Abstract
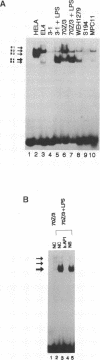
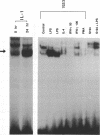
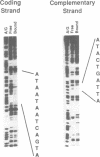
Expression of the kappa immunoglobulin light chain gene requires developmental- and tissue-specific regulation by trans-acting factors which interact with two distinct enhancer elements. A new protein-DNA interaction has been identified upstream of the intron enhancer, within the matrix-associated region of the J-C intron. The binding activity is greatly inducible in pre-B cells by bacterial lipopolysaccharide and interleukin-1 but specific complexes are found at all stages of B cell development tested. The footprinted binding site is homologous to the consensus AP1 motif. The protein components of this complex are specifically competed by an AP1 consensus motif and were shown by supershift to include c-Jun and c-Fos, suggesting that this binding site is an AP1 motif and that the Jun and Fos families of transcription factors play a role in the regulation of the kappa light chain gene. Mutation of the AP1 motif in the context of the intron enhancer was shown to decrease enhancer-mediated activation of the promoter in both pre-B cells induced with LPS and constitutive expression in mature B cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Luk D., Curran T. Transcriptional regulation by Fos and Jun in vitro: interaction among multiple activator and regulatory domains. Mol Cell Biol. 1991 Jul;11(7):3624–3632. doi: 10.1128/mcb.11.7.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annweiler A., Müller U., Wirth T. Functional analysis of defined mutations in the immunoglobulin heavy-chain enhancer in transgenic mice. Nucleic Acids Res. 1992 Apr 11;20(7):1503–1509. doi: 10.1093/nar/20.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cockerill P. N., Garrard W. T. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell. 1986 Jan 31;44(2):273–282. doi: 10.1016/0092-8674(86)90761-0. [DOI] [PubMed] [Google Scholar]
- Diamond M. I., Miner J. N., Yoshinaga S. K., Yamamoto K. R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990 Sep 14;249(4974):1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- Dickinson L. A., Joh T., Kohwi Y., Kohwi-Shigematsu T. A tissue-specific MAR/SAR DNA-binding protein with unusual binding site recognition. Cell. 1992 Aug 21;70(4):631–645. doi: 10.1016/0092-8674(92)90432-c. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton R., Van Ness B. Kappa immunoglobulin promoters and enhancers display developmentally controlled interactions. Nucleic Acids Res. 1993 Oct 25;21(21):4941–4947. doi: 10.1093/nar/21.21.4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Baltimore D. Cell-type specificity of immunoglobulin gene expression is regulated by at least three DNA sequence elements. Cell. 1985 Jul;41(3):885–897. doi: 10.1016/s0092-8674(85)80069-6. [DOI] [PubMed] [Google Scholar]
- Hadman M., Loo M., Bos T. J. In vivo viral and cellular Jun complexes exhibit differential interaction with a number of in vitro generated 'AP-1- and CREB-like' target sequences. Oncogene. 1993 Jul;8(7):1895–1903. [PubMed] [Google Scholar]
- Jensen D. E., Frankis R. C., Jr, Sando J. J. Defective induction of Jun and Fos-related proteins in phorbol ester-resistant EL4 mouse thymoma cells. Oncogene. 1991 Jul;6(7):1219–1225. [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Lenardo M., Pierce J. W., Baltimore D. Protein-binding sites in Ig gene enhancers determine transcriptional activity and inducibility. Science. 1987 Jun 19;236(4808):1573–1577. doi: 10.1126/science.3109035. [DOI] [PubMed] [Google Scholar]
- Li L., Chambard J. C., Karin M., Olson E. N. Fos and Jun repress transcriptional activation by myogenin and MyoD: the amino terminus of Jun can mediate repression. Genes Dev. 1992 Apr;6(4):676–689. doi: 10.1101/gad.6.4.676. [DOI] [PubMed] [Google Scholar]
- Max E. E., Maizel J. V., Jr, Leder P. The nucleotide sequence of a 5.5-kilobase DNA segment containing the mouse kappa immunoglobulin J and C region genes. J Biol Chem. 1981 May 25;256(10):5116–5120. [PubMed] [Google Scholar]
- McBride K., Robitaille L., Tremblay S., Argentin S., Nemer M. fos/jun repression of cardiac-specific transcription in quiescent and growth-stimulated myocytes is targeted at a tissue-specific cis element. Mol Cell Biol. 1993 Jan;13(1):600–612. doi: 10.1128/mcb.13.1.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muegge K., Williams T. M., Kant J., Karin M., Chiu R., Schmidt A., Siebenlist U., Young H. A., Durum S. K. Interleukin-1 costimulatory activity on the interleukin-2 promoter via AP-1. Science. 1989 Oct 13;246(4927):249–251. doi: 10.1126/science.2799385. [DOI] [PubMed] [Google Scholar]
- Murphy J. J., Norton J. D. Phorbol ester induction of early response gene expression in lymphocytic leukemia and normal human B-cells. Leuk Res. 1993 Aug;17(8):657–662. doi: 10.1016/0145-2126(93)90070-2. [DOI] [PubMed] [Google Scholar]
- Murphy J. J., Tracz M., Norton J. D. Patterns of nuclear proto-oncogene expression during induced differentiation and proliferation of human B chronic lymphocytic leukaemia cells. Immunology. 1990 Mar;69(3):490–493. [PMC free article] [PubMed] [Google Scholar]
- Nelms K., Hromas R., Van Ness B. Identification of a second inducible DNA-protein interaction in the kappa immunoglobulin enhancer. Nucleic Acids Res. 1990 Feb 25;18(4):1037–1043. doi: 10.1093/nar/18.4.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. W., Gifford A. M., Baltimore D. Silencing of the expression of the immunoglobulin kappa gene in non-B cells. Mol Cell Biol. 1991 Mar;11(3):1431–1437. doi: 10.1128/mcb.11.3.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radler-Pohl A., Gebel S., Sachsenmaier C., König H., Krämer M., Oehler T., Streile M., Ponta H., Rapp U., Rahmsdorf H. J. The activation and activity control of AP-1 (fos/jun). Ann N Y Acad Sci. 1993 Jun 11;684:127–148. doi: 10.1111/j.1749-6632.1993.tb32277.x. [DOI] [PubMed] [Google Scholar]
- Ryseck R. P., Bravo R. c-JUN, JUN B, and JUN D differ in their binding affinities to AP-1 and CRE consensus sequences: effect of FOS proteins. Oncogene. 1991 Apr;6(4):533–542. [PubMed] [Google Scholar]
- Serfling E., Barthelmäs R., Pfeuffer I., Schenk B., Zarius S., Swoboda R., Mercurio F., Karin M. Ubiquitous and lymphocyte-specific factors are involved in the induction of the mouse interleukin 2 gene in T lymphocytes. EMBO J. 1989 Feb;8(2):465–473. doi: 10.1002/j.1460-2075.1989.tb03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt L. M., Lenardo M. J. Immunoglobulin gene transcription. Annu Rev Immunol. 1991;9:373–398. doi: 10.1146/annurev.iy.09.040191.002105. [DOI] [PubMed] [Google Scholar]
- Stein B., Baldwin A. S., Jr, Ballard D. W., Greene W. C., Angel P., Herrlich P. Cross-coupling of the NF-kappa B p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J. 1993 Oct;12(10):3879–3891. doi: 10.1002/j.1460-2075.1993.tb06066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilzey J. F., Chiles T. C., Rothstein T. L. Jun-B gene expression mediated by the surface immunoglobulin receptor of primary B lymphocytes. Biochem Biophys Res Commun. 1991 Feb 28;175(1):77–83. doi: 10.1016/s0006-291x(05)81202-1. [DOI] [PubMed] [Google Scholar]
- van Wijnen A. J., Bidwell J. P., Fey E. G., Penman S., Lian J. B., Stein J. L., Stein G. S. Nuclear matrix association of multiple sequence-specific DNA binding activities related to SP-1, ATF, CCAAT, C/EBP, OCT-1, and AP-1. Biochemistry. 1993 Aug 24;32(33):8397–8402. doi: 10.1021/bi00084a003. [DOI] [PubMed] [Google Scholar]
- van Wijnen A. J., Bidwell J. P., Fey E. G., Penman S., Lian J. B., Stein J. L., Stein G. S. Nuclear matrix association of multiple sequence-specific DNA binding activities related to SP-1, ATF, CCAAT, C/EBP, OCT-1, and AP-1. Biochemistry. 1993 Aug 24;32(33):8397–8402. doi: 10.1021/bi00084a003. [DOI] [PubMed] [Google Scholar]