Background: UV-tolerant rice strains exhibit higher photolyase DNA repair of UV-induced cyclobutane pyrimidine dimers (CPDs).
Results: The first eukaryotic CPD photolyase structure reveals differences in active-site, flavin hydrogen-bonding, and electron transfer and allows mapping of UV-resistance polymorphisms.
Conclusion: Critical functional features are conserved by convergent evolution.
Significance: This structure provides a paradigm for light-dependent DNA repair in higher organisms and development of UV-resistant plants.
Keywords: DNA Repair, Flavoproteins, Photoreceptors, Plant, X-ray Crystallography
Abstract
Ozone depletion increases terrestrial solar ultraviolet B (UV-B; 280–315 nm) radiation, intensifying the risks plants face from DNA damage, especially covalent cyclobutane pyrimidine dimers (CPD). Without efficient repair, UV-B destroys genetic integrity, but plant breeding creates rice cultivars with more robust photolyase (PHR) DNA repair activity as an environmental adaptation. So improved strains of Oryza sativa (rice), the staple food for Asia, have expanded rice cultivation worldwide. Efficient light-driven PHR enzymes restore normal pyrimidines to UV-damaged DNA by using blue light via flavin adenine dinucleotide to break pyrimidine dimers. Eukaryotes duplicated the photolyase gene, producing PHRs that gained functions and adopted activities that are distinct from those of prokaryotic PHRs yet are incompletely understood. Many multicellular organisms have two types of PHR: (6-4) PHR, which structurally resembles bacterial CPD PHRs but recognizes different substrates, and Class II CPD PHR, which is remarkably dissimilar in sequence from bacterial PHRs despite their common substrate. To understand the enigmatic DNA repair mechanisms of PHRs in eukaryotic cells, we determined the first crystal structure of a eukaryotic Class II CPD PHR from the rice cultivar Sasanishiki. Our 1.7 Å resolution PHR structure reveals structure-activity relationships in Class II PHRs and tuning for enhanced UV tolerance in plants. Structural comparisons with prokaryotic Class I CPD PHRs identified differences in the binding site for UV-damaged DNA substrate. Convergent evolution of both flavin hydrogen bonding and a Trp electron transfer pathway establish these as critical functional features for PHRs. These results provide a paradigm for light-dependent DNA repair in higher organisms.
Introduction
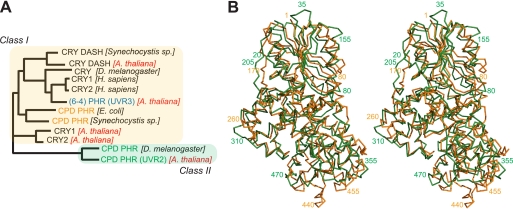
Sunlight ultraviolet (UV) components trigger damaging chemical reactions in DNA that must be repaired for cell survival. To maintain genetic integrity, diverse DNA repair systems have evolved, ranging from UvrA, -B, and -C in Escherichia coli, to XPA through XPG in mammalian nucleotide excision repair (1). Key diversity occurs even within the standalone photolyase (PHR) DNA repair enzymes, which are critical for repair of UV damage in plants and bacteria. PHRs use flavin adenine dinucleotide (FAD) to catalyze the unique light-dependent restoration of normal bases from the two major photoproducts in UV-damaged DNA: cyclobutane pyrimidine dimer (CPD)4 and (6-4) photoproduct. Three distinct, but homologous, PHR families have been identified (see Fig. 1A): (6-4) PHR in eukaryotes and CPD PHRs, divided into Class I in single-cell organisms and Class II in multicellular organisms (2, 3). Although Class II CPD PHRs (CPD2PHRs) from eukaryotes can functionally complement bacterial Class I CPD PHRs, the two enzyme classes differ significantly in sequence (4). Remarkably, despite their different substrates, Class I CPD and (6-4) PHRs show much higher sequence similarities with each other and with their cryptochrome (CRY) homologs than with their Class II counterparts (see Fig. 1A), suggesting that structural information for CPD2PHRs would be of great value.
FIGURE 1.
The PHR/CRY family consists of two major classes. A, shown is the unrooted phylogenetic tree of 12 representative members of the PHR/CRY protein family. CPD2PHR is the outlier in the family. B, overlaid structures of Class I and II CPD PHRs revealed diversity in the catalytic C-terminal domain.
For DNA base excision repair, combined structural and mutational analyses have provided key insights into mechanisms and biological activities leading to recognition of conserved and variable modes of damage recognition and a unified understanding of many base repair processes (5, 6). Similarly, recent structures and mutational analyses have provided insights into links between base excision repair and nucleotide excision repair and the impacts of nucleotide repair processes on cell biology (7–9). Although less is understood about structure/function relationships in PHRs than in base excision repair or nucleotide excision repair enzymes, crystallographic structures of (6-4) (10, 11) and bacterial CPD PHRs (12, 13) have been determined and analyzed with respect to their damage recognition and repair mechanisms (14, 15); understanding of CPD2PHRs, however, has been hampered by the absence of a structure.
To date structures of blue light and photo-induced DNA damage responses for plants and bacteria have led to improved understandings of cell biology including the discovery of PAS domains and motifs acting in DNA repair PHRs and clock CRYs (11, 16, 17). Plants, being unable to escape photo damage by movement, have evolved elegant systems to both benefit and protect themselves from sunlight. Indeed, both CPD and (6-4) PHRs are essential for plant growth, as shown in Arabidopsis thaliana (mustard cress) and the crop plant Oryza sativa (rice) (18–20). Among isolated Arabidopsis mutants exhibiting decreased UV resistance (UVR), UVR2 and UVR3 genes were, respectively, identified as CPD and (6-4) PHR (18, 21, 22). Studies in rice confirmed these fundamental assignments and identified polymorphisms among rice strains selected for UV tolerance (20, 23, 24), supporting the value of an improved knowledge of CPD2PHRs for understanding plant cell biology.
Here, we report the determination and analysis of the crystal structure of Class II CPD photolyase (a UVR2 homolog) from O. sativa (Sasanishiki cultivar) at 1.7 Å resolution. This structure and analysis reveals new mechanistic information about how rice strains tune light usage by CPD2PHR to gain UV tolerance. Furthermore, this first structure of a eukaryotic CPD2PHR together with (6-4) PHR structures, provides a foundation for understanding UV resistance mechanisms in higher organisms.
EXPERIMENTAL PROCEDURES
Sample Preparation
GST-tagged O. sativa (Sasanishiki) CPD2PHR was expressed and purified as described previously (25). For crystallographic studies, GST was cleaved by treatment with thrombin at 25 °C for 2 h and removed by using a heparin column with a gradient of 0–0.5 m sodium chloride in 50 mm Tris-HCl (pH 8.0) and 5% glycerol. The protein was stored in 50 mm Tris-HCl (pH 8.0), 50 mm NaCl, 1 mm DTT, and 5% glycerol.
Crystallization, Data Collection, and Structure Determination
Crystals of CPD2PHR were obtained at room temperature under two different conditions, designed as form I and II. Form I was crystallized by hanging drop vapor diffusion against 100 mm sodium cacodylate (pH 6.5), 200 mm ammonium sulfate, and 30% w/v polyethylene glycol 8000, whereas form II was obtained against 32% w/v polyethylene glycol 4000, 5% urea, 100 mm imidazole/malate (pH 7.4). The data were collected at SSRL beam line 9.0.2 and ALS beam line 5.0.2 for form I and ALS beam line 8.2.2 for form II and processed with Denzo/Scalepack (26). Forms I and II have two molecules per asymmetric unit in space groups P212121 (a = 93.8, b = 107.8, c = 108.5 Å) and P21 (a = 53.4, b = 109.4, c = 97.7 Å, β = 93°), respectively. Form I crystals were twinned; however, initial phases were obtained after soaking crystals in 10 mm KPtCl2, re-collecting data with multiple wavelengths, and running SOLVE (27). For form II, initial phases were obtained by using AMoRe (28) with three overlaid bacterial CPD PHRs (PDB codes 1DNP, 1QNF, and 1IQR) as search probes. A correlation factor of 18.9% and an R-factor of 52.1% in the resolution range 20–3.85 Å resulted. Building the CPD2PHR model was done manually with TURBO FRODO (29), Xtalview (30) and COOT (31). Refinement was carried out using CNS and PHENIX (32, 33). Data collection and refinement statistics are summarized in Table 1.
TABLE 1.
Crystallographic and refinement statistics
| Measurement | Value |
|---|---|
| X-ray data | |
| Space group | P21 |
| a = 53.35 Å | |
| b = 109.36 Å | |
| c = 97.74 Å | |
| β = 93.59° | |
| Resolution | 50.0-1.70 (1.76-1.70) Å |
| Total number of reflections | 1,448,785 |
| Unique reflections | 119,326 |
| Number of reflections used | 105,768 |
| Completeness (%) | 98.5 (98.0) |
| Average I/δ | 23.1 (2.87) |
| Rsym | 5.6 (34.1) |
| Refinement | |
| Resolution | 50.0-1.70 Å |
| Number of protein atoms | 7535 |
| Number of water atoms | 1286 |
| Rwork (%) | 18.1 |
| Rfree (%) | 21.7 |
| Bond length | 0.006 Å |
| Bond angles | 1.058° |
| Favored (%) | 95.26 |
| Allowed (%) | 4.19 |
Electrophoresis Mobility Shift Assay (EMSA)
A synthesized 49-mer oligonucleotide with a single CPD was annealed to a complementary strand 5′-tagged with IRDye® 700 (Integrated DNA Technologies, Inc., San Diego, CA). The damaged strand sequence, 5′-d(AGCTACCATGCCTGCACGAAXXAAGCAATTCGTAATCATGGTCATAGCT)-3′ (XX = CPD) was designed to have the photoproduct at a MseI site as described previously (14, 15). Electrophoresis indicated that 28.5% of the dyed complementary strand was incorporated to the damaged strand. Therefore, the resulting double-stranded DNA was purified by electrophoresis in a 6.5% polyacrylamide gel and extracted into pure water. The purified double-stranded DNA (0.5 nm) was incubated with 0–20 nm CPD2PHR in solution (20 mm Tris-HCl (pH 7.5) and 20 mm NaCl) on ice overnight. Subsequent electrophoresis of the mixtures in a 6.5% polyacrylamide gel was used to assay DNA binding. Protein-free nucleotides and the protein-DNA complex were quantified with bioimaging analyzer Odyssey® Infrared Imaging System (LI-COR Biotechnology).
DNA Distortion Assay
Oligonucleotides containing the normal thymine dimer TT or CPD, d(GCAAGXXGGAG; XX = TT or CPD), were hybridized to a complementary strand containing 2-aminopurine (Ap), d(CTCC-Ap-ACTTGC), as described (34). These duplexes were dissolved at 0.3 μm in a buffer containing 50 mm Tris-HCl (pH 8.0), 100 mm NaCl, 1 mm 2-mercaptoethanol. Fluorescence spectra were measured at 5 °C in the absence or presence of 0.35 μm E. coli or O. sativa CPD PHRs. The excitation wavelength was set to 313 nm. Fluorescence detection of DNA distortion upon enzyme binding was performed as demonstrated previously (35).
RESULTS AND DISCUSSION
Eukaryotic Class II CPD Photolyase Structure
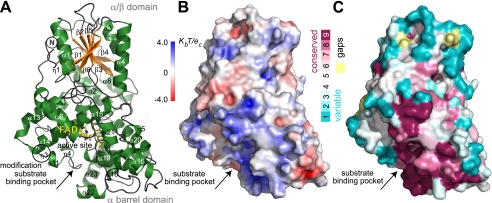
To better understand the mechanisms of UV tolerance in eukaryotes and features distinguishing CPD2PHRs from other PHRs, we expressed, purified, crystallized, and solved the structure of CPD2PHR from O. sativa (Os) Sasanishiki. We determined and refined (Rwork = 18.1%; Rfree = 21.7%) the crystal structure at 1.7 Å resolution (Table 1). Despite very low sequence similarity (20.9% identity with E. coli PHR), this eukaryotic CPD2PHR strikingly maintains the overall fold of bacterial CPD PHRs (Fig. 1). PHR structures have an N-terminal α/β domain and a C-terminal α-helical domain, connected by a long loop.
The extended N terminus unique to eukaryotic CPD2PHRs packs between the two domains and then contributes an initial anti-parallel β-strand to the otherwise parallel β-sheet (Fig. 2, supplemental Fig. 1). Within the C-terminal domain, the “FAD triangle” of helices frames a concave pocket (25 Å across, 7 Å deep) that holds the FAD cofactor in the U-shaped conformation shared by other PHRs (Figs. 3 and 4). Conserved insertions and deletions in CPD2PHRs relative to other family members, cluster three-dimensionally in the protein lobe between the FAD binding cavity and the protein C terminus (Fig. 2C). Surprisingly, although both Class I and II CPD PHRs repair the same damaged substrate, their structural differences lie predominantly within the catalytic C-terminal domain rather than the non-catalytic N-terminal domain (Figs. 1B and 3, A and B).
FIGURE 2.
Crystal structure of rice CPD2PHR. A, ribbon diagrams show the folds of the N-terminal α/β domain (top) with additional leading β-strand and C-terminal FAD-binding α-helical domain (bottom) connected by a long loop with an extra C-terminal α-helix (α8) not found in other CRY/PHR structures. B, the electrostatic potential surface shows a positively charged DNA binding groove above FAD binding cavity. C, a cross-species amino acid conservation map of CPD2PHRs using sequences in supplemental Fig. 2 highlights DNA binding groove and substrate binding pocket.
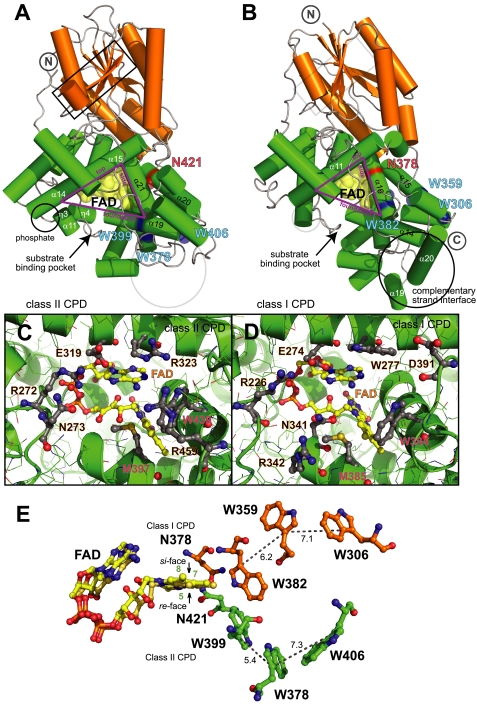
FIGURE 3.
Comparison of eukaryotic Os Class II (A, C, and E) and bacterial E. coli Class I (B, D, and E) CPD PHR structures. A and B, distinct differences in catalytic C-terminal α-helical domains (green) of the overall fold surround the functionally conserved triangular frame enclosing FAD (yellow) at the functionally conserved binding pocket for catalytic CPD repair (C and D). E, the FAD-tuning Asn (red in A and B) and Trp electron-transfer pathways (blue in A and B) for Class II (green) and Class I (orange) are structurally distinct and diverge from opposing faces of FAD.
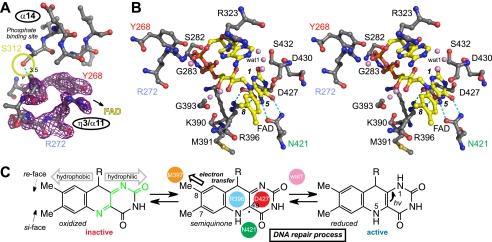
FIGURE 4.
Phosphate and FAD binding sites in Os CPD2PHR. A, the omit electron density map (purple mesh, contoured at 1σ) for residues near the Ser-312 phosphorylation site were identified by mass spectrometry (25). B, shown is a stereoview of FAD binding site residues (gray) and FAD (yellow) with red oxygen and blue nitrogen atoms. Only selected hydrogen bonds (dashed lines) and nearest water molecules (pink) are shown. C, schematic highlighting key structural, chemical, and redox properties of FAD for function within enzyme environment are shown. a.u., absorbance units.
To examine the basis for damage specificity, we superimposed our eukaryotic CPD2PHR structure on bacterial CPD PHR structures with (PDB code 1TEZ) and without (PDB code 1DNP) bound DNA product (Fig. 1B, supplemental Fig. 2) as well as (6-4) PHR (11). The CPD binding pocket formed by the top (α14-α15), foundation (α19) and side (α21) helices of the FAD triangle differs significantly between Class I and Class II CPD PHRs (Fig. 3, A and B) despite their common substrate. In Os CPD2PHR (Fig. 3C, supplemental Fig. 2), the flipped out thymine bases abut the Trp-439 indole ring (side helix) and are sandwiched between the Arg-323 guanidinium (top) and the Met-397 sulfur (foundation), which also packs with the terminal C8 methyl of FAD. Trp-439 (corresponding to Trp-384 in E. coli PHR) is completely conserved in all PHRs (10–13). Arg-323 of Os CPD2PHR, in contrast, replaces a second active-site Trp conserved in both Class I CPD PHRs (E. coli Trp-277; Fig. 3D) and (6-4) PHRs despite their different substrates. This second Trp is proposed to support efficient electron transfer between FAD and the respective substrates (36).
Active-site Met-397 lies within a nearly invariant (18/19 identical residues) sequence among CPD2PHRs, encompassing the foundation helix of the FAD triangle (supplemental Fig. 1). This Met, which corresponds to the second active-site His in (6-4) PHRs (11, 15), is conserved in CPD PHRs and proposed to mediate reversible electron transfer between cofactor and substrate (37). CRYs, PHR homologs without DNA-repair activity (Fig. 1A), also lack this Met (16).
Residues located in the second and third shells flanking the substrate binding pocket differ between Class II and Class I CPD PHRs. Behind Arg-323 in Os CPD2PHR lies Ala-185, with its small side chain replacing an aromatic residue (Phe or Tyr) conserved in all other CRY/PHR family members. In Os CPD2PHR, salt bridges joining residues conserved among CPD2PHRs occupy this aromatic ring position (Arg-185 to Asp-318) and underlie conserved Trp-439 (Arg-459 to Asp-446). Os CPD2PHR Met-461, flanking Met-397, replaces yet another aromatic side chain conserved in both Class I CPD (Phe-399 in E. coli) and (6-4) PHRs (Tyr 422 in Arabidopsis). Overall, the substrate binding pocket in eukaryotic CPD2PHRs has fewer aromatic side chains, an additional conserved Met, and more Arg-containing, second-shell salt bridges than those of bacterial CPD PHRs despite the shared underlying FAD triangle framework.
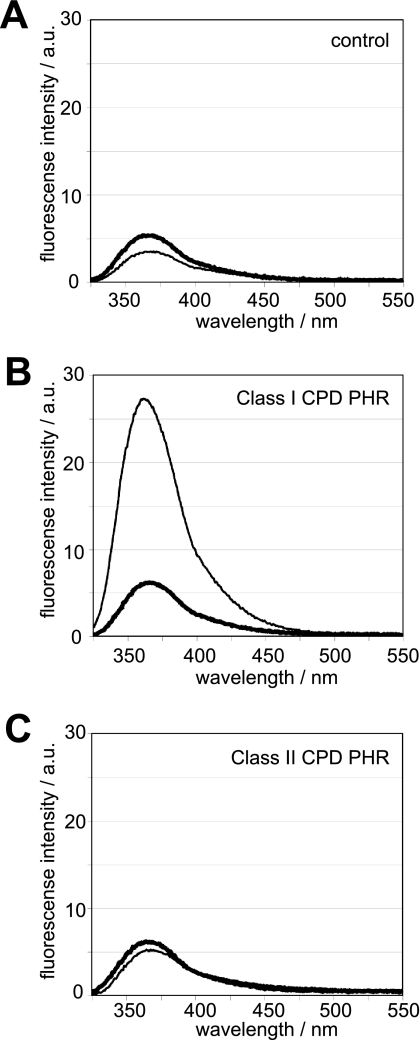
Distinctive features in and near the Os CPD2PHR active site suggest that damaged DNA recognition differs between Class I and II CPD PHRs. Besides the differences local to the substrate binding pocket, as described above, Os CPD2PHR lacks two C-terminal helices of E. coli PHR that provide the interface with the complementary undamaged DNA strand (Fig. 3, A and B). The results of DNA distortion assays using a fluorescent nucleic acid analog as a probe are consistent with this structural analysis. The increase in fluorescence intensity upon CPD binding, indicating distortion of the substrate DNA double helix, was much greater for E. coli Class I than for Os Class II CPD PHR (Fig. 5). Thus, our data suggest that eukaryotic Class II and prokaryotic Class I PHRs bind CPD damage in similar active sites but interact differently with the DNA duplex.
FIGURE 5.
DNA distortion upon enzyme binding. Fluorescence detection of DNA distortion upon enzyme binding was done by using double-stranded oligonucleotides containing 2-aminopurine in the absence (A) or the presence of E. coli Class I (B) and O. sativa Class II CPD PHRs (C). Thick and thin lines represent the fluorescence spectra of 2-aminopurine located opposite the normal TT and the CPD, respectively.
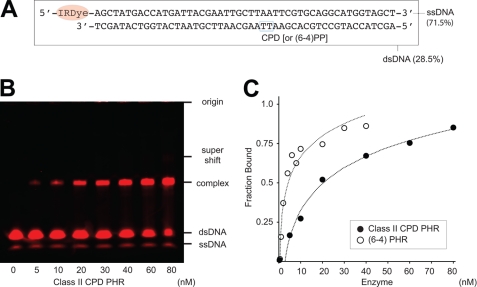
We also compared the binding of eukaryotic photolyase repair enzymes to damaged DNA containing each of the two major UV-induced photoproducts: CPDs and (6-4) photoproducts. Bacteria, represented by E. coli, have only Class I CPD PHR, whereas many higher organisms, such as fly, fish, and frog, have two types of photoactive repair enzymes: (6-4) and Class II CPD PHRs. These two enzymes are individually well conserved in the amino acid sequence but are phylogenetically distinct (Fig. 1A). Plants, including rice (38), have both enzymes, as first identified from isolated mutant strains of Arabidopsis; UVR2 is CPD2PHR, UVR3 is (6-4) PHR. Before the evolution of more complex multiprotein DNA repair systems, such as nucleotide excision repair, these two PHR enzymes with different substrate specificities would cooperatively function in higher eukaryotic cells to maintain overall genomic integrity. To compare damaged DNA binding by CPD2PHR (Fig. 6) to that for (6-4) PHR (14), we used an electrophoresis mobility shift assay (EMSA) with damaged DNA of the same length. The results indicate that damaged DNA strands bind to CPD2PHR with ∼10× less affinity than to (6-4) PHR (Fig. 6C).
FIGURE 6.
Electrophoresis mobility shift assay of CPD2PHR with 49-mer oligonucleotide carrying a single CPD with infrared imaging system. A, red fluorescence-tagged oligonucleotide sequence containing a single CPD is shown. B, detection of Os CPD2PHR binding to the damaged DNA is shown. C, shown is a comparison of affinities to 49-mer double-stranded DNA carrying a single photoproduct between Class I type (6-4) PHR (open circle) (14) and Class II CPD PHR (closed circle).
In UV-induced photoproducts, CPD damage is dominant (70–80%) and mutagenic, whereas (6-4) photoproducts, which have lost genetic information by hydroxyl or amino group transfer, are lethal. Compared with (6-4) photoproduct, CPD is also a poor substrate for nucleotide excision repair. To compensate, the lower affinity for damaged DNA (Fig. 6) and fewer interfaces for the complementary strand likely enable quicker turnover for CPD2PHR. Thus different accessibilities and interfaces to DNA may promote the cooperative functioning of two distinct photolyases in a single eukaryotic cell.
FAD Cofactor Interactions and Tuning
Our crystallographic structure revealed the FAD binding site in detail, indicating key amino acid differences between the two classes of CPD PHRs despite overall conservation of the flavin environment (Fig. 4; supplemental Fig. 1). The U-shaped FAD is positioned with the isoalloxazine ring buried and the adenine ring solvent-exposed beneath the substrate binding pocket (Figs. 2A, 3C, and 4). A salt bridge (Arg-396 to Asp-427) across the isoalloxazine ring orients the guanidinium to stabilize a semiquinone radical at the C4a position (Fig. 4, B and C). This salt bridge is invariant among all structurally known PHRs and CRYs, suggesting a role for the flavin radical in all family members (11). These features are consistent with ENDOR spectroscopy, suggesting that plant CPD2PHRs share the FAD binding mode of the bacterial enzymes (39) despite sequence dissimilarities (4).
However, specific interactions of FAD with Os CPD2PHR reveal interesting variations. The Asn-421 side-chain hydrogen bonds directly to N5 and O4 of FAD and to Asp-422 and indirectly to Glu-425 via a water molecule. FAD N3 hydrogen-bonds with the Asp-427 backbone, and FAD O2 is tethered via water molecules to Asp-430 and Ser-432 side chains. Hydrogen bonds also link FAD hydroxyl groups Met-391 and Gly-393 backbone via water molecules, and FAD phosphates with Tyr-268, Ser-282, and Lys-390 side chains and Ser-282, Gly-283, and Ser-285 main chain.
In Class I CPD and (6-4) PHRs and most homologs, including CRY DASH and mammalian clock CRYs (Fig. 1A), FAD is tuned by an invariant Asn (side helix) that hydrogen-bonds to the redox-active N5 position (11, 40, 41). Indeed, an Asn → Ser mutant of Class I CPD PHR has defects in DNA repair (40). Remarkably, the equivalent residue in Class II is Gly-433; the Asn-421 side chain that hydrogen-bonds to the N5 position of FAD is instead contributed from a distinct structural location and approaches the FAD isoalloxazine ring from the opposite face (Fig. 3E) compared with all other structurally characterized CRY/PHR proteins. Asn-421, unlike its counterparts, also hydrogen-bonds to FAD O4. Despite its the unique orientation, this Asn along with the invariant salt bridge tunes FAD redox properties for DNA repair as in the Class I CPD and (6-4) PHRs.
Remarkably, the Trp electron-transfer pathway, which allows reduction of oxidized FAD to restore CPD PHR activity, exhibits convergent evolution in Os CPD2PHR (Fig. 3E). A unique Trp triad (Os CPD2PHR Trp-399–Trp-378–Trp-406), invariant among CPD2PHRs (supplemental Fig. 1) forms a pathway from the re face of FAD to the protein surface. A different and otherwise invariant Trp triad (E. coli PHR Trp-382–Trp-359–Trp-306) on the si side of FAD is shared by all other CRY/PHR proteins (11, 42, 43) but CPD2PHRs. In Class I CPD and (6-4) PHRs, FAD oxidation can be reversed by stepwise light-dependent reduction via this Trp pathway (42). Os CPD2PHR exhibits photoreduction kinetics resembling those of the class I enzymes (39), indicating functional substitution by the alternative Trp pathway.
Phosphate Binding of Endogenous Enzyme
Endogenously purified rice CPD2PHR exhibits two different molecular weights, whereas the same enzyme recombinantly expressed in E. coli is homogenous (25). Mass spectrometry analyses of endogenous protein indicate that phosphate may be attached at Ser-312 in vivo. Phosphate binding might account for the better substrate binding and CPD photorepair exhibited by native rice CPD2PHR relative to recombinant protein shows. In the Os CPD2PHR structure, Ser-312 is located near the N terminus of the foundation helix of the FAD triangle (Figs. 2A, 3A, and 4A). The Ser-312 side-chain hydroxyl group faces the N-terminal end of helix η3/α11, which directs Tyr-268 and Arg-272 toward FAD (Fig. 4B). The larger phosphate-bound Ser could cap this helix to rigidify or reorder the active site, potentially enhancing substrate binding and photorepair. In the Arabidopsis (6-4) PHR structure, a similarly positioned phosphate anion is encircled by a phosphate binding motif of the protein that constricts the entrance to the substrate binding cavity and directs a Lys side chain inward to hydrogen-bond with the N7 atom of the FAD adenine moiety (11). Thus, positioning a phosphate ion near the substrate and cofactor binding sites may provide both eukaryotic PHR families with control mechanisms for constraining and regulating their active sites. Phosphate-induced protein conformational changes could adjust intra- or intermolecular hydrogen bonds to aid electron transfer, DNA distortion, base flipping, or product release during the repair process (44).
UV-resistant Polymorphisms
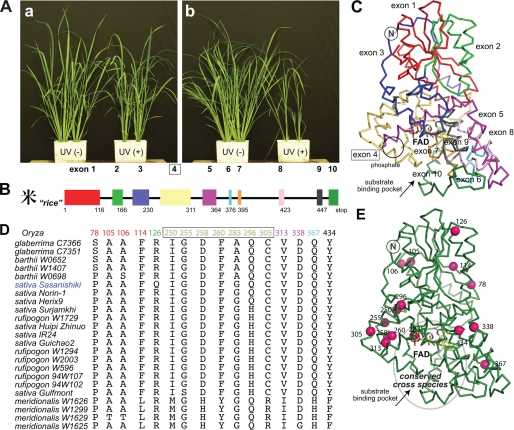
For plants, repeated and inevitable sunlight exposure makes DNA repair of UV damage essential. Regardless of habitat or species, plants use both (6-4) and CPD PHRs. Mechanistic studies of these enzymes have been initiated with both model (Arabidopsis) and crop (Oryza) plants (18, 19). CPD may produce more mutations; (6-4) photoproducts block polymerases, whereas error-prone polymerases can overcome CPD by low fidelity (1). Recently, UV sensitivity was examined in 12 rice strains belonging to two cultivated (O. sativa and Oryza glaberrima) and three wild (Oryza barthii, Oryza meridionalis. and Oryza rufipogon) species (24). Rice, including these examined strains, conserves the CPD2PHR genomic structure, although genotype and phenotype vary even within species (Fig. 7). Surprisingly, some strains show significantly higher photorepair activity and enhanced UV resistance (19, 24). We used sequence alignments to identify polymorphisms potentially responsible for this enzymatic robustness (Fig. 7D); however, most strains exhibit multiple amino acid differences. So the effect of an individual mutation cannot be simply connected to plant phenotype (24).
FIGURE 7.
Polymorphisms in the amino acid sequence of rice CPD2PHR. A, different cultivars vary significantly in their resistance (Sasanishiki) (a) versus sensitivity (Norin-1) (b) to UV irradiation despite the highly conserved genomic structure (B and C). Half of the sequence polymorphisms identified in 23 rice strains (D) lie within exon 4 (yellow) and the beginning of exon 5 (magenta) and map onto a lobe of the protein forming one bank (left) of the active-site groove leading to the FAD-binding pocket (E).
Therefore, mutations of available rice CPD2PHR genes in addition to the sequences of characterized strains were mapped onto the Os CPD2PHR structure. These spontaneous mutations map predominantly onto a lobe of the protein forming one side of the active-site groove leading to FAD (Fig. 7E). Remarkably, a single substitution divides the 23 characterized strains into two groups; the more UV-tolerant cultivars, including the Sasanishiki strain used in this study, have Gln-296, whereas the UV-sensitive cultivars have His (Fig. 7D). The longer, more flexible Gln side chain can form hydrogen bonds to cross-brace the Os CPD2PHR-fold by linking the C-terminal, FAD binding, α-helical domain to Val-201 in the long inter-domain loop and Trp-54 in the N-terminal α/β domain. The Trp-54 ring is thus fixed to stack between Phe-203 and Arg-25 from the ∼30-residue N-terminal extension unique to CPD2PHRs. Other members of the CRY/PHR family lack this N-terminal extension but conserve Arg at the Gln/His-296 position of Os CPD2PHR. Our structure thus provides a basis for understanding how polymorphisms in CPD2PHR can produce improved UV resistance for plants to counter increasing solar UV-B from loss of ozone protection.
Conclusions
We determined the crystal structure of a UVR2 homolog, the prototypic CPD2PHR essential for UV resistance to DNA damage in plants. Our structure-based comparisons reveal the convergent evolution of both the Trp pathway and the flavin hydrogen bonding, establishing these as critical functional features for PHRs. Furthermore, mapping mutations onto this structure provides an informed basis for understanding the structure-activity relationship of CPD2PHRs as additional genotypic and phenotypic information become available. This structure provides a new paradigm for light usage and DNA repair mechanisms in higher organisms. Furthermore, the collective results build the foundation for the eventual design of improved UV-resistant plant cultivars for better harvests.
Supplementary Material
Acknowledgments
We thank A. Pratt and Drs. Christie, Kumagai, Weber, Toh, and Daiyasu for valuable discussions and ALS and SSRL beamline staff for technical assistance. We also thank Dr. Groocock for assistance with EMSA.
Note Added in Proof
While this paper was under revision, an archaeal Class II CPD PHR structure was reported in an advanced online publication of the EMBO J. (2011) 30, 4437–4449. Both studies are completely independent and complement one another.
This work was supported, in whole or in part, by National Institutes of Health Grants. GM37684 (to E. D. G.) and in part by Grant GM046312 (to J. A. T.).
The atomic coordinates and structure factors (code 3UMV) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).

This article contains supplemental Figs. 1 and 2.
- CPD
- cyclobutane pyrimidine dimer
- UVR
- UV resistance
- Os
- O. sativa.
REFERENCES
- 1. Friedberg E. C., Walker G. C, Siede W., Wood R. D., Schultz R. A., Ellenberger T. (2005) DNA Repair and Mutagenesis, American Society for Microbiology, Washington, D. C [Google Scholar]
- 2. Kato T., Jr., Todo T., Ayaki H., Ishizaki K., Morita T., Mitra S., Ikenaga M. (1994) Cloning of a marsupial DNA photolyase gene and the lack of related nucleotide sequences in placental mammals. Nucleic Acids Res. 22, 4119–4124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yasui A., Eker A. P., Yasuhira S., Yajima H., Kobayashi T., Takao M., Oikawa A. (1994) A new class of DNA photolyases present in various organisms including aplacental mammals. EMBO J. 13, 6143–6151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kanai S., Kikuno R., Toh H., Ryo H., Todo T. (1997) Molecular evolution of the photolyase blue light photoreceptor family. J. Mol. Evol. 45, 535–548 [DOI] [PubMed] [Google Scholar]
- 5. Huffman J. L., Sundheim O., Tainer J. A. (2005) DNA base damage recognition and removal. New twists and grooves. Mutat. Res. 577, 55–76 [DOI] [PubMed] [Google Scholar]
- 6. Hitomi K., Iwai S., Tainer J. A. (2007) The intricate structural chemistry of base excision repair machinery. Implications for DNA damage recognition, removal, and repair. DNA Repair 6, 410–428 [DOI] [PubMed] [Google Scholar]
- 7. Tubbs J. L., Latypov V., Kanugula S., Butt A., Melikishvili M., Kraehenbuehl R., Fleck O., Marriott A., Watson A. J., Verbeek B., McGown G., Thorncroft M., Santibanez-Koref M. F., Millington C., Arvai A. S., Kroeger M. D., Peterson L. A., Williams D. M., Fried M. G., Margison G. P., Pegg A. E., Tainer J. A. (2009) Flipping of alkylated DNA damage bridges base and nucleotide excision repair. Nature 459, 808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fan L., Fuss J. O., Cheng Q. J., Arvai A. S., Hammel M., Roberts V. A., Cooper P. K., Tainer J. A. (2008) XPD helicase structures and activities. Insights into the cancer and aging phenotypes from XPD mutations. Cell 133, 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fuss J. O., Tainer J. A. (2011) XPB and XPD helicases in TFIIH orchestrate DNA duplex opening and damage verification to coordinate repair with transcription and cell cycle via CAK kinase. DNA Repair 10, 697–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maul M. J., Barends T. R., Glas A. F., Cryle M. J., Domratcheva T., Schneider S., Schlichting I., Carell T. (2008) Crystal structure and mechanism of a DNA (6-4) photolyase. Angew. Chem. Int. Ed. Engl. 47, 10076–10080 [DOI] [PubMed] [Google Scholar]
- 11. Hitomi K., DiTacchio L., Arvai A. S., Yamamoto J., Kim S. T., Todo T., Tainer J. A., Iwai S., Panda S., Getzoff E. D. (2009) Functional motifs in the (6-4) photolyase crystal structure make a comparative framework for DNA repair photolyases and clock cryptochromes. Proc. Natl. Acad. Sci. U.S.A. 106, 6962–6967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park H. W., Kim S. T., Sancar A., Deisenhofer J. (1995) Crystal structure of DNA photolyase from Escherichia coli. Science 268, 1866–1872 [DOI] [PubMed] [Google Scholar]
- 13. Tamada T., Kitadokoro K., Higuchi Y., Inaka K., Yasui A., de Ruiter P. E., Eker A. P., Miki K. (1997) Crystal structure of DNA photolyase from Anacystis nidulans. Nat. Struct. Biol. 4, 887–891 [DOI] [PubMed] [Google Scholar]
- 14. Hitomi K., Kim S. T., Iwai S., Harima N., Otoshi E., Ikenaga M., Todo T. (1997) Binding and catalytic properties of Xenopus (6–4) photolyase. J. Biol. Chem. 272, 32591–32598 [DOI] [PubMed] [Google Scholar]
- 15. Hitomi K., Nakamura H., Kim S. T., Mizukoshi T., Ishikawa T., Iwai S., Todo T. (2001) Role of two histidines in the (6-4) photolyase reaction. J. Biol. Chem. 276, 10103–10109 [DOI] [PubMed] [Google Scholar]
- 16. Brudler R., Hitomi K., Daiyasu H., Toh H., Kucho K., Ishiura M., Kanehisa M., Roberts V. A., Todo T., Tainer J. A., Getzoff E. D. (2003) Identification of a new cryptochrome class. Structure, function, and evolution. Mol. Cell 11, 59–67 [DOI] [PubMed] [Google Scholar]
- 17. Pellequer J. L., Wager-Smith K. A., Kay S. A., Getzoff E. D. (1998) Photoactive yellow protein. A structural prototype for the three-dimensional fold of the PAS domain superfamily. Proc. Natl. Acad. Sci. U.S.A. 95, 5884–5890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiang C. Z., Yee J., Mitchell D. L., Britt A. B. (1997) Photorepair mutants of Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 94, 7441–7445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hidema J., Kumagai T., Sutherland B. (2000) UV radiation-sensitive norin 1 rice contains defective cyclobutane pyrimidine dimer photolyase. Plant Cell 12, 1569–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hidema J., Taguchi T., Ono T., Teranishi M., Yamamoto K., Kumagai T. (2007) Increase in CPD photolyase activity functions effectively to prevent growth inhibition caused by UVB radiation. Plant J. 50, 70–79 [DOI] [PubMed] [Google Scholar]
- 21. Ahmad M., Jarillo J. A., Klimczak L. J., Landry L. G., Peng T., Last R. L., Cashmore A. R. (1997) An enzyme similar to animal type II photolyases mediates photoreactivation in Arabidopsis. Plant Cell 9, 199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakajima S., Sugiyama M., Iwai S., Hitomi K., Otoshi E., Kim S. T., Jiang C. Z., Todo T., Britt A. B., Yamamoto K. (1998) Cloning and characterization of a gene (UVR3) required for photorepair of 6-4 photoproducts in Arabidopsis thaliana. Nucleic Acids Res. 26, 638–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hidema J., Teranishi M., Iwamatsu Y., Hirouchi T., Ueda T., (2005) Spontaneously occurring mutations in the cyclobutane pyrimidine dimer photolyase gene cause different sensitivities to ultraviolet-B in rice. Plant J. 43, 57–67 [DOI] [PubMed] [Google Scholar]
- 24. Iwamatsu Y., Aoki C., Takahashi M., Teranishi M., Ding Y., Sun C., Kumagai T., Hidema J. (2008) UVB sensitivity and cyclobutane pyrimidine dimer (CPD) photolyase genotypes in cultivated and wild rice species. Photochem. Photobiol. Sci. 7, 311–320 [DOI] [PubMed] [Google Scholar]
- 25. Teranishi M., Nakamura K., Morioka H., Yamamoto K., Hidema J. (2008) The native cyclobutane pyrimidine dimer photolyase of rice is phosphorylated. Plant Physiol. 146, 1941–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Otwinowski Z., Minor W. (1997) Processing of x-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 27. Terwilliger T. C., Berendzen J. (1999) Automated MAD and MIR structure solution. Acta Crystallogr. D Biol. Crystallogr. 55, 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Navaza J. (1994) AMoRe: An automated package for molecular replacement. Acta Crystallogr. A. 50, 157–163 [Google Scholar]
- 29. Roussel A., Cambillau C. (1989) TURBO-FRODO, Silicon Graphics Geometry Partners Directory (Silicon Graphics), Mountai View, CA [Google Scholar]
- 30. McRee D. (1999) XtalView/Xfit. A versatile program for manipulating atomic coordinates and electron density. J. Struct. Biol. 125, 156–165 [DOI] [PubMed] [Google Scholar]
- 31. Emsley P., Lohkamp B., Scott W. G., Cowtan K. (2010) Features and development of Coot. Acta Crystallogr. D. Biol. Crystallogr. 66, 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brünger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., Read R. J., Rice L. M., Simonson T., Warren G. L. (1998) Crystallography and NMR system. A new software suite for macromolecular structure determination. Acta Crystallogr. D. 54, 905–921 [DOI] [PubMed] [Google Scholar]
- 33. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., Zwart P. H. (2010) PHENIX. A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamamoto J., Hitomi K., Todo T., Iwai S. (2006) Chemical synthesis of oligodeoxyribonucleotides containing the Dewar valence isomer of the (6-4) photoproduct and their use in (6-4) photolyase studies. Nucleic Acids Res. 34, 4406–4415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Christine K. S., MacFarlane A. W., IV, Yang K., Stanley R. J. (2002) Cyclobutylpyrimidine dimer base flipping by DNA photolyase. J. Biol. Chem. 277, 38339–38344 [DOI] [PubMed] [Google Scholar]
- 36. Kim S. T., Li Y. F., Sancar A. (1992) The third chromophore of DNA photolyase. Trp-277 of Escherichia coli DNA photolyase repairs thymine dimers by direct electron transfer. Proc. Natl. Acad. Sci. U.S.A. 89, 900–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miyazawa Y., Nishioka H., Yura K., Yamato T. (2008) Discrimination of class I cyclobutane pyrimidine dimer photolyase from blue light photoreceptors by single methionine residue. Biophys J. 94, 2194–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. International Rice Genome Sequencing Project (2005) The map-based sequence of the rice genome. Nature 436, 793–800 [DOI] [PubMed] [Google Scholar]
- 39. Okafuji A., Biskup T., Hitomi K., Getzoff E. D., Kaiser G., Batschauer A., Bacher A., Hidema J., Teranishi M., Yamamoto K., Schleicher E., Weber S. (2010) Light-induced activation of class II cyclobutane pyrimidine dimer photolyases. DNA Repair 9, 495–505 [DOI] [PubMed] [Google Scholar]
- 40. Xu L., Mu W., Ding Y., Luo Z., Han Q., Bi F., Wang Y., Song Q. (2008) Active site of Escherichia coli DNA photolyase. Asn-378 is crucial both for stabilizing the neutral flavin radical cofactor and for DNA repair. Biochemistry 47, 8736–8743 [DOI] [PubMed] [Google Scholar]
- 41. Iwata T., Zhang Y., Hitomi K., Getzoff E. D., Kandori H. (2010) Key dynamics of conserved asparagine in a cryptochrome/photolyase family protein by fourier transform infrared spectroscopy. Biochemistry 49, 8882–8891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aubert C., Vos M. H., Mathis P., Eker A. P., Brettel K. (2000) Intraprotein radical transfer during photoactivation of DNA photolyase. Nature 405, 586–590 [DOI] [PubMed] [Google Scholar]
- 43. Biskup T., Schleicher E., Okafuji A., Link G., Hitomi K., Getzoff E. D., Weber S. (2009) Direct observation of a photoinduced radical pair in a cryptochrome blue-light photoreceptor. Angew. Chem. Int. Ed. Engl. 48, 404–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kondoh M., Hitomi K., Yamamoto J., Todo T., Iwai S., Getzoff E. D., Terazima M. (2011) Light-induced conformational change and product release in DNA repair by (6-4) photolyase. J. Am. Chem. Soc. 133, 2183–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.