Background: CMTM8 is a chemokine-like factor-like MARVEL transmembrane domain-containing protein with tumor suppressor-like activities.
Results: CMTM8 down-regulation induces epithelial-to-mesenchymal transition (EMT)-like phenotypes via c-MET/ERK signaling.
Conclusion: CMTM8 may inhibit EMT-like processes by interfering with c-MET/ERK signaling in some tumor cell types.
Significance: Being a novel EMT regulator, CMTM8 may be used as a therapeutic target of cancer with hyperactivated c-MET/ERK signaling.
Keywords: Cell Migration, ERK, MAP Kinases (MAPKs), Ras, Receptor Endocytosis, Receptor Regulation, Receptor Tyrosine Kinase, Signal Transduction, Tumor Metastases, Tumor Suppressor Gene
Abstract
The acquisition of an invasive phenotype is a critical turning point for malignant tumor cells. CMTM8, a potential tumor suppressor, is frequently down-regulated in solid tumors, and its overexpression induces tumor cell apoptosis. Here, we identify a new role for CMTM8 in regulating tumor cell migration. Reducing CMTM8 expression in HepG2 hepatocellular carcinoma cells results in the acquisition of epithelial-to-mesenchymal transition (EMT) features, including a morphological change from organized epithelial sheets to scattered fibroblast-like shapes, reduction of the epithelial marker E-cadherin, and an increased invasive and migratory ability. These phenotypic changes are mediated in large part by the ERK-MAPK pathway, as the MEK inhibitor U0126 and shRNA-mediated knockdown of ERK2 significantly reversed these phenotypes. Hepatocyte growth factor binding to the c-MET receptor is known to induce EMT in HepG2 cells. We found that CMTM8 knockdown in HepG2 cells induced c-MET signaling and ERK activation. Inhibition of c-MET signaling with the small molecule inhibitor SU11274 or c-MET RNAi blocked the EMT-like changes following CMTM8 knockdown. CMTM8 overexpression in HepG2 cells inhibited hepatocyte growth factor-induced EMT-like morphological changes and cell motility. Down-regulation of CMTM8 also promoted an EMT-like change in MCF-10A cells, indicating a broader role for CMTM8 in regulating cellular transformation.
Introduction
Cancer cell invasion and metastasis are the main causes of cancer patient mortality (1–3). Epithelial-to-mesenchymal transition (EMT)4 appears to play a role in the progression of some in situ carcinomas to aggressive metastatic cancers. During EMT, a transcriptionally induced program causes a reduction in the differentiated epithelial cell characteristics, including cell-cell adhesion and apical-basal polarity. The program induces mesenchymal cell characteristics, including increased motility and invasiveness and a heightened resistance to apoptosis (2, 4). Thus, EMT has been found to contribute to invasion, metastatic dissemination, and acquisition of therapeutic resistance (5).
The Ras-ERK1/2-MAPK pathway plays a critical role in numerous cellular processes, including proliferation, differentiation, survival, and motility (6–8). Deregulation of the Ras-ERK pathway has been implicated in multiple types of human cancers, including hepatocellular carcinoma (9–12). Previous studies have established a critical role for receptor tyrosine kinase signaling and the Ras pathway in EMT (13, 14). ERK activation is necessary for EMT downstream of Ras (15, 16). The DEF (docking site for ERK, FXF) motif and D-domain (docking domain) are found within ERK1/2 interactors and promote ERK interaction with and phosphorylation of substrates. ERK1/2 interactors containing only DEF motifs, D-domains, or a combination of the two motifs have been described, thus allowing for intricate regulation of downstream biological processes (17, 18). ERK contains a DEF motif-binding pocket and a common D-domain that bind DEF motifs and D-domains, respectively. Single point mutations on the ERK2 DEF motif-binding pocket or the ERK2 common D-domain can impair the signaling to DEF motif- or D-domain-containing interactors of ERK2, respectively, without greatly affecting its overall kinase activity (19). A recent study revealed that ERK2 (but not ERK1) overexpression is sufficient to induce EMT via its DEF motif signaling in human breast epithelial cells (16).
Hepatocyte growth factor (HGF) is a mesenchymal cell-derived protein that is mitogenic for primary hepatocytes as well as other cell types (9, 20, 21). HGF binding to the c-MET receptor activates c-MET tyrosine kinase activity and the phosphorylation of multiple sites on the receptor. These phosphorylation events recruit adaptor proteins such as the GRB2/SOS (Son of Sevenless) complex to the plasma membrane, which promotes Ras GTP loading and initiates a protein kinase cascade leading to activation of ERK through Raf and MEK (22–25). HGF induces EMT in hepatocellular carcinoma cell lines, and recent studies have begun to elucidate the molecular mechanism mediating this event (26, 27). In HepG2 and Huh7 hepatocellular carcinoma cell lines, HGF-induced EMT phenotypes are significantly inhibited by the Raf inhibitor sorafenib and the MEK inhibitor U0126, but not the PI3K inhibitor wortmannin (27). This finding suggests that the Raf-ERK-MAPK pathway mediates the signal between c-MET activation and EMT induction in hepatocellular carcinoma cell lines.
CMTM8 (chemokine-like factor-like MARVEL transmembrane domain-containing 8) belongs to the CMTM family, which consists of nine members: chemokine-like factor-1 and CMTM1–8 (1). The CMTM8 gene encodes two isoforms. Generally, the longer isoform is the predominant isoform expressed in human cell lines (28) and normal human tissue.5 The longer isoform promotes EGF receptor (EGFR) internalization and attenuates EGFR-mediated signaling for cell growth (29). When overexpressed, both isoforms induce apoptosis in multiple tumor cell lines (30, 31). In this work, unless specifically described, CMTM8 refers to both isoforms.
In this study, we demonstrate that down-regulation of CMTM8 in HepG2 cells causes EMT-like phenotypic changes that are blocked by chemical MEK inhibition or ERK2 knockdown. We found that c-MET was a main contributor to the EMT-like phenotype, as inhibition of c-MET signaling with the small molecule inhibitor SU11274 or c-MET RNAi suppressed the acquisition of an EMT-like phenotype following CMTM8 knockdown. Moreover, when CMTM8 expression was reduced, the protein levels of c-MET, both on the surface and in cells, were elevated, and HGF-induced c-MET/ERK signaling was enhanced and sustained. Transient overexpression of the longer CMTM8 isoform antagonized HGF-induced cell scattering and decreased the motility of HepG2 cells toward HGF, accompanied by inhibition of c-MET/ERK signaling. Together, these findings suggest that CMTM8 functions as a negative regulator of HGF/c-MET signaling to ERK and hence may be a useful target for treatment of cancers with aberrant activation of this pathway.
EXPERIMENTAL PROCEDURES
Cell Culture, siRNA, and Transfection
HepG2 human hepatocyte carcinoma cells were cultured at 37 °C and 5% CO2 in Eagle's minimal essential medium (American Type Culture Collection, Manassas, VA) supplemented with 10% FBS (Biochrom) and 2 mm l-glutamine (Sigma). MCF-10A immortalized human breast epithelial cells were cultured as described previously (32). HEK293T cells and HEK293GPG packaging cells were cultured at 37 °C and 5% CO2 in DMEM supplemented with 10% FBS (Biochrom) and 2 mm l-glutamine (Sigma). The siRNAs for CMTM8 and c-MET knockdown were both purchased from Qiagen. The siRNA target sequences were as follows: CMTM8 siRNA1, CAGCAGCAGCTTCGCCTACGA; and CMTM8 siRNA2, GTGCCTTCGTCTTGTACCT. They target the sequences transcribed from exons 1 and 3, respectively. The two siRNAs targeting c-MET were functionally validated siRNAs (catalog nos. SI00300860 and SI00300897). The transfection of the siRNAs was performed with Lipofectamine 2000 (Invitrogen) using 20 nm mixtures of duplexes.
Antibodies, Inhibitors, Growth Factors, and Other Reagents
The antibodies used in immunoblotting were obtained from the indicated commercial sources: anti-ZEB1 and anti-c-MET (Santa Cruz Biotechnology); anti-GAPDH (Ambion); anti-E-cadherin (BD Biosciences); anti-α-smooth muscle actin (Dako, Carpintaria, CA); HA.11 (Covance); anti-phospho-ERK (pTEpY), and anti-tubulin (Sigma); and anti-ERK, anti-phospho-c-MET (Tyr1234/Tyr1235), and anti-H-Ras (Cell Signaling Technology). The antibodies used in immunofluorescence include anti-E-cadherin and anti-β-catenin (BD Biosciences), and those used in FACS include anti-c-MET (R&D Biosystems) and anti-EGFR (Calbiochem). Secondary antibodies used for immunoblotting and immunofluorescence were from LI-COR Biosciences and Invitrogen, respectively. Rabbit anti-CMTM8 antibody was prepared and purified in our laboratory. U0126 and LY294002 were from BIOMOL, and AG1478, SU11274, and SB431542 were purchased from Calbiochem. EGF was purchased from PeproTech. Human HGF and human TGFβ1 were purchased from R&D Systems.
Plasmids and Adenovirus
The lentiviral shRNA constructs for generating ERK2 stable knockdown cell lines were kindly provided by Dr. William C. Hahn (Dana-Farber Institute). The pBabe-H-RasV12 constructs and the pBabe-HA-ERK2 constructs (ERK2-WT, ERK2-D319N, and ERK2-Y261A) were described previously (16). Adenovirus for CMTM8 (longer isoform) overexpression was purchased from Vector Gene Technology Co. Ltd. (Beijing, China).
RT-PCR Analysis
Total RNA was purified from cultured cells using the RNeasy minikit (Qiagen) following the manufacturer's protocol. After a 50 °C/50-min reverse transcription step, nest PCR with 28 cycles for each round was performed for CMTM8 amplification. PCR products were analyzed on 1% agarose gels. The sequences of primers for amplification were as follows: CMTM8 external primers, 5′-GGAGGAGCCGCAGCGCG-3′ (forward) and 5′-CTGTATGGTCCTGGATCTCC-3′ (reverse); CMTM8 internal primers, 5′-GCAGAGAACTTCTCCAC-3′ (forward) and 5′-TAGCAGATGGTGACCAG-3′ (reverse); GAPDH, 5′-ACCACAGTCCATGCCATCAC-3′ (forward) and 5′-TCCACCACCCTGTTGCTGTA-3′ (reverse).
Western Blotting
Cell lysates were extracted with lysis buffer (1% Triton X-100, 20 mm Tris-HCl (pH 7.4), 150 mm NaCl, 2 mm MgCl2, 1 mm EGTA (pH 8.0), 2 mm Na3VO4, 2 mm NaF, and protease inhibitor mixture tablets (Roche Applied Bioscience)) and cleared by centrifugation at 16,000 × g for 15 min at 4 °C. The total protein concentration of the cell lysate was measured using a BCA protein assay kit (Pierce) with bovine serum albumin as the standard. Proteins were resolved by SDS-PAGE and transferred to nitrocellulose membranes (HybondTM ECLTM, Amersham Biosciences). For detecting CMTM8 expression, the cell lysates were not boiled before loading. After blocking in ODYSSEY blocking buffer (927-40003, LI-COR Biosciences) at room temperature for 1 h, membranes were incubated with primary antibodies at 4 °C overnight and then with IRDye 680/800-labeled IgG secondary antibody at room temperature for 1 h. The IR fluorophores on the membranes were detected with the LI-COR infrared imaging system and analyzed with ODYSSEY software.
Transwell Migration and Invasion Assays
HepG2 cells were detached with 5 mm EDTA, washed three times with prewarmed assay medium (minimal essential medium containing 0.2% BSA), and resuspended in the same wash medium at a density of 5 × 105 cells/ml. For migration assays, assay medium supplemented with growth factors (1 ng/ml HGF or 5 ng/ml EGF) was added to the bottom chamber of Transwell chambers (8-μm pore size; BD Biosciences) in a 24-well plate. 100-μl cell suspensions were then added to the top chamber. After 5 h of incubation, the cells that had migrated to the lower surface of the membrane were fixed with methanol and stained with 0.2% crystal violet in 2% ethanol. The number of migrated cells was quantified by counting five random distinct fields under a microscope at ×40 magnification. For invasion assays, BD BioCoat invasion chambers coated with Matrigel were used. Invasion chambers were prepared according to the manufacturer's specifications, and assays were performed as described for migration assays, except the incubation time was increased to ∼15 h, and NIH3T3 culture medium rather than diluted growth factors was added to the bottom chamber.
Immunofluorescence
Immunofluorescence for β-catenin imaging was carried out as described previously (8). The method for E-cadherin staining was the same, except fixation was done in a 1:1 mixture of methanol and acetone. Images within each experiment were taken with a Nikon Ti spinning disc confocal microscope using identical exposure times and were scaled equivalently.
Generation of Stable Cell Lines
Cell lines stably expressing H-RasV12, wild-type ERK2, and mutant ERK2 and the ERK2 stable knockdown cell lines were generated as described previously (16).
Flow Cytometric Analysis
Analysis was completed with a FACSCalibur (BD Biosciences) as described previously (33). Briefly, cells were detached with 5 mm EDTA and washed twice with ice-cold FACS buffer (PBS containing 0.2% BSA). Aliquots of cells were incubated with either primary antibody or isotype-matched control IgG. The cells were washed three times with ice-cold FACS buffer and then incubated with secondary antibodies (Invitrogen) for 1 h at 4 °C. After three washes, the cells were resuspended in FACS buffer and analyzed using a FACSCalibur flow cytometer.
Statistical Analysis
For the migration and invasion assays, two-tailed unpaired t tests were used to calculate significance between experimental and control groups with GraphPad Prism 5 software.
RESULTS
Down-regulation of CMTM8 Induces an EMT-like Phenotype in HepG2 Cells
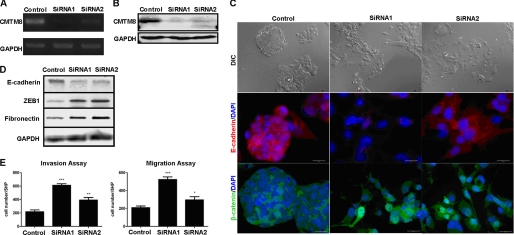
We tested the knockdown efficiency of two CMTM8 siRNAs by RT-PCR (Fig. 1A) and Western blotting (Fig. 1B). Both siRNAs blocked CMTM8 expression at the mRNA and protein levels (assayed at 72 and 96 h, respectively). Notably, the efficiency of the first siRNA (siRNA1) was better than that of the second one (siRNA2).
FIGURE 1.
Down-regulation of CMTM8 in HepG2 cells induces EMT-like phenotype. A, RT-PCR analysis of the mRNA level of CMTM8 72 h post-siRNA transfection. B, analysis of the protein level of CMTM8 96 h post-siRNA transfection by Western blotting. For both experiments, GAPDH was used as an internal control. C, upper panels, differential interference contrast (DIC) images showing the morphology of HepG2 cells transfected with non-silencing RNA (control) or siRNAs for CMTM8 knockdown (scale bars = 30 μm); middle and lower panels, confocal microscopy images of indirect immunofluorescence of HepG2 cells stained with anti-E-cadherin (scale bars = 20 μm) and anti-β-catenin (scale bars = 30 μm) antibodies, respectively. D, immunoblot of E-cadherin, ZEB1, and fibronectin. GAPDH was used as an internal control. Relative quantities were calculated by normalizing to the internal control. E, Transwell migration and invasion assays. Error bars indicate S.E. (n = 3). ***, p < 0.001; **, p < 0.01; *, p < 0.05 (using a two-tailed unpaired t test). 5HP indicates five high-powered fields of view.
Interestingly, when the expression of CMTM8 was reduced via siRNA, the morphology of HepG2 cells dramatically changed from round organized epithelial sheets to a spindle-shaped scattered pattern (Fig. 1C). This morphology change began to appear 60 h post-transfection, became pronounced after 72 h, and lasted longer than 120 h. As this change in morphology was reminiscent of cells undergoing an EMT transition, we investigated this possibility. We first tested the expression of EMT markers by indirect immunofluorescence and Western blotting. E-cadherin is an epithelial marker that functions in epithelial cell-cell adhesion and is down-regulated or mislocalized both in EMT cell models and at the invasive edge of some human tumor biopsies (2, 15, 16, 34, 35). In HepG2 cells with CMTM8 knockdown, E-cadherin was indeed down-regulated, which was verified by both immunofluorescence (Fig. 1C) and Western blot assays (Fig. 1D). Additionally, CMTM8 knockdown induced β-catenin translocation from the plasma membrane into the nucleus (Fig. 1C). Nuclear β-catenin can serve as a subunit of a transcriptional complex that helps to induce the expression of many genes, including the EMT-inducing transcription factors (36). CMTM8 knockdown also increased ZEB1 and fibronectin protein levels. ZEB1 is an EMT transcriptional regulator known to repress E-cadherin expression. Fibronectin is another EMT marker (Fig. 1D) (37). Finally, Transwell assays revealed that CMTM8 knockdown increased HepG2 cell invasion and migration (Fig. 1E). CMTM8 down-regulation also resulted in a fibroblast-like morphological change and reduction of E-cadherin in MCF-10A cells (supplemental Fig. S1).
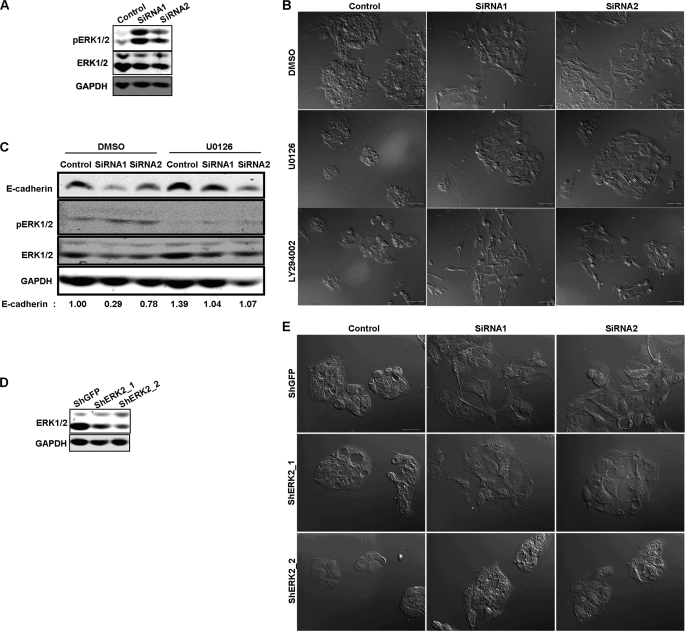
ERK-MAPK Pathway Is Required for the EMT-like Phenotype Induced by CMTM8 Knockdown in HepG2 Cells
We sought to investigate the cause of the EMT-like phenotype upon reduction of CMTM8 expression in HepG2 cells. We noted that ERK1/2 was activated when CMTM8 was knocked down (Fig. 2A), so we determined if ERK activity is required for the EMT-like phenotype induced by CMTM8 knockdown. We used the small molecule inhibitor U0126 to block MEK-mediated ERK activation. Indeed, the morphological change induced by CMTM8 knockdown was almost completely abrogated by U0126 (Fig. 2B), as was the reduction of E-cadherin expression (Fig. 2C). The PI3K inhibitor LY294002 failed to reverse the morphology change caused by CMTM8 knockdown, indicating that the PI3K-AKT pathway is not involved in this process in HepG2 cells (Fig. 2B).
FIGURE 2.
ERK-MAPK pathway is primary contributor to EMT-like phenotype induced by CMTM8 knockdown in HepG2 cells. A, immunoblot of cell lysates showing the levels of phosphorylated ERK. B, DIC images showing the morphology of HepG2 cells transfected with control or CMTM8 siRNAs and treated with Me2SO, U0126 (10 μm), or LY294002 (50 μm). Scale bars = 50 μm. C, immunoblot of cell lysates from HepG2 cells transfected with control or CMTM8 siRNAs and treated with Me2SO or U0126 (10 μm). The quantification of E-cadherin normalized to GAPDH is indicated. D, immunoblot of ERK1/2 showing the knockdown efficiency of the shRNA targeting ERK2 expression in HepG2 cells. E, DIC images showing the morphology of HepG2 cells stably expressing the vector control (shGFP) or shRNA for ERK2 knockdown (ShERK2) and transfected with control or CMTM8 siRNAs. Scale bar = 30 μm.
ERK2 (but not ERK1) induces EMT via DEF motif signaling in several mammalian breast epithelial cell types (16). To confirm the role of the ERK2 isoform in causing the EMT-like phenotype in HepG2 cells, we stably knocked down endogenous ERK2 using lentiviral shRNA constructs (Fig. 2D) and then knocked down CMTM8 expression with siRNAs. Consistent with our inhibitor data, partial knockdown of endogenous ERK2 largely impaired the induction of fibroblast-like morphology following knockdown of CMTM8 (Fig. 2E).
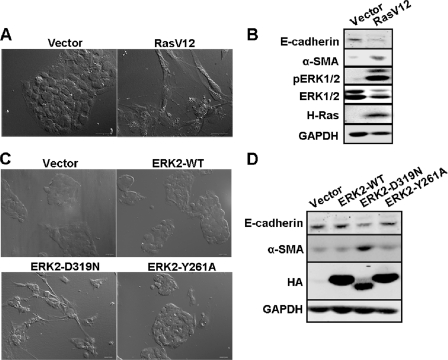
Activation of the Ras-ERK-MAPK Pathway Induces EMT in HepG2 Cells
We have reported previously that the Ras-ERK-MAPK pathway induces EMT in mammalian breast epithelial cells (16). Because the ERK-MAPK pathway was required for the EMT-like phenotypic change caused by CMTM8 knockdown, we asked if activation of the Ras-ERK-MAPK pathway alone could induce EMT in HepG2 cells. We first generated stable cell lines expressing the vector or RasV12 and monitored changes in morphology and EMT makers by Western blotting. We found that cells expressing RasV12 displayed dramatic alterations, including fibroblast-like morphological changes (Fig. 3A), increased expression of the mesenchymal marker α-smooth muscle actin, a reduction in E-cadherin, and an increase in ZEB1 expression (Fig. 3B and supplemental Fig. S3). To determine whether ERK2 signaling is sufficient to induce EMT-like features in HepG2 cells, we then ectopically overexpressed ERK2-WT, ERK2-D319N (common D-domain mutant ERK2 allele), and ERK2-Y261A (DEF motif-binding pocket mutant ERK2 allele). Only ERK2-D319N induced EMT-like phenotypic changes, although not to the extent of RasV12 (Fig. 3, C and D). Although expressed at lower levels, ERK2-D319N also induced expression of α-smooth muscle actin and reduced the expression of E-cadherin (Fig. 3D). However, we were unable to detect changes in ZEB1 (data not shown). It is not clear why ERK2-D319N, which signals to DEF motif-containing substrates, could not be expressed at levels equivalent to ERK2-WT and ERK2-Y261A, which, despite higher expression, induced no EMT-like changes. These results reveal that activation of the Ras-ERK-MAPK pathway is required for EMT induction in HepG2 cells and that ERK DEF motif signaling contributes significantly but alone is not sufficient to induce the full RasV12 phenotype.
FIGURE 3.
Activation of Ras-ERK-MAPK pathway induces EMT in HepG2 cells. A, DIC images showing the morphology of HepG2 cells expressing the empty vector or RasV12. Scale bars = 30 μm. B, immunoblot of cell lysates from HepG2 cells expressing the empty vector or RasV12. C, DIC images showing the morphology of HepG2 cells expressing the empty vector, ERK2-WT, ERK2-D319N, or ERK2-Y261A. Scale bars = 30 μm. D, immunoblot of cell lysates from HepG2 cells expressing the empty vector, ERK2-WT, ERK2-D319N, or ERK2-Y261A.
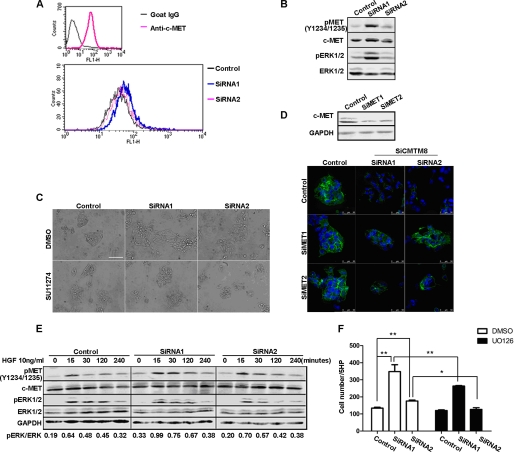
HGF/c-MET Signaling Is an Important Contributor to the EMT-like Phenotype Induced by CMTM8 Knockdown in HepG2 Cells
Our previous studies showed CMTM8 can accelerate the internalization of EGFR and hence attenuate its downstream signaling to ERK1/2 (29). Therefore, we first determined whether EGFR signaling to ERK is responsible for the EMT-like phenotypic change induced by CMTM8 knockdown in HepG2 cells. We found that EGFR surface levels were elevated in the cells with CMTM8 knockdown (supplemental Fig. S2A), and those cells exhibited greater migration toward EGF (5 ng/ml) (supplemental Fig. S2B). However, pharmacologically blocking EGFR activity by the small molecule inhibitor AG1478 did not noticeably reverse the morphological change observed in HepG2 cells with CMTM8 knockdown (supplemental Fig. S2C), indicating the EGFR might not be the main contributor of the EMT-like phenotype in the HepG2 system.
ERK is the downstream effector of multiple receptor tyrosine kinases. We asked if other receptor tyrosine kinases regulated by CMTM8 play a critical role in the induction of the EMT-like phenotype in HepG2 cells upon CMTM8 down-regulation. We focused on c-MET, which is abundantly expressed in HepG2 cells (Fig. 4A). It has been reported that c-MET is frequently overexpressed and hyperactivated in hepatocellular carcinoma (38, 39). HGF, the natural ligand of c-MET, was also reported to induce EMT in hepatocellular carcinoma cell lines (26, 27). In our studies, we found increased expression of c-MET both on the surface of HepG2 cells and in the whole cell lysates when CMTM8 expression was reduced (Fig. 4, A and B). Pharmacologically blocking c-MET activity with the small molecule inhibitor SU11274 (5 μm) almost completely reversed the fibroblast-like morphological change (Fig. 4C). To confirm this, we also performed experiments utilizing siRNAs to knock down endogenous c-MET. As shown by confocal image analysis, in cells cotransfected with siRNAs to CMTM8 and c-MET, E-cadherin expression were not altered (Fig. 4D). Furthermore, cells with greater CMTM8 knockdown (siRNA1) exhibited enhanced and slightly more sustained levels of phosphorylated c-MET and ERK when stimulated with HGF (Fig. 4E, see quantitation). These same cells exhibited increased migration toward HGF, which was blocked by pretreatment with U0126 at 10 μm for 2 h (Fig. 4F). Together, these data suggest that CMTM8 inhibition promotes increased c-MET/ERK signaling, which correlates with the observed EMT-like changes and cell motility.
FIGURE 4.
HGF/c-MET signaling is activated and required for EMT-like phenotypic alterations in HepG2 cells with CMTM8 knockdown. A, flow cytometric analysis of c-MET on the surface of HepG2 cells transfected with non-silencing RNA or siRNAs for CMTM8 knockdown. B, immunoblot of cell lysates from HepG2 cells transfected with control or CMTM8 siRNAs. C, phase-contrast images showing the morphology of HepG2 cells transfected with non-silencing siRNA or siRNAs for CMTM8 knockdown and treated with Me2SO or SU11274 (5 μm). Scale bar = 100 μm. D, confocal images of indirect immunofluorescence of HepG2 cells cotransfected with control or CMTM8 siRNAs (SiCMTM8) and control or c-MET siRNAs (SiMET) and stained with anti-E-cadherin antibody. Scale bars = 50 μm. The immunoblot of c-MET shows the knockdown efficiency of c-MET siRNAs in HepG2 cells. E, immunoblot of cell lysates from HepG2 cells transfected with control or CMTM8 siRNAs, serum-starved, and stimulated with 10 ng/ml HGF for the indicated times. F, migration assay. 1 ng/ml HGF was used as the chemoattractant for HepG2 cells transfected with control or CMTM8 siRNAs and pretreated with Me2SO or U0126 (10 μm) for 2 h. Error bars indicate S.E. (n = 3). **, p < 0.01; *, p < 0.05 (two-tailed unpaired t test). 5HP indicates five high-powered fields of view.
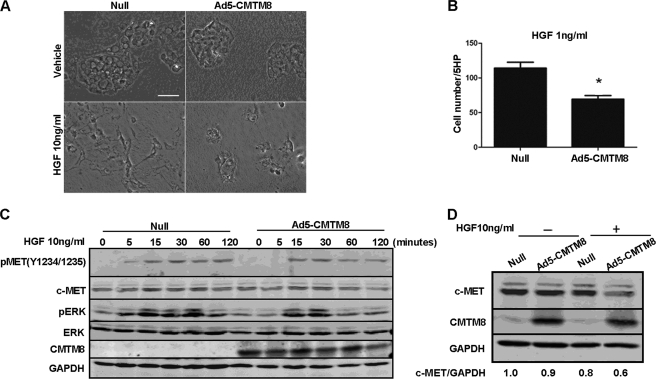
CMTM8 Overexpression Suppresses Fibroblast-like Morphological Change and Cell Motility of HepG2 Cells Induced by HGF/c-MET/ERK Signaling
The administration of HGF induces EMT in HepG2 cells, which is ERK-dependent (27). To further investigate the relationship between CMTM8 and c-MET signaling, we overexpressed CMTM8 in HepG2 cells and found that this resulted in partial reversion of the fibroblast-like morphological change induced by HGF addition (Fig. 5A) and dramatically inhibited cell motility using HGF (1 ng/ml) as the chemoattractant (Fig. 5B). CMTM8 overexpression suppressed the sustained ERK phosphorylation normally observed after stimulation of HepG2 cells with HGF (Fig. 5C), further supporting its role in inhibiting HGF/c-MET/ERK signaling. Furthermore, CMTM8 overexpression promoted the decrease in c-MET protein levels 12 h post-HGF stimulation (Fig. 5D, fourth lane), indicating that the inhibitory function of CMTM8 in HGF/c-MET/ERK signaling may due in part to its regulation of c-MET protein levels. These data further support that CMTM8 can function as an antagonistic force of EMT-like changes induced by HGF/c-MET/ERK signaling.
FIGURE 5.
CMTM8 overexpression suppresses fibroblast-like morphological change and cell motility of HepG2 cells induced by HGF/c-MET/ERK signaling. A, phase-contrast images showing the morphology of HepG2 cells infected with the control adenovirus (Null) or the adenovirus carrying CMTM8 (Ad5-CMTM8) and then treated 24 h later with a vehicle control or HGF (10 ng/ml) for 48 h. B, migration assay. HGF (1 ng/ml) was used as the chemoattractant for HepG2 cells infected with the control adenovirus or Ad5-CMTM8. Error bars indicate S.E. (n = 3). *, p < 0.05 (two-tailed unpaired t test). 5HP indicates five high-powered fields of view. C, immunoblot of cell lysates from HepG2 cells infected with the control adenovirus or Ad5-CMTM8, serum-starved, and stimulated with HGF (10 ng/ml) for the indicated times. D, immunoblot of cell lysates from HepG2 cells infected with the control adenovirus or Ad5-CMTM8 and treated with or without HGF (10 ng/ml) for 12 h. The quantification of c-MET normalized to GAPDH is indicated. Each experiment was done at least three times with similar results.
DISCUSSION
Our studies show that down-regulation of CMTM8 results in morphological and molecular changes that resemble EMT. These changes require enhanced c-MET and ERK signaling.
Unlike the results obtained with expression of activated RasV12, CMTM8 knockdown did not elevate the expression of α-smooth muscle actin (data not shown), which is a mesenchymal marker reported for EMT in hepatocyte-originated cell models (35). This difference may be due to incomplete knockdown with the available CMTM8 siRNAs, which are not capable of maintaining full knockdown during the course of extended EMT experiments. Because we could not determine whether the partial EMT phenotype (increased cell motility with a change in some EMT markers) that we observed is due to partial CMTM8 knockdown or if complete CMTM8 loss would generate the same phenotype, we describe the morphological and biochemical changes as EMT-like.
We have shown that the EMT-like phenotype induced by CMTM8 knockdown is mediated mainly by ERK (but not AKT) signaling. Although ERK and AKT are both downstream effectors of Ras, it has been demonstrated that the ERK (but not PI3K-AKT) pathway mediates the induction of EMT by oncogenic Ras (15, 16), which is consistent with our results. However, it is important to note that although knockdown of ERK2 indicates an important requirement of ERK2 signaling, ERK2 overexpression, specifically ERK2-D319N overexpression, generated a less pronounced EMT phenotype than RasV12. We have observed similar results in MCF-10A breast epithelial cells for ERK2 signaling via DEF motifs (16).
Signaling by receptor tyrosine kinases to Ras-ERK-MAPK has been shown to participate in EMT (40). Among them, EGFR was shown previously to be regulated by CMTM8 (29). However, in this study, we found that CMTM8 regulation of c-MET (not EGFR) was the main contributor to the EMT-like phenotypic alterations observed after CMTM8 knockdown in HepG2 cells. Similarly, in MCF-10A cells, an EGFR-dependent cell line, EGF stimulation did not induce visible morphological changes consistent with EMT, and AG1478 failed to block the EMT-like morphological change induced by CMTM8 knockdown (data not shown). Thus, although CMTM8 regulation of EGFR internalization may control cell survival (29), its regulation of c-MET is likely to contribute to the control of cell motility and invasion.
c-MET signaling promotes invasive growth and can drive cancer malignancy (41). In vitro studies have revealed that c-MET positively regulates invasion and migration of hepatocellular carcinoma cells, as well as other types of cancer cell lines (28, 42). Dysregulation of c-MET is associated with various molecular-genetic factors, and overexpression has been linked with increased tumor grade and poor prognosis (38, 39, 43–45). This study suggests that in addition to c-MET amplification and activating mutations, CMTM8 down-regulation is an additional mechanism by which tumors can induce c-MET signaling. Future studies on the inverse correlation between CMTM8 and c-MET expression in different tumor types will determine whether CMTM8 expression can be used as a biomarker to classify tumors most likely to respond to anti-c-MET therapeutics. CMTM8 could also be used as a potential therapeutic target of tumors with hyperactive c-MET/ERK signaling.
Supplementary Material
Acknowledgments
We thank Sang-Oh Yoon, Sejeong Shin, Yonghao Yu, Jamie Dempsey, and Emrah Er in the Blenis laboratory for helpful discussions regarding this work.
This work was supported, in whole or in part, by National Institutes of Health Grant R37CA46595 from NCI (to J. B.). This work was also supported by National Natural Science Foundation of China Grants 81071749, 30872292, and 90813025; National Basic Research Program of China Grant 2012CB518000; National Key New Drug Creation Program of China Grant 2009ZX09503-004; the China Scholarship Council; and Susan G. Komen for the Cure (to M. C. M.).

This article contains supplemental Figs. S1–S4.
W. Zhang, H. Qi, and Y. Wang, unpublished data.
- EMT
- epithelial-to-mesenchymal transition
- HGF
- hepatocyte growth factor
- EGFR
- EGF receptor
- DIC
- differential interference contrast.
REFERENCES
- 1. Pramanik R., Qi X., Borowicz S., Choubey D., Schultz R. M., Han J., Chen G. (2003) p38 isoforms have opposite effects on AP-1-dependent transcription through regulation of c-Jun. The determinant roles of the isoforms in the p38 MAPK signal specificity. J. Biol. Chem. 278, 4831–4839 [DOI] [PubMed] [Google Scholar]
- 2. Thiery J. P., Acloque H., Huang R. Y., Nieto M. A. (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 [DOI] [PubMed] [Google Scholar]
- 3. Thiery J. P., Sleeman J. P. (2006) Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 7, 131–142 [DOI] [PubMed] [Google Scholar]
- 4. Wang S. P., Wang W. L., Chang Y. L., Wu C. T., Chao Y. C., Kao S. H., Yuan A., Lin C. W., Yang S. C., Chan W. K., Li K. C., Hong T. M., Yang P. C. (2009) p53 controls cancer cell invasion by inducing the MDM2-mediated degradation of Slug. Nat. Cell Biol. 11, 694–704 [DOI] [PubMed] [Google Scholar]
- 5. Polyak K., Weinberg R. A. (2009) Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat. Rev. Cancer 9, 265–273 [DOI] [PubMed] [Google Scholar]
- 6. Roux P. P., Blenis J. (2004) ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 68, 320–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anjum R., Blenis J. (2008) The RSK family of kinases: emerging roles in cellular signaling. Nat. Rev. Mol. Cell Biol. 9, 747–758 [DOI] [PubMed] [Google Scholar]
- 8. Mendoza M. C., Er E. E., Zhang W., Ballif B. A., Elliott H. L., Danuser G., Blenis J. (2011) ERK-MAPK drives lamellipodial protrusion by activating the WAVE2 regulatory complex. Mol. Cell 41, 661–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoshida T., Hisamoto T., Akiba J., Koga H., Nakamura K., Tokunaga Y., Hanada S., Kumemura H., Maeyama M., Harada M., Ogata H., Yano H., Kojiro M., Ueno T., Yoshimura A., Sata M. (2006) Spreds, inhibitors of the Ras/ERK signal transduction, are dysregulated in human hepatocellular carcinoma and linked to the malignant phenotype of tumors. Oncogene 25, 6056–6066 [DOI] [PubMed] [Google Scholar]
- 10. Hwang Y. H., Choi J. Y., Kim S., Chung E. S., Kim T., Koh S. S., Lee B., Bae S. H., Kim J., Park Y. M. (2004) Overexpression of c-raf-1 proto-oncogene in liver cirrhosis and hepatocellular carcinoma. Hepatol. Res. 29, 113–121 [DOI] [PubMed] [Google Scholar]
- 11. Hoshino R., Chatani Y., Yamori T., Tsuruo T., Oka H., Yoshida O., Shimada Y., Ari-i S., Wada H., Fujimoto J., Kohno M. (1999) Constitutive activation of the 41/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene 18, 813–822 [DOI] [PubMed] [Google Scholar]
- 12. Schmitz K. J., Wohlschlaeger J., Lang H., Sotiropoulos G. C., Malago M., Steveling K., Reis H., Cicinnati V. R., Schmid K. W., Baba H. A. (2008) Activation of the ERK and AKT signaling pathway predicts poor prognosis in hepatocellular carcinoma, and ERK activation in cancer tissue is associated with hepatitis C virus infection. J. Hepatol. 48, 83–90 [DOI] [PubMed] [Google Scholar]
- 13. Thiery J. P. (2003) Epithelial-mesenchymal transitions in development and pathologies. Curr. Opin. Cell Biol. 15, 740–746 [DOI] [PubMed] [Google Scholar]
- 14. McCubrey J. A., Steelman L. S., Chappell W. H., Abrams S. L., Wong E. W., Chang F., Lehmann B., Terrian D. M., Milella M., Tafuri A., Stivala F., Libra M., Basecke J., Evangelisti C., Martelli A. M., Franklin R. A. (2007) Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation, and drug resistance. Biochim. Biophys. Acta 1773, 1263–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Janda E., Lehmann K., Killisch I., Jechlinger M., Herzig M., Downward J., Beug H., Grünert S. (2002) Ras and TGFβ cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J. Cell Biol. 156, 299–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shin S., Dimitri C. A., Yoon S. O., Dowdle W., Blenis J. (2010) ERK2 but not ERK1 induces epithelial-to-mesenchymal transformation via DEF motif-dependent signaling events. Mol. Cell 38, 114–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murphy L. O., MacKeigan J. P., Blenis J. (2004) A network of immediate early gene products propagates subtle differences in mitogen-activated protein kinase signal amplitude and duration. Mol. Cell. Biol. 24, 144–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murphy L. O., Smith S., Chen R. H., Fingar D. C., Blenis J. (2002) Molecular interpretation of ERK signal duration by immediate early gene products. Nat. Cell Biol. 4, 556–564 [DOI] [PubMed] [Google Scholar]
- 19. Dimitri C. A., Dowdle W., MacKeigan J. P., Blenis J., Murphy L. O. (2005) Spatially separate docking sites on ERK2 regulate distinct signaling events in vivo. Curr. Biol. 15, 1319–1324 [DOI] [PubMed] [Google Scholar]
- 20. Zarnegar R., Muga S., Enghild J., Michalopoulos G. (1989) NH2-terminal amino acid sequence of rabbit hepatopoietin A, a heparin-binding polypeptide growth factor for hepatocytes. Biochem. Biophys. Res. Commun. 163, 1370–1376 [DOI] [PubMed] [Google Scholar]
- 21. Yeh J. J., Routh E. D., Rubinas T., Peacock J., Martin T. D., Shen X. J., Sandler R. S., Kim H. J., Keku T. O., Der C. J. (2009) KRAS/BRAF mutation status and ERK1/2 activation as biomarkers for MEK1/2 inhibitor therapy in colorectal cancer. Mol. Cancer Ther. 8, 834–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schlessinger J. (2000) New roles for Src kinases in control of cell survival and angiogenesis. Cell 100, 293–296 [DOI] [PubMed] [Google Scholar]
- 23. Ponzetto C., Bardelli A., Zhen Z., Maina F., dalla Zonca P., Giordano S., Graziani A., Panayotou G., Comoglio P. M. (1994) A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell 77, 261–271 [DOI] [PubMed] [Google Scholar]
- 24. Tsukada Y., Miyazawa K., Kitamura N. (2001) High intensity ERK signal mediates hepatocyte growth factor-induced proliferation inhibition of the human hepatocellular carcinoma cell line HepG2. J. Biol. Chem. 276, 40968–40976 [DOI] [PubMed] [Google Scholar]
- 25. Kondo A., Hirayama N., Sugito Y., Shono M., Tanaka T., Kitamura N. (2008) Coupling of Grb2 to Gab1 mediates hepatocyte growth factor-induced high intensity ERK signal required for inhibition of HepG2 hepatoma cell proliferation. J. Biol. Chem. 283, 1428–1436 [DOI] [PubMed] [Google Scholar]
- 26. Togawa A., Sfakianos J., Ishibe S., Suzuki S., Fujigaki Y., Kitagawa M., Mellman I., Cantley L. G. (2010) Hepatocyte growth factor-stimulated cell scattering requires ERK and Cdc42-dependent tight junction disassembly. Biochem. Biophys. Res. Commun. 400, 271–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nagai T., Arao T., Furuta K., Sakai K., Kudo K., Kaneda H., Tamura D., Aomatsu K., Kimura H., Fujita Y., Matsumoto K., Saijo N., Kudo M., Nishio K. (2011) Sorafenib inhibits the hepatocyte growth factor-mediated epithelial mesenchymal transition in hepatocellular carcinoma. Mol. Cancer Ther. 10, 169–177 [DOI] [PubMed] [Google Scholar]
- 28. Salvi A., Arici B., Portolani N., Giulini S. M., De Petro G., Barlati S. (2007) In vitro c-met inhibition by antisense RNA and plasmid-based RNAi down-modulates migration and invasion of hepatocellular carcinoma cells. Int. J. Oncol. 31, 451–460 [PubMed] [Google Scholar]
- 29. Jin C., Ding P., Wang Y., Ma D. (2005) Regulation of EGF receptor signaling by the MARVEL domain-containing protein CKLFSF8. FEBS Lett. 579, 6375–6382 [DOI] [PubMed] [Google Scholar]
- 30. Li D., Jin C., Yin C., Zhang Y., Pang B., Tian L., Han W., Ma D., Wang Y. (2007) An alternative splice form of CMTM8 induces apoptosis. Int. J. Biochem. Cell Biol. 39, 2107–2119 [DOI] [PubMed] [Google Scholar]
- 31. Jin C., Wang Y., Han W., Zhang Y., He Q., Li D., Yin C., Tian L., Liu D., Song Q., Ma D. (2007) CMTM8 induces caspase-dependent and -independent apoptosis through a mitochondria-mediated pathway. J. Cell. Physiol. 211, 112–120 [DOI] [PubMed] [Google Scholar]
- 32. Debnath J., Mills K. R., Collins N. L., Reginato M. J., Muthuswamy S. K., Brugge J. S. (2002) The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell 111, 29–40 [DOI] [PubMed] [Google Scholar]
- 33. Li N., Hill K. S., Elferink L. A. (2008) Analysis of receptor tyrosine kinase internalization using flow cytometry. Methods Mol. Biol. 457, 305–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schmalhofer O., Brabletz S., Brabletz T. (2009) E-cadherin, β-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 28, 151–166 [DOI] [PubMed] [Google Scholar]
- 35. Lahsnig C., Mikula M., Petz M., Zulehner G., Schneller D., van Zijl F., Huber H., Csiszar A., Beug H., Mikulits W. (2009) ILEI requires oncogenic Ras for the epithelial-to-mesenchymal transition of hepatocytes and liver carcinoma progression. Oncogene 28, 638–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vincan E., Barker N. (2008) The upstream components of the Wnt signaling pathway in the dynamic EMT and MET associated with colorectal cancer progression. Clin. Exp. Metastasis 25, 657–663 [DOI] [PubMed] [Google Scholar]
- 37. Kalluri R., Weinberg R. A. (2009) The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaposi-Novak P., Lee J. S., Gòmez-Quiroz L., Coulouarn C., Factor V. M., Thorgeirsson S. S. (2006) Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. J. Clin. Invest. 116, 1582–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ueki T., Fujimoto J., Suzuki T., Yamamoto H., Okamoto E. (1997) Expression of hepatocyte growth factor and its receptor c-met proto-oncogene in hepatocellular carcinoma. Hepatology 25, 862–866 [DOI] [PubMed] [Google Scholar]
- 40. Whittaker S., Marais R., Zhu A. X. (2010) The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene 29, 4989–5005 [DOI] [PubMed] [Google Scholar]
- 41. Trusolino L., Bertotti A., Comoglio P. M. (2010) MET signaling: principles and functions in development, organ regeneration, and cancer. Nat. Rev. Mol. Cell Biol. 11, 834–848 [DOI] [PubMed] [Google Scholar]
- 42. Lee S. A., Ladu S., Evert M., Dombrowski F., De Murtas V., Chen X., Calvisi D. F. (2010) Synergistic role of Sprouty2 inactivation and c-Met up-regulation in mouse and human hepatocarcinogenesis. Hepatology 52, 506–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tavian D., De Petro G., Benetti A., Portolani N., Giulini S. M., Barlati S. (2000) u-PA and c-MET mRNA expression is coordinately enhanced while hepatocyte growth factor mRNA is down-regulated in human hepatocellular carcinoma. Int. J. Cancer 87, 644–649 [PubMed] [Google Scholar]
- 44. Sattler M., Salgia R. (2007) c-Met and hepatocyte growth factor: potential as novel targets in cancer therapy. Curr. Oncol. Rep. 9, 102–108 [DOI] [PubMed] [Google Scholar]
- 45. Sattler M., Salgia R. (2009) The MET axis as a therapeutic target. Update Cancer Ther. 3, 109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.