Background: Connexin hemichannels are Ca2+-permeable plasma membrane channels that are controlled by [Ca2+]i; therefore, they may contribute to Ca2+ oscillations.
Results: Ca2+ oscillations triggered by bradykinin in connexin-expressing cells were inhibited by blocking hemichannel opening or by preventing their closure at high [Ca2+]i; ATP-triggered oscillations were unaffected.
Conclusion: Hemichannels contribute to oscillations by controlling Ca2+ entry.
Significance: Hemichannels together with InsP3 receptors help shape agonist-induced Ca2+ oscillations.
Keywords: ATP; Calcium; Connexin; Gap Junctions; Inositol 1,4,5-Trisphosphate; Bradykinin; Calcium Entry; Calcium Oscillations; Connexin Hemichannel
Abstract
Many cellular functions are driven by changes in the intracellular Ca2+ concentration ([Ca2+]i) that are highly organized in time and space. Ca2+ oscillations are particularly important in this respect and are based on positive and negative [Ca2+]i feedback on inositol 1,4,5-trisphosphate receptors (InsP3Rs). Connexin hemichannels are Ca2+-permeable plasma membrane channels that are also controlled by [Ca2+]i. We aimed to investigate how hemichannels may contribute to Ca2+ oscillations. Madin-Darby canine kidney cells expressing connexin-32 (Cx32) and Cx43 were exposed to bradykinin (BK) or ATP to induce Ca2+ oscillations. BK-induced oscillations were rapidly (minutes) and reversibly inhibited by the connexin-mimetic peptides 32Gap27/43Gap26, whereas ATP-induced oscillations were unaffected. Furthermore, these peptides inhibited the BK-triggered release of calcein, a hemichannel-permeable dye. BK-induced oscillations, but not those induced by ATP, were dependent on extracellular Ca2+. Alleviating the negative feedback of [Ca2+]i on InsP3Rs using cytochrome c inhibited BK- and ATP-induced oscillations. Cx32 and Cx43 hemichannels are activated by <500 nm [Ca2+]i but inhibited by higher concentrations and CT9 peptide (last 9 amino acids of the Cx43 C terminus) removes this high [Ca2+]i inhibition. Unlike interfering with the bell-shaped dependence of InsP3Rs to [Ca2+]i, CT9 peptide prevented BK-induced oscillations but not those triggered by ATP. Collectively, these data indicate that connexin hemichannels contribute to BK-induced oscillations by allowing Ca2+ entry during the rising phase of the Ca2+ spikes and by providing an OFF mechanism during the falling phase of the spikes. Hemichannels were not sufficient to ignite oscillations by themselves; however, their contribution was crucial as hemichannel inhibition stopped the oscillations.
Introduction
Ca2+ is a universal and versatile intracellular messenger controlling a large variety of basic cellular functions that include fertilization, gene expression, cell differentiation, exocytosis, muscle contraction, cell survival, and cell death (1, 2). Ca2+ is released from the endoplasmic reticulum (ER),3 the cell's major Ca2+ store, after activation of Gαq-protein-coupled receptors (GPCR) on the plasma membrane with subsequent activation of phospholipase Cβ (PLCβ), hydrolysis of phosphatidylinositol 4,5-bisphosphate, and production of inositol 1,4,5-trisphosphate (InsP3). Stimulation of InsP3 receptors (InsP3Rs) on the ER membrane triggers the release of ER Ca2+, thereby increasing the cytosolic Ca2+ concentration ([Ca2+]i). [Ca2+]i elevation is followed by a recovery phase that is mediated by Ca2+ sequestration back into the ER lumen through sarcoplasmic/endoplasmic Ca2+ ATPases and by Ca2+ extrusion out of the cell through plasma membrane Ca2+ ATPases (3, 4). This is followed by store-operated Ca2+ entry (SOCE) to replenish the ER with Ca2+ and attain pre-spike ER Ca2+ content (5). Physiological Ca2+ signals can take the form of a single, transient elevation in [Ca2+]i but may also appear as repeated [Ca2+]i transients, called Ca2+ oscillations (2, 6). The simplest model of Ca2+ oscillations is based on positive and negative modulatory effects of Ca2+ on the open probability of InsP3R channels, which display a typical bell-shaped dependence (7, 8). When [Ca2+]i is below a certain threshold (∼300 nm), Ca2+ potentiates InsP3-triggered Ca2+ release (7), resulting in Ca2+-induced Ca2+ release (9). A further rise in [Ca2+]i above this level results in negative feedback, marking the start of the decaying phase of the Ca2+ spike (7). After restoration of the ER Ca2+ content (by sarcoplasmic/endoplasmic Ca2+ ATPase pumps and SOCE) the cycle repeats to induce the next Ca2+ spike. An important condition for Ca2+ oscillations to occur is the necessity for kinetic differences between positive and negative Ca2+ feedback, which means the positive feedback action should be faster than the negative one (8, 10–12), a condition that is fulfilled for InsP3R channels (13, 14). Continued Ca2+ oscillations necessitate a slightly elevated intracellular InsP3 concentration that sets a certain degree of Ca2+ excitability of InsP3R channels, making them sensitive to small local Ca2+ increases that can fire the next Ca2+ spike through Ca2+-induced Ca2+ release (10). When the InsP3 concentration is elevated consequent to stronger GPCR stimulation, the oscillation frequency generally increases (10, 15). In addition, the intracellular InsP3 concentration is not solely determined by the level of GPCR stimulation but may also be influenced by direct or indirect feedback actions of [Ca2+]i on Ca2+- or PKC-sensitive PLC isoforms (δ, ζ, η or β, γ isoforms, respectively) (16, 17), thereby generating oscillations in the InsP3 concentration. These InsP3 oscillations may modulate the Ca2+ oscillations (by augmenting Ca2+-induced Ca2+ release (11)) but may also take the lead and provide the primary driving force for the Ca2+ oscillations (18), depending on the GPCRs involved. Additionally, other feedback actions on the InsP3 metabolism (11, 19) and on Ca2+ entry (20–24) provide supplementary tools to modulate and shape the oscillatory signal (25).
Evidence is accruing that connexin channels play a role in Ca2+ oscillations. Connexins form two kinds of channels, hemichannels and gap junction channels, the latter resulting from the head-to-head interaction of two hemichannels. Gap junction channels connect the cytoplasm of adjacent cells, whereas unapposed hemichannels, when open, link the cytoplasm with the extracellular fluid. Both types of channels are permeable to substances with a molecular mass below 1–1.5 kDa (26, 27). Kawano et al. (28) reported that octanol, a nonspecific connexin channel blocker, inhibited spontaneous Ca2+ oscillations in human mesenchymal stem cells. This work suggested the opening of hemichannels followed by ATP diffusing out of the cell and acting in an autocrine way on plasma membrane P2Y1 receptors, thereby activating PLCβ and generating InsP3. Verma et al. (29) reported that 43Gap26 and 37/43Gap27, two synthetic peptides that mimic short sequences in, respectively, the first extracellular loop of connexin 43 (Cx43) and the second extracellular loop of Cx37/Cx43, inhibited Ca2+ oscillations in connexin-expressing HeLa cells and in cardiac myocytes. 43Gap26 and 37/43Gap27 peptides are inhibitors of Cx43 gap junctions and have been reported to inhibit Cx43 hemichannels with faster kinetics (30–33). Verma et al. (29) proposed that Gap inhibition of Ca2+ oscillations was mediated by reducing Ca2+ entry via hemichannels thereby affecting ER Ca2+ release. We recently reported that 37/43Gap27 inhibits bradykinin (BK)-triggered Ca2+ oscillations in blood-brain barrier endothelial cells and thereby prevents a subsequent increase in barrier permeability (34). In addition to the fact that hemichannel-mediated ATP release and Ca2+ entry may play a role in Ca2+ oscillations, hemichannel opening is controlled by [Ca2+]i (35–37). Because hemichannels both influence and are influenced by [Ca2+]i, we examined the mechanisms by which hemichannels contribute to Ca2+ oscillations. We specifically aimed to determine whether hemichannel-[Ca2+]i interactions constitute a mechanism supporting oscillatory activity in a manner analogous to the InsP3R-[Ca2+]i link by using tools that selectively target either InsP3Rs or connexin channels. We found that hemichannels contribute to InsP3R-based oscillations by providing a Ca2+ entry pathway and by shutting down this Ca2+ entry pathway by inhibiting hemichannel activity when [Ca2+]i increases above ∼500 nm. This contribution of hemichannels was essential as the Gap peptides blocked the BK-induced oscillations. Hemichannels were not involved in ATP-induced Ca2+ oscillations, indicating that they may help in shaping distinct Ca2+ response patterns to different agonists.
EXPERIMENTAL PROCEDURES
Cell Culture
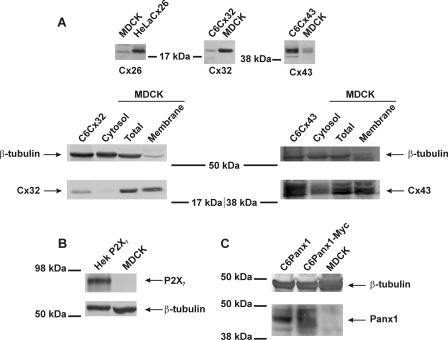
MDCK cells (up to passage 15) and C6-glioma cells stably transfected with Cx43 (C6Cx43), Panx1 (C6Panx1), or Panx1-Myc (C6Panx1-Myc) (C6 were kindly provided by Dr. Christian C. Naus, University of British Columbia) were maintained in Dulbecco's modified Eagle's medium/Ham's F-12 (1:1) supplemented with 10% fetal calf serum (FCS) and 2 mm glutamine, 10 units/ml penicillin, 10 μg/ml streptomycin, and 0.25 μg/ml Fungizone (all from Invitrogen) at 37 °C and 5% CO2. Rat brain endothelial (RBE4) cells were maintained in α-minimal essential medium/Ham's F-10 supplemented with 10% FCS, 2 mm l-glutamine, 300 μg/ml G418 (Invitrogen), and 1 ng/ml human recombinant basic fibroblast growth factor (Roche Diagnostics). MDCK and RBE4 cells were grown on collagen-coated recipients (rat-tail collagen, Roche Diagnostics).
Chemicals and Reagents
Arachidonyl trifluoromethyl ketone, 4-bromo-A23187, ATP, apyrase grade VI and VII, bradykinin, carbenoxolone (Cbx), cytochrome c (CytC) from equine heart, digitonin, EGTA, 8-(p-sulfophenyl)theophylline, and staurosporine were purchased from Sigma. 1,2-Bis(2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid tetrakis-acetoxymethyl ester (BAPTA-AM), calcein-AM, 5-carboxyfluorescein diacetate-AM, 10-kDa dextran Texas Red (DTR), 10-kDa dextran fluorescein, d-myo-inositol 1,4,5-trisphosphate, P4(5)-1-(2-nitrophenyl)ethyl ester (“caged InsP3”), fluo3-AM, fura2-AM, Hoechst 33342, Mitotracker Green FM, o-nitrophenyl-EGTA-AM (“Caged Ca2+”), propidium iodide (PI), RhodFF-AM and thapsigargin were from Molecular probes (Invitrogen). Xestospongin-C and U73122 were from Tocris Bioscience (Bristol, UK). 32Gap27 (SRPTEKTVFT, amino acids 182–191 in the second extracellular loop of Cx32), 43Gap26 (VCYDKSFPISHVR, amino acids 64–76 in the first extracellular loop of Cx43), CT9 (RPRPDDLEI, last 9 amino acids of the C-terminal tail of Cx43), CT9ΔI (RPRPDDLE), the reversed CT9 peptide (CT9Rev, IELDDPRPR), YGRKKRRQRRR-CT9 (Tat-CT9), and Tat-CT9Rev were synthesized by LifeTein at >80% purity. The C-terminal peptide IP3RCYT, corresponding to amino acids 2621–2636 of InsP3R1 (DNKTVTFEEHIKEEHNC) (38), was a kind gift of Dr. Darren F. Boehning (University of Texas Medical Branch).
Ca2+ Imaging
MDCK cells were seeded onto 18-mm-diameter glass coverslips (Knittel Glaser, Novolab, Geraardsbergen, Belgium), and experiments were performed at subconfluency the next day. The cells were loaded with 10 μm fluo3-AM in HBSS-Hepes (1 mm CaCl2, 0.81 mm MgSO4, 13 mm NaCl, 0.18 mm Na2HPO4, 5.36 mm KCl, 0.44 mm KH2PO4, 5.55 mm d-glucose, and 25 mm Hepes) for 1 h at room temperature (RT). After 1 h, coverslips were washed and then left for an additional 30 min at RT in HBSS-Hepes to allow for de-esterification. Cells were thereafter transferred to an inverted epifluorescence microscope (Eclipse TE 300, Nikon Belux, Brussels, Belgium) equipped with a superfusion system that allowed changing the bath solution within ∼1 min (bath volume ∼300 μl). Superfusion was switched off during the registration of oscillatory activity. Images were taken every second with a ×40 oil-immersion objective and an electron-multiplying CCD camera (Quantem 512SC, Photometrics, Tucson, AZ). We used a Lambda DG-4 filterswitch (Sutter Instrument Company, Novato, CA) to deliver excitation at 482 nm and captured emitted light via a 505-nm long-pass dichroic mirror and a 535-nm bandpass filter (35-nm bandwidth). Cells were loaded with fura2-AM (5 μm) in a similar manner. Excitation was alternated between 340 and 380 nm at a rate of one image pair every second. Emitted light was captured using a 430-nm long-pass dichroic mirror and a 510-nm bandpass-filter (40-nm bandwidth). Fura2 in situ calibration was performed in zero Ca2+ medium (10 mm EGTA) containing 10 μm A23187 (Rmin) and a saturating Ca2+ solution (10 mm CaCl2) containing 40 μm digitonin (Rmax). [Ca2+]i was calculated from Kd·Q·[(R − Rmin)/(Rmax− R)], where R is the F340/F380 ratio, Q is Fmin/Fmax at 380 nm, and Kd 224 nm (39). Mitochondrial Ca2+ measurements were performed with the mitochondrial Ca2+ indicator RhodFF as we described previously (40). MDCK cells were loaded with RhodFF-AM (5 μm) for 1 h at RT followed by 30 min of de-esterification. Imaging was performed in a similar manner as fluo3 imaging but with excitation at 556 nm and long-pass filtering at 590 nm. A punctate distribution that matches the distribution of Mitotracker Green (100 nm, 1 h, RT) confirmed the mitochondrial localization of the dye (supplemental Figs. S1). Recordings and analysis were done with custom-developed QuantEMframes and Fluoframes software written in Microsoft Visual C++ 6.0. Oscillatory activity was recorded in a 10-min observation window, and only Ca2+ spikes minimally 10% above baseline were considered in the analysis. To calculate the percentage of oscillating cells, an oscillating cell was defined as a cell displaying at least two Ca2+ spikes subsequent to the initial spike. The percentage of cells oscillating was calculated relative to the total number of cells in view. The oscillation frequency is the average frequency of all cells in view including non-oscillating cells.
Electroporation Loading
MDCK cells were loaded with CytC or CT peptides by electroporation. Cells, seeded the day before the experiment, were rinsed with a low conductivity electroporation buffer and placed on the microscope stage. Thereafter, a small volume (10 μl) of CytC (3 μm)/DTR (100 μm) or CT9 peptide (300 μm)/PI (12 μm) dissolved in electroporation buffer was added to a parallel wire Pt-Ir electrode positioned 400 μm above the cells. DTR (10 kDa) or PI (668 Da) have molecular masses approaching that of CytC (12 kDa) or CT9 (1 kDa), respectively, and were added to visualize the electroporated cells. In experiments using the Ca2+ dye RhodFF, we visualized the CytC-loaded zone with 10-kDa dextran fluorescein. The IP3RCYT peptide (15 μm) was added to the CytC (3 μm) solution (see above) 30 min before electroporation to allow for interaction. Electroporation was performed after fluo3-AM loading with 50-kHz bipolar pulses at a field strength of 1000 V/cm, applied as 15 trains of 10 pulses of 2-ms duration each (41, 42). Electroporation did not result in loss of fluo3/fura2/RhodFF from the cells.
Caged Compound Loading and Photoliberation
Cells seeded on coverslips the day before the experiment were electroporated with cell-impermeant, caged InsP3 (30 μm), and DTR (100 μm) as described above. Caged Ca2+ was loaded into the cells by ester-loading, similar to the Ca2+-sensitive dyes. Thereafter, the coverslips were transferred to an inverted epifluorescence microscope. Photoliberation of InsP3 was done by spot (20 μm diameter) illumination with 1-kHz pulsed UV light (349 nm UV laser Explorer, Spectra-Physics, Newport, Utrecht, The Netherlands) applied during 20 ms (20 pulses of 90 μJ energy measured at the entrance of the microscope epifluorescence tube). For uncaging of Ca2+ we applied UV illumination with different flash durations.
Hemichannel Assays
Hemichannel opening was investigated by calcein (623 Da) release, which is based on the efflux of the preloaded dye via open hemichannels (43). Subconfluent cultures of MDCK cells grown on glass coverslips (18 mm diameter), were preloaded with 50 μm calcein-AM in HBSS-HEPES for 1 h at RT. Subsequently, the remaining calcein-AM was removed; cells were left for an additional 30 min at RT in HBSS-Hepes to allow the AM ester to de-esterify and were then transferred to the stage of an inverted epifluorescence microscope. For analysis, we measured the decrease in calcein fluorescence in function of time. The first 5 min, baseline leakage in HBSS-Hepes was measured (control); thereafter, the trigger solution was added, and efflux of calcein was further evaluated. The slope of the curve, calculated by linear regression, was used as a parameter describing the loss of dye in time. Calcein efflux in the presence of trigger is presented as % of control.
Gap Junction Dye Coupling Studies
Dye coupling via gap junctions was determined using fluorescence recovery after photobleaching. MDCK cell cultures were grown to confluence on 9.2 cm2 Petri dishes (Techno Plastic Products, Trasadingen, Switzerland) and loaded with the gap junction-permeable fluorescent dye 5-carboxyfluorescein diacetate-AM (532 Da, 10 μm) in HBSS-Hepes for 1 h at RT. After de-esterification, cells were transferred to a custom-made video-rate confocal laser scanning microscope with a ×40 water immersion objective (CFI Plan Fluor) and a 488-nm laser excitation source (Cyan CW Laser, 488 nm, 100 milliwatt; Newport Spectra-Physics, Utrecht, The Netherlands). After 1 min of recording, the cell in the middle of the field was photobleached by spot exposure (1 s) to increased power of the 488 nm laser, and fluorescence recovery, caused by dye influx from neighboring non-bleached cells, was recorded during an additional 5-min period. The fluorescence recovery trace was then analyzed for the recovery of the signal expressed relative to the starting level before photobleaching.
Ectonucleotidase Activity
Ectonucleotidase activity was assessed by measuring the breakdown of ATP added to the cells via the decline of luciferin/luciferase luminescence in the medium above the cells. MDCK and RBE4 cells were seeded at a density of 40,000 cells/cm2 in 24-well plates. ATP (100 μm) together with an ATP bioluminescent assay mix (luciferin/luciferase, 625-fold dilution, Sigma) was prepared in ATP assay mix dilution buffer (Sigma). Photon flux was counted using a multilabel counter (Victor-3, type 1420, PerkinElmer Life Sciences). The time constant (τ) of the exponential luminescence decay was used as a parameter to express ectonucleotidase activity.
Apoptosis Assay
Annexin V staining detects the flip-flop of phosphatidylserine toward the outer plasma membrane leaflet that occurs during apoptosis. After electroporation with 10-kDa DTR (100 μm) and CytC (3 μm), cells were rinsed with PBS and incubated for 15 min (RT) with annexin V-FITC (1:50; Roche Diagnostics) and Hoechst 33342 (2 μg/ml) in annexin V buffer (140 mm NaCl, 5 mm CaCl2, 10 mm HEPES, pH 7.4). The cultures were subsequently washed with PBS and transferred to a Nikon TE300 epifluorescence microscope, equipped with a ×10 objective (Plan Apo, NA 0.4, Nikon). Ten images inside the electroporation zone were taken, and annexin V-positive cells were counted. The number of apoptotic cells was expressed as the percentage of annexin V-positive cells relative to the total number of cells (determined by Hoechst 33342 staining).
siRNA Treatment
MDCK cells were seeded onto 18-mm diameter glass coverslips at a density of 20,000 cells/cm2 and transfected the following day using Dharmafect1 lipid reagent (Dharmacon, Thermo Fisher Scientific). We used two siRNA duplexes (125 nm) directed against the canine Cx43 gene gja1 (siCx43–1, 5′-GUUCAAGUAUGGAAUUGAA-dTdT-3′; siCx43–2, 5′-UUCAAUUCCAUACUUGAAC-dTdT-3′). On day 3, medium was refreshed, and on day 4, cells were used for experiments. Transfection efficiency on day 3 was 52 ± 4.1% (n = 11), as determined with the fluorescent indicator siGLO. Control conditions were untreated cultures, mock-treated cultures (lipid reagent alone), and a negative control consisting of cultures transfected with a scrambled version of siCx43–1 (siCx43–1Scr: 5′-GCGUAUAUAUGAGUAAGUA-dTdT-3′). All siRNA duplexes were synthesized and annealed by Eurogentec (Luik, Belgium) after selective designing against Canis familiaris and screening for off-target sequences using Batch RNAi selector (44).
Electrophoresis and Western Blot Analysis
For Western blots, cells were seeded in 75-cm2 flasks. Total MDCK cell lysates were extracted with radioimmune precipitation assay buffer (25 mm Tris, 50 mm NaCl, 0.5% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 1 mm DTT, 0.055 g/ml β-glycerol phosphate, 30 μl/ml phosphatase inhibitor mixture, and 20 μl/ml mini EDTA-free protease inhibitor mixture). For separation of Triton X-100 soluble (cytosol) and insoluble (membrane) fractions, cells were harvested in 1% Triton X-100 supplemented with 50 mm NaF and 1 mm Na3VO4, and centrifuged at 16,000 g for 10 min. The Triton X-100 insoluble pellets were resuspended in 1× Laemmli sample buffer. Protein concentration was determined using a Bio-Rad DC protein assay (Bio-Rad), and absorbance was measured with a 590-nm long-pass filter. Lysates were separated by electrophoresis over a 10% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane (Amersham Biosciences). Membranes were subsequently blocked with TBS containing 5% nonfat milk and 0.1% Tween20. After blocking, blots were probed with rabbit anti-Cx43 antibody (Sigma), rabbit anti-Cx32 antibody (Sigma), rabbit anti-Cx26 antibody (Zymed Laboratories Inc., Invitrogen), rabbit anti-phospho-Cx43 (Ser(P)-368) (Cell Signaling Technology, Inc., Danvers, MA), rabbit anti-P2X7 antibody (Alomone Labs, Jerusalem, Israel), anti-Panx1 antibody (a kind gift of Dr. Dale W. Laird, University of Western Ontario), and rabbit anti-β-tubulin antibody (Abcam, Cambridge, UK) as a loading control. Membranes were subsequently incubated with an alkaline phosphatase-conjugated goat anti-rabbit IgG antibody (Sigma), and detection was done using the nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate reagent (Zymed Laboratories Inc., Invitrogen). Quantification was done by drawing a rectangular window around the concerned band and determining the signal intensity using ImageJ software. Background correction was done by the same procedure applied to nitrocellulose membranes where protein was absent.
Statistical Analysis
Data are expressed as the mean ± S.E. with n giving the number of independent experiments. Multiple groups were compared by one-way analysis of variance and Bonferroni post-test, making use of Graphpad Instat software. Two groups were compared with an unpaired Student's t test and two-tail p value. Results were considered statistically significant when p < 0.05 (one symbol for p < 0.05, two for p < 0.01, and three for p < 0.001).
RESULTS
Concentration and InsP3 Dependence of BK- and ATP-induced Ca2+ Oscillations
We first characterized BK- and ATP-induced Ca2+ oscillations in MDCK cells and determined the concentration dependence of the percentage of oscillating cells and of the oscillation frequency. We used non-confluent MDCK cell cultures to this purpose to limit the degree of gap junctional coupling. BK and ATP triggered an initial [Ca2+]i transient followed by repetitive Ca2+ spikes (recorded over a 10-min period) with quite a different profile (Fig. 1, A and B). BK concentrations ranging from 0.05 to 100 μm all triggered Ca2+ oscillations in an invariable percentage of cells (∼72%) and a similar oscillation frequency (∼5 spikes/10 min, measured over all cells in view) (Fig. 1C). By contrast, oscillations triggered by ATP concentrations between 0.5 μm and 2 mm were characterized by a bell-shaped concentration-response curve for the percentage of oscillating cells and oscillation frequency (Fig. 1D). The maximum number of oscillating cells (89 ± 3.4%; n = 5) and the maximal oscillation frequency (13 ± 1.1 spikes/10 min; n = 5) were observed with 10 μm ATP. These markedly different patterns of concentration dependence indicate distinct oscillation mechanisms for ATP and BK. We chose 10 μm ATP (a concentration located between the two peaks depicted in Fig. 1D) and 0.5 μm BK (relative location within the range of concentrations tested comparable with ATP) for further analysis of the differences between the oscillations triggered by these two agonists. We first determined whether the amplitude of the initial [Ca2+]i transient triggered by BK (0.5 μm) or ATP (10 μm) was different but found they were very similar (650 ± 59 and 783 ± 110 nm, respectively, n = 8, p > 0.05) which suggests that the intracellular InsP3 elevation is comparable with the two stimuli. Yet the area under the curve (AUC) for both triggers differed markedly with BK exhibiting a much larger AUC compared with ATP (Fig. 1, E and F). Because the amplitudes were not different, it follows that the BK-triggered [Ca2+]i transient is longer than the one triggered by ATP (see example traces in Fig. 1A). The longer duration of the BK-induced [Ca2+]i transient is likely to be related to Ca2+ entry from the extracellular space. In line with this, withdrawal of extracellular Ca2+ (zero extracellular Ca2+) did not much affect the initial [Ca2+]i transient elicited by ATP, whereas it largely reduced the one triggered by BK (Fig. 1, E and F). Further probing of SOCE by reintroducing extracellular Ca2+ after the [Ca2+]i transient in zero extracellular Ca2+ conditions showed that SOCE was much larger for BK than for ATP (Fig. 1G).
FIGURE 1.
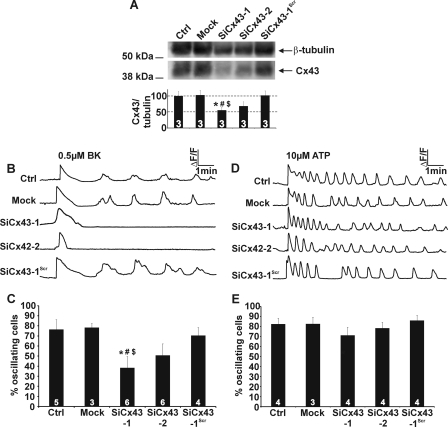
Concentration dependence of Ca2+ oscillations triggered by BK and ATP. A and B, representative traces demonstrate that exposure of MDCK cells to 0.5 μm BK or 10 μm ATP elicited Ca2+ oscillations. C, challenging MDCK cells with different concentrations of BK gave an almost flat response curve for both the percentage of oscillating cells and the oscillation frequency. D, the concentration dependence of ATP-induced oscillations was bell-shaped with a maximum at ∼10 μm ATP. E and F, comparison of the initial [Ca2+]i transients (AUC and amplitude) triggered by BK (0.5 μm) and ATP (10 μm) under control (1 mm extracellular Ca2+) and extracellular zero Ca2+ (no added Ca2+ + 1 mm EGTA) conditions. A.U., arbitrary units. G, ATP and BK transients were triggered in zero Ca2+ medium after which Ca2+ (1 mm) was reintroduced to the bathing medium. SOCE (AUC) triggered in this manner was significantly larger for BK compared with ATP. H and I, example traces illustrate the effect of photolytically releasing InsP3 (indicated by the star) on Ca2+ oscillations induced by BK (0.5 μm) or ATP (10 μm). J and K, the bar chart summarizes the percentage of cells displaying delayed or accelerated spiking activity following photorelease of InsP3.
Sneyd et al. (45) described a method to distinguish between oscillations characterized by a constant level of intracellular InsP3 and those associated with oscillatory InsP3 fluctuations by recording the response to an applied InsP3-concentration step. If InsP3 fluctuates during the Ca2+ oscillations, induction of a sudden InsP3 increase will introduce a delay to the next Ca2+ spike. The delay is caused by the fact that InsP3 has to recover to a level that is again compatible with Ca2+ oscillations; thereafter, the oscillation frequency stabilizes again. If InsP3 is constant during the Ca2+ oscillations, an induced InsP3 elevation will temporarily increase the oscillation frequency, giving accelerated oscillations (45, 46). We performed InsP3 elevation experiments with flash photolysis of caged InsP3 and found that the majority of the cells (∼48%) showed a delayed response for both BK- and ATP-induced oscillations (Fig. 1, H–K). A smaller fraction of the cells displayed an accelerated response: ∼10% for BK and ∼22% for ATP oscillations. In the remainder of the cells InsP3 elevation had no effect. These data indicate that oscillations triggered by BK and ATP are similar, at least with respect to the occurrence of InsP3 oscillations.
Connexin Channel Blockers Inhibit BK-induced Ca2+ Oscillations but Not Those Triggered by ATP
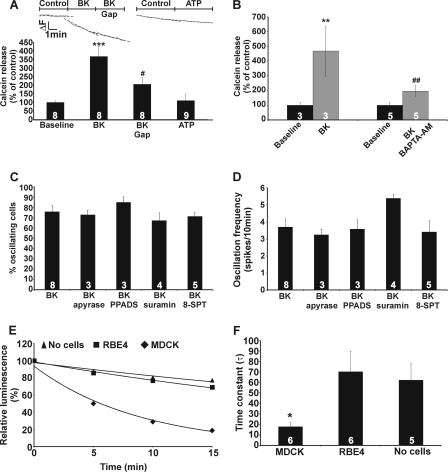
SDS-PAGE and Western blot analysis revealed the presence of Cx32 and Cx43 in MDCK cells, with a small background expression of Cx26. Both Cx32 and Cx43 were present in the plasma membrane, whereas their presence in the cytosolic fraction was limited (Fig. 2A). MDCK cells did not express Panx1 or P2X7, which is linked to Panx1 channels (47) (Fig. 2, B and C). When BK-triggered oscillations were elicited in the presence of the general connexin channel blocker Cbx (25 μm, 30-min preincubation and present during the 10-min observation window), the initial Ca2+ spike remained, but the subsequent oscillations disappeared. We further applied two peptide connexin channel inhibitors, 32Gap27 and 43Gap26, that target Cx32 and Cx43 channels, respectively (36, 48). Application of 32Gap27/43Gap26 (“Gap,” 200 μm, 30-min preincubation and present during the recording) also inhibited the Ca2+ oscillations without perturbing the initial peak (Fig. 3A). 32Gap27 and 43Gap26, either separately or in an equimolar mix, reduced the percentage of oscillating cells to ∼1/3, and Cbx reduced it to ∼1/7 (Fig. 3B); a similar degree of inhibition was observed for the oscillation frequency (Fig. 3C). Superfusion experiments showed that inhibition by 32Gap27/43Gap26 was rapid, within ∼1 min, and that oscillations reappeared upon wash-out of the peptides (Fig. 3D). We next tested the effect of Gap peptides and Cbx on Ca2+ oscillations triggered by ATP (10 μm) and found they had no effect on the percentage of oscillating cells or the oscillation frequency (Fig. 3, E-G). Thus, only BK-triggered Ca2+ oscillations are influenced by Cbx or Gap peptides. The BK-triggered Ca2+ oscillations were not synchronized in neighboring cells, pointing to absence of gap junctional or other synchronizing mechanisms (49–51). The rapid block of oscillations by Gap peptides suggests an effect at the level of hemichannels, as generally longer incubations are needed to also influence gap junctions (30, 32, 34). In line with this, 32Gap27/43Gap26 (200 μm, 60 min) had no effect on gap junction dye coupling studied with fluorescence recovery after photobleaching (Fig. 3H). Knockdown of Cx43 RNA showed that suppressing Cx43 expression prevented BK-triggered Ca2+ oscillations, in line with inhibition of oscillations by 43Gap26 added alone (without 32Gap27). Western blot analysis indicated a ∼50% reduction of Cx43 expression in cells transfected with SiCx43–1 (125 nm, 48 h) (Fig. 4A), and the number of oscillating cells in the presence of BK was reduced to a similar extent (Fig. 4, B and C). SiCx43–2 was less efficient and gave proportionally less inhibition (Fig. 4, A and C). Importantly, neither SiCx43–1 nor SiCx43–2 reduced Cx32 expression (data not shown); therefore, the oscillations that remain after Cx43 silencing may result from incomplete Cx43 knockdown or from the contribution of Cx32 hemichannels. Cx43-gene silencing did not influence ATP-triggered Ca2+ oscillations (Fig. 4, D and E).
FIGURE 2.
Connexin, pannexin, and P2X7 expression in MDCK cells. A, Western blot analysis shows expression of Cx32 and Cx43, with a small background of Cx26. Cx32 and Cx43 were mainly present in membrane fractions, whereas their presence in the cytosol fraction was much lower. C6Cx32, C6Cx43, or HeLaCx26 were used as positive controls, and β-tubulin was a loading control. B, MDCK cells do not express the purinoceptor P2X7. HEK cells stably transfected with P2X7 were used as a positive control. C, Panx1 is not expressed in MDCK cells. C6 cells stably transfected with Panx1 or Panx1-Myc are used as positive controls.
FIGURE 3.
Gap peptides and Cbx block BK-induced Ca2+ oscillations. A, representative traces of [Ca2+]i responses to BK application (0.5 μm) illustrating that Cbx (25 μm) and 32Gap27/43Gap26 (Gap, 200 μm) inhibit the oscillations. B, effect of Cbx and Gap peptides on the percentage of oscillating cells. C, effect of Cbx and Gap peptides on oscillation frequency. D, superfusion experiment illustrating that BK-triggered oscillations almost immediately disappeared after the addition of the Gap peptides. Summary data of experiments are shown below. Stars indicate significance compared with BK alone. E, example traces of Ca2+ responses triggered by ATP (10 μm) illustrate the absence of any effect of Cbx and Gap on the oscillations. F, the percentage of cells displaying oscillations in response to ATP was not altered by Cbx and Gap. G, the oscillation frequency with ATP stimulation was not significantly altered by Cbx and Gap. H, Gap junctional dye coupling was unaffected when 32Gap27/43Gap26 (Gap) were incubated for 1 h but strongly declined when these peptides were present for 24 h or when the aspecific connexin channel blocker Cbx (25 μm, 15 min) was used.
FIGURE 4.
Effect of Cx43 gene silencing on Ca2+ oscillations. A, silencing of Cx43 gene expression in MDCK cells demonstrates significantly reduced expression to ∼50% of the control signals (the star compares to Ctrl, the number sign compares to Mock-treated cultures, and the dollar sign compares to cells transfected with a scrambled sequence). B, example traces of Ca2+ responses triggered by BK (0.5 μm) for the different treatment conditions shown in panel A. C, Cx43 knockdown in MDCK cells approximately halved the number of oscillating cells in the presence of 0.5 μm BK (significance signs as in panel A). D and E, Cx43 gene silencing did not influence Ca2+ oscillations triggered by 10 μm ATP.
Lowering Extracellular Ca2+ Differentially Affects BK- and ATP-induced Oscillations
Hemichannels may contribute to the oscillations via ATP release acting in an autocrine manner (28, 34) or via Ca2+ entry (29). We first set out to find evidence for hemichannel opening in response to BK. Exposure of cells preloaded with calcein (a hemichannel-permeable fluorescent dye with a molecular mass of 623 Da (43)) to BK (0.5 μm) increased the calcein efflux rate, and this effect was counteracted by 32Gap27/43Gap26 (Fig. 5A). In contrast, ATP (10 μm) did not accelerate dye efflux (Fig. 5A). BK-triggered calcein release could be furthermore reduced by buffering increases in [Ca2+]i using the Ca2+ chelator BAPTA-AM (Fig. 5B). Phospholipase A2 is reported to be activated by BK (52, 53) and is involved in connexin hemichannel opening (36); however, phospholipase A2 inhibition by arachidonyl trifluoromethyl ketone had no effect on the percentage of cells displaying oscillations in response to BK (63 ± 7% oscillating cells in control versus 65 ± 7% oscillating cells in arachidonyl trifluoromethyl ketone-treated cells, n = 4), further emphasizing that [Ca2+]i changes are necessary for hemichannel opening. We next tested whether ATP release through open hemichannels played a role in BK-induced Ca2+ oscillations. We applied apyrase VI/VII (to degrade extracellular ATP), PPADS, or suramin (to inhibit purinergic P2 receptors), and 8-(p-sulfophenyl)theophylline (to inhibit adenosine A1/A2B receptors), but these agents did not significantly influence the percentage of oscillating cells or the oscillation frequency (Fig. 5, C and D). We recently reported that ATP release via hemichannels was involved in BK-induced oscillations in RBE4 brain endothelial cells (34), and we speculated that MDCK cells display a stronger ectonucleotidase activity than RBE4 cells. In line with this, we found that ATP added to the incubation solution above the cells was more rapidly degraded in MDCK cells as compared with RBE4 cell cultures (Fig. 5, E and F), indicating stronger ectonucleotidase activity in MDCK cells. Thus, ATP released via BK-induced hemichannel opening likely has no downstream effects on purinergic receptors or Ca2+ oscillations in MDCK cells due to its rapid degradation.
FIGURE 5.
Calcein hemichannel studies and involvement of ATP release. A, BK (0.5 μm) stimulated the rate of calcein release from MDCK cells, and this was rapidly inhibited by 32Gap27/43Gap26 (Gap, 200 μm). ATP did not influence the rate of calcein release. B, BK-triggered calcein release was inhibited by [Ca2+]i chelation with BAPTA-AM (5 μm, 1 h preincubation). Asterisks indicate significance compared with baseline, and number signs indicate a significant difference compared with BK-triggered calcein release. C and D, apyrase (5 units/ml, 30 min preincubation), PPADS (75 μm, 30 min preincubation), suramin (200 μm, 30 min preincubation), or 8-(p-sulfophenyl)theophylline (8-SPT, 100 μm, 30 min preincubation) did not significantly influence BK-induced Ca2+ oscillations. E, example traces illustrate the spontaneous decrease of ATP concentration after the addition of exogenous ATP to the medium above the cells. In MDCK cells, ATP decreased much more rapidly compared with rat brain endothelial cells (RBE4) or no cells being present. F, a bar chart summarizes analysis of experiments shown in E. The data indicate prominent ectonucleotidase activity in MDCK cells.
Because ATP release does not contribute to BK-triggered Ca2+ oscillations in MDCK cells, we evaluated the role of Ca2+ entry through hemichannels. To this purpose we decreased the driving force for Ca2+ entry by lowering the extracellular Ca2+ concentration. The latter may result in hemichannel opening (54–56), compromising the interpretation of the intended experiments. However, concentrations below ∼0.2 mm are reported to open hemichannels (57); thus, we applied 0.5 mm instead of the normal extracellular Ca2+ concentration of 1 mm. Calcein release experiments confirmed that exposure of MDCK cells to 0.5 mm extracellular Ca2+ did not trigger hemichannel opening, whereas a further reduction of extracellular Ca2+ to 0.2 mm or the subnanomolar range indeed provoked the opening of hemichannels (Fig. 6, A and B). Application of 0.5 mm extracellular Ca2+ during BK exposure interrupted the Ca2+ oscillations, and re-addition of normal extracellular Ca2+ restored the oscillations (Fig. 6C). The number of oscillating cells was strongly reduced by 0.5 mm extracellular Ca2+ (Fig. 6D). Interestingly, Ca2+ oscillations induced by ATP decreased in amplitude but were not suppressed by switching to 0.5 mm extracellular Ca2+, and the percentage of oscillating cells was not significantly altered (Fig. 6, E and F). Thus, BK-induced oscillations are dependent on extracellular Ca2+, indicating a contribution of SOCE or Ca2+ entry via hemichannels to the oscillation mechanism. Importantly, hemichannels did not contribute to SOCE, as 32Gap27/43Gap26, added during reintroduction of extracellular Ca2+ in an experiment as shown in Fig. 1G, did not influence SOCE after BK stimulation (data not shown).
FIGURE 6.
Depletion of extracellular Ca2+ inhibits BK-induced Ca2+ oscillations. A, applying 0.5 mm Ca2+ solution did not trigger calcein release in MDCK cells, whereas 0.2 mm or ∼1 nm free extracellular Ca2+ solutions did. B, the bar chart summarizes the effect of low extracellular Ca2+ on calcein release. The asterisk indicates a significant difference from the corresponding control. C, switching to 0.5 mm extracellular Ca2+ during BK-induced Ca2+ oscillations immediately interrupted the oscillations, and the effect was reversible upon switching to normal extracellular Ca2+. D, low extracellular Ca2+ (0.5 mm) strongly reduced the number of oscillating cells. E and F, low extracellular Ca2+ (0.5 mm) had no effect on ATP-induced oscillations. *, significantly different from 0.5 μm BK before Ca2+ depletion; # significantly different from oscillations after restoration of extracellular Ca2+.
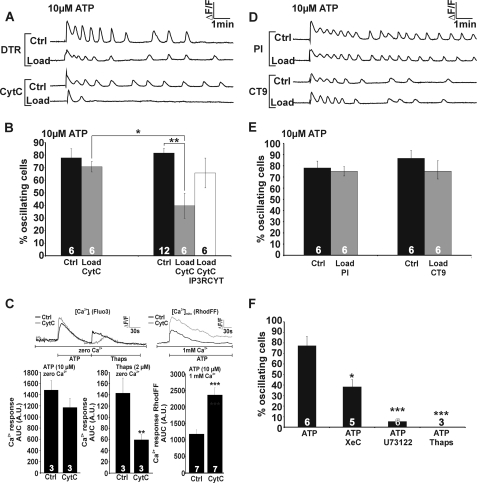
ATP-induced Ca2+ Oscillations Are Inhibited by CytC, whereas BK-induced Oscillations Are Inhibited by Both CytC and Cx43-targeting CT9 Peptide
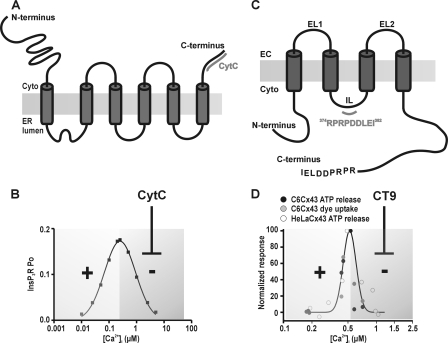
A critical and essential factor in the generation of Ca2+ oscillations is the presence of positive and negative feedback actions (11, 12, 15). In the classical scheme of InsP3-triggered Ca2+ oscillations, this feedback acts at InsP3Rs, with low [Ca2+]i stimulating ER Ca2+ release and higher concentrations being inhibitory (7) (Fig. 7B). Based on dye uptake and ATP release studies, hemichannels composed of Cx32 and Cx43 have also been demonstrated to display a bell-shaped [Ca2+]i dependence for opening (35, 36) (Fig. 7D). There is an interesting set of tools available to influence the bell-shaped Ca2+ dependence of InsP3Rs and hemichannels. Negative feedback of Ca2+ on InsP3Rs can be alleviated by CytC (58) (Fig. 7, A and B). In fact, this is an important mechanism contributing to apoptotic cell death because CytC binding to the InsP3 receptor removes the brake on ER Ca2+ release, resulting in Ca2+ accumulation in mitochondria that amplifies CytC release in a vicious circle (59–61). Inhibiting the declining phase of InsP3R activity is expected to disrupt oscillations because of the essential role of negative feedback as an OFF signal in the oscillation cycle. We recently reported that a synthetic peptide composed of the last 10 amino acids of the C-terminal (CT) tail of Cx43 prevented the inhibitory phase of the bell-shaped [Ca2+]i dependence of hemichannel opening (37) (Fig. 7, C and D). If this bell-shaped [Ca2+]i dependence contributes as a hemichannel-related mechanism in the oscillations, it is expected (similar as for the InsP3R) that such CT peptide would inhibit the oscillations by removing the OFF signal. We used CytC and CT9 peptide (last nine amino acids of the Cx43 CT) to selectively interfere with the negative feedback of Ca2+ on InsP3Rs and connexin hemichannels, respectively, and examined their effect on the BK-induced Ca2+ oscillations. CytC binds to InsP3R type 1 and 3 (58), which are both present in MDCK cells (62). CT9 and CytC are plasma membrane-impermeable, and we used in situ electroporation to load these substances into the cells without disturbing cell function or viability (41, 42). To identify the cells loaded with these agents, we included the fluorescent markers DTR/dextran fluorescein and PI that have molecular masses in the range of CytC (∼12 kDa) and CT9 (∼1 kDa), respectively. Electroporation loading allows analysis of the Ca2+ oscillations in loaded as well as non-loaded (control) cells (Fig. 8, A and F). After applying BK (0.5 μm), cells loaded with CytC (∼1 μm intracellular concentration) displayed significantly less oscillatory activity as compared with control cells in the same culture and as compared with cells loaded with vehicle-only (Fig. 8, B and C). The decreased oscillations were not caused by apoptosis (triggered by CytC), as CytC exposure was short (10 min), and annexin V staining to detect early apoptotic cells was negative (data not shown). We further applied the IP3RCYT peptide that corresponds to the CytC binding residues of the InsP3R1 (located on the C terminus), thereby preventing the binding of CytC to InsP3R (38, 63). Inclusion of the IP3RCYT peptide (∼5 μm intracellular concentration) prevented the CytC-mediated decrease in percentage of cells displaying Ca2+ oscillations (Fig. 8C). To further document the involvement of InsP3 signaling in BK-triggered oscillations, we tested inhibition of PLC with U73122, inhibition of InsP3Rs with xestospongin C (XeC), and pre-emptying of thapsigargin-sensitive Ca2+ stores; all these conditions suppressed BK-triggered oscillations, as expected (Fig. 8E). Loading cells with CytC did not affect the AUC (Fig. 8D) or peak amplitude (not shown) of the initial [Ca2+]i transient triggered by BK under zero extracellular Ca2+ conditions, indicating no effect of CytC on the Ca2+ dynamics associated with the initial [Ca2+]i transient triggered by BK. However, the addition of thapsigargin after BK stimulation (still under zero extracellular Ca2+ conditions) released less Ca2+ from CytC-loaded cells than from control cells (Fig. 8D). Hence, Ca2+ store emptying was more complete with CytC, and this substance thus potentiates BK-triggered ER Ca2+ release. This effect of CytC is in line with its expected action as a stimulator of InsP3Rs, which is the result of a decreased InsP3R inhibition by high [Ca2+]i (38). Because CytC did not influence the amplitude of the [Ca2+]i transient, we anticipated that the larger ER Ca2+ release flux was more effectively taken up by mitochondria. Accordingly, we found that the mitochondrial Ca2+ response (measured with RhodFF) after BK stimulation was larger in CytC-loaded cells as compared with control (Fig. 8D).
FIGURE 7.
CytC and CT9 influence InsP3Rs and connexin hemichannels, respectively. A, CytC binds to the C-terminal domain of InsP3R. B, InsP3Rs have a bell-shaped [Ca2+]i dependence of the open probability (Po) with below 250 nm concentrations potentiating InsP3R opening and higher concentrations acting inhibitory. The bell-shaped dose response curve is derived from parameters and equations described in Ref. 97 for InsP3R type 1. CytC binds to InsP3R and removes the inhibitory effects of high [Ca2+]i on InsP3R open probability. C, CT9 peptide is composed of the last 9 amino acids of the Cx43 C terminus and interacts with a sequence on the intracellular loop (IL). EC, extracellular. D, Ca2+ activation of hemichannels composed of Cx43 are characterized by a bell-shaped [Ca2+]i dependence (hemichannel ATP release and dye uptake studies, derived from data in Ref. 36). CT9 peptide removes the declining phase at [Ca2+]i above 500 nm (37).
FIGURE 8.
CytC and CT9 inhibit BK-induced Ca2+ oscillations. A, cells were loaded with CytC or with vehicle only (DTR) by in situ electroporation. Loaded cells are in red (Load), and non-loaded cells are gray (Ctrl). The scale bar is 25 μm. B, representative traces of CytC loaded cells (Load) and control cells (Ctrl). Parallel experiments were done with the cells loaded with vehicle only (DTR). C, average data of experiments as illustrated in the previous panel. The loading procedure resulted by itself in some suppressive effect on the oscillations (percentage of oscillating cells). CytC strongly reduced the oscillations and co-loading together with IP3RCYT peptide removed the inhibitory effect of CytC. The stars indicate a significant difference between the bars indicated. D, CytC did not affect the initial [Ca2+]i transient triggered by 0.5 μm BK (2 min in zero Ca2+ medium). Subsequent addition of thapsigargin (2 μm, 3 min, in zero Ca2+ medium) liberated less Ca2+ from intracellular stores in CytC-loaded cells compared with their controls, indicating more complete store emptying. Mitochondrial Ca2+ uptake was larger in cells containing CytC (mitochondrial Ca2+ concentration, [Ca2+]mito, was measured with RhodFF). E, pretreatment with Xestospongin-C (XeC; 10 μm, 1 h), U73122 (10 μm, 1 h), and thapsigargin (Thaps; 2 μm, 10 min) reduced the number of oscillating cells triggered by BK. F, cells were loaded with CT9 or with vehicle only (PI, similar to panel A). G, representative traces of loaded and control cells. H, CT9 peptide and CT9ΔI peptide significantly reduced the percentage of oscillating cells, in contrast to CT9Rev. Significant differences are indicated as explained in panel C. I, Western blot analysis for Cx43-specific phosphorylated Ser-368 and total levels of Cx43 in control cells and cells treated with Tat-CT or Tat-CTRev (100 μm, 30 min) in the presence or absence of BK (0.5 μm). β-Tubulin was used as a loading control. The bar chart summarizes data derived from different Western blots.
When cells were loaded with the Cx43-targeting CT9 peptide (last 9 amino acids of the Cx43 CT; ∼100 μm intracellular concentration), BK-induced oscillations were significantly inhibited compared with non-loaded control cells in the same culture and to cells loaded with vehicle-only (Fig. 8, G and H). By contrast, CT9Rev peptide (reversed sequence) did not affect the number of oscillating cells (Fig. 8H). The last isoleucine residue of Cx43 is essential for interaction with the scaffolding protein ZO-1 (64), and CT9 peptide is thus expected to bind to ZO-1 and prevent Cx43/ZO-1 binding (37, 65). To investigate whether Ca2+ oscillations are suppressed by dissociation of the Cx43/ZO-1 complex, we used a peptide similar to CT9 that is lacking the last isoleucine residue (CT9ΔI). This peptide does not disrupt Cx43/ZO-1 interaction (37) but was equally potent in inhibiting BK-triggered Ca2+ oscillations (Fig. 8H). Therefore, the CT9-induced block of Ca2+ oscillations is not likely caused by altering Cx43/ZO-1 interactions. As observed for CytC, the AUC or peak amplitude of the first [Ca2+]i increase was not influenced by CT9 or CT9ΔI, indicating that the InsP3 production and initial InsP3R responses were not affected by these treatments. In addition, baseline [Ca2+]i was not affected by CytC or CT9: 58 ± 5 nm for CytC and 56 ± 4 nm for CT9 compared with 57 ± 5 nm for control (n = 4). Calcein release was not different in CT9- or CytC-loaded cells from their controls outside the loading zone (88 ± 8 and 107 ± 31%, respectively, versus 100% in control, n = 3), indicating that CT9 and CytC by themselves do not trigger hemichannel opening.
Recently, O'Quinn et al. (66) showed that the CT9 peptide triggers a PKC-mediated phosphorylation of the Cx43 Ser-368 consensus site in cardiomyocytes, but we could not observe such an effect when MDCK cells were treated with cell-permeable Tat-CT9 peptide (Fig. 8I).
We next tested CytC and CT9 peptide on Ca2+ oscillations elicited by ATP (Fig. 9) and found that CytC was inhibitory and inclusion of the IP3RCYT peptide removed this inhibition. Again, Ca2+ store emptying was more complete, and mitochondrial Ca2+ response was larger with CytC (Fig. 9C). Also, inhibition of PLC and InsP3R as well as pre-emptying of thapsigargin-sensitive Ca2+ stores suppressed oscillatory activity (Fig. 9F). Importantly, CT9 peptide had no effect on ATP-induced Ca2+ oscillations. These experiments indicate that ATP-induced oscillations rely on InsP3Rs, whereas those induced by BK rely on both InsP3Rs and hemichannels.
FIGURE 9.
ATP-induced Ca2+ oscillations are inhibited by CytC but not by CT9 peptide. Experiments as in Fig. 8 but now performed with ATP as the oscillation-inducing stimulus. A, example traces of vehicle/CytC-loaded cells and their controls. B, CytC inhibited the oscillatory activity, and this effect was suppressed by co-loading with IP3RCYT peptide. C, less Ca2+ was liberated from intracellular stores in the presence of CytC, indicating more complete store emptying; accordingly, mitochondrial Ca2+ uptake was larger. D, representative traces of vehicle/CT9-loaded cells. E, CT9 peptide had no effect on ATP-triggered oscillations. F, Xestospongin-C, U73122, and thapsigargin inhibited ATP-triggered oscillations. Significance symbols are as defined in Fig. 8.
We further tested whether hemichannels were sufficient as a mechanism to obtain oscillations without a contribution of InsP3Rs. Hemichannel opening can be triggered by [Ca2+]i elevation without increasing InsP3 and thus without activating InsP3Rs and InsP3R-based oscillations. [Ca2+]i elevation triggered by photolytic release of Ca2+ did not trigger Ca2+ oscillations (n = 4), indicating that hemichannels are not sufficient as an oscillatory mechanism and InsP3Rs are the dominant mechanism leading to oscillations.
DISCUSSION
The present data demonstrate that BK- and ATP-induced Ca2+ oscillations display distinct properties in MDCK cells. Oscillations induced by BK (i) showed a flat concentration dependence of frequency and percentage of responding cells, (ii) were inhibited by Cbx, Gap peptides, and Cx43 gene silencing, (iii) disappeared when extracellular Ca2+ was lowered, and (iv) were inhibited by CytC and CT9 peptide. By contrast, ATP-induced oscillations (i) showed a bell-shaped ATP concentration dependence, (ii) were not influenced by Cbx, Gap peptides, or Cx43 silencing, (iii) were not abolished by lowering extracellular Ca2+, and (iv) were inhibited by CytC but not by CT9 peptide. Some data point to the involvement of hemichannels: (i) BK potentiated calcein dye release, whereas ATP did not, (ii) BK-triggered calcein release was rapidly (∼1 min) inhibited by Gap peptides, whereas 60 min of incubation with these peptides had no effect on dye coupling, and (iii) BK-induced Ca2+ oscillations were inhibited by CT9 peptide that removes the Ca2+ inhibition of Cx43 hemichannels but not by CT9rev peptide. The fact that the oscillations were not synchronized between cells furthermore argues against a contribution of gap junctions.
ATP-induced Ca2+ oscillations displayed a bell-shaped concentration dependence. An S-shaped concentration dependence is more typical (13, 67–69), but it is well known that oscillations can only occur in a limited range of InsP3 concentrations so the disappearance of the oscillations at high ATP concentration may well result from too high an intracellular InsP3 level (45, 70, 71). BK-induced oscillations had a flat concentration dependence, a finding also reported by others (72–74). Despite the importance of encoding the strength of agonist stimulation in the frequency of oscillations, it is not uncommon that spiking frequencies are independent of agonist concentration. According to the dynamic desensitization mechanism that occurs upon stimulation of mGluR5 (75) or the M3 muscarinic receptor (76), elevated levels of [Ca2+]i and diacylglycerol activate PKC that phosphorylates and desensitizes the receptor, thereby limiting InsP3 production and stabilizing the oscillation frequency. BK does activate PKC (77), and PKC phosphorylation consensus sites in BK receptors have been identified (78), but further studies are required to determine whether dynamic desensitization is involved here.
Ca2+ oscillations were previously found to be inhibited by octanol, palmitoleic acid, and 18αGA (28) by antibodies directed against connexins and by Gap peptides (29, 34), all known blockers of connexin channels. Conversely, stimulating hemichannel responses with the anti-arrhythmic peptide AAP (79) has been reported to induce Ca2+ oscillations (80). Ca2+ oscillations triggered by BK were inhibited by 32Gap27/43Gap26 peptides within 1 min, as reported by others (81), and the Gap peptides did not influence the initial [Ca2+]i transient triggered by BK. The latter indicates that the InsP3-triggered Ca2+ release was not influenced by the Gap peptides, which is in accordance with our observation that these peptides do not influence [Ca2+]i transients triggered by photolytic release of InsP3 (34). BK potentiated calcein dye efflux from MDCK cells, indicating hemichannel opening. This opening is likely caused by the elevation of [Ca2+]i to values in the 500 nm range that are appropriate for activation of Cx32 and Cx43 hemichannels (35, 36). BK-triggered calcein efflux was rapidly inhibited by the Gap peptides, in line with previous experimental work (34) and complemented by recent single-channel electrophysiological studies that indicate Gap peptide inhibition of unitary currents through Cx43 hemichannels with a time constant in the order of minutes.4 The contribution of hemichannel opening to the Ca2+ oscillations appeared to be related to Ca2+ entry, as indicated by the low extracellular Ca2+ experiments, and was not related to hemichannel ATP release. We cannot exclude hemichannel ATP release, but our results indicate rapid ATP degradation at the surface of MDCK cells, lowering its concentration below the threshold for purinergic receptor activation. A role for hemichannels as a non-selective Ca2+-entry route has been postulated under varying circumstances including alkalinization (Cx43 (82)) and metabolic inhibition (Cx32 (83) and Cx43 (84)). Ca2+ entry contributes to reloading of ER Ca2+ stores, and the entry rate can influence the interspike interval and thus the oscillation frequency (85). At low agonist concentrations, hardly any Ca2+ is lost from the ER (86) and Ca2+ entry may, under those circumstances, act to sensitize the InsP3R to produce a regenerative Ca2+ spike (87) or exert positive feedback on PLC-mediated InsP3 production (19, 88). The present study shows that Ca2+ entry, related to SOCE or as a consequence of hemichannel opening (or a combination of both), is more prominent with BK than with ATP as a stimulus.
Ca2+-mediated feedback actions residing at different levels of the Ca2+ signaling cascade (GPCR, InsP3R, or PLC) lie at the basis of Ca2+ oscillations (10, 18–23). Ca2+ activation of hemichannel-dye uptake and ATP release is, like the InsP3R open probability, characterized by a bell-shaped [Ca2+]i response curve (35, 36) (Fig. 7D), and recent evidence shows that unitary currents through Cx43 hemichannels display a similar bimodal [Ca2+]i dependence.4 This bimodal [Ca2+]i dependence may introduce additional positive and negative feedback that contributes to the Ca2+ oscillations. Thus, we used CT9 peptide that overcomes Cx43 hemichannel inhibition at high [Ca2+]i without influencing hemichannel activity at lower [Ca2+]i (37). Ca2+-dependent activation of hemichannels has been proposed to involve binding of the C-terminal tail to the intracellular loop of Cx43. Conversely, hemichannel inhibition at high [Ca2+]i is mediated by Ca2+ activation of actomyosin that disrupts the loop/tail interaction. The addition of CT9 peptide will substitute for the disrupted C-terminal tail binding by interacting with the cytoplasmic loop and thereby preventing high [Ca2+]i inhibition of hemichannels (37). Here, we show that CT9 but not the reversed sequence inhibits Ca2+ oscillations induced by BK. CT9 inhibition of BK-induced oscillations is mediated by an effect on Cx43 but not on Cx32. Unfortunately, a CT9 analog for Cx32 is not yet available. Both Cx43 and Cx32 appear to be essential in the BK-triggered Ca2+ oscillations, as interfering with each of these connexins individually prevented the oscillations. This is in line with the fact that hemichannels composed of Cx32 also have, like those composed of Cx43, a bell-shaped [Ca2+]i dependence.
Mathematical modeling has demonstrated that positive and negative feedback on voltage-gated Ca2+ entry is by itself sufficient to generate Ca2+ oscillations (89). In contrast, the present experiments show that a forced [Ca2+]i change does not induce Ca2+ oscillations, indicating that the Ca2+-sensitive and Ca2+ entry-mediating hemichannels are not sufficient as an oscillation mechanism. Consistent with this is the observation that BK-triggered Ca2+ oscillations largely rely on InsP3Rs (Fig. 8). CytC removes InsP3R inactivation at high [Ca2+]i, giving increased ER Ca2+ release leading to a gradually increasing [Ca2+]i, mitochondrial Ca2+ overload, and apoptosis (58). We did not observe [Ca2+]i elevation after CytC application, but this is probably related to the relatively short (10 min) time window used to record Ca2+ oscillations. Additionally, high [Ca2+]i inhibition of InsP3Rs is not the only OFF mechanism mediating [Ca2+]i restoration to the resting level; Ca2+ pumps, like sarcoplasmic/endoplasmic and plasma membrane Ca2+ ATPases, which are not affected by CytC (58), will ensure proper maintenance of normal [Ca2+]i within the 10-min time frame of our recordings. Importantly, our data are the first demonstration in intact cells that CytC promotes ER Ca2+ release, supporting its proposed action as an antagonist of high [Ca2+]i inhibition of InsP3Rs (38). CytC also stimulated mitochondrial Ca2+ uptake, but this may be a direct consequence of the increased ER Ca2+ release.
Interestingly, ATP-induced oscillations were also inhibited by CytC but not by CT9 peptide, indicating that these oscillations thrive exclusively on InsP3-based mechanisms. Thus, BK-triggered oscillations are based on InsP3Rs with an additional hemichannel component. Because hemichannels can be activated by moderate (<500 nm) [Ca2+]i elevation, we hypothesize that these channels open with each Ca2+ spike and contribute with Ca2+ entry during the rising phase. When the spike amplitude rises above 500 nm, hemichannels close again and contribute with an OFF mechanism that adds to the OFF mechanism of InsP3R channels. The fact that hemichannels are by themselves not sufficient to mediate oscillations probably results from a slower kinetics for opening than for closing. Previous work has indeed suggested that hemichannel activation by Ca2+ is characterized by several intermediate signaling steps pointing to slow activation kinetics (36). A point of notice is that the duration of the primary Ca2+ peak was different for both triggers: ∼40 s for 0.5 μm BK and 15 s for 10 μm ATP (see example traces in Fig. 1). Preliminary modeling using the activation kinetics of hemichannel opening presented in Refs. 35 and 36 indeed indicates that a [Ca2+]i rise of 40 s (BK) can trigger the opening of twice as much hemichannels compared with a peak that lasts only 15 s (ATP). Additionally, the Ca2+ spikes of BK-triggered oscillations were generally of longer duration than those triggered by ATP (see example traces in Fig. 1) making it possible that these Ca2+ transients were more efficient in inducing hemichannel opening. We speculate that the more prominent contribution of SOCE with BK stimulation is more effective in triggering hemichannel opening because it occurs more localized and in close proximity of the hemichannels in the plasma membrane.
Our experiments indicate that hemichannels actively contribute to BK-induced Ca2+ oscillations by providing a Ca2+ entry pathway characterized by a bimodal [Ca2+]i dependence. This contribution is crucial as inhibition of this pathway blocks the oscillations. Several compounds like the InsP3R inhibitor 2-APB used to explore the mechanism of Ca2+ oscillations (90) are known to inhibit connexin hemichannels (91). Additionally, the non-selective Ca2+ channel blocker La3+ has frequently been used to investigate Ca2+ entry during Ca2+ oscillations, also in cells that express connexins (92–94), but these trivalent ions also inhibit hemichannels (31, 95, 96). In conclusion, connexin hemichannels may contribute to Ca2+ oscillations in connexin-expressing cells. Interestingly, connexin-expressing cells may also display hemichannel-independent Ca2+ oscillations, as exemplified here with ATP. This differential contribution of hemichannels to Ca2+ oscillations may result in distinct downstream response patterns to this fundamental cell signal, as recently observed in brain endothelial cells (34).
Supplementary Material
Acknowledgments
We gratefully thank Dr. C. C. Naus for supplying C6 glioma cells and Dr. D. F. Boehning for sharing the IP3RCYT peptide. Special thanks go to K. Leurs, E. Tack, C. Mabilde, T. Vanthuyne, B. Blanckaert, and M. Yüksel for outstanding technical support.
This work was supported by the Institute for the Promotion of Innovation of Science and Technology in Flanders (IWT Vlaanderen) Grant 63352 (to M. D. B.), the Fund for Scientific Research Flanders (FWO) grants G.0140.08 and 3G.0134.09 (to L. L.) and G.0298.11 (L. L. and G. B.), and the Interuniversity Attraction Poles Program (Belgian Science Policy) P6/31 (to L. L.) and P6/28 (to G. B.).

This article contains supplemental Fig. S1.
N. Wang and L. Leybaert, unpublished observation.
- ER
- endoplasmic reticulum
- AUC
- area under the curve
- BK
- bradykinin
- CT
- C-terminal peptide
- Cx
- connexin
- CytC
- cytochrome c
- DTR
- dextran Texas Red
- GPCR
- G-protein-coupled receptor
- InsP3
- inositol 1,4,5 trisphosphate
- InsP3R
- InsP3 receptor
- MDCK
- Madin-Darby canine kidney
- PI
- propidium iodide
- PLC
- phospholipase C
- RT
- room temperature
- SOCE
- store-operated Ca2+ entry
- Cbx
- carbenoxolone
- HBSS
- Hanks' balanced salt solution
- PPADS
- Pyridoxalphosphate-6-azophenyl-2',4'-disulfonic acid.
REFERENCES
- 1. Case R. M., Eisner D., Gurney A., Jones O., Muallem S., Verkhratsky A. (2007) Evolution of calcium homeostasis. from birth of the first cell to an omnipresent signaling system. Cell Calcium 42, 345–350 [DOI] [PubMed] [Google Scholar]
- 2. Dupont G., Combettes L., Leybaert L. (2007) Calcium dynamics. Spatio-temporal organization from the subcellular to the organ level. Int. Rev. Cytol. 261, 193–245 [DOI] [PubMed] [Google Scholar]
- 3. Berridge M. J., Lipp P., Bootman M. D. (2000) The versatility and universality of calcium signaling. Nat. Rev. Mol. Cell Biol. 1, 11–21 [DOI] [PubMed] [Google Scholar]
- 4. Berridge M. J., Bootman M. D., Roderick H. L. (2003) Calcium signaling. Dynamics, homeostasis, and remodeling. Nat. Rev. Mol. Cell Biol. 4, 517–529 [DOI] [PubMed] [Google Scholar]
- 5. Putney J. W., Bird G. S. (2008) Cytoplasmic calcium oscillations and store-operated calcium influx. J. Physiol. 586, 3055–3059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uhlén P., Fritz N. (2010) Biochemistry of calcium oscillations. Biochem. Biophys. Res. Commun. 396, 28–32 [DOI] [PubMed] [Google Scholar]
- 7. Bezprozvanny I., Watras J., Ehrlich B. E. (1991) Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351, 751–754 [DOI] [PubMed] [Google Scholar]
- 8. Keizer J., De Young G. W. (1992) Two roles of Ca2+ in agonist stimulated Ca2+ oscillations. Biophys. J. 61, 649–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldbeter A., Dupont G., Berridge M. J. (1990) Minimal model for signal-induced Ca2+ oscillations and for their frequency encoding through protein phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 87, 1461–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sneyd J., Keizer J., Sanderson M. J. (1995) Mechanisms of calcium oscillations and waves. A quantitative analysis. FASEB J. 9, 1463–1472 [DOI] [PubMed] [Google Scholar]
- 11. Young K. W., Nash M. S., Challiss R. A., Nahorski S. R. (2003) Role of Ca2+ feedback on single cell inositol 1,4,5-trisphosphate oscillations mediated by G-protein-coupled receptors. J. Biol. Chem. 278, 20753–20760 [DOI] [PubMed] [Google Scholar]
- 12. Politi A., Gaspers L. D., Thomas A. P., Höfer T. (2006) Models of IP3 and Ca2+ oscillations. Frequency encoding and identification of underlying feedbacks. Biophys. J. 90, 3120–3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parker I., Ivorra I. (1990) Inhibition by Ca2+ of inositol trisphosphate-mediated Ca2+ liberation. A possible mechanism for oscillatory release of Ca2+. Proc. Natl. Acad. Sci. U.S.A. 87, 260–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dawson A. P. (1997) Calcium signaling. How do IP3 receptors work? Curr. Biol. 7, R544–R547 [DOI] [PubMed] [Google Scholar]
- 15. Hajnóczky G., Thomas A. P. (1997) Minimal requirements for calcium oscillations driven by the IP3 receptor. EMBO J. 16, 3533–3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rebecchi M. J., Pentyala S. N. (2000) Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol. Rev. 80, 1291–1335 [DOI] [PubMed] [Google Scholar]
- 17. Fukami K., Inanobe S., Kanemaru K., Nakamura Y. (2010) Phospholipase C is a key enzyme regulating intracellular calcium and modulating the phosphoinositide balance. Prog. Lipid Res. 49, 429–437 [DOI] [PubMed] [Google Scholar]
- 18. Nash M. S., Young K. W., Challiss R. A., Nahorski S. R. (2001) Intracellular signaling. Receptor-specific messenger oscillations. Nature 413, 381–382 [DOI] [PubMed] [Google Scholar]
- 19. Thore S., Dyachok O., Tengholm A. (2004) Oscillations of phospholipase C activity triggered by depolarization and Ca2+ influx in insulin-secreting cells. J. Biol. Chem. 279, 19396–19400 [DOI] [PubMed] [Google Scholar]
- 20. Thomas A. P., Bird G. S., Hajnóczky G., Robb-Gaspers L. D., Putney J. W., Jr. (1996) Spatial and temporal aspects of cellular calcium signaling. FASEB J. 10, 1505–1517 [PubMed] [Google Scholar]
- 21. Reetz G., Reiser G. (1996) [Ca2+]i oscillations induced by bradykinin in rat glioma cells associated with Ca2+ store-dependent Ca2+ influx are controlled by cell volume and by membrane potential. Cell Calcium 19, 143–156 [DOI] [PubMed] [Google Scholar]
- 22. Shuttleworth T. J., Mignen O. (2003) Calcium entry and the control of calcium oscillations. Biochem. Soc. Trans. 31, 916–919 [DOI] [PubMed] [Google Scholar]
- 23. Jones B. F., Boyles R. R., Hwang S. Y., Bird G. S., Putney J. W. (2008) Calcium influx mechanisms underlying calcium oscillations in rat hepatocytes. Hepatology 48, 1273–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sacks R. S., Firth A. L., Remillard C. V., Agange N., Yau J., Ko E. A., Yuan J. X. (2008) Thrombin-mediated increases in cytosolic Ca2+ involve different mechanisms in human pulmonary artery smooth muscle and endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 295, 1048–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dupont G., Combettes L., Bird G. S., Putney J. W. (2011) Calcium oscillations. Cold Spring Harb. Perspect. Biol. 3, pii: a004226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loewenstein W. R. (1981) Junctional intercellular communication. The cell-to-cell membrane channel. Physiol. Rev. 61, 829–913 [DOI] [PubMed] [Google Scholar]
- 27. Alexander D. B., Goldberg G. S. (2003) Transfer of biologically important molecules between cells through gap junction channels. Curr. Med. Chem. 10, 2045–2058 [DOI] [PubMed] [Google Scholar]
- 28. Kawano S., Otsu K., Kuruma A., Shoji S., Yanagida E., Muto Y., Yoshikawa F., Hirayama Y., Mikoshiba K., Furuichi T. (2006) ATP autocrine/paracrine signaling induces calcium oscillations and NFAT activation in human mesenchymal stem cells. Cell Calcium 39, 313–324 [DOI] [PubMed] [Google Scholar]
- 29. Verma V., Hallett M. B., Leybaert L., Martin P. E., Evans W. H. (2009) Perturbing plasma membrane hemichannels attenuates calcium signaling in cardiac cells and HeLa cells expressing connexins. Eur. J. Cell Biol. 88, 79–90 [DOI] [PubMed] [Google Scholar]
- 30. Braet K., Vandamme W., Martin P. E., Evans W. H., Leybaert L. (2003) Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26. Cell Calcium 33, 37–48 [DOI] [PubMed] [Google Scholar]
- 31. Braet K., Aspeslagh S., Vandamme W., Willecke K., Martin P. E., Evans W. H., Leybaert L. (2003) Pharmacological sensitivity of ATP release triggered by photoliberation of inositol-1,4,5-trisphosphate and zero extracellular calcium in brain endothelial cells. J. Cell Physiol. 197, 205–213 [DOI] [PubMed] [Google Scholar]
- 32. Leybaert L., Braet K., Vandamme W., Cabooter L., Martin P. E., Evans W. H. (2003) Connexin channels, connexin mimetic peptides and ATP release. Cell Commun. Adhes. 10, 251–257 [DOI] [PubMed] [Google Scholar]
- 33. Evans W. H., De Vuyst E., Leybaert L. (2006) The gap junction cellular internet. Connexin hemichannels enter the signaling limelight. Biochem. J. 397, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Bock M., Culot M., Wang N., Bol M., Decrock E., De Vuyst E., da Costa A., Dauwe I., Vinken M., Simon A. M., Rogiers V., De Ley G., Evans W. H., Bultynck G., Dupont G., Cecchelli R., Leybaert L. (2011) Connexin channels provide a target to manipulate brain endothelial calcium dynamics and blood-brain barrier permeability. J. Cereb. Blood Flow Metab. 31, 1942–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Vuyst E., Decrock E., Cabooter L., Dubyak G. R., Naus C. C., Evans W. H., Leybaert L. (2006) Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J. 25, 34–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De Vuyst E., Wang N., Decrock E., De Bock M., Vinken M., Van Moorhem M., Lai C., Culot M., Rogiers V., Cecchelli R., Naus C. C., Evans W. H., Leybaert L. (2009) Ca2+ regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell Calcium 46, 176–187 [DOI] [PubMed] [Google Scholar]
- 37. Ponsaerts R., De Vuyst E., Retamal M., D'hondt C., Vermeire D., Wang N., De Smedt H., Zimmermann P., Himpens B., Vereecke J., Leybaert L., Bultynck G. (2010) Intramolecular loop/tail interactions are essential for connexin 43-hemichannel activity. FASEB J. 24, 4378–4395 [DOI] [PubMed] [Google Scholar]
- 38. Boehning D., van Rossum D. B., Patterson R. L., Snyder S. H. (2005) A peptide inhibitor of cytochrome c/inositol 1,4,5-trisphosphate receptor binding blocks intrinsic and extrinsic cell death pathways. Proc. Natl. Acad. Sci. U.S.A. 102, 1466–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grynkiewicz G., Poenie M., Tsien R. Y. (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440–3450 [PubMed] [Google Scholar]
- 40. Van Moorhem M., Decrock E., Coussee E., Faes L., De Vuyst E., Vranckx K., De Bock M., Wang N., D'Herde K., Lambein F., Callewaert G., Leybaert L. (2010) l-β-ODAP alters mitochondrial Ca2+ handling as an early event in excitotoxicity. Cell Calcium 47, 287–296 [DOI] [PubMed] [Google Scholar]
- 41. De Vuyst E., De Bock M., Decrock E., Van Moorhem M., Naus C., Mabilde C., Leybaert L. (2008) In situ bipolar electroporation for localized cell loading with reporter dyes and investigating gap junctional coupling. Biophys. J. 94, 469–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Decrock E., De Vuyst E., Vinken M., Van Moorhem M., Vranckx K., Wang N., Van Laeken L., De Bock M., D'Herde K., Lai C. P., Rogiers V., Evans W. H., Naus C. C., Leybaert L. (2009) Connexin 43 hemichannels contribute to the propagation of apoptotic cell death in a rat C6 glioma cell model. Cell Death Differ. 16, 151–163 [DOI] [PubMed] [Google Scholar]
- 43. Anselmi F., Hernandez V. H., Crispino G., Seydel A., Ortolano S., Roper S. D., Kessaris N., Richardson W., Rickheit G., Filippov M. A., Monyer H., Mammano F. (2008) ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc. Natl. Acad. Sci. U.S.A. 105, 18770–18775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iyer S., Deutsch K., Yan X., Lin B. (2007) Batch RNAi selector. A stand-alone program to predict specific siRNA candidates in batches with enhanced sensitivity. Comput. Methods Programs Biomed 85, 203–209 [DOI] [PubMed] [Google Scholar]
- 45. Sneyd J., Tsaneva-Atanasova K., Reznikov V., Bai Y., Sanderson M. J., Yule D. I. (2006) A method for determining the dependence of calcium oscillations on inositol trisphosphate oscillations. Proc. Natl. Acad. Sci. U.S.A. 103, 1675–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Swann K., Yu Y. (2008) The dynamics of calcium oscillations that activate mammalian eggs. Int. J. Dev. Biol. 52, 585–594 [DOI] [PubMed] [Google Scholar]
- 47. Pelegrin P., Surprenant A. (2006) Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 25, 5071–5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vinken M., Decrock E., De Vuyst E., De Bock M., Vandenbroucke R. E., De Geest B. G., Demeester J., Sanders N. N., Vanhaecke T., Leybaert L., Rogiers V. (2010) Connexin32 hemichannels contribute to the apoptotic-to-necrotic transition during Fas-mediated hepatocyte cell death. Cell Mol. Life Sci. 67, 907–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. De Blasio B. F., Røttingen J. A., Sand K. L., Giaever I., Iversen J. G. (2004) Global, synchronous oscillations in cytosolic calcium and adherence in bradykinin-stimulated Madin-Darby canine kidney cells. Acta Physiol. Scand. 180, 335–346 [DOI] [PubMed] [Google Scholar]
- 50. Bindschadler M., Sneyd J. (2001) A bifurcation analysis of two coupled calcium oscillators. Chaos 11, 237–246 [DOI] [PubMed] [Google Scholar]
- 51. Koizumi S. (2010) Synchronization of Ca2+ oscillations. Involvement of ATP release in astrocytes. FEBS J. 277, 286–292 [DOI] [PubMed] [Google Scholar]
- 52. Easton A. S., Abbott N. J. (2002) Bradykinin increases permeability by calcium and 5-lipoxygenase in the ECV304/C6 cell culture model of the blood-brain barrier. Brain Res. 953, 157–169 [DOI] [PubMed] [Google Scholar]
- 53. Kennedy C., Proulx P. R., Hébert R. L. (1997) Bradykinin-induced translocation of cytoplasmic phospholipase A2 in MDCK cells. Can J. Physiol. Pharmacol. 75, 563–567 [PubMed] [Google Scholar]
- 54. Gómez-Hernández J. M., de Miguel M., Larrosa B., González D., Barrio L. C. (2003) Molecular basis of calcium regulation in connexin-32 hemichannels. Proc. Natl. Acad. Sci. U.S.A. 100, 16030–16035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thimm J., Mechler A., Lin H., Rhee S., Lal R. (2005) Calcium-dependent open/closed conformations and interfacial energy maps of reconstituted hemichannels. J. Biol. Chem. 280, 10646–10654 [DOI] [PubMed] [Google Scholar]
- 56. Ye Z. C., Wyeth M. S., Baltan-Tekkok S., Ransom B. R. (2003) Functional hemichannels in astrocytes. A novel mechanism of glutamate release. J. Neurosci. 23, 3588–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stridh M. H., Tranberg M., Weber S. G., Blomstrand F., Sandberg M. (2008) Stimulated efflux of amino acids and glutathione from cultured hippocampal slices by omission of extracellular calcium. Likely involvement of connexin hemichannels. J. Biol. Chem. 283, 10347–10356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Boehning D., Patterson R. L., Sedaghat L., Glebova N. O., Kurosaki T., Snyder S. H. (2003) Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat. Cell Biol. 5, 1051–1061 [DOI] [PubMed] [Google Scholar]
- 59. Szalai G., Krishnamurthy R., Hajnóczky G. (1999) Apoptosis driven by IP3-linked mitochondrial calcium signals. EMBO J. 18, 6349–6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pacher P., Hajnóczky G. (2001) Propagation of the apoptotic signal by mitochondrial waves. EMBO J. 20, 4107–4121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Boehning D., Patterson R. L., Snyder S. H. (2004) Apoptosis and calcium. New roles for cytochrome c and inositol 1,4,5-trisphosphate. Cell Cycle 3, 252–254 [PubMed] [Google Scholar]
- 62. Colosetti P., Tunwell R. E., Cruttwell C., Arsanto J. P., Mauger J. P., Cassio D. (2003) The type 3 inositol 1,4,5-trisphosphate receptor is concentrated at the tight junction level in polarized MDCK cells. J. Cell Sci. 116, 2791–2803 [DOI] [PubMed] [Google Scholar]
- 63. Monaco G., Decrock E., Akl H., Ponsaerts R., Vervliet T., Luyten T., De Maeyer M., Missiaen L., Distelhorst C. W., De Smedt H., Parys J. B., Leybaert L., Bultynck G. (2012) Selective regulation of IP3 receptor-mediated Ca2+ signaling and apoptosis by the BH4 domain of Bcl-2 versus Bcl-Xl. Cell Death Differ 19, 295–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Giepmans B. N., Moolenaar W. H. (1998) The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr. Biol. 8, 931–934 [DOI] [PubMed] [Google Scholar]
- 65. Hunter A. W., Barker R. J., Zhu C., Gourdie R. G. (2005) Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol. Biol. Cell 16, 5686–5698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. O'Quinn M. P., Palatinus J. A., Harris B. S., Hewett K. W., Gourdie R. G. (2011) A peptide mimetic of the connexin43 carboxyl terminus reduces gap junction remodeling and induced arrhythmia after ventricular injury. Circ. Res. 108, 704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tai C. J., Kang S. K., Leung P. C. (2001) Adenosine triphosphate-evoked cytosolic calcium oscillations in human granulosa-luteal cells. Role of protein kinase C. J. Clin. Endocrinol. Metab. 86, 773–777 [DOI] [PubMed] [Google Scholar]
- 68. Bergner A., Sanderson M. J. (2002) ATP stimulates Ca2+ oscillations and contraction in airway smooth muscle cells of mouse lung slices. Am. J. Physiol. Lung Cell Mol. Physiol. 283, L1271–L1279 [DOI] [PubMed] [Google Scholar]
- 69. Berridge M. J., Cobbold P. H., Cuthbertson K. S. (1988) Spatial and temporal aspects of cell signaling. Philos. Trans. R Soc. Lond B Biol. Sci. 320, 325–343 [DOI] [PubMed] [Google Scholar]
- 70. Davis R. J., Challiss J., Nahorski S. R. (1999) Enhanced purinoceptor-mediated Ca2+ signaling in L-fibroblasts overexpressing type 1 inositol 1,4,5-trisphosphate receptors. Biochem. J. 341, 813–820 [PMC free article] [PubMed] [Google Scholar]
- 71. Røttingen J. A., Camerer E., Mathiesen I., Prydz H., Iversen J. G. (1997) Synchronized Ca2+ oscillations induced in Madin-Darby canine kidney cells by bradykinin and thrombin but not by ATP. Cell Calcium 21, 195–211 [DOI] [PubMed] [Google Scholar]
- 72. Sage S. O., Adams D. J., van Breemen C. (1989) Synchronized oscillations in cytoplasmic free calcium concentration in confluent bradykinin-stimulated bovine pulmonary artery endothelial cell monolayers. J. Biol. Chem. 264, 6–9 [PubMed] [Google Scholar]
- 73. Carter T. D., Bogle R. G., Bjaaland T. (1991) Spiking of intracellular calcium ion concentration in single cultured pig aortic endothelial cells stimulated with ATP or bradykinin. Biochem. J. 278, 697–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. De Blasio B. F., Iversen J. G., Røttingen J. A. (2004) Intercellular calcium signaling in cultured renal epithelia. A theoretical study of synchronization mode and pacemaker activity. Eur. Biophys. J. 33, 657–670 [DOI] [PubMed] [Google Scholar]
- 75. Nash M. S., Schell M. J., Atkinson P. J., Johnston N. R., Nahorski S. R., Challiss R. A. (2002) Determinants of metabotropic glutamate receptor-5-mediated Ca2+ and inositol 1,4,5-trisphosphate oscillation frequency. Receptor density versus agonist concentration. J. Biol. Chem. 277, 35947–35960 [DOI] [PubMed] [Google Scholar]
- 76. Bird G. S., Rossier M. F., Obie J. F., Putney J. W., Jr. (1993) Sinusoidal oscillations in intracellular calcium requiring negative feedback by protein kinase C. J. Biol. Chem. 268, 8425–8428 [PubMed] [Google Scholar]
- 77. Boarder M. R., Challiss R. A. (1992) Role of protein kinase C in the regulation of histamine- and bradykinin-stimulated inositol polyphosphate turnover in adrenal chromaffin cells. Br. J. Pharmacol. 107, 1140–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Willars G. B., Müller-Esterl W., Nahorski S. R. (1999) Receptor phosphorylation does not mediate cross-talk between muscarinic M(3) and bradykinin B(2) receptors. Am. J. Physiol. 277, C859–C869 [DOI] [PubMed] [Google Scholar]
- 79. Clarke T. C., Williams O. J., Martin P. E., Evans W. H. (2009) ATP release by cardiac myocytes in a simulated ischemia model. Inhibition by a connexin mimetic and enhancement by an antiarrhythmic peptide. Eur. J. Pharmacol. 605, 9–14 [DOI] [PubMed] [Google Scholar]
- 80. Zhang S. W., Liu Y., Huang G. Z., Liu L. (2007) Aconitine alters connexin43 phosphorylation status and Ca2+ oscillation patterns in cultured ventricular myocytes of neonatal rats. Toxicol. In Vitro 21, 1476–1485 [DOI] [PubMed] [Google Scholar]
- 81. Romanov R. A., Rogachevskaja O. A., Bystrova M. F., Jiang P., Margolskee R. F., Kolesnikov S. S. (2007) Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 26, 657–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Schalper K. A., Sánchez H. A., Lee S. C., Altenberg G. A., Nathanson M. H., Sáez J. C. (2010) Connexin 43 hemichannels mediate the Ca2+ influx induced by extracellular alkalinization. Am. J. Physiol. Cell Physiol. 299, C1504–C1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sánchez H. A., Orellana J. A., Verselis V. K., Sáez J. C. (2009) Metabolic inhibition increases activity of connexin-32 hemichannels permeable to Ca2+ in transfected HeLa cells. Am. J. Physiol. Cell Physiol. 297, C665–C678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shintani-Ishida K., Uemura K., Yoshida K. (2007) Hemichannels in cardiomyocytes open transiently during ischemia and contribute to reperfusion injury after brief ischemia. Am. J. Physiol. Heart Circ. Physiol. 293, H1714–H1720 [DOI] [PubMed] [Google Scholar]
- 85. Shuttleworth T. J. (1999) What drives calcium entry during [Ca2+]i oscillations? Challenging the capacitative model. Cell Calcium 25, 237–246 [DOI] [PubMed] [Google Scholar]
- 86. Martin S. C., Shuttleworth T. J. (1994) Ca2+ influx drives agonist-activated [Ca2+]i oscillations in an exocrine cell. FEBS Lett. 352, 32–36 [DOI] [PubMed] [Google Scholar]
- 87. Shuttleworth T. J., Thompson J. L. (1996) Ca2+ entry modulates oscillation frequency by triggering Ca2+ release. Biochem. J. 313, 815–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Meyer T., Stryer L. (1988) Molecular model for receptor-stimulated calcium spiking. Proc. Natl. Acad. Sci. U.S.A. 85, 5051–5055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fioretti B., Franciolini F., Catacuzzeno L. (2005) A model of intracellular Ca2+ oscillations based on the activity of the intermediate-conductance Ca2+-activated K+ channels. Biophys. Chem 113, 17–23 [DOI] [PubMed] [Google Scholar]
- 90. Maruyama T., Kanaji T., Nakade S., Kanno T., Mikoshiba K. (1997) 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J. Biochem. 122, 498–505 [DOI] [PubMed] [Google Scholar]
- 91. Tao L., Harris A. L. (2007) 2-Aminoethoxydiphenyl borate directly inhibits channels composed of connexin26 and/or connexin32. Mol. Pharmacol. 71, 570–579 [DOI] [PubMed] [Google Scholar]
- 92. Hu R., He M. L., Hu H., Yuan B. X., Zang W. J., Lau C. P., Tse H. F., Li G. R. (2009) Characterization of calcium signaling pathways in human preadipocytes. J. Cell. Physiol. 220, 765–770 [DOI] [PubMed] [Google Scholar]
- 93. Estrada M., Espinosa A., Gibson C. J., Uhlen P., Jaimovich E. (2005) Capacitative calcium entry in testosterone-induced intracellular calcium oscillations in myotubes. J. Endocrinol. 184, 371–379 [DOI] [PubMed] [Google Scholar]
- 94. Pizzo P., Burgo A., Pozzan T., Fasolato C. (2001) Role of capacitative calcium entry on glutamate-induced calcium influx in type-I rat cortical astrocytes. J. Neurochem. 79, 98–109 [DOI] [PubMed] [Google Scholar]
- 95. Contreras J. E., Sáez J. C., Bukauskas F. F., Bennett M. V. (2003) Gating and regulation of connexin 43 (Cx43) hemichannels. Proc. Natl. Acad. Sci. U.S.A. 100, 11388–11393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Retamal M. A., Froger N., Palacios-Prado N., Ezan P., Sáez P. J., Sáez J. C., Giaume C. (2007) Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J. Neurosci. 27, 13781–13792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tu H., Wang Z., Bezprozvanny I. (2005) Modulation of mammalian inositol 1,4,5-trisphosphate receptor isoforms by calcium. A role of calcium sensor region. Biophys. J. 88, 1056–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.