Anti-microtubule-associated protein1 light chain 3B (LC3) antibody was from nanoTools (Hamburg, Germany). Antibodies against HA, Beclin 1, lysosomal-associated membrane protein-1 (LAMP-1), β-actin, and FITC-conjugated anti-rat IgG antibody were from Santa Cruz Biotechnology (Santa Cruz, CA). Cy3-conjugated anti-rabbit IgG was from Jackson ImmunoResearch Laboratories. Lipofectamine PlusTM reagent was obtained from Invitrogen (Carlsbad, CA).
Mice
Male C57BL/6 mice (8–12 week of age) were from Jackson Laboratory (Bar Harbor, ME). Beclin 1+/− and beclin 1+/+ mice were kindly provided by Dr. Beth Levine (University of Texas Southwestern Medical Center) (35). Mitogen-activated protein kinase kinase 3 null (mkk3−/−) mice and wild-type (mkk3+/+) mice were previously described (36, 37). All experimental protocols were approved by the Institutional Animal Care and Use Committee at Harvard Medical School.
Cell Culture
MMC from mkk3−/− and mkk3+/+ mice were previously established (37). MMC were obtained from C57BL/6, GFP-LC3 transgenic (Riken Laboratories, Hirosawa, Japan), beclin 1+/−, and beclin 1+/+ mice using procedures as described previously (37). Cells were maintained in RPMI 1640 containing 15% FBS and antibiotics as described (37) and used for experiments at passages 12–20. Cells were rendered quiescent in media containing 0.5% FBS for 24 h prior to treatment with TGF-β1.
Small Interfering RNA (siRNA) and Transfection
MMC were transiently transfected with Beclin 1 siRNA or non-targeting control siRNA according to the manufacturer's protocol (Santa Cruz Biotechnology, Santa Cruz, CA). Mouse MKK3 construct (pcDNA-V5-MKK3) was previously described (38).
Immunofluorescence Microscopy
Cells were fixed with 4% paraformaldehyde and analyzed by immunofluorescence staining protocol as previously described (33) using anti-Col-I (Calbiochem, San Diego, CA) or anti-LAMP-1 (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies. Images were captured using a Nikon D-eclipse C1 confocal fluorescence microscope. Exposure settings were unchanged throughout acquisition. For quantitation of colocalization, z-stack images of a mean of ten cells for each condition per experiment were analyzed and Mander's coefficients were determined using the NIS-Elements AR 3.10 software.
Western Blot Analysis
Immunoblotting was carried out as described previously (33, 34). To evaluate for aggregate formation of Col-I, a centrifugal fractionation assay followed by immunoblotting was performed as described by to Ishida et al. (9). Briefly, proteins were extracted with buffer containing 0.05 m Tris-HCl. pH 8.0, 0.15 m NaCl, 5.0 mm EDTA, 1% Nonidet P-40, and protease inhibitors (2.0 mm N-ethylmaleimide, 2.0 mm 4-(2-aminoethyl)-benzenesulfonyl fluoride, 1 μg/ml leupeptin, and pepstatin) at 4 °C. After centrifugation (14,000 rpm, 20 min), supernatant (detergent soluble), and pellet (detergent insoluble) fractions were collected and subjected to Western blotting as described previously (9, 33) and target proteins were detected by using LumiGLO (Cell Signaling Technology, Danvers, MA) and exposed to x-ray films.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and Quantitative Real-time PCR
RT-PCR was carried out as described previously (33). The PCR primer sets for mouse Col-I α1: (forward) 5′-GACGCCATCAAGGTCTACTG-3′, (reverse) 5′-ACGGGAATCCATCGGTCA-3′ and mouse GAPDH: (forward) 5′-GCATTGTGGAAGGGCTCA-3′; (reverse) 5′-GGGTAGGAACACGGAAGG-3′. For real-time PCR, 7900 Real-Time PCR System (Applied Biosystems, Foster, CA) with SYBR green PCR reagent (Qiagen, Hilden, Germany) was used according to the manufacturer's instructions. Triplicate samples were run at each time, and detected levels of target mRNAs were calculated using the ΔΔCt method and normalized to GAPDH in arbitrary units.
CO Exposures
For low-dose CO exposure, cells were incubated with 250 ppm concentration of exogenous CO along with 5% CO2 in humidified modular chambers maintained at 37 °C, and CO levels in the chambers were monitored as described previously (34). In CO donor experiments, MMC were incubated in media containing 0.5% FBS for 24 h with various concentrations of CORM-2 (0 to 20 mm). The cell lysates were subsequently analyzed with antibodies against Col-I, Beclin 1, and LC3. For in vivo CO exposures, 1% CO mixed with room air (RA; 21% oxygen) was directed into a 3.7-ft3 glass exposure chamber at a flow rate of 121/min, as described previously (34). To maintain the CO level at 250 ppm in the chamber, a CO analyzer (Interscan, Chartsworth, CA) was used. The mice were exposed to 250 ppm concentration of CO for the indicated time periods and mice not undergoing CO exposures were maintained in normal RA. After exposure for the indicated times, the animals (n = 3 for each group of animals) were sacrificed, and the kidneys were harvested for histology and Western blot analysis.
Statistical Analysis
Statistical significance of the experimental data from three independent experiments was derived by the Student's t test or ANOVA, and p values < 0.05 were considered significant. All of the experiments were performed at least three times. Densitometric analysis for the quantitation of Western blot and RT-PCR data were carried out by using Bio-Rad Quantity One software.
RESULTS
Heterozygous Deletion of Beclin 1 Results in Increased Collagen Deposition in the Kidney
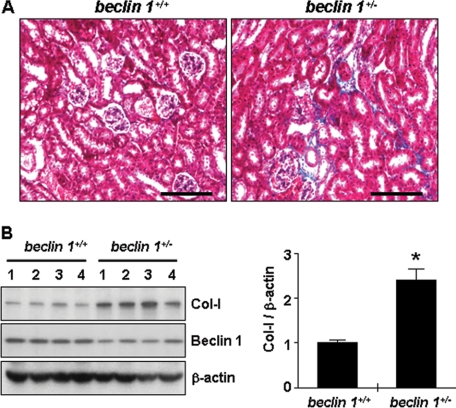
Although the homozygous beclin 1−/−-null mice are not viable, the heterozygous beclin 1+/− mice exhibit ∼50% reduction in autophagic capacity (26) and reduced basal autophagic flux compared with beclin 1+/+ littermates (39). To evaluate our hypothesis that autophagy is a process that promotes collagen degradation, we first examined whether a reduction in autophagic activity led to increased collagen deposition in the kidney of mice with heterozygous deletion of beclin 1. As shown in Fig. 1A, Masson's trichrome staining revealed increased collagen deposition in the kidneys from beclin 1+/− mice compared with beclin 1+/+ mice. We also analyzed the total kidney tissue lysates by Western blotting for Col-I. As shown in Fig. 1B, ∼2.4-fold increase in Col-I protein expression was observed in the kidneys from beclin 1+/− mice compared with beclin 1+/+ mice. We confirmed by immunoblotting that Beclin 1 protein expression was decreased in the kidneys of beclin 1+/− mice. These data show that reduced Beclin 1 expression leads to increased collagen levels, suggesting that Beclin 1 functions to prevent an excess deposition of collagen in the kidney.
FIGURE 1.
Beclin 1 heterozygous deletion in mice leads to increased collagen deposition in the kidney. A, Masson's trichrome staining of representative kidney sections from beclin 1+/− mice and age-matched beclin 1+/+ littermates. Scale bar, 100 μm. Similar results were observed for samples from four different mice of each genotype. B, expression levels of type I collagen (Col-I) and Beclin 1 by Western blot analysis of kidney tissue extracts from beclin 1+/− mice and age-matched beclin 1+/+ littermates. Each lane represents protein lysates from the kidney of a single animal (data shown are from 4 different mice per group). Immunoblotting for β-actin was used as protein loading control. The levels of Col-I were quantitated as ratio to β-actin by densitometry. Data are presented as the mean ± S.E. of four independent experiments (*, p < 0.05 versus beclin 1+/+ mice).
Reduced Expression of Beclin 1 by Heterozygous Deletion of Beclin 1 or by siRNA Knockdown Results in Increased Col-I Levels in Cultured Mesangial Cells
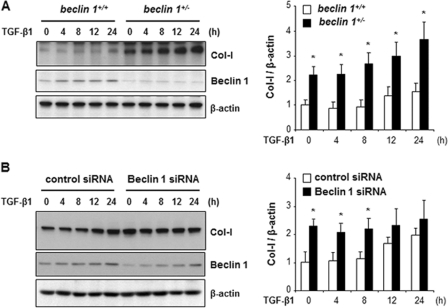
We next determined whether reduction of Beclin 1 expression leads to increased Col-I levels. Cell lysates of MMC obtained from the kidneys of beclin 1+/+ and beclin 1−/− mice were analyzed for Col-I and Beclin 1 expression by Western blotting. As shown in Fig. 2A, MMC from beclin 1+/− mice, with reduced Beclin 1 expression, exhibited high basal level of Col-1 compared with MMC from beclin 1+/+ mice (lane 1 versus lane 6). In addition, quantitative analysis demonstrated that Col-I protein levels induced by TGF-β1 stimulation were significantly increased in the beclin 1+/− MMC (solid bars) in a time-dependent manner compared with the beclin 1+/+ MMC (open bars). Similarly, in Fig. 2B, transient knockdown of Beclin 1 with specific siRNA in the beclin 1+/+ MMC also resulted in increased protein levels of Col-I (solid bars) compared with control cells (open bars), although the difference in Col-I levels becomes less apparent with time, which is associated with recovery of Beclin 1 expression in the Beclin 1 siRNA-transfected cells. Taken together, these data provide further support that reduction in the expression of Beclin 1 increases collagen protein levels, and suggest that the process of autophagy may serve as a mechanism to suppress the excess accumulation of Col-I induced by TGF-β1 stimulation.
FIGURE 2.
Reduced expression of Beclin 1 increases Col-I levels in mesangial cells. A, Western blot analysis of type I collagen (Col-I) and Beclin 1 in MMC obtained from beclin 1+/+ and beclin 1−/− mice. Cells were treated with TGF-β1 (2 ng/ml) for the indicated time periods. B, expression levels of Col-I and Beclin 1 in beclin 1+/+ MMC transfected with either control siRNA or beclin 1 siRNA and treated with TGF-β1 (2 ng/ml) for the indicated time periods. Immunoblotting for β-actin was used as protein loading control (A and B). The levels of Col-I were quantitated as ratio to β-actin by densitometry. Data are presented as the mean ± S.E. of three independent experiments (for each time point *, p < 0.05 versus beclin 1+/+ MMC in A, and *, p < 0.05 versus control siRNA in B).
Disruption of Autophagic Activity Suppresses Intracellular Degradation of Col-I
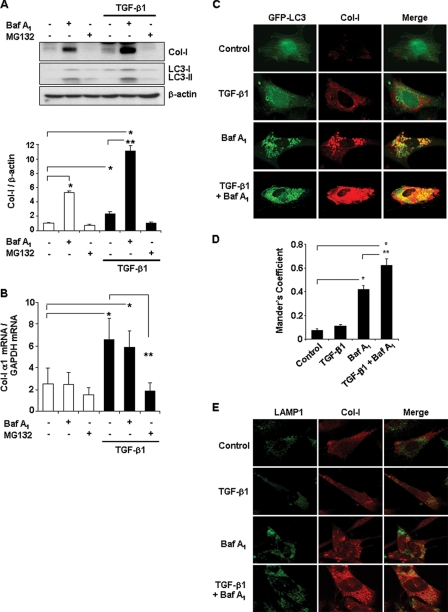
Beclin 1, present on isolation membranes, is implicated in the initiation of autophagic process, and Beclin 1 deficiency inhibits autophagic activity (25, 26). Our findings above indicate that heterozygous disruption of beclin 1 or knockdown of Beclin 1 by siRNA leads to increased Col-I protein levels in MMC. We next examined whether inhibition of autolysosomal protein degradation also results in increased Col-I levels. To this end, we utilized bafilomycin A1, a specific inhibitor of the vacuolar type H+-ATPase (V-ATPase) in cells, that has been demonstrated to inhibit autophagosome-lysosome fusion (40) or affect intralysosomal degradation by inhibiting acidification within the lysosome (41). As shown in Fig. 3A, Col-I protein levels were significantly increased in MMC by bafilomycin A1 treatment (lane 2, top panel) and further increased upon stimulation of Col-I production by TGF-β1 (lane 5, top panel). We also monitored endogenous levels of autophagic vesicle-associated-form LC3-II in the same samples in Fig. 3A and confirmed the accumulation of LC3-II upon bafilomycin A1 treatment (lanes 2 and 5, middle panel), as LC3-II is degraded by autophagy and bafilomycin A1 impairs LC3-II degradation (41, 42). In contrast, treatment with MG132, an inhibitor of ubiquitin-proteasome system (UPS), failed to significantly increase Col-I levels (Fig. 3A, lanes 3 and 6, top panel). Thus, these data suggest that autolysosomal protein degradation pathway, but not UPS, may be responsible for the intracellular degradation of Col-I protein.
FIGURE 3.
Inhibition of autolysosomal protein degradation pathway results in the accumulation of Col-I in mesangial cells. A, Western blot analysis of type I collagen (Col-I) and LC3 in MMC, incubated in the absence or presence of TGF-β1 (2 ng/ml) and bafilomycin A1 (Baf A1, 10 nm) or MG132 (10 μm), as indicated, for 24 h. Immunoblotting for β-actin was used as protein loading control. The levels of Col-I were quantitated as ratio to β-actin by densitometry. Data are presented as the mean ± S.E. of three independent experiments (*, p < 0.05 versus untreated control cells; **, p < 0.01 versus TGF-β1 alone treated cells). B, real-time PCR for Col-I α1 mRNA expression in MMC, incubated in the absence or presence of TGF-β1 (2 ng/ml) and bafilomycin A1 (Baf A1, 10 nm) or MG132 (10 μm), as indicated, for 24 h. Triplicate samples were run for each experiment. Data are presented as the mean ± S.E. of three independent experiments. (*, p < 0.05 versus untreated control cells; **, p < 0.01 versus TGF-β1 alone treated cells). C, immunofluorescence staining for Col-I (red) in MMC obtained from GFP-LC3 (green) transgenic mice. Cells were incubated in the absence (Control) or presence of TGF-β1 (2 ng/ml) and bafilomycin A1 (Baf A1, 10 nm), as indicated, for 24 h. D, Mander's coefficient of colocalization for GFP-LC3 (green) with respect to Col-I (red) was determined by analyzing z-stack images of a mean of ten cells for each condition. (*, p < 0.01 versus untreated control cells; **, p < 0.05 versus Baf A1 alone treated cells). E, coimmunofluorescence staining for Col-I (red) and LAMP-1 (green) in MMC incubated in the absence (control) or presence of TGF-β1 (2 ng/ml) and bafilomycin A1 (Baf A1, 10 nm), as indicated, for 24 h. Images were captured using a Nikon D-eclipse C1 confocal microscope (C–E).
To assure that the increases in Col-I protein levels observed with bafilomycin A1 treatment in MMC were not due to corresponding increases in Col-I α1 mRNA expression, we performed quantitative real-time PCR, and representative results are shown in Fig. 3B. No significant increases in the levels of Col-I α1 mRNA were detected in MMC upon treatment with bafilomycin A1 compared with untreated cells. Stimulation with TGF-β1 significantly increased Col-I α1 mRNA, as expected, but cotreatment with bafilomycin A1 did not further increase Col-I α1 mRNA. Thus, bafilomycin A1 increased Col-I protein levels without similarly inducing Col-I α1 mRNA expression, indicating that the increase in Col-1 protein levels is a result of inhibition of its degradation rather than increased synthesis. The effects of bafilomycin A1 alone suggest that there is some basal autophagic activity. Indeed, the increase in LC3II levels with bafilomycin A1 treatment provides further evidence for basal autophagic activity. In contrast to bafilomycin A1, we observed that the inhibition of the proteasome by treatment with MG132 repressed the expression of Col-I α1 mRNA in MMC. Similar effect of MG132 has been previously reported in human dermal fibroblasts (43) and rat cardiac fibroblasts (44).
We next determined whether intracellular Col-I is colocalized with LC3 puncta, which represent autophagosome formation (42). A frequently utilized approach to detect puncta formation is the transient transfection of cells with GFP-LC3 construct. In our colocalization studies, rather than using this overexpression system, we employed primary mesangial cells obtained from GFP-LC3 transgenic mice. Hartleben et al. (45) have demonstrated that in the GFP-LC3 transgenic mouse kidneys, GFP-LC3 is highly expressed in the podocytes but not in other renal glomerular cells. We assessed for punctate distribution of GFP-LC3 (green) visualized by confocal fluorescence microscopy together with immunofluorescence staining of Col-I (red), and representative images are shown in Fig. 3C. We observed that treatment with bafilomycin A1 alone or with TGF-β1 increased the abundance of autophagosomes (green dots) as well as Col-I staining compared with the non-stimulated control cells. These results are consistent with the Western blot analysis (Fig. 3A) detecting increases in endogenous LC3-II. Moreover, bafilomycin A1 alone or with TGF-β1 resulted in increased colocalization of GFP-LC3 and Col-I, seen as orange-colored dots in the merged images (Fig. 3C), which was reflected in increased Mander's coefficient (Fig. 3D), compared with the non-stimulated control cells. We also assessed for colocalization of Col-I and LAMP-1, a lysosomal marker. As shown in Fig. 3E, in wild-type MMC, coimmunofluorescence staining for Col-I (red) and LAMP-1 (green) demonstrated that colocalization of Col-I and LAMP-1 was also increased after treatment with bafilomycin A1 alone or with TGF-β1, compared with non-stimulated control cells. Together, our data suggest that Col-I levels are regulated by intracellular degradation through the autolysosome-mediated degradation pathway in MMC.
TFP, an Activator of Autophagy, Reduces Protein Levels of Col-I Induced by TGF-β1
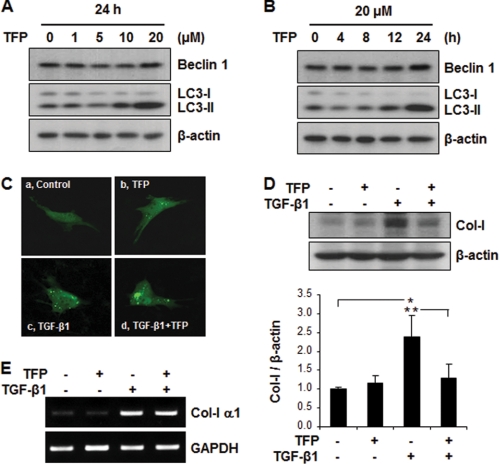
To further determine whether activation of autophagy promotes intracellular degradation of Col-I, we examined the effects of TFP, a known activator of autophagy (46). We first demonstrated that treatment of MMC with TFP, at concentrations of up to 20 μm for 24 h, resulted in significantly increased expression of LC3-II, the lipidated membrane-bound form of LC3 used as a marker of autophagic activity (Fig. 4, A and B). On the other hand, the effect of TFP on the protein levels of Beclin 1 was not as apparent, similar to the findings reported by Kiyono et al. in human hepatocyte HuH7 cells treated with TGF-β1 (47). We also determined that TFP-induced LC3-II expression was associated with increased autophagosome formation as demonstrated by the punctate formation of GFP-LC3 (Fig. 4C, panel b), compared with untreated control cells (Fig. 4C, panel a). Cotreatment with TFP and TGF-β1 further enhanced the puncta formation (Fig. 4C, panel d). We next evaluated whether the induction of autophagy by TFP promoted degradation of Col-I stimulated by TGF-β1. As shown in Fig. 4D, Western blot analysis revealed that TGF-β1-induced Col-I expression was significantly reduced by cotreatment with TFP in MMC (lane 3 versus lane 4). To determine that the reduction in protein levels of Col-I in TFP-treated MMC was not due to down-regulation of Col-I α1 mRNA, we analyzed for Col-I α1 mRNA expression by RT-PCR and representative results are shown in Fig. 4E. As expected, stimulation with TGF-β1 increased Col-I α1 mRNA levels (Fig. 4E, lane 3), but TFP treatment did not alter the levels of Col-I α1 mRNA (Fig. 4E, lane 4). Together, our findings suggest that the reduction in Col-I protein levels in TFP-treated MMC is due to autophagy-induced intracellular degradation of Col-I, and that increases in autophagic activity may be a mechanism that prevents accumulation of Col-I protein induced by TGF-β1.
FIGURE 4.
TFP-induced autophagy suppresses Col-I induced by TGF-β1 stimulation. A, Western blot analysis of Beclin 1 and LC3 in MMC incubated in the absence or presence of varying concentrations of TFP for 24 h, as indicated. B, Western blot analysis of Beclin 1 and LC3 in MMC incubated in the absence or presence of TFP (20 μm) for varying time periods, as indicated. C, punctate formation of GFP-LC3 visualized by confocal fluorescence microscopy. MMC transfected with GFP-LC3 were incubated in the absence (panel a) or presence of 20 μm of TFP (panel b), or 2 ng/ml of TGF-β1 (panel c), or both (panel d) for 24 h. Images were captured using a Nikon D-eclipse C1 confocal fluorescence microscope. D, protein levels of Col-I in MMC incubated in the absence or presence of 20 μm of TFP, or 2 ng/ml of TGF-β1, or both for 24 h. Immunoblotting for β-actin was used as protein loading control (A, B, and D). The levels of Col-I were quantitated as ratio to β-actin by densitometry. Data are presented as the mean ± S.E. of three independent experiments (*, p < 0.01 versus untreated control cells; **, p < 0.05 versus TFP and TGF-β1 treated cells). E, Col-I α1 mRNA expression analyzed by RT-PCR in MMC incubated in the absence or presence of 20 μm of TFP, or 2 ng/ml of TGF-β1, or both for 24 h. Data shown are representative of three independent experiments with similar results.
Carbon monoxide (CO)-induced Autophagy Stimulates Degradation of Col-I Induced by TGF-β1
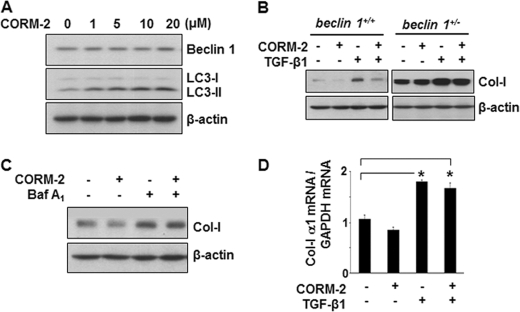
Recent studies provide evidence that low-dose CO (250 ppm) induces autophagy (48). We have previously reported that exposure to low-dose CO (250 ppm) markedly reduced collagen deposition in the kidneys after UUO in mice (34). Moreover, low-dose CO treatment inhibited the induction of α-smooth muscle actin and major ECM proteins, Col-I and fibronectin, in the mouse kidneys after UUO. Thus, we reasoned that low-dose CO may, at least in part, suppress collagen deposition in the UUO kidneys through autophagy. As shown in Fig. 5A, we determined that in mice, low-dose CO (250 ppm) exposure for 1 and 3 days induced the expression of Beclin 1 and LC3-I/LC3-II in the kidneys. We also investigated whether low-dose CO (250 ppm) induced Beclin 1 and LC3 expression in cultured MMC (Fig. 5B). The expression of LC3-I/LC3-II was significantly induced by CO in a time-dependent manner. Similar to the TFP effects (Fig. 4, A and B), the induction of protein levels of Beclin 1 by low-dose CO exposure was not as apparent. We also examined whether CO releasing molecule-2 (CORM-2), which has been employed as pharmacological method of CO delivery to cells (49), exerted similar effects as exposure to gaseous CO. As shown in Fig. 6A, treatment with CORM-2, at varying concentrations from 1–20 μm, for 24 h induced LC3 expression in MMC, and Beclin 1 expression to a much lesser degree. Taken together, these data indicate that CO also has the ability to induce autophagy in vivo in the kidney and in cultured MMC.
FIGURE 5.

Low-dose CO induces autophagy. A, Western blot analysis of Beclin 1 and LC3 in kidney tissue extracts from C57BL6 mice exposed to room air (lanes 1–3), or low-dose CO (250 ppm) for 1 day (lanes 4–6) and 3 days (lanes 7–9). Each lane represents protein lysates from the kidney of a single animal (data shown are from 3 different mice per group). B, expression levels of Beclin 1 and LC3 in MMC exposed to room air (lane 1), or low-dose CO (250 ppm) for the indicated time periods. Immunoblotting for β-actin was used as protein loading control (A and B).
FIGURE 6.
CORM-2-induced autophagy suppresses Col-I induced by TGF-β1 stimulation. A, expression levels of Beclin 1 and LC3 in MMC incubated without of with increasing concentrations of CORM-2 (1–20 μm) for 24 h. B, protein levels of Col-I in MMC obtained from beclin 1+/+ and beclin 1−/− mice. Cells were treated with or without CORM-2 (10 μm) for 24 h in the presence or absence of TGF-β1 (2 ng/ml), as indicated. C, Col-I protein levels in beclin 1+/+ MMC treated with or without CORM-2 (10 μm) for 24 h in the presence or absence of bafilomycin A1 (Baf A1, 10 nm), as indicated. Immunoblotting for β-actin was used as protein loading control (A–C). D, real-time PCR for Col-I α1 mRNA expression in beclin 1+/+ MMC, incubated in the absence or presence of CORM-2 (10 μm) for 24 h, and without or with TGF-β1 (2 ng/ml), as indicated. Triplicate samples were run for each experiment. Data are presented as the mean ± S.E. of three independent experiments. (*, p < 0.05 versus untreated control cells).
We next examined the effects of CO-induced autophagy on accumulation of Col-I stimulated by TGF-β1, utilizing autophagy-deficient beclin 1+/− MMC. As shown in Fig. 6B, treatment with CORM-2 (10 μm) for 24 h attenuated the protein levels of Col-I stimulated by TGF-β1 in beclin 1+/+ MMC (top left panel, lanes 3 versus 4), whereas in autophagy-deficient beclin 1+/− MMC, Col-I protein levels were unaffected by CORM-2 treatment (top right panel, lanes 3 versus 4). Interestingly, CORM-2 also reduced Col-I levels in the absence of TGF-β1 (Fig. 6B, lanes 1 versus 2) in beclin 1+/+ MMC, but not in autophagy-deficient beclin 1+/− MMC. Therefore, CO regulates basal as well as TGF-β1 stimulated Col-I protein expression. When we blocked the autophagic process in the beclin 1+/+ MMC by inhibiting the autolysosomal protein degradation pathway using bafilomycin A1, we observed that treatment with CORM-2 (10 μm) for 24 h no longer attenuated the protein levels of Col-I stimulated by TGF-β1 (Fig. 6C, lanes 3 versus 4). We also determined that the reduction in protein levels of Col-I by CORM-2 in the TGF-β1-treated beclin 1+/+ MMC (Fig. 6B, top left panel, lane 4) was not due to down-regulation of Col-I α1 mRNA (Fig. 6D). Stimulation with TGF-β1 significantly increased Col-I α1 mRNA, as expected, but cotreatment with CORM-2 did not repress Col-I α1 mRNA in the beclin 1+/+ MMC. Taken together, these findings suggest that CO inhibits accumulation of Col-I protein induced by TGF-β1, at least in part, through induction of autophagy.
TGF-β1 Induces Autophagy via TAK1-MKK3 Signaling Pathway in Mesangial Cells
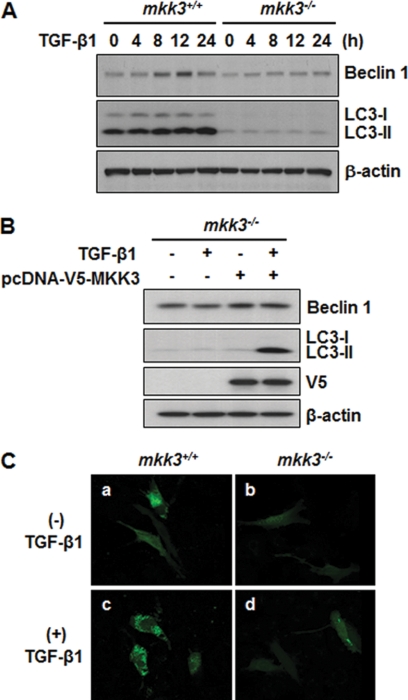
TGF-β1 is well known to exert pleiotropic actions, and there is now a growing body of evidence that TGF-β1 activates autophagy. TGF-β1 has been reported to induce autophagy in certain human cancer cells (47), and in normal bovine mammary epithelial cells (50). However, the mechanism of TGF-β1 signaling and autophagy induction remains incompletely understood, and is likely cell type specific. We have previously reported that TGF-β1 induces autophagy in MMC and that the activation of TAK1 is critical for TGF-β1-induced LC3 expression (33). Here, we investigated whether the MKK3, one of immediate downstream signaling molecules of TAK1, mediates TGF-β1 induction of autophagic process through modulation of Beclin 1 and LC3 expression. We examined the effects of TGF-β1 treatment in MMC from mkk3+/+ and mkk3−/− mice. As shown in Fig. 7A, the basal levels of Beclin 1 and LC3 expression were significantly lower in the mkk3−/− MMC compared with mkk3+/+ MMC (lane 1 versus lane 6). Upon TGF-β1 stimulation, there was a time-dependent increase in LC3-I/LC3-II expression in the mkk3+/+ MMC, whereas in the mkk3−/− MMC, the TGF-β1 effects were abrogated. Similarly, TGF-β1 induced Beclin 1 expression in the mkk3+/+ MMC, but not in the mkk3−/− MMC. We note that the induction of Beclin 1 protein levels is relatively small, compared with induction of LC3. Similar findings were reported by Kiyono et al. in human hepatocyte HuH7 cells treated with TGF-β1 (47). Reconstitution of MKK3 in the mkk3−/− MMC effectively restored the responsiveness to induction by TGF-β1 (Fig. 7B, lane 4). We also determined that TGF-β1 stimulation induced punctate formation of ectopically expressed GFP-LC3 in mkk3+/+ MMC (Fig. 7C, panel c), but not in mkk3−/− MMC (Fig. 7C, panel d). Based on our present data and our previous studies (33) TGF-β1 induces autophagy in MMC via the TAK1-MKK3 signaling pathway.
FIGURE 7.
Involvement of the MKK3 signaling pathway in TGF-β1-induced autophagy. A, Western blot analysis of Beclin 1 and LC3 in MMC obtained from mkk3+/+ and mkk3−/− mice. Cells were incubated in the absence or presence of TGF-β1 (2 ng/ml) for varying time periods, as indicated. B, reconstitution of MKK3 in mkk3−/− MMC. Cells from mkk3−/− mice, transfected with mouse MKK3 expression vector construct (pcDNA-V5-MKK3) or empty vector pcDNA, were incubated in the absence or presence of TGF-β1 (2 ng/ml) for 24 h. Expression levels of Beclin 1 and LC3 were detected by immunoblotting. Transfection and ectopic expression of MKK3 were confirmed by Western blot analysis with anti-V5. Immunoblotting for β-actin served as loading control. (A and B). C, punctate formation of GFP-LC3 visualized by confocal fluorescence microscopy. MMC from mkk3+/+ and mkk3−/− mice were transfected with GFP-LC3, and incubated in the absence (panels a, b) or presence of 2 ng/ml of TGF-β1 (panels c, d) for 24 h. Images were captured using a Nikon D-eclipse C1 confocal fluorescence microscope.
Autophagy Promotes Degradation of Aggregated Collagen
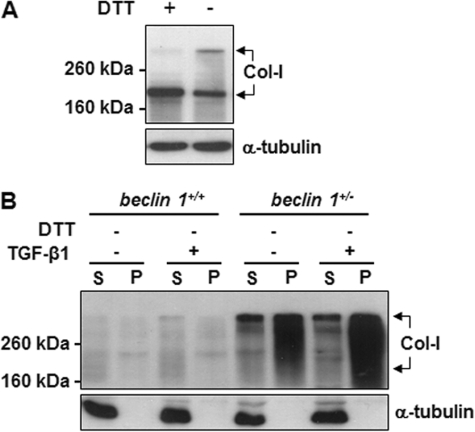
Our data indicate that autophagy induces intracellular degradation of Col-I in MMC. We sought to understand how autophagy sequestrates Col-I for degradation. Recently, it has been noted that, in heat shock protein 47 null (Hsp47−/−) fibroblasts, misfolded trimeric forms of type I procollagen accumulate as aggregates in the endoplasmic reticulum (ER) and eliminated through autophagy (9). In the present study, we examined whether the aggregated trimeric forms of collagen are increased in the autophagy-deficient beclin 1+/− MMC. As shown in Fig. 8A, trimeric forms were detectable when the cell extracts were prepared in lysis buffer without DTT (lane 2) and analyzed by SDS-PAGE followed by Western blotting with anti-Col-I antibody. We also compared cell lysates from beclin 1+/+ and beclin 1+/− MMC subjected to centrifugal fractionation assay described by Ishida et al. (9) followed by SDS-PAGE under nonreducing conditions and Western blot analysis with anti-Col-I antibody. As shown in Fig. 8B, in the beclin 1+/+ MMC, low levels of trimeric forms of Col-I were detected in the detergent soluble (S, supernatant) fraction, and much lower levels were detectable in detergent insoluble (P, pellet) fraction. In contrast, significantly higher levels of trimeric forms of Col-I were detected in the both detergent soluble and insoluble fractions obtained from beclin 1+/− MMC compared with beclin 1+/+ MMC. Moreover, in the beclin 1+/− MMC, the Col-I levels in the detergent insoluble pellet fractions were more abundant than the detergent soluble fractions, and this difference was further enhanced with TGF-β1 treatment. These data indicate that inhibition of autophagic activity stimulates accumulation of aggregated collagen in the ER. Thus, collagen degradation appears to be highly dependent on autophagy.
FIGURE 8.
Aggregated collagen is increased in Beclin 1-deficient mesangial cells. A, detection of trimeric form of collagen in wild-type MMC by Western blot analysis with anti-Col-I antibody, in SDS-PAGE loading buffer with or without DTT (100 mm), as indicated. Immunoblotting for α-tubulin was used as protein loading control. B, MMC obtained from beclin 1+/+ and beclin 1+/− mice were treated without or with TGF-β1 (2 ng/ml) for 24 h. Cell lysates were analyzed by centrifugal fractionation assay followed by SDS-PAGE under nonreducing conditions (without DTT) and Western blot analysis with anti-Col-I antibody. S, supernatant (detergent soluble); P, pellet (detergent insoluble). Immunoblotting for α-tubulin in soluble fractions was used as protein loading control.
DISCUSSION
The balance between synthesis and degradation of ECM proteins is central to ECM remodeling in tissues. Proteolysis of ECM by MMPs is a major mechanism by which tissues eliminate excess ECM protein accumulation and prevent tissue fibrosis. Recent evidence suggests an additional mechanism by which Col-I, one of the major ECM components, can undergo intracellular degradation via induction of autophagy (9, 10). Autophagy represents a fundamental cellular homeostatic process to degrade and recycle cellular proteins and damaged organelles. However, the functional role of autophagy in the kidney remains largely understudied. Beclin 1 is required for the initiation of autophagy (24), and our present study demonstrates that inhibition of autophagy through heterozygous inactivation of beclin 1 resulted in a profibrotic phenotype in the kidney, suggesting that Beclin 1 functions to prevent excess deposition of collagen in the kidney.
Autophagy is emerging as a key cellular stress response involved in a variety of disease states (18). The role of autophagy as an anti-fibrotic response is intriguing. A recent report by Tannous et al. has also shown that heterozygous inactivation of beclin 1 resulted in a 3-fold increase in interstitial fibrosis in a model of severe desmin-related cardiomyopathy (29). The beclin 1+/− mice have previously been shown to exhibit ∼50% reduction in autophagic capacity (26). Haspel et al. have more recently reported that the beclin 1+/− mice had reduced basal autophagic flux compared with beclin 1+/+ littermates (39). Reduced Beclin 1 expression has been described in the affected brain regions in early Alzheimer disease, and linked to amyloid-β accumulation (51). A common feature to this neurodegenerative disease was the aberrant intracellular accumulation of misfolded proteins within the affected tissues (52). Thus, these findings suggest that perturbation of the autophagic process mediated by the reduction of Beclin 1 function may cause an insufficient degradation of disease-related proteins.
We employed two separate strategies to reduce Beclin 1 expression in cultured MMC, through genetic disruption of beclin 1 by heterozygous deletion or knockdown by specific siRNA, and confirmed that reduction in the expression of Beclin 1 increases collagen protein levels. Furthermore, the inhibition of autolysosomal protein degradation with bafilomycin A1 also significantly increased protein levels of Col-I, without alterations in the expression of Col-I α1 mRNA. Bafilomycin A1 also increased the colocalization of Col-I with LC3, an autophagy marker, or with LAMP-1, a lysosome marker. Taken altogether, our data strongly suggest that autophagy participates in the intracellular degradation of Col-I in MMC.
A key feature of autophagy is its dynamic regulation. Autophagic activity or flux, which is generally low under basal conditions, can be induced by various stimuli. Nutrient starvation is a well-established classic inducer of autophagy. We have previously reported that serum deprivation rapidly induces autophagy in MMC (33). Besides starvation, however, autophagy can also be induced by other stress stimuli. Here, we show that TGF-β1 is also an inducer of autophagy. Our data provide the first evidence that TGF-β1 promotes the intracellular degradation of Col-I via autophagic process to regulate the levels of Col-I. Others had previously reported that autophagy induced by the activation of β2-adrenergic receptors enhanced the degradation of collagen in cardiac fibroblasts (10). Rapamycin, which induces autophagy by inhibiting mTOR, enhanced elimination of aggregated procollagen (9). In addition, we utilized another known inducer of autophagy, TFP (46), and demonstrated that treatment with TFP resulted in significant decrease of protein levels of Col-I induced by TGF-β1 stimulation, without an alteration of Col-I α1 mRNA expression. These data provide a strong link between induction of autophagy and intracellular degradation of Col-I.
Autophagy is increasingly appreciated as a protective mechanism against the progression of certain human diseases (18) and recent studies implicate a cytoprotective role of autophagy in kidney injury (31, 32). CO is an endogenous product of heme oxygenase activity that has been shown to exert potent cytoprotection against tissue injury including kidney fibrosis (34), and recently CO has been shown to induce autophagy (48). Low-dose CO (250 ppm) increased the expression and activation of the autophagic protein LC3 in the mouse lung, and in cultured human alveolar (A549) or bronchial epithelial cells in a time-dependent manner (48). Our present study also demonstrated that CO induced Beclin 1 and LC3 expression in the mouse kidneys. We have previously reported that CO suppressed Col-I accumulation and development of renal fibrosis in the kidneys after UUO (34). In cultured MMC, CO administered either as 250 ppm of gaseous CO or pharmacological CO releasing molecule CORM-2, induced autophagic proteins. Moreover, CORM-2 suppressed basal as well as TGF-β1 stimulated Col-I protein expression. Blockade of autophagic activity, either by genetic disruption of beclin 1 by heterozygous deletion or treatment with bafilomycin A1, abrogated the CO effects, indicating that CO, at least in part, suppressed Col-I accumulation through autophagy. Thus, induction of autophagy promotes intracellular degradation of Col-I, and thereby providing a cytoprotective mechanism against kidney fibrosis.
TGF-β1 is a prototypic multifunctional cytokine. Its actions as a potent inducer of the synthesis of ECM proteins including Col-I are well established. We and others have now reported that TGF-β1 is also an inducer of autophagy in normal bovine mammary epithelial cells (50), in hepatocellular and mammary carcinoma cells (47), and in primary MMC (33). The apparent duality of TGF-β1 functions as both an inducer of Col-I synthesis and an inducer of autophagy and Col-I degradation in MMC underscores the multifunctionality of TGF-β1, akin to its well-known dual functions as both tumor suppressor and tumor promoter (53). We propose that TGF-β1-induced autophagy plays a crucial role in the intracellular degradation of Col-1 and thereby limiting the net accumulation of Col-I whose expression is induced by TGF-β1. Interestingly, Gewin et al. (54) recently described that deletion of TGF-β type II receptor in the murine collecting duct resulted in paradoxic increase in interstitial fibrosis associated with Col-I deposition rather than the predicted attenuation of fibrosis after UUO. Beside the profibrotic effects, TGF-β1 also has an essential role in maintaining cellular homeostasis. The induction of autophagy may serve as a cytoprotective mechanism to suppress the excess accumulation of Col-I induced by TGF-β1 and maintain cellular homeostasis.
TGF-β1 signaling pathway activating autophagy is not well understood. TGF-β1 signals in a cell-type and context specific manner. The present study demonstrates that TGF-β1 induction of autophagy in MMC involves activation of the TAK1-MKK3-p38 signaling axis (33). TAK1 can activate MKK4/7-JNK, MKK3/6-p38, NF-κB, and AMPK in a cell-specific manner (8, 55–57) and, intriguingly, all of these molecules are known to be involved in either the expression of certain Atg proteins or the activation of autophagy. For instance, JNK is implicated in the expression and activation of Beclin 1 (58, 59), whereas p38 is necessary for LPS or oxidative stress-induced formation of autophagosomes in C2C12 myoblasts (60) and in HL-1 cardiomyocytes (61). In addition, ER stress-induced activation of autophagy and trafficking of Atg9 are regulated by the activation of p38 (62). We have previously demonstrated that MKK3 is the immediate upstream activator of p38 in MMC (37) and in the present study, mkk3 null mutation abrogated TGF-β1 induction of autophagy, whereas reconstitution of MKK3 in the mkk3−/− MMC effectively restored the responsiveness to induction by TGF-β1, indicating the involvement of the TAK1-MKK3-p38 signaling pathway in the induction of autophagy.
We observed that the inhibition of the proteasome by treatment with MG132 repressed the expression of Col-I α1 mRNA in MMC. This negative regulatory effect of MG132, in fact, has been previously shown in human dermal fibroblasts (43) and rat cardiac fibroblasts (44). However the precise mechanism by which proteasome inhibitor MG132 represses Col-I α1 mRNA expression remains to be determined. The transcriptional regulation of Col-I α1 mRNA is known to be highly complex that involves various enhancers and repressors. The studies by Fineschi et al. (43), suggest that although JNK is activated by MG132, JNK activity may not be involved in the regulation of Col-I α1 mRNA transcription, since inhibition of JNK failed to reverse the negative regulatory effect on Col-I expression. Interestingly, there have been several reports that proteasome inhibitors activate transcription factor NF-κB in certain cell types (63–66), and NF-κB has been shown to inhibit Col-I α1 promoter activity (67).
Assembly and correct folding of procollagen I (pro-Col-I) occur within the ER and require coordinated action of a large number of ER-resident enzymes and molecular chaperones such as protein disulfide isomerase, prolyl 4-hydroxylase, and Hsp47 (68). The accumulation of unfolded or misfolded proteins caused by mutations in collagen genes, or saturation of protein folding capacity in ER induces ER stress, which is a potent trigger of autophagy (15). A recent study demonstrated that the interruption of correct folding of the pro-Col-I by the depletion of Hsp47 function or misfolded pro-Col-I induced their aggregation in ER, and the aggregates underwent autophagy-mediated degradation (9). Our studies suggest that misfolded and/or unfolded Col-I aggregates are main target to be eliminated by autophagy, however, we cannot rule out the possibility that autophagy-mediated degradation of collagen is not just limited to misfolded or unfolded Col-I degradation. In the present study, heterozygous deletion of beclin 1 resulted in increased accumulation of aggregated Col-I even under nonstimulated conditions, and stimulation with TGF-β1 further increased aggregated Col-I. During autophagy, synthesis of PI3P by Vps34 complex is a key early event in autophagosome formation and PI3P-rich membrane structures called omegasomes form at subdomains of ER membranes and autophagosomes form from within omegasomes (21). Therefore, it is plausible that heterozygous deletion of beclin 1 may reduce the formation of omegasomes and the autophagic process, leading to the accumulation of the aggregated Col-I. The high molecular weight aggregates are thought to correspond to trimeric forms of pro-Col-I, as described by Ishida et al. (9). In contrast, the high molecular weight aggregated Col-I was barely detectable in wild-type MMC, suggesting that in beclin 1+/+ cells the Col-I aggregates were rapidly degraded. Thus, these data indicate that certain portions of pro-Col-I form aggregates and are eliminated through degradation by autophagy.
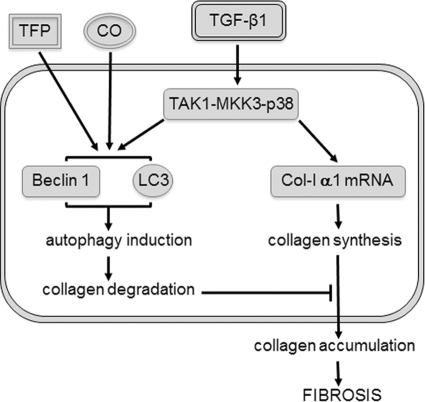
In summary, our data provide evidence that TGF-β1 induces autophagy in MMC via TAK1-MKK3-p38 signaling pathway, and autophagy promotes intracellular degradation of Col-I (Fig. 9). The dual functions of TGF-β1 as both an inducer of Col-I synthesis and an inducer of autophagy and Col-I degradation underscore the multifunctional nature of TGF-β1. TFP or low-dose CO, shown to exert cytoprotection against renal fibrosis, induces autophagy to suppress the accumulation of Col-I. Taken together, our findings suggest a novel role of autophagy as a cytoprotective mechanism to negatively regulate and limit excess collagen accumulation in the kidney, and suggest that autophagy may be a new therapeutic target to prevent or mitigate renal fibrosis.
FIGURE 9.
Model for regulation of autophagy-mediated Col-I degradation in mesangial cells. TGF-β1 acts as both an inducer of Col-I synthesis and an inducer of autophagy and Col-I degradation, via TAK1-MKK3-p38 signaling pathway. TFP or low-dose CO, shown to exert cytoprotection against renal fibrosis, induces autophagy to suppress the accumulation of Col-I.
Acknowledgment
We thank Soo Young Jun for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 DK57661 from the NIDDK and the M. James Scherbenske Grant from the American Society of Nephrology (to M. E. C.); Carl W. Gottschalk Research Scholar Grant from the American Society of Nephrology (ASN) and Beginning Grant-in-Aid 0665379U from the American Heart Association (AHA) (to S. I. K.).
- ECM
- extracellular matrix
- MMC
- mesangial cell
- MMP
- matrix metalloproteinase
- Atg
- autophagy-related gene
- PI3P
- phosphatidylinositol 3-phosphate
- UUO
- unilateral ureteral obstruction
- TFP
- trifluoperazine.
REFERENCES
- 1. Border W. A., Noble N. A. (1994) Transforming growth factor β in tissue fibrosis. N. Engl. J. Med. 331, 1286–1292 [DOI] [PubMed] [Google Scholar]
- 2. Sharma K., Ziyadeh F. N. (1995) Hyperglycemia and diabetic kidney disease. The case for transforming growth factor-β as a key mediator. Diabetes 44, 1139–1146 [DOI] [PubMed] [Google Scholar]
- 3. Vu T. H., Werb Z. (2000) Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 14, 2123–2133 [DOI] [PubMed] [Google Scholar]
- 4. Nagase H., Woessner J. F. (1999) Matrix metalloproteinases. J. Biol. Chem. 274, 21491–21494 [DOI] [PubMed] [Google Scholar]
- 5. Ignotz R. A., Massagué J. (1986) Transforming growth factor-β stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J. Biol. Chem. 261, 4337–4345 [PubMed] [Google Scholar]
- 6. Yoshioka K., Tohda M., Takemura T., Akano N., Matsubara K., Ooshima A., Maki S. (1990) Distribution of type I collagen in human kidney diseases in comparison with type III collagen. J. Pathol. 162, 141–148 [DOI] [PubMed] [Google Scholar]
- 7. Chin B. Y., Mohsenin A., Li S. X., Choi A. M., Choi M. E. (2001) Stimulation of pro-α(1)(I) collagen by TGF-β(1) in mesangial cells: role of the p38 MAPK pathway. Am. J. Physiol. Renal Physiol. 280, F495–F504 [DOI] [PubMed] [Google Scholar]
- 8. Kim S. I., Kwak J. H., Zachariah M., He Y., Wang L., Choi M. E. (2007) TGF-β-activated kinase 1 and TAK1-binding protein 1 cooperate to mediate TGF-β1-induced MKK3-p38 MAPK activation and stimulation of type I collagen. Am. J. Physiol. Renal Physiol. 292, F1471–F1478 [DOI] [PubMed] [Google Scholar]
- 9. Ishida Y., Yamamoto A., Kitamura A., Lamandé S. R., Yoshimori T., Bateman J. F., Kubota H., Nagata K. (2009) Autophagic elimination of misfolded procollagen aggregates in the endoplasmic reticulum as a means of cell protection. Mol. Biol. Cell 20, 2744–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aránguiz-Urroz P., Canales J., Copaja M., Troncoso R., Vicencio J. M., Carrillo C., Lara H., Lavandero S., Díaz-Araya G. (2011) β(2)-adrenergic receptor regulates cardiac fibroblast autophagy and collagen degradation. Biochim. Biophys. Acta 1812, 23–31 [DOI] [PubMed] [Google Scholar]
- 11. Xie Z., Klionsky D. J. (2007) Autophagosome formation: core machinery and adaptations. Nat. Cell Biol. 9, 1102–1109 [DOI] [PubMed] [Google Scholar]
- 12. Ecker N., Mor A., Journo D., Abeliovich H. (2010) Induction of autophagic flux by amino acid deprivation is distinct from nitrogen starvation-induced macroautophagy. Autophagy 6, 879–890 [DOI] [PubMed] [Google Scholar]
- 13. Liang J., Shao S. H., Xu Z. X., Hennessy B., Ding Z., Larrea M., Kondo S., Dumont D. J., Gutterman J. U., Walker C. L., Slingerland J. M., Mills G. B. (2007) The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat. Cell Biol. 9, 218–224 [DOI] [PubMed] [Google Scholar]
- 14. Meijer A. J., Codogno P. (2011) Autophagy: regulation by energy sensing. Curr. Biol. 21, R227–229 [DOI] [PubMed] [Google Scholar]
- 15. Nijholt D. A., de Graaf T. R., van Haastert E. S., Oliveira A. O., Berkers C. R., Zwart R., Ovaa H., Baas F., Hoozemans J. J., Scheper W. (2011) Endoplasmic reticulum stress activates autophagy but not the proteasome in neuronal cells: implications for Alzheimer's disease. Cell Death Differ. 18, 1071–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Majmundar A. J., Wong W. J., Simon M. C. (2010) Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 40, 294–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scherz-Shouval R., Shvets E., Fass E., Shorer H., Gil L., Elazar Z. (2007) Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 26, 1749–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levine B., Kroemer G. (2008) Autophagy in the pathogenesis of disease. Cell 132, 27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mizushima N. (2009) Physiological functions of autophagy. Curr. Top. Microbiol. Immunol. 335, 71–84 [DOI] [PubMed] [Google Scholar]
- 20. Klionsky D. J., Cregg J. M., Dunn W. A., Jr., Emr S. D., Sakai Y., Sandoval I. V., Sibirny A., Subramani S., Thumm M., Veenhuis M., Ohsumi Y. (2003) A unified nomenclature for yeast autophagy-related genes. Dev. Cell 5, 539–545 [DOI] [PubMed] [Google Scholar]
- 21. Axe E. L., Walker S. A., Manifava M., Chandra P., Roderick H. L., Habermann A., Griffiths G., Ktistakis N. T. (2008) Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 182, 685–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takeshige K., Baba M., Tsuboi S., Noda T., Ohsumi Y. (1992) Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J. Cell Biol. 119, 301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakamura N., Matsuura A., Wada Y., Ohsumi Y. (1997) Acidification of vacuoles is required for autophagic degradation in the yeast, Saccharomyces cerevisiae. J. Biochem. 121, 338–344 [DOI] [PubMed] [Google Scholar]
- 24. Burman C., Ktistakis N. T. (2010) Autophagosome formation in mammalian cells. Semin. Immunopathol. 32, 397–413 [DOI] [PubMed] [Google Scholar]
- 25. Yue Z., Jin S., Yang C., Levine A. J., Heintz N. (2003) Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl. Acad. Sci. U.S.A. 100, 15077–15082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qu X., Yu J., Bhagat G., Furuya N., Hibshoosh H., Troxel A., Rosen J., Eskelinen E. L., Mizushima N., Ohsumi Y., Cattoretti G., Levine B. (2003) Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Invest. 112, 1809–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arsov I., Li X., Matthews G., Coradin J., Hartmann B., Simon A. K., Sealfon S. C., Yue Z. (2008) BAC-mediated transgenic expression of fluorescent autophagic protein Beclin 1 reveals a role for Beclin 1 in lymphocyte development. Cell Death Differ. 15, 1385–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pickford F., Masliah E., Britschgi M., Lucin K., Narasimhan R., Jaeger P. A., Small S., Spencer B., Rockenstein E., Levine B., Wyss-Coray T. (2008) The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β accumulation in mice. J. Clin. Invest. 118, 2190–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tannous P., Zhu H., Johnstone J. L., Shelton J. M., Rajasekaran N. S., Benjamin I. J., Nguyen L., Gerard R. D., Levine B., Rothermel B. A., Hill J. A. (2008) Autophagy is an adaptive response in desmin-related cardiomyopathy. Proc. Natl. Acad. Sci. U.S.A. 105, 9745–9750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suzuki K., Kirisako T., Kamada Y., Mizushima N., Noda T., Ohsumi Y. (2001) The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 20, 5971–5981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Periyasamy-Thandavan S., Jiang M., Wei Q., Smith R., Yin X. M., Dong Z. (2008) Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int. 74, 631–640 [DOI] [PubMed] [Google Scholar]
- 32. Pallet N., Bouvier N., Legendre C., Gilleron J., Codogno P., Beaune P., Thervet E., Anglicheau D. (2008) Autophagy protects renal tubular cells against cyclosporine toxicity. Autophagy 4, 783–791 [DOI] [PubMed] [Google Scholar]
- 33. Ding Y., Kim J. K., Kim S. I., Na H. J., Jun S. Y., Lee S. J., Choi M. E. (2010) TGF-β1 protects against mesangial cell apoptosis via induction of autophagy. J. Biol. Chem. 285, 37909–37919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang L., Lee J. Y., Kwak J. H., He Y., Kim S. I., Choi M. E. (2008) Protective effects of low-dose carbon monoxide against renal fibrosis induced by unilateral ureteral obstruction. Am. J. Physiol. Renal Physiol. 294, F508–F517 [DOI] [PubMed] [Google Scholar]
- 35. Liang X. H., Jackson S., Seaman M., Brown K., Kempkes B., Hibshoosh H., Levine B. (1999) Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402, 672–676 [DOI] [PubMed] [Google Scholar]
- 36. Lu H. T., Yang D. D., Wysk M., Gatti E., Mellman I., Davis R. J., Flavell R. A. (1999) Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 18, 1845–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang L., Ma R., Flavell R. A., Choi M. E. (2002) Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for activation of p38α and p38δ MAPK isoforms by TGF-β1 in murine mesangial cells. J. Biol. Chem. 277, 47257–47262 [DOI] [PubMed] [Google Scholar]
- 38. Kim S. I., Kwak J. H., Wang L., Choi M. E. (2008) Protein phosphatase 2A is a negative regulator of transforming growth factor-β1-induced TAK1 activation in mesangial cells. J. Biol. Chem. 283, 10753–10763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haspel J., Shaik R. S., Ifedigbo E., Nakahira K., Dolinay T., Englert J. A., Choi A. M. (2011) Characterization of macroautophagic flux in vivo using a leupeptin-based assay. Autophagy 7, 629–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamamoto A., Tagawa Y., Yoshimori T., Moriyama Y., Masaki R., Tashiro Y. (1998) Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct. Funct. 23, 33–42 [DOI] [PubMed] [Google Scholar]
- 41. Klionsky D. J., Elazar Z., Seglen P. O., Rubinsztein D. C. (2008) Does bafilomycin A1 block the fusion of autophagosomes with lysosomes? Autophagy 4, 849–950 [DOI] [PubMed] [Google Scholar]
- 42. Mizushima N., Yoshimori T., Levine B. (2010) Methods in mammalian autophagy research. Cell 140, 313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fineschi S., Reith W., Guerne P. A., Dayer J. M., Chizzolini C. (2006) Proteasome blockade exerts an antifibrotic activity by coordinately down-regulating type I collagen and tissue inhibitor of metalloproteinase-1 and up-regulating metalloproteinase-1 production in human dermal fibroblasts. FASEB J. 20, 562–564 [DOI] [PubMed] [Google Scholar]
- 44. Meiners S., Hocher B., Weller A., Laule M., Stangl V., Guenther C., Godes M., Mrozikiewicz A., Baumann G., Stangl K. (2004) Down-regulation of matrix metalloproteinases and collagens and suppression of cardiac fibrosis by inhibition of the proteasome. Hypertension 44, 471–477 [DOI] [PubMed] [Google Scholar]
- 45. Hartleben B., Gödel M., Meyer-Schwesinger C., Liu S., Ulrich T., Köbler S., Wiech T., Grahammer F., Arnold S. J., Lindenmeyer M. T., Cohen C. D., Pavenstädt H., Kerjaschki D., Mizushima N., Shaw A. S, Walz G., Huber T. B. (2010) Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J. Clin. Invest. 120, 1084–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang L., Yu J., Pan H., Hu P., Hao Y., Cai W., Zhu H., Yu A. D., Xie X., Ma D., Yuan J. (2007) Small molecule regulators of autophagy identified by an image-based high-throughput screen. Proc. Natl. Acad. Sci. U.S.A. 104, 19023–19028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kiyono K., Suzuki H. I., Matsuyama H., Morishita Y., Komuro A., Kano M. R., Sugimoto K., Miyazono K. (2009) Autophagy is activated by TGF-β and potentiates TGF-β-mediated growth inhibition in human hepatocellular carcinoma cells. Cancer Res. 69, 8844–8852 [DOI] [PubMed] [Google Scholar]
- 48. Lee S. J., Ryter S. W., Xu J. F., Nakahira K., Kim H. P., Choi A. M., Kim Y. S. (2011) Carbon monoxide activates autophagy via mitochondrial reactive oxygen species formation. Am. J. Respir. Cell Mol. Biol. 45, 867–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Motterlini R., Mann B. E., Foresti R. (2005) Therapeutic applications of carbon monoxide-releasing molecules. Expert. Opin. Investig. Drugs 14, 1305–1318 [DOI] [PubMed] [Google Scholar]
- 50. Gajewska M., Gajkowska B., Motyl T. (2005) Apoptosis and autophagy induced by TGF-β1 in bovine mammary epithelial BME-UV1 cells. J. Physiol. Pharmacol. 56, Suppl. 3, 143–157 [PubMed] [Google Scholar]
- 51. Wong E., Cuervo A. M. (2010) Autophagy gone awry in neurodegenerative diseases. Nat. Neurosci. 13, 805–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Forloni G., Terreni L., Bertani I., Fogliarino S., Invernizzi R., Assini A., Ribizzi G., Negro A., Calabrese E., Volonté M. A., Mariani C., Franceschi M., Tabaton M., Bertoli A. (2002) Protein misfolding in Alzheimer's and Parkinson's disease: genetics and molecular mechanisms. Neurobiol. Aging 23, 957–976 [DOI] [PubMed] [Google Scholar]
- 53. Bierie B., Moses H. L. (2006) Tumour microenvironment: TGFβ: the molecular Jekyll and Hyde of cancer. Nat. Rev. Cancer 6, 506–520 [DOI] [PubMed] [Google Scholar]
- 54. Gewin L., Bulus N., Mernaugh G., Moeckel G., Harris R. C., Moses H. L., Pozzi A., Zent R. (2010) TGF-β receptor deletion in the renal collecting system exacerbates fibrosis. J. Am. Soc. Nephrol. 21, 1334–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shirakabe K., Yamaguchi K., Shibuya H., Irie K., Matsuda S., Moriguchi T., Gotoh Y., Matsumoto K., Nishida E. (1997) TAK1 mediates the ceramide signaling to stress-activated protein kinase/c-Jun N-terminal kinase. J. Biol. Chem. 272, 8141–8144 [DOI] [PubMed] [Google Scholar]
- 56. Sakurai H., Suzuki S., Kawasaki N., Nakano H., Okazaki T., Chino A., Doi T., Saiki I. (2003) Tumor necrosis factor-α-induced IKK phosphorylation of NF-κB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J. Biol. Chem. 278, 36916–36923 [DOI] [PubMed] [Google Scholar]
- 57. Xie M., Zhang D., Dyck J. R., Li Y., Zhang H., Morishima M., Mann D. L., Taffet G. E., Baldini A., Khoury D. S., Schneider M. D. (2006) A pivotal role for endogenous TGF-β-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc. Natl. Acad. Sci. U.S.A. 103, 17378–17383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Scarlatti F., Bauvy C., Ventruti A., Sala G., Cluzeaud F., Vandewalle A., Ghidoni R., Codogno P. (2004) Ceramide-mediated macroautophagy involves inhibition of protein kinase B and up-regulation of beclin 1. J. Biol. Chem. 279, 18384–18391 [DOI] [PubMed] [Google Scholar]
- 59. Wei Y., Pattingre S., Sinha S., Bassik M., Levine B. (2008) JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell 30, 678–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Doyle A., Zhang G., Abdel Fattah E. A., Eissa N. T., Li Y. P. (2011) Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin-proteasome and autophagy-lysosome pathways. FASEB J. 25, 99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yuan H., Perry C. N., Huang C., Iwai-Kanai E., Carreira R. S., Glembotski C. C., Gottlieb R. A. (2009) LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cytoprotection. Am. J. Physiol. Heart Circ. Physiol. 296, H470–H479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Webber J. L., Tooze S. A. (2010) New insights into the function of Atg9. FEBS Lett. 584, 1319–1326 [DOI] [PubMed] [Google Scholar]
- 63. Li C., Chen S., Yue P., Deng X., Lonial S., Khuri F. R., Sun S. Y. (2010) Proteasome inhibitor PS-341 (bortezomib) induces calpain-dependent IκB(α) degradation. J. Biol. Chem. 285, 16096–16104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hideshima T., Ikeda H., Chauhan D., Okawa Y., Raje N., Podar K., Mitsiades C., Munshi N. C., Richardson P. G., Carrasco R. D., Anderson K. C. (2009) Bortezomib induces canonical nuclear factor-κB activation in multiple myeloma cells. Blood 114, 1046–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dolcet X., Llobet D., Encinas M., Pallares J., Cabero A., Schoenenberger J. A., Comella J. X., Matias-Guiu X. (2006) Proteasome inhibitors induce death but activate NF-kappaB on endometrial carcinoma cell lines and primary culture explants. J. Biol. Chem. 281, 22118–22130 [DOI] [PubMed] [Google Scholar]
- 66. Calvaruso G., Giuliano M., Portanova P., De Blasio A., Vento R., Tesoriere G. (2006) Bortezomib induces in HepG2 cells IκBα degradation mediated by caspase-8. Mol. Cell Biochem. 287, 13–19 [DOI] [PubMed] [Google Scholar]
- 67. Ghosh A. K. (2002) Factors involved in the regulation of type I collagen gene expression: implication in fibrosis. Exp. Biol. Med. 227, 301–314 [DOI] [PubMed] [Google Scholar]
- 68. Lamandé S. R., Bateman J. F. (1999) Procollagen folding and assembly: the role of endoplasmic reticulum enzymes and molecular chaperones. Semin. Cell Dev. Biol. 10, 455–464 [DOI] [PubMed] [Google Scholar]