Background: The cross-talk between CXCL12/CXCR4 axis and PI3K/mTOR pathway in migration of gastric carcinoma cells is unknown.
Results: p110β provided a conduit for CXCL12-stimulated signaling and targeting PI3K/mTOR blocked CXCL12-activated cell motility.
Conclusion: Targeting PI3K/mTOR pathway inhibited CXCL12-mediated cell migration.
Significance: Drugs targeting mTOR pathway may be used for the therapy of metastatic gastric cancer expressing high levels of CXCL12.
Keywords: Cell Motility, CXCR4, mTOR, PI 3-Kinase (PI3K), Tumor Metastases, CXCL12
Abstract
CXCL12/CXCR4 plays an important role in metastasis of gastric carcinoma. Rapamycin has been reported to inhibit migration of gastric cancer cells. However, the role of mTOR pathway in CXCL12/CXCR4-mediated cell migration and the potential of drugs targeting PI3K/mTOR pathway remains unelucidated. We found that CXCL12 activated PI3K/Akt/mTOR pathway in MKN-45 cells. Stimulating CHO-K1 cells expressing pEGFP-C1-Grp1-PH fusion protein with CXCL12 resulted in generation of phosphatidylinositol (3,4,5)-triphosphate, which provided direct evidence of activating PI3K by CXCL12. Down-regulation of p110β by siRNA but not p110α blocked phosphorylation of Akt and S6K1 induced by CXCL12. Consistently, p110β-specific inhibitor blocked the CXCL12-activated PI3K/Akt/mTOR pathway. Moreover, CXCR4 immunoprecipitated by anti-p110β antibody increased after CXCL12 stimulation and Gi protein inhibitor pertussis toxin abrogated CXCL12-induced activation of PI3K. Further studies demonstrated that inhibitors targeting the PI3K/mTOR pathway significantly blocked the chemotactic responses of MKN-45 cells triggered by CXCL12, which might be attributed primarily to inhibition of mTORC1 and related to prevention of F-actin reorganization as well as down-regulation of active RhoA, Rac1, and Cdc42. Furthermore, rapamycin inhibited the secretion of CXCL12 and the expression of CXCR4, which might form a positive feedback loop to further abolish upstream signaling leading to cell migration. Finally, we found cells expressing high levels of cxcl12 were sensitive to rapamycin in its activity inhibiting migration as well as proliferation. In summary, we found that the mTOR pathway played an important role in CXCL12/CXCR4-mediated cell migration and proposed that drugs targeting the mTOR pathway may be used for the therapy of metastatic gastric cancer expressing high levels of cxcl12.
Introduction
Gastric carcinoma is a disease with a high death rate, making it the second most common cause of cancer death worldwide following lung cancer (1). Metastasis is a frequent cause of death in patients with advanced gastric carcinoma, and the prognosis of metastatic gastric cancer is poor with a six month survival rate of <15%. Recent research revealed that CXCL12 (chemokine (CXC motif) ligand 12) could be an independent prognostic factor in gastric cancer, with CXCL12-positive gastric cancer showing highly aggressive behavior, including metastasis (2).
CXCL12 is a 68-amino acid small cytokine that belongs to the CXC chemokine family, playing an important role in mediating tumor metastasis (3). CXCL12 is the only known ligand for chemokine (CXC motif) receptor 4 (CXCR4), which activates the receptor CXCR4 and attracts circulating CXCR4-expressing cells to peripheral tissues. The CXCL12/CXCR4 axis regulates a wide variety of downstream signal pathways related to chemotaxis, cell survival, and/or proliferation (4). PI3K is the major downstream transducer in CXCR4-mediated chemotaxis, which in turn regulates divergent signaling pathways (4). mTOR (mammalian target of rapamycin), a conserved serine/threonine kinase, sits in the center of the PI3K-mediated signaling pathways and regulates multiple processes, including mRNA translation, cell cycle progression, cell survival, and motility (5, 6). Although it has been reported that CXCL12 activates mTOR (7, 8), the precise mechanism underlying activation of the mTOR triggered by CXCL12 in gastric cancer cells remains elusive.
mTOR exists in two protein complexes, mTORC1 (mTOR, mLST8, and raptor) and mTORC2 (mTOR, mLST8, mSIN1, and Rictor). mTORC1 integrates signals from growth factor or nutritional status and controls cap-dependent mRNA translation by phosphorylating its substrates 4E-BP1 and S6K1;4 mTORC2 phosphorylates AKT and SGK1 at a C-terminal site known as the hydrophobic motif and regulates the organization of the actin cytoskeleton, but the biological significance of these activities is not well understood (6, 9, 10). mTOR plays a critical role in the regulation of tumor cell motility and cancer metastasis (11). However, the underlying mechanism of mTOR regulating cell motility and mTOR inhibitors inhibiting tumor cell motility is understood poorly and controversial (12, 13).
In the present study, we studied the role of mTOR signaling pathway in cell migration mediated by CXCL12/CXCR4 axis in gastric cancer cells. We found that CXCL12 activated the CXCR4 receptor, which coupled Gi protein and interacted with p110β catalytic subunit and activated its activity and downstream signaling pathway, including phosphorylation of AKT and p70S6K1. Inhibitors targeting PI3K or/and mTOR inhibited cell migration induced by CXCL12, which is attributed primarily to inhibition of mTORC1 and related to decreased activity of RhoA, Cdc42 and Rac1 as well as prevention of F-actin reorganization. Interestingly, rapamycin decreased the secretion of CXCL12 and expression of its receptor CXCR4, which might form a positive feedback loop to further block cell migration. We also found that cells expressing high levels of CXCL12 are more sensitive to rapamycin in its ability in inhibiting cells migration and proliferation. We proposed that inhibitors targeting the mTOR pathway may be used for therapy of metastatic gastric cancer expressing high levels of CXCL12.
MATERIALS AND METHODS
Cell Culture and Reagents
MKN-28, MKN-45, and SGC-7901 human gastric cancer cells were obtained from the Cancer Research Foundation of Japan and were cultured in RPIM-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen), l-glutamine (2 mm), penicillin (100 international units/ml), streptomycin (100 μg/ml), and HEPES (10 mm, pH 7.4). Cells were incubated in a humidified atmosphere of 95% air plus 5% CO2 at 37 °C. AMD-3100, PI-103, TGX-221, rapamycin, PP-242, and pertussis toxin were obtained from Sigma-Aldrich, Cayman Chemical (Ann Arbor, MI), LC Laboratories (Woburn, MA), respectively. CXCL12 was purchased from R&D Systems (Minneapolis, MN).
Immunoblotting, Immunoprecipitation, and Immunofluorescence
Immunoblotting, immunoprecipitation, and immunofluorescence were conducted using standard procedures, using antibodies against S6K1, phosphorylated S6K1, 4EBP1, phosphorylated 4EBP1, Akt, phosphorylated Akt, and p110 α (Cell Signaling Technology, Beverly, MA); p110β and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA); and CXCR4 (Abcam, Cambridge, MA). Texas Red-X® phalloidin was obtained from Invitrogen. Alexa Fluor® 488 IgG was used as a fluorescent secondary antibody.
AKT Translocation Assay
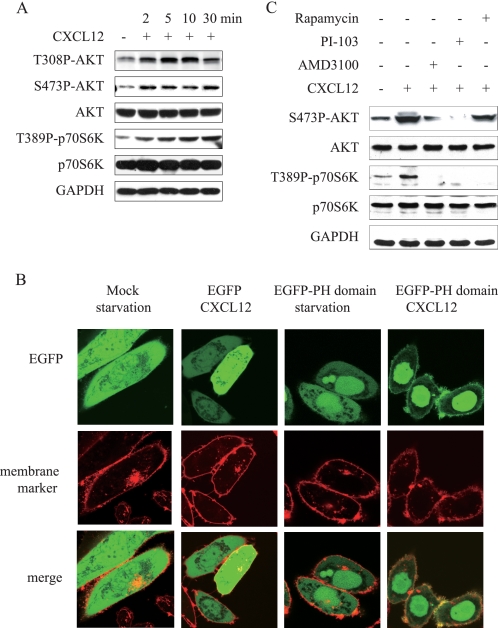
CHO-K1 transfected with pEGFP-C1-Grp1-PH or pEGFP-C1 were seeded and further incubated in serum-free medium for 24 h. The cell was stained with CellMaskTM (Invitrogen) deep red plasma membrane stain (5 μg/ml) diluted in culture medium for 5 min at 37 °C. Then the stain solution was washed out and replaced with fresh medium. After addition of CXCL12 (300 ng/ml) for 2 min, the fluorescent images of the cell were captured with a confocal microscopy (FV1000-SIM; Olympus, Tokyo, Japan). The term co-localization refers to the coincidence of green and red fluorescence.
Real-time Quantitative PCR
Total RNA was extracted with TRIzol according to the manufacturer's instructions and was transcribed using Prime ScriptTM RT reagent kit (TaKaRa, Dalian, China). The cDNA template was amplified by real-time PCR using the SYBR-Premix Ex TaqTM kit (TaKaRa). The primer sequences were as follows: 5′-CTGGGCAAAGCTAGTGAAG-3′ (forward) and 5′-AGAACGTGGAGGATGTGGAG-3′ (reverse) for cxcl12; 5′-AGCTGTTGGTGAAAAGGTGGTCTATG-3′ (forward) and 5′-GCGCTTCTGGTGGCCCTTGGAGTGTG-3′ (reverse) for CXCR4; and 5′-GCACCGTCAAGGCTGAGAAC-3′ (forward) and 5′-GCCTTCTCCATGGTGGTGAA-3′ (reverse) for GAPDH. Thermal cycling was programmed as follows: 95 °C for 30 s followed by 40 cycles of 95 °C for 5 s, 60 °C for 20 s, and 72 °C for 15 s, and then 72 °C for 10 min. Gene expression was assessed by ΔCt method, and mRNA levels of cxcl12 were normalized to those of the GAPDH internal standard.
Transfection of siRNA
The synthetic siRNAs targeting p110 catalytic subunit, Raptor, and Rictor were purchased from Shanghai GenePharma Co., Ltd with sequences as follows: 5′-GGUGGACCACGAAGAGUUATT-3′ (p110α); 5′-CGCAUUUGGUGGACCCAAATT-3′ (p110β); 5′- CAGCGGAGAGCUCAUCAACAATT-3′ (Raptor siRNA1; 5′- GACACGGAAGAUGUUCGACAATT-3′ (Raptor siRNA2); 5′- GCGAGCUGAUGUAGAAUUATT-3′ (Rictor siRNA1); and 5′- GGGAAUACAACUCCAAAUATT-3′ (Rictor siRNA2). Scrambled siRNA (5′-UUCUCCGAACGUGUCACGUTT-3′) was used as a negative control. The siRNA were transfected into MKN-45 cell using OligofectamineTM reagent (Invitrogen) according to the instructions of the manufacturer.
Transwell Assay
Cell migration was evaluated using an 8-mm pore size Transwell system (Costar, Cambridge, MA). Briefly, cells were resuspended in serum-free RPMI 1640 medium at a density of 2 × 105 cells/ml. The top chamber of the Transwell was loaded with 100 μl of cell suspension containing tested compounds, and the bottom chamber was loaded with 0.6 ml of RPMI 1640 medium containing CXCL12 (50 ng/ml) or 10% FBS. The total migrated cells to the lower chamber were fixed, stained with 0.1% crystal violet, and photographed after treatment. Crystal violet stained cells were dissolved with 10% acetic acid, and OD value was measured at 595 nm.
Pulldown Analysis of RhoA, Rac1, and Cdc42
Quantification of GTP-bound RhoA, Rac1, and Cdc42 was performed using the BK124, BK128, and BK127 G-LISA assay as instructed by the manufacturer (Cytoskeleton). Briefly, MNK-45 cells after treatment were suspended in lysis buffer. Cell extracts were incubated in separate wells of the G-LISA plate. The wells were probed with anti-RhoA, Rac1, or Cdc42 monoclonal antibodies and a secondary antibody. Finally, the plate was developed with a colorimetric substrate, and the absorbance was read at 490 nm with a multiwell plate reader.
Statistical Methods for Analysis of Rapamycin Activity and Gene Expression Data
Gene expression patterns for the NCI-60 cell lines assessed with HG-U133A Affymetrix chip. The probe sets were filtered to delete those with very little pattern (i.e. S.D. < 0.2 log2 units). The concentrations required to achieve 50% growth inhibition of the NCI-60 cells were obtained from the Developmental Therapeutics Program (http://www.dtp.nci.nih.gov/docs/dtp_search.html). Statistical analyses were performed by using the R package as described previously (14).
RESULTS
Activation of PI3K/AKT/mTOR Pathway by CXCL12
We first detected whether CXCL12 activates mTOR pathway in human gastric carcinoma MKN-45 cells. As shown in Fig. 1A, phosphorylated Akt at Thr-308 and Ser-473 as well as phosphorylated S6K1 increased significantly within 2 min after stimulation with CXCL12. As Akt may be phosphorylated by PDK1 at Thr-308 and by TORC2 at Ser-473 after PI3K activation, these results suggested that CXCL12 may stimulate PI3K and mTOR in a cascade.
FIGURE 1.
CXCL12 activated PI3K/Akt/mTOR signaling cascade. A, CXCL12 enhanced phosphorylation of Akt and p70S6K in MKN-45 cells. Serum-deprived MKN-45 cells were treated with CXCL12 (100 ng/ml) for the indicated times. Total Akt and p70S6K protein, as well as their phosphorylated forms were detected in whole cell lysates. GAPDH was employed as a loading control. B, CXCL12 increased PIP3 generation. CHO-K1 cells transfected with pEGFP-C1-Grp1-PH or pEGFP-C1 were incubated in serum-free media for 24 h. The cell was stained with CellMaskTM deep red plasma membrane stains. Then, the stain solution was washed out and replaced with fresh medium. After addition of CXCL12 (300 ng/ml) for 2 min, the fluorescent images of the cell were captured with confocal microscopy. C, pretreatment of AMD3100 (100 ng/ml), PI-103 (100 nmol/liter), and rapamycin (10 nmol/liter) abrogated enhanced phosphorylation of Akt and/or p70S6K induced by CXCL12. Serum-deprived MKN-45 cells were treated with aforementioned inhibitors for 1 h and then stimulated with CXCL12 for 5 min. Phosphorylated Akt and p70S6K were detected by immunoblotting. GAPDH was employed as a loading control. Data shown are representative of at least three independent experiments.
To further verify that phosphorylation of Akt and S6K1 is resulted from activation of PI3K in gastric cancer cells, the redistribution of the Grp1-PH domain after stimulation of CXCL12 was determined in CHO-K1 cells expressing Grp1-PH fused to EGFP (15). The Grp1-PH domain is shown a specific binding affinity to PtdIns(3,4,5)P3 rather than phosphatidylinositol 4,5-bisphosphate in cells and aggregates around the cell membrane responding to the recruitment of PtdIns(3,4,5)P3 (15). As shown in Fig. 1B, the EGFP-Grp1-PH distributed in the cytoplasm in serum-deprived cells. However, CXCL12 stimulation resulted in the translocation of GFP-Grp1-PH to cellular membrane, which was indicated as the fluorescent foci of EGFP (green) co-localized with cell membrane stain (red), indicating that stimulation of cells with CXCL12 activates PI3K and results in production of PtdIns(3,4,5)P3 on the cellular membrane. On the other hand, the fluorescent foci of EGFP has distributed equally in the cytoplasm in cells transfected with EGFP despite CXCL12 stimulation.
Accordingly, pretreatment of CXCR4 antagonist AMD-3100, PI3K pan-inhibitor PI-103, or mTOR inhibitor rapamycin abrogated phosphorylation of Akt at Ser-473 and/or S6K1 at Thr-389 stimulated by CXCL12 (Fig. 1C). It appears that CXCL12 interacted with and activated CXCR4 receptor, which in turn activated PI3K and generated PtdIns(3,4,5)P3 and further activates the Akt/mTOR signaling cascade.
CXCL12/CXCR4 Axis Interacting with p110β Catalytic Isoform and Activating PI3K Signaling
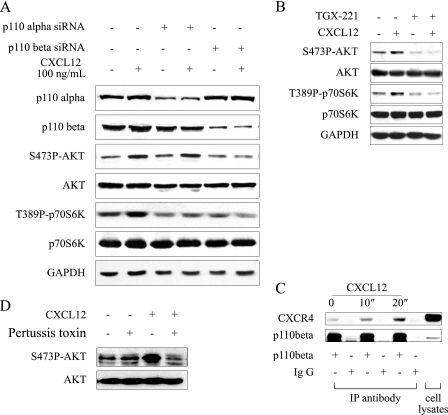
The class I PI3K are divided into class 1A (α, β, δ) and class 1B (γ) according to their different catalytic isoforms, which are activated by various extra- or intracellular signal molecules (16). We next investigated which catalytic isoform would provide a conduit for the CXCL12-activated mTOR pathway. As p110α and p110β are the two main isoforms, which are expressed in MKN45 cells, we examined the CXCL12-stimulated mTOR pathway after down-regulation of p110α or p110β by transient transfection of siRNA targeting p110α or p110β. As shown in Fig. 2A, transfection of isoform-specific siRNA in MKN-45 cells reduced the protein level of p110α or p110β without interference with each other. The p110α-specific siRNA did not abrogate CXCL12-induced phosphorylation of Akt and S6K1 in MKN-45 cells, although it should be noticed that phosphorylation of S6K1 reduced in cells transfected with p110α-specific siRNA compared with those wild type cells. By contrast, p110β-specific siRNA blocked the CXCL12-stimulated Akt/mTOR signaling cascade. Consistently, phosphorylation of Akt and S6K1 induced by CXCL12 was abrogated by pretreatment of a p110β-specific inhibitor TGX-221 in MKN-45 cells (Fig. 2B), suggesting that p110β may be the main PI3K isoform coupled with the CXCL12/CXCR4 axis in gastric cancer cells.
FIGURE 2.
CXCL12/CXCR4 axis interacted with p110β catalytic subunits and activated PI3K/mTOR signaling pathway. A, down-regulation of p110 subunit by transient transfection of siRNA targeting p110β but not p110α blocked phosphorylation Akt and p70S6K induced by CXCL12. After transfected with respective siRNA for 48 h, MKN-45 cells were treated with CXCL12 (100 ng/ml) for 5 min. Phosphorylated Akt and p70S6K were analyzed by immunoblotting; GAPDH was employed a loading control. B, p110β-specific inhibitor TGX-221 abrogated CXCL12-triggered PI3K/mTOR signaling. Serum-deprived MKN-45 cells were pretreated with TGX-221 for 1 h and then stimulated with CXCL12 for 5 min. C, CXCL12 enhanced the association between p110β and CXCR4. Serum-deprived MKN-45 cells were treated with vehicle or CXCL12 for 5 min. For immunoprecipitation, the primary antibody (1 μg) against p110β was added to 200 μl of cell lysates (2 × 106 cells) and incubated with rotation at 4 °C for 2 h. Rabbit IgG was used as a negative control. After precipitation with agarose A/G, proteins were resolved in 100 μl 1×SDS loading buffer, and 20 μl of each sample was separated with 12% SDS-PAGE and analyzed by immunoblotting using the antibody against CXCR4. Protein samples from whole cell lysate were used to indicate the position of the protein examined. D, involvement of Gi protein in CXCL12-induced phosphorylation of AKT. Serum-deprived MKN-45 cells were pretreated with pertussis toxin (100 ng/ml) for 2 h and then stimulated with CXCL12 for 5 min. Data shown are representative of at least three independent experiments.
To further verify the obtained results, we analyzed the association of CXCR4 with p110β upon CXCL12 stimulation in MKN-45 cells with an immunoprecipitation assay. As shown in Fig. 2C, CXCR4 pulled down by an anti-p110β antibody significantly increased in MKN-45 cells after stimulation with CXCL12 for 20 s, indicating a significant increase in the association of CXCR4 with p110β. Thus, these results demonstrated that CXCR4 interacted with p110β and activated its downstream signaling. However, it is unknown whether CXCR4 interacts with p110β directly, which deserves to be further studied.
CXCR4 is a G protein-coupled receptor. We next investigated whether activation of p110β by the CXCL12/CXCR4 axis required G protein. As shown in Fig. 2D, Gi protein inhibitor pertussis toxin pretreatment abrogated phosphorylation of Akt at Ser-473 induced by CXCL12, indicating that G protein is required for activation of PI3K by CXCR4.
PI3K/mTOR Pathway Inhibitor Blocking Cell Migration Induced by CXCL12
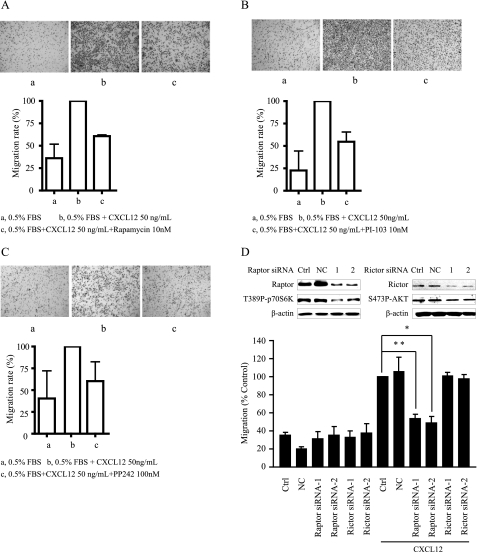
Because CXCL12 promotes the motility of cells and we also validated that the CXCL12/CXCR4 axis activated the PI3K/mTOR signaling pathway in gastric cancer cells, we examined whether compounds targeting the PI3K/mTOR pathway would attenuate the motility of gastric cancer cells induced by CXCL12. To this end, a Transwell assay was utilized to detect migration of MKN-45 cell stimulated by CXCL12 or FBS in the presence of PI3K/mTOR dual inhibitor PI-103, mTORC1 inhibitor rapamycin or mTORC1/2 inhibitor PP-242. As shown in Fig. 3A, treatment of rapamycin at 10 nmol/liter for 24 h significantly inhibited CXCL12-stimulated migration of MKN-45 cells, with an inhibitory rate of ∼40%. Similarly, PI-103 (10 nmol/liter) or PP-242 (100 nmol/liter) treatment for 8 h inhibited CXCL12-stimulated MKN-45 cells migration by ∼45% (Fig. 3, B and C). These results indicated that compounds targeting the PI3K/mTOR pathway inhibited the motility of gastric cancer cells mediated by the CXCL12/CXCR4 axis.
FIGURE 3.
Compounds targeting PI3K/mTOR pathway inhibited cell motility stimulated by CXCL12. Motility of MKN-45 cells in the presence of rapamycin (10 nm; A), PI-103 (10 nm; B), or PP-242 (100 nm; C) was determined by the classic Transwell assay as described under “Materials and Methods.” Cells migrating to the lower aspect of the Boyden chamber filter were stained and photographed. Top, representative images of at least three independent experiments. Bottom, quantitative analysis of cell migration. Columns indicate mean of three experiments. Bars, S.D. D, down-regulation of Raptor impeded CXCL12-induced MKN-45 cells migration. After transfection with respective siRNA for 48 h, MKN-45 cells were put into a Transwell assay, and the cells that migrated to the lower chamber were detected after 24 h. Upper panel, down-regulation of Raptor or Rictor after transfection with respective siRNAs. Lower panel, quantitative analysis of cell migration. Columns indicate the mean of three experiments; Bars, S.D. Ctrl, control. NC, negative control.
To further distinguish the role of mTORC1 and mTORC2 in CXCL12-stimulated cell motility, we examined the migration of gastric cancer cells induced by CXCL12 after down-regulation of Raptor or Rictor by transiently transfection of respective siRNAs. As shown in the Fig. 3D, down-regulation of Raptor significantly inhibited CXCL12-stimulated migration of MKN-45 cells, whereas down-regulation of Rictor had little effect on this process. These data indicated that mTORC1 were more important in regulating the motility of gastric cancer MKN-45 cells.
Compounds Targeting PI3K/mTOR Pathway Prevented F-actin Reorganization and Down-regulated Active RhoA and RAC
Cell migration is a multistep cellular event, including cell polarization/protrusion (F-actin reorganization), adhesion, and de-adhesion, whereas the rapid reorganization of the actin cytoskeleton characterized as an early event of cell migration (17). Recently, it has been shown that rapamycin inhibition of insulin-like growth factor-induced cell motility is associated with its prevention of F-actin reorganization (18). We thus determined whether stimulation of CXCL12 resulted in F-actin reorganization and whether blocking PI3K/mTOR signaling prevented F-actin reorganization induced by CXCL12 in MKN-45 cells.
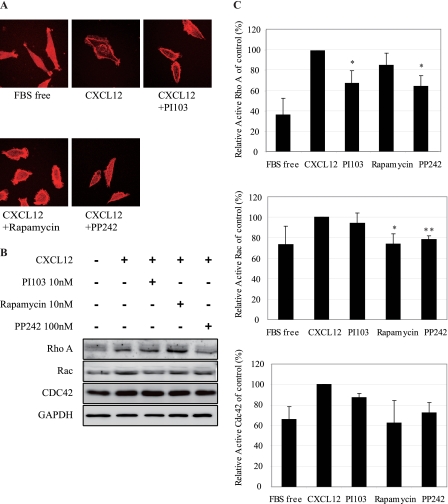
As shown in Fig. 4A, F-actin distributed randomly across cells in serum-deprived MKN-45 cells with few lamellipodia formation, which was demonstrated by staining with Texas Red®-X phalloidin. CXCL12 stimulation resulted in the formation of lamellipodia and filopodia. Within the structure of lamellipodia, the condensation of F-actin at the leading edge indicated that stimulation of cells with CXCL12 results in F-actin reorganization. Pretreatment with PI-103 or PP-242 significantly abrogated F-actin reorganization at the leading edge induced by CXCL12. These results indicated that inhibition of cell motility by targeting the PI3K/mTOR pathway is correlated to disturbance of F-actin reorganization and lamellipodia formation.
FIGURE 4.
Compounds targeting PI3K/mTOR pathway inhibited cell motility by preventing F-actin reorganization and reducing activity of RhoA, CDC42, and Rac. Serum-starved MKN-45 cells were pretreated with PI-103 (10 nm), rapamycin (10 nm), or PP-242 (100 nm) for 6 h, followed by stimulation with CXCL12 (300 ng/ml) for 10 min. A, inhibition of F-actin reorganization. F-actin was stained with Texas Red®-X phalloidin. Actin filaments (F-actin) were visualized and photographed with an Olympus BX51 microscope. B, protein levels of RhoA, Rac1, and Cdc42 were analyzed by immunoblotting. GAPDH was employed as a loading control. C, down-regulation of the active form of RhoA, Rac1, and Cdc42. Cells were harvested for assays detecting the GTP-bound RhoA (upper panel), Rac1 (middle panel), and Cdc42 (lower panel) per the manufacturer's instructions. Data shown are mean ± S.D. from three independent experiments. *, p < 0.05; **, p < 0.01, difference with CXCL12 group.
The Rho family of small GTPase, in particular Rac1, Cdc42, and RhoA, consists of molecular switches that control the organization and dynamics of the actin cytoskeleton. It has been reported recently that rapamycin inhibits cytoskeleton reorganization and cell motility stimulated by insulin-like growth factor 1 via suppressing RhoA expression and activity (19). We thus detected the protein levels of Rac1, Cdc42, and RhoA in MKN-45 cells pretreated with PI-103, rapamycin, or PP242 for 6 h before CXCL12 stimulation. As shown in 4B, PI-103, and rapamycin had little effect on the protein expression of these GTPases, whereas PP-242 slightly inhibited protein expression of RhoA and Rac1. The inconsistency between our results and previous report (19) might reflect different cell types used and time of treatment in the studies. We next examined the active form of RhoA, Rac, and Cdc42 upon inhibition of PI3K and mTOR signaling using a pulldown assay that specifically recognizes the active GTP-bound forms. As shown in Fig. 4C, CXCL12 stimulation activates RhoA, Rac, and Cdc42 in MKN-45 cells. Pretreatment of PI-103, rapamycin, or PP-242 significantly abrogated the activation of the three GTPases, indicating that inhibition of cell motility stimulated by CXCL12/CXCR4 by targeting the PI3K/mTOR pathway is mostly due to decreased active GTPases, especially RhoA.
Compounds Targeting PI3K/mTOR Pathway Down-regulated Expression of CXCl12 and CXCR4
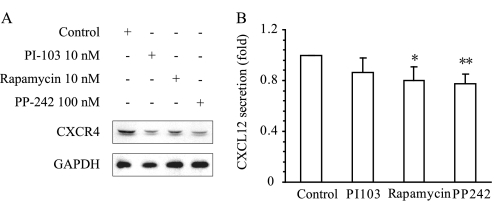
TORC1 plays a critical role in protein synthesis by phosphorylating S6 kinase and the translational inhibitor 4E-BP1. Phosphorylation of 4E-BP1 activates cap-dependent translation by dissociating with eIF-4E. Inhibition of TORC1 by rapamycin has been reported to down-regulate the expression of a number of proteins. We thus detected the effect of rapamycin on the expression of CXCR4 and CXCL12. As shown in Fig. 5A, CXCL12 enhanced the expression of CXCR4, whereas CXCR4 protein levels decreased significantly upon treatment with rapamycin for 6 h. The similar results were obtained with PI-103 and PP-242. Moreover, rapamycin, PI-103, and PP-242 inhibited the secretion of CXCL12 (Fig. 5B). These results indicated that inhibition of mTOR might form a positive feedback loop to further abolish upstream signaling leading to cell migration.
FIGURE 5.
Down-regulation of the expression of CXCL12 and CXCR4 by inhibiting mTOR pathway in MKN-45 cells. A, compounds targeting the PI3K/mTOR pathway down-regulated CXCR4 protein expression. MKN-45 cells were treated with PI-103, rapamycin, or PP-242 for 6 h, and CXCR4 protein was analyzed by immunoblotting. GAPDH was employed as a loading control. Data shown are representative of at least three independent experiments. B, PI3K pathway inhibitors inhibited the secretion of CXCL12. The supernatant was collected from the culture of MKN-45 cells after treatment with inhibitors for 24 h, and the CXCL12 level was assessed by human CXCL12 ELISA kit. The data (mean ± S.D. of three independent experiments) shown are fold change relative to the CXCL12 level in control group. *, p < 0.05; **, p < 0.01, difference with control group.
Cells Expressing High Levels of CXCL12 Sensitive to Rapamycin Anti-proliferative or Anti-migratory Activities
As rapamycin inhibited CXCL12-stimulated cell migration, we investigated whether there is association between the CXCL12 expression profile and the anti-migratory activity of rapamycin in gastric cancer cells.
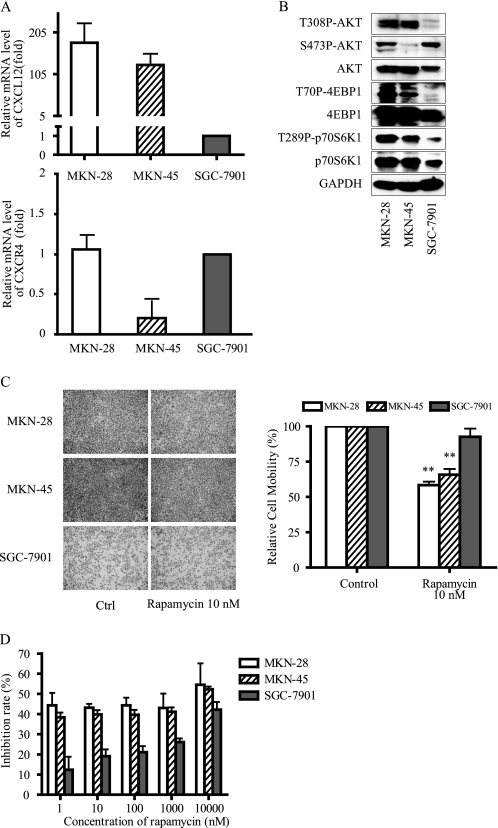
Real-time PCR was employed to detect cxcl12 and cxcr4 expression profile in three gastric cancer cell lines. As shown in Fig. 6A, MKN-45, MKN-28 cells expressed high levels of cxcl12, whereas SGC-7901 cells expressed very low levels of cxcl12. Meanwhile, mRNA level of cxcr4 in MKN45 cells are less than that in MKN-28 and SGC-7901 cells. Interestingly, it appeared that the PI3K/mTOR pathway was more activated in MKN-45 and MKN-28 cells expressing high levels of cxcl12 (Fig. 6B), i.e. high levels of phosphorylated of Akt, 4EBP1, and S6K1 were observed, which further suggested the association between the CXCL12 and PI3K/mTOR signaling pathway. We also observed that the phosphorylation level of AKT at Ser-473 did not correlate with that of AKT at Thr-308 in MKN-45 and SGC-7901 cells, which is interesting and deserves further study.
FIGURE 6.
CXCL12 expression profiles and rapamycin anti-migratory or anti-proliferative activities in gastric cancer cells. A, mRNA levels of CXCL12 and CXCR4 in gastric cancer cells. Basal levels of CXCL12 or CXCR4 (normalized to GAPDH levels) were measured by real-time PCR. Data shown are fold-change relative to the CXCL12 or CXCR4 levels in SGC-7901 cells. B, activation status of the PI3K/AKT/mTOR pathway in gastric cancer cells were analyzed by immunoblotting. GAPDH was employed as a loading control. C, anti-proliferative activity was assessed by SRB assay after exposure to rapamycin for 72 h. D, effects of rapamycin on the cell migration was determined by the Transwell assay. Representative images (B and D) and quantitative data (A, C, and D; mean ± S.D. of three independent experiments) were shown. *, p < 0.05, difference with control (Ctrl) group.
The motility of MKN-45, MKN-28, and SGC-7901 cells was detected by Transwell assay. As shown in Fig. 6C, MKN-28 and MKN-45 cells displayed higher motility than SGC-7901, with more cells migrated to the lower chamber. Furthermore, rapamycin is more potent in inhibiting the migration of MKN-28 and MKN-45 cells expressing high levels of cxcl12 with inhibitory rates of ∼40%, whereas rapamycin exhibited little activity in SGC-7901 cells expressing low levels of cxcl12 with an inhibitory rate of ∼5%. No such correlation was found with CXCR4 expression.
It has been reported that CXCL12/CXCR4 axis is involved in the survival and proliferation of tumor cells (20). We next detected the anti-proliferative activities of rapamycin in these cells by sulphorhodamine B assays. MKN-45 and MKN-28 cells, expressing high levels of cxcl12, were sensitive to rapamycin (Fig. 6D), with inhibitory rates 40∼50% after exposure to 1 nm rapamycin for 72 h. By contrast, SGC-7901 cells expressing low levels of cxcl12, were resistant to rapalogs. Rapamycin inhibited the proliferation of those cells by ∼10% at the same concentration (Fig. 6D).
To investigate whether expression of cxcl12 is correlated with the anti-proliferative activity of rapamycin, we computed pairwise Pearson correlation coefficients (r) between rapamycin activity and genes expression patterns in NCI-60 cells. Analysis of 9,706 gene probes, including both proliferative and non-proliferative genes, discovered that expression of cxcl12 significantly correlate with rapamycin activity (r = 0.395, p = 0.0011). Ten most positively correlated genes were listed in Table 1. Although the cell line samples in the present study are small, the results obtained provided a clue that mTOR inhibitor may be used for therapy of metastatic gastric cancer expressing high levels of cxcl12.
TABLE 1.
Pearson correlations (r) between rapamycin activity (−lgGI50) and gene expression patterns in the NCI-60 cell lines based on data from the Affymetrix HG-U133A chip
| Rank | Gene name | Gene description | r | p value |
|---|---|---|---|---|
| 1 | CDKN1B | Cyclin-dependent kinase inhibitor 1B (p27, Kip1) | 0.473 | 0.0000 |
| 2 | ATP8A2 | ATPase, aminophospholipid transporter-like, class I, type 8A, member 2 | 0.436 | 0.0005 |
| 3 | CYFIP2 | Cytoplasmic FMR1-interacting protein 2 | 0.408 | 0.0010 |
| 4 | SOX1 | SRY (sex determining region Y)-box 1 | 0.402 | 0.0011 |
| 5 | C5orf13 | Chromosome 5 open reading frame 13 | 0.399 | 0.0007 |
| 6 | CXCL12 | Chemokine (CXC motif) ligand 12 (stromal cell-derived factor 1) | 0.395 | 0.0011 |
| 7 | PIP5K2A | Phosphatidylinositol 4-phosphate 5-kinase type II α | 0.384 | 0.0009 |
| 8 | LOC283567 | Hypothetical protein LOC283567 | 0.382 | 0.0008 |
| 9 | MGC16169 | Hypothetical protein MGC16169 | 0.382 | 0.0010 |
| 10 | NEK11 | NIMA (never in mitosis gene a)-related kinase 11 | 0.3792 | 0.0031 |
DISCUSSION
In the present study, we elucidated that the CXCL12/CXCR4 axis activated PI3K and revealed that p110β catalytic isoform provided a conduit for CXCL12-activated PI3K signaling pathway in gastric cancer cells. Inhibitors targeting the PI3K/mTOR pathway blocked cell migration induced by CXCL12, which was attributed to decrease in the activity of Rac, Cdc42, and RhoA and inhibition of F-actin reorganization. Rapamycin also decreased the secretion of CXCL12 and expression of its receptor CXCR4, which might form a positive feedback loop to further abolish upstream signaling leading to cell migration. We also found a positive correlation between cxcl12 expression and rapamycin activity in inhibiting cells migration and proliferation. Taken together, we proposed that inhibitors targeting the mTOR pathway may be used for therapy of metastatic gastric cancers expressing high levels of cxcl12.
The chemokine CXCL12 binds primarily to the CXCR4 receptor and constitutes a biological axis, which is related to tumor progression, angiogenesis, metastasis, and survival (3). Blocking CXCL12/CXCR4 interaction or inhibiting downstream intracellular signaling pathway may be useful for cancer therapy (4). We found that CXCL12 stimulation activated PI3K/Akt/mTOR signaling cascade in gastric cancer MKN-45 cells, which is consistent with the previous report, where phosphorylated Akt was utilized as an indirect marker for the activation of PI3K (7, 22–25). By employing CHO-K1 cells stably expressing pEGFP-C1-Grp1-PH fusion protein, we demonstrated that CXCL12 stimulation resulted in generation of PtdIns(3,4,5)P3 and the translocation of protein contained PH domain to cellular membrane, providing direct evidence that CXCL12 activated PI3K. We further revealed that p110β was the main PI3K isoform coupled with the CXCL12/CXCR4 axis in gastric cancer cells, which was further supported by the fact that CXCL12 enhanced the association between CXCR4 and p110β. It has been reported that CXCL12 activate the activity of PI3K in Gi protein inhibitor pertussis toxin-treated cells, indicating both Gαi and Gα13 proteins can mediate PI3K activation or this activation is G protein-independent (8). However, Gi protein was reported to be required for the activation of PI3K by CXCL12 in T lymphocytes (26). In aforementioned studies, only p110γ was detected to be associated with CXCR4 after CXCL12 stimulation. Recently, it has been reported p110β isoform signals downstream of G protein-coupled receptors, including CXCR4, and is functionally redundant with p110γ (27). We found that pertussis toxin pretreatment abrogated phosphorylation of Akt at Ser-473 induced by CXCL12, indicating that Gi protein is required for activation of PI3K by CXCR4. Although how CXCR4 interacts with p110β deserves to be further studied, our data shed new light on the understanding of the precise signaling underlying the activation of mTOR by CXCL12 in gastric cancer cells.
It has been reported that expression of CXCR4 and CXCL12 was associated with lymph node and liver metastasis in intestinal-type gastric cancer (28). The CXCL12/CXCR4 axis and its downstream signaling could be therapeutic targets for preventing metastasis of gastric cancer. Moreover, it has been reported that both mTORC1 and mTORC2 play critical roles in the regulation of tumor cell motility and cancer metastasis (12, 19). We found that the CXCL12/CXCR4 axis was able to activate the PI3K/mTOR pathway and stimulate cell migration in human MKN-45 gastric cancer cells. On the other hand, inhibition of PI3K/mTOR pathway by the PI3K/mTOR dual inhibitor PI-103, TORC1 inhibitor rapamycin, or TORC1/2 inhibitor PP-242 significantly blocked the chemotactic responses of MKN-45 cells triggered by CXCL12, which is related to prevention of F-actin reorganization and down-regulation of active RhoA, Rac1, and Cdc42. Interestingly, inhibition of cell migration by compounds targeting the PI3K/mTOR pathway was primarily attributed to their action against mTORC1, as down-regulation of Raptor but not Rictor impeded migration of MKN-45 cells.
Furthermore, rapamycin treatment simultaneously decreased the secretion of chemokine CXCL12 and the expression of its receptor CXCR4 in MKN cells (Fig. 5). It has been reported that CXCR4 is a transcriptional target of HIF-1α (25), and Song et al. reported that PDGF-BB induces CXCL12 expression through HIF-1α activation (29). Inhibition of mTOR by rapamycin results in down-regulation of HIF-1α via block of cap-dependent translation. Therefore, decreased CXCR4 and CXCL12 expression by rapamycin could either due to global inhibition of protein translation or decreased transcription mediated by HIF-1α, which deserves further exploration. Autocrine of CXCL12 from tumor cells is able to activate CXCR4 receptor, which will promote cell motility as well as the viability of tumor cells at distant metastasis (2). Taken together, rapamycin not only inhibited CXCL12/CXCR downstream signaling but also down-regulated the expression of the chemokine and its receptor, which might form a positive feedback loop to block cell migration. Targeting the PI3K/mTOR pathway may be a new strategy for preventing gastric tumor metastasis mediated by the CXCL12/CXCR4 axis, even though additional preclinical and clinical studies are required to validate this proposal.
Recent study revealed that CXCL12 could be an independent prognostic factor in gastric cancer, with CXCL12-positive gastric cancer showing more aggressive behavior, including metastasis (2). We found that rapamycin-inhibited CXCL12 stimulation activated the PI3K/mTOR pathway and displayed selectivity to CXCL12-stimulated cell migration. These results were consistent with the observation that cells expressing high levels of CXCL12 were more sensitive to rapamycin-induced anti-metastatic activity. Meanwhile, we also found the positive correlation of cxcl12 expression and rapamycin anti-proliferative activity in gastric cancer cells. This point was also confirmed by studying the correlation between cxcl12 gene expression profiles and rapamycin activity in NCI-60 cell lines. Sporadic studies indicated that CXCL12 mediated the proliferation of cancer cells, which may be related to activation of Akt, Erk, Akt-1/FOXO-3α, Stat-3, and etc. (7, 20, 21, 30, 31). Although additional study is warranted to elucidate the correlation between rapamycin anti-proliferation activity and cxcl12 expression, this finding further supports that mTOR inhibitor may be useful for gastric cancer therapy.
In conclusion, we have shown that mTOR pathway play an important role in cell migration mediated by the CXCL12/CXCR4 axis and inhibitors targeting mTOR pathway could inhibit CXCL12-stimulated cell migration. On the other hand, cells that express high levels of cxcl12 were more sensitive to rapamycin in its activity in inhibiting both cell migration and proliferation. We proposed that drugs targeting the mTOR pathway may be used for the therapy of metastatic gastric cancer expressing high levels of cxcl12.
This work was supported in part by National Science & Technology Major Project “Key New Drug Creation and Manufacturing Program” 2012ZX09301-001, National Natural Science Foundation of China Grant 81021062′81173079, and Knowledge Innovation Program of Chinese Academy of Sciences Grant KSCX2-EW-Q-3.
- S6K1
- S6 kinase 1
- PtdIns(3,4,5)P3
- phosphatidylinositol 3,4,5-trisphosphate
- PH
- pleckstrin homology
- EGFP
- enhanced GFP.
REFERENCES
- 1. Boku N. (2008) Chemotherapy for metastatic gastric cancer in Japan. Int. J. Clin. Oncol. 13, 483–487 [DOI] [PubMed] [Google Scholar]
- 2. Ishigami S., Natsugoe S., Okumura H., Matsumoto M., Nakajo A., Uenosono Y., Arigami T., Uchikado Y., Setoyama T., Arima H., Hokita S., Aikou T. (2007) Clinical implication of CXCL12 expression in gastric cancer. Ann. Surg. Oncol. 14, 3154–3158 [DOI] [PubMed] [Google Scholar]
- 3. Balkwill F. (2004) Cancer and the chemokine network. Nat. Rev. Cancer 4, 540–550 [DOI] [PubMed] [Google Scholar]
- 4. Teicher B. A., Fricker S. P. (2010) CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin. Cancer Res. 16, 2927–2931 [DOI] [PubMed] [Google Scholar]
- 5. Baselga J., Semiglazov V., van Dam P., Manikhas A., Bellet M., Mayordomo J., Campone M., Kubista E., Greil R., Bianchi G., Steinseifer J., Molloy B., Tokaji E., Gardner H., Phillips P., Stumm M., Lane H. A., Dixon J. M., Jonat W., Rugo H. S. (2009) Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J. Clin. Oncol. 27, 2630–2637 [DOI] [PubMed] [Google Scholar]
- 6. Guertin D. A., Sabatini D. M. (2007) Defining the role of mTOR in cancer. Cancer Cell 12, 9–22 [DOI] [PubMed] [Google Scholar]
- 7. Hashimoto I., Koizumi K., Tatematsu M., Minami T., Cho S., Takeno N., Nakashima A., Sakurai H., Saito S., Tsukada K., Saiki I. (2008) Blocking on the CXCR4/mTOR signaling pathway induces the anti-metastatic properties and autophagic cell death in peritoneal disseminated gastric cancer cells. Eur. J. Cancer 44, 1022–1029 [DOI] [PubMed] [Google Scholar]
- 8. Monterrubio M., Mellado M., Carrera A. C., Rodríguez-Frade J. M. (2009) PI3Kγ activation by CXCL12 regulates tumor cell adhesion and invasion. Biochem. Biophys. Res. Commun. 388, 199–204 [DOI] [PubMed] [Google Scholar]
- 9. Easton J. B., Houghton P. J. (2006) mTOR and cancer therapy. Oncogene 25, 6436–6446 [DOI] [PubMed] [Google Scholar]
- 10. Yang Q., Guan K. L. (2007) Expanding mTOR signaling. Cell Res. 17, 666–681 [DOI] [PubMed] [Google Scholar]
- 11. Zhou H., Huang S. (2010) mTOR signaling in cancer cell motility and tumor metastasis. Crit. Rev. Eukaryot. Gene Expr. 20, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jacinto E., Loewith R., Schmidt A., Lin S., Rüegg M. A., Hall A., Hall M. N. (2004) Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin-insensitive. Nat. Cell Biol. 6, 1122–1128 [DOI] [PubMed] [Google Scholar]
- 13. Liu L., Li F., Cardelli J. A., Martin K. A., Blenis J., Huang S. (2006) Rapamycin inhibits cell motility by suppression of mTOR-mediated S6K1 and 4E-BP1 pathways. Oncogene 25, 7029–7040 [DOI] [PubMed] [Google Scholar]
- 14. Chen G., Yang N., Wang X., Zheng S. Y., Chen Y., Tong L. J., Li Y. X., Meng L. H., Ding J. (2010) Identification of p27/KIP1 expression level as a candidate biomarker of response to rapalog therapy in human cancer. J. Mol. Med. 88, 941–952 [DOI] [PubMed] [Google Scholar]
- 15. Li T., Wang J., Wang X., Yang N., Chen S. M., Tong L. J., Yang C. H., Meng L. H., Ding J. (2010) WJD008, a dual phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin inhibitor, prevents PI3K signaling and inhibits the proliferation of transformed cells with oncogenic PI3K mutant. J. Pharmacol. Exp. Ther. 334, 830–838 [DOI] [PubMed] [Google Scholar]
- 16. Engelman J. A., Luo J., Cantley L. C. (2006) The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 7, 606–619 [DOI] [PubMed] [Google Scholar]
- 17. Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. (2003) Cell migration: Integrating signals from front to back. Science 302, 1704–1709 [DOI] [PubMed] [Google Scholar]
- 18. Liu L., Chen L., Chung J., Huang S. (2008) Rapamycin inhibits F-actin reorganization and phosphorylation of focal adhesion proteins. Oncogene 27, 4998–5010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu L., Luo Y., Chen L., Shen T., Xu B., Chen W., Zhou H., Han X., Huang S. (2010) Rapamycin inhibits cytoskeleton reorganization and cell motility by suppressing RhoA expression and activity. J. Biol. Chem. 285, 38362–38373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barbero S., Bonavia R., Bajetto A., Porcile C., Pirani P., Ravetti J. L., Zona G. L., Spaziante R., Florio T., Schettini G. (2003) Stromal cell-derived factor 1α stimulates human glioblastoma cell growth through the activation of both extracellular signal-regulated kinases 1/2 and Akt. Cancer Res. 63, 1969–1974 [PubMed] [Google Scholar]
- 21. Wald O., Izhar U., Amir G., Kirshberg S., Shlomai Z., Zamir G., Peled A., Shapira O. M. (2011) Interaction between neoplastic cells and cancer-associated fibroblasts through the CXCL12/CXCR4 axis: Role in non-small cell lung cancer tumor proliferation. J. Thorac Cardiovasc. Surg. 141, 1503–1512 [DOI] [PubMed] [Google Scholar]
- 22. Zhao M., Mueller B. M., DiScipio R. G., Schraufstatter I. U. (2008) Akt plays an important role in breast cancer cell chemotaxis to CXCL12. Breast Cancer Res. Treat. 110, 211–222 [DOI] [PubMed] [Google Scholar]
- 23. Chinni S. R., Sivalogan S., Dong Z., Filho J. C., Deng X., Bonfil R. D., Cher M. L. (2006) CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: The role of bone microenvironment-associated CXCL12. Prostate 66, 32–48 [DOI] [PubMed] [Google Scholar]
- 24. Leelawat K., Leelawat S., Narong S., Hongeng S. (2007) Roles of the MEK1/2 and AKT pathways in CXCL12/CXCR4-induced cholangiocarcinoma cell invasion. World J. Gastroenterol. 13, 1561–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Phillips R. J., Mestas J., Gharaee-Kermani M., Burdick M. D., Sica A., Belperio J. A., Keane M. P., Strieter R. M. (2005) Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia-inducible factor-1α. J. Biol. Chem. 280, 22473–22481 [DOI] [PubMed] [Google Scholar]
- 26. Sotsios Y., Whittaker G. C., Westwick J., Ward S. G. (1999) The CXC chemokine stromal cell-derived factor activates a Gi-coupled phosphoinositide 3-kinase in T lymphocytes. J. Immunol. 163, 5954–5963 [PubMed] [Google Scholar]
- 27. Guillermet-Guibert J., Bjorklof K., Salpekar A., Gonella C., Ramadani F., Bilancio A., Meek S., Smith A. J., Okkenhaug K., Vanhaesebroeck B. (2008) The p110β isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110γ. Proc. Natl. Acad. Sci. U.S.A. 105, 8292–8297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iwasa S., Yanagawa T., Fan J., Katoh R. (2009) Expression of CXCR4 and its ligand SDF-1 in intestinal-type gastric cancer is associated with lymph node and liver metastasis. Anticancer Res. 29, 4751–4758 [PubMed] [Google Scholar]
- 29. Song N., Huang Y., Shi H., Yuan S., Ding Y., Song X., Fu Y., Luo Y. (2009) Overexpression of platelet-derived growth factor-BB increases tumor pericyte content via stromal-derived factor-1α/CXCR4 axis. Cancer Res. 69, 6057–6064 [DOI] [PubMed] [Google Scholar]
- 30. Ehtesham M., Mapara K. Y., Stevenson C. B., Thompson R. C. (2009) CXCR4 mediates the proliferation of glioblastoma progenitor cells. Cancer Lett. 274, 305–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu Y., Peng H., Cui M., Whitney N. P., Huang Y., Zheng J. C. (2009) CXCL12 increases human neural progenitor cell proliferation through Akt-1/FOXO3a signaling pathway. J. Neurochem. 109, 1157–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]