Background: eNOS regulation involves a number of post-translational events.
Results: GIT1 binds to and post-translationally modulates eNOS function, and moreover its expression is itself down-regulated in disease.
Conclusion: GIT1 plays an important role in eNOS function, in both normal and abnormal pathophysiologic states.
Significance: Recovery of GIT function in the injured state is likely to improve eNOS function.
Keywords: Endothelial Cell, Endothelial Dysfunction, Liver, Nitric Oxide, Nitric-oxide Synthase
Abstract
Endothelial cell nitric-oxide (NO) synthase (eNOS), the enzyme responsible for synthesis of NO in the vasculature, undergoes extensive post-translational modifications that modulate its activity. Here we have identified a novel eNOS interactor, G-protein-coupled receptor (GPCR) kinase interactor-1 (GIT1), which plays an unexpected role in GPCR stimulated NO signaling. GIT1 interacted with eNOS in the endothelial cell cytoplasm, and this robust association was associated with stimulatory eNOS phosphorylation (Ser1177), enzyme activation, and NO synthesis. GIT1 knockdown had the opposite effect. Additionally, GIT1 expression was reduced in sinusoidal endothelial cells after liver injury, consistent with previously described endothelial dysfunction in this disease. Re-expression of GIT1 after liver injury rescued the endothelial phenotype. These data emphasize the role of GPCR signaling partners in eNOS function and have fundamental implications for vascular disorders involving dysregulated eNOS.
Introduction
Nitric oxide (NO) is generated in endothelial cells from conversion of l-arginine to l-citrulline by the enzymatic action of endothelial nitric-oxide synthase (eNOS).3 It is well established that eNOS is regulated by post-translational modifications, including phosphorylation by serine/threonine kinase Akt (1, 2) and myristoylation/palmitoylation (3, 4), and by interaction with protein partners (5–8). In addition, eNOS activity can be regulated by each of its cellular localization as well as its intracellular calcium level (9, 10).
Impaired endothelial NO release has been observed in a number of disease states, including pulmonary hypertension (11, 12), congestive heart failure (13), and portal hypertension (14, 15). We have previously demonstrated that impaired eNOS activity in sinusoidal endothelial cells in animals with portal hypertension results from abnormal post-translational modifications (16).
A variety of G-protein-coupled receptors (GPCRs) signal to eNOS in endothelial cells, including muscarinic receptors, bradykinin receptors, angiogenesis II receptors, thrombin receptors, endothelin-B (ET-B) receptors, and purinergic receptors (17–19). Cognate GPCR ligands (i.e. acetylcholine, bradykinin, angiotensin II, thrombin, endothelin-1, and ATP) stimulate GPCRs, activate phospholipase C, and mobilize intracellular calcium to lead to eNOS activity (20–22). Additionally, endothelial GPCR-dependent signaling is also coupled by Gβγ to PI3K/Akt to phosphorylate and activate eNOS (23, 24).
G-protein-coupled receptor kinase interactor-1 (GIT1) is a ubiquitous expressed multidomain protein that contains an N-terminal ARF-GAP domain (residues 1–124), ankyrin repeats (residues 130–254), a Spa2 homology domain (residues 264–374), and a C terminus that includes paxillin-binding domain (residues 624–770). GIT1 is a GTPase-activating protein for the ADP-ribosylation factor family of small GTP-binding proteins, and functions as part of a scaffolding protein complex that also includes the PIX family of Rho guanine nucleotide-exchange factors (25). GIT/PIX recruitment to diverse cellular locations such as focal adhesions, the plasma membrane, and synapses is accomplished through binding to partners including paxillin (26), scribble (27), piccolo (28), and liprin-α (29, 30). GIT-PIX complexes scaffold a variety of signaling molecules, including the G-protein-coupled receptor kinases (31), p21-activated kinases (32), focal adhesion kinase (FAK) (33, 34), mitogen-activated protein kinase kinase 1 (MEK1) (35), mitogen-activated protein kinases (ERK1/2), and phospholipase Cγ (36).
We postulated that GIT1 might regulate eNOS activity in sinusoidal endothelial cells. In this study, we have demonstrated that GIT1 itself is a novel eNOS interactor, and more importantly that it is regulated after liver injury, indicating that it plays a critical role in modulating the biological function of eNOS.
EXPERIMENTAL PROCEDURES
Cell Isolation and Culture
Sinusoidal endothelial cells were isolated from male Sprague-Dawley rats (450–500 g) (Harlan, Indianapolis, IN). In brief, after in situ perfusion of the liver with 20 mg % Pronase (Roche Molecular Biochemicals, Indianapolis, IN), followed by collagenase (Worthington Biochemical Corporation, Lakewood, NJ), dispersed cell suspensions were removed from a layered discontinuous density gradient of 8.2 and 15.6% Accudenz (Accurate Chemical and Scientific, Westbury, NY), further purified by centrifugal elutriation (18 ml/min flow), and grown in medium containing 20% serum (10% horse/calf). The purity of endothelial cells was documented by their uptake of fluorescently labeled di-I-acetoacetylated low density lipoprotein. Only primary sinusoidal endothelial isolates of >95% purity were used for study.
siRNA
siRNA duplexes targeting GIT1 (Santa Cruz Biotechnology, Santa Cruz, CA) were as follows: 1) sense 5′-GCACUCAGCAACCGGCUCUTT-3′, antisense 5′-AGAGCCGGUUGCUGAGUGCTT-3′; 2) sense 5′-CCACCUUGAUCAUCGACAUTT-3′, antisense 5′-AUGUCGAUGAUCAAGGUGGTT-3′; and 3) sense 5′-GACUUGAAGGGAAGCGAUUTT-3′, antisense 5′-AAUCGCUUCCCUUCAAGUCTT-3′. Negative control siRNAs (Dharmacon RNAi Technologies, Lafayette, Co) were as follows: sense, 5′-UAGCGACUAAACACAUCAAUU-3′ and antisense, 5′-UUGAUGUUUAGUCGCUAUU-3′.
Adenovirus
The Ad-GFP-GIT1 was a kind gift of Greg Helm (University of Virginia). For adenoviral amplification, adenovirus (37) particles were purified from infected 293 cells by lysis in virus storage buffer followed by two sequential rounds of cesium chloride density gradient ultracentrifugation. We confirmed the efficiency of adenovirus infection of sinusoidal endothelial cells in vivo as described previously (38). Sinusoidal endothelial cells were exposed to adenovirus in 2% serum for 16 h, and medium was exchanged; cells were then harvested at the specified time points. For in vivo animal experiments, adenovirus was injected into the femoral vein 4 days after bile duct ligation in 200 ml of phosphate-buffered saline (PBS) at a concentration of 1 × 1010 plaque-forming units/kg.
Plasmids
pBK(Δ)-rat GIT1/FLAG, GIT1/HA, GIT1 (R39A), GIT1 (ΔABC), GIT1(K663E/K758E), and GIT1(Y554F) have been described previously (25, 31, 39). Akt-CA and Akt-DN plasmids were a kind gift of Matthew Ringel (Ohio State University, Columbus, OH) (40). For transient transfection, plasmid DNA encoding GIT1 (or an empty vector as control) was transfected into sinusoidal endothelial cells or NIH 3T3 cells using Lipofectamine 2000 (Invitrogen) as per the manufacturer's instructions. Transfection reagents and vectors were removed 4 h after transfection. Analysis of transfected proteins was routinely carried out 24–36 h after transfection. Transfection efficiency was confirmed using anti-HA, anti-FLAG, or anti-GFP antibodies.
Immunoprecipitation, Immunoblotting, and Immunofluorescence Microscopy
Immunoprecipitation assays were used to investigate the interaction of eNOS with GIT1 in cells. Briefly, DNA encoding GIT1 and eNOS (or empty vector controls) were co-transfected into sinusoidal endothelial cells or NIH 3T3 cells as described above. Twenty-four h after transfection, cells were harvested and cell lysates (200 μg of total protein) were subjected to immunoprecipitation with antibody to GIT1 (Santa Cruz Biotechnology) or antibody to eNOS (BD Transduction Laboratories, San Jose, CA) overnight. A control immunoprecipitation including IgG was utilized in each experiment. Immunocomplexes were bound by incubating protein samples with protein A beads for 2 h at 4 °C. Immunoprecipitated proteins were separated by SDS-PAGE.
Immunoblotting was performed by using specified primary antibodies, including anti-eNOS antibody (1:1,000; BD Transduction Laboratories, San Jose, CA), anti-phospho-eNOS-Ser1177 (1:1,000; BD Transduction Laboratories or Cell Signaling Technology), anti-Akt, anti-phospho-Akt (1:1,000; Cell Signaling Technology), anti-GIT1 antibody H-170 (1:1,000, Santa Cruz Biotechnology), anti-PKL (1:1,000; BD Transduction Laboratories), anti-GFP antibody (1:1,000, Molecular Probes, Carlsbad, CA), and horseradish peroxidase-conjugated secondary antibody. Specific signals were visualized using enhanced chemiluminescence as per the manufacturer's instructions, and were scanned and quantitated with standard software. Immunoblot images shown are representative of at least 3.
Immunofluorescence microscopy was used to investigate the co-localization of eNOS with GIT1 in sinusoidal endothelial cells. Briefly, primary sinusoidal endothelial cells from normal and injured livers were fixed in 4% paraformaldehyde/PBS and permeabilized in 0.2% Triton X-100, and then labeled with monoclonal anti-eNOS antibody (1:200; BD Transduction Laboratories), followed by Alexa Fluor 488 donkey anti-mouse IgG secondary antibody (Molecular Probes) and polyclonal anti-GIT1 antibody (1:200, Santa Cruz Biotechnology), followed by Alexa Fluor 555 donkey anti-rabbit IgG secondary antibody (Molecular Probes). eNOS and GIT1 were visualized using an LSM-510 Zeiss confocal microscope, and images were overlaid with LSM-510 software.
Animal Model of Liver Injury and Portal Hypertension
Liver injury and portal hypertension was induced by performing bile duct ligation in 450–500-g male retired breeder Sprague-Dawley rats as described (41). Briefly, bile duct ligation was performed by surgical isolation and ligation of the common bile duct. This model creates a portal based fibrogenic response and portal hypertension 10–14 days after surgery (41). In sham-operated rats, laparotomy without isolation and section of the bile duct was performed. All animals received humane care according to National Institutes of Health guidelines; studies were approved by the local Institutional Animal Care and Use Committees.
Portal Pressure Measurement
Portal pressure measurement was as described (41). In brief, after induction of anesthesia (nembutal at 50 mg/kg intraperitoneally), laparotomy was performed and intestines were displaced laterally onto moist gauze. An 18-gauge IV catheter (Becton Dickinson Vascular Access, Sandy, Utah) was introduced into the portal vein and secured with a silk tie. A calibrated low pressure transducer (Digi-Med, Louisville, KY) was connected to the portal vein catheter via polyethylene tubing and portal pressure was recorded continuously using the Digi-Med System Integrator 200 (Digi-Med).
Nitric Oxide Measurement
To assess NO production, we analyzed the release of nitrite, the stable breakdown product of NO, using a Sievers Chemiluminescence NO Analyzer (Sievers Instruments, Inc., Boulder, CO) as per the manufacturer's instructions. Briefly, conditioned medium was injected into a refluxing glass reaction chamber containing 0.1 m vanadium chloride, and then carried in nitrogen gas to the chemiluminescence detector. Measurements of known concentrations of nitrite were used to generate a standard curve between 25 and 500 pmol of nitrite.
NOS Activity Assays
NOS activity from recombinant eNOS protein and sinusoidal endothelial cell lysates was assessed by measuring the conversion of 3H-labeled l-arginine to 3H-labeled l-citrulline as described (5, 6, 42) per the manufacturer's instructions (Cayman Chemical Co., Ann Arbor, MI). Briefly, GST fusion proteins containing fragments of individual domains or GST alone were eluted from glutathione-Sepharose beads. To determine NOS activity, triplicate samples of recombinant proteins or duplicate samples of cell lysates were incubated with a reaction buffer containing 3 pmol of l-[3H]arginine (45 Ci/mmol), 0–50 μm arginine, 1 mm NADPH, 100 nm calmodulin (CaM), 2 mm CaCl2, and 30 μm BH4 in the final reaction volume of 50–100 μl at room temperature for 30 min and at 37 °C for an additional 30 min in the presence and absence of 1 mm l-NAME. The reaction mixture was terminated by the addition of stop buffer and passed over a Dowex AG 50WX-8 resin column. Radiolabeled counts per min of generated l-citrulline were measured and used to determine l-NAME inhabitable NOS activity. Each experiment includes total counts and background counts.
Reductase Activity
NADPH-cytochrome c reductase activity was measured as a change in absorbance at 550 nm and using an extinction coefficient of 0.021/μm/cm, per the manufacturer's instructions (Sigma). In brief, 40 mm HEPES (pH 7.4), 10 units/ml of superoxide dismutase, 10 units/ml of catalase, 5 μm FMN, 5 μm FAD, 0.5 mm EDTA, 1.0 mg/ml of BSA, 0.3 mm NADPH, and 100 μm cytochrome c were mixed together, and in some cases 0.7 mm CaCl2 and 10 μg/ml of CaM were also added. eNOS protein concentrations were 25 and 100 nm in the cytochrome c reaction, and the GIT1 or vehicle were preincubated as described above prior to initiating the reaction with NADPH.
Recombinant Protein GIT1/His6 (r-GIT1) and His6-eNOS (r-eNOS)
Rat GIT1/His6 was purified from recombinant baculovirus-infected Sf9 cells as described (31). The pcWori-His6 eNOS plasmid was the kind gift of Paul Ortiz de Montellano (University of California, San Francisco, CA). Recombinant bovine His6-eNOS was expressed in E. coli BL21(DE3) strain and purified as described (43).
Expression and Purification of GST-GIT1 Fusion Proteins
GST fusion proteins containing defined domain fragments of GIT1 in the pGEX-4T-1 vector have been described previously (25). Briefly, GST fusions of the GIT1 domain were expressed in the E. coli BL21 strain, and purified on glutathione-agarose beads. Beads were washed, and eluted with glutathione. GST-GIT1 fragments were visualized by SDS-PAGE and staining with Coomassie Blue.
Statistical Analyses
All experiments were performed in replicates using cells isolated from different rats. All results were expressed as the mean ± S.E. We performed statistical analysis using the two-tailed Student t test; Vmax and Km were obtained from the direct fits to the data according to a Michaelis-Menten equation on SigmaPlot 12 software; p < 0.05 was considered statistically significant.
RESULTS
GIT1 Expression and Function in Sinusoidal Endothelial Cells
We first examined endogenous eNOS and GIT1 expression in normal sinusoidal endothelial cells; both proteins were present (Fig. 1A). To determine the effect of GIT1 on eNOS activity, we introduced full-length GIT1 into normal cells (Fig. 1A). GIT1 overexpression was associated with increased eNOS Ser1177 phosphorylation, but did not affect total eNOS expression (Fig. 1A). It is noteworthy that this serine residue in the reductase domain (Ser1177) is associated with eNOS activation (1, 2). Finally, GIT1 overexpression caused a dramatic increase in NO production (Fig. 1A), consistent with its effect on phosphorylation at Ser1177. The effect of GIT1 appeared to be dependent on its absolute abundance because transfection of increasing amounts of GIT1 into normal cells led to progressive increases in eNOS Ser1177 phosphorylation and NO synthesis (supplemental Fig. S1, A and B). We examined the effect of GIT1 knockdown on eNOS function (we also specifically evaluated siRNA transfection efficiency, demonstrating that it reached ∼60% in sinusoidal endothelial cells (supplemental Fig. S2)). Knockdown of endogenous GIT1 reduced eNOS Ser1177 phosphorylation but not total eNOS expression; it also reduced NO production (Fig. 1B).
FIGURE 1.
GIT1 expression and its effect on eNOS activity in normal sinusoidal endothelial cells. A, sinusoidal endothelial cells were transfected with cDNA encoding full-length GIT1 (GIT1, 2 μg) or a cognate empty vector (EV). Phospho-eNOS (Ser1177), total eNOS, and GIT1 were detected in cell lysates by immunoblotting, and representative images are shown. Nitrite was measured in conditioned medium from the same cells and is shown in the right graph (n = 5, *, p < 0.001 for GIT1 transfected cells compared with control). B, GIT1 siRNA at the indicated concentrations was transfected into sinusoidal endothelial cells, and cell lysates were subjected to immunoblotting with the indicated antibodies (upper panels). A scrambled siRNA (Scr) was used as a negative control siRNA. The graph below representative images provides quantitative data for specific bands corresponding to GIT1, normalized to β-actin (n = 3, *, p < 0.05 for GIT1 siRNA compared with no transfection or Scr). Nitrite levels from the same cells are shown in the bottom graph (n = 3, *, p < 0.01 for GIT1 siRNA compared with no transfection or Scr). C, one representative example of 10 others of immunohistochemical localization of eNOS (upper, green) and GIT1 (middle, red) and a merged image (lower) in sinusoidal endothelial cells. The bar is 10 μm in length.
We next examined the involvement of known modifiers of eNOS function. First, we found that the eNOS enzyme inhibitor, l-NAME, blocked GIT1 induced NO production (supplemental Fig. S3A). Interestingly, GIT1 stimulated NO production was not calcium/CaM dependent because the CaM inhibitor W13 had no effect on GIT1 induced NO production (supplemental Fig. S3B). Furthermore, GIT1 expression did not have any effect on iNOS expression as well as eNOS expression (supplemental Figs. S1A and S3C).
GIT1 and eNOS Interact in Sinusoidal Endothelial Cells
Because GIT1 has been shown to be serve as a scaffold through its interaction with a variety of proteins (25, 35, 36, 44), we reasoned that its effect on eNOS might also be due to direct interaction, and thus we investigated whether GIT1 interacted with eNOS. We performed dual-immunolabeling experiments to detect eNOS and GIT1 (Fig. 1C); both eNOS and GIT1 were detected in the perinuclear region and also at the plasma membrane. eNOS and GIT1 were co-localized predominantly in the perinuclear region of normal cells. eNOS and GIT1 localization were further evaluated by immunolabeling with an antibody directed against a Golgi marker (anti-GM130) or against caveolin-1 (supplemental Fig. S4, A and B). As previously described (45, 46), eNOS colocalized with caveolin-1 in the Golgi. Given the apparent GIT1-eNOS colocalization, we explored the interaction biochemically, and found that regardless of whether eNOS or GIT1 were immunoprecipitated, eNOS and GIT1 were associated with each other (Fig. 2A). Additionally, by controlling for the quantity of endogenous eNOS or GIT1 included in cell lysates subjected to immunoprecipitation, we determined that ∼5.8% of GIT1 was immunoprecipitated together with 57.7% of lysate eNOS and 5.8% of eNOS was immunoprecipitated together with 54.2% of lysate GIT1. Overexpression of full-length eNOS or GIT1 increased the level of co-immunoprecipitation (Fig. 2B). To determine whether the GIT1-eNOS interaction played a role in eNOS activation, we investigated eNOS phosphorylation in GIT1-associated eNOS (Fig. 2C); GIT1 overexpression or knockdown modified Ser1177 phosphorylation.
FIGURE 2.
GIT1 and eNOS interact in sinusoidal endothelial cells. A, GIT1 or eNOS from normal sinusoidal endothelial cells was immunoprecipitated (IP) and the presence of associated eNOS (left panel and middle panel) or GIT1 (right panel) protein was assessed by immunoblotting. Immunoprecipitation with IgG as a control and the expression of eNOS and GIT1 from 10% of the total cell lysate used for immunoprecipitation are also shown (left, middle and right panels, respectively). B, cDNA encoding full-length eNOS (1 μg, left panel) or GIT1 (1 μg, right panel) was overexpressed in cells and eNOS or GIT1 were detected in cell lysates by immunoblotting (upper panels); eNOS or GIT1 were immunoprecipitated and the presence of associated of GIT1 (left middle panel) or eNOS (right middle panel) was assessed by immunoblotting (IB). Bands corresponding to GIT1 or eNOS were quantitated, normalized, and shown in the lower graphs (n = 3, *, p < 0.01 for eNOS or GIT1 transfected cells compared with control or EV). C, cells were transfected with GIT1 (1 μg) or GIT1 siRNA (50 nm) and GIT1 from sinusoidal endothelial cells were immunoprecipitated and the presence of associated phospho-eNOS (left panel) or eNOS (right panel) protein was assessed by immunoblotting. Bands corresponding to phospho-eNOS and eNOS were quantified, normalized, and shown in the graphs below the images (n = 3, *, p < 0.01 for GIT1 compared with no transfectants; **, p < 0.05 for GIT1 siRNA compared with no transfectants).
GIT1 and eNOS Directly Interact in Vitro
To test whether GIT1 and eNOS directly interact, we first confirmed the purity of purified recombinant eNOS and GIT1 proteins used in our assay system (supplemental Fig. S5A); we detected no other proteins, including HSP 90, a ubiquitously expressed and well known eNOS molecular chaperone protein (supplemental Fig. S5B). We then examined in vitro binding of these recombinant proteins. We combined purified GIT1 (r-GIT1) with purified eNOS (r-eNOS), and immunoprecipitated GIT1 or eNOS. We found that GIT1 and eNOS associated together directly in the absence of other cellular proteins (Fig. 3A). We next explored the biological significance of this protein-protein interaction by incubating recombinant eNOS protein with recombinant GIT1 protein, and assessing NOS activity. We found that addition of GIT1 significantly increased NOS activity, which was inhibited by l-NAME (Fig. 3B). We compared eNOS activities and measured the Michaelis-Menten values (Vmax and Km) of eNOS for l-arginine in the absence and presence of GIT1. We found that eNOS exhibited a higher velocity of NO synthesis in the presence of GIT1. The Vmax value for l-arginine was increased 1.65-fold by GIT1 (from 179.4 ± 8.5 to 295.2 ± 11.8 nmol/min/mg of protein for eNOS in the absence or presence of GIT1, respectively). Moreover, GIT1 increased the affinity of eNOS for l-arginine, as the Km value for l-arginine was 5.93 ± 6.4 μm in the absence of GIT1 and 2.55 ± 0.4 μm in the presence of GIT1 (Fig. 3C). Because the rate of electron flux from the reductase domain to the oxygenase domain is critical for NOS activity, we also examined whether this increase in eNOS activity by GIT1 could be attributed to enhanced NOS reductase activity. We measured a significant increase in eNOS NADPH-cytochrome c reductase activity in the presence of GIT1 compared with the absence of GIT1 (Fig. 3D), suggesting that one potential mechanism for the increased activity may lie within the reductase domain. Furthermore, both GIT1-dependent and -independent activity was further stimulated by the presence of CaM (Fig. 3D). Cytochrome c reductase activity of eNOS was increased by CaM in the basal condition as well as after addition GIT1. The proportionally equivalent increases in calcium/CaM-stimulated cytochrome c reductase activity of eNOS without and with GIT1 suggests that GIT1 and calcium/CaM-activate eNOS independently. To identify GIT1 domains involved in activating eNOS, we mixed purified eNOS with GST-GIT1 fragments, and measured eNOS activity. Fragments containing the GIT1 carboxyl terminus (483–647 and 647–770), containing the synaptic localization domain and paxillin-binding domain, respectively, were able to stimulate eNOS activity (Fig. 3E). In contrast, a GST-GIT2 carboxyl terminus construct did not affect eNOS activity (Fig. 3E). To delineate functional GIT1 domains important for eNOS activation, we introduced a series of GIT1 mutants (Fig. 3F) into sinusoidal endothelial cells and determined their ability to augment NOS activity (Fig. 3G). GIT1(R39A), which lacks Arf GAP activity due to mutation of the arginine finger residue, was as effective as wild type GIT1. The GIT1 (ΔABC) mutant, which lacks both Spa2 homology repeats and the coiled-coil domain found in the central region of GIT1 and does not bind to PIX (25), also stimulated eNOS activity, indicating that the PIX interaction is not required for eNOS stimulation. On the other hand, GIT1 bearing carboxyl terminus mutations that render it unable to bind paxillin, GIT1(K663E,K758E) (39), did not augment eNOS activity. Similarly, GIT1(Y554F), which removes a site for Src-mediated regulatory phosphorylation (47) also failed to stimulate eNOS activity.
FIGURE 3.
Biochemical features of the GIT1 and eNOS interaction. A, immunoprecipitation (IP) of recombinant GIT1 or eNOS (r-GIT1, 2 μg; r-eNOS, 1 μg) reveals that eNOS binds GIT1 (upper panels) or GIT1 binds eNOS (lower panels). Immunoprecipitation with IgG as a control is also shown (upper left and lower left panels). B, the activity of r-eNOS (2 μg) was examined after incubation without or with r-GIT1 (4 μg) in the presence or absence of l-NAME (1 mm). The activity of r-eNOS alone was arbitrarily set to 100; n = 3, *, p < 0.005, and **, p < 0.001 compared with r-eNOS alone. C, r-eNOS (2 μg) was incubated with vehicle (control) or r-GIT1 (2 μg) in the presence of 10 μm Ca2+ and 100 nm CaM, and eNOS activity was determined as a function of l-arginine concentration (n = 3, *, p < 0.001 and **, p < 0.01 compared with r-eNOS alone). D, r-eNOS (2 μg) was incubated with vehicle (control) or r-GIT1 (2 μg) in the presence or absence of CaM (100 nm), and then NADPH-dependent cytochrome c reductase activity was determined at 23 °C for 3 min (n = 3, *, p < 0.01, and **, p < 0.1 compared with r-eNOS alone). E, GST fusion proteins containing fragments of individual GIT1 domains (4 μg) were incubated with r-eNOS (2 μg) and eNOS activity was measured (note, GST-GIT1 encoding full-length GIT1 is misfolded and insoluble when expressed in E. coli and so was not tested). The activity of eNOS + GST alone was arbitrarily set to 100; n = 3, *, p < 0.05 and **, p < 0.01 compared with eNOS + GST alone. F, schematic diagram showing the GIT1 domains and highlighting specific GIT1 mutations. G, the indicated FLAG-tagged constructs were transfected into normal sinusoidal cells and NOS activity was measured in cell lysates after normalization to control (EV) (n = 3, *, p < 0.01 versus EV). IB, immunoblot.
Liver Injury Leads to Reduced GIT1 Expression
To explore the biological relevance of the GIT1-eNOS interaction, we utilized an in vivo model system in which disruption of endothelial function is prominent. We and others have shown that eNOS function is reduced after liver injury and this is associated with reduced NO production by sinusoidal endothelial cells as well as increased intrahepatic vascular resistance (14, 15). Thus, we asked whether GIT1 expression and the GIT1-eNOS interaction were altered in sinusoidal endothelial cells after liver injury. Cells were isolated after liver injury (bile duct ligation, BDL) and immediately examined. First, immunolabeling experiments revealed that GIT1 levels appeared to be reduced in injured cells, although eNOS was not notably changed (Fig. 4A) (see also Fig. 1C for comparison). Consequently, GIT1/eNOS colocalization in the perinuclear region was markedly reduced. Furthermore, immunoblotting revealed that GIT1 expression in injured cells was markedly reduced relative to normal cells, although total eNOS and GIT2 expression were unchanged (Fig. 4B). Additionally, the interaction of GIT1 with eNOS (Fig. 4C) was reduced after injury.
FIGURE 4.
Liver injury leads to reduced GIT1 expression and interaction with eNOS. A, immunohistochemical localization of GIT1 and eNOS in sinusoidal endothelial cells isolated directly after BDL; eNOS (left, green), GIT1 (middle, red), and a merged image (right) are shown. Representative images of 10 others are shown; the bar is 10 μm in length. B, cell lysates from normal and injured (i.e. BDL) sinusoidal endothelial cells were subjected to immunoblotting (IB) to detect eNOS, GIT1, and/or GIT2 (anti-GIT1 and eNOS antibodies were as described in the legend to Fig. 1; anti-PKL antibody was used to recognize GIT1 and GIT2 simultaneously). Specific bands corresponding to GIT1 were quantitated and presented graphically below representative blots (n = 3, *, p < 0.05 versus normal). C, the interaction of eNOS and GIT1 in normal and BDL sinusoidal endothelial cells was measured by immunoprecipitation (IP) with antibody to GIT1 and immunoblotting with antibody to eNOS. The level of eNOS from 10% of the total cell lysate used for immunoprecipitation is also shown (left lane). Bands corresponding to eNOS were quantitated, normalized to the level of immunoprecipitated GIT1, and presented in the graph shown below the immunoblot (n = 3, *, p < 0.005 for cells from BDL compared with that from normal).
Overexpression of GIT1 in Injured Endothelial Cells Restores eNOS Function
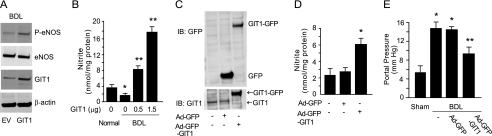
Given the apparent critical nature of the GIT1 and eNOS interaction, and the decreased expression of GIT1 after liver injury, we hypothesized that restoration of GIT1 expression in injured cells would restore eNOS activity and result in a physiologically relevant increase in portal pressure. First, we transfected full-length GIT1 into injured cells, which restored phospho-eNOS levels (Fig. 5A) and significantly increased NO production (Fig. 5B). To directly examine the role of GIT1 in vivo, recombinant adenovirus encoding full-length GIT1 (Ad-GFP-GIT1) was administered to rats that had undergone BDL. Exposure of livers to the adenoviral construct led to an increase in GIT1 expression in injured sinusoidal endothelial cells (Fig. 5C). Additionally, overexpression of GIT1 in injured sinusoidal endothelial cells led to enhanced NO production (Fig. 5D). The ability to achieve high level GIT1 expression in sinusoidal endothelial cells after injury is consistent with previous work demonstrating high level gene expression in non-parenchymal cells after adenovirus infection (38). Finally, overexpression of GIT1 in the injured liver led to a significant reduction in portal pressure (Fig. 5E). These data suggest that depressed GIT1 expression and impaired eNOS phosphorylation resulting from inadequate GIT1 and eNOS interaction in portal hypertensive endothelial cells leads to impaired eNOS signaling.
FIGURE 5.
Overexpression of GIT1 in injured liver endothelial cells enhances NO production and ameliorates portal hypertension. A, sinusoidal endothelial cells isolated after BDL were transfected with GIT1 or EV, and cell lysates were subjected to immunoblotting (IB) with the indicated antibodies (representative immunoblots of 3 are shown). B, sinusoidal endothelial cells from BDL were transfected with GIT1 (0.5 to 1.5 μg) and nitrite levels from conditioned medium were measured (n = 3, *, p < 0.01 versus normal; **, p < 0.001 versus BDL not transduced with GIT1). C–E, rats were subjected to BDL, and 4 days later, Ad-GFP or Ad-GFP-GIT1 (1 × 1010 plaque-forming units/kg) were administered as described under ”Experimental Procedures.“ After 10 additional days, sinusoidal endothelial cells were isolated. C, cell lysates were subjected to immunoblotting with the indicated antibodies, immunoblots representative of 3 others are shown. D, conditioned media from the same cells was collected and nitrite levels were measured (n = 3, *, p < 0.01 versus rats not receiving adenovirus or receiving Ad-GFP alone). E, portal pressure in rats as in C and D was measured as described under ”Experimental Procedures.“ Some rats had sham surgery as under ”Experimental Procedures“ (n = 5, *, p < 0.005 versus sham BDL; **, p < 0.01 versus BDL alone or BDL with Ad-GFP). IP, immunoprecipitation.
Akt Potentiates GIT1/eNOS/NO Signaling
Akt is known to regulate eNOS phosphorylation in a post-translational manner. We found that constitutively active Akt (Akt-CA) not only increased NO production (Fig. 6A), but also enhanced GIT1-eNOS interaction and eNOS phosphorylation (Fig. 6B). Dominant-negative Akt (Akt-DN) reduced NO production (Fig. 6A) and reduced the GIT1-eNOS interaction (Fig. 6C). Immunolabeling experiments also revealed that overexpression of active Akt led to increased GIT1 and eNOS colocalization in the perinuclear region (Fig. 6D). In contrast, dominant-negative Akt markedly reduced GIT1 and eNOS colocalization in the perinuclear region; additionally, eNOS was detected at low levels and colocalization with GIT1 in the plasma membrane (Fig. 6D). Moreover, we found that knockdown of endogenous GIT1 by siRNA reduced eNOS Ser1177 phosphorylation but not total eNOS in the presence of constitutively active Akt (Ad-myrAkt) (Fig. 6E). These results emphasize the importance of appropriate localization of Akt, GIT1, and eNOS in eNOS signaling and the impact of Akt on eNOS function.
FIGURE 6.
Effect of Akt on GIT1/eNOS/NO signaling. A, sinusoidal endothelial cells were isolated from normal rat livers and transfected with cDNA encoding a dominant active Akt (Akt-CA), a dominant-negative Akt (Akt-DN), or an EV, all 1 μg, and nitrite levels were measured in conditioned medium (n = 3, *, p < 0.005 compared with control; **, p < 0.01 compared with control). B, GIT1 was immunoprecipitated (IP) from normal sinusoidal endothelial cell lysates transfected with Akt-CA or EV followed by immunoblotting (IB) to detect either phospho-eNOS or eNOS (representative immunoblots are shown). C, sinusoidal endothelial cells were transfected with Akt-CA, Akt-DN, or EV as in A, and in the upper panel, lysates were immunoblotted to detect eNOS. In the middle panel, lysates were immunoprecipitated with anti-GIT1, then immunoblotted to detect eNOS; specific eNOS bands were quantitated and normalized to the level of immunoprecipitated GIT1 and presented in the graph in the lower panel (n = 3, *, p < 0.05 versus EV or Akt-DN). D, sinusoidal endothelial cells were transfected with Akt-CA or Akt-DN; representative examples (of >10 others) of immunolocalization of eNOS (left, green) and GIT1 (middle, red) in sinusoidal endothelial cells are shown. Merged images after overexpression Akt-CA, Akt-DN, or EV are shown. The bar is 10 μm in length. E, sinusoidal endothelial cells were transfected with GIT1 siRNA (50 nm) and then with adenovirus (multiplicity of infection of 250) encoding constitutively active Akt (Ad-myrAkt) or empty adenovirus without a cDNA insert (left lane in all immunoblots). After 48 h, cell lysates were subjected to immunoblotting with the indicated antibodies. A scrambled siRNA was used as a negative control siRNA (left and middle lanes in all immunoblots). Specific bands corresponding to phospho-eNOS, GIT1, and phospho-Akt were quantitated and presented graphically (n = 3, *, p < 0.05 versus Ad-myrAkt alone, #, p < 0.001 versus empty virus alone).
Agonist-mediated GIT1 and eNOS Association and Signaling
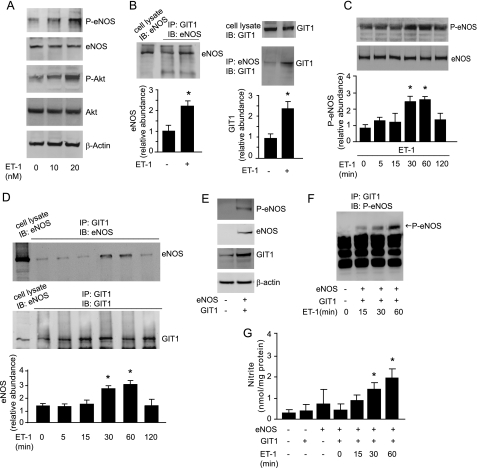
We next tested the responsiveness of the GIT1 signaling system to agonist stimulation. Because the G-protein-coupled receptor ligand, endothelin-1 (ET-1), is well known to stimulate eNOS activation via an ET-B receptor pathway, we hypothesized that ET-1 may stimulate Akt to affect GIT1 regulation of eNOS activity. We exposed normal sinusoidal endothelial cells to ET-1 and measured whether this affected the GIT1-eNOS interaction. ET-1 caused a clear increase in Akt and eNOS phosphorylation (Fig. 7A). It also led to enhanced association of eNOS and GIT1 (Fig. 7B), most prominent from 30 min to 1 h after ET-1 exposure (Fig. 7, C and D), suggesting dynamic modulation of the eNOS and GIT1 interaction. To provide further support for the GIT1-eNOS interaction and GIT1-mediated regulation of eNOS activity, we tested whether GIT1 could activate eNOS in a heterologous cell system. NIH 3T3 cells lack endogenous eNOS but possess endogenous GIT1 (Fig. 7E) as well as ET receptors (48, 49). ET-1 increased the interaction of overexpressed eNOS and GIT1 (Fig. 7F). Furthermore, in a heterologous system in which the cell type in question did not possess endogenous eNOS, when eNOS and GIT1 were co-expressed and stimulated with ET-1, significant time-dependent increases in NO production were apparent (Fig. 7G).
FIGURE 7.
Agonist stimulation of GIT1 and eNOS interaction. A, sinusoidal endothelial cells were exposed to ET-1 (10 to 20 nm) for 1 h, and cell lysates were subjected to immunoblotting (IB) with the indicated antibodies. B, cells were exposed to ET-1 (10 nm) for 1 h, and cell lysates were subjected to immunoprecipitation (IP) with antibody to GIT1 (left panel) or eNOS (right panel) and immunoblotted with antibody to eNOS or GIT1; specific eNOS or GIT1 bands were quantitated and normalized to the level of immunoprecipitated GIT1 or eNOS and presented in the graph in the lower panel (n = 3, *, p < 0.05 versus no ET-1). The expression of eNOS or GIT1 from 10% of the total cell lysate used for immunoprecipitation is also shown (left panel, left band, and right top panel). C and D, cells were exposed to ET-1 (10 nm) from 0 to 120 min. C, cell lysates were subjected to immunoblotting to detect phospho-eNOS or total eNOS (upper panel); specific phospho-eNOS bands are depicted graphically (lower panel, n = 3, *, p < 0.01 compared with time “0”). D, GIT1 or eNOS from the same cells as in C was immunoprecipitated and the presence of associated eNOS or GIT1 protein was assessed by immunoblotting (upper panel) and specific eNOS bands were quantified and normalized to the level of immunoprecipitated GIT1 (lower panel, n = 3, *, p < 0.05 compared with time 0). In E–G, NIH 3T3 cells were co-transfected with GIT1 or eNOS as indicated. E, cell lysates were subjected to immunoblotting with the indicated antibodies. F and G, NIH 3T3 cells were stimulated with ET-1 for the indicated times, GIT1 was immunoprecipitated and the presence of phospho-eNOS protein was assessed by immunoblotting (F) and conditioned medium was collected and nitrite levels were measured (G, n = 3, *, p < 0.01 compared with no ET-1 or to time 0).
DISCUSSION
The major finding of the present study is that GIT1 interaction with eNOS in liver (sinusoidal) endothelial cells leads to direct eNOS activation. This new signal transduction pathway appears to be activated dynamically by ET-1 and Akt activation, but to be independent of calcium/CaM. Moreover, this pathway appears to be critical in a highly relevant model of injury, emphasizing its pathophysiological importance (Fig. 8). In the normal state, ET-1 activates its cognate G-protein-coupled receptor (ET-B receptor) and activates Gβγ and Gα subunits, which go on to activate Akt and then eNOS (Fig. 8). In the proposed model, activated Akt promotes GIT1-eNOS interaction in the perinuclear region and eNOS activation (Fig. 6). In contrast, in injured sinusoidal endothelial cells, the relative lack of activated Akt (16) and GIT1 reduces GIT1-eNOS interaction and subsequent eNOS phosphorylation and activity (Fig. 4). Taken together, our data highlight a regulatory signaling complex involving Akt, GIT1, and eNOS. Importantly, each component appears to be important in determining ultimate eNOS function.
FIGURE 8.
A proposed model of GIT1-mediated eNOS activation in sinusoidal endothelial cells. In normal sinusoidal endothelial cells (left panel), ET-1 activates its cognate G-protein-coupled receptor (ET-B receptor) and activates Gβγ and Gα subunits. This is followed by Gβγ stimulation of Akt (and desensitization of the receptor by GRK2 is not shown). Activated Akt also subsequently facilitates GIT1 binding to eNOS and the direct GIT1-eNOS interaction enhances eNOS activity and NO release. In contrast, in injured sinusoidal endothelial cells (right panel), impaired Akt diminishes the ability of (also reduced levels of) GIT1 to activate eNOS. The ultimate consequence of altered expression and signaling by GIT1 in injured sinusoidal endothelial cells is reduced eNOS activation and NO production.
Our findings are highly consistent with a substantial body of literature that emphasizes regulation of eNOS activity by virtue of post-translational modifications including multisite phosphorylation, myristoylation, and palmitoylation, and through interaction with a number of specific regulatory proteins. For example, partners that exert a stimulatory effect include Akt (1) and CaM (9, 42), whereas those exerting an inhibitory effect include caveolin-1 (9, 50) and NOSIP (51). We now add GIT1 to this list of stimulatory partners.
Our work is also important in the context of the molecular mechanism by which eNOS is activated when present in different regions of the cell. In canonical eNOS biology, eNOS appears to be tethered to the cell membrane by its myristoylation and palmitoylation (52), where it appears to bind to caveolin-1 (53, 54). Upon its dissociation from the cell membrane and caveolin-1, and movement to the cytosol, eNOS can be phosphorylated and activated (10, 55); this process appears to be calcium/CaM dependent (56). Importantly, independent of its localization, eNOS may be activated by Akt (57, 58) both in the perinuclear region and in the plasma membrane (57). Our work suggests that perinuclear localization of eNOS appears to be critical for the GIT1-eNOS interaction and subsequent eNOS phosphorylation/activation. Furthermore, Akt appears to be involved in GIT1 localization (Fig. 6). It was noteworthy that the Akt-DN appeared to push GIT1 to the membrane, where eNOS was inactive. In contrast, active Akt (Akt-CA) led to a perinuclear localization of GIT1, where it could interact with and activate eNOS (Fig. 6). The localization of GIT1 in our system appeared to be PI 3-kinase/Akt-dependent, which is consistent with previous work (27, 31, 59).
The current data raise several important questions. The first has to do with the interaction between GIT1 and eNOS. GIT1 has multiple domains including an amino-terminal zinc finger ADP-ribosylation factor-GTPase-activating protein domain, three ankyrin repeats, two repeated regions of homology with the yeast protein Spa2 that interacts with several partners, a coiled-coil region responsible for dimerization, a region that mediates synaptic localization, and a focal adhesion targeting domain, as well as multiple tyrosine residues phosphorylated by Src and other kinases (47). Each of these domains is recognized as a specific signal transduction domain and/or protein-binding site. Purified recombinant GIT1 interacts with recombinant eNOS and activates it, indicating that this interaction is direct and functional. Experiments with fragments of GIT1 suggest that elements of the carboxyl-terminal are the most important for eNOS activation, specifically the region encompassing the coiled-coil region, synaptic localization domain, and focal adhesion targeting domain. Another important remaining issue has to do with the detailed mechanism by which GIT1 leads to eNOS activation. We have shown that increasing NO production by overexpression of GIT1 does not require calcium in intact cells (supplemental Fig. S3), and GIT1 increases the reductase activity of purified eNOS in the absence of CaM, whereas the NOS reductase activity is further increased following the addition of CaM (Fig. 3). These data raise the possibility that the calcium/CaM regulatory domain of eNOS is not involved in the GIT1-eNOS interaction. GIT1 enhances both the Km and Vmax of purified eNOS for arginine, but also leads to elevated stimulatory phosphorylation of eNOS in cells. Although beyond the scope of the current study, two critical areas for further investigation include to elucidate specifically how the GIT1 carboxyl-terminal domain and still undefined eNOS domains interact to promote eNOS function and how GIT1 binding enhances or stabilizes eNOS phosphorylation. Experiments using domain deletions in full-length GIT1 (Fig. 3) suggest that GIT1 activation of eNOS in cells may be complex, and may involve other GIT1 partners such as paxillin, or those that stimulate GIT1 tyrosine phosphorylation, such as Src. Additionally, it is possible that GIT1 helps coordinate other eNOS activators such as Akt and/or calcium/CaM.
We have also shown that GIT1 protein levels were decreased in sinusoidal endothelial cells after injury, revealing that GIT1 expression is regulated in a disease state, leading to reduced in GIT1-eNOS interaction and NO production (Fig. 4). These data are consistent thematically with previous information about GIT1 in disease pathophysiology. GIT1 appears to be important in a variety of cellular morphological processes, and has been proposed to be important in dendritic synapse formation as well as neuronal cell signaling involving Rac, PIX, and p21-activated kinase, the latter two of which may be involved in cognition in mental retardation (32). Studies with GIT1-deficient mice have revealed roles in lung capillary development and in cardiomyocyte mitochondria biogenesis (60, 61). Finally, studies in vascular endothelial cells (61) have shown that GIT1 distribution changes as cells undergo thrombin or sphingosine 1-phosphate-stimulated cortical actin rearrangements associated with altered endothelial barrier function (33, 62). GIT1 is also an important mediator of Slit2-Robo signaling to Arf6 in endothelial cells during neovascularization and vascular leak (63). Our finding that GIT1 is regulated in a vascular endothelial liver injury model (Fig. 5) substantially extends this previous work and further suggests that GIT1 could be important in vascular signaling and disease.
Supplementary Material
Acknowledgments
We thank Xianjie Zeng for valuable assistance with primary cell isolation, cell culture, and animal models, and Robert Schmalzigaug for assistance with confocal microscopy and transfection of plasmids.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 DK 57830 (to D. C. R.).

This article contains supplemental Figs. S1–S5.
- eNOS
- endothelial nitric-oxide synthase
- CaM
- calmodulin
- NOS
- nitric-oxide synthase
- GIT
- G-protein-coupled receptor kinase interactor
- ET-1
- endothelin-1
- ET-B
- endothelin-B
- BDL
- bile duct ligation
- Akt-CA
- constitutively active Akt
- Akt-DN
- dominant-negative Akt
- GPCR
- G-protein-coupled receptor
- EV
- empty vector
- Ad
- adenovirus.
REFERENCES
- 1. Fulton D., Gratton J. P., McCabe T. J., Fontana J., Fujio Y., Walsh K., Franke T. F., Papapetropoulos A., Sessa W. C. (1999) Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399, 597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dimmeler S., Fleming I., Fisslthaler B., Hermann C., Busse R., Zeiher A. M. (1999) Activation of nitric-oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399, 601–605 [DOI] [PubMed] [Google Scholar]
- 3. Busconi L., Michel T. (1993) Endothelial nitric-oxide synthase. N-terminal myristoylation determines subcellular localization. J. Biol. Chem. 268, 8410–8413 [PubMed] [Google Scholar]
- 4. García-Cardeña G., Fan R., Stern D. F., Liu J., Sessa W. C. (1996) Endothelial nitric-oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. J. Biol. Chem. 271, 27237–27240 [DOI] [PubMed] [Google Scholar]
- 5. Cao S., Yao J., McCabe T. J., Yao Q., Katusic Z. S., Sessa W. C., Shah V. (2001) Direct interaction between endothelial nitric-oxide synthase and dynamin-2. Implications for nitric-oxide synthase function. J. Biol. Chem. 276, 14249–14256 [DOI] [PubMed] [Google Scholar]
- 6. García-Cardeña G., Fan R., Shah V., Sorrentino R., Cirino G., Papapetropoulos A., Sessa W. C. (1998) Dynamic activation of endothelial nitric-oxide synthase by Hsp90. Nature 392, 821–824 [DOI] [PubMed] [Google Scholar]
- 7. Freedman N. J., Ament A. S., Oppermann M., Stoffel R. H., Exum S. T., Lefkowitz R. J. (1997) Phosphorylation and desensitization of human endothelin A and B receptors. Evidence for G protein-coupled receptor kinase specificity. J. Biol. Chem. 272, 17734–17743 [DOI] [PubMed] [Google Scholar]
- 8. Dudzinski D. M., Michel T. (2007) Life history of eNOS. Partners and pathways. Cardiovasc. Res. 75, 247–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Michel J. B., Feron O., Sacks D., Michel T. (1997) Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J. Biol. Chem. 272, 15583–15586 [DOI] [PubMed] [Google Scholar]
- 10. Prabhakar P., Thatte H. S., Goetz R. M., Cho M. R., Golan D. E., Michel T. (1998) Receptor-regulated translocation of endothelial nitric-oxide synthase. J. Biol. Chem. 273, 27383–27388 [DOI] [PubMed] [Google Scholar]
- 11. Bakir S., Mori T., Durand J., Chen Y. F., Thompson J. A., Oparil S. (2000) Estrogen-induced vasoprotection is estrogen receptor dependent. Evidence from the balloon-injured rat carotid artery model. Circulation 101, 2342–2344 [DOI] [PubMed] [Google Scholar]
- 12. Xue C., Johns R. A. (1995) Endothelial nitric-oxide synthase in the lungs of patients with pulmonary hypertension. N. Engl. J. Med. 333, 1642–1644 [DOI] [PubMed] [Google Scholar]
- 13. Fleming I., Fisslthaler B., Dimmeler S., Kemp B. E., Busse R. (2001) Phosphorylation of Thr495 regulates Ca2+/calmodulin-dependent endothelial nitric-oxide synthase activity. Circ. Res. 88, E68–75 [DOI] [PubMed] [Google Scholar]
- 14. Rockey D. C., Chung J. J. (1998) Reduced nitric oxide production by endothelial cells in cirrhotic rat liver. Endothelial dysfunction in portal hypertension. Gastroenterology 114, 344–351 [DOI] [PubMed] [Google Scholar]
- 15. Shah V., Toruner M., Haddad F., Cadelina G., Papapetropoulos A., Choo K., Sessa W. C., Groszmann R. J. (1999) Impaired endothelial nitric-oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology 117, 1222–1228 [DOI] [PubMed] [Google Scholar]
- 16. Liu S., Premont R. T., Kontos C. D., Zhu S., Rockey D. C. (2005) A crucial role for GRK2 in regulation of endothelial cell nitric-oxide synthase function in portal hypertension. Nat. Med. 11, 952–958 [DOI] [PubMed] [Google Scholar]
- 17. Dessy C., Kelly R. A., Balligand J. L., Feron O. (2000) Dynamin mediates caveolar sequestration of muscarinic cholinergic receptors and alteration in NO signaling. EMBO J. 19, 4272–4280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dudzinski D. M., Igarashi J., Greif D., Michel T. (2006) The regulation and pharmacology of endothelial nitric-oxide synthase. Annu. Rev. Pharmacol. Toxicol. 46, 235–276 [DOI] [PubMed] [Google Scholar]
- 19. Whalen E. J., Foster M. W., Matsumoto A., Ozawa K., Violin J. D., Que L. G., Nelson C. D., Benhar M., Keys J. R., Rockman H. A., Koch W. J., Daaka Y., Lefkowitz R. J., Stamler J. S. (2007) Regulation of β-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell 129, 511–522 [DOI] [PubMed] [Google Scholar]
- 20. Goetz R. M., Thatte H. S., Prabhakar P., Cho M. R., Michel T., Golan D. E. (1999) Estradiol induces the calcium-dependent translocation of endothelial nitric-oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 96, 2788–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Isshiki M., Ando J., Korenaga R., Kogo H., Fujimoto T., Fujita T., Kamiya A. (1998) Endothelial Ca2+ waves preferentially originate at specific loci in caveolin-rich cell edges. Proc. Natl. Acad. Sci. U.S.A. 95, 5009–5014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loscalzo J., Welch G. (1995) Nitric oxide and its role in the cardiovascular system. Prog. Cardiovasc. Dis. 38, 87–104 [DOI] [PubMed] [Google Scholar]
- 23. Liu S., Premont R. T., Kontos C. D., Huang J., Rockey D. C. (2003) Endothelin-1 activates endothelial cell nitric-oxide synthase via heterotrimeric G-protein βγ subunit signaling to protein kinase B/Akt. J. Biol. Chem. 278, 49929–49935 [DOI] [PubMed] [Google Scholar]
- 24. Naga Prasad S. V., Barak L. S., Rapacciuolo A., Caron M. G., Rockman H. A. (2001) Agonist-dependent recruitment of phosphoinositide 3-kinase to the membrane by β-adrenergic receptor kinase 1. A role in receptor sequestration. J. Biol. Chem. 276, 18953–18959 [DOI] [PubMed] [Google Scholar]
- 25. Premont R. T., Perry S. J., Schmalzigaug R., Roseman J. T., Xing Y., Claing A. (2004) The GIT/PIX complex. An oligomeric assembly of GIT family ARF GTPase-activating proteins and PIX family Rac1/Cdc42 guanine nucleotide exchange factors. Cell Signal. 16, 1001–1011 [DOI] [PubMed] [Google Scholar]
- 26. Manabe R., Kovalenko M., Webb D. J., Horwitz A. R. (2002) GIT1 functions in a motile, multimolecular signaling complex that regulates protrusive activity and cell migration. J. Cell Sci. 115, 1497–1510 [DOI] [PubMed] [Google Scholar]
- 27. Meyer M. Z., Déliot N., Chasserot-Golaz S., Premont R. T., Bader M. F., Vitale N. (2006) Regulation of neuroendocrine exocytosis by the ARF6 GTPase-activating protein GIT1. J. Biol. Chem. 281, 7919–7926 [DOI] [PubMed] [Google Scholar]
- 28. Kim S., Ko J., Shin H., Lee J. R., Lim C., Han J. H., Altrock W. D., Garner C. C., Gundelfinger E. D., Premont R. T., Kaang B. K., Kim E. (2003) The GIT family of proteins forms multimers and associates with the presynaptic cytomatrix protein Piccolo. J. Biol. Chem. 278, 6291–6300 [DOI] [PubMed] [Google Scholar]
- 29. Nayal A., Webb D. J., Horwitz A. F. (2004) Talin. An emerging focal point of adhesion dynamics. Curr. Opin. Cell Biol. 16, 94–98 [DOI] [PubMed] [Google Scholar]
- 30. Ko J., Kim S., Valtschanoff J. G., Shin H., Lee J. R., Sheng M., Premont R. T., Weinberg R. J., Kim E. (2003) Interaction between liprin-α and GIT1 is required for AMPA receptor targeting. J. Neurosci. 23, 1667–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Premont R. T., Claing A., Vitale N., Freeman J. L., Pitcher J. A., Patton W. A., Moss J., Vaughan M., Lefkowitz R. J. (1998) β2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proc. Natl. Acad. Sci. U.S.A. 95, 14082–14087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang H., Webb D. J., Asmussen H., Niu S., Horwitz A. F. (2005) A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J. Neurosci. 25, 3379–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shikata Y., Birukov K. G., Birukova A. A., Verin A., Garcia J. G. (2003) Involvement of site-specific FAK phosphorylation in sphingosine-1 phosphate- and thrombin-induced focal adhesion remodeling. Role of Src and GIT. FASEB J. 17, 2240–2249 [DOI] [PubMed] [Google Scholar]
- 34. Zhao Z. S., Manser E., Loo T. H., Lim L. (2000) Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol. Cell Biol. 20, 6354–6363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yin G., Haendeler J., Yan C., Berk B. C. (2004) GIT1 functions as a scaffold for MEK1-extracellular signal-regulated kinase 1 and 2 activation by angiotensin II and epidermal growth factor. Mol. Cell Biol. 24, 875–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haendeler J., Yin G., Hojo Y., Saito Y., Melaragno M., Yan C., Sharma V. K., Heller M., Aebersold R., Berk B. C. (2003) GIT1 mediates Src-dependent activation of phospholipase Cγ by angiotensin II and epidermal growth factor. J. Biol. Chem. 278, 49936–49944 [DOI] [PubMed] [Google Scholar]
- 37. Lieu A. S., Li J. Z., Webb D. J., Hankins G. R., Howng S. L., Helm G. A. (2006) Functions of G-protein-coupled receptor kinase interacting protein 1 in human neuronal (N2TN) cells. J. Neurosurg. 105, 103–110 [DOI] [PubMed] [Google Scholar]
- 38. Yu Q., Que L. G., Rockey D. C. (2002) Adenovirus-mediated gene transfer to nonparenchymal cells in normal and injured liver. Am. J. Physiol. Gastrointest. Liver Physiol. 282, G565–572 [DOI] [PubMed] [Google Scholar]
- 39. Schmalzigaug R., Garron M. L., Roseman J. T., Xing Y., Davidson C. E., Arold S. T., Premont R. T. (2007) GIT1 utilizes a focal adhesion targeting-homology domain to bind paxillin. Cell Signal. 19, 1733–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saji M., Vasko V., Kada F., Allbritton E. H., Burman K. D., Ringel M. D. (2005) Akt1 contains a functional leucine-rich nuclear export sequence. Biochem. Biophys. Res. Commun. 332, 167–173 [DOI] [PubMed] [Google Scholar]
- 41. Connelly L., Madhani M., Hobbs A. J. (2005) Resistance to endotoxic shock in endothelial nitric-oxide synthase (eNOS) knock-out mice. A proinflammatory role for eNOS-derived NO in vivo. J. Biol. Chem. 280, 10040–10046 [DOI] [PubMed] [Google Scholar]
- 42. Busse R., Mülsch A. (1990) Calcium-dependent nitric-oxide synthesis in endothelial cytosol is mediated by calmodulin. FEBS Lett. 265, 133–136 [DOI] [PubMed] [Google Scholar]
- 43. Nishida C. R., Ortiz de Montellano P. R. (1998) Electron transfer and catalytic activity of nitric-oxide synthases. Chimeric constructs of the neuronal, inducible, and endothelial isoforms. J. Biol. Chem. 273, 5566–5571 [DOI] [PubMed] [Google Scholar]
- 44. Hoefen R. J., Berk B. C. (2006) The multifunctional GIT family of proteins. J. Cell Sci. 119, 1469–1475 [DOI] [PubMed] [Google Scholar]
- 45. Fernández-Hernando C., Fukata M., Bernatchez P. N., Fukata Y., Lin M. I., Bredt D. S., Sessa W. C. (2006) Identification of Golgi-localized acyltransferases that palmitoylate and regulate endothelial nitric-oxide synthase. J. Cell Biol. 174, 369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Govers R., van der Sluijs P., van Donselaar E., Slot J. W., Rabelink T. J. (2002) Endothelial nitric-oxide synthase and its negative regulator caveolin-1 localize to distinct perinuclear organelles. J. Histochem. Cytochem. 50, 779–788 [DOI] [PubMed] [Google Scholar]
- 47. Webb D. J., Mayhew M. W., Kovalenko M., Schroeder M. J., Jeffery E. D., Whitmore L., Shabanowitz J., Hunt D. F., Horwitz A. F. (2006) Identification of phosphorylation sites in GIT1. J. Cell Sci. 119, 2847–2850 [DOI] [PubMed] [Google Scholar]
- 48. Dulin N. O., Sorokin A., Reed E., Elliott S., Kehrl J. H., Dunn M. J. (1999) RGS3 inhibits G protein-mediated signaling via translocation to the membrane and binding to Gα11. Mol. Cell Biol. 19, 714–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nambi P., Mattern M. R., Wu H. L., Pullen M., Nuthulaganti P., Hofmann G. A., Kumar C. (1997) Absence of endothelin receptors and receptor mRNA in mammalian fibroblasts transformed with SV40 or ras oncogene. Mol. Cell Biochem. 175, 29–35 [DOI] [PubMed] [Google Scholar]
- 50. Ju H., Zou R., Venema V. J., Venema R. C. (1997) Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J. Biol. Chem. 272, 18522–18525 [DOI] [PubMed] [Google Scholar]
- 51. Dedio J., König P., Wohlfart P., Schroeder C., Kummer W., Müller-Esterl W. (2001) NOSIP, a novel modulator of endothelial nitric-oxide synthase activity. FASEB J. 15, 79–89 [DOI] [PubMed] [Google Scholar]
- 52. Shaul P. W. (2002) Regulation of endothelial nitric-oxide synthase. Location, location, location. Annu. Rev. Physiol. 64, 749–774 [DOI] [PubMed] [Google Scholar]
- 53. Prabhakar P., Cheng V., Michel T. (2000) A chimeric transmembrane domain directs endothelial nitric-oxide synthase palmitoylation and targeting to plasmalemmal caveolae. J. Biol. Chem. 275, 19416–19421 [DOI] [PubMed] [Google Scholar]
- 54. Shaul P. W., Smart E. J., Robinson L. J., German Z., Yuhanna I. S., Ying Y., Anderson R. G., Michel T. (1996) Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. J. Biol. Chem. 271, 6518–6522 [DOI] [PubMed] [Google Scholar]
- 55. Figueroa X. F., González D. R., Martínez A. D., Durán W. N., Boric M. P. (2002) ACh-induced endothelial NO synthase translocation, NO release and vasodilatation in the hamster microcirculation in vivo. J. Physiol. 544, 883–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pott C., Steinritz D., Bölck B., Mehlhorn U., Brixius K., Schwinger R. H., Bloch W. (2006) eNOS translocation but not eNOS phosphorylation is dependent on intracellular Ca2+ in human atrial myocardium. Am. J. Physiol. Cell Physiol. 290, C1437–1445 [DOI] [PubMed] [Google Scholar]
- 57. Fulton D., Fontana J., Sowa G., Gratton J. P., Lin M., Li K. X., Michell B., Kemp B. E., Rodman D., Sessa W. C. (2002) Localization of endothelial nitric-oxide synthase phosphorylated on serine 1179 and nitric oxide in Golgi and plasma membrane defines the existence of two pools of active enzyme. J. Biol. Chem. 277, 4277–4284 [DOI] [PubMed] [Google Scholar]
- 58. Gallis B., Corthals G. L., Goodlett D. R., Ueba H., Kim F., Presnell S. R., Figeys D., Harrison D. G., Berk B. C., Aebersold R., Corson M. A. (1999) Identification of flow-dependent endothelial nitric-oxide synthase phosphorylation sites by mass spectrometry and regulation of phosphorylation and nitric oxide production by the phosphatidylinositol 3-kinase inhibitor LY294002. J. Biol. Chem. 274, 30101–30108 [DOI] [PubMed] [Google Scholar]
- 59. Vitale N., Patton W. A., Moss J., Vaughan M., Lefkowitz R. J., Premont R. T. (2000) GIT proteins. A novel family of phosphatidylinositol 3,4,5-trisphosphate-stimulated GTPase-activating proteins for ARF6. J. Biol. Chem. 275, 13901–13906 [DOI] [PubMed] [Google Scholar]
- 60. Pang J., Xu X., Getman M. R., Shi X., Belmonte S. L., Michaloski H., Mohan A., Blaxall B. C., Berk B. C. (2011) G protein-coupled receptor kinase 2 interacting protein 1 (GIT1) is a novel regulator of mitochondrial biogenesis in heart. J. Mol. Cell Cardiol. 51, 769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pang J., Hoefen R., Pryhuber G. S., Wang J., Yin G., White R. J., Xu X., O'Dell M. R., Mohan A., Michaloski H., Massett M. P., Yan C., Berk B. C. (2009) G-protein-coupled receptor kinase interacting protein-1 is required for pulmonary vascular development. Circulation 119, 1524–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shikata Y., Birukov K. G., Garcia J. G. (2003) S1P induces FA remodeling in human pulmonary endothelial cells. Role of Rac, GIT1, FAK, and paxillin. J. Appl. Physiol. 94, 1193–1203 [DOI] [PubMed] [Google Scholar]
- 63. Jones C. A., Nishiya N., London N. R., Zhu W., Sorensen L. K., Chan A. C., Lim C. J., Chen H., Zhang Q., Schultz P. G., Hayallah A. M., Thomas K. R., Famulok M., Zhang K., Ginsberg M. H., Li D. Y. (2009) Slit2-Robo4 signaling promotes vascular stability by blocking Arf6 activity. Nat. Cell Biol. 11, 1325–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.