Background: Besides inhibiting osteoblast differentiation of MSC, noggin may induce adipogenesis.
Results: Noggin induces adipogenic differentiation of MSC via a novel mechanism. Individuals with high BMI have elevated circulating noggin levels in plasma.
Conclusion: Noggin regulates both osteoblast and adipocyte differentiation of MSC and, hence, is a master regulator of MSC plasticity.
Significance: Noggin may be a novel biomarker for obesity.
Keywords: Adipogenesis, Bone, Mesenchymal Stem Cells, Obesity, Osteoblasts, Noggin
Abstract
Noggin is a glycosylated-secreted protein known so far for its inhibitory effects on bone morphogenetic protein (BMP) signaling by sequestering the BMP ligand. We report here for the first time a novel mechanism by which noggin directly induces adipogenesis of mesenchymal stem cells independently of major human adipogenic signals through C/EBPδ, C/EBPα and peroxisome proliferator-activated receptor-γ. Evaluation of a possible mechanism for noggin-induced adipogenesis of mesenchymal stem cells identified the role of Pax-1 in mediating such differentiation. The relevance of elevated noggin levels in obesity was confirmed in a preclinical, immunocompetent mouse model of spontaneous obesity and in human patients with higher body mass index. These data clearly provide a novel role for noggin in inducing adipogenesis and possibly obesity and further indicates the potential of noggin as a therapeutic target to control obesity.
Introduction
Ever since obesity was recognized as a major health problem, considerable efforts have been invested in identifying its causes, which affects individuals all over the world. A variety of factors play a role in obesity, thus making it a complex health issue to address. The earlier belief that obesity is caused by uncoupling of energy intake and expenditure has been further delineated in the last decade at a molecular level (1, 2). Consequently, roles of various adipokines such as adiponectin, leptin, and hormones like testosterone, adrenalin, and thyroid hormones in promoting obesity have been established (3). However, not much information exists on the key molecular mechanism(s) that triggers adipogenesis.
Adipocytes originate from multipotent mesenchymal stem cells (MSC), which also give rise to other lineages including osteoblasts, chondrocytes, and myocytes (4). Within the bone marrow, the differentiation of MSC into either osteoblasts or adipocytes is delicately balanced and influenced by several growth factors. The processes of osteoblastogenesis and adipogenesis are reciprocally associated (5). The balance is tilted toward adipogenesis with age and in several bone diseases with progressive bone loss such as osteoporosis. MSC from osteoporosis patients have increased adipogenic potential as shown by increased PPAR-γ2 levels, which is a major transcription factor for adipocyte differentiation (6).
Studies using murine models of obesity and aging clearly show decreased osteoblastogenesis and increased adipogenic potential of bone marrow MSC (7, 8). Ex vivo cultures of MSC from these mice showed increased numbers of fully differentiated marrow adipocytes compared with age-matched control (8). Both these mice models also exhibit decreased osteoblast numbers and functions (7, 8). Decreased osteoblast function in such models was not due to impaired levels of bone morphogenetic protein (BMP), which is known to induce osteoblast differentiation and function, but was rather due to increased noggin levels. Noggin is a glycoprotein that was discovered for its ability to induce secondary axis formation in Xenopus (9). Classically, noggin is well known as a potent inhibitor of BMPs and thus of osteoblast differentiation (10, 11). Very little is known about other functions of noggin, if any. As the processes of osteoblastogenesis and adipogenesis are strikingly interdependent, we hypothesized that in addition to sequestering BMP from interacting with its receptor, noggin may have a dual role in inducing adipogenesis and consequently obesity.
This study systematically established the role of noggin in inducing adipogenesis of MSC. Noggin-mediated adipocytic differentiation was found to be independent of the classical adipogenic signals but effected via Pax-1. Analysis of MSC from obese mice and plasma from obese individuals clearly indicated significantly elevated noggin levels. Collectively, the data clearly provide a novel role for noggin in inducing adipogenesis and possibly obesity and further indicates the potential of noggin as a therapeutic target to control obesity.
EXPERIMENTAL PROCEDURES
Cell Lines and Reagents
Murine MSC from normal and C57BL/6 mice with spontaneous obesity were isolated as described before (12). Briefly, bone marrow was collected by flushing of femurs and tibiae and culturing of these cells in Stemline Mesenchymal Expansion medium (Sigma) supplemented with 10% fetal bovine serum (Sigma.) and 1% penicillin-streptomycin, 1.5 mg/liter l-glutamine (Invitrogen), and the medium was changed every day for removal of non-adherent cells. Adherent cells were trypsinized and stained with biotinylated antibody to CD11b. Using anti-biotin microbeads (Miltenyi Biotec, Auburn, CA), we collected CD11b− cells and cultured them in Stemline Mesenchymal Expansion medium supplemented with EGF and platelet-derived growth factor AA. The presence of MSC was further confirmed by staining with antibodies to CD11b and CD45. MSC stain negative for both these markers. Human MSC were a kind gift from Dr. Larisa Pereboeva from the Department of Pathology, University of Alabama at Birmingham. Recombinant noggin, recombinant BMP-2, protein kinase A inhibitor, LY294002, and rapamycin were obtained from Sigma. Antibodies used were rabbit anti-mouse noggin antibody (Chemicon, Billerica, MA), rabbit anti-mouse Pax-1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and goat anti-rabbit horseradish peroxidase (HRP)-conjugated antibody (GE Healthcare).
Generation of MSC with Stable Knockdown of Pax-1 Using shRNA Plasmid
Custom-designed shRNA expression vector for Pax-1 was obtained from Sigma. MSC were transfected with Pax-1 shRNA plasmid or a scrambled shRNA sequence using Lipofectamine 2000 (Invitrogen) as per the manufacturer's directions and incubated 37 °C in the presence of 5% CO2. After 24 h, the transfection medium was replaced with normal medium, and after another 24 h, the cells were trypsinized and split 1:4 and cultured in selection medium containing 2 μg/ml puromycin. Cell lysates from the clonal derivatives were screened by Western blot to confirm abrogation of Pax-1 expression.
Generation of MSC with Transient Knockdown of Phosphoinositide 3-Kinase (PI3K)
Commercially available siRNA for PI3K were obtained from Cell Signaling. MSC were transfected with PI3K-specific siRNA or a control siRNA using Lipofectamine 2000. To maintain down-regulation of PI3K until differentiation of MSC into adipocytes, this procedure was repeated every 3 days.
Adipocyte Differentiation and Inhibitor Studies
For adipocyte differentiation, 104 MSC were seeded per well in a 24-well dish (Corning Inc., Corning, NY). Once the cells were confluent, the medium was replaced with adipocyte differentiation medium (ADM) containing 10−8 m dexamethasone, 0.5 μg/ml insulin, 0.5 mm isobutylmethylxanthine, and 10% horse serum in MSC culture medium (12, 13). The medium was replenished every 2 days. Recombinant noggin was added at a concentration of 50 ng/ml. After 14 days adipocytes were detected by Oil Red O staining. Briefly, cells were fixed in 60% isopropyl alcohol followed by staining with Oil Red O dye for 30 min. Cells were washed in phosphate-buffered saline (Sigma). 100% isopropyl alcohol was then added to individual wells, and the intensity of the color was measured at an A500. For the inhibition studies, 20 μm protein kinase A inhibitor (14), 20 μm N6-benzoyl-cAMP, sodium salt (6-Bz-cAMP) (15), 20 μm LY294002 (16), 100 nm Wortmannin (17), and 1 nm rapamycin (16) were added individually or in combination to ADM along with noggin.
Osteoblast Differentiation of MSC
For differentiation of MSC into osteoblasts, 104 cells/ml were cultured in osteoblast differentiation medium in 6-well tissue culture plates containing 10−8 m dexamethasone, 10 mm glycerophosphate, and 0.3 mm ascorbic acid for 10 days (12). The medium was changed every 2 days. 100 ng/ml BMP-2 was added alone or along with noggin during osteoblast differentiation. After 10 days, cultures were stained for alkaline phosphatase using commercially available kit (Sigma) to detect osteoblasts.
Glycerol-3-phosphate Dehydrogenase (GPDH) Activity Assay
For assessing GPDH activity, MSC were cultured with noggin alone in ADM, in ADM containing inhibitors, and in ADM containing inhibitors and noggin as described above. Combinations of any two inhibitors were used for this study. GPDH assay was carried out as described previously (18, 19). Briefly, after 14 days in culture, cells were washed with PBS (pH 7.4) and harvested in prechilled 25 mmol/liter Tris-HCl buffer containing 1 mmol/liter EDTA (pH 7.4) and 1 mmol/liter β-mercaptoethanol. After sonication, aliquots of the cell extracts were added to an assay mixture containing 100 mmol/liter triethanolamine-HCl (pH 7.5), 2.5 mmol/liter EDTA, 0.12 mmol/liter NADH, and 0.1 mmol/liter β- mercaptoethanol, and GPDH activity was measured spectrophotometrically at 340 nm. The reactions were started by adding 0.2 mmol/liter dihydroxyacetone phosphate. The GPDH activity was normalized to the total protein content for each sample and expressed as milliunits/μg of total protein.
RNA Isolation and Analysis
Total RNA was isolated on days 0, 3, 4, and 7 from MSC, cultured in ADM and noggin, in the presence or absence of various inhibitors using TRIzol reagent (Invitrogen). For detecting expression of functional adipocyte markers, RNA was isolated after 14 days of culture under conditions described earlier. cDNA was prepared using iScript cDNA synthesis kit from Bio-Rad as per the instructions and was further used for RT-PCR analysis using SYBR Green (Sigma) to detect expression levels of CCAAT enhancer binding protein a (C/EBP-α) and CCAAT enhancer binding protein δ (C/EBP-δ), and PPAR-γ with GAPDH as the internal control. Expression of adiponectin, leptin, and β-3 adreno receptor, which are markers expressed by functional adipocytes, were detected by semiquantitative RT-PCR. The primer sequences used in PCR reactions were: C/EBPα forward (5′-CCGGGAGAACTCTAACTC-3′) and C/EBPα reverse (5′-GATGTAGGCGCTGATGT-3′); C/EBPδ forward (5′-ACGACGAGAGCGCCATC-3′) and C/EBPδ (reverse, 5′-TCGCCGTCGCCCCAGTC-3′); PPARγ forward (5′-AGGCCGAGAAGGAGAAGCTGTTG-3′) and PPARγ reverse (5′-TGGCCACCTCTTTGCTCTGCTC-3′); adiponectin forward (5′-GCACTGGCAAGTTCTACTGCAACA-3′) and adiponectin reverse (5′-AGAGAACGGCCTTGTCCTTCT-3′); leptin forward (5′-AGCAGTGCCTATCCAGAAAGTCCA-3′) and leptin reverse (5′-AATGAAGTCCAAGCCAGTGACCCT-3′); β-3 adreno receptor forward (5′-CTGCTGTTCCTTTGCCTCCAACAT-3′) and β-3 adreno receptor reverse (5′-AGCCACAACGAACACTCGAGCATC-3′); GAPDH forward (5′-TCAACAGCAACTCCCACTCTTCCA-3′) and GAPDH reverse (5′-ACCCTGTTGCTGTAGCCGTATTCA-3′).
Western Blot Analysis
The presence of noggin, BMP-2, and Pax-1 from MSC lysates were detected by Western blot using rabbit anti-mouse noggin antibody (Chemicon), rabbit anti- mouse BMP-2 antibody (R&D Biosystems), and rabbit anti-mouse Pax-1 antibody (Santa Cruz Biotechnology), respectively. A goat anti-rabbit HRP-conjugated secondary antibody was used for further detection.
Immunocytochemical Staining for Noggin
MSC were grown on slide chambers, washed with PBS, and fixed with 10% formalin containing 0.1% Triton X-100 for 20 min followed by washing in PBS containing Ca2+ and Mg2+ and blocked with 2% BSA. Cells were then stained with an anti-noggin antibody overnight at 4 °C and washed with PBS containing 0.05% Tween 20 and stained with a FITC-conjugated secondary antibody (Invitrogen) for 1 h and washed with PBS (3×, 10 min each). Nuclei were stained using DAPI. The cells were imaged at 200× original magnification on a Leica DMI 4000B fluorescent microscope (Leica Microsystems Inc., Bannockburn, IL) to qualitatively determine noggin levels.
Dual-energy X-ray Absorptiometry, Microcomputed Tomography (Micro-CT), and Histology
For dual-energy x-ray absorptiometry analysis, animals were briefly anesthetized with an isoflurane (2%), oxygen mixture and placed in a prostrate position on the imaging plate. Body weight, fat mass, lean mass, and percent fat was assessed in vivo by dual-energy x-ray absorptiometry (GE-Lunar PIXImus, Version 1.45; Madison, WI). Histomorphometric parameters, including bone volume, trabecular connectivity, trabecular thickness, trabecular separation, and degree of anisotropy were evaluated using high resolution micro-CT imaging system (μCT40; SCANCO Medical, Wayne, PA).
Formalin-fixed tissues were decalcified in EDTA solution for 2 weeks and embedded in paraffin. Longitudinal sections of 5-μm thicknesses were cut from paraffin-embedded blocks of frontal sections of tibia using a Leica 2265 microtome. Sections were then stained with hematoxylin and eosin for histological evaluation.
Analysis of Noggin Levels in Human Plasma Samples
The study subjects were recruited in the Webb Clinical Research Facility in the University of Alabama at Birmingham Department of Nutrition Sciences using an approved protocol by the Institutional Review Board. Those meeting inclusion requirements were subsequently enrolled. Participating individuals were grouped into two based on their BMI. Individuals with BMIs less than 27 were grouped in the lean group, and individuals with BMI higher than 27 were grouped in the obese group. Near equal numbers of males and females and Caucasians and African Americans were included in the study samples. The final study group comprised 25 volunteers in each group with ages between 21 and 55. Plasma samples from lean and obese individuals were collected and used to detect noggin levels using commercially available Human Noggin ELISA kit (catalog ABIN415152, Life Science Inc., Atlanta, GA) as per the manufacturer's instructions.
Statistical Analysis
Data were analyzed by one-way analysis of variance. A Tukey test was also applied for multiple comparisons wherever applicable. Values provided are the mean ± S.E., and the differences were considered significant if p < 0.05.
RESULTS
Noggin Induces Adipocyte Differentiation of MSC Independently of Known Adipocyte Differentiation Mechanisms
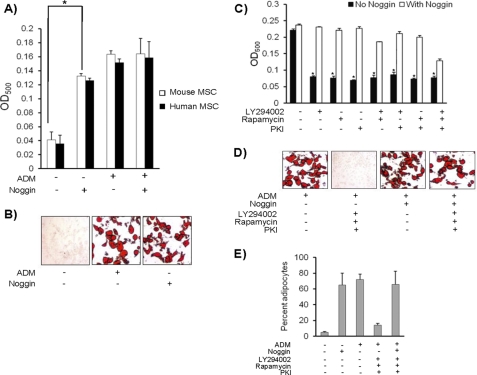
Bone marrow-derived MSC from mouse and human were differentiated in ADM in the presence or absence of noggin. There was a significant increase in the numbers of adipocytes when MSC were cultured in the presence of only noggin (Fig. 1A). When cultured in adipocyte differentiation medium alongside noggin, there was no further increase in adipocytic potential of MSC (Fig. 1B). We cultured MSC with the suboptimal concentration of noggin along with ADM to assess if there was any additive effect. But the results clearly showed the absence of additive effect (data not shown). Before inducing adipocyte differentiation of MSC in the presence of noggin, the cells were cultured in the presence of BMP-2 and osteoblast differentiation medium to assess if the recombinant noggin protein was biologically active. Upon the addition of noggin to osteogenic media, noggin was able to inhibit BMP-2-induced osteoblast differentiation (supplemental Fig. S1).
FIGURE 1.
Noggin induces adipocyte differentiation of MSC. A, to determine the possible role of noggin in inducing adipocyte differentiation of MSC, 104 MSC derived from mice and humans were cultured in the presence of 500 ng/ml concentrations of recombinant Noggin only, noggin + ADM, and ADM only for 14 days. The presence of adipocytes was detected by Oil Red O staining as mentioned under “Experimental Procedures.” B, shown are representative micrographs of adipocyte differentiation of MSC in the presence of noggin. C, 104 MSC were cultured in ADM in the presence or absence of noggin. Three different inhibitors of adipocyte differentiation (LY294002, rapamycin, and protein kinase A inhibitor (PKI)) were added either individually or in the indicated combinations during the culture. After 14 days, Oil Red O staining was performed to detect the presence of adipocytes. D, representative micrographs of adipocyte differentiation of MSC in the presence of noggin and inhibitors is shown. All the experiments were repeated three times independently (n = 3; *, p < 0.05). E, percent adipocytes in total number of cells is indicated for each experimental group (n = 3; *, p < 0.05).
Adipocytic differentiation of MSC involves induction of PI3K, mTOR/AKT, and cAMP. To further define the role of noggin in inducing adipocytic differentiation of MSC and to determine whether adipocyte differentiation of MSC is signaled through known pathways of adipogenesis, murine MSC were cultured with noggin and inhibitors of adipogenesis. The inhibitors used in this study were LY294002, rapamycin, and protein kinase A inhibitor, which prevent activation of PI3K, mTOR/AKT, and cAMP, respectively. To characterize the effects through a specific pathway of adipocyte differentiation, MSC were cultured with the inhibitors either individually or in combinations in the presence of noggin. Data from this study indicated that noggin induces adipocyte differentiation in the presence of all inhibitors tested (Fig. 1, C and D, supplemental Fig. S2). These experiments were also repeated by using additional inhibitors of PI3K (wortmannin) and cAMP (N6-benzoyl-cAMP, sodium salt) (supplemental Fig. S3). Similar results were observed that further confirmed the role of noggin in adipocyte differentiation. Although presence of the three inhibitors together caused cell death, the percent of adipocytes formed in the presence of noggin remained the same (Fig. 1E). Alternatively, to rule out further cellular toxicity by use of inhibitors, MSC transfected with siRNA for PI3K were differentiated into adipocytes under identical conditions. Similar to the above observations, the presence of noggin induced adipocyte differentiation of MSC transfected with PI3K siRNA (supplemental Fig. S3B). This data clearly indicates that noggin-mediated adipogenesis is independent of known adipogenic mechanisms. Because use of all three inhibitors was toxic and caused cell death, subsequent experiments were performed using a combination of any two inhibitors.
Noggin Induces Expression of Transcription Factors Required for Adipocyte Differentiation
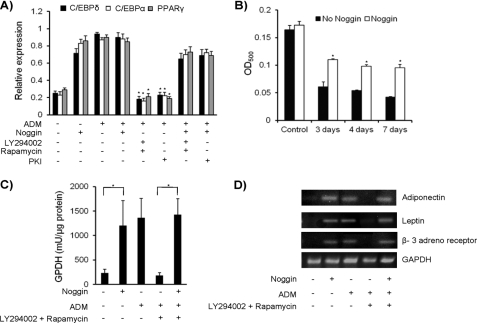
Three major transcription factors involved in adipocyte differentiation are C/EBPδ, C/EBPα, and PPARγ, which are expressed on days 3, 4, and 7, respectively, during adipocyte differentiation of MSC. When MSC were cultured in ADM in the presence of any two inhibitors that could block PI3K, mTOR/AKT, or cAMP activation, the expression of transcription factors and adipogenesis was inhibited. But when noggin was added to the same culture, interestingly, their expression was restored, and MSC readily differentiated into adipocytes (Fig. 2A). Next, the cells were treated with the three inhibitors either individually or in combinations for 3, 4, and 7 days before the addition of noggin to assess its effect on adipocyte differentiation. Results of this study demonstrated that noggin was still able to induce adipocyte differentiation of MSC when added even at later time points of adipogenic differentiation (Fig. 2B).
FIGURE 2.
Noggin-mediated adipocyte differentiation of MSC is independent of known mechanisms. A, MSC were cultured with noggin, ADM, ADM + inhibitors, and ADM + noggin + inhibitors. Total RNA was isolated on days 3, 4, and 7 to detect expression of C/EBPδ, C/EBPα, and PPARγ, respectively, by real time RT-PCR. Results are presented as relative expression compared with their expression in MSC on day 0. B, MSC were treated with LY294002 and rapamycin for 3, 4, and 7 days in ADM before the addition of noggin. The treatment with inhibitors was continued until the end of experiment at which point the presence of adipocytes was determined by Oil Red O staining. C, MSC were cultured with noggin, ADM, ADM + inhibitors (LY294002 + rapamycin) and ADM + noggin + inhibitors. After 14 days of culture, cell lysates were prepared and used for assaying GPDH activity as described under “Experimental Procedures.” D, RNA was isolated from MSC cultured with noggin, ADM, ADM + inhibitors, and ADM + noggin + inhibitors after 14 days of culture. Expressions of adiponectin, leptin, and β-3 adreno receptor were detected by semi quantitative RT-PCR analysis. All the experiments were performed three times independently (n = 3; *, p < 0.05).
Functional assessment of noggin-induced adipocytes was performed by determining GPDH activity and expression of adiponectin, leptin, and β-3 adreno receptor. MSC were cultured in the presence of noggin only, in the ADM, in the ADM containing combination of any two inhibitors (data shown for LY294002 and rapamycin; data not shown for other combinations), and in ADM along with inhibitors and noggin. As expected, MSC cultured in ADM differentiated into functional adipocytes, assessed by high GPDH activity and expression of adiponectin, leptin, and β-3 adreno receptor (Fig. 2, C and D). Similarly, results were obtained when MSC were cultured with noggin alone, thus confirming that noggin-induced differentiation of functional adipocytes. MSC cultured in ADM and inhibitors did not show elevated GPDH activity and lacked expression of functional adipocyte markers, as they did not differentiate into adipocyte. But this phenotype was rescued by the addition of noggin (Fig. 2, C and D).
Elevated Noggin Levels in Obese Individuals
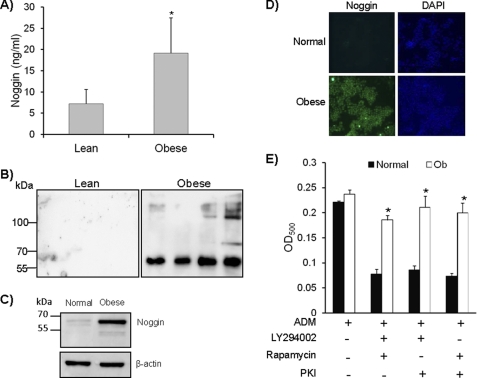
Clinical significance of the above observation was further confirmed by assaying plasma noggin levels from individuals with a body mass index (BMI) <27, which was considered lean, and from individuals with BMI >27, which was considered obese. Twenty-five samples were included for each group. Obese individuals showed strikingly higher plasma noggin levels compared with individuals with BMIs <27 (Figs. 3, A and B). The mean BMI for the lean and obese individuals were 24.4 and 35.1, respectively. Inclusion of subjects in the cohorts had equal numbers of adult males and females (age 21–55) and also equal numbers of Caucasians and African American population, indicating the results were not due to without gender or racial variables.
FIGURE 3.
Noggin levels are elevated in obese individuals and obese mice. A, plasma samples from lean individuals (BMI < 27) and obese individuals (BMI > 27) were used to detect the levels of noggin using a commercially available ELISA kit (n = 25; p = 0.008). B, a Western blot for detecting presence of noggin was carried out on lean and obese plasma samples using anti-mouse Noggin antibody (n = 25). C, MSC were isolated from BM of obese mice. Cell lysates were prepared from both normal and obese mice MSC, and noggin expression was by Western blot using anti-noggin antibody. A representative blot is presented here (n = 3). D, MSC from normal and obese (Ob) mice were grown on chamber slides and stained using anti-noggin antibody followed by FITC-labeled secondary antibody to detect the presence of noggin inside the cells. A representative image from each sample is shown here. Nuclei were stained using DAPI. Magnification is 200× (n = 3). E, MSC from the obese mice were cultured in the presence of inhibitors for adipocyte differentiation. Adipocytes were detected as mentioned before. The experiment was repeated three times (n = 3; *, p < 0.05).
To further identify if the elevated noggin level is associated with obesity in mouse models of spontaneous obesity and if higher expression of noggin is found in the MSC of mice with spontaneous obesity, MSC from C57BL/6 mice with spontaneous obesity were isolated. Once again, irrespective of the gender of these mice, noggin levels were elevated in their MSC compared with age-matched controls (Fig. 3, C and D). MSC from the obese mice also spontaneously differentiated into adipocytes in culture without the need of exogenous noggin (Fig. 3E). Interestingly, when MSC from obese mice were cultured in the presence of known adipogenic inhibitors, there was no effect on adipogenic differentiation.
Elevated Noggin Levels in Obese Mice Are Associated with Increased Body Fat and Decreased Bone Density
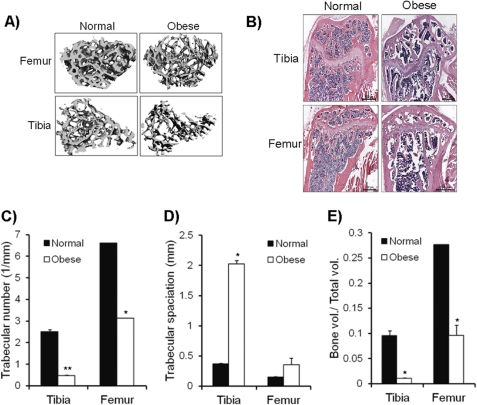
We measured total body fat of the spontaneously obese mice for elevated noggin levels. Results of this analysis indicated that the obese mice had double the amount of body fat compared with the gender- and age- matched controls, which do not show elevated noggin levels. These data further support our claim that noggin induces adipogenesis (Table 1). Furthermore, these mice also had significantly reduced bone mass index. The bone marrow of these mice contained excessive adipocytes along with significantly reduced trabecular bone thickness in both femur and tibia (Fig. 4, A and B). Micro-CT analysis of both the femur and tibia from these obese mice showed a dramatic reduction in trabecular numbers, trabecular spaciation, and total bone volume (Fig. 4, C–E).
TABLE 1.
Obese mice exhibit twice the amount of fat compared to the normal mice
Normal and obese mice were subjected to DXA analysis to measure total body fat content. n = 3.
| Animals | Weight ± S.E. | Lean ± S.E. | Fat ± S.E. | Total ± S.E. | % Fat ± S.E. |
|---|---|---|---|---|---|
| g | g | g | g | ||
| Normal | 25.86 ± 0.54 | 19.5 ± 0.35 | 3.3 ± 0.31 | 22.8 ± 1.2 | 14.4 ± 0.89 |
| Obese | 32.78 ± 1.02 | 19.9 ± 0.69 | 9.55 ± 0.24 | 29.45 ± 1 | 32.5 ± 0.21 |
FIGURE 4.
Obese mice with elevated noggin have reduced bone mineral density. A, femurs and tibiae from normal and obese mice were used for micro-CT analysis of trabecular bone. A representative image is shown. B, histology of bone sections for femur and tibia from normal and obese mice after hematoxylin and eosin staining are shown. Various parameters such as trabecular number (1/mm) (C), trabecular speciation (mm) (D), and bone volume/total volume (E) were analyzed based on micro-CT data. Data are representative of micro-CT analysis from three different mice (n = 3; *, p < 0.05).
Noggin Mediates Adipocyte Differentiation via Pax-1
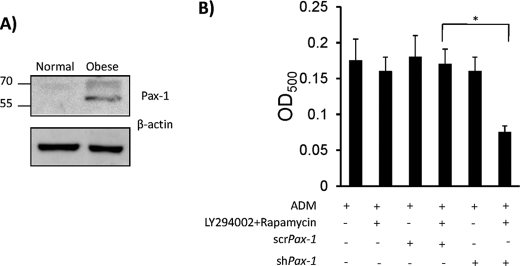
Next, we sought to understand the molecular pathway through which noggin mediated adipogenesis. It is known that mice with noggin haploinsufficiency exhibit reduced Paired box gene-1 (Pax-1) levels (20, 21). MSC from spontaneously obese mice, which exhibit high noggin levels, were found to have significantly elevated levels of Pax-1, thus raising the possibility of a novel adipocyte differentiation pathway of noggin through Pax-1 (Fig. 5A). To confirm the hypothesis that noggin-mediated adipocyte differentiation occurs via Pax-1, expression of Pax-1 in MSC derived from obese mice was abrogated in situ using Pax-1 shRNA (supplemental Fig. S4). As a control, MSC were transfected with a scramble shRNA construct. Adipocyte differentiation was carried out in the ADM along with inhibitors of classical adipocyte differentiation pathways. This segregated noggin-mediated adipogenesis from the canonical pathways and further facilitated to study if Pax-1 has any role in this process. Exogenous noggin was not added, as MSC from obese mice have elevated endogenous noggin. Obese MSC expressing either scr or shPax-1 differentiated into adipocytes as expected. But in the presence of inhibitors, when only noggin mediated pathway was active, obese MSC expressing shPax-1 failed to differentiate into adipocytes, whereas those expressing scrPax-1 differentiated into adipocytes (Fig. 5B). These data demonstrate that noggin-mediated adipocyte induction is through Pax-1 activation and thus provides evidence for a novel adipocyte differentiation pathway.
FIGURE 5.
Noggin mediates adipocyte differentiation via Pax-1. A, cell lysates obtained from normal and obese MSC were detected for presence of Pax-1 by Western blot using anti-Pax-1 antibody. A representative blot is shown here. B, MSC with Pax-1 abrogation by stable transfection using Pax-1 shRNA (MSCshPax-1) were subjected to adipocyte differentiation in the presence and absence of LY294002 and rapamycin. As a control, MSC stably transfected with a scramble construct (MSCscrPax-1) was used. After 14 days, the presence of adipocytes was detected by Oil Red O staining. All the experiments were repeated three times (n = 3; *, p < 0.05).
DISCUSSION
We demonstrate here for the first time a novel role for noggin as an inducer of adipogenesis. Significantly elevated noggin levels in obese individuals clearly signify that in addition to being a potential target, noggin may also serve as a potential surrogate biomarker for obesity. Results of the current study also indicate that noggin acts as a key regulator balancing bone formation and adipogenesis. Reduced bone density has been previously reported in other murine obesity models as well (7, 8). Initially, it was thought that increased BMI protects against osteoporosis and bone fractures (22), but recent studies prove otherwise (23). Obese individuals show increased bone fat along with reduced trabecular bone mass. Aged women and women with osteoporosis also show increased bone fat levels and are susceptible to fractures (22). From our data, it is highly suggestive that increased noggin levels could result in increased bone fat and reduced BMD, and thus noggin could act as a molecular switch controlling the fate of MSC differentiation.
This study identified that noggin-mediated adipogenesis of MSC is independent of known adipogenesis pathways, which involve activation of PI3K, mTOR/AKT, and cAMP. Signals from these activators lead to expression of C/EBPδ, C/EBPα, and PPAR-γ, which are key transcription factors of adipogenesis. Noggin was able to induce expression of all these three transcription factors during adipocytic differentiation of MSC. PPARγ and C/EBPα cross-regulate each other to maintain gene expression. Both these transcription factors either alone or in cooperation with each other induce the transcription of many adipocyte genes encoding proteins and enzymes involved in creating and maintaining the adipocyte phenotype (24).
Based on our novel observation, up-regulation of noggin marks an independent mechanism of inducing adipogenesis. The factors that lead to noggin up-regulation remain unexplored. But there are some possible mechanisms that may be considered. Insulin-like growth factor-1 (IGF-1), an important differentiation factor for osteoblasts, suppresses noggin expression (25). It is very important for maintenance of bone homeostasis. During obesity, IGF-1 is drastically reduced. Hence, the interplay between IGF-1 and noggin may be a key to understand noggin-mediated adipogenesis (23). Further studies need to elucidate the molecular mechanism of noggin-induced Pax-1 up-regulation. Pax-1 encodes a DNA-binding protein with transcriptional activating properties and plays a role during embryonic patterning (20, 21). Mice with noggin haplo- insufficiency exhibit reduced Pax-1 levels. Recently Pax-1 was mapped to the locus chromosome 2 in mice and chromosome 20p11.1 in humans, both of which also contain various obesity genes (26). Mutation in Pax-1 gene in mice results in substantial decrease in adiposity index (26). Further support for the role of Pax-1 in adipogenesis is also suggested from the promoter analysis of PPAR-γ, C/EBP-α, and C/EBP-δ genes. Promoter regions of genes encoding these transcription factors display putative Pax-1 binding sites, indicating a possible role of Pax-1 in noggin-mediated adipogenesis. Further studies are needed to elucidate the molecular mechanism of noggin-induced Pax-1 up-regulation. The role of noggin and Pax-1 has been studied well during the early stages of embryonic development. Noggin induces Pax-1 expression during sclerotome development in the early somite stage (27). Mutation in noggin completely abrogates Pax-1 expression and further results in reduced survival of sclerotome (28). Besides, BMP-2 and BMP-4 are potent inhibitors of Pax-1 induction and are known to inhibit sclerotome growth and development (29). Thus, inhibition of BMP signaling by noggin is important for somite development in vertebrates (30). BMPs induce osteoblast differentiation, and expression of BMPs results in reduced adipogenesis of MSC (31). Because the results of this study indicate that noggin induces adipogenesis, we speculated that MSC from obese mice, with elevated noggin and Pax-1 levels, may have reduced BMP-2 expression compared with normal MSC. Interestingly, when tested for this possibility, MSC from obese mice in fact indicated drastically low levels of BMP-2 (supplemental Figs. S5). Thus, down-regulation of BMP-2 by noggin may also be a mechanism for elevated Pax-1 expression to facilitate adipogenesis.
As noggin is a secreted protein, it can be speculated that it functions in a paracrine manner leading to obesity. It remains to be determined how noggin may act in a paracrine fashion. One of the possibilities is that noggin may act through BMP receptors. It is known that signaling via BMP receptor Ia leads to adipocyte differentiation, whereas signaling via BMP receptor Ib leads to osteoblast differentiation (32). BMP receptors commonly signal through activation of Smad family of proteins. Smad1, which is a receptor-regulated Smad protein, undergoes phosphorylation during adipogenesis via BMP receptor (33, 34). Binding of phosphorylated Smad1 to Smad4, a common mediator Smad, induces expression of PPAR-γ and CEBP-α. Overexpression of Smad6, a natural antagonist of Smad1, blocks Smad1 activation and thus inhibits adipogenesis (35). Smad3, which is also a receptor-regulated Smad, inhibits adipogenic differentiation of MSC (36, 37). Besides roles played by Smad proteins in adipogenesis, activation of p38 kinase also leads to adipocytes formation (33, 34). Thus, further studies to delineate possible roles of these proteins in noggin-mediated adipogenesis would provide more details. p38 kinase is known to greatly enhance transactivation of Pax family of proteins, mainly Pax-5 and Pax-6 (38). It will also be interesting to investigate if p38 kinase has a similar effect of Pax-1 activation. It needs to be determined if adipocytes can secrete noggin and whether there is a paracrine effect leading to progressive obesity. Thus, further understanding of noggin-mediated adipocyte pathways may have a profound impact in developing therapies for treating obesity.
Supplementary Material
Acknowledgment
We thank Dr. Robert Hardy for insightful comments.
This work was supported, in whole or in part, by National Institutes of Health Grants AR050251, AR060948 (to S. P.), DK083562 (to W. T. G.), and DK038765 (to W. T. G.). This work was also supported by the University of Alabama at Birmingham Diabetes Research and Training Center Grant P60 DK079626 (to W. T. G.).

This article contains supplemental Figs. S1–S5.
- PPAR
- peroxisome proliferator-activated receptor
- MSC
- mesenchymal stem cell
- Pax-1
- Paired box gene-1
- BMP
- bone morphogenetic protein
- ADM
- adipocyte differentiation medium
- GPDH
- glycerol-3-phosphate dehydrogenase
- micro-CT
- micro-computed tomography
- BMI
- body mass index.
REFERENCES
- 1. Haslam D. W., James W. P. (2005) Obesity. Lancet 366, 1197–1209 [DOI] [PubMed] [Google Scholar]
- 2. Barness L. A., Opitz J. M., Gilbert-Barness E. (2007) Obesity: genetic, molecular, and environmental aspects. Am. J. Med. Genet. A 143A, 3016–3034 [DOI] [PubMed] [Google Scholar]
- 3. Magni P., Dozio E., Galliera E., Ruscica M., Corsi M. M. (2010) Molecular aspects of adipokine-bone interactions. Curr. Mol. Med. 10, 522–532 [DOI] [PubMed] [Google Scholar]
- 4. Takada I., Kouzmenko A. P., Kato S. (2009) Molecular switching of osteoblastogenesis versus adipogenesis. Implications for targeted therapies. Expert Opin. Ther. Targets 13, 593–603 [DOI] [PubMed] [Google Scholar]
- 5. Muruganandan S., Roman A. A., Sinal C. J. (2009) Adipocyte differentiation of bone marrow-derived mesenchymal stem cells. Cross-talk with the osteoblastogenic program. Cell Mol. Life Sci. 66, 236–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodríguez J. P., Astudillo P., Ríos S., Pino A. M. (2008) Involvement of adipogenic potential of human bone marrow mesenchymal stem cells (MSC) in osteoporosis. Curr. Stem Cell Res. Ther. 3, 208–218 [DOI] [PubMed] [Google Scholar]
- 7. Wu X. B., Li Y., Schneider A., Yu W., Rajendren G., Iqbal J., Yamamoto M., Alam M., Brunet L. J., Blair H. C., Zaidi M., Abe E. (2003) Impaired osteoblastic differentiation, reduced bone formation, and severe osteoporosis in noggin-overexpressing mice. J. Clin. Invest. 112, 924–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kajkenova O., Lecka-Czernik B., Gubrij I., Hauser S. P., Takahashi K., Parfitt A. M., Jilka R. L., Manolagas S. C., Lipschitz D. A. (1997) Increased adipogenesis and myelopoiesis in the bone marrow of SAMP6, a murine model of defective osteoblastogenesis and low turnover osteopenia. J. Bone Miner Res. 12, 1772–1779 [DOI] [PubMed] [Google Scholar]
- 9. Valenzuela D. M., Economides A. N., Rojas E., Lamb T. M., Nuñez L., Jones P., Lp N. Y., Espinosa R., 3rd, Brannan C. I., Gilbert D. J. (1995) Identification of mammalian noggin and its expression in the adult nervous system. J. Neurosci. 15, 6077–6084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang L., Zhang X., Guo Y., Chen X., Li R., Liu L., Shi C., Guo C., Zhang Y. (2010) Involvement of BMPs/Smad signaling pathway in mechanical response in osteoblasts. Cell Physiol. Biochem. 26, 1093–1102 [DOI] [PubMed] [Google Scholar]
- 11. Gazzerro E., Gangji V., Canalis E. (1998) Bone morphogenetic proteins induce the expression of noggin, which limits their activity in cultured rat osteoblasts. J. Clin. Invest. 102, 2106–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tropel P., Noël D., Platet N., Legrand P., Benabid A. L., Berger F. (2004) Isolation and characterization of mesenchymal stem cells from adult mouse bone marrow. Exp. Cell Res. 295, 395–406 [DOI] [PubMed] [Google Scholar]
- 13. Ng F., Boucher S., Koh S., Sastry K. S., Chase L., Lakshmipathy U., Choong C., Yang Z., Vemuri M. C., Rao M. S., Tanavde V. (2008) platelet-derived growth factor, TGF-β, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSC). Transcriptional profiling can identify markers and signaling pathways important in differentiation of MSC into adipogenic, chondrogenic, and osteogenic lineages. Blood 112, 295–307 [DOI] [PubMed] [Google Scholar]
- 14. Yang D. C., Tsay H. J., Lin S. Y., Chiou S. H., Li M. J., Chang T. J., Hung S. C. (2008) cAMP/PKA regulates osteogenesis, adipogenesis, and the ratio of RANKL/OPG mRNA expression in mesenchymal stem cells by suppressing leptin. PLoS One 3, e1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beraldo F. H., Almeida F. M., da Silva A. M., Garcia C. R. (2005) Cyclic AMP and calcium interplay as second messengers in melatonin-dependent regulation of Plasmodium falciparum cell cycle. J. Cell Biol. 170, 551–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu W., Chen Z., Zhang J., Zhang L., Ke H., Huang L., Peng Y., Zhang X., Li S., Lahn B. T., Xiang A. P. (2008) Critical role of phosphoinositide 3-kinase cascade in adipogenesis of human mesenchymal stem cells. Mol. Cell. Biochem. 310, 11–18 [DOI] [PubMed] [Google Scholar]
- 17. Forte G., Minieri M., Cossa P., Antenucci D., Sala M., Gnocchi V., Fiaccavento R., Carotenuto F., De Vito P., Baldini P. M., Prat M., Di Nardo P. (2006) Hepatocyte growth factor effects on mesenchymal stem cells. Proliferation, migration, and differentiation. Stem Cells 24, 23–33 [DOI] [PubMed] [Google Scholar]
- 18. van Harmelen V., Dicker A., Rydén M., Hauner H., Lönnqvist F., Näslund E., Arner P. (2002) Increased lipolysis and decreased leptin production by human omental as compared with subcutaneous preadipocytes. Diabetes 51, 2029–2036 [DOI] [PubMed] [Google Scholar]
- 19. Rydén M., Dicker A., Götherström C., Aström G., Tammik C., Arner P., Le Blanc K. (2003) Functional characterization of human mesenchymal stem cell-derived adipocytes. Biochem. Biophys. Res. Commun. 311, 391–397 [DOI] [PubMed] [Google Scholar]
- 20. Walther C., Guenet J. L., Simon D., Deutsch U., Jostes B., Goulding M. D., Plachov D., Balling R., Gruss P. (1991) Pax: a murine multigene family of paired box-containing genes. Genomics 11, 424–434 [DOI] [PubMed] [Google Scholar]
- 21. Chalepakis G., Fritsch R., Fickenscher H., Deutsch U., Goulding M., Gruss P. (1991) The molecular basis of the undulated/Pax-1 mutation. Cell 66, 873–884 [DOI] [PubMed] [Google Scholar]
- 22. Holecki M., Wiecek A. (2010) Relationship between body fat mass and bone metabolism. Polskie Archiwum Medycyny Wewntrznej 120, 361–367 [PubMed] [Google Scholar]
- 23. Bredella M. A., Torriani M., Ghomi R. H., Thomas B. J., Brick D. J., Gerweck A. V., Rosen C. J., Klibanski A., Miller K. K. (2011) Obesity 19, 49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ntambi J. M., Young-Cheul K. (2000) Adipocyte differentiation and gene expression. J. Nutr. 130, 3122S–3126S [DOI] [PubMed] [Google Scholar]
- 25. Kim J. S., Ellman M. B., An H. S., van Wijnen A. J., Borgia J. A., Im H. J. (2010) Insulin-like growth factor 1 synergizes with bone morphogenetic protein 7-mediated anabolism in bovine intervertebral disc cells. Arthritis Rheum. 62, 3706–3715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Warden C. H., Stone S., Chiu S., Diament A. L., Corva P., Shattuck D., Riley R., Hunt S. C., Easlick J., Fisler J. S., Medrano J. F. (2004) Identification of a congenic mouse line with obesity and body length phenotypes. Mamm. Genome 15, 460–471 [DOI] [PubMed] [Google Scholar]
- 27. DiPaola C. P., Farmer J. C., Manova K., Niswander L. A. (2005) Molecular signaling in intervertebral disk development. J. Orthop. Res. 23, 1112–1119 [DOI] [PubMed] [Google Scholar]
- 28. Fan C. M., Porter J. A., Chiang C., Chang D. T., Beachy P. A., Tessier-Lavigne M. (1995) Long range sclerotome induction by sonic hedgehog. Direct role of the amino-terminal cleavage product and modulation by the cyclic AMP signaling pathway. Cell 81, 457–465 [DOI] [PubMed] [Google Scholar]
- 29. Monsoro-Burq A. H., Duprez D., Watanabe Y., Bontoux M., Vincent C., Brickell P., Le Douarin N. (1996) The role of bone morphogenetic proteins in vertebral development. Development 122, 3607–3616 [DOI] [PubMed] [Google Scholar]
- 30. McMahon J. A., Takada S., Zimmerman L. B., Fan C. M., Harland R. M., McMahon A. P. (1998) Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 12, 1438–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Choi K. S., Ahn S. Y., Kim T. S., Kim J., Kim B. G., Han K. H., Ban S. J., Kim H. S., Choi Y., Lim C. J. (2011) Characterization and biodistribution of human mesenchymal stem cells transduced with lentiviral-mediated BMP2. Arch. Pharm. Res. 34, 599–606 [DOI] [PubMed] [Google Scholar]
- 32. Chen D., Ji X., Harris M. A., Feng J. Q., Karsenty G., Celeste A. J., Rosen V., Mundy G. R., Harris S. E. (1998) Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J. Cell Biol. 142, 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee J. S., Park J. H., Kwon I. K., Lim J. Y. (2011) Retinoic acid inhibits BMP4-induced C3H10T1/2 stem cell commitment to adipocyte via down-regulating Smad/p38MAPK signaling. Biochem. Biophys. Res. Commun. 409, 550–555 [DOI] [PubMed] [Google Scholar]
- 34. Hata K., Nishimura R., Ikeda F., Yamashita K., Matsubara T., Nokubi T., Yoneda T. (2003) Differential roles of Smad1 and p38 kinase in regulation of peroxisome proliferator-activating receptor γ during bone morphogenetic protein 2-induced adipogenesis. Mol. Biol. Cell 14, 545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Choy L., Skillington J., Derynck R. (2000) Roles of autocrine TGF-β receptor and Smad signaling in adipocyte differentiation. J. Cell Biol. 149, 667–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Choy L., Derynck R. (2003) Transforming growth factor-β inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J. Biol. Chem. 278, 9609–9619 [DOI] [PubMed] [Google Scholar]
- 37. Marchildon F, St.-Louis C., Akter R, Roodman V, Wiper-Bergeron NL. (2010) Transcription factor Smad3 is required for the inhibition of adipogenesis by retinoic acid. J. Biol. Chem. 285, 13274–13284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yan Q., Liu W. B., Qin J., Liu J., Chen H. G., Huang X., Chen L., Sun S., Deng M., Gong L., Li Y., Zhang L., Liu Y., Feng H., Xiao Y., Liu Y., Li D. W. (2007) Protein phosphatase-1 modulates the function of Pax-6, a transcription factor controlling brain and eye development. J. Biol. Chem. 282, 13954–13965 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.