Background: HIF-1α tumor pro-survival activity requires functional microtubules.
Results: Microtubules and the dynein motor protein transport HIF-1α protein toward the nucleus enabling its transcriptional activity in taxane-sensitive cell lines.
Conclusion: Renal cell carcinoma suffers from loss of microtubule-dependent HIF-1α regulation underlying taxane resistance.
Significance: The integrity of the microtubule-HIF-1 axis contributes to taxane activity and may determine clinical response.
Keywords: Dynein, Hypoxia-inducible Factor (HIF), Microtubules, Nuclear Translocation, Trafficking
Abstract
Disruption of the microtubule cytoskeleton impairs tumor angiogenesis by inhibiting the hypoxia-inducible factor (HIF-1α) pathway. However, the signaling cascade linking microtubule disruption to HIF-1α inactivation has not been elucidated. Here, we show that microtubule-targeting drug (MTD) treatment impaired HIF-1α protein nuclear translocation, which significantly down-regulated HIF transcriptional activity. We provide strong evidence that HIF-1α protein associates with polymerized microtubules and traffics to the nucleus, with the aid of the dynein motor protein. Together, these data suggest that microtubules are critically involved in the nuclear trafficking and transcriptional activity of HIF-1α. We also show that the connection between the microtubule cytoskeleton and HIF-1α regulation is lost in renal cell carcinoma (RCC), where HIF-1α is overexpressed because of mutations in the von Hippel Lindau (VHL) tumor suppressor protein. Specifically, we show that MTD treatment of RCC cells did not impair HIF-1α nuclear accumulation or transcriptional activity, and had no effect on the polysome association profile of HIF-1α. Interestingly, we found that HIF-1α protein did not bind microtubules in RCC. Moreover, restoration of VHL function failed to restore the ability of MTDs to inhibit HIF-1α, suggesting that VHL does not contribute to this phenotype. Together, these results suggest that HIF-1α regulation is microtubule-independent, and likely contributes to the chemoresistant nature of RCCs. Further understanding of the microtubule-dependent HIF-1α regulation, and lack thereof in RCC, is essential given the importance of HIF-1α in tumor biology, and the widespread use of MTDs in clinical oncology.
Introduction
Hypoxia is a common characteristic of the tumor microenviroment that triggers angiogenesis via activation of the hypoxia-inducible factor (HIF-1)4 pathway. HIF-1α, the oxygen-regulated subunit of HIF-1, is a major pro-survival and pro-angiogenic transcription factor that has been found overexpressed in over 70% of human tumors and their metastases (1). Thus, HIF-1α inhibition represents an attractive strategy for cancer therapy (2, 3). We have previously shown that clinically relevant microtubule-targeting drugs (MTDs), such as Taxol and 2-methoxyestradiol (2ME2), impair angiogenesis through repression of HIF-1α translation (4–6). Importantly, we showed that the inhibition of HIF-1α translation occurs downstream of microtubule disruption, and is reversed following microtubule repolymerization (4). These findings provide solid proof of a functional relationship between the anti-tubulin and anti-angiogenic effects of MTDs.
Though MTDs are traditionally thought of as anti-mitotic agents, we and others (7, 8) have shown that these drugs affect important cellular functions during interphase. These functions include intracellular trafficking and nuclear targeting of transcription factors, such as the p53 tumor suppressor protein and the androgen receptor (8–11). Thus, MTD-induced microtubule damage significantly impairs the nuclear accumulation, and hence function, of these transcription factors. As we have shown that microtubules control HIF-1α mRNA transport and subsequent HIF translation, we wondered whether HIF-1α protein trafficking to the nucleus, a requirement for transcription, was also microtubule dependent.
Here, we show that HIF-1α protein associates with polymerized microtubules, using them as tracks to facilitate its nuclear translocation following hypoxic induction. We also show that the microtubule-associated motor protein, dynein, is critically involved in this transport mechanism. Treatment with taxanes and other MTDs inhibits HIF-1α nuclear accumulation and transcriptional activity as a result of microtubule disruption. This new mechanism adds HIF-1α to the list of transcription factors that are transported on interphase microtubules, and reveals that microtubules regulate HIF-1α at both the level of protein synthesis and protein trafficking.
Further understanding of the microtubule-dependent regulation of HIF-1α will have important therapeutic implications for the identification of patients most likely to respond to MTDs. Though MTDs are used against a wide variety of tumor types, and have the broadest range of indications among all classes of cancer chemotherapeutics, certain tumor types, such as renal cell carcinoma (RCC), do not respond clinically to anti-tubulin therapies (12). At the same time, HIF-1α is critically important for RCC disease progression, as ∼70–80% of RCCs have loss-of-function mutations in the von Hippel Lindau (VHL) protein (13), which is responsible for HIF-1α degradation in normoxia. As a result, HIF-1α and its target genes are constitutively expressed in RCC, regardless of oxygen tension, resulting in hypervascularity and aggressive tumor phenotypes. Therefore, HIF inhibition has been proposed as an important therapeutic strategy for RCC treatment.
This work, together with our previous data, demonstrates that MTDs inhibit the activity of HIF-1α by two distinct mechanisms. MTDs first impair HIF-1α protein nuclear accumulation, which is a prerequisite for transcription, and second, suppress HIF-1α translation, completely abolishing HIF activity. Therefore, the apparent disconnect between the up-regulation of HIFs in RCC and the lack of clinical activity of MTDs led us to investigate the integrity of the microtubule-HIF axis in this tumor type. Surprisingly, we found that RCCs represent the only tumor type we have tested to date where HIF-1α is not regulated by microtubules, and therefore not inhibited by MTDs. Specifically, we show that MTDs do not inhibit HIF-1α translation, nor do they affect the nuclear accumulation of HIF-1α protein and its downstream transcriptional activity. We also show that HIF-1α protein does not associate with microtubules in RCC cells, implying that in contrast to other tumor models, microtubule-dependent trafficking of HIF-1α does not occur in RCCs. Importantly, we found that the lack of MTD-induced HIF-1α inhibition occurs in both the presence and absence of functional VHL protein. This work reveals important insights into RCC chemoresistance. Further understanding of the mechanism responsible for the uncoupling of the microtubule cytoskeleton from HIF-1α activity in RCC will lead to the development of more effective therapies for this disease.
EXPERIMENTAL PROCEDURES
Cell Lines
PC3, MCF7, MDA-MB-231, ACHN, and CAKI-1 cells are from ATCC. The LN229-V6R glioblastoma cell line stably expressing the pBI-GL-V6L vector (14) was a gift from Erwin Van Meir (Emory University, Atlanta, GA). The RCC2 (mutant VHL) and RCC2-VHL (VHL restored) cells were obtained from Patrick Maxwell (Imperial College, UK), and the RCC4 (mutant VHL) and RCC4-VHL (VHL restores) cells were obtained from Leonard Neckers (National Cancer Institute, Bethesda, MD). Cells were cultured in RPMI 1640 (Cellgro), supplemented with 10% FBS and antibiotics, and cultured at 37 °C with 5% CO2. For hypoxic exposure, cells were placed in a sealed modular incubator chamber (Billups-Rothenberg) and flushed with 1% 02, 5% CO2, and 94% N2.
Chemicals, Antibodies, and Vectors
2ME2 was a gift from EntreMed Inc, Paclitaxel (Taxol) and docetaxel (Taxotere) were purchased from Sigma, Vincristine from MP Biomedicals, and Epothilone B from Calbiochem. Primary antibodies were: rat anti-α-tubulin (YL1/2, Chemicon International), mouse anti-α-tubulin (DM1α, Sigma), mouse anti-HIF-1α (BD Biosciences), mouse anti-dynein intermediate chain (IC74, Covance), mouse anti-importin α1 (karyopherin α2, BD Biosciences), and rabbit anti-actin (Sigma). Secondary antibodies were: horseradish peroxidase-conjugated (Amersham Biosciences) and Alexa Fluor 488, 568, 680, and 800 fluorescent antibodies (Molecular Probes). The pBI-GL-V6L vector was a gift from Erwin Van Meir (Emory University), the pCMVβ-gal vector was from Invitrogen, and the pCMVH50dynamitin vector was provided by Richard Vallee (University of Massachusetts, Worcester, MA) (15).
Linear Sucrose Gradient Fractionation
Polysome profile analyses were performed following linear sucrose gradient (LSG) fractionation, as previously described (16). Briefly, cells were lysed with 500 μl of LSG lysis buffer (100 mm KCl, 20 mm Tris 7.5, 5 mm MgCl2, 0.4% Nonidet P-40, 100 μg/ml cyclohexamide, 0.1 units of RNasin, and complete mini-EDTA free protease inhibitors; Roche) and pre-cleared by centrifugation (14,000 rpm, 15 min, 4 °C). The supernatant was loaded onto 5–45% sucrose gradients (in buffer containing 5 mm MgCl2, 80 mm NaCl, and 20 mm Tris-HCl, pH 7.5) and spun (36,000 rpm, 1.5 h, 4 °C) in an SW41 ultracentrifuge rotor. 12 equal-volume (1 ml) fractions were collected using the Gradient Station (BioComp Model 153). To assess the translational status of HIF-1α and GAPDH, we pooled the mRNAs extracted from fractions 1–6, versus those from fractions 7–12.
Quantitative Real-time PCR
Total RNA was isolated using the RNeasy Mini kit (Qiagen). cDNA was synthesized with the ProtoScript First Strand cDNA Synthesis Kit (New England BioLabs). qRT-PCR was performed using iQ SYBR green Supermix (Bio-Rad) and primers: HIF-1α (F:5′-TGG TGA CAT GAT TTA CAT TTC TGA-3′; R:5′-AAG GCC ATT TCT GTG TGT AAG C-3′); GAPDH (F:5′-GGA GTC AAC GGA TTT GGT CG-3′; R:5′-CTT GAT TTT GGA GGG ATC TCG-3′); VEGF (F:5′-CAA CAT CAC CAT GCA GAT TAT GC-3′; R:5′-TCG GCT TGT CAC ATT TTT CTT GT-3′); p53 (F:5′-TCA CAG CAC ATG ACG GAG GTT-3′; R:5′-TCG GAT AAG ATG CTG AGG AGG-3′).
Immunofluorescence
Immunostaining was performed as previously described (5, 6). Cells were imaged using a Zeiss 5 LIVE (Carl Zeiss, Inc.) or Zeiss 700 confocal microscope, with a 100×/1.4 Plan APOCHOMAT objective. Percent co-localization was calculated using MetaMorph image analysis software (Molecular Devices) in original images as well as in images where the HIF-1α (red) channel was shifted by 3 pixels, both horizontally and vertically. Pixel shifting was done in small areas in the cell periphery, where individual microtubules are more clearly visible. Percent nuclear HIF was quantified as (nuclear HIF fluorescence intensity/total HIF fluorescence intensity) × 100, using MetaMorph.
Western Blot
Total cell extracts were immunoblotted with the indicated antibodies as previously described (5). Immunoreactivity was visualized by the LI-COR Odyssey Infrared Imaging System (LI-COR Biosciences).
Transient Transfections and Reporter Gene Assay
Cells were transfected with 1 μg/well reporter plasmid using Fugene-6 (Roche) for 24 h, and luciferase reporter assays were performed as previously described (17).
Transmission Electron Microscopy
Cells were plated on 12 mm Thermanox plastic coverslips (Nunc), subjected to 6 h hypoxia, and fixed with complete PHEMO buffer (6) for 10 min at room temperature. Coverslips were blocked in 10% goat serum/PBS for 10 min and double immunolabeled with mouse anti-HIF-1α (1:300), followed by 6 nm colloidal gold goat anti-mouse secondary antibody (Jackson ImmunoResearch, 1:40 dilution) and rat anti α-tubulin antibody (1:500), followed by 12 nm colloidal gold goat anti-rat secondary antibody (Jackson ImmunoResearch, 1:40 dilution). Cells were then fixed in 2% glutaraldehyde for 4 h at room temperature, rinsed twice in distilled water, postfixed in 1% OsO4 in 0.1 m sodium cacodylate buffer (pH 7.4) at 4 °C for 1 h, and rinsed in distilled water as above. Samples were dehydrated through an ethanol series (30, 50, 60, 80, 90, 100%) followed by two changes of propylene oxide (10 min each). Samples were infiltrated with Embed 812 (Electron Microscopy Science) for 3 days according to the manufacturer's instructions. Blocks were cut to 1 × 1 mm using a diamond knife and RMC MT-7000 ultramicrotome. Sections were collected on 200 mesh copper grids. Grids were poststained with 10% uranyl acetate and then 2% lead citrate for 20 min each. Sections were analyzed with a two electron microscope.
Co-Immunoprecipitation
Cells were lysed in TNES buffer (50 mm Tris, pH 7.5, 2 mm EDTA, 100 mm NaCl, 1% Nonidet P-40) and centrifuged (14,000 rpm, 15 min, 4 °C). 1 mg of pre-cleared protein was incubated with primary antibody (1 μg of Ab/mg protein) for 1 h at room temperature followed by overnight incubation with 15 μl of protein A- or G Plus-agarose beads at 4 °C. Beads were washed 5× with 1 ml of TNES buffer, resuspended in 20 μl of 1× SDS buffer, and loaded onto SDS-PAGE gels.
Statistical Analyses
Data are presented as mean ± S.D. Student's t-tests were used to determine statistical significance using Stata for Windows (release 10).
RESULTS
Microtubule Disruption Prevents HIF-1α Nuclear Accumulation
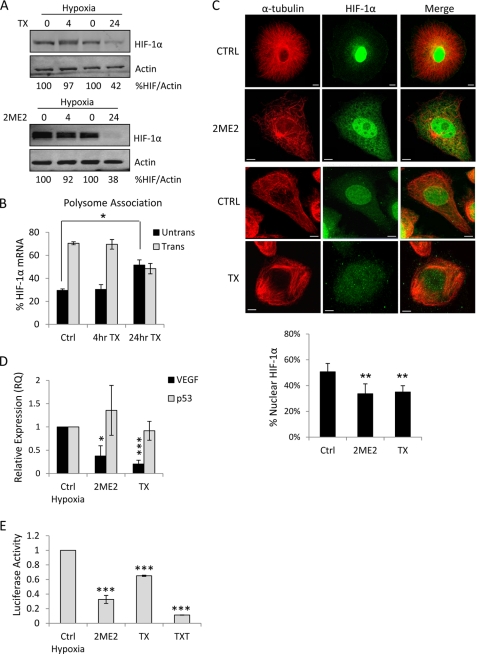
We have recently shown that interphase microtubule perturbation, by taxanes and other MTDs, impairs the active translation of HIF-1α mRNA by sequestering HIF-1α mRNA to P-bodies, which are cytoplasmic sites of translational repression (4). In addition, we showed that once the integrity of the microtubule cytoskeleton is restored, upon repolymerization, HIF-1α mRNA re-enters active translation, reinstating HIF-1α protein expression and activity. While these results indicate that microtubule dynamics tightly and reversibly regulate the fate of HIF-1α mRNA, the temporal relationship between microtubule disruption, HIF-1α translation and HIF-1α protein function is not well elucidated. To investigate this we treated PC3 prostate cancer cells with the microtubule-stabilizing drug, Taxol, or the microtubule-depolymerizing drug, 2ME2, in the presence of hypoxia for 4 and 24 h and assessed HIF-1α activity by immunoblot and immunofluorescence. We observed inhibition of HIF-1α protein levels at 24, but not 4 h post MTD treatment, suggesting that microtubule disruption is indirectly affecting HIF-1α translation (Fig. 1A). To investigate the time-course of HIF-1α translational repression, we assessed the polysome association profile of HIF-1α in Taxol-treated PC3 cells following sucrose gradient centrifugation. In this assay, we divided each gradient into 12 equal-volume fractions and monitored the position of non-translating ribosomal subunits (1–6) or translationally active polyribosomes (7–12) with continuous A254 measurements (supplemental Fig. S1A). The distribution of HIF-1α mRNA between polyribosomes and quiescent ribosomal subunits is plotted (Fig. 1B). As shown in the figure, no change in the translation status of HIF-1α was observed following a short-term (4 h) Taxol treatment, as the majority of HIF-1α mRNA co-fractionated with actively translating polysomes (∼70%), similar to untreated cells. Consistent with our previous results, Taxol treatment for 24 h resulted in a significant shift of HIF-1α polyribosome-association profile toward inactive translation (55%) (Fig. 1B). No shift was observed in the translation status of the unrelated message, GAPDH, at either time point (supplemental Fig. S1B).
FIGURE 1.
Microtubule disruption impairs HIF-1α nuclear translocation and transcriptional activity. A, immunoblot of HIF-1α and actin following 4 or 24 h treatment with 25 μm 2ME2 or 25 nm Taxol (TX) under hypoxia (1% oxygen). The percent HIF/actin was calculated using densitometry. B, polysome association profile of PC3 cells after 4 or 24 h treatment with 25 nm TX. Following linear sucrose gradient centrifugation, RNA was extracted from each fraction, and qRT-PCR for HIF-1α and GAPDH was performed. The distribution of each mRNA between untranslated (fractions 1–6) and translated (fractions 7–12) fractions is plotted as mean ± S.D. C, representative confocal microscopy images of PC3 cells treated as in A and processed for HIF-1α (green) and α-tubulin (red) immunofluorescence. Scale bars: 10 μm. The percent of nuclear HIF-1α protein is plotted for each condition as mean ± S.D. **, p < 0.01, t test. D, VEGF mRNA levels quantified by qRT-PCR following treatment of PC3 cells with 100 μm 2ME2 or 100 nm TX for 4 h under hypoxia. E, MCF7 cells were transiently transfected with the pBI-GL-V6L vector encoding luciferase driven by 6 HRE elements from the VEGF promoter, subjected to hypoxia for 4 h in the presence or absence of 50 μm 2ME2, 1 μm TX, or 1 μm TXT and processed for luciferase reporter assay. *, p < 0.05; ***, p < 0.001, t test.
While the 4 h MTD treatment had no effect on total HIF protein levels, it affected the intracellular distribution of HIF-1α by impairing its nuclear accumulation. Taxol or 2ME2 treatment at concentrations that stabilized or depolymerized microtubules, respectively, resulted in significant cytoplasmic retention of HIF-1α in hypoxia, in sharp contrast to the intense nuclear HIF-1α accumulation observed in untreated cells (Fig. 1C, p < 0.01).
This result suggested that the transcriptional activity of HIF-1α would be inhibited as a result of its impaired nuclear accumulation. To examine HIF-1α transcriptional activity following a 4 h MTD treatment, we assessed the expression of the well established HIF-target gene, vascular endothelial growth factor (VEGF), by quantitative RT-PCR (qRT-PCR). In agreement with our results in Fig. 1C, 4 h treatment of PC3 cells with Taxol or 2ME2 under hypoxia resulted in a significant decrease in VEGF mRNA but not in p53 mRNA, used here as a negative control for hypoxia-inducible gene (Fig. 1D and supplemental Fig. S1C). This result strongly suggests that the transcriptional activity of HIF-1α is impaired following short-term treatment with MTDs, even though HIF-1α protein levels remain unchanged.
To extend and corroborate these results, we also performed hypoxia response element (HRE)-luciferase reporter assays to assess HIF-transcriptional activity. For these experiments, MCF7 breast cancer cells were transiently transfected with a pBI-GL-V6L luciferase reporter vector, which is under the control of six copies of the HRE from the VEGF promoter. A decrease in HIF-1α transcriptional activity was observed following treatment with either stabilizing or depolymerizing MTDs, and hypoxic stabilization (Fig. 1E and supplemental Fig. S1D). Similar results were obtained using the glioblastoma cell line, LN229-V6R, which stably expresses the pBI-GL-V6L vector encoding HIF-HRE-luciferase reporter (14) (supplemental Fig. S1, E–G).
Together, these results suggest that microtubule disruption has a profound effect on the activity of HIF-1α by two distinct mechanisms. At early time-points, microtubule disruption impairs the nuclear translocation of HIF-1α protein, while prolonged microtubule disruption signals for repression of HIF-1α translation.
HIF-1α Protein Associates with Polymerized Microtubules
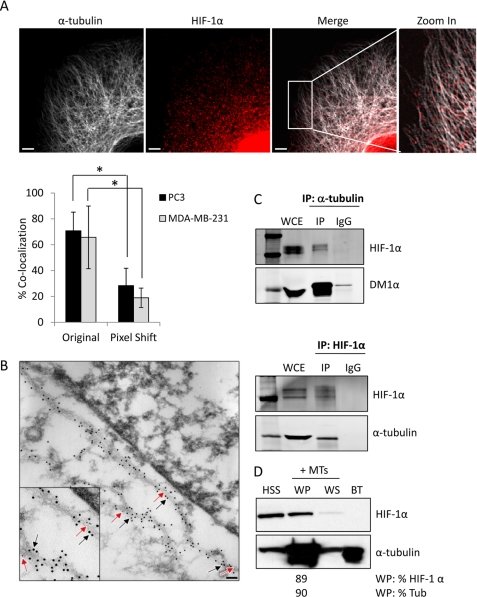
The observation that microtubule disruption resulted in HIF-1α cytoplasmic retention led us to hypothesize that HIF-1α protein utilizes microtubules as cellular tracks to facilitate its nuclear translocation. To test this hypothesis, we analyzed the subcellular localization of HIF-1α protein by confocal microscopy. Our results showed clear co-localization of hypoxia-stabilized HIF-1α with interphase microtubules in PC3 prostate cancer and MDA-MB-231 breast cancer cells (Fig. 2A and data not shown). To ensure that the observed co-localization between HIF-1α and tubulin was specific and not due to random overlap, we performed pixel shifting analyses. If the co-localization were random, we would observe a similar percent co-localization between the two proteins before and after the pixel shift. On the contrary, our results showed a significant decrease in the co-localization of HIF-1α and tubulin following a shift of the HIF-1α (red) channel by 3 pixels, both horizontally and vertically, confirming that the association was specific (Fig. 2A). Next, we performed transmission electron microscopy (TEM) using antibodies specific for HIF-1α and tubulin, to enhance the spatial resolution between the two proteins. PC3 cells were subjected to hypoxia and double immunostained with antibodies against HIF-1α and α-tubulin, followed by colloidal gold secondary antibodies of different sizes (6 nm gold particles for HIF-1α and 12 nm gold particles for tubulin). HIF-1α particles (red arrows) decorated microtubule polymers (black arrows) in the cell cytoplasm (Fig. 2B).
FIGURE 2.
HIF-1α protein associates with microtubules. A, confocal microscopy images of PC3 cells subjected to hypoxia for 6 h and labeled with anti-HIF-1α (red) and anti-α-tubulin (white) antibodies. Right panel shows a higher magnification of the marked cytoplasmic area. Scale bars: 2 μm. The bar graph represents percent co-localization between α-tubulin and HIF-1α protein in PC3 or MDA-MB-231 cells in original and pixel-shifted images (mean ± S.D. *, p < 0.05, t test). B, representative immuno-TEM image of PC3 cells fixed and processed for colloidal gold immunolabeling using primary antibodies against HIF-1α (6 nm colloidal gold secondary antibody, red arrows) or α-tubulin (12 nm colloidal gold secondary antibody, black arrows), following 6 h hypoxia. Inset: higher magnification showing co-localization between HIF-1α protein and microtubules. Scale bar: 0.2 μm. C. PC3 cells were exposed to 4 h hypoxia, lysed, and subjected to immunoprecipitation using a rat anti-α-tubulin antibody or matching rat IgG. Immunoprecipitates and 50 μg of whole cell extract (WCE) were resolved by SDS-PAGE and immunoblotted with antibodies against HIF-1α or mouse α-tubulin (DM1α) (top panel). MCF7 cells exposed to hypoxia for 4 h and subjected to immunoprecipitation using an antibody against HIF-1α and immunoblotted with antibodies against HIF-1α or rat α-tubulin (YL1/2 antibody) (bottom panel). D, microtubule co-sedimentation assay of PC3 cells lysates exposed to a 6 h hypoxia and incubated with exogenous purified bovine brain tubulin (BT) for 30 min at 37 °C in the presence of Taxol. Briefly, the pre-cleared high speed supernatant (HSS) was used as input for the assay and was subjected to a 100,000 × g centrifugation at 37 °C to separate the microtubule polymers (warm pellet, WP) from the soluble tubulin dimers (warm supernatant, WS). All fractions were resolved by SDS-PAGE and immunoblotted for the presence of HIF-1α and tubulin. The distribution of HIF-1α in each fraction was assessed by densitometry.
To further investigate the physical interaction between the two proteins, we immunoprecipitated α-tubulin from total PC3 cell lysates and assessed the presence of HIF-1α protein by immunoblot. Our results show that HIF-1α was immunoprecipitated with the α-tubulin antibody, but not by the control, species matched IgG, ensuring the specificity of the interaction (Fig. 2C, top). Similarly, α-tubulin was immunoprecipitated with a HIF-1α antibody in MCF7 cells, further confirming the physical association between the two proteins (Fig. 2C, bottom). To examine whether HIF-1α protein associates preferentially with the soluble (α/β-tubulin dimers) or polymerized form of tubulin, we performed a microtubule co-sedimentation assay. Cell lysates from PC3 cells subjected to hypoxia were first pre-cleared by centrifugation. The high speed supernatant (HSS), containing the majority of cellular proteins, was subjected to a tubulin polymerization cycle in the presence of exogenous purified bovine brain tubulin protein (BT) and Taxol, to accelerate tubulin polymerization. Our results indicate that the majority of HIF-1α co-fractionated with the microtubule warm pellets (WP) following the polymerization cycle (Fig. 2D). HIF-1α was not detected in the exogenous BT control, excluding the possibility that HIF-1α was associated with BT during the tubulin purification process, or that there was any nonspecific staining for HIF-1α. This result suggests that HIF-1α protein binds preferentially to polymerized tubulin, further suggesting that HIF protein may utilize microtubules for its intracellular trafficking.
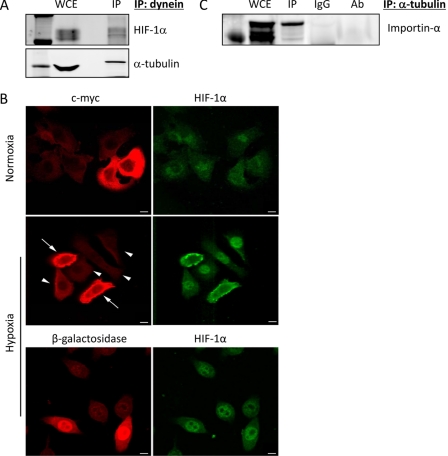
Dynein Mediates the Microtubule Association of HIF-1α
To investigate whether HIF-1α protein utilizes microtubules as tracks for trafficking, similar to our previous results regarding p53 and the androgen receptor (8, 10, 11), we examined HIF association with the microtubule-based minus-end directed motor protein, dynein (18, 19). Thus, we immunoprecipitated dynein from PC3 cell lysates and found that HIF-1α was indeed associated with the motor protein (Fig. 3A). Tubulin was also co-precipitated with dynein and HIF-1α, as expected (Fig. 3A). Next, we set out to investigate whether dynein was the primary motor protein mediating HIF-1α trafficking. To do so, we inhibited dynein-dependent transport by overexpressing dynamitin, a dynein-associated protein whose overexpression disrupts dynein-cargo interactions (15, 20). Using this approach, we transfected PC3 cells with either a pCMVH50myc vector (encoding a c-Myc-tagged human p50dynamitin) or a control vector expressing β-galactosidase, prior to hypoxic exposure and analyzed HIF subcellular localization by confocal microscopy. Dynamitin overexpression (Fig. 3B, arrows) clearly inhibited the hypoxia-stimulated nuclear accumulation of HIF-1α, in contrast to neighboring non-transfected cells (Fig. 3B, arrowheads), which displayed intense HIF-1α nuclear staining. No effect on HIF nuclear accumulation was observed with a β-galactosidase expressing vector, ruling out potential transfection artifacts.
FIGURE 3.
The dynein motor protein mediates the microtubule association of HIF-1α. A, PC3 cells were exposed to 4 h hypoxia and subjected to immunoprecipitation using an antibody against the intermediate chain of dynein. Immunoprecipitates and 50 μg of whole cell extract (WCE) were resolved by SDS-PAGE and immunoblotted with antibodies against HIF-1α or α-tubulin. B, PC3 cells were transiently transfected with pCMVH50 dynamitin vector (encoding a c-Myc-tagged human dynamitin), or the unrelated pCMVβ-galactosidase vector as a control, and subjected to 6 h hypoxia. Cells were fixed and processed for double immunofluorescence labeling using antibodies against HIF-1α (green) or c-Myc (red). Solid arrows point to cells overexpressing dynamitin. Arrowheads point to non-transfected cells. Scale bars: 5 μm. C, importin-α co-precipitates with tubulin. PC3 cells stably expressing mCherry-tubulin were subjected to immunoprecipitation using an antibody against rat α-tubulin (YL1/2) or matching rat IgG. Immunoprecipitates were resolved on SDS-PAGE along with 50 μg of WCE and immunoblotted with an antibody against importin-α1 (karyopherin α2). 1 μg of the antibody used for the IP was also loaded as a control for the heavy chains (Ab).
One question that remains to be answered is how HIF-1α is transported through the nuclear pore complex following its dynein-mediated arrival at the microtubule organizing center, which lies just outside the nuclear envelope. HIF-1α contains a nuclear localization signal (NLS), and is transported into the nucleus by the importin α/β pathway (21). Thus, we sought to determine whether importin-α might be associated with cellular microtubules. As shown in Fig. 3C, tubulin co-precipitation brought down importin-α1 specifically, suggesting that microtubules serve as the scaffold where HIF-1α associates with dynein and importin-α, for its efficient and directional nuclear import.
Microtubule Disruption Does Not Repress HIF-1α Activity in RCC
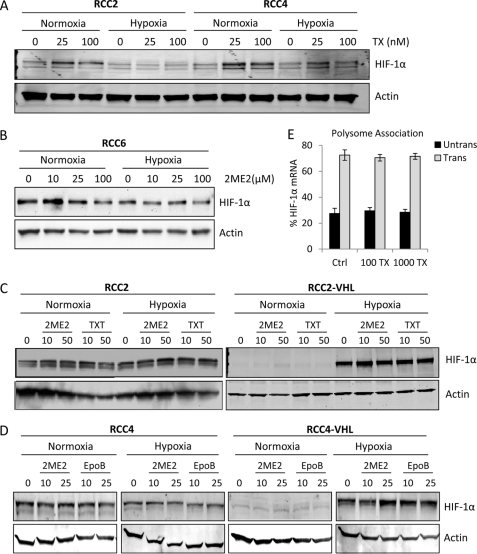
Our results suggest that inhibition of HIF-1α activity, at both the level of nuclear accumulation and protein synthesis, may mediate MTD sensitivity. Therefore, we hypothesized that tumor types with high expression of HIF-1α protein might be more susceptible to MTD treatment. To test this hypothesis, we monitored the effects of MTDs in several RCC cell lines (RCC2, RCC4, and RCC6) that express high levels of HIF-1α protein, even under normoxia, because of loss of VHL function. Surprisingly, we found that overnight Taxol treatment had no effect on HIF-1α protein levels, independently of oxygen tension, even when very high drug concentrations (1000 nm) were used (Fig. 4A and supplemental Fig. S2A). Similarly, treatment with the microtubule depolymerizing drug 2ME2, did not down-regulate HIF-1α protein (Fig. 4B).
FIGURE 4.
Microtubule-targeting drugs do not inhibit HIF-1α translation in RCC cells. A–D, HIF-1α immunoblot in different RCC cell lines, following the indicated MTD treatments in normoxia or 4 h hypoxia. E, polysome association profile of HIF-1α mRNA from RCC2 cells treated with 100 nm or 1 μm TX. The percent of HIF-1α mRNA associated with actively translating polysomes (gray bars) or non-translating ribosomal subunits (black bars) is plotted as mean ± S.D.
These results suggested that HIF-1α was not susceptible to MTD treatment in RCC cells with loss of VHL function. Given that VHL is reported to associate with microtubules and regulate microtubule stability (22, 23), we next sought to determine whether the loss of VHL accounted for the dissociation between HIF-1α regulation and microtubule disruption in these cells. Thus, we used two pairs of isogenic RCC cell lines in which functional VHL has been stably introduced, namely, RCC2/RCC2-VHL, and RCC4/RCC4-VHL. Surprisingly, both microtubule-stabilizing agents, Taxotere (TXT) and Epothilone B (EpoB), and the microtubule-depolymerizing agent, 2ME2, were unable to inhibit HIF-1α or HIF-2α protein levels, regardless of VHL status (Fig. 4, C and D and supplemental Fig. S2C). To rule out the possibility that RCC cells with loss of VHL had also lost factors required to link HIF-1α inhibition with microtubule disruption, we tested the SN12C, ACHN, and CAKI cell lines which harbor endogenous wild-type VHL. Again, our results revealed a lack of MTD-mediated inhibition of HIF-1α (supplemental Fig. S2D). These results suggested that unlike the other cancer cell lines we have tested to date (4–6), there is a clear loss of microtubule-dependent regulation of HIF-1α in RCC, regardless of VHL status.
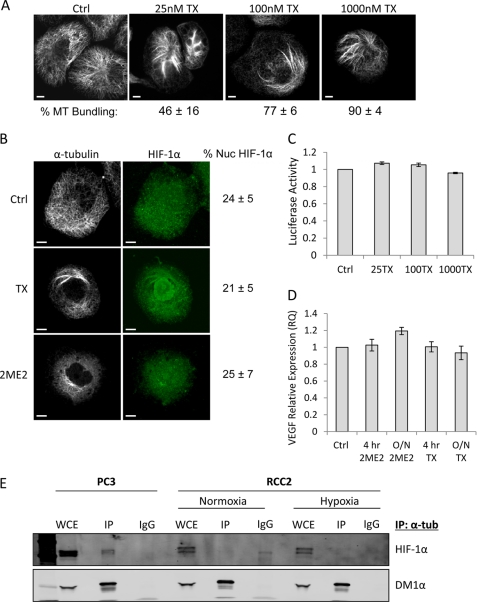
To understand the mechanism underlying the uncoupling HIF-1α and microtubules in RCC, we first examined the effect of MTD treatment on HIF-1α translation. The polysome association profile of HIF-1α in RCC2 cells following overnight treatment with Taxol showed that HIF-1α mRNA co-fractionated with the actively translating polysomes in both untreated and drug treated cells (Fig. 4E). The translation status of the control message, GAPDH, was also unaffected (supplemental Fig. S2B). Given that HIF-1α translation was not affected by MTD treatment, we wondered whether the microtubule network might not have been sufficiently disrupted by the drug treatment in these cells. Therefore, we treated RCC cells with Taxol and quantified the drug-induced microtubule bundling, a hallmark of efficient drug-target engagement. Our data revealed that Taxol treatment led to efficient and dose-dependent microtubule stabilization/bundling in the RCC2 cells (Fig. 5A), suggesting that the lack of HIF-1α inhibition in RCC does not stem from a loss of efficient drug-target engagement.
FIGURE 5.
HIF-1α protein and activity is uncoupled from microtubule disruption in RCC. A, immunofluorescence labeling of tubulin in RCC2 cells treated with 25, 100, or 1000 nm TX overnight. Scale bars: 10 μm. The percent of cells with microtubule (MT) bundling is shown as mean ± standard deviation for each treatment. B, confocal microscopy images of RCC2 cells treated with 100 nm TX or 100 μm 2ME2 overnight, and labeled with anti-HIF-1α (green) and anti-α-tubulin (white) antibodies. The percent of nuclear HIF-1α protein is shown for each condition (mean ± S.D.). C, HRE-luciferase reporter assay of RCC2 cells transfected with the pBI-GL-V6L HRE-luciferase vector, and treated with 25, 100, or 1000 nm TX overnight (data represented as mean ± S.D.). D, VEGF mRNA expression quantified by qRT-PCR following treatment of RCC2 cells with 100 nm TX or 100 μm 2ME2 for 4 h or overnight. Data plotted as mean ± S.D. E, PC3 or RCC2 cells processed for immunoprecipitation with a rat α-tubulin antibody in normoxia or following 4 h hypoxia. Immunoprecipitates were resolved by SDS-PAGE and immunoblotted with antibodies against HIF-1α or mouse α-tubulin (DM1α).
Next, we sought to determine whether microtubule disruption leads to a change in the subcellular localization of HIF-1α protein in RCC cells. Interestingly, confocal microscopy revealed that there was no change in the percentage of nuclear HIF-1α protein following Taxol or 2ME2 treatment, even though microtubules were efficiently stabilized or depolymerized, respectively (Fig. 5B). Consequently, we did not observe any change in HIF-1α transcriptional activity in RCC2 cells, as assessed by HRE-luciferase activity (Fig. 5C) or VEGF mRNA production (Fig. 5D). To examine whether HIF-1α protein remained associated with microtubules despite the uncoupling between HIF's trafficking and microtubule disruption, we immunoprecipitated α-tubulin from RCC2 cell lysates, under either normoxic or hypoxic conditions, and assessed HIF-1α co-precipitation by immunoblot. Our results clearly showed that HIF-1α protein was not present in the α-tubulin immunoprecipitates from RCC cells (Fig. 5E). In contrast, we observed co-immunoprecipitation between tubulin and HIF-1α in PC3 cells which were included as a positive control, in agreement with our results in Fig. 2. These results indicate that the interaction between microtubules and HIF-1α is specifically lost in renal cell carcinoma. These data strongly suggest that HIF-1α localization and activity are not susceptible to MTD treatment in renal cell carcinoma.
DISCUSSION
Microtubule-targeting drugs are the most effective class of chemotherapeutics, and their success in clinical oncology argues that tubulin is one of the best drug targets identified to date (24, 25). However, despite the presence of tubulin in all cancer cells, there are several tumor types that do not respond to MTD treatment, including renal cell carcinoma. Given that microtubules have critical roles in cell biology outside of mitosis, perharps MTD efficacy can be better explained if attributed to inhibition of interphase pathways that depend on functional microtubules, and are therefore impaired following microtubule disruption. As different tumor types are driven by distinct pathways, ranging from androgen or estrogen receptor signaling in prostate and breast cancer, respectively, and VHL loss-of-function and HIF overexpression in RCC, elucidation of the pathways regulated by the chemomechanics of microtubules will help us understand MTD clinical activity and resistance. The work reported here suggests that a key determinant of clinical response to MTD treatment is the ability of these drugs to inhibit the pro-survival and pro-angiogenic transcription factor, HIF-1α.
The microtubule cytoskeleton was once thought to solely provide a structural framework for the cell. However, it is now understood that microtubules are critical for processes such as cell movement, vesicle and organelle transport and mitosis. It is also clear that microtubules are essential for the intracellular trafficking of viruses (26), protein complexes and transcriptions factors, such as p53 (8), the androgen receptor (11), the parathyroid hormone (PTH) (27) and the glucocorticoid receptor β (GRβ) (28).
In this work, we hypothesized that HIF-1α protein is also being transported by microtubules. We sought to build upon previous work where we showed that both microtubule stabilization and depolymerization inhibited HIF-1α translation (4–6). Here, we demonstrate that MTDs inhibit HIF activity by inhibiting the nuclear accumulation of HIF-1α protein, in addition to repressing HIF translation. Interestingly, there is a temporal separation between these two modes of action. First, shortly after microtubule damage, the trafficking of HIF-1α protein is impaired, while inhibition of HIF translation occurs later, following at least 8–12 h of MTD treatment. These results suggest that HIF-1α protein utilizes microtubules, almost exclusively, for efficient nuclear targeting and that any microtubule perturbation has a significant impact on its nuclear translocation and activity. On the other hand, microtubule-dependent repression of HIF translation requires prolonged microtubule damage, which likely triggers additional signaling events that affect the synthesis of HIF-1α, and potentially other messages, via selective recruitment to Argonaute-2 in P-bodies.
Given that HIF-1α is essential for cell survival in hypoxic microenvironments, it is not surprising that both its transport and translation are tightly linked to microtubule dynamics. In this way, cells can immediately adapt to hypoxic stress by having a mechanism in place for quick and directional transport of HIF-1α to the nucleus, where HIF exerts its effects on transcription. Microtubule-dependent transport is a faster, more efficient way of trafficking, as compared to passive diffusion through the cytoplasm to the nuclear envelope, and is a process that is conserved in organisms ranging from plants (29), to neurons (30) and epithelial cancer cells (8, 9, 11, 27, 28).
Cytoplasmic dynein, the minus-end directed motor protein, utilizes microtubules to drive a wide variety of cytoplasmic motor activities, ranging from movement of ER-Golgi complexes, late endosomes and lipid droplets (31, 32) to viral capsids (26) and chromosomes (33, 34). To control such a variety of motile functions, dynein works in concert with various accessory factors, including dynactin, a massive protein complex that directs and coordinates dynein activities (35). Here we show that HIF-1α is transported along microtubules via dynein, and that the dynein-HIF association is required for HIF nuclear accumulation with the aid of importin-α (Fig. 3). However, the dynamics and temporal relationships between these protein-protein associations remain to be elucidated, as well as whether there are additional partner proteins involved in the integrity of the microtubule-HIF-1α axis. Our data, combined with a recent study showing that hsp90-immunophilins link p53 to dynein during its microtubule-based transport to the nucleus (9), highlight the importance of microtubule-dynein dependent trafficking for transcription factors that require rapid and targeted nuclear translocation upon specific stimuli.
Taken together, our results highlight the importance of the microtubule cytoskeleton in the regulation of HIF-1 pathway and suggest that the inhibition of HIF-1α is a key determinant of taxane clinical activity. Surprisingly, we also show in this report that this principle does not apply to RCC treatment, making RCC the only example to date, where the expression and activity of HIF-1α is microtubule-independent. Indeed, we showed that treatment with taxanes and other MTDs does not affect HIF-1α translation, protein trafficking or transcriptional activity (Figs. 4 and 5 and supplemental Fig. S2). Furthermore we show that HIF-1α does not bind microtubules in RCC, providing a potential explanation for the lack of microtubule-based transport. Identification of the protein(s) that normally mediate HIF-1α binding to microtubules is underway in an effort to identify cellular factors that are missing or deregulated in RCC.
These results can potentially explain the lack MTD clinical activity in RCC, a disease driven by constitutive overexpression of HIF-α, which results from VHL loss of function (13). Recent studies have shown that VHL is a microtubule-associated protein (22) involved in regulating microtubule dynamics (23), spindle orientation (36), and ciliogenesis (37, 38). These reports prompted us to hypothesize that VHL loss was responsible for the uncoupling between HIF and microtubules. Surprisingly, we found the opposite, as treatment with Taxol and other clinically relevant MTDs, such as Epothilone B, did not inhibit HIF-1α expression or activity in RCC cells in which wild-type VHL was stably reintroduced (Fig. 4). Similarly, we found that MTD treatment of RCC cell lines with endogenous wild-type VHL had no anti-HIF activity (supplemental Fig. S2D). In agreement with these observations, a recent phase II clinical trial of Ixabepilone (an Epothilone B analog) in RCC showed no correlation between VHL status and clinical response to treatment (39). Together these results suggest that there are additional inherent differences in RCC cells that contribute to taxane insensitivity. Several potential mechanisms of MTD resistance have been proposed, including increased drug efflux due to overexpression of the membrane transporter, P-glycoprotein (MDR-1). However, no correlation between P-glycoprotein expression and response to MTDs has been found in renal cancer cell lines (40). Another possible mechanism of resistance is the presence of β-tubulin mutations in the respective MTD drug binding sites; however, to date, no β-tubulin mutations have been identified in renal tumors (41). Interestingly, overexpression of the neuronal-specific βIII tubulin isotype, which contains HREs and is driven by HIF-1α (42), has been clinically correlated with taxane resistance in several epithelial tumor types (43). Earlier studies have also shown that the ability of Taxol to induce tubulin polymerization in vitro was significantly impaired in βIII-enriched purified microtubules (44). However, the expression of βIII tubulin in RCC has not been thoroughly investigated and there is no study correlating the presence of βIII tubulin with response to microtubule-targeted agents.
A final possibility is that there is an additional protein(s) involved in the mechanism of microtubule-dependent regulation of HIF-1α that is lost in RCC cells. Future studies will be aimed at determining if there are any HIF-1α-binding proteins that are lost or mutated in RCCs, using proteomics approaches.
Collectively, our data show that microtubule dynamics regulate HIF-1α activity by enabling the nuclear accumulation of HIF protein and by regulating its active translation. This mechanism has important clinical implications as HIF-1α inhibition mediates, at least in part, the clinical activity of MTD-based chemotherapy. A better understanding of the cellular pathways regulating the integrity of the microtubule-HIF axis will help the development of more effective anticancer therapies.
Supplementary Material
Acknowledgments
We dedicate this work to the memory of Dr. Robert P. Apkarian. We would like to thank Jeannette V. Taylor and Dr. Robert Apkarian for their superb technical assistance in TEM experiments.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 CA114335, R01 CA100202, R01 CA137020, NCI U54 CA143876, NCI Supplement to R01 CA86335; Entremed, Inc. Grant 659342 (to P. G.), and Grant TL1RR024998 of the Weill Cornell Medical College (WCMC) Clinical and Translational Science Center (to M. C.).

This article contains supplemental Figs. S1 and S2.
- HIF
- hypoxia inducible factor
- MTDs
- microtubule-targeting drugs
- 2ME2
- 2-methoxyestradiol
- PTX
- paclitaxel
- BT
- brain tubulin
- WP
- warm pellets
- WS
- warm supernatants
- HSS
- high speed supernatants
- MT
- microtubule
- HRE
- hypoxia response elements
- PTH
- parathyroid hormone
- GRβ
- glucocorticoid receptor β
- GFP
- green fluorescence protein
- VEGF
- vascular endothelial growth factor
- RCC
- renal cell carcinoma
- VHL
- von Hippel Lindau.
REFERENCES
- 1. Semenza G. L. (2003) Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3, 721–732 [DOI] [PubMed] [Google Scholar]
- 2. Escuin D., Simons J. W., Giannakakou P. (2004) Exploitation of the HIF axis for cancer therapy. Cancer Biol. Ther. 3, 608–611 [DOI] [PubMed] [Google Scholar]
- 3. Poon E., Harris A. L., Ashcroft M. (2009) Targeting the hypoxia-inducible factor (HIF) pathway in cancer. Expert Rev. Mol. Med. 11, e26. [DOI] [PubMed] [Google Scholar]
- 4. Carbonaro M., O'Brate A., Giannakakou P. (2011) Microtubule disruption targets HIF-1α mRNA to cytoplasmic P-bodies for translational repression. J. Cell Biol. 192, 83–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Escuin D., Kline E. R., Giannakakou P. (2005) Both microtubule-stabilizing and microtubule-destabilizing drugs inhibit hypoxia-inducible factor-1α accumulation and activity by disrupting microtubule function. Cancer Res. 65, 9021–9028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mabjeesh N. J., Escuin D., LaVallee T. M., Pribluda V. S., Swartz G. M., Johnson M. S., Willard M. T., Zhong H., Simons J. W., Giannakakou P. (2003) 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell 3, 363–375 [DOI] [PubMed] [Google Scholar]
- 7. Gascoigne K. E., Taylor S. S. (2008) Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell 14, 111–122 [DOI] [PubMed] [Google Scholar]
- 8. Giannakakou P., Sackett D. L., Ward Y., Webster K. R., Blagosklonny M. V., Fojo T. (2000) p53 is associated with cellular microtubules and is transported to the nucleus by dynein. Nat. Cell Biol. 2, 709–717 [DOI] [PubMed] [Google Scholar]
- 9. Galigniana M. D., Harrell J. M., O'Hagen H. M., Ljungman M., Pratt W. B. (2004) Hsp90-binding immunophilins link p53 to dynein during p53 transport to the nucleus. J. Biol. Chem. 279, 22483–22489 [DOI] [PubMed] [Google Scholar]
- 10. Giannakakou P., Nakano M., Nicolaou K. C., O'Brate A., Yu J., Blagosklonny M. V., Greber U. F., Fojo T. (2002) Enhanced microtubule-dependent trafficking and p53 nuclear accumulation by suppression of microtubule dynamics. Proc. Natl. Acad. Sci. U.S.A. 99, 10855–10860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Darshan M. S., Loftus M. S., Thadani-Mulero M., Levy B. P., Escuin D., Zhou X. K., Gjyrezi A., Chanel-Vos C., Shen R., Tagawa S. T., Bander N. H., Nanus D. M., Giannakakou P. (2011) Taxone-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 71, 6019–6029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Motzer R. J., Russo P. (2000) Systemic therapy for renal cell carcinoma. J. Urol. 163, 408–417 [PubMed] [Google Scholar]
- 13. Kaelin W. G., Jr., Maher E. R. (1998) The VHL tumour-suppressor gene paradigm. Trends Genet. 14, 423–426 [DOI] [PubMed] [Google Scholar]
- 14. Post D. E., Van Meir E. G. (2001) Generation of bidirectional hypoxia/HIF-responsive expression vectors to target gene expression to hypoxic cells. Gene Ther. 8, 1801–1807 [DOI] [PubMed] [Google Scholar]
- 15. Echeverri C. J., Paschal B. M., Vaughan K. T., Vallee R. B. (1996) Molecular characterization of the 50-kDa subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 132, 617–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feng Y., Absher D., Eberhart D. E., Brown V., Malter H. E., Warren S. T. (1997) FMRP associates with polyribosomes as an mRNP, and the I304N mutation of severe fragile X syndrome abolishes this association. Mol. Cell 1, 109–118 [DOI] [PubMed] [Google Scholar]
- 17. Mabjeesh N. J., Post D. E., Willard M. T., Kaur B., Van Meir E. G., Simons J. W., Zhong H. (2002) Geldanamycin induces degradation of hypoxia-inducible factor 1α protein via the proteosome pathway in prostate cancer cells. Cancer Res. 62, 2478–2482 [PubMed] [Google Scholar]
- 18. Schnapp B. J., Reese T. S. (1989) Dynein is the motor for retrograde axonal transport of organelles. Proc. Natl. Acad. Sci. U.S.A. 86, 1548–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schroer T. A., Steuer E. R., Sheetz M. P. (1989) Cytoplasmic dynein is a minus end-directed motor for membranous organelles. Cell 56, 937–946 [DOI] [PubMed] [Google Scholar]
- 20. Burkhardt J. K., Echeverri C. J., Nilsson T., Vallee R. B. (1997) Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 139, 469–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Depping R., Steinhoff A., Schindler S. G., Friedrich B., Fagerlund R., Metzen E., Hartmann E., Köhler M. (2008) Nuclear translocation of hypoxia-inducible factors (HIFs): involvement of the classical importin αβ pathway. Biochim. Biophys. Acta 1783, 394–404 [DOI] [PubMed] [Google Scholar]
- 22. Hergovich A., Lisztwan J., Barry R., Ballschmieter P., Krek W. (2003) Regulation of microtubule stability by the von Hippel-Lindau tumour suppressor protein pVHL. Nat. Cell Biol. 5, 64–70 [DOI] [PubMed] [Google Scholar]
- 23. Thoma C. R., Matov A., Gutbrodt K. L., Hoerner C. R., Smole Z., Krek W., Danuser G. (2010) Quantitative image analysis identifies pVHL as a key regular of microtubule dynamic instability. J. Cell Biol. 190, 991–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jordan M. A., Kamath K. (2007) How do microtubule-targeted drugs work? An overview. Curr. Cancer Drug Targets 7, 730–742 [DOI] [PubMed] [Google Scholar]
- 25. Zhou J., Giannakakou P. (2005) Targeting microtubules for cancer chemotherapy. Curr. Med. Chem. Anticancer Agents 5, 65–71 [DOI] [PubMed] [Google Scholar]
- 26. Leopold P. L., Pfister K. K. (2006) Viral strategies for intracellular trafficking: motors and microtubules. Traffic 7, 516–523 [DOI] [PubMed] [Google Scholar]
- 27. Lam M. H., Thomas R. J., Loveland K. L., Schilders S., Gu M., Martin T. J., Gillespie M. T., Jans D. A. (2002) Nuclear transport of parathyroid hormone (PTH)-related protein is dependent on microtubules. Mol. Endocrinol. 16, 390–401 [DOI] [PubMed] [Google Scholar]
- 28. Zhang X. Y., Clark A. F., Yorio T. (2005) Regulation of glucocorticoid responsiveness in glaucomatous trabecular meshwork cells by glucocorticoid receptor-β. Invest. Ophthalmol. Vis. Sci. 46, 4607–4616 [DOI] [PubMed] [Google Scholar]
- 29. Smith H. M., Raikhel N. V. (1999) Protein targeting to the nuclear pore. What can we learn from plants? Plant Physiol. 119, 1157–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ambron R. T., Schmied R., Huang C. C., Smedman M. (1992) A signal sequence mediates the retrograde transport of proteins from the axon periphery to the cell body and then into the nucleus. J. Neurosci. 12, 2813–2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goodson H. V., Skube S. B., Stalder R., Valetti C., Kreis T. E., Morrison E. E., Schroer T. A. (2003) CLIP-170 interacts with dynactin complex and the APC-binding protein EB1 by different mechanisms. Cell Motil. Cytoskeleton 55, 156–173 [DOI] [PubMed] [Google Scholar]
- 32. Valetti C., Wetzel D. M., Schrader M., Hasbani M. J., Gill S. R., Kreis T. E., Schroer T. A. (1999) Role of dynactin in endocytic traffic: effects of dynamitin overexpression and colocalization with CLIP-170. Mol. Biol. Cell 10, 4107–4120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eckley D. M., Gill S. R., Melkonian K. A., Bingham J. B., Goodson H. V., Heuser J. E., Schroer T. A. (1999) Analysis of dynactin subcomplexes reveals a novel actin-related protein associated with the arp1 minifilament pointed end. J. Cell Biol. 147, 307–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Quintyne N. J., Gill S. R., Eckley D. M., Crego C. L., Compton D. A., Schroer T. A. (1999) Dynactin is required for microtubule anchoring at centrosomes. J. Cell Biol. 147, 321–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kardon J. R., Vale R. D. (2009) Regulators of the cytoplasmic dynein motor. Nat. Rev. Mol. Cell Biol. 10, 854–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thoma C. R., Toso A., Gutbrodt K. L., Reggi S. P., Frew I. J., Schraml P., Hergovich A., Moch H., Meraldi P., Krek W. (2009) VHL loss causes spindle misorientation and chromosome instability. Nat. Cell Biol. 11, 994–1001 [DOI] [PubMed] [Google Scholar]
- 37. Schermer B., Ghenoiu C., Bartram M., Müller R. U., Kotsis F., Höhne M., Kühn W., Rapka M., Nitschke R., Zentgraf H., Fliegauf M., Omran H., Walz G., Benzing T. (2006) The von Hippel-Lindau tumor suppressor protein controls ciliogenesis by orienting microtubule growth. J. Cell Biol. 175, 547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thoma C. R., Frew I. J., Hoerner C. R., Montani M., Moch H., Krek W. (2007) pVHL and GSK3β are components of a primary cilium-maintenance signaling network. Nat. Cell Biol. 9, 588–595 [DOI] [PubMed] [Google Scholar]
- 39. Zhuang S. H., Agrawal M., Edgerly M., Bakke S., Kotz H., Thambi P., Rutt A., Balis F. M., Bates S., Fojo T. (2005) A Phase I clinical trial of ixabepilone (BMS-247550), an epothilone B analog, administered intravenously on a daily schedule for 3 days. Cancer 103, 1932–1938 [DOI] [PubMed] [Google Scholar]
- 40. Ferguson R. E., Jackson S. M., Stanley A. J., Joyce A. D., Harnden P., Morrison E. E., Patel P. M., Phillips R. M., Selby P. J., Banks R. E. (2005) Intrinsic chemotherapy resistance to the tubulin-binding antimitotic agents in renal cell carcinoma. Int. J. Cancer 115, 155–163 [DOI] [PubMed] [Google Scholar]
- 41. Ferguson R. E., Taylor C., Stanley A., Butler E., Joyce A., Harnden P., Patel P. M., Selby P. J., Banks R. E. (2005) Resistance to the tubulin-binding agents in renal cell carcinoma: no mutations in the class I β-tubulin gene but changes in tubulin isotype protein expression. Clin. Cancer Res. 11, 3439–3445 [DOI] [PubMed] [Google Scholar]
- 42. Raspaglio G., Filippetti F., Prislei S., Penci R., De Maria I., Cicchillitti L., Mozzetti S., Scambia G., Ferlini C. (2008) Hypoxia induces class III β-tubulin gene expression by HIF-1α binding to its 3′-flanking region. Gene 409, 100–108 [DOI] [PubMed] [Google Scholar]
- 43. Kavallaris M. (2010) Microtubules and resistance to tubulin-binding agents. Nat. Rev. Cancer 10, 194–204 [DOI] [PubMed] [Google Scholar]
- 44. Derry W. B., Wilson L., Khan I. A., Luduena R. F., Jordan M. A. (1997) Taxol differentially modulates the dynamics of microtubules assembled from unfractionated and purified β-tubulin isotypes. Biochemistry 36, 3554–3562 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.