Background: Survival of neonatal hippocampal neurons during developmental neuronal death requires integrin.
Results: Levels of laminin increase because levels of matrix metalloproteinase-9 decrease during developmental neuronal death.
Conclusion: Matrix metalloproteinase-9 regulates survival of neurons by regulating laminin-integrin β1 signaling during developmental neuronal death.
Significance: This is the first report to show a role of matrix metalloproteinases in the survival mechanism in neurons during a critical developmental process.
Keywords: Akt, Akt PKB, Extracellular Matrix, Laminin, Matrix Metalloproteinase (MMP), Developmental Death
Abstract
The number of neurons in the adult rodent brain is strongly influenced by events in early postnatal life that eliminate approximately half of the neurons. Recently, we reported that neurotrophins induced survival of neonatal rat hippocampal neurons by promoting neural activity and activation of the Ser/Thr kinase, Akt. The survival of neurons also depended on integrin signaling, but a role for the extracellular matrix (ECM) in this mechanism was yet to be explored. Here, we show that levels of the matrix metalloproteinase-9 (MMP9) decrease, and the level of the ECM protein laminin increases in rat hippocampus during the period of neuronal death. Hippocampi from MMP9 null mice showed higher levels of laminin expression than wild type at P1 and no further increase at P10. In vitro, the matrix metalloproteinase inhibitor FN-439 promoted survival of neurons in a laminin-integrin β1-dependent manner. Blocking laminin signaling attenuated activation of Akt by depolarization. In vivo, injecting FN-439 into the neonatal hippocampus increased the level of laminin and promoted neuronal survival through an integrin-dependent mechanism. These results show signals from the ECM are not simply permissive but rather actively regulated, and they interact with neuronal activity to control the number of hippocampal neurons. This work is the first to report a role for MMP9 in regulating neuronal survival through the developmental process that establishes the functional brain.
Introduction
Extracellular matrix (ECM)2 is composed of proteins that include laminin, fibronectin, and collagen. Integrins serve as receptors for ECM proteins and consist of heterodimers (α and β (1)). Integrin-ECM interactions provide cells with signaling cues and play essential roles in fundamental aspects of cell biology, such as morphogenesis, migration, and survival. Matrix metalloproteinases (MMPs) regulate cell-ECM interactions through the cleavage of ECM proteins (2, 3). In the adult brain, proteolysis of ECM is a major cause of excitotoxin-induced neuronal death (4, 5). Excitotoxic stimuli up-regulate MMP activity (6) and induce changes in the electrophysiological properties of neurons (7, 8). However, the role of MMPs and their substrate ECM proteins during development and neuronal maturation is unknown.
Neuron number is controlled by a process of cell death throughout the central nervous system during postnatal development. In the rodent hippocampus, this developmental death occurs during the first postnatal week (9, 10). Spontaneous neural network activity that is thought to be important for the development of functional neuronal circuits occurs during this time in the neonatal hippocampus (11). Recently, we reported that the development of active networks plays a key role in the survival of neonatal hippocampal neurons (12). We found that neurotrophins promote neurons to join active networks such that calcium influx through L-type voltage-gated calcium channels regulates survival through sustained activation of the serine-threonine kinase Akt. This sustained activation of Akt also requires integrin β1 (12). These results demonstrate the importance of an integrin-dependent signal in the survival of neonatal neurons. However, whether this signal is merely permissive or actively regulates neuronal survival was unknown.
Here, we report that MMP9 is expressed at high levels in the immediate postnatal hippocampus and that their expression levels fall just as laminin levels increase. Delivering an MMP inhibitor to the hippocampus in vitro and in vivo controls neuron numbers through a laminin-dependent process. These results assign a critical role to MMP9 and ECM in the activity-dependent regulation of neuron numbers in the developing hippocampus and may also help illuminate disease conditions in adult brain.
EXPERIMENTAL PROCEDURES
Reagents
FN-439 was purchased from Calbiochem. DQ gelatin from pig skin and fluorescein conjugate were purchased from Molecular Probes. 4′,6′-Diamidino-2-phenylindole dihydrochloride (DAPI), laminin, and p-aminophenylmercuric acetate were purchased from Sigma. Mouse/rat recombinant MMP2 (rMMP2), rat recombinant MMP9 (rrMMP9), and fibronectin were purchased from R & D Systems.
Animals
Sprague-Dawley rats were purchased from Charles River. FVB/NJ control mice and MMP9 null mice were purchased from The Jackson Laboratory.
Antibodies
Antibodies were used for Western blot and immunostaining with the following dilutions: polyclonal rabbit anti-cleaved caspase 3 (c-cas3), anti-phospho-Ser-473 Akt, and anti-pan-Akt (Cell Signaling Technology), 1:500; monoclonal mouse anti-NeuN (Millipore); polyclonal rabbit anti-MMP2 and anti-MMP9 (Torrey Pines Biolabs), 1:200; polyclonal rabbit anti-phospho-Ser-166 murine double minute 2 (MDM2) and anti-p53 (Santa Cruz Biotechnology), 1:200; polyclonal rabbit anti-laminin, monoclonal mouse anti-MAP2, and anti-β-actin (Sigma), 1:1000; Alexa 488-conjugated goat anti-mouse IgG, Alexa 546-conjugated goat anti-rabbit IgG, Alexa 488-conjugated donkey anti-goat IgG, and Alexa 546-conjugated donkey anti-mouse IgG (Molecular Probes), 1:500. For function blocking, hamster anti-integrin β1 (50 mg/ml; BD Biosciences) and rabbit anti-laminin (50 mg/ml; Sigma) were used.
Antibody Pre-absorption
Prior to pre-absorption, 100 ml of 0.5 mg/ml rabbit anti-laminin (Sigma) was dialyzed to remove sodium azide with 1 liter of phosphate-based saline (PBS) overnight at 4 °C using Slide-A-Lyzer dialysis cassette G2 (Thermo Scientific). Culture dishes were coated with 3 μg/ml laminin (Sigma) or fibronectin (R & D) in PBS for 2 h followed by washing with distilled water. The dialyzed antibody was diluted to 50 μg/ml with Neurobasal and B27 (Invitrogen), applied to the laminin- or fibronectin-coated culture dishes, and incubated for 2 h at 4 °C. To confirm the efficiency of pre-absorption, media containing antibody were applied to a gel for SDS-PAGE, and rabbit IgG was detected with Western blot analysis.
Dissociated Cell Cultures
Culture was prepared as described previously (13). Hippocampi from embryonic day 18 (E18) Sprague-Dawley rat embryos were used for both astrocyte (plated at a density of 80,000 cells/ml) and neuron (density, 200,000 cells/ml) cultures. Astrocytes were cultured in Neurobasal (Invitrogen) with 5% fetal bovine serum (FBS) in 5% CO2 at 37 °C for 14 days. Medium was changed completely twice a week. Neurons were plated on confluent astrocyte beds and cultured in Neurobasal and B27 in 5% CO2 at 37 °C. Half of the medium was changed every 2 days.
Western Blot
Hippocampi were taken from a P1 or P10 rat or FVB/NJ control mouse or MMP9 null mouse (The Jackson Laboratory), weighed, and homogenized in 300% (v/w) lysis buffer (150 mm NaCl, 1% Nonidet P-40, 50 mm Tris-HCl, pH 8.0) containing a protease inhibitor mixture (Roche Applied Science) on ice. Homogenates were then diluted with an equal volume of 2× SDS loading buffer. Samples from dissociated culture were collected with 1× SDS loading buffer (60 μl per one 24-well culture dish). The samples were boiled for 5 min, and then applied to a 4–10% gradient SDS gel (Bio-Rad). The proteins were transferred to a nitrocellulose membrane. The membranes were blocked with 4% skim milk in PBS for 30 min. Incubation with antibodies was performed in the blocking solution. Membranes were washed with Tris-buffered saline with 0.05% Tween 20. The proteins were visualized with SuperSignal West Pico System (Pierce), detected, and analyzed with a BioChemi System (UVP BioImaging Systems). Means ± S.E. are plotted.
Immunoprecipitation
Hippocampi were taken from P1 or P10 rat pups and homogenized in 6.5 ml of lysis buffer (150 mm NaCl, 1% Nonidet P-40, and 50 mm Tris-HCl, pH 8.0), containing a protease inhibitor mixture (Roche Applied Science) per 1 g of tissue, and centrifuged at 12,000 × g for 10 min. Supernatants were pre-absorbed with 10% (v/v) protein A-conjugated Sepharose beads (Amersham Biosciences) for 1 h and then centrifuged at 3000 × g for 3 min. The supernatant was incubated with 1% (v/v) antibodies for 2 h followed by 10% (v/v) protein A-conjugated Sepharose beads for 1 h. The beads were then washed twice with the lysis buffer. Proteins were eluted with 10 times (v/v) nonreducing SDS sample buffer. Procedure was done at 4 °C.
Gel Zymography
m/rrMMP2 and rrMMP9 were incubated with 1 mm p-aminophenylmercuric acetate at 37 °C for 6 h for activation (15). 10 ng/lane recombinant MMPs or equal volumes (30 μl) of immunoprecipitation samples were applied to Ready Gel Zymogram Gel (Bio-Rad; 10% SDS-PAGE containing gelatin) under nonreducing condition. Gels were washed two times for 15 min with 2.5% Triton X-100 with/without 50 μm FN-439 and then incubated in 50 mm Tris-HCl, pH 7.5, with 10 mm CaCl2, 1 mm ZnCl2, 1% Triton X-100, 0.02% sodium azide (and 50 μm FN-439) at 37 °C for 2 days. Gelatinolysis was visualized with Coomassie Brilliant Blue R-250 staining followed by destaining solution (Bio-Rad).
In Vivo Injection
In vivo injection to CA1 was described previously (16). Briefly, Sprague-Dawley rat pups (P2) of either sex were anesthetized by hypothermia (in ice for 5 min) prior to the surgery. The anesthetized animal was placed on ice in a stereotaxic instrument. The stereotaxic coordinates from bregma are as follows: anterior-posterior +1.5; midline, ±1.8; ventral-dorsal, −1.8 mm. 0.3 μl of reagents were delivered at a rate of 0.1 μl/min using a Hamilton syringe with an LASI needle attached to a pump. FN-439 was injected at 720 μm. Hamster anti-integrin β1 antibody has been reported to block β1 subunit-containing integrins (17). Anti-integrin β1 antibody was injected at 0.5 mg/ml. PBS was used as control. Pups were kept at 37 °C for 1–2 h to recover from anesthesia, and then returned to their mother and kept for 2 days.
In Situ Zymography
In situ zymography was performed following the method of Oh et al. (18). Brains from P4 pups were quickly dissected and frozen in dry ice. The frozen brains were then immersed in ornithine carbamoyltransferase compound (Tissue-Tek) on dry ice. Hippocampal slices of 300 μm thickness were incubated with 50 mm Tris-HCl, pH 7.5, with 150 mm NaCl, 5 mm CaCl2 and 0.02% sodium azide (and 50 μm FN-439) containing 40 μg/ml DQ gelatin fluorescein conjugate at 37 °C overnight. Proteolysis by gelatinases cleaves intramolecularly quenched DQ gelatin-FITC into fluorescent peptides. Brain sections were washed with PBS three times and fixed with 4% paraformaldehyde on ice for 15 min. All fluorescence images were taken using the same exposure time, and the fluorescence intensities of the CA3 region were analyzed using ImageJ software (National Institutes of Health).
Reverse Transcription (RT)-PCR
Hippocampi were taken from P1 or P10 rat, weighed, and homogenized in 300% (v/w) lysis buffer (150 mm NaCl, 1% Nonidet P-40, 50 μm Tris-HCl, pH 8.0) containing a protease inhibitor mixture (Roche Applied Science) on ice. RNA was isolated from the homogenates using TriPure isolation reagent (Roche Applied Science). RT-PCR was performed using SuperScript first-strand synthesis system for RT-PCR (Invitrogen). Using 5 μg of total RNA, first-strand cDNA synthesis reaction by reverse transcriptase was done using oligo(dT)12–18 as primers. PCR was performed using Taq polymerase (Roche Applied Science). The sequences of the primers are the following: CCACACTTTCTACAATGAGC and CCGTCAGGATCTTCATGAGG for β-actin; CTATTCTGTCAGCACTTTGG and CAGACTTTGGTTCTCCAACTT for MMP2; AAATGTGGGTGTACACAGGC and TTCACCCGGTTGTGGAAACT for MMP9; and TGAAGTCGAACAGCTCT and TGTCTGCAGTGACTTTA for laminin β1 chain. Conditions for PCRs are as follows: 35 cycles at 95 °C (30 s), 57 °C (30 s), and 72 °C (2 min) for MMP2 and β-actin; 35 cycles at 95 °C (30 s), 62 °C (30 s), and 72 °C (2 min) for MMP9; and 35 cycles at 95 °C (30 s), 60 °C (30 s), and 72 °C (2 min) for laminin β1 chain. The primers yield ∼300-bp products. The PCR products were separated in 2% agarose gel.
Immunostaining
Cultures were fixed with 4% paraformaldehyde, permeabilized in 0.5% Triton X-100, and blocked with 4% normal goat serum (NGS, Vector Laboratories). For immunohistochemistry, pups were perfused with PBS and then 4% paraformaldehyde. Brains were fixed with 4% paraformaldehyde for 2 days followed by incubation with 20% sucrose for 1 day at 4 °C and then frozen in dry ice-chilled 2-methyl butane for 45 s. The frozen brains were then immersed in ornithine carbamoyltransferase compound (Tissue-Tek) on dry ice. Coronal cryostat 16-μm sections were blocked with 4% NGS in PBS with 0.1% Triton X-100. Antibodies were diluted in 4% NGS. Fluorescent images were taken with an Apotome (Zeiss, Thornwood, NY, USA) with 10 or 40× lenses. Each image was taken at 1384 × 1040 pixels with 150 pixels/inch resolution. To compare intensities, the same exposure time was used for all samples. Resolution of fluorescence intensity was 254 levels.
Cleavage of Hippocampal Laminin by MMPs
Coronal cryostat 16-μm sections of P10 brains were first incubated at 65 °C overnight to reverse formaldehyde cross-links (19). The sections were then incubated with PBS containing 10 ng/ml activated mouse/rat rMMP2 or rrMMP9 (and 50 μm FN-439) at 37 °C for 24 h and washed twice with PBS. Sections were re-fixed with 4% paraformaldehyde at 4 °C for 20 min, permeabilized in 0.5% Triton X-100, and blocked with 4% NGS and then immunostained.
Cell Quantification
Cell quantification was performed as described previously (12). Neurons were visualized by immunostaining neuron-specific microtubule-associated protein 2 (MAP2) (20). Fluorescence images were obtained from the following five fields; one from the center of the coverslip and two vertically and two horizontally 400–3000 μm from the center. We avoided sampling the edge of coverslips where densities of neurons were higher than other regions. Each coverslip was defined as an individual culture. Numbers represent means ± S.E. All results were obtained from multiple cultures. All analyses were done blind.
To quantify the number of apoptotic neurons in the neonatal hippocampus, every fourth sequential section was used for immunohistochemistry. Identification of individual cells was confirmed by DAPI staining (to visualize nuclei), and the cell type was determined by immunostaining with the neuron-specific nuclear protein, NeuN (21). Ratio of apoptotic (c-cas3+) neurons (NeuN+) over total neurons in one hippocampus was defined as n = 1. All analyses were done blind.
Statistical Analyses
Statistical significance between two groups was determined with a two-tailed paired Student's t test. For multiple groups, statistical comparisons were made by ANOVA followed by individual group tests with Bonferroni corrections for multiple comparisons.
RESULTS
Levels of MMP2 and MMP9 Decrease during the First 10 Postnatal Days
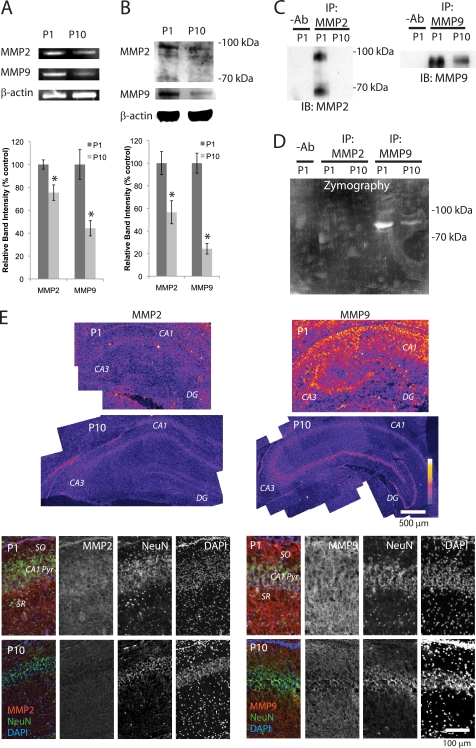
MMP2 and MMP9 are expressed in adult hippocampus (6). To determine whether these MMPs are expressed in rat neonatal hippocampus, we first performed reverse transcription (RT)-PCR analyses. The results of RT-PCR showed both MMP2 and MMP9 were highly expressed at postnatal day 1 (P1) but less expressed at P10 (Fig. 1A), suggesting that transcriptions of these genes decreases with age. To test whether levels of protein changed during this time, we next performed Western blot analyses. As we had reported previously (6), the antibody against MMP2 detected the expected band at ∼70 kDa and, in addition, a band with a high molecular weight (98 kDa) from P1 hippocampus (Fig. 1B). MMP9 (∼95 kDa) was also present in P1 hippocampus (Fig. 1B). However, levels of both MMPs had decreased significantly by P10 (Fig. 1B). We used gel zymography to test whether these MMPs were active. When MMP2 and MMP9 were immunoprecipitated with their specific antibodies (6), we detected both active and latent forms of MMP2 in P1 hippocampus (Fig. 1C), although we observed very weak enzymatic activity (Fig. 1D). We detected strong activity of MMP9 in the P1 hippocampus, which had dramatically decreased by P10 (Fig. 1, C and D). Consistent with the Western blot results, immunohistochemical analysis of P1 and P10 hippocampal regions showed a decrease in levels of MMP2 and MMP9 between the two time points (Fig. 1E). Double staining of the CA1 region with a neuronal marker, NeuN (21), showed MMP2 and MMP9 were both present at P1 in the pyramidal cell layer, the striatum oriens (SO), and the stratum radiatum, but by P10 the amounts had decreased dramatically (Fig. 1E). These results show levels of MMPs are developmentally regulated during the first postnatal 10 days.
FIGURE 1.
Levels of MMP2 and MMP9 decrease during the first 10 postnatal days. A, RT-PCR analyses of MMP2 and MMP9 in P1 and P10 hippocampi. β-Actin was used as a control (n = 4). Means ± S.E. are plotted. Asterisk, p < 0.0001 (Student's t test). B, Western blot analyses of MMP2 and MMP9 in P1 and P10 hippocampi. β-Actin was used as a control (n = 4). Means ± S.E. are plotted. Asterisk, p < 0.0001 (Student's t test). C, immunoprecipitation (IP) of MMP2 and MMP9 from P1 and P10 hippocampi. −Ab, homogenate from P1 hippocampi was incubated with protein-A agarose without antibody. IB, immunoblot. D, gel zymography of MMP2 and MMP9 immunoprecipitated from P1 and P10 hippocampi. E, immunostaining of MMP2 and MMP9. Upper panel, P1 and P10 hippocampi. Fluorescence intensity is represented by a color scale. Lower panel, CA1 regions from P1 and P10 are shown. MMP2 or MMP9 (red); the neuronal marker protein NeuN (green); and DAPI nuclear stain (blue).
Levels of Laminin in Hippocampal Neurons Increase during the First 10 Postnatal Days
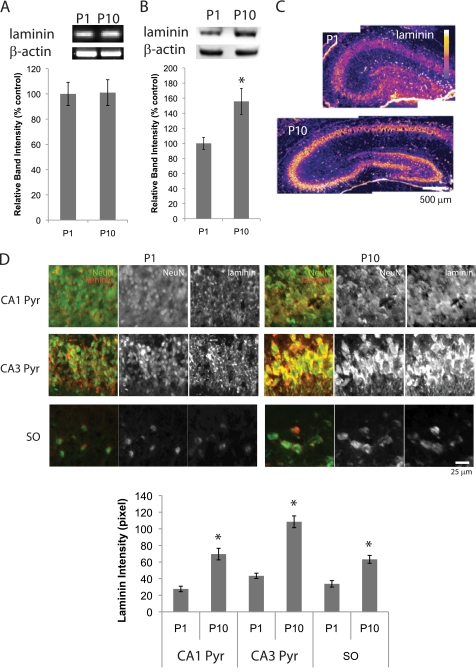
Because levels of MMPs decrease during the first 10 postnatal days, we tested whether components of the ECM, such as laminin, a substrate for MMPs, change expression levels. Laminin β1 is expressed in both neonatal and adult hippocampus (22). When the transcription of laminin β1 was analyzed by RT-PCR (23), we found no changes in message level between P1 and P10 (Fig. 2A). However, Western blot analyses of P1 and P10 hippocampus clearly showed that laminin levels increased between P1 and P10 (Fig. 2B). To check which cells express laminin in the neonatal hippocampus, we next performed immunohistochemistry. As shown previously in adult hippocampus (4), laminin was expressed mainly in the cell body of hippocampal neurons (Fig. 2, C and D). Consistent with the results of the Western blots, immunohistochemical analyses showed that between P1 and P10, neurons in hippocampus expressed increasing levels of laminin (Fig. 2, C and D). In addition to CA1 and CA3 pyramidal cells, interneurons in the SO also expressed laminin mainly in cell bodies and increased the levels of laminin expression between P1 and P10 (Fig. 2D). These results show that hippocampal neurons increase levels of laminin during the first 10 postnatal days.
FIGURE 2.
Levels of laminin increase during the first 10 postnatal days. A, RT-PCR analyses of laminin β1 chain. Graph shows relative band intensities of laminin β1 chain standardized against β-actin bands (n = 4). Means ± S.E. are plotted. B, Western blot analyses of laminin in P1 and P10 hippocampal tissue. Graph shows relative band intensities of laminin standardized against β-actin bands (n = 4). Means ± S.E. are plotted. Asterisk, p < 0.0001 (Student's t test). C, immunostaining of laminin. P1 and P10 hippocampi. Fluorescence intensity is represented by a color scale. D, immunostaining of laminin (red) and NeuN (green). Top, CA1 and CA3 pyramidal cell layers, and CA1 SO neurons of P1 and P10 hippocampi. Bottom, intensities of laminin immunostaining. Pyramidal cell layer and soma region of SO interneurons were determined using NeuN staining. Fluorescent intensity of laminin staining from NeuN− region was subtracted to plot laminin intensities (n = 5; means ± S.E.). Asterisks, p < 0.001 (Student's t test).
MMP9 Regulates Laminin Levels in Developing Neurons
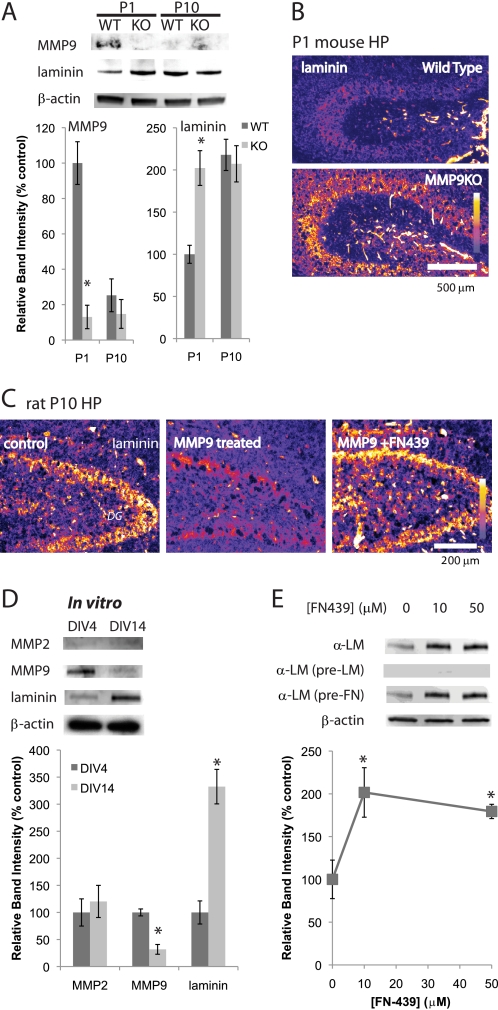
Analyses of neonatal hippocampus revealed decreasing levels of MMPs and concomitant increasing levels of laminin. Because we detected only low enzymatic activity from MMP2 in P1 hippocampus, we tested, by comparing wild type and MMP9 null mice, whether MMP9 plays a major role in regulating laminin levels. As shown in Fig. 3A, profiles of MMP9 and laminin levels were similar in both wild type mice and rats. However, MMP9 null mice showed higher levels of laminin expression than wild type at P1; this level showed no further increase at P10 (Fig. 3, A and B). These results suggest that MMP9 plays a major role in regulating laminin levels in neonatal hippocampus.
FIGURE 3.
MMP9 and laminin are developmentally regulated in vitro. A, Western blot analyses of MMP9 and laminin in the wild type and MMP9KO mouse hippocampus. C, cleavage of laminin by rrMMP9. Immunostaining of laminin in P10 dentate gyrus region is shown. Fluorescence intensity is represented by a color scale. Samples were incubated with 10 ng/ml rrMMP9 at 37 °C for 24 h. 50 μm FN-439 was used. Graph shows relative band intensities standardized against β-actin bands (n = 4). Means ± S.E. were plotted. Asterisk, p < 0.0001 (Student's t test). B, immunostaining of laminin. P1 hippocampi of wild type (top panel) and MMP9KO (bottom panel) mice. Fluorescence intensity is represented by a color scale. D, Western blot analyses of MMP2, MMP9, and laminin, comparing expression in DIV4 and DIV14. β-Actin was used as a loading control. Graph shows relative band intensities standardized against β-actin bands (n = 4). Means ± S.E. were plotted. Asterisk, p < 0.0001 (Student's t test). E, Western blot analysis shows laminin expression is regulated by MMPs. Neurons were cultured with 0, 10, or 50 μm FN-439 from DIV4 to DIV6. Western blot was performed using laminin antibody (α-LM), laminin-pre-absorbed laminin antibody (α-LM (pre-LM)), or fibronectin-pre-absorbed laminin antibody (α-LM (pre-FN)). β-Actin was used as a loading control. Analyses were done using the same membrane repeatedly stripped by 100 mm glycine-HCl, pH 2.5, and reprobed. Graph shows relative band intensities standardized against β-actin bands (n = 4). Means ± S.E. are plotted. Asterisk, p < 0.0001 (one-way ANOVA).
To directly evaluate the effects of enzymatic activity of MMP9 on laminin, we treated P10 hippocampal slices with MMP9 prior to immunostaining. Whereas a strong laminin signal could be detected in the cell bodies of dentate gyrus neurons under control conditions, only very weak signals were detected from the rrMMP9-treated slice (Fig. 3C). The effects of treatment with MMP9 were completely blocked in the presence of the MMP inhibitor, FN-439 (Fig. 3C). These results confirm that enzymatic activity of MMP9 degrades rather than activates laminin.
To further test whether MMP9 plays a role in regulating laminin levels, we used dissociated cultured hippocampal neurons. We previously showed that dissociated cultured neurons, like those in vivo, go through developmental death between days in vitro 5 (DIV5) to DIV9 (12). We performed Western blot analyses using samples from DIV4 and DIV14. The MMP2 expression levels were very low in these samples (Fig. 3D). However, MMP9 was present at DIV4 but decreased at DIV14, whereas laminin levels increased between DIV4 and DIV14, just as we had observed in samples from neonatal hippocampus (Fig. 3D).
We then tested whether MMPs can influence levels of laminin expression. Gel zymography confirmed that FN-439 efficiently inhibits the enzymatic activity of MMP9 (data not shown). We then tested whether FN-439 also changed laminin levels. Indeed, incubation with FN-439 for 2 days between DIV4 and DIV6 increased the levels of laminin (Fig. 3E). The antibody against laminin was specific; antibody that had been pre-absorbed with laminin did not yield these bands, but antibody pre-absorbed with fibronectin was able to detect laminin (Fig. 3E). These data show MMP9 activity changes laminin expression levels.
Laminin Is Critical for Depolarization-triggered Phosphorylation of Ser-473 Akt
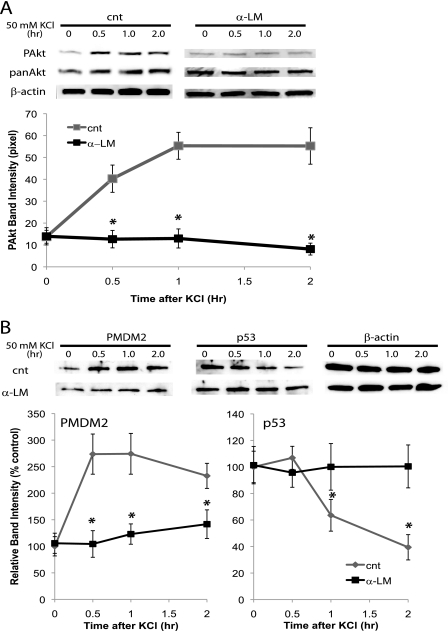
Next, we tested whether laminin is involved in survival signaling in developing neurons. The serine/threonine kinase Akt responds to various extracellular cues and induces cell survival signaling (24). Akt has two phosphorylation sites, Thr-308 and Ser-473, and each mediates distinct cellular responses (25). As we reported previously (12), spontaneous network activity allows calcium influx through L-type voltage-gated calcium channels, which then induces the phosphorylation of Ser-473 of Akt, thereby activating the critical signal for survival. Here, we found depolarizing hippocampal neurons by briefly exposing them to elevated potassium efficiently induced phosphorylation of both sites (Fig. 4A). However, preincubating the neurons with laminin antibody abolished depolarization-induced Ser(P)-473 without affecting Thr(P)-308 (Fig. 4A). These results suggest laminin signaling is required specifically for Ser(P)-473 Akt triggered by neuronal activity.
FIGURE 4.
A, activating the serine/threonine kinase, Akt, by depolarizing neurons requires laminin. Graph shows band intensities of pAkt (n = 4; means ± S.E.). cnt, control. B, activation of MDM2 and down-regulation of p53 induced by depolarization was blocked by inhibition of laminin signaling. Neurons (DIV14) were depolarized by a brief (2 min) exposure to HBS containing 50 mm KCl. Neurons were preincubated with 50 μg/ml of laminin antibody for 2 h prior to the depolarization. Graphs show relative band intensities of PMDM2 and p53 (n = 4; means ± S.E.). Asterisks, p < 0.001 (Student's t test).
To further analyze the effects of laminin blockade, we monitored one of the targets of Akt, MDM2 (26). A brief depolarization by high potassium phosphorylated MDM2 (Fig. 4B). The phosphorylation of MDM2 induced degradation of p53 (27). We detected a decrease in levels of p53 upon brief depolarization (Fig. 4B). Preincubation with laminin antibody abolished these changes (Fig. 4B). These results suggest the laminin blockade affects events downstream of Akt activation.
Inhibition of MMPs and Laminin Regulates Survival of Neurons
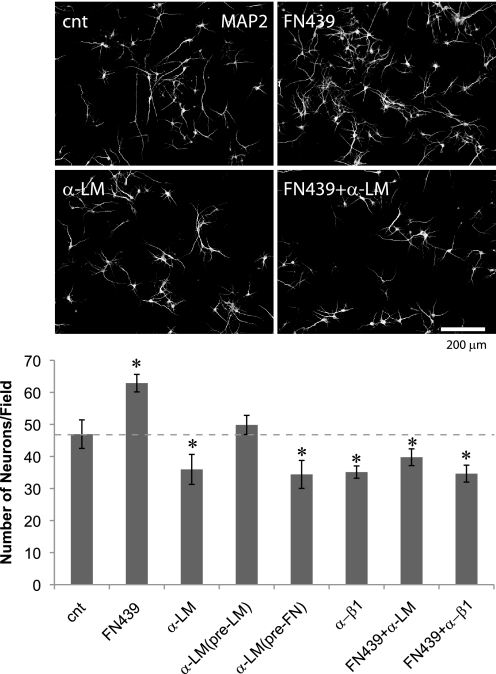
To examine whether activity of MMPs and laminin signaling are involved in the survival of developing neurons, from DIV5–7 we grew cultures in the presence of the MMP inhibitor FN-439 and laminin antibody (Fig. 5). Incubation with FN-439 significantly increased the number of surviving neurons, whereas incubation with laminin antibody significantly decreased the number of neurons (Fig. 5). Numbers of astrocytes were unchanged by these treatments (data not shown). Pre-absorption with laminin (but not fibronectin) completely abolished the effect of laminin antibody; thus, the antibody exerts its effect by inhibiting laminin signaling (Fig. 5). Incubation with activated 10 ng/ml rrMMP9 from DIV5–7 resulted in a severe loss of both neurons and astrocytes (data not shown), suggesting multiple pathways, in addition to a loss of laminin signaling, can lead to the death of neurons when they are exposed to high MMP activity. As we reported previously (12), a function blocking antibody against integrin β1 reduced the number of surviving neurons to a degree similar to that caused by treatment with laminin antibody (Fig. 5). Finally, the MMP inhibitor failed to promote the survival of neurons when incubated with laminin antibody or integrin β1 antibody (Fig. 5), suggesting that the survival effect is due to an increase in laminin-integrin signaling. These results show that MMP activity and laminin signaling are both involved in the survival of developing neurons.
FIGURE 5.
Inhibition of MMPs and laminin signaling regulate neuronal survival in vitro. Upper panel, MAP2 immunostaining of control (cnt), FN439-treated, laminin antibody (α-LM)-treated cultures, and cultures treated with both FN-439 and α-LM. Lower panel, quantification of neuron numbers (n = 10; means ± S.E.) Asterisks, p < 0.05 (one-way ANOVA). Neurons were incubated from DIV5 to DIV7. 50 μm FN439, 50 μg/ml α-LM, and 50 μg/ml anti-integrin β1 antibody (α-β1) were used.
MMP Inhibitor FN-439 Increases Laminin Levels and Decreases the Number of Apoptotic Neurons
The experiments performed in vitro suggested that regulation of MMPs controls the number of neurons during the period of developmental death via laminin signaling. To test this possibility in vivo, we injected the MMP inhibitor FN-439 into P2 hippocampi. We first prepared hippocampal slices and used in situ zymography to test whether the drug was spread effectively and was functional throughout the hippocampus. The presence of active MMPs in the CA3 region of P4 control animals was revealed by a strong fluorescence signal. This signal emanates from gelatin conjugated with a quenched fluorophore that dramatically increases its fluorescence when the gelatin is cleaved by active MMPs (Fig. 6A). Hippocampal slices from the FN-439-treated P2 animals, however, showed a strong decrease in the fluorescence signal, confirming that the injection of FN-439 effectively inhibited MMP activity in vivo (Fig. 6A).
FIGURE 6.
In vivo injection of FN-439 inhibits MMP activity and suppresses apoptosis. A, in situ zymography. FN-439 was injected at P2, and its effect was analyzed at P4. Left panel, CA3 region of control and FN-439-injected animal. DAPI staining (left) and gelatin-FITC (right). Right panel, fluorescence intensities of gelatin-FITC. CA3 regions of the control and FN-439-injected animals were compared (n = 5; means ± S.E.). Asterisk, p < 0.01 (Student's t test). B, immunostaining of laminin. Left panel, CA3 regions of the control and FN-439-treated animals are shown. Resolution of fluorescent intensities is 0–254 levels, with the color code for intensity shown as a scale bar. Right panel, intensity of laminin immunostaining signals (n = 6; means ± S.E.). Asterisk, p < 0.0001 (Student's t test). C, c-cas3+ neurons in the control, FN-439-injected and anti-integrin β1 antibody (α-β1)-injected animals were analyzed for CA1, CA3 pyramidal cells, and CA1 SO interneurons (n = 10; means ± S.E.). Asterisks, p < 0.05 (one-way ANOVA). cnt, control.
Next, we tested the effect of MMP inhibitor on levels of laminin. Hippocampi treated with FN-439 showed significantly higher laminin immunofluorescence than hippocampi receiving a control treatment (Fig. 6B). These results show that levels of laminin, the integrin ligand, increased when MMP function was inhibited.
To determine the effect of MMP inhibition on neuronal death, we counted the number of c-cas3+ neurons between P2 and P4. When MMP was inhibited, the number of c-cas3+ neurons decreased significantly in both CA1 and CA3 pyramidal cells and in the interneurons in SO of the CA1 region (Fig. 6C). However, when FN-439 was co-injected with the function blocking anti-integrin β1 antibody, the number of apoptotic neurons increased compared with control (Fig. 6C), suggesting the effect of FN-439 depends on integrin β1 signaling. These results are consistent with our results obtained in vitro that signals from the extracellular matrix positively regulate the survival of hippocampal neurons in neonatal animals.
DISCUSSION
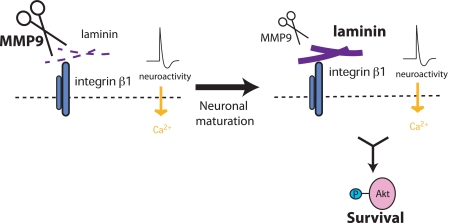
This study shows that the levels of MMP9 decrease during the first 10 postnatal days, a time when large numbers of neurons are eliminated. Immunohistochemistry tests show that laminin levels increase during postnatal 10 days. Because proteoglycans such as dystroglycan are known to bind to laminin (28), abundant interactions with those molecules in P1 hippocampus could potentially mask the binding site, preventing the antibody from recognizing laminin. However, this is unlikely because the results of Western blot analyses followed by separation of proteins with SDS-PAGE are consistent with the immunohistochemistry results. Alternatively, post-translational modification of laminin, such as glycosylation (29), could potentially mask the epitope. However, we observed no change in the mobility of laminin species in SDS-PAGE between P1 and P10 hippocampal samples, suggesting that levels of post-translational modification are equivalent. Taken together, it is likely that total levels of laminin increase between P1 and P10. The MMP inhibitor FN-439 increased levels of laminin both in vivo and in vitro. Consistently, the levels of laminin in MMP9 null mouse hippocampus were high at P1 and showed no further increases with age. Application of an MMP inhibitor increases neuron numbers through a laminin-dependent mechanism. Laminin is required for depolarization-induced Akt activation, which we recently reported is critical for the survival of neurons (12). Laminin blockade attenuated down-regulation of the tumor suppressor p53 by Akt activation, which we also recently reported is the key regulator of survival (30). Here, with results obtained in vitro and in vivo, we provide the first report that MMP9 is involved in the regulation of developmental neuronal death (Fig. 7).
FIGURE 7.
As neurons mature, levels of MMP9 and laminin change, regulating critical survival signaling triggered by neuronal activity. In early postnatal neurons, levels of MMP9 that cleave laminin are high. As neurons mature, the levels of MMP9 decline, and laminin expression increases. Laminin-integrin signaling is required for depolarization-induced (L-type calcium-dependent) Ser(P)-473 Akt, which is critical for neuronal survival during the developmental period of neuronal death.
The serine/threonine kinase Akt has been widely studied because of its important biological functions (24). Akt has two phosphorylation sites that mediate distinct cellular responses, and phosphorylation of Ser-473 is particularly involved in cell survival (25). Our new results show that phosphorylation of Ser-473 Akt by depolarization is blocked by inhibiting laminin signaling. Similarly, echistatin, a type of disintegrin that blocks β1/β3-containing integrins (31), and anti-integrin β1 antibody (but not anti-integrin β3 antibody) attenuated depolarization-triggered Ser(P)-473 Akt (12). These results show that laminin is critically involved in the induction of neuronal activity-dependent survival signaling.
The isoforms of laminin involved in activation of Akt remain to be elucidated. In adult hippocampus, a major isoform that is degraded by excitotoxic injury is laminin-10 (5), whose receptors contain a β1 subunit (32). Although integrins containing a β1 subunit are known to bind ligands other than ECM proteins (33), our new results suggest that laminin-integrin β1 interactions directly mediate survival. These results point to a need for further analysis of the molecular events at the cell surface that control the interactions among neurotrophins, activity, and the ECM to initiate survival responses.
Downstream events that mediate survival are also of great interest. Akt is known to induce degradation of the tumor suppressor p53 through the activation of MDM2, the E3 ubiquitin ligase (26, 27). Sustained activation of Akt triggered by neurotrophins effectively reduces levels of p53 and its pro-apoptotic target, Bax (30). Inhibiting integrin β1 completely attenuates the effects of neurotrophin on p53 and Bax. Conversely, induction of neuronal death by inhibition of integrin β1 signaling is blocked by a genetic deletion of p53. Transcription of MMPs, in turn, is regulated by p53 (34, 35). Thus, survival signals may exert feed-forward effects through p53-dependent transcription of MMPs. These results suggest a molecular model in which ECM/integrin signals play a key role alongside neurotrophins and activity in controlling neuron numbers.
Neurons are thought to undergo multiple stages of maturation during early postnatal life. Understanding how MMPs are regulated developmentally during the period of neuronal death may lead to new strategies for preventing neurodegenerative diseases. Genes for MMPs are associated with neurodegenerative diseases (36), and up-regulation of MMPs is reported in patients with Alzheimer disease (37, 38) and in animal models of Parkinson disease (39, 40). The experimental system we have developed may provide a model to define the molecular mechanism linking ECM signals to survival of neurons in disease.
This work was supported by a National Institutes of Health grant from the Intramural Research Program, NINDS.
- ECM
- extracellular matrix
- MMP
- matrix metalloproteinase
- c-cas3
- cleaved caspase 3
- P1
- postnatal day 1
- DIV
- days in vitro
- SO
- striatum oriens
- α-LM
- laminin antibody
- ANOVA
- analysis of variance
- rr
- rat recombinant
- NGS
- normal goat serum
- SR
- stratum radiatum.
REFERENCES
- 1. Hynes R. O. (2002) Integrins. Bidirectional, allosteric signaling machines. Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 2. Werb Z. (1997) ECM and cell surface proteolysis. Regulating cellular ecology. Cell 91, 439–442 [DOI] [PubMed] [Google Scholar]
- 3. Lukashev M. E., Werb Z. (1998) ECM signaling. Orchestrating cell behavior and misbehavior. Trends Cell Biol. 8, 437–441 [DOI] [PubMed] [Google Scholar]
- 4. Chen Z. L., Strickland S. (1997) Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell 91, 917–925 [DOI] [PubMed] [Google Scholar]
- 5. Indyk J. A., Chen Z. L., Tsirka S. E., Strickland S. (2003) Laminin chain expression suggests that laminin-10 is a major isoform in the mouse hippocampus and is degraded by the tissue plasminogen activator/plasmin protease cascade during excitotoxic injury. Neuroscience 116, 359–371 [DOI] [PubMed] [Google Scholar]
- 6. Szklarczyk A., Lapinska J., Rylski M., McKay R. D., Kaczmarek L. (2002) Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. J. Neurosci. 22, 920–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gu Z., Cui J., Brown S., Fridman R., Mobashery S., Strongin A. Y., Lipton S. A. (2005) A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J. Neurosci. 25, 6401–6408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilczynski G. M., Konopacki F. A., Wilczek E., Lasiecka Z., Gorlewicz A., Michaluk P., Wawrzyniak M., Malinowska M., Okulski P., Kolodziej L. R., Konopka W., Duniec K., Mioduszewska B., Nikolaev E., Walczak A., Owczarek D., Gorecki D. C., Zuschratter W., Ottersen O. P., Kaczmarek L. (2008) Important role of matrix metalloproteinase 9 in epileptogenesis. J. Cell Biol. 180, 1021–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gould E., Woolley C. S., McEwen B. S. (1991) Naturally occurring cell death in the developing dentate gyrus of the rat. J. Comp. Neurol. 304, 408–418 [DOI] [PubMed] [Google Scholar]
- 10. Ferrer I., Tortosa A., Blanco R., Martín F., Serrano T., Planas A., Macaya A. (1994) Naturally occurring cell death in the developing cerebral cortex of the rat. Evidence of apoptosis-associated internucleosomal DNA fragmentation. Neurosci. Lett. 182, 77–79 [DOI] [PubMed] [Google Scholar]
- 11. Ben-Ari Y., Khazipov R., Leinekugel X., Caillard O., Gaiarsa J. L. (1997) GABAA, NMDA, and AMPA receptors. A developmentally regulated “ménage à trois.” Trends Neurosci. 20, 523–529 [DOI] [PubMed] [Google Scholar]
- 12. Murase S., Owens D. F., McKay R. D. (2011) In the newborn hippocampus, neurotrophin-dependent survival requires spontaneous activity and integrin signaling. J. Neurosci. 31, 7791–7800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murase S., McKay R. D. (2006) A specific survival response in dopamine neurons at most risk in Parkinson disease. J. Neurosci. 26, 9750–9760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deleted in proof
- 15. Suzuki K., Enghild J. J., Morodomi T., Salvesen G., Nagase H. (1990) Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin). Biochemistry 29, 10261–10270 [DOI] [PubMed] [Google Scholar]
- 16. Cunningham M. G., McKay R. D. (1993) A hypothermic miniaturized stereotaxic instrument for surgery in newborn rats. J. Neurosci. Methods 47, 105–114 [DOI] [PubMed] [Google Scholar]
- 17. Mendrick D. L., Kelly D. M. (1993) Temporal expression of VLA-2 and modulation of its ligand specificity by rat glomerular epithelial cells in vitro. Lab. Invest. 69, 690–702 [PubMed] [Google Scholar]
- 18. Oh L. Y., Larsen P. H., Krekoski C. A., Edwards D. R., Donovan F., Werb Z., Yong V. W. (1999) Matrix metalloproteinase-9/gelatinase B is required for process outgrowth by oligodendrocytes. J. Neurosci. 19, 8464–8475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Impey S., McCorkle S. R., Cha-Molstad H., Dwyer J. M., Yochum G. S., Boss J. M., McWeeney S., Dunn J. J., Mandel G., Goodman R. H. (2004) Defining the CREB regulon, A genome-wide analysis of transcription factor regulatory regions. Cell 119, 1041–1054 [DOI] [PubMed] [Google Scholar]
- 20. Izant J. G., McIntosh J. R. (1980) Microtubule-associated proteins. A monoclonal antibody to MAP2 binds to differentiated neurons. Proc. Natl. Acad. Sci. U.S.A. 77, 4741–4745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mullen R. J., Buck C. R., Smith A. M. (1992) NeuN, a neuronal specific nuclear protein in vertebrates. Development 116, 201–211 [DOI] [PubMed] [Google Scholar]
- 22. Sharif K. A., Baker H., Gudas L. J. (2004) Differential regulation of laminin b1 transgene expression in the neonatal and adult mouse brain. Neuroscience 126, 967–978 [DOI] [PubMed] [Google Scholar]
- 23. Plateroti M., Freund J. N., Leberquier C., Kedinger M. (1997) Mesenchyme-mediated effects of retinoic acid during rat intestinal development. J. Cell Sci. 110, 1227–1238 [DOI] [PubMed] [Google Scholar]
- 24. Toker A., Newton A. C. (2000) Cellular signaling: pivoting around PDK-1. Cell 103, 185–188 [DOI] [PubMed] [Google Scholar]
- 25. Jacinto E., Facchinetti V., Liu D., Soto N., Wei S., Jung S. Y., Huang Q., Qin J., Su B. (2006) SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127, 125–137 [DOI] [PubMed] [Google Scholar]
- 26. Mayo L. D., Donner D. B. (2001) A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc. Natl. Acad. Sci. U.S.A. 98, 11598–11603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grossman S. R., Perez M., Kung A. L., Joseph M., Mansur C., Xiao Z. X., Kumar S., Howley P. M., Livingston D. M. (1998) p300-MDM2 complexes participate in MDM2-mediated p53 degradation. Mol. Cell 2, 405–415 [DOI] [PubMed] [Google Scholar]
- 28. Hewitt J. E. (2009) Abnormal glycosylation of dystroglycan in human genetic disease. Biochim. Biophys. Acta 1792, 853–861 [DOI] [PubMed] [Google Scholar]
- 29. Morita A., Sugimoto E., Kitagawa Y. (1985) Post-translational assembly and glycosylation of laminin subunits in parietal endoderm-like F9 cells. Biochem. J. 229, 259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murase S., Poser S. W., Joseph J., McKay R. D. (2011) p53 controls neuronal death in the CA3 region of the newborn mouse hippocampus. Eur. J. Neurosci. 34, 374–381 [DOI] [PubMed] [Google Scholar]
- 31. Pfaff M., McLane M. A., Beviglia L., Niewiarowski S., Timpl R. (1994) Comparison of disintegrins with limited variation in the RGD loop in their binding to purified integrins αIIbβ3, αVβ3 and α5β1 and in cell adhesion inhibition. Cell Adhes. Commun. 2, 491–501 [DOI] [PubMed] [Google Scholar]
- 32. Kikkawa Y., Sanzen N., Fujiwara H., Sonnenberg A., Sekiguchi K. (2000) Integrin binding specificity of laminin-10/11. Laminin-10/11 are recognized by α3β1, α6β1, and α6β4 integrins. J. Cell Sci. 113, 869–876 [DOI] [PubMed] [Google Scholar]
- 33. Staniszewska I., Sariyer I. K., Lecht S., Brown M. C., Walsh E. M., Tuszynski G. P., Safak M., Lazarovici P., Marcinkiewicz C. (2008) Integrin α9β1 is a receptor for nerve growth factor and other neurotrophins. J. Cell Sci. 121, 504–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bian J., Sun Y. (1997) Transcriptional activation by p53 of the human type IV collagenase (gelatinase A or matrix metalloproteinase 2) promoter. Mol. Cell. Biol. 17, 6330–6338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Staun-Ram E., Goldman S., Shalev E. (2009) p53 mediates epidermal growth factor (EGF) induction of MMP-2 transcription and trophoblast invasion. Placenta 30, 1029–1036 [DOI] [PubMed] [Google Scholar]
- 36. Shibata N., Ohnuma T., Higashi S., Usui C., Ohkubo T., Kitajima A., Ueki A., Nagao M., Arai H. (2005) Genetic association between matrix metalloproteinase MMP-9 and MMP-3 polymorphisms and Japanese sporadic Alzheimer disease. Neurobiol. Aging 26, 1011–1014 [DOI] [PubMed] [Google Scholar]
- 37. Yoshiyama Y., Asahina M., Hattori T. (2000) Selective distribution of matrix metalloproteinase-3 (MMP-3) in Alzheimer disease brain. Acta Neuropathol. 99, 91–95 [DOI] [PubMed] [Google Scholar]
- 38. Horstmann S., Budig L., Gardner H., Koziol J., Deuschle M., Schilling C., Wagner S. (2010) Matrix metalloproteinases in peripheral blood and cerebrospinal fluid in patients with Alzheimer disease. Int. Psychogeriatr. 22, 966–972 [DOI] [PubMed] [Google Scholar]
- 39. Sung J. Y., Park S. M., Lee C. H., Um J. W., Lee H. J., Kim J., Oh Y. J., Lee S. T., Paik S. R., Chung K. C. (2005) Proteolytic cleavage of extracellular secreted α-synuclein via matrix metalloproteinases. J. Biol. Chem. 280, 25216–25224 [DOI] [PubMed] [Google Scholar]
- 40. Choi D. H., Kim E. M., Son H. J., Joh T. H., Kim Y. S., Kim D., Flint Beal M., Hwang O. (2008) A novel intracellular role of matrix metalloproteinase-3 during apoptosis of dopaminergic cells. J. Neurochem. 106, 405–415 [DOI] [PubMed] [Google Scholar]