Background: Skeletal muscle-derived myokines modulate metabolic, inflammatory, and other processes.
Results: Myonectin, a novel myokine whose expression and circulating levels are regulated by diet, metabolic state, and exercise, functions to control lipid metabolism.
Conclusion: Myonectin is a potential nutrient-responsive metabolic regulator secreted by muscle.
Significance: Myonectin links muscle to systemic lipid metabolism via its action on adipocytes and hepatocytes, providing insight into complex tissue cross-talk.
Keywords: Adipokines, Exercise, Fatty acid transport, Lipid metabolism, Skeletal muscle, Skeletal muscle metabolism, myokine
Abstract
Skeletal muscle plays important roles in whole-body glucose and fatty acid metabolism. However, muscle also secretes cytokines and growth factors (collectively termed myokines) that can potentially act in an autocrine, a paracrine, and/or an endocrine manner to modulate metabolic, inflammatory, and other processes. Here, we report the identification and characterization of myonectin, a novel myokine belonging to the C1q/TNF-related protein (CTRP) family. Myonectin transcript was highly induced in differentiated myotubes and predominantly expressed by skeletal muscle. Circulating levels of myonectin were tightly regulated by the metabolic state; fasting suppressed, but refeeding dramatically increased, its mRNA and serum levels. Although mRNA and circulating levels of myonectin were reduced in a diet-induced obese state, voluntary exercise increased its expression and circulating levels. Accordingly, myonectin transcript was up-regulated by compounds (forskolin, epinephrine, ionomycin) that raise cellular cAMP or calcium levels. In vitro, secreted myonectin forms disulfide-linked oligomers, and when co-expressed, forms heteromeric complexes with other members of the C1q/TNF-related protein family. In mice, recombinant myonectin administration reduced circulating levels of free fatty acids without altering adipose tissue lipolysis. Consistent with this, myonectin promoted fatty acid uptake in cultured adipocytes and hepatocytes, in part by up-regulating the expression of genes (CD36, FATP1, Fabp1, and Fabp4) that promote lipid uptake. Collectively, these results suggest that myonectin links skeletal muscle to lipid homeostasis in liver and adipose tissue in response to alterations in energy state, revealing a novel myonectin-mediated metabolic circuit.
Introduction
A large proportion of diet-derived glucose is taken up by skeletal muscle in response to insulin, and excess glucose is stored in muscle as glycogen until mobilized (1). In addition, muscle burns large amounts of fat via mitochondrial β-oxidation in response to energy demands (2). Insulin resistance in skeletal muscle has long been recognized to be an important underlying mechanism of type 2 diabetes (3). Although the importance of skeletal muscle in controlling whole-body glucose and lipid metabolism is well established, its role as an endocrine tissue that secretes biologically active polypeptide hormones and cytokines (collectively termed myokines) involved in modulating metabolic, inflammatory, and other physiological processes in nonmuscle tissues has only recently been appreciated (4).
Recent proteomics studies focusing on the secretome (the entire complement of secreted proteins) of cultured mouse or human myotubes have revealed a large number (∼250 in human and ∼600 in mouse) of muscle cell-derived secretory proteins with potential autocrine, paracrine, and/or endocrine functions (5, 6). IL-6 (4), FGF-21 (7, 8), insulin-like 6 (Insl6) (9), follistatin-like 1 (Fstl-1; also known as TSC-36) (10), leukemia inhibitory factor (LIF) (11), IL-7 (12), IL-15 (13), and musclin (14) are the currently characterized myokines. These myokines act locally in an autocrine/paracrine manner and/or as endocrine factors linking skeletal muscle to regulation of physiological processes in other tissues. IL-6 is the first myokine described and remains the best studied (15). Secreted by skeletal muscle fiber in response to exercise, IL-6 has been shown to improve whole-body insulin sensitivity and dampen inflammation, providing a link between exercise and the improvement in systemic metabolic parameters (15, 16). In mice, skeletal muscle-derived Fstl-1 promotes endothelial cell function and revascularization in ischemic tissue (10). Elevated serum levels of IL-15 via transgenic expression in mouse skeletal muscle reduce fat mass and decrease adiposity in response to high-fat feeding (13). These studies highlight the potential roles of myokines in mediating tissue cross-talk to control integrated physiology.
However, the expression of all myokines described to date is not restricted to skeletal muscle; they are generally expressed by a variety of cell types, and most are, in fact, expressed at much higher levels by nonmuscle tissues (17–19). Prior to this study, no myokine has been discovered to be preferentially expressed by skeletal muscle.
While characterizing the metabolic function of the CTRP5 family of proteins we recently uncovered (20–26), we identified myonectin (CTRP15) as a novel member of the family on the basis of sequence homology in the shared C1q domain, the signature that defines this protein family. Here, we provide the first molecular, biochemical, and functional characterization of myonectin and show that it is a novel nutrient-responsive myokine secreted by skeletal muscle to regulate whole-body fatty acid metabolism.
MATERIALS AND METHODS
Antibodies and Chemicals
Mouse monoclonal anti-FLAG M2 antibody was obtained from Sigma, and rat monoclonal anti-HA (clone 3F10) antibody was obtained from Roche Applied Science. AICAR (an AMP-activated protein kinase activator) (27) was obtained from Calbiochem; insulin, isoproterenol, ionomycin, and epinephrine were from Sigma; forskolin was from Cell Signaling Technology.
Animals
Eight-week-old male or female wild-type C57BL/6J mice were purchased from The Jackson Laboratory and were housed in polycarbonate cages on a 12-h light-dark photocycle with free access to water and standard laboratory chow diet (Lab Diet 5001, PMI Nutrition International, St. Louis, MO) throughout the study period. Separate cohorts of mice were fed a high-fat diet (60% kcal derived from fat; D12492) or an isocaloric matched low-fat diet (10% kcal derived from fat; D12450B) purchased from Research Diets Inc. (New Brunswick, NJ). High-fat diet was provided for a period of 12–14 weeks, starting at 4–5 weeks of age, to make mice diet-induced obese. Blood samples were collected for serum analysis. Tissues were collected, snap-frozen in liquid nitrogen, and kept at −80 °C. All animal protocols were approved by the Institutional Animal Care and Use Committee of Johns Hopkins University School of Medicine.
Cloning of Mouse Myonectin
A search for CTRP-like proteins in the National Center for Biotechnology Information (NCBI) GenBankTM databases identified a novel cDNA encoding a previously undescribed member of the CTRP family, which we designated as CTRP15/myonectin. Based on genomic DNA and EST sequences, a PCR approach was used to clone the entire coding region of mouse myonectin from a skeletal muscle cDNA library (Clontech). For mouse myonectin, the primer pair used was 5′-CAGCATGGCCTCGACCCGCCGCCCCGTCGGAG-3′ and 5′-CAGCTGCTGCAGGCTCTTACCCTTA-3′. The PCR product was agarose gel-purified and cloned into the pCR2.1 TOPO vector (Invitrogen). The entire cDNA insert was sequenced.
cDNA Constructs
C-terminal FLAG epitope (DYKDDDDK)-tagged mouse myonectin cDNA was generated by PCR, cloned into the mammalian expression vector (pCDNA3.1; Invitrogen), and verified by DNA sequencing. C-terminal HA epitope (YPYDVPDYA)-tagged mouse adiponectin and all other CTRPs used in this study were generated as described previously (20–23).
Generation of Myonectin-specific Antibody
Rabbit polyclonal anti-peptide antibody that can specifically recognize mouse myonectin (epitope 77KQSDKGINSKRRSKARR93) was generated (YenZym Antibodies, LLC) and tested against conditioned medium harvested from myonectin-transfected HEK 293T cells.
Protein Purification
Recombinant full-length mouse myonectin, containing a C-terminal FLAG-tagged epitope, was produced in mammalian cells as described previously (22). The presence of carbohydrate moieties and the formation of higher order oligomers necessitate that recombinant myonectin be produced in mammalian cells to ensure biologically active protein. Briefly, HEK 293T cells (GripTiteTM cells, Invitrogen) were cultured in DMEM containing 10% (v/v) bovine calf serum supplemented with antibiotics. Transfections were performed in HEK 293T cells using the calcium phosphate method (28). At 48 h after transfection, medium was replaced with serum-free Opti-MEM (Invitrogen) supplemented with vitamin C (0.1 mg/ml). Supernatants were collected three times, every 48 h, pooled, and purified using an anti-FLAG affinity gel according to the manufacturer's protocol (Sigma) and then eluted with 150 μg/ml FLAG peptide (Sigma). Purified proteins were dialyzed against 20 mm Hepes buffer (pH 8.0) containing 135 mm NaCl in a 10-kDa cut-off Slide-A-Lyzer dialysis cassette (Pierce). Protein concentration was determined using a Coomassie Plus protein assay reagent (Thermo Scientific), and samples were stored at −80 °C.
Recombinant Protein Injection
Experiments were carried out as described previously (22, 26). Briefly, food was removed in the morning (around 8–9 a.m.), 2 h prior to recombinant protein injection; drinking water was supplied for the duration of the experiment. Recombinant myonectin (5 μg/g of body weight) or the equivalent volume of vehicle buffer (20 mm Hepes (pH 8.0) containing 135 mm NaCl) was injected intraperitoneally into 10-week-old C57BL/6 mice (n = 6). Serum samples were harvested by tail bleeding at baseline (time 0) and every hour for 5 h after injection and separated using Microvette® CB 300 (Sarstedt). Glucose concentrations were also measured using a glucometer (BD Pharmingen) when tail blood was collected at the indicated time points.
Isolation of Skeletal Muscle
Mice were sacrificed, and soleus and plantaris muscles were immediately isolated and snap-frozen in liquid nitrogen. Homogenized muscle cell lysates were prepared in lysis buffer (T-PER, Thermo Scientific) containing protease and phosphatase inhibitor cocktails (Sigma). Protein content was quantified using Coomassie Plus protein reagent (Thermo Scientific).
Cell Culture
Mouse C2C12 myocytes and mouse 3T3-L1 preadipocytes were cultured and differentiated into myotubes and adipocytes, respectively, as described previously (23, 26). Rat H4IIE hepatocytes were also cultured as described previously (23). Differentiated cells were stimulated with insulin (100 nm), AICAR (1 mm), epinephrine (1 μm), ionomycin (1 μm), or forskolin (1 μm) for the indicated time, and total RNAs were isolated and subjected to quantitative real-time PCR analysis for myonectin expression.
Fatty Acid Uptake Assay
Cells were washed twice in PBS and placed in stimulation media (0.5% BSA for 3T3-L1 adipocytes and 0.1% BSA for H4IIE hepatocytes in high-glucose, fatty acid-free DMEM) at 37 °C and 5% CO2 in an incubator for 2 h. Next, media were changed to the same DMEM (with 0.5 and 0.1%, respectively, fatty acid-free BSA) containing vehicle control, recombinant myonectin (1, 2.5, 5, or 10 μg/ml), or insulin (50 nm) overnight. Cells were transferred to a 37 °C water bath where 1 μCi/well (in a 24-well format) of [3H]palmitate (dissolved previously for 1 h in the fatty acid-free BSA DMEM) was added for 0, 30, or 60 s. Media were then aspirated out, and cells were washed twice in cold PBS. Cells were lysed in 10% SDS and transferred to a scintillation vial. Radioactive counts were measured and normalized to protein concentration of final cell lysate.
Palmitate and Glucose Treatment
Differentiated mouse C2C12 myotubes were washed twice with PBS followed by the addition of 0.1% fatty acid-free BSA (Sigma) in serum- and glucose-free DMEM for 2 h. Next, the same solution was added with or without 25 mm glucose or 1 μm palmitic acid. The palmitic acid and fatty acid-free BSA mixture was made 1 h prior to addition to cells and kept at 37 °C to completely dissolve into solution. Total RNA was harvested from cells treated for 18 h.
Running Wheel-induced Exercise
C57BL/6 male mice were placed individually in a cage with a running wheel or a locked wheel (control) for a period of 2 weeks. They were given ad libitum food access. At the end of the 2-week period, mice were fasted overnight (12 h), and serum and skeletal muscle were harvested for analysis.
Intragastric Gavage
Mice were fasted for 12 h and gavaged with 10% glucose solution (10 μl/g of body weight) or 20% emulsified Intralipid (soybean oil; Sigma; 10 μl/g of body weight). Sera were collected before and after gavage for blood chemistry and Western blot analysis.
Serum and Blood Chemistry Analysis
Mouse serum samples were harvested by tail bleed and separated using a Microvette® CB 300 (Sarstedt). Glucose concentration was determined at the time of collection with a glucometer (BD Biosciences). Serum triglycerides (Thermo Fisher), nonesterified free fatty acid (NEFA) (Wako), and insulin (Millipore) were determined using commercially available kits.
Quantitative Real-time PCR Analysis
The tissue expression profile of myonectin was determined using mouse tissue cDNA panels (Clontech). Otherwise, total RNAs were isolated from tissues or cell lines using TRIzol® and reverse-transcribed using SuperScript II RNase H-reverse transcriptase (Invitrogen). Primers used in real-time PCR included the following: myonectin forward 5′-TGCTTGGATGCTGTTCGTCAA-3′ and reverse 5′-CAGATGGGATAAAGGGGCCTG-3′; CD36 forward 5′-ATGGGCTGTGATCGGAACTG-3′ and reverse 5′-AGCCAGGACTGCACCAATAAC-3′; FATP1 forward 5′-CTGGGACTTCCGTGGACCT-3′ and reverse 5′-TCTTGCAGACGATACGCAGAA-3′; caveolin-1 (Cav1) forward 5′-GGACATCTCTACACTGTTCCCA-3′ and reverse 5′-CGCGTCATACACTTGCTTCT-3′; Fabp1 forward 5′-ATGAACTTCTCCGGCAAGTACC-3′ and reverse 5′-GGTCCTCGGGCAGACCTAT-3′; Fabp4 forward 5′-ATCAGCGTAAATGGGGATTTGG-3′ and reverse 5′-GTCTGCGGTGATTTCATCGAA-3′. Quantitative real-time PCR analyses were performed on an Applied Biosystems Prism 7500 sequence detection system. Samples were analyzed in 25-μl reactions according to the standard protocol provided for the SYBR® Green PCR master mix (Applied Biosystems). All expression was normalized to 18 S rRNA in each sample.
Immunoblot Analysis
Serum samples were diluted 1:20 in SDS loading buffer (50 mm Tris-HCl, pH 7.4, 2% SDS w/v, 6% glycerol w/v, 1% 2-mercaptoethanol v/v, and 0.01% bromphenol blue w/v) and were separated on 10% Bis-Tris NuPAGE gel (Invitrogen). Each well was loaded with an equivalent of 1 μl of serum. For skeletal muscle lysates, 10 μg of protein were loaded and separated on a 10% Bis-Tris NuPAGE gel. Fractionated proteins were then transferred to Protran BA8 nitrocellulose membranes (Whatman), blocked in 2% nonfat milk for 1 h, and probed with primary antibodies in the presence of 2% nonfat milk overnight. Immunoblots were washed three times (10 min each) in PBS containing 0.1% Tween 20 and incubated with horseradish peroxidase-conjugated secondary antibody (Amersham Biosciences) (1:2000) for 1 h. Blots were washed three times (10 min each) in PBS containing 0.1% Tween 20, developed in ECL reagent (Millipore) for 2–5 min, and visualized with MultiImage III FluorChem® Q (Alpha Innotech). Quantifications of signal intensity were performed using Alphaview Software (Alpha Innotech).
Native Gel Electrophoresis
Nondenaturing, nonreducing gel electrophoresis was carried out as described previously (29).
Adipose Tissue and 3T3-L1 Adipocyte Lipolysis
Experiments were performed as described previously (30). Food was removed from mice 2 h prior to the isolation of epididymal fat pads. Fat pads were cut into 20-mg sections and placed in 500 μl (per piece) of DMEM (high-glucose) containing 0.5% fatty acid-free BSA, with or without 1 μm isoproterenol or purified myonectin (5 μg/ml). Tissue samples in media were kept in a 24-well cell culture dish at 37 °C and 5% CO2. Medium was collected at various time points, and NEFA content was measured using an absorption-based HR series NEFA kit (Wako Diagnostics). In the case of differentiated 3T3-L1 adipocytes, cells were plated in a 24-well culture dish. Prior to treatment, cells were washed once with PBS and placed in high-glucose DMEM containing 0.5% fatty acid-free BSA, with or without 1 μm isoproterenol or purified myonectin (5 μg/ml). Media were collected prior to and 1 h following treatment, and NEFA content was measured.
Statistical Analysis
Comparisons were performed using two-tailed Student's t tests. Values were considered to be significant at p < 0.05. All data are presented as mean ± S.E.
RESULTS
Identification of Myonectin
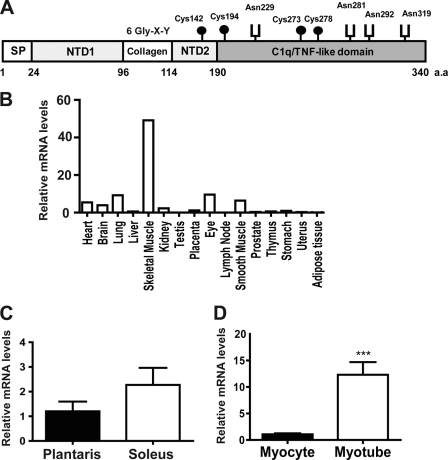
We cloned a previously undescribed member of the CTRP family from mouse skeletal muscle on the basis of sequence homology shared between their C1q domains (Fig. 1A). We designated this novel cDNA and its encoded protein CTRP15/myonectin. While current work was ongoing, Lim et al. (31) reported on a distantly related but distinct C1q family member, CTRP5 (C1qTNF5), in human and had inadvertently coined the term myonectin for CTRP5, although that term was not used in a previous CTRP5 study by the same authors (32). Given that mouse myonectin cDNA and protein sequences (HQ285249 and ADP00570, respectively) were deposited in the GenBank database and released to the public prior to the study by Lim et al. (31), we propose that CTRP5 retains its original designation (20, 33) and CTRP15 retains its current designation as myonectin to prevent confusion in nomenclature. The deduced mouse myonectin protein consists of five domains: a signal peptide for secretion, an N-terminal domain-1 (NTD1), a short collagen domain with six Gly-X-Y repeats, an N-terminal domain-2 (NTD2), and a C-terminal C1q/TNF-like domain. This protein consists of 340 amino acids and contains four Cys residues and four potential N-linked glycosylation sites that conform to the consensus sequence N-X-(Ser/Thr) (34). The 7.8-kb mouse myonectin gene is located on chromosome 1 (contig NC_000002.11) and contains eight exons.
FIGURE 1.
The deduced myonectin protein and its expression in skeletal muscle and cultured myotubes. A, the deduced domain structure of mouse myonectin. SP, signal peptide; NTD1, N-terminal domain-1; NTD2, N-terminal domain-2; a.a., amino acids. B, expression profile of myonectin in mouse tissues. C, expression of myonectin transcript in isolated plantaris and soleus muscle fiber type (n = 5 per group). D, expression of myonectin transcript in undifferentiated mouse C2C12 myocytes and differentiated myotubes (n = 8 per group). All quantitative real-time PCR data were normalized to 18 S rRNA and expressed as mean ± S.E. (***, p < 0.005).
Expression of Myonectin in Myotubes and Skeletal Muscle
In mice, myonectin transcript was predominantly expressed by skeletal muscle, with significantly lower expression in other tissues (Fig. 1B). Within skeletal muscle, soleus (a predominantly slow-twitch, oxidative muscle fiber type) tended to have higher expression of myonectin transcript as compared with plantaris (a predominantly fast-twitch, glycolytic muscle fiber type) (Fig. 1C). Consistent with preferential skeletal muscle expression, myonectin was greatly induced in differentiated mouse C2C12 myotubes as compared with undifferentiated myoblasts (Fig. 1D), suggesting that myonectin is produced by skeletal muscle fiber and not satellite cells. These data indicate that myonectin is a novel myokine.
Myonectin Forms Disulfide-linked Oligomers and Heteromeric Complexes with Other CTRPs
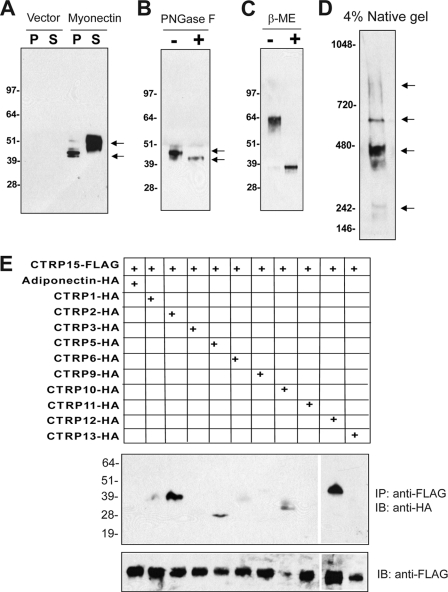
Consistent with the presence of a signal peptide, myonectin was robustly secreted into the conditioned medium when expressed in vitro (Fig. 2A). Secreted myonectin contains N-linked glycans; treatment with N-glycosidase F (an N-glycosidase) reduced the apparent molecular weight of myonectin on immunoblot (Fig. 2B), confirming that one or more of the conserved Asn residues are glycosylated. In the absence of reducing agent (β-mercaptoethanol), myonectin migrated as a dimer on immunoblot (Fig. 2C), indicating the presence of intermolecular disulfide bond. On a nonreducing, nondenaturing, native gel immunoblot, myonectin existed as a multimeric complex, revealing its higher-order oligomeric structure (Fig. 2D). Formation of oligomers is a biochemical feature shared by all CTRPs and proteins of the C1q family (21, 22, 35, 36). Further, heteromeric complex formation between different CTRPs has been previously demonstrated (21–23, 37), suggesting a potential mechanism to generate an expanded repertoire of functionally distinct complexes. Similarly, when co-expressed in mammalian cells (HEK 293T), myonectin formed heteromeric complexes with CTRP2 and CTRP12, and, to a lesser extent, with CTRP5 and CTRP10 (Fig. 2E).
FIGURE 2.
Myonectin is secreted as a multimeric protein that can form heteromeric complexes with other CTRP family members. A, immunoblot analysis of cell pellet (P) or supernatant (S) from transfected HEK 293T cells expressing pCDNA3.1 control vector or FLAG epitope-tagged myonectin. B and C, immunoblot analysis of myonectin subjected to N-glycosidase F (PNGase F) (B) or β-mercaptoethanol (β-ME) (C) treatment. D, native gel immunoblot analysis of myonectin. Arrows in panels A and B, indicate possible isoforms of myonectin resulting from differential glycosylation. Arrows in panel D indicate different oligomeric forms of myonectin. E, immunoprecipitation (IP) followed by immunoblot (IB) analysis of supernatants from HEK 293T cells expressing a combination of FLAG-tagged myonectin and HA-tagged adiponectin or CTRPs.
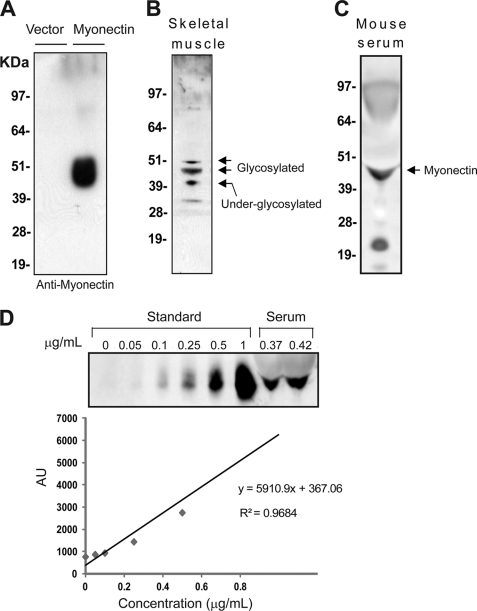
Myonectin Circulates in Blood
To examine the endogenous expression of myonectin and to address whether myonectin is a plasma protein and hence may function as an endocrine factor, we generated an anti-peptide antibody that can specifically recognize mouse myonectin secreted from the conditioned medium of transfected cells (Fig. 3A). In mouse skeletal muscle lysate, endogenous myonectin was detected in multiple isoforms, with apparent molecular masses between 40 and 50 kDa on immunoblot (Fig. 3B), likely reflecting different degrees of glycosylation (Fig. 2, A and B) due to the presence of multiple conserved Asn residues (Fig. 1A). Western blot analysis of mouse serum revealed the presence of myonectin (Fig. 3C), confirming that it is a secreted protein that circulates in blood. Using purified recombinant myonectin as a standard, serum concentration of myonectin in ad libitum wild-type 12-week-old male mice was determined to be ∼0.4 μg/ml (Fig. 3D).
FIGURE 3.
Myonectin is produced by skeletal muscle and circulates in plasma. A, immunoblot analysis of supernatant from HEK 293T cells expressing control vector or FLAG-tagged myonectin using a rabbit anti-myonectin antibody. B, immunoblot detection of myonectin in mouse skeletal muscle lysate. Arrows indicate possible isoforms of myonectin resulting from differential glycosylation. C, immunoblot detection of myonectin in mouse serum. D, estimation of serum concentration of myonectin in wild-type 12-week-old C57BL/6J male mice. Purified recombinant myonectin was used to construct a standard curve. AU, arbitrary units.
Regulation of Myonectin Expression in Myotubes
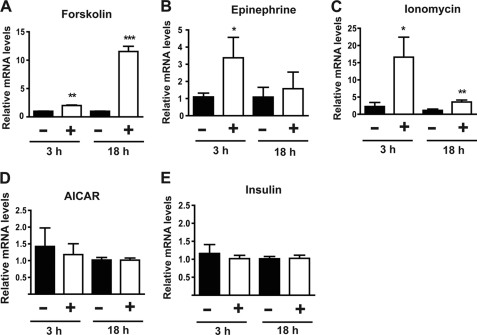
Expression of myonectin under different cellular conditions was examined to address whether factors that are known to regulate skeletal muscle physiology also affect myonectin expression. Raising intracellular levels of cAMP (by forskolin and epinephrine) or calcium (by ionomycin) substantially induced expression of myonectin transcript in mouse C2C12 myotubes (Fig. 4, A–C). In contrast, insulin and AICAR (an AMP-activated protein kinase activator) had no effect on myonectin expression in myotubes (Fig. 4, D and E).
FIGURE 4.
Myonectin in expression in myotubes is up-regulated by increase in cellular cAMP or calcium levels. A–E, quantitative real-time PCR analysis of myonectin expression in mouse C2C12 myotubes treated with vehicle control or 1 μm forskolin (A); 1 μm epinephrine (B); 1 μm ionomycin (C); 1 mm AICAR (D); or 100 nm insulin (E). n = 8 for each experiment. All expression data were normalized to 18 S rRNA. All data are presented as mean ± S.E. relative to vehicle control (*, p < 0.05; **, p < 0.01; ***, p < 0.005).
Exercise Increases Myonectin mRNA Expression and Circulating Levels
The in vitro results of myonectin expression in myotubes suggest that exercise-induced rises in intracellular calcium levels may also up-regulate myonectin expression in intact skeletal muscle. To test this, we subjected a cohort of mice to voluntary exercise for 2 weeks. In contrast, control mice had access to a locked wheel. An average of 86 ± 29 revolutions/day was recorded for mice with access to a running wheel. Exercise was confirmed by the expected reduction of fast-twitch muscle transcripts (troponin C2, myosin heavy chain polypeptide IIB (MHCIIB), and myosin heavy polypeptide 4 (MyoH4)) resulting from exercise-induced fiber type switch (supplemental Fig. S1) (38). In support of the in vitro data, myonectin expression was significantly induced in soleus and plantaris of mice given access to a running wheel for 2 weeks (Fig. 5A). Consistent with enhanced mRNA expression in skeletal muscle of mice subjected to voluntary exercise, circulating levels of myonectin also increased (Fig. 5B), suggesting a potential role of myonectin in exercise-induced physiology.
FIGURE 5.
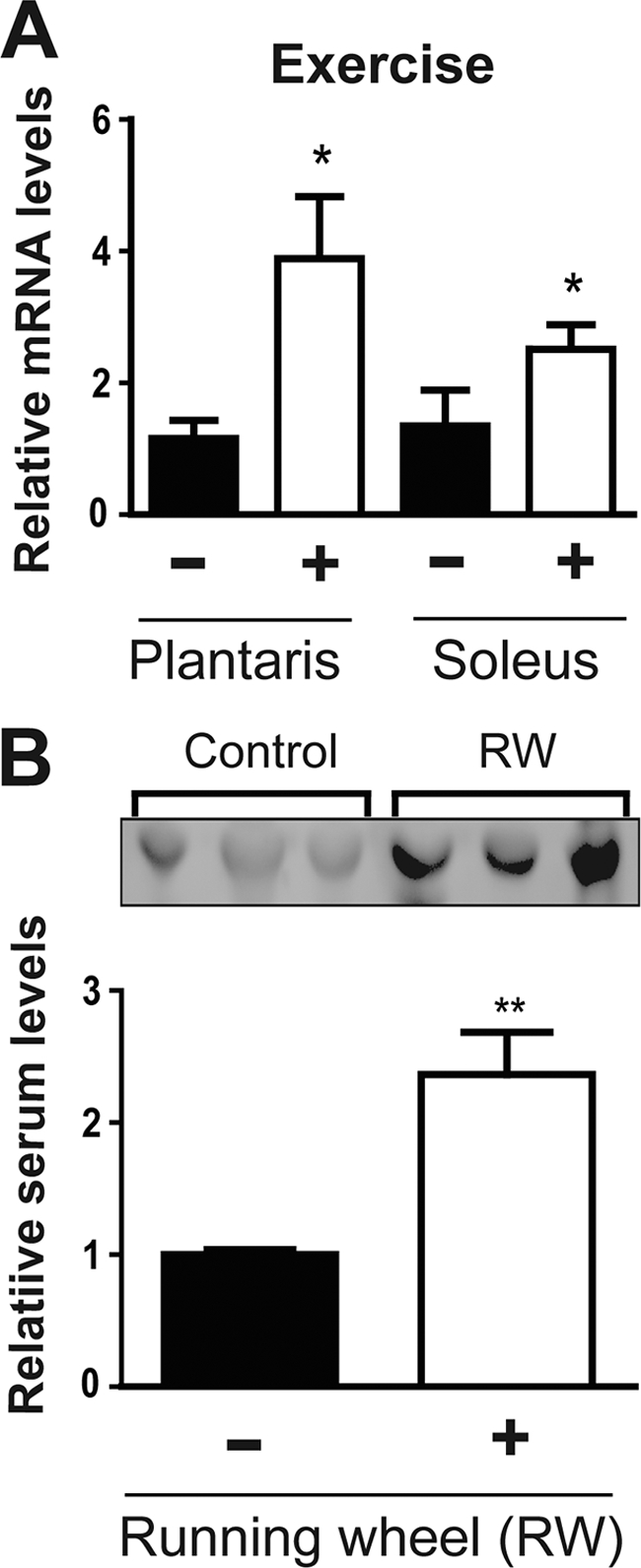
Exercise increases myonectin expression in skeletal muscle as well as circulating levels. A, quantitative real-time PCR analysis of myonectin expression in plantaris and soleus muscle from mice given access to a running wheel (RW) for 2 weeks or matched controls with access to locked wheel. All expression data were normalized to 18 S rRNA. B, immunoblot analysis of serum myonectin from the same cohort of mice. All data are presented as mean ± S.E. relative to control mice (n = 6 mice/group; *, p < 0.05; **, p < 0.01).
Metabolic State Regulates Myonectin Expression and Circulating Levels
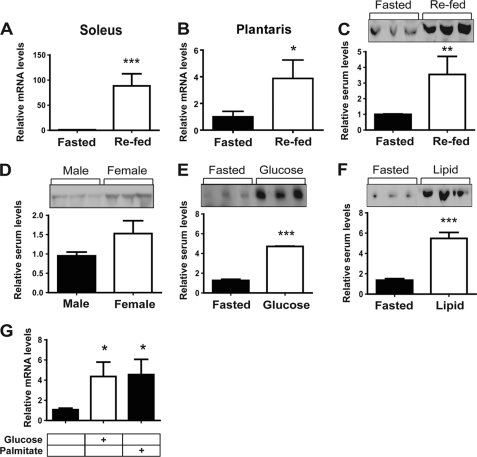
Given that exercise induces myonectin expression in skeletal muscle, we next addressed whether short- and long-term changes in nutritional/metabolic state also regulate myonectin expression and circulating levels. Surprisingly, an overnight fast greatly suppressed myonectin expression, but a 2-h refeeding period (following an overnight fast) dramatically up-regulated its mRNA expression in skeletal muscle (Fig. 6, A and B). Intriguingly, refeeding induced myonectin mRNA expression to a much greater extent in soleus than in plantaris muscle fiber of both male and female mice (data not shown), suggesting that myonectin expression may be regulated differentially depending on muscle fiber type. Consistent with the mRNA data, fasting reduced, but refeeding substantially increased, circulating levels of myonectin (Fig. 6C). Interestingly, fasted females also tended to have higher circulating levels of myonectin as compared with male mice (Fig. 6D), a trend that persisted upon refeeding (data not shown). In a separate cohort of mice, we determined whether the carbohydrate or lipid component within the diet was responsible for the induction of myonectin expression and secretion in the fasted/re-fed state. Overnight fasted male mice were gavaged with a bolus of glucose or emulsified Intralipid. Sera were collected from these animals 2 h after gavage and subjected to blood chemistry and Western blot analysis. As expected, blood glucose and serum triglycerides rose significantly in response to glucose and intralipid gavage, respectively (supplemental Fig. S2). It appeared that both glucose and lipid were equally potent at increasing circulating levels of myonectin in the re-fed state (Fig. 6, E and F). These data raised the possibility that the presence of nutrient (carbohydrate or lipid) in the gastrointestinal tract induced the secretion of a gut-derived hormone (e.g. incretins such as glucagon-like protein-1 (GLP-1) and glucose-induced insulinotropic polypeptide (GIP)) that, in turn, up-regulated myonectin expression and secretion from the skeletal muscle. To rule out this possibility, differentiated C2C12 myotubes cultured in serum-free media were starved of glucose and lipid and then stimulated with glucose or free fatty acid (palmitate). In the absence of any potential gut-derived hormones, both glucose and free fatty acid were able to acutely up-regulate myonectin expression in vitro (Fig. 6G). These data suggest that nutrient flux through muscle cells may directly regulate myonectin expression.
FIGURE 6.
Nutritional state regulates expression and circulating levels of myonectin. A–C, quantitative PCR analysis of myonectin expression in soleus (A) and plantaris muscle (B), as well as immunoblot quantification of serum levels (C) after a 12-h fast (Fasted) or fasted followed by 2 h of unrestricted food access (Re-fed) (n = 10 mice/group). All PCR data were normalized to 18 S rRNA. D, immunoblot quantification of serum myonectin levels in male and female mice subjected to a 12-h fast (n = 10 mice/group). E and F, immunoblot quantification of serum myonectin levels in mice subjected to a 12-h fast (Fasted) or fasted and gavaged with glucose (E) or emulsified intralipid (F) (n = 10 mice/group). G, quantitative PCR analysis of myonectin expression in C2C12 myotubes cultured in serum-free media containing no glucose/lipids (control) or treated with 25 mm glucose or 1 μm palmitate for 18 h (n = 8/group). All data are presented as mean ± S.E. relative to fasted mice or vehicle control (*, p < 0.05; **, p < 0.01; ***, p < 0.005).
Myonectin Expression and Circulating Levels Are Reduced in the Obese State
To test whether myonectin expression and circulating levels are responsive to long-term chronic alteration in whole-body energy balance, we examined its mRNA and serum levels in diet-induced obese mice. As compared with mice fed an isocaloric matched low-fat diet, mice fed a high-fat diet had lower myonectin mRNA and serum levels (Fig. 7), suggesting that obesity-induced alteration in energy balance may be linked to dysregulation of myonectin-mediated processes in the obese state.
FIGURE 7.
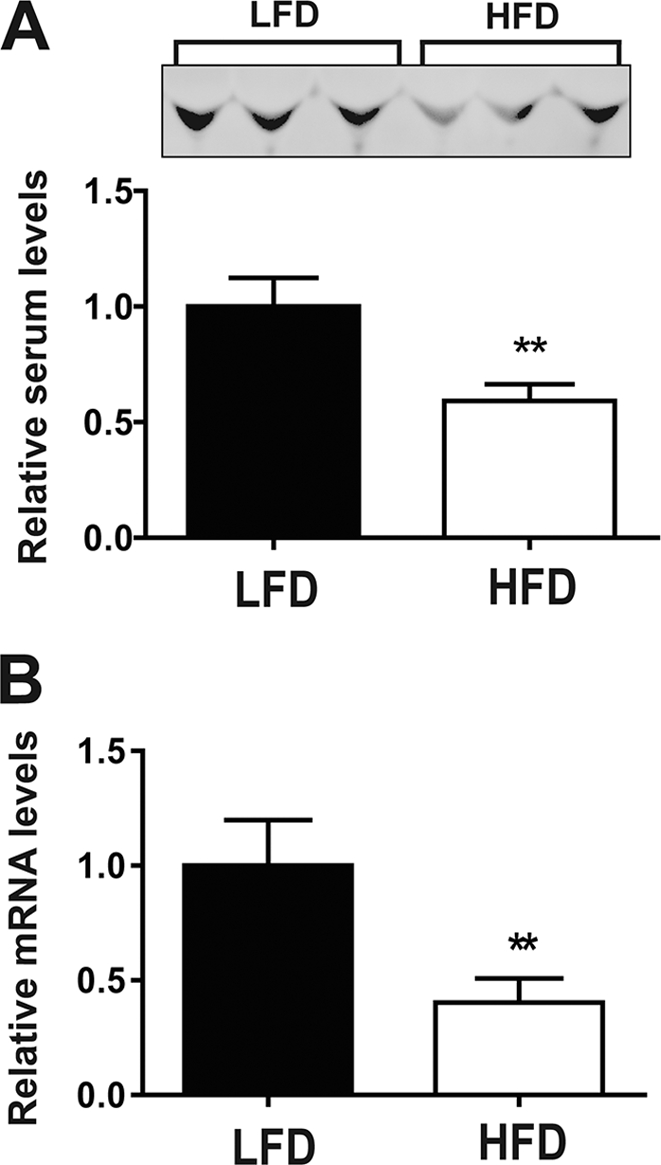
High-fat diet reduces myonectin expression and its circulating levels. A, immunoblot quantification of serum myonectin levels in mice fed a high-fat diet (HFD) or an isocaloric matched low-fat diet (LFD) for 12 weeks. B, quantitative PCR analysis of myonectin expression in calf muscle isolated from low-fat diet- or high-fat diet-fed male mice. All data are presented as mean ± S.E. relative to low-fat diet-fed mice (n = 8 mice/group; **, p < 0.01).
Recombinant Myonectin Administration Lowers Circulating Free Fatty Acids Levels
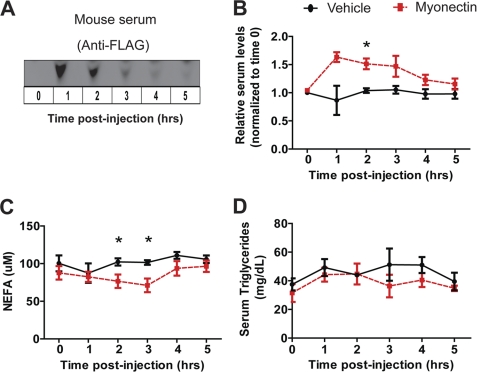
To address the possible metabolic function of myonectin in vivo, we administered purified recombinant myonectin to wild-type male mice. Due to multiple posttranslational modifications (glycosylation and oligomerization), recombinant myonectin was produced in mammalian cells to ensure production of biologically active protein. When injected intraperitoneally into mice at a dose of 5 μg/g of body weight, circulating levels of recombinant myonectin reached their maximum at 1 h and gradually declined over time (Fig. 8A). At the injected dose, we could raise serum levels of myonectin by 60–70% above endogenous steady-state levels (Fig. 8B). Elevating the circulating level of myonectin did not result in lowering of blood glucose over time as compared with mice injected with vehicle control (data not shown). In contrast, a relatively modest rise in serum myonectin levels was sufficient to lower (by ∼30%) NEFA levels over time relative to vehicle-injected controls (Fig. 8C). However, no significant difference was observed in serum triacylglycerol levels between the two groups of mice (Fig. 8D). These data suggest a potential role of myonectin in regulating systemic fatty acid metabolism.
FIGURE 8.
Recombinant myonectin administration reduces serum nonesterified free fatty acid levels in mice. A, immunoblot detection of FLAG epitope-tagged myonectin in mouse serum before and after recombinant protein injection. B, immunoblot quantification revealed an ∼60% elevation in serum myonectin levels above normal baseline levels after recombinant protein injection (n = 5 per group). C and D, male mice were injected intraperitoneally with vehicle or myonectin (5 μg/g of body weight), and sera were harvested every hour for 5 h following recombinant protein administration. Food was removed 2 h prior to protein injection. Serum nonesterified fatty acid (C) and triglyceride (D) levels were quantified (n = 5 mice/group). All data are presented as mean ± S.E. (*, p < 0.05).
Recombinant Myonectin Promotes Fatty Acid Uptake but Not Adipose Tissue Lipolysis
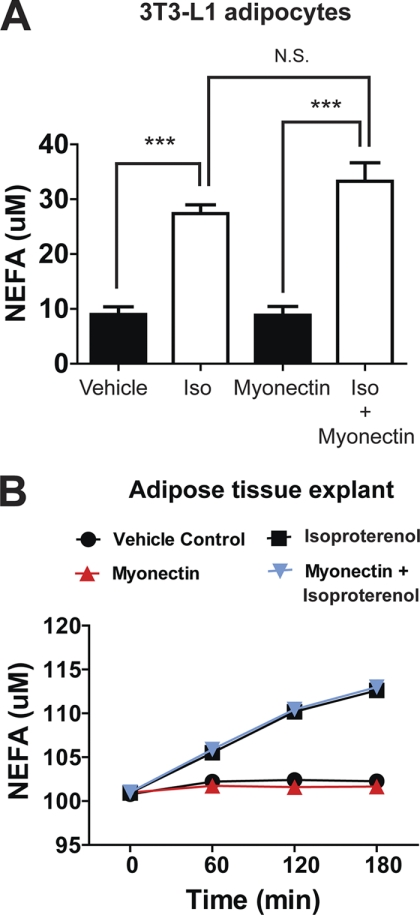
Myonectin can lower circulating NEFA levels via two possible mechanisms: suppressing adipose tissue lipolysis or promoting fatty acid uptake into cells. Treatment of 3T3-L1 adipocytes in vitro or primary adipose tissue (epididymal fat pads) ex vivo with recombinant myonectin had no effect on lipolysis (Fig. 9). Myonectin also had no effect in suppressing isoproterenol-induced lipolysis in cultured 3T3-L1 adipocytes or primary adipose tissue explants (Fig. 9). Thus, the suppression of adipose tissue lipolysis is likely not the mechanism by which myonectin lowers serum NEFA levels in mice.
FIGURE 9.
Recombinant myonectin has no effect on adipocytes or adipose tissue lipolysis. A, NEFA concentration in media of 3T3-L1 adipocytes treated for 1 h with vehicle, isoproterenol (1 μm), myonectin (5 μg/ml), or a combination of myonectin and isoproterenol (Iso, n = 12 per group). N.S., not significant. All data are presented as mean ± S.E. relative to vehicle control (***, p < 0.005). B, time course of NEFA release into conditioned media of adipose tissue (epididymal fat pad) explants treated with vehicle, isoproterenol (1 μm), myonectin (5 μg/ml), or a combination of myonectin and isoproterenol (n = 6 per group).
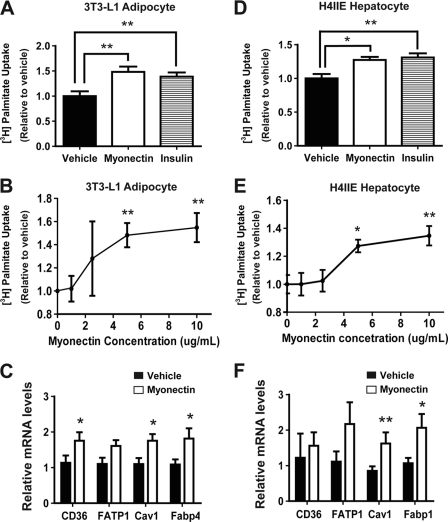
Next, we investigated whether myonectin promotes fatty acid uptake in differentiated mouse 3T3-L1 adipocytes. As expected, treatment of adipocytes with a saturating dose of insulin (50 nm) led to a maximum 50% increase in fatty acid uptake (Fig. 10A). Similarly, treatment of adipocytes with recombinant myonectin (5 μg/ml) also enhanced fatty acid uptake to the same extent as insulin. A dose-response curve indicated that maximum fatty acid uptake in adipocytes can be achieved with 5 μg/ml myonectin (Fig. 10B). Several proteins, such as CD36, FATP1, Cav1, and Fabp4, are known to play important roles in fatty acid uptake into cells (39–41). Quantitative real-time PCR analysis showed that expression of these genes was up-regulated in adipocytes by recombinant myonectin (Fig. 10C). To determine whether myonectin-mediated enhancement of lipid uptake is specific to adipocytes, we also tested the effect of myonectin on lipid uptake in rat H4IIE hepatocytes. We observed a modest (∼25%) but consistent increase in fatty acid uptake into hepatocytes stimulated with myonectin (5 μg/ml), an effect similar to cells treated with a saturating dose of insulin (50 nm) (Fig. 10D). A dose-response curve indicated that maximum fatty acid uptake in hepatocytes can also be achieved with 5 μg/ml myonectin (Fig. 10E). Enhanced lipid uptake is coupled to increased expression of Cav1 and Fabp1 in H4IIE hepatocytes (Fig. 10F). Together, these results indicate that myonectin promotes lipid uptake into adipocytes and hepatocytes via transcriptional up-regulation of genes involved in fatty acid uptake.
FIGURE 10.
Myonectin enhances fatty acid uptake in 3T3-L1 adipocytes and H4IIE hepatocytes via transcriptional mechanism. A and D, mouse 3T3-L1 adipocytes (A) or rat H4IIE hepatocytes (D) were treated overnight with vehicle buffer, recombinant myonectin (5 μg/ml), or insulin (50 nm) and subjected to [3H]palmitate uptake assay for 10, 30, or 60 s (n = 8/group). Data represent cumulative uptake over 60 s. B and E, dose-response curves of [3H]palmitate uptake in 3T3-L1 adipocytes (B) and H4IIE hepatocytes (E) stimulated with various concentrations of myonectin. C and F, quantitative real-time PCR analysis of CD36, FATP1, Cav1, and FABP4 or FABP1 expression in adipocytes (C) or hepatocytes (F) treated with vehicle buffer or myonectin (5 μg/ml) for 12 h (n = 8/group). All expression data were normalized to 18 S rRNA. All data are presented as mean ± S.E. relative to vehicle control (*, p < 0.05; **, p < 0.01).
DISCUSSION
We provide the first characterization of myonectin, with in vitro and in vivo evidence that it is a novel myokine with important metabolic function. Unlike the other CTRPs characterized to date (21–23), myonectin (CTRP15) is expressed and secreted predominantly by skeletal muscle. Although some CTRPs function as adipokines linking adipose tissue to regulation of systemic insulin sensitivity and energy metabolism (24, 25, 26), myonectin functions as a myokine that mediates cross-talk between skeletal muscle and other metabolic compartments (e.g. adipose tissue and liver) to coordinate the integration of whole-body metabolism. Consistent with this notion, the expression and secretion of myonectin by skeletal muscle are highly responsive to acute nutritional and metabolic changes (e.g. fast/re-fed cycle and exercise), as well as chronic alteration in the energy state of the animals (e.g. diet-induced obesity).
A rise in intracellular cAMP or calcium levels due to muscle contraction or pharmacologic agents (forskolin, epinephrine, or ionomycin) increases expression of myonectin. In contrast, insulin and AICAR (an AMP-activated protein kinase activator) have no apparent effect on the transcription of myonectin in myotubes. Intriguingly, in starved myotubes cultured in serum-free medium, the presence of glucose or free fatty acid is sufficient to induce the expression of myonectin transcript (4-fold), analogous to overnight-fasted mice gavaged with a bolus of glucose or lipid (Fig. 6, E and F). The observed in vitro results implicate a direct effect of glucose and/or fatty acids in inducing myonectin expression in myotubes, rather than through an indirect effect of incretin hormones (e.g. GLP-1 and GIP) secreted in response to the presence of nutrients in the gastrointestinal tract. These results suggest that myonectin is a nutrient-responsive metabolic regulator secreted by skeletal muscle in response to changes in cellular energy state resulting from glucose or fatty acid fluxes.
Many metabolically relevant secreted proteins (e.g. adiponectin, leptin, resistin, and RBP4) and the signaling pathways they regulate in tissues are known to be dysregulated in the condition of obesity (42–45). The reduction in expression and circulating levels of myonectin in the obese state may represent yet another component of the complex metabolic circuitry dysregulated by excess caloric intake. Although exercise has long been known to have profound positive impacts on systemic insulin sensitivity and energy balance, the underlying mechanisms remain incompletely understood. That voluntary exercise dramatically increases the expression and circulating levels of myonectin to promote fatty acid uptake into cells may underlie one of the beneficial effects of physical exercise.
A modest rise in the circulating levels of myonectin resulting from recombinant protein administration is sufficient to lower serum NEFA without altering serum triglyceride levels. Unlike CTRP1 (22), CTRP3 (26) and CTRP12 (24), injection of recombinant myonectin into mice appears to have no glucose-lowering effect. Reduction in circulating NEFA is not due to suppression of adipose tissue lipolysis; rather, it results from increased fatty acid uptake by adipocytes and hepatocytes. Although the myonectin-mediated enhancement of lipid uptake in vitro appears modest (25–50%), in fact, the magnitude of this effect is comparable with cells stimulated with 50 nm insulin, a saturating dose that leads to maximum increase in fatty acid uptake. Even in primary muscle cells that overexpress molecules that promote fatty acid uptake (e.g. CD36, FATP1, and FATP4), only a 20–60% increase in lipid uptake is observed as compared with control cells (46). Stimulation of adipocytes with a combination of insulin and myonectin does not result in further increases in fatty acid uptake (data not shown), suggesting that myonectin and insulin likely activate similar pathway(s) in cells to enhance lipid uptake. In support, recombinant myonectin treatment up-regulates the expression of CD36, Cav1, and Fabp1/Fapb4 in adipocytes and hepatocytes, similar to insulin (data not shown), to mediate its effect on fatty acid uptake. In accordance with myonectin mediating its metabolic effect through a transcriptional mechanism, a reduction in circulating NEFA in mice occurred only 2 h after recombinant protein injection, a lag period presumably required for mRNA and protein synthesis.
In summary, myonectin is a novel multimeric protein of the C1q family that is synthesized and secreted by skeletal muscle, with its expression subjected to complex metabolic regulation. Circulating myonectin functions as a myokine linking skeletal muscle to lipid metabolism in liver and adipose tissue, providing insights into tissue cross-talk that underlies the integrated control of whole-body metabolism.
Supplementary Material
Acknowledgments
We thank the Paul A. Watkins laboratory for help with the fatty acid uptake assay and Wan Fang Han for help with food gavage in mice. We also thank Jessica M. Ellis for help with lipolysis assay using primary adipose tissue explants.
This work was supported, in whole or in part, by National Institutes of Health Grant DK084171 (to G. W. W.). This work was also supported in part by American Heart Association Grant SDG2260721 (to G. W. W.).

This article contains supplemental Figs. S1 and S2.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBankTM/EBI Data Bank with accession number(s) HQ285249.
- CTRP
- C1q-TNF-related protein
- AICAR
- aminoimidazole carboxamide ribonucleotide
- FATP1
- fatty acid transport protein-1
- FATP4
- fatty acid transport protein-4
- Cav1
- caveolin-1
- FABP1
- hepatocyte-specific fatty acid binding protein-1
- FABP4
- adipocyte-specific fatty acid binding protein-4
- MyoH4
- myosin heavy polypeptide 4
- NEFA
- nonesterified free fatty acid
- contig
- group of overlapping clones
- Bis-Tris
- 2-(bis(2-hydroxyethyl)amino)-2-(hydroxymethyl)propane-1,3-diol.
REFERENCES
- 1. Wasserman D. H., Ayala J. E. (2005) Interaction of physiological mechanisms in control of muscle glucose uptake. Clin. Exp. Pharmacol. Physiol. 32, 319–323 [DOI] [PubMed] [Google Scholar]
- 2. Rasmussen B. B., Wolfe R. R. (1999) Regulation of fatty acid oxidation in skeletal muscle. Annu. Rev. Nutr. 19, 463–484 [DOI] [PubMed] [Google Scholar]
- 3. Petersen K. F., Shulman G. I. (2002) Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am. J. Cardiol. 90, 11G–18G [DOI] [PubMed] [Google Scholar]
- 4. Pedersen B. K. (2009) Edward F. Adolph distinguished lecture: muscle as an endocrine organ: IL-6 and other myokines. J. Appl. Physiol. 107, 1006–1014 [DOI] [PubMed] [Google Scholar]
- 5. Henningsen J., Rigbolt K. T., Blagoev B., Pedersen B. K., Kratchmarova I. (2010) Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol. Cell. Proteomics 9, 2482–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Norheim F., Raastad T., Thiede B., Rustan A. C., Drevon C. A., Haugen F. (2011) Proteomic identification of secreted proteins from human skeletal muscle cells and expression in response to strength training. Am. J. Physiol. Endocrinol. Metab. 301, E1013–E1021 [DOI] [PubMed] [Google Scholar]
- 7. Hojman P., Pedersen M., Nielsen A. R., Krogh-Madsen R., Yfanti C., Akerstrom T., Nielsen S., Pedersen B. K. (2009) Fibroblast growth factor-21 is induced in human skeletal muscles by hyperinsulinemia. Diabetes 58, 2797–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Izumiya Y., Bina H. A., Ouchi N., Akasaki Y., Kharitonenkov A., Walsh K. (2008) FGF21 is an Akt-regulated myokine. FEBS Lett. 582, 3805–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zeng L., Akasaki Y., Sato K., Ouchi N., Izumiya Y., Walsh K. (2010) Insulin-like 6 is induced by muscle injury and functions as a regenerative factor. J. Biol. Chem. 285, 36060–36069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ouchi N., Oshima Y., Ohashi K., Higuchi A., Ikegami C., Izumiya Y., Walsh K. (2008) Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J. Biol. Chem. 283, 32802–32811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Broholm C., Laye M. J., Brandt C., Vadalasetty R., Pilegaard H., Pedersen B. K., Scheele C. (2011) LIF is a contraction-induced myokine stimulating human myocyte proliferation. J. Appl. Physiol. 111, 251–259 [DOI] [PubMed] [Google Scholar]
- 12. Haugen F., Norheim F., Lian H., Wensaas A. J., Dueland S., Berg O., Funderud A., Skålhegg B. S., Raastad T., Drevon C. A. (2010) IL-7 is expressed and secreted by human skeletal muscle cells. Am. J. Physiol. Cell Physiol. 298, C807–C816 [DOI] [PubMed] [Google Scholar]
- 13. Quinn L. S., Anderson B. G., Strait-Bodey L., Stroud A. M., Argilés J. M. (2009) Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am. J. Physiol. Endocrinol. Metab. 296, E191–E202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nishizawa H., Matsuda M., Yamada Y., Kawai K., Suzuki E., Makishima M., Kitamura T., Shimomura I. (2004) Musclin, a novel skeletal muscle-derived secretory factor. J. Biol. Chem. 279, 19391–19395 [DOI] [PubMed] [Google Scholar]
- 15. Pedersen B. K., Febbraio M. A. (2008) Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol. Rev. 88, 1379–1406 [DOI] [PubMed] [Google Scholar]
- 16. Steensberg A., Toft A. D., Schjerling P., Halkjaer-Kristensen J., Pedersen B. K. (2001) Plasma interleukin-6 during strenuous exercise: role of epinephrine. Am. J. Physiol. Cell Physiol. 281, C1001–C1004 [DOI] [PubMed] [Google Scholar]
- 17. Kharitonenkov A., Shiyanova T. L., Koester A., Ford A. M., Micanovic R., Galbreath E. J., Sandusky G. E., Hammond L. J., Moyers J. S., Owens R. A., Gromada J., Brozinick J. T., Hawkins E. D., Wroblewski V. J., Li D. S., Mehrbod F., Jaskunas S. R., Shanafelt A. B. (2005) FGF-21 as a novel metabolic regulator. J. Clin. Invest. 115, 1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lok S., Johnston D. S., Conklin D., Lofton-Day C. E., Adams R. L., Jelmberg A. C., Whitmore T. E., Schrader S., Griswold M. D., Jaspers S. R. (2000) Identification of INSL6, a new member of the insulin family that is expressed in the testis of the human and rat. Biol. Reprod. 62, 1593–1599 [DOI] [PubMed] [Google Scholar]
- 19. Mashimo J., Maniwa R., Sugino H., Nose K. (1997) Decrease in the expression of a novel TGF β1-inducible and ras-recision gene, TSC-36, in human cancer cells. Cancer Lett. 113, 213–219 [DOI] [PubMed] [Google Scholar]
- 20. Wong G. W., Wang J., Hug C., Tsao T. S., Lodish H. F. (2004) A family of Acrp30/adiponectin structural and functional paralogs. Proc. Natl. Acad. Sci. U.S.A. 101, 10302–10307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wong G. W., Krawczyk S. A., Kitidis-Mitrokostas C., Ge G., Spooner E., Hug C., Gimeno R., Lodish H. F. (2009) Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J. 23, 241–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wong G. W., Krawczyk S. A., Kitidis-Mitrokostas C., Revett T., Gimeno R., Lodish H. F. (2008) Molecular, biochemical, and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-γ agonist, cysteine-mediated oligomerizations, combinatorial associations, and metabolic functions. Biochem. J. 416, 161–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wei Z., Peterson J. M., Wong G. W. (2011) Metabolic regulation by C1q/TNF-related protein-13 (CTRP13): activation OF AMP-activated protein kinase and suppression of fatty acid-induced JNK signaling. J. Biol. Chem. 286, 15652–15665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wei Z., Peterson J. M., Lei X., Cebotaru L., Wolfgang M. J., Baldeviano G. C., Wong G. W. (January 14, 2012) C1q/TNF-related protein-12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. J. Biol. Chem. 10.1074/jbc.M111.303651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peterson J. M., Aja S., Wei Z., Wong G. W. (2012) CTRP1 protein enhances fatty acid oxidation via AMP-activated protein kinase (AMPK) activation and acetyl-CoA carboxylase (ACC) inhibition. J. Biol. Chem. 287, 1576–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peterson J. M., Wei Z., Wong G. W. (2010) C1q/TNF-related protein-3 (CTRP3), a novel adipokine that regulates hepatic glucose output. J. Biol. Chem. 285, 39691–39701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sullivan J. E., Brocklehurst K. J., Marley A. E., Carey F., Carling D., Beri R. K. (1994) Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 353, 33–36 [DOI] [PubMed] [Google Scholar]
- 28. Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y., Axel R. (1977) Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell 11, 223–232 [DOI] [PubMed] [Google Scholar]
- 29. Deans M. R., Peterson J. M., Wong G. W. (2010) Mammalian otolin: a multimeric glycoprotein specific to the inner ear that interacts with otoconial matrix protein otoconin-90 and cerebellin-1. PLoS One 5, e12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang H., Bell M., Sreenevasan U., Hu H., Liu J., Dalen K., Londos C., Yamaguchi T., Rizzo M. A., Coleman R., Gong D., Brasaemle D., Sztalryd C. (2011) Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J. Biol. Chem. 286, 15707–15715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lim S., Choi S. H., Koo B. K., Kang S. M., Yoon J. W., Jang H. C., Choi S. M., Lee M. G., Lee W., Shin H., Kim Y. B., Lee H. K., Park K. S. (2012) Effects of aerobic exercise training on C1q tumor necrosis factor α-related protein isoform 5 (myonectin): association with insulin resistance and mitochondrial DNA density in women. J. Clin. Endocrinol. Metab. 97, E88–E93 [DOI] [PubMed] [Google Scholar]
- 32. Park S. Y., Choi J. H., Ryu H. S., Pak Y. K., Park K. S., Lee H. K., Lee W. (2009) C1q tumor necrosis factor α-related protein isoform 5 is increased in mitochondrial DNA-depleted myocytes and activates AMP-activated protein kinase. J. Biol. Chem. 284, 27780–27789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hayward C., Shu X., Cideciyan A. V., Lennon A., Barran P., Zareparsi S., Sawyer L., Hendry G., Dhillon B., Milam A. H., Luthert P. J., Swaroop A., Hastie N. D., Jacobson S. G., Wright A. F. (2003) Mutation in a short-chain collagen gene, CTRP5, results in extracellular deposit formation in late-onset retinal degeneration: a genetic model for age-related macular degeneration. Hum. Mol. Genet. 12, 2657–2667 [DOI] [PubMed] [Google Scholar]
- 34. Yan A., Lennarz W. J. (2005) Unraveling the mechanism of protein N-glycosylation. J. Biol. Chem. 280, 3121–3124 [DOI] [PubMed] [Google Scholar]
- 35. Kishore U., Gaboriaud C., Waters P., Shrive A. K., Greenhough T. J., Reid K. B., Sim R. B., Arlaud G. J. (2004) C1q and tumor necrosis factor superfamily: modularity and versatility. Trends Immunol. 25, 551–561 [DOI] [PubMed] [Google Scholar]
- 36. Briggs D. B., Jones C. M., Mashalidis E. H., Nuñez M., Hausrath A. C., Wysocki V. H., Tsao T. S. (2009) Disulfide-dependent self-assembly of adiponectin octadecamers from trimers and presence of stable octadecameric adiponectin lacking disulfide bonds in vitro. Biochemistry 48, 12345–12357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peterson J. M., Wei Z., Wong G. W. (2009) CTRP8 and CTRP9B are novel proteins that hetero-oligomerize with C1q/TNF family members. Biochem. Biophys. Res. Commun. 388, 360–365 [DOI] [PubMed] [Google Scholar]
- 38. Rasbach K. A., Gupta R. K., Ruas J. L., Wu J., Naseri E., Estall J. L., Spiegelman B. M. (2010) PGC-1α regulates a HIF2α-dependent switch in skeletal muscle fiber types. Proc. Natl. Acad. Sci. U.S.A. 107, 21866–21871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Coburn C. T., Knapp F. F., Jr., Febbraio M., Beets A. L., Silverstein R. L., Abumrad N. A. (2000) Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J. Biol. Chem. 275, 32523–32529 [DOI] [PubMed] [Google Scholar]
- 40. Wu Q., Ortegon A. M., Tsang B., Doege H., Feingold K. R., Stahl A. (2006) FATP1 is an insulin-sensitive fatty acid transporter involved in diet-induced obesity. Mol. Cell. Biol. 26, 3455–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ring A., Le Lay S., Pohl J., Verkade P., Stremmel W. (2006) Caveolin-1 is required for fatty acid translocase (FAT/CD36) localization and function at the plasma membrane of mouse embryonic fibroblasts. Biochim. Biophys. Acta 1761, 416–423 [DOI] [PubMed] [Google Scholar]
- 42. Kadowaki T., Yamauchi T., Kubota N., Hara K., Ueki K., Tobe K. (2006) Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 116, 1784–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Friedman J. M., Halaas J. L. (1998) Leptin and the regulation of body weight in mammals. Nature 395, 763–770 [DOI] [PubMed] [Google Scholar]
- 44. Steppan C. M., Bailey S. T., Bhat S., Brown E. J., Banerjee R. R., Wright C. M., Patel H. R., Ahima R. S., Lazar M. A. (2001) The hormone resistin links obesity to diabetes. Nature 409, 307–312 [DOI] [PubMed] [Google Scholar]
- 45. Yang Q., Graham T. E., Mody N., Preitner F., Peroni O. D., Zabolotny J. M., Kotani K., Quadro L., Kahn B. B. (2005) Serum retinol-binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436, 356–362 [DOI] [PubMed] [Google Scholar]
- 46. Nickerson J. G., Alkhateeb H., Benton C. R., Lally J., Nickerson J., Han X. X., Wilson M. H., Jain S. S., Snook L. A., Glatz J. F., Chabowski A., Luiken J. J., Bonen A. (2009) Greater transport efficiencies of the membrane fatty acid transporters FAT/CD36 and FATP4 as compared with FABPpm and FATP1 and differential effects on fatty acid esterification and oxidation in rat skeletal muscle. J. Biol. Chem. 284, 16522–16530 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.