Background: Development of mature myelinating oligodendrocytes requires the co-ordinated migration, proliferation, and differentiation of oligodendrocyte progenitor cells (OPCs).
Results: OPCs lacking PTPα show enhanced proliferation and altered activity and/or expression of several signaling molecules.
Conclusion: PTPα-dependent signaling limits OPC proliferation.
Significance: This provides insight into the molecular events that promote the cessation of proliferation to position OPCs for differentiation.
Keywords: Cell Proliferation, Cell Signaling, Oligodendrocytes, Phosphotyrosine Signaling, Protein Phosphatase, Tyrosine-protein Phosphatase (Tyrosine Phosphatase), Fyn, PTPRA
Abstract
Tightly controlled termination of proliferation determines when oligodendrocyte progenitor cells (OPCs) can initiate differentiation and mature into myelin-forming cells. Protein-tyrosine phosphatase α (PTPα) promotes OPC differentiation, but its role in proliferation is unknown. Here we report that loss of PTPα enhanced in vitro proliferation and survival and decreased cell cycle exit and growth factor dependence of OPCs but not neural stem/progenitor cells. PTPα−/− mice have more oligodendrocyte lineage cells in embryonic forebrain and delayed OPC maturation. On the molecular level, PTPα-deficient mouse OPCs and rat CG4 cells have decreased Fyn and increased Ras, Cdc42, Rac1, and Rho activities, and reduced expression of the Cdk inhibitor p27Kip1. Moreover, Fyn was required to suppress Ras and Rho and for p27Kip1 accumulation, and Rho inhibition in PTPα-deficient cells restored expression of p27Kip1. We propose that PTPα-Fyn signaling negatively regulates OPC proliferation by down-regulating Ras and Rho, leading to p27Kip1 accumulation and cell cycle exit. Thus, PTPα acts in OPCs to limit self-renewal and facilitate differentiation.
Introduction
Oligodendrocytes (OLs)2 are the myelin-forming cells of the CNS. The development of oligodendrocyte progenitor cells (OPCs) into mature OLs is a complex process requiring cessation of proliferation, cell cycle exit, expression of OL-specific genes, and extension of processes and myelin sheets. Various growth factors have been shown to regulate OL development, such as platelet-derived growth factor (PDGF) and basic fibroblast growth factor (bFGF) (1) that co-operatively promote self-renewal and survival and inhibit differentiation of OPCs (2–4). PDGF induces OPCs to proliferate for a number of divisions, thereby preventing premature differentiation (5, 6). bFGF blocks terminal differentiation and myelin gene expression at the late progenitor stage (7, 8). Moreover, bFGF can maintain a high level of expression of the PDGF receptor α, (PDFGRα), the only PDGF receptor expressed in OLs (8, 9).
Receptor-like protein-tyrosine phosphatase α (PTPα) is a widely expressed transmembrane protein with two intracellular catalytic domains. The major substrates of PTPα are Src family kinases (SFKs) (10), which are activated by PTPα-catalyzed dephosphorylation of the inhibitory phosphotyrosine near the C terminus (11–14). PTPα-mediated SFK activation is often carried out in response to ligand binding to receptors that themselves do not possess catalytic activity (such as F3/contactin, NCAM, NB-3, CHL1, integrins) (14–17), but also occurs upon ligand activation of growth factor receptor-tyrosine kinases (such as c-Kit, EGFR, PDGFR) (18, 19).
PTPα promotes OPC differentiation upon cessation of proliferation (21). This raises the question of whether PTPα regulates other processes that appropriately position OPCs to differentiate, such as cell cycle exit. Indeed, PTPα has been shown to have positive and negative roles in growth control, which apparently depend on cell context. For example, PTPα overexpression activates Src and confers tumorigenic properties on fibroblasts (11), while inducing G1 arrest in breast cancer cells that reduces proliferation and delays tumor formation in vivo (20). In OPCs, PTPα regulates Fyn activation and signaling during differentiation (21). Fyn promotes growth arrest and differentiation of keratinocytes (22) and neuroblastoma cells (23), but its role in OPC proliferation is not well defined. Fyn is reported to not be required for PDGF-mediated proliferation nor to be activated by FGF or PDGF treatment of OPCs (24, 25). However, Fyn expression and autophosphorylation in oligodendroglial cells is increased by apotransferrin (26), which inhibits the mitogenic action of PDGF (27). We therefore investigated the role of PTPα, and PTPα-mediated Fyn signaling, in proliferation and cell cycle regulation of OPCs.
EXPERIMENTAL PROCEDURES
Mice
The 129SvEv PTPα−/− mice (13) were backcrossed with C57BL/6 mice for 10 generations. PTPα−/− and wild type (WT) C57BL/6 mice were housed under specific pathogen-free conditions. Animal care and use followed the guidelines of the University of British Columbia and the Canadian Council on Animal Care, and were reviewed and approved by the University of British Columbia.
Cell Line and Primary Cell Cultures
The CG4 cell line was kindly provided by Dr. Y. Feng (Emory University School of Medicine) and maintained as described (21) in CG4 proliferation medium (DMEM, 1% FBS, 5 μg/ml insulin, 50 μg/ml transferrin, 30 nm sodium selenite, 100 μm putrescine, 20 nm progesterone, 10 ng/ml biotin, 10 ng/ml PDGF, 10 ng/ml bFGF). Primary mouse oligospheres and OPCs were generated from neurospheres prepared from wild-type and PTPα−/− mice as described (21) and maintained in proliferation medium (DMEM/F12, 25 μg/ml insulin, 100 μg/ml apo-transferrin, 20 nm progesterone, 60 μm putrescine, and 30 nm sodium selenite, 20 ng/ml PDGF-AA, 20 ng/ml bFGF) as oligospheres in suspension or as adherent OPCs on poly-dl-ornithine (PDLO, 50 ng/ml)-coated dishes or chamber slides.
Reagents, Antibodies, and Growth Factors
Reagents were obtained from Sigma-Aldrich Canada (Oakville, ON, Canada) unless otherwise indicated. DNase I was purchased from Invitrogen Canada (Burlington, ON, Canada). Anti-PTPα antiserum has been described previously (28). Antibodies to PCNA, Olig2, O4, NG2, Ras, PDGFRα, and phosphotyrosine (4G10) were purchased from Millipore (Billerica, MA). Antibodies to phosphoTyr527-Src was purchased from BIOSOURCE (Camarillo, CA). Antibodies to Fyn, FAK, Rac1, Cdc42, and p27 were purchased from BD Transduction Laboratories (San Jose, CA). Antibodies to cleaved caspase-3, phosphoSer473-Akt, Akt, phosphor-Thr202/Tyr204-ERK1/2, ERK, phosphor-Thr183/Tyr185-JNK were purchased from Cell Signaling. Antibody to p120RasGAP and p21Cip/WAF1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody to actin were purchased from Sigma-Aldrich Canada. Antibody to Rho was purchased from Stressgen Biotechnologies (Victoria, BC, Canada). Antibody to Ki-67 was purchased from Dako Canada (Burlington, ON, Canada). Secondary antibodies conjugated with Alexa Fluor 488 or 594 (Molecular Probes) were purchased from Invitrogen Canada. Human recombinant PDGF-AA, bFGF, and EGF were purchased from PeproTech (Rocky Hill, NJ).
BrdU Incorporation Assay
BrdU incorporation assay was performed using the In Situ Cell Proliferation Kit, FLUOS (Roche, Mannheim, Germany).
Immunofluorescence Labeling, Immunoblotting, Immunoprecipitation
These procedures were performed as previously described (21). Cell lysates were prepared with RIPA lysis buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 0.5% sodium deoxycholate, 1% Nonidet P-40, 0.1% SDS, 1 mm EDTA, 2 mm sodium orthovanadate, 50 mm sodium fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mm PMSF) or Nonidet P-40 lysis buffer (RIPA lysis buffer without sodium deoxycholate and SDS).
siRNA Transfection
The following siRNAs (Dharmacon, Chicago, IL) were used: Control (siCONTROL Non-Targeting siRNA Pool 2 D-001206-14-20), PTPα (ON-TARGETplus SMARTpool l-080089-01-0050, Rat PTPRA, NM_012763) and Fyn (ON-TARGETplus SMARTpool l-089444-00-0010, Rat Fyn, NM_012755). CG4 cells were seeded in CG4 proliferation medium (3 × 104/cm2). After overnight attachment, cells were incubated with 20 nm siRNA and Lipofectamine RNAiMax (Invitrogen Canada) in OPTI-MEM I (Cat. 31985, Invitrogen) for 16–18 h followed by incubation in CG4 proliferation medium for indicated times.
Ras and Rho Family GTPase Activity Assays
Ras activity was measured by GST-Raf1 RBD (Ras-binding domain) pull-down assays performed using the Ras Activation Assay Kit (Upstate, Temecula, CA). Rho activity was measured by GST-RhBD (Rhotekin-binding domain) pull-down assays performed using the Rho Activation Assay Kit (Upstate). Rac1 and Cdc42 activities were measured by GST-PBD (PAK-binding domain) pull-down assays (29). Cells were lysed on ice by adding RIPA lysis buffer directly onto the cells. Cell lysates (50–100 μg) were incubated with 10 μg of GST-PBD bound to glutathione-Sepharose beads. Samples were washed with lysis buffer and then immunoblotted with anti-Rac1 and Cdc42 antibodies. Lysates were directly immunoblotted to determine the total amount of Ras, Rho, Rac1, or Cdc42 proteins. Levels of active Ras, Rho, Rac1, and Cdc42 were normalized to those of total Ras, Rho, Rac1, and Cdc42.
Data Analysis
Densitometric quantification of immunoblots and cell differentiation data were statistically analyzed using ANOVA (single factor). Data obtained from three independent experiments are expressed as the mean ± S.D. *, p < 0.05; **, p < 0.01.
RESULTS
PTPα Negatively Regulates OPC Growth and Growth Factor Dependence
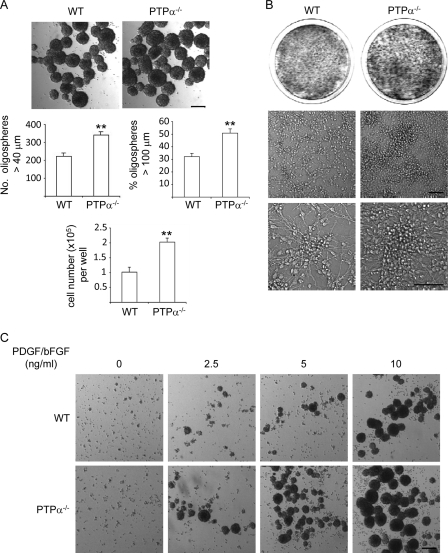
Cell proliferation and differentiation are highly coordinated processes during development. If cells persist in the cell cycle, they do not differentiate properly. We used cultured primary OPCs isolated from WT and PTPα-null (PTPα−/−) mouse embryos to investigate the function of PTPα in progenitor cells as a regulator of proliferation, cell cycle exit, and survival. We first examined the growth of WT and PTPα−/− OPCs as oligospheres. Dissociated oligospheres (OPCs) were cultured in OPC proliferation medium for 5 days, and the newly formed oligospheres were examined. As shown in Fig. 1A, PTPα−/− oligospheres were larger than WT oligospheres. The oligospheres were counted and their size was measured, revealing that PTPα−/− cells formed more oligospheres, and that large (>100 μm) oligospheres comprised a significantly higher proportion of the total PTPα−/− oligosphere population than of the WT oligosphere population, suggesting that loss of PTPα may increase OPC self-renewal capability. The original oligospheres were derived from dissociated neurospheres, and neurospheres are motile and aggregate in culture (30). To rule out the possibility that the larger PTPα−/− oligospheres resulted from enhanced aggregation, or from the association of larger sized but similar numbers of individual cells, WT and PTPα−/− oligospheres were collected and dissociated, and total cell numbers were counted. The total cell number obtained from the dissociated PTPα−/− oligosphere population was higher than that obtained from the total WT oligosphere population (Fig. 1A), indicating a faster rate of PTPα−/− cell growth. To verify that PTPα−/− OPCs also grow faster than WT OPCs in adherent culture, oligospheres were dissociated and seeded on PDLO-coated dishes in OPC proliferation medium for 2 weeks. PTPα−/− OPCs grew to a higher density and formed larger colonies compared with WT OPCs (Fig. 1B). These results indicate that the growth rate of PTPα−/− OPCs is faster than WT OPCs.
FIGURE 1.
Increased growth and self-renewal of OPCs in the absence of PTPα. A, dissociated oligospheres were seeded in proliferation medium for 5 days. Bright-field images were taken and the numbers of oligospheres larger than 40 μm and 100 μm were counted. For cell counting, oligospheres were dissociated and live cells were counted by trypan blue exclusion. Scale bar, 140 μm. B, dissociated oligospheres were seeded on PDLO-coated dishes in proliferation medium for 2 weeks. Cells were washed and stained with crystal violet (upper panel) and bright-field images were taken (lower panel). Scale bar, 70 μm. C, dissociated oligospheres were seeded in medium with 0, 2.5, 5, and 10 ng/ml each of PDGF and bFGF for 5 days. The bright-field images shown are representative of two independent experiments. Scale bar, 140 μm.
The best-characterized mitogens for OPCs are PDGF and bFGF (1), and both PDGF and bFGF are survival factors for OPCs (2, 4). In the oligosphere culture system, cells undergo self-renewing cell division in OPC proliferation medium, which contains PDGF and bFGF. We determined if PTPα−/− OPCs are hypersensitive to PDGF/bFGF stimulation. As shown in Fig. 1C, WT and PTPα−/− OPCs cannot form new spheres in the absence of these growth factors, but PTPα−/− OPCs can form more and larger new spheres at lower concentrations of growth factors than WT OPCs. This indicates that PTPα−/− OPCs are hypersensitive to PDGF/bFGF, but that PTPα loss did not render oligosphere formation growth factor-independent.
PTPα Negatively Regulates OPC Proliferation, Cell Cycle Entry, and Survival in Response to PDGF/bFGF
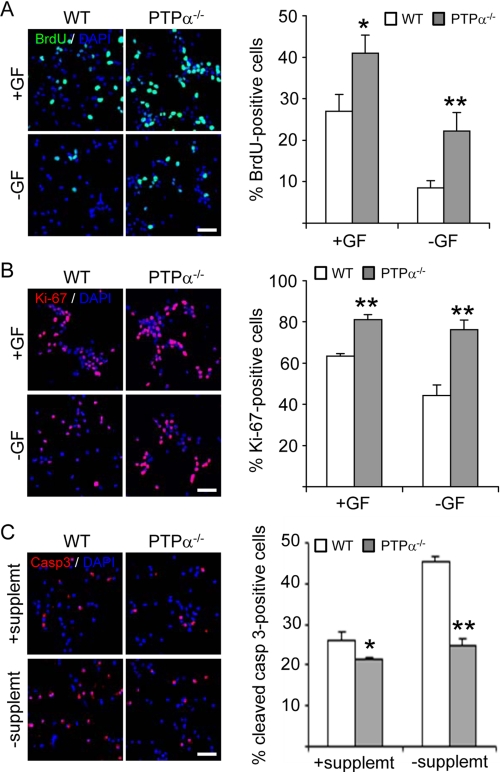
The accumulation of large numbers of PTPα−/− OPCs may result from an increase in cell proliferation, a decrease in cell cycle exit and/or a decrease in cell death. To address which mechanism is affected by PTPα to regulate OPC growth, we assessed these parameters in WT and PTPα−/− OPCs. WT and PTPα−/− oligospheres were dissociated and seeded on PDLO-coated chamber slides in proliferation medium for 2 days followed by culture in fresh medium with or without PDGF/bFGF for another 24 h. BrdU incorporation was significantly increased in PTPα−/− OPCs compared with WT OPCs in the presence or absence of PDGF/bFGF (Fig. 2A). Ki-67 is a protein associated with proliferation that is present in cells that are within the cell cycle but not in cells that have exited the cell cycle. To address whether PTPα regulates cell cycle exit, immunofluorescent staining with antibody against Ki-67 was performed. This demonstrated that more PTPα−/− cells than WT cells are in the cell cycle in the presence of PDGF/bFGF (Fig. 2B). In addition, the percentage of Ki-67-positive WT cells decreased by 19.1% when the cells were cultured in the absence of PDGF/bFGF, while the percentage of Ki-67-positive PTPα−/− cells only decreased by 4.7% when these growth factors were absent (Fig. 2B). This indicates that PTPα−/− cells are more resistant to PDGF/bFGF withdrawal-induced cell cycle exit. We next determined if PDGF/bFGF-mediated survival is altered in PTPα−/− OPCs. For this, apoptosis was induced by withdrawal of supplement (including insulin) for 24 h while maintaining PDGF/bFGF, and the cells were labeled with the apoptosis marker, cleaved caspase-3. While the percentages of apoptotic cells in WT and PTPα−/− OPCs cultured under normal conditions (with supplement) showed a significant but small difference (WT, 26%; PTPα−/−, 21%), the withdrawal of supplement differentially enhanced apoptosis in the two cell types. The percentage of apoptotic WT OPCs increased to 45%, while the percentage of apoptotic PTPα−/− OPCs rose to only 25% (Fig. 2C). Therefore, PDGF/bFGF-mediated survival is increased in PTPα−/− OPCs.
FIGURE 2.
Increased proliferation, decreased cell cycle exit and apoptosis of OPCs in the absence of PTPα. A, WT and PTPα−/− OPCs were seeded on PDLO-coated chamber slides in proliferation medium for 2 days. Cells were cultured in the presence (+GF) or absence (−GF) of PDGF/bFGF for 24 h. BrdU was added 4 h prior to fixation. Cells were immunostained with antibodies against BrdU. B, WT and PTPα−/− OPCs were seeded on PDLO-coated chamber slides in proliferation medium for 2 days. Cells were cultured in the presence (+GF) or absence (−GF) of PDGF/bFGF for 24 h and immunostained with antibody against the proliferation marker Ki-67. C, WT and PTPα−/− OPCs were seeded on PDLO-coated chamber slides in proliferation medium for 2 days. Cells were cultured in the presence or absence of supplement for 24 h. Cells were immunostained with antibody against cleaved caspase-3. A–C, at least 1500 cells were counted for each group. Scale bar, 40 μm.
Lack of PTPα Does Not Affect Proliferation of Neural Stem/Progenitor Cells
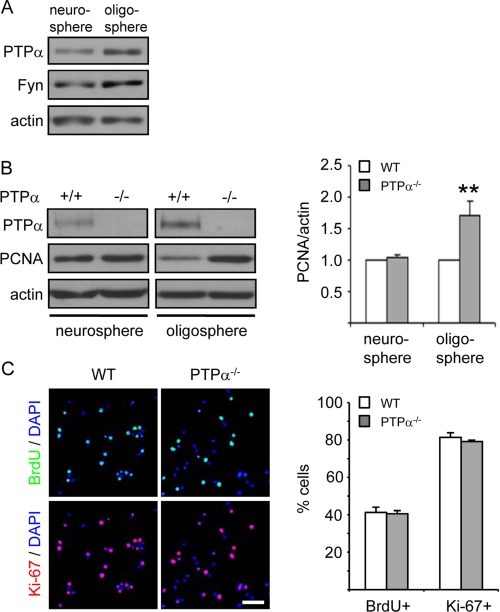
The inhibitory effect of PTPα on OPC proliferation raised the question of how early PTPα is expressed during development and whether it regulates the proliferation and cell cycle exit of cells at an earlier stage of differentiation. It has been shown that PTPα mRNA is not detectable in undifferentiated mouse embryonic pluripotent stem cells (P19 embryonic carcinoma cells), but is expressed in neuroectoderm-like cells (retinoic acid-treated P19 derivatives) (12). Therefore, the expression of PTPα protein in neurospheres composed of neural stem/progenitor cells was determined and compared with that in oligospheres composed of OPCs. PTPα protein and its potential substrate Fyn were detectable in neurospheres, but at lower levels than in oligospheres (3.3-fold less PTPα, 1.9-fold less Fyn; n = 2) (Fig. 3A). We next examined the expression of the proliferation marker PCNA in WT and PTPα−/− neurospheres and oligospheres. As shown in Fig. 3B, in contrast to the different expression levels of PCNA in WT and PTPα−/− oligospheres, PCNA expression levels are similar in WT and PTPα−/− neurospheres, suggesting that PTPα does not have a significant role in regulating proliferation of neural stem/progenitor cells in response to EGF/bFGF. This finding was confirmed by determining BrdU incorporation and Ki-67 positivity, revealing that PTPα ablation does not significantly affect proliferation and cell cycle exit of neural stem/progenitor cells (Fig. 3C).
FIGURE 3.
Lack of PTPα does not affect proliferation and cell cycle exit in neural stem cells. A, PTPα protein level is higher in oligospheres than in neurospheres. PTPα, Fyn, and actin protein amounts were determined by immunoblotting cell lysates. The results shown are representative of those from two independent experiments. B, PTPα, PCNA, and actin protein amounts were determined by immunoblotting cell lysates. The band intensity of PCNA was normalized to that of actin. C, WT and PTPα−/− neurospheres were dissociated and seeded on PDLO/gelatin-coated chamber slides in neural growth medium for 1 day. BrdU was added 2 h prior to fixation. Cells were immunostained with antibodies against BrdU and Ki-67. At least 600 cells were counted for each group. Scale bar, 40 μm.
PTPα Negatively Regulates the Activities of Ras and the Rho Family Small GTPases Cdc42, Rac1, and Rho, in Primary OPCs
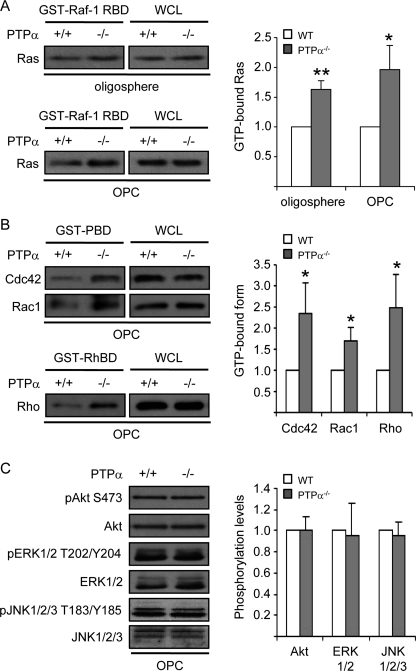
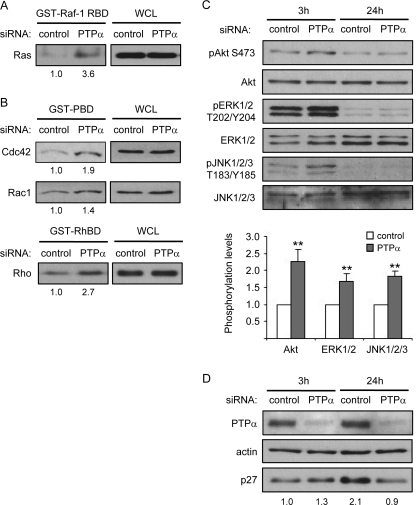
Activation of PDGFRα promotes proliferation and inhibits premature differentiation of OPCs (5, 6). No difference in PDGFRα expression was detected between PTPα−/− and WT oligospheres or OPCs (data not shown). Ras proteins are important downstream effectors of growth factor receptor-tyrosine kinase signaling pathways (31), and Ras plays an important role in oligodendrocyte development (32–34). We found that Ras activities were increased in both PTPα−/− oligospheres and OPCs compared with the WT cells (Fig. 4A), suggesting that PTPα negatively regulates Ras activity in OPCs.
FIGURE 4.
Increased activities of Ras and Rho family small GTPases, Cdc42, Rac1, and Rho, in PTPα−/− OPCs. A, amounts of activated Ras isolated from cell lysates by GST-Raf-1 RBD pull-downs (left panel), or of total Ras in WCL (right panel), were determined by immunoprobing with anti-Ras antibody. The band intensity of GTP-bound Ras was normalized to that of Ras in WCL. B, amounts of activated Cdc42 and Rac1 isolated from cell lysates by GST-PBD pull-downs (left panels), or of total Cdc42 and Rac1 in WCL (right panels), were determined by immunoprobing with anti-Cdc42 and Rac1 antibodies. The amounts of activated Rho isolated from cell lysates by GST-RhBD pull-downs (left panels), or of total Rho in WCL (right panels), were determined by immunoprobing with anti-Rho antibody. The band intensities of GTP-bound Cdc42, Rac1, and Rho were normalized to that of Cdc42, Rac1, and Rho in WCL, respectively. C, phosphorylation of ERK1/2 at T202/Y204, that of Akt at S473 and that of JNK1/2/3 at T183/Y185 were determined by immunoblotting with anti-ERK1/2 phospho-T202/Y204, anti-Akt phospho-S473 and anti-JNK1/2/3 phospho-T183/Y185 antibodies, respectively. The band intensity of phospho-ERK1/2, that of phospho-Akt and that of phospho-JNK1/2/3 was normalized to that of ERK1/2, Akt, and JNK1/2/3, respectively.
Ras can activate at least three types of downstream effectors/signaling pathways; the Rho family small GTPases, mitogen-activated protein kinase (MAPK) and Akt pathways (35). We next determined which of these were affected by PTPα ablation. Using GST-PBD pull-down assays to measure the levels of active GTP-bound Cdc42 and Rac1 demonstrated that PTPα−/− OPCs exhibit increased activities of Cdc42 and Rac1 (Fig. 4B). Also, Rho activity was significantly increased in PTPα−/− OPCs, as detected using GST-RBD pull-down assays (Fig. 4B). However, the phosphorylation of Akt at Ser-473, that of ERK (extracellular signal-regulated kinase 1/2) at Thr202/Tyr204 and that of JNK at Thr183/Tyr185 were not affected in OPCs by the absence of PTPα after culturing in proliferation medium for 2 days (Fig. 4C). The p38MAPK phosphorylation was low in the OPCs (data not shown), as has been described by others (36), and could not be reliably analyzed. These results indicate that PTPα negatively regulates the activities of the Rho GTPases Rho, Rac1, and Cdc42, but does not affect the phosphorylation status of Akt, ERK, and JNK in this cell culture condition and time point of investigation.
PTPα Up-regulates the Expression of p27Kip1 Protein
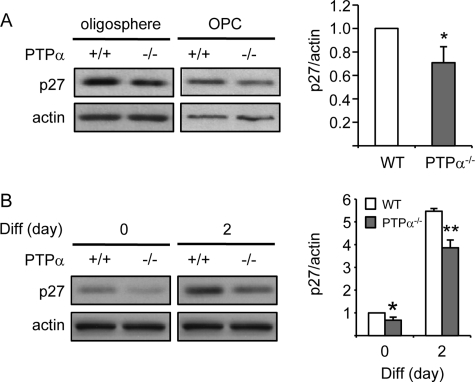
The cell cycle inhibitory protein p27Kip1 (p27) plays a critical role in cell cycle regulation in response to factors in the extracellular environment, such as growth factors and the extracellular matrix. As cultured OPCs proliferate, p27 progressively accumulates and eventually promotes cell cycle exit (37, 38). Signaling pathways active in proliferating cells can limit p27 up-regulation. For example, Ras activation is required for the suppression of p27 levels throughout the cell cycle (39). On the other hand, p27 can also regulate Ras activation by preventing Grb2-SOS complex formation (40). Overexpression of p27 reduces cell proliferation and self-renewal and promotes cell death in neural progenitor cells (41). In oligodendrocyte lineage cells, p27 is a crucial regulator of the decision to withdraw from the cell cycle (42). Therefore, we determined if p27 expression is altered in PTPα−/− OPCs. As shown in Fig. 5A, decreased (by about 30%) p27 expression was detected in PTPα−/− oligospheres and OPCs, indicating that PTPα may up-regulate p27 expression to promote cell cycle exit. Since accumulation of p27 is required for OPC differentiation (38, 42–44) and since PTPα−/− OPCs fail to differentiate with proper timing (21), p27 expression was monitored before and after differentiation. As shown in Fig. 5B, differentiation induction by mitogen-withdrawal and T3 exposure induced p27 accumulation in both WT and PTPα−/− cells, but a lower expression of p27 was observed in both PTPα−/− OPCs and differentiating OLs compared with WT cells. These results suggest that PTPα promotes OPC differentiation, at least partially, by facilitating p27 accumulation and cell cycle exit, leading to decreased self-renewing proliferation and increased cell fate commitment.
FIGURE 5.
Decreased expression of p27 in PTPα−/− OPCs and differentiating OLs. A, p27 expression was determined by immunoblotting with anti-p27 and anti-actin antibodies, and quantified (graph) for OPCs. B, WT and PTPα−/− mouse oligospheres were dissociated and seeded on PDLO-coated dishes for 2 days in OPC proliferation medium (day 0) followed by incubation for the indicated times in OPC differentiation medium (day 2). The expression of p27 was determined by immunoblotting with anti-p27 and anti-actin antibodies. A and B, band intensity of p27 was normalized to that of actin.
PTPα Negatively Regulates Multiple Signaling Pathways and Is Required for p27 Accumulation in CG4 OPCs
CG4 cells were used for subsequent investigation of PTPα-dependent signaling in oligodendrocyte precursor/progenitor cells because of the following reasons. First, some of the primary OPCs differentiate spontaneously, leading to a heterogeneous population of cells. Second, a large number of the limited and difficult to prepare primary OPCs is required for biochemical studies to elucidate molecular signaling mechanisms. Third, it is difficult to synchronize the primary OPCs without inducing differentiation, therefore, some signaling events are difficult to manipulate and monitor. To study PTPα-dependent signaling in response to PDGF/bFGF stimulation, CG4 cells were transfected with control siRNA and PTPα siRNA and starved overnight, followed by stimulation with CG4 proliferation medium for 3 h. Unaltered PDGFR expression (data not shown) and the increased activities of Ras and the Rho GTPases, Rac1, Cdc42, and Rho were confirmed in PTPα-knockdown CG4 cells (Fig. 6, A and B). Phosphorylation of Akt, ERK, and JNK was also determined by immunoblotting. In contrast to the results obtained with primary OPCs, the phosphorylation of Akt at Ser-473, that of ERK at Thr202/Tyr204 and that of JNK at Thr183/Tyr185 were up-regulated 3 h after stimulation in PTPα-knockdown CG4 cells (Fig. 6C). However, after stimulation for 24 h, the phosphorylation of these molecules decreased compared with 3 h, and exhibited no significant difference between control and PTPα-knockdown CG4 cells. These results suggest that PTPα negatively and transiently regulates growth factor-mediated phosphorylation of Akt, ERK, and JNK in CG4 cells. The expression of p27 was also monitored in CG4 cells stimulated for 3 h and 24 h. As shown in Fig. 6D, the p27 expression level is similar in control siRNA and PTPα siRNA-treated CG4 cells after stimulation for 3 h. However, after 24 h stimulation, the p27 protein level increased in control CG4 cells, but not in PTPα-knockdown CG4 cells (Fig. 6D). Therefore, in accord with findings in OPCs, PTPα negatively regulates the activities of Ras and Rho GTPases, Rac1, Cdc42, and Rho, and is required for p27 accumulation in OPCs.
FIGURE 6.
PTPα negatively regulates Ras, Rho GTPases, MAPK, and Akt and is required for p27 accumulation in CG4 cells. A and B, control siRNA or PTPα siRNA treated cells were stimulated with CG4 proliferation medium for 3 h. The amount of Ras, Rac1/Cdc42, and Rho in the pull-down assay as well as in the WCL was determined by immunoblotting with anti-Ras, anti-Rac1, anti-Cdc42, and ant-Rho antibodies, respectively. The numbers at the bottom show the relative amounts of GTP-bound protein normalized to the amount of that protein in the lysate as calculated after densitometric quantification. C, control siRNA or PTPα siRNA-treated cells were stimulated with CG4 proliferation medium for 3 h or 24 h. Phosphorylation of ERK1/2 at T202/Y204, Akt at S473 and JNK1/2/3 at T183/Y185 at 3 h was determined by immunoblotting with anti-ERK1/2 phospho-T202/Y204, anti-Akt phospho-S473, and anti-JNK1/2/3 phospho-T183/Y185 antibodies, respectively. D, control siRNA or PTPα siRNA-treated cells were stimulated with CG4 proliferation medium for 3 h or 24 h. PTPα and p27 expression was determined by immunoblotting with anti-PTPα, anti-p27 and anti-actin antibodies. The numbers at the bottom show the relative band intensities of p27 after normalization to the actin signal.
Fyn Is Required for Ras and Rho Inactivation and Accumulation of p27 and p21 in CG4 Cells
Because SFKs are substrates of PTPα, we investigated if SFKs are responsible for the inhibition of OPC growth. Treatment of CG4 cells with the SFK inhibitors SU6656 (2 μm and 10 μm) or PP2 (2 μm and 10 μm) resulted in a decrease in the number of cells, suggesting that SFKs are required for CG4 growth (data not shown). This result is similar to previous findings that SFKs are required for PDGF-induced OPC proliferation (45, 46).
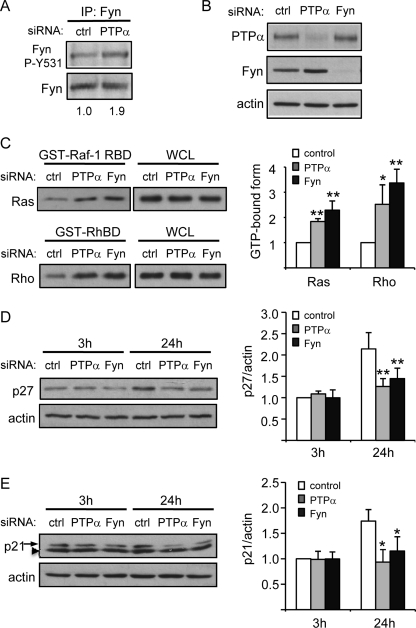
Fyn has been reported to promote growth arrest and differentiation of keratinocytes (22) and neuroblastoma cells (23), suggesting a role of Fyn in cell cycle regulation. It has been shown that fewer oligodendroglial cells (O4-positive pro-OLs) developed in Fyn−/− mixed glia cell cultures (47), suggesting that Fyn may be required for oligodendrocyte lineage commitment. Because Fyn is an important substrate of PTPα and we have shown that Fyn activity is decreased in proliferating PTPα−/− primary OPCs (as evidenced by the ∼2-fold higher phosphorylation at the inhibitory Tyr-528 site) (21), we next determined if Fyn is involved in PTPα-mediated signaling that inhibits CG4 OPC proliferation and promotes cell cycle exit. We first confirmed that Fyn activity is reduced in PTPα-knockdown CG4 cells stimulated with growth factors (Fig. 7A). We then determined if ablation of Fyn results in hyperactivation of Ras and Rho. As shown in Fig. 7, B and C, Ras and Rho activity increased in both PTPα-knockdown and Fyn-knockdown CG4 cells, suggesting that PTPα may act through Fyn to inactivate Ras and Rho.
FIGURE 7.
PTPα-Fyn signaling negatively regulates the activaties of Ras and Rho and promotes p27 and p21 accumulation in CG4 cells. A, control siRNA or PTPα siRNA-treated cells were stimulated with CG4 proliferation medium for 3 h. Fyn phosphorylation at Tyr-531 was analyzed by immunoprecipitation with anti-Fyn antibody followed by probing with anti-Src phospho-Tyr527 and anti-Fyn antibodies. The numbers at the bottom show Fyn phosphorylation per unit Fyn as calculated after densitometric quantification. B, control siRNA, PTPα siRNA, or Fyn siRNA-treated CG4 cells were stimulated with CG4 proliferation medium for 3 h, and cell lysates were probed for PTPα, Fyn, and actin. C, control siRNA, PTPα siRNA, or Fyn siRNA-treated cells were stimulated with CG4 proliferation medium for 3 h. The amounts of activated Ras isolated from cell lysates by GST-Raf-1 RBD pull-downs (left panels), or of total Ras in WCL (right panels), were determined by immunoprobing with anti-Ras antibody. The amounts of activated Rho isolated from cell lysates by GST-RhBD pull-downs (left panels), or of total Rho in WCL (right panels), were determined by immunoprobing with anti-Rho antibody. The band intensities of GTP-bound Ras and Rho were normalized to that of Ras and Rho in WCL, respectively. D and E, impaired up-regulation of p27 and p21 in PTPα-knockdown and Fyn-knockdown CG4 cells. Control siRNA, PTPα siRNA, or Fyn siRNA-treated cells were stimulated with CG4 proliferation medium for 3 h or 24 h. The expression of p27 and p21 was determined by immunoblotting with antibodies against p27, p21, and actin. The band intensities of p27 and p21 were normalized to that of actin. *, nonspecific binding.
Fyn, Ras, and Rho have been implicated in regulating p27 expression. Overexpression of Fyn results in increased expression of p27 (22). Microinjection of oncogenic Ras reduces p27 expression (39). Inhibition of Rho, by either lovastatin or C3 exoenzyme, increases the translational efficiency of p27 mRNA (48). Therefore, it is possible that PTPα activates Fyn to inactivate Ras and Rho, leading to increased p27 protein expression. In keeping with this scenario, p27 protein levels were similar in control cells and cells in which PTPα and Fyn expression was silenced at the earlier time of 3 h stimulation, but at 24 h stimulation the p27 level more than doubled in control cells, but did not significantly increase in PTPα-knockdown or Fyn-knockdown CG4 cells. Moreover, the p27 level was significantly lower in PTPα-knockdown and in Fyn-knockdown CG4 cells compared with control cells at 24 h stimulation (Fig. 7D). These results suggest that PTPα may act through Fyn to promote Ras and Rho inactivation and p27 accumulation.
Another cyclin-dependent kinase inhibitor, p21Cip1 (p21), is required for the differentiation of OPCs independently of cell cycle withdrawal (49). Overexpression of Fyn leads to elevated expression of p21 (22) and Rho is required for Ras-mediated suppression of p21 expression (50). We determined if p21 expression also decreased in PTPα- and Fyn-deficient CG4 cells. As shown in Fig. 7E, the p21 level was significantly lower in PTPα-knockdown and in Fyn-knockdown CG4 cells compared with control cells at 24 h stimulation. These results suggest that PTPα may act through Fyn to down-regulate the activities of Ras and Rho, leading to p21 accumulation and OPC differentiation.
Inhibition of Rho Relieves the Suppression of p27 Expression in PTPα-silenced CG4 Cells
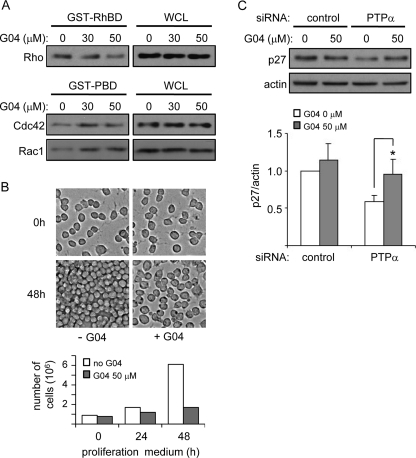
To investigate if PTPα-regulated p27 expression was mediated by PTPα signaling through Rho, we determined if Rho inhibition affected PTPα-dependent p27 expression. We first tested the efficacy and specificity of the Rho inhibitor G04,3 and found that 50 μm G04 inhibited Rho but not Rac1 or Cdc42 in CG4 cells (Fig. 8A). In fact, G04 activated Rac1 and Cdc42. Treatment with G04 inhibited CG4 cell proliferation (Fig. 8B). While perhaps involving additional factors besides p27 up-regulation, this observation supports a role for Rho in regulating OPC proliferation. CG4 cells were treated with control or PTPα-directed siRNA in the presence of G04 followed by stimulation with CG4 proliferation medium, and then p27 expression was determined. G04 had no effect on p27 expression in the control cells, but restored the reduced p27 expression in PTPα-silenced cells to a level comparable to control cells (Fig. 8B). This suggests that inhibition of Rho by G04 counteracts the elevated Rho activity resulting from ablated PTPα expression and thus inhibits Rho-mediated suppression of p27.
FIGURE 8.
Pharmacological inhibition of Rho rescues p27 expression in PTPα-deficient CG4 cells. A, CG4 cells were starved in the presence of G04 for 18 h, followed by stimulation with CG4 proliferation medium for 3 h. The amount of activated Rho isolated from cell lysates by GST-RhBD pull-downs (left panels), or of total Rho in WCL (right panels), was determined by immunoprobing with anti-Rho antibody. The amount of activated Cdc42 and Rac1 isolated from cell lysates by GST-PBD pull-downs (left panels), or of total Cdc42 and Rac1 in WCL (right panels), was determined by immunoprobing with anti-Cdc42 and Rac1 antibodies. B, as in A, but the CG4 cells were cultured in proliferation medium for 48 h. Cells were counted at the end of the starvation period (0 h), and at 24 and 48 h after addition of proliferation medium. Representative photographs (10× magnification) of the cells at 0 and 48 h are shown (top), and cell numbers per 10 cm2 are depicted in the graph. C, CG4 cells were transfected with control or PTPα siRNA in the presence of G04 while undergoing starvation for 18 h, followed by stimulation with CG4 proliferation medium for 24 h. The expression of p27 was determined by immunoblotting with antibodies against p27 and actin. The band intensities of p27 were normalized to that of actin.
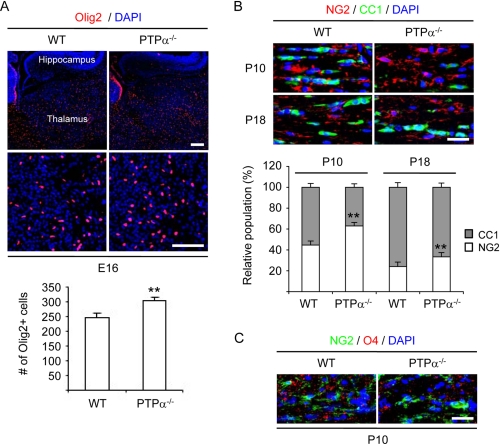
Loss of PTPα Results in Increased Number of Oligodendrocyte Lineage Cells in Embryonic Mouse Forebrain and Delayed Maturation of OPC into Myelin-forming Cells in Developing Mouse Brain
We have shown that PTPα promotes oligodendrocyte differentiation by activating Fyn signaling and that fewer myelinated fibers are detected in PTPα−/− mouse brain as determined by immunostaining of myelin basic protein (21). To determine whether the growth of oligodendrocyte lineage cells was abnormal and the maturation of OPCs into myelin-forming cells was impaired in vivo, immunophenotypic analysis of oligodendrocyte lineage cells in brain sections of WT and PTPα−/− mouse embryos at embryonic day 16 and that of WT and PTPα−/− mice at postnatal days 10 and 18 was conducted. Immunofluorescence with antibody against Olig2 was used to identify oligodendrocyte lineage cells. Double immunofluorescence with antibodies against NG2 and CC1 was used to identify progenitors and mature oligodendrocytes, respectively. Images were acquired, the numbers of Olig2-positive, NG2-positive and CC1-positive cells were counted, and the relative proportion of NG2-positive and CC1-positive cells was calculated as a percentage of the total oligodendrocyte lineage population. At E16, more oligodendrocyte lineage cells in the forebrain of PTPα−/− mouse embryos were observed compared with that of WT mouse embryos (Fig. 9A), suggesting that lack of PTPα leads to increased proliferation and/or survival of oligodendrocyte lineage cells. At P10, the oligodendrocyte lineage population in the corpus callosum of WT mice was composed of 55.3% ± 3.2% CC1-positive cells while in PTPα−/− mice only 37% ± 6.8% of the cells were CC1-positive (Fig. 9B). However, the difference in CC1-positive populations between WT and PTPα−/− mice decreased by P18 (76.1% ± 3.1% versus 66.7% ± 3.4%, respectively) compared with P10, suggesting that the lack of PTPα delayed but did not completely block the maturation of oligodendrocyte lineage cells. This result was confirmed by immunofluoresence using antibodies specific for NG2 and for the lipid sulfatide recognized by O4 (Fig. 9C).
FIGURE 9.
Higher number of oligodendrocyte lineage cells in prenatal PTPα−/− mouse brain and delayed maturation of oligodendrocytes in postnatal PTPα−/− mouse brain. A, embryonic day 16 (E16) PTPα−/− mouse embryos have more oligodendrocyte lineage cells in the forebrain compared with E16 WT mouse embryos. Brain sagittal sections of E16 mouse embryos were immunostained with antibodies against Olig2. The numbers of Olig2-positive cells in the thalamus region were counted. Scale bar, 85 μm. B, postnatal day 10 (P10) PTPα−/− mice have fewer mature oligodendrocytes in the corpus callosum compared with P10 WT mice. Brain coronal sections of P10 and postnatal day 18 (P18) mice were immunostained with antibodies against NG2 and adenomatous poliposis coli (APC, clone CC1). The numbers of NG2-positive and CC1-positive cells in the central region of the corpus callosum (about 0.03 mm2) were counted. Scale bar, 30 μm. A and B, two sections/animal were counted. The values indicate mean ± S.D. cell counts obtained from two sections from three mice of each genotype per time point. C, P10 PTPα−/− mice have less O4 immunoreactivity in corpus callosum compared with P10 WT mice. Brain coronal sections of P10 mice were immunostained with antibodies against NG2 and oligodendrocyte marker O4. Scale bar, 30 μm.
DISCUSSION
In this study we investigated the role of PTPα in proliferating OPCs prior to the induction of differentiation. PTPα−/− OPC proliferation is enhanced compared with WT OPCs, with more PTPα−/− cells entering S phase and fewer exiting the cell cycle. In addition, fewer apoptotic PTPα−/−OPCs than WT cells are detectable in proliferating cultures. PTPα−/− OPCs have enhanced sensitivity to and reduced dependence on the mitogens PDGF and bFGF, as they form new oligospheres in response to lower concentrations of PDGF/bFGF than WT OPCs, and are more resistant to PDGF/bFGF withdrawal-induced cell cycle exit. Also, PTPα-null OPCs are more resistant to supplement withdrawal-induced apoptosis. Altogether, these observations indicate that PTPα functions as a negative regulator of PDGF/bFGF-mediated OPC proliferation and survival. Proliferation of neurospheres, the progenitors of the oligospheres/OPCs, was unaffected by the loss of PTPα expression. This suggests that this role of PTPα is cell lineage stage-specific or specific to the PDGF/bFGF-regulated signaling events that maintain proliferating oligospheres/OPCs, in contrast to the distinct EGF/bFGF-dependent signaling operating during neural stem/progenitor cell proliferation and survival. More OL lineage cells were present in the thalamus of PTPα-null mouse embryos, supporting our observations that primary PTPα−/− OPCs grew faster in culture and generated more cells than WT OPCs. Moreover, in keeping with enhanced OPC growth and proliferation inhibiting the onset of differentiation, the appearance of maturing OLs in corpus callosum was delayed in PTPα−/− mice.
To elucidate the molecular mechanisms by which PTPα exerts these effects, we examined the effects of PTPα ablation on several signaling molecules and pathways in primary WT and PTPα−/− OPCs and in the readily available and easier to manipulate rat CG4 OPC cell line. Ras was identified as a target for PTPα, as in both cell systems Ras activity was up-regulated in the absence or upon the loss of PTPα. Silencing PTPα expression resulted in reduced Fyn activity in proliferating CG4 cells, in accord with the reduced Fyn activation in PTPα−/− primary OPCs prior to differentiation (21). Furthermore, directly abolishing Fyn activity in CG4 cells by silencing Fyn expression resulted in enhanced Ras activity. These results implicate Fyn as an intermediate in PTPα-dependent negative regulation of Ras. How PTPα-activated Fyn suppresses Ras activity in proliferating OPCs is unclear, but could involve up-regulation or relocalization of RasGAP activities through phosphorylation of RasGAP-binding proteins. We eliminated focal adhesion kinase (FAK) (52) as a mediator of increased sequestration of p120RasGAP in PTPα−/− OPCs (data not shown), but other candidates such as Cbp/PAG (53) require investigation. Enhanced Ras activity due to ablation of the RasGAP neurofibromin (NF1) results in OPC hyperproliferation and increased OPC numbers in vivo (32, 54). This suggests that the analogous enhancement of Ras activity resulting from ablated PTPα-Fyn signaling in PTPα−/− OPCs is a key contributor to their increased proliferation in vitro and the higher OPC numbers in PTPα-null mouse embryonic forebrain.
Ras signaling to promote cell proliferation and survival typically involves activation of the MAPK ERK and PI3K/Akt. PDGF induces the phosphorylation and activation of the MAPKs ERK, JNK, and p38 in OPCs (36). Silencing PTPα in CG4 OPCs resulted in transiently increased ERK, Akt, and JNK activities. However, ERK, Akt, and JNK activation appeared normal in primary PTPα−/− OPCs. Similarly, enhanced Ras activity in hyperproliferating NF1−/− OPCs does not increase ERK activity or phosphorylation of the PI3K/Akt/mTOR target S6 (54). It is possible that we might have missed a window of altered activity outside of which these kinase activities are tightly limited by opposing negative modulators. Further investigations of the temporal activities of these kinases in proliferating OPCs are needed to determine their roles in PTPα- and Ras-dependent proliferation and survival.
PTPα−/− OPCs exhibited enhanced activation of Rho, Rac1, and Cdc42, indicating that PTPα negatively regulates these GTPases in the proliferating cells. Interestingly, in differentiating OLs we previously showed that PTPα positively regulates Rac1 and Cdc42 activation while still suppressing Rho activation (21), demonstrating that PTPα differentially controls Rac1 and Cdc42 activities in a process-specific (proliferation versus differentiation) manner. The roles of Rho, Rac1, and Cdc42 in OPC proliferation and survival are unknown, although Cdc42 activity is not critical for these processes (55). Rho functions in PDGF-induced proliferation of glioma cells (56), and we find that the Rho inhibitor G04 inhibits CG4 cell proliferation. Increased activities of these GTPases correlate with increased proliferation, survival, and decreased cell cycle exit in PTPα-null OPCs, suggesting that they are PTPα-regulated downstream effectors of PDGF or bFGF that promote these processes in OPCs. Silencing Fyn in CG4 cells mimicked the silencing of PTPα to induce elevated Rho activity. Thus, PTPα-directed Fyn-dependent signaling limits both Ras and Rho activities in proliferating OPCs.
Expression of p27, a member of the CIP/KIP family of cyclin-dependent kinase inhibitors (CKIs), is inhibited in PTPα-deficient OPCs and CG4 cells. Increased levels of p27 that accumulate during OPC proliferation promote G1 arrest and cell cycle withdrawal that is impaired in cultured p27-null OPCs, and p27−/− mice have more proliferating OPCs (37, 38, 42, 57). Conversely, p27 overexpression induces cell cycle arrest even in the presence of mitogens, but is insufficient for differentiation (58–60). Accordingly, the reduced p27 accumulation in the absence of PTPα may be insufficient to permit OPC cell cycle withdrawal, thus resulting in or contributing to the enhanced proliferation of PTPα−/− OPCs. Both p27−/− OPCs and PTPα−/− OPCs exhibit enhanced mitogen sensitivity (37), further supporting a functional linkage between PTPα and p27.
PTPα silencing in CG4 cells suppresses the expression of another CIP/KIP protein, p21, suggesting that PTPα-mediated elevation of p21 may play a role in promoting growth arrest or differentiation. However, p21 was present at a very low level in primary WT OPCs (data not shown) and its detection was unreliable, so we were unable to determine if PTPα−/− OPCs showed a relative decrease in p21 to validate this protein as PTPα target. Despite p21 promoting growth arrest in many cell types, its role in regulating OPC cell cycle progression is not well understood. FGF2-up-regulated p21 may promote S-phase entry in OPCs (61). On the other hand, cultured p21−/− OPCs exit the cell cycle normally and exist in normal numbers in p21−/− mouse cerebellum, although their differentiation is impaired (49).
The PTPα substrate Fyn promotes growth arrest and causes up-regulation of p27 in keratinocytes (22). Moreover, active Fyn induces G1 arrest and differentiation of neuroblastoma cells (23), suggesting a role of Fyn in suppressing or terminating proliferation prior to differentiation. Ablation of Fyn in CG4 cells results in decreased p27 protein level, indicating that PTPα may down-regulate p27 protein expression through dephosphorylating and activating Fyn. The mechanisms by which Fyn regulates p27 expression remain unclear. Since RhoA can down-regulate p27 protein levels post-transcriptionally and post-translationally (48, 62), one possibility is that Fyn may up-regulate p27 protein level by phosphorylating RhoGAP to inhibit Rho-mediated suppression of p27 expression. Indeed, ablation of Fyn resulted in increased Rho activity in CG4 cells. Although we did not observe impaired phosphorylation of p190RhoGAP in PTPα-null OPCs (21), PTPα-activated Fyn might phosphorylate the brain-specific p200/p250RhoGAP (63, 64). Additional evidence implicating PTPα and Fyn as regulators of Rho-dependent p27 expression is provided by our demonstration that the Rho inhibitor G04 counteracts the suppression of p27 expression that occurs upon silencing PTPα. Together these results lead us to propose that PTPα activates Fyn to down-regulate Rho and enhance p27 expression, thus promoting cell cycle exit and limiting OPC proliferation. Another non-exclusive mechanism by which PTPα-Fyn signaling may regulate p27 involves the RNA-binding protein QKI. Phosphorylation of QKI by Fyn alters QKI binding with mRNA (65). QKI-6 and -7 induce G0/G1 cell cycle arrest in OPCs and upregulate p27 expression by stabilizing p27 mRNA (51). Therefore, Fyn may phosphorylate QKI to promote QKI-mediated protection of p27 mRNA. In this scenario PTPα is also envisaged to function as an activator of Fyn.
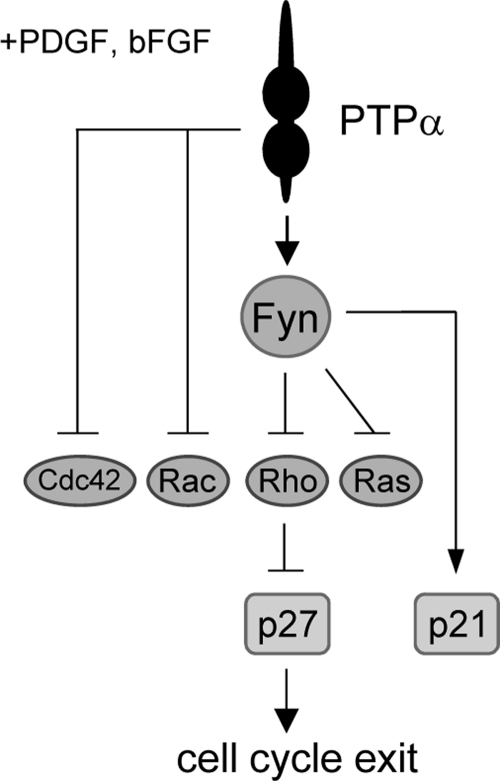
In summary, our study demonstrates an important role of PTPα in negatively regulating growth factor-mediated proliferation, cell cycle entry and survival, and thereby self-renewal, in OPCs. PTPα is required for Fyn activation in proliferating OPCs and CG4 cells, and we have identified Ras and the Rho GTPases Rac1, Cdc42 and Rho (and possibly Akt, ERK, and JNK) as PTPα negative regulatory targets in proliferating OPCs and/or CG4 cells. Furthermore, we propose that PTPα inhibits these processes at least partially by acting through Fyn to negatively regulate growth factor-mediated activation of Ras and Rho, leading to accumulation of p27 (schematically summarized in Fig. 10). Therefore, loss of PTPα leads to enhanced self-renewal and impaired differentiation of OPCs, thus affecting myelination. In these ways, PTPα could also affect remyelination in oligodendrocyte-related diseases or development of oligodendrogliomas.
FIGURE 10.
Schematic diagram of PTPα-dependent signaling events in proliferating OPCs. In PDGF/bFGF-stimulated OPC proliferation, PTPα activates Fyn to reduce the activities of Ras and Rho, and PTPα acts through unknown mechanisms to inhibit Rac1 and Cdc42 activities. PTPα-Fyn signaling through unknown intermediates up-regulates the expression of p21. The PTPα-Fyn-mediated suppression of Rho activity up-regulates the expression of p27, and this is likely to play a role in promoting cell cycle exit and limiting proliferation.
Acknowledgments
We thank Yue Feng, Emory University School of Medicine, for CG4 cells, and Suzanne Cheng for assistance with data quantification.
This work was supported by Grant MOP-62759 from the Canadian Institutes of Health Research (to C. J. P.). P.-S. Wang was supported by a studentship from the Multiple Sclerosis Society of Canada. C. J. Pallen is the recipient of an investigator award from the Child and Family Research Institute.
X. Shang, F. Marchioni, N. Sipes, C. R. Evelyn, M. Jerabek-Willemsen, S. Duhr, W. Seibel, and Y. Zheng, submitted manuscript.
- OL
- oligodendrocyte
- bFGF
- basic fibroblast growth factor
- EGF
- epidermal growth factor
- FAK
- focal adhesion kinase
- GAP
- GTPase-activating protein
- GST
- glutathione S-transferase
- OPC
- oligodendrocyte progenitor cell
- PBD
- PAK-binding domain
- PDGF
- platelet-derived growth factor
- PDGFR
- platelet-derived growth factor receptor
- PDLO
- poly-dl-ornithine
- PTP
- protein-tyrosine phosphatase
- RBD
- Ras-binding domain
- RhBD
- Rhotekin-binding domain
- SFK
- src family kinase
- siRNA
- small interfering RNA
- WCL
- whole cell lysate.
REFERENCES
- 1. Baron W., Metz B., Bansal R., Hoekstra D., de Vries H. (2000) PDGF and FGF-2 signaling in oligodendrocyte progenitor cells: regulation of proliferation and differentiation by multiple intracellular signaling pathways. Mol. Cell Neurosci. 15, 314–329 [DOI] [PubMed] [Google Scholar]
- 2. Barres B. A., Hart I. K., Coles H. S., Burne J. F., Voyvodic J. T., Richardson W. D., Raff M. C. (1992) Cell death and control of cell survival in the oligodendrocyte lineage. Cell 70, 31–46 [DOI] [PubMed] [Google Scholar]
- 3. Bögler O., Wren D., Barnett S. C., Land H., Noble M. (1990) Cooperation between two growth factors promotes extended self-renewal and inhibits differentiation of oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. Proc. Natl. Acad. Sci. U.S.A. 87, 6368–6372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yasuda T., Grinspan J., Stern J., Franceschini B., Bannerman P., Pleasure D. (1995) Apoptosis occurs in oligodendroglial lineage, and is prevented by basic fibroblast growth factor. J. Neurosci. Res. 40, 306–317 [DOI] [PubMed] [Google Scholar]
- 5. Raff M. C., Lillien L. E., Richardson W. D., Burne J. F., Noble M. D. (1988) Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature 333, 562–565 [DOI] [PubMed] [Google Scholar]
- 6. Noble M., Murray K., Stroobant P., Waterfield M. D., Riddle P. (1988) Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature 333, 560–562 [DOI] [PubMed] [Google Scholar]
- 7. Bansal R., Pfeiffer S. E. (1994) Inhibition of protein and lipid sulfation in oligodendrocytes blocks biological responses to FGF-2 and retards cytoarchitectural maturation, but not developmental lineage progression. Dev. Biol. 162, 511–524 [DOI] [PubMed] [Google Scholar]
- 8. McKinnon R. D., Matsui T., Dubois-Dalcq M., Aaronson S. A. (1990) FGF modulates the PDGF-driven pathway of oligodendrocyte development. Neuron 5, 603–614 [DOI] [PubMed] [Google Scholar]
- 9. Hart I. K., Richardson W. D., Heldin C. H., Westermark B., Raff M. C. (1989) PDGF receptors on cells of the oligodendrocyte-type-2 astrocyte (O-2A) cell lineage. Development 105, 595–603 [DOI] [PubMed] [Google Scholar]
- 10. Pallen C. J. (2003) Protein tyrosine phosphatase α (PTPα): a Src family kinase activator and mediator of multiple biological effects. Curr. Top Med. Chem. 3, 821–835 [DOI] [PubMed] [Google Scholar]
- 11. Zheng X. M., Wang Y., Pallen C. J. (1992) Cell transformation and activation of pp60c-src by overexpression of a protein-tyrosine phosphatase. Nature 359, 336–339 [DOI] [PubMed] [Google Scholar]
- 12. den Hertog J., Pals C. E., Peppelenbosch M. P., Tertoolen L. G., de Laat S. W., Kruijer W. (1993) Receptor protein-tyrosine phosphatase α activates pp60c-src and is involved in neuronal differentiation. EMBO J. 12, 3789–3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ponniah S., Wang D. Z., Lim K. L., Pallen C. J. (1999) Targeted disruption of the tyrosine phosphatase PTPα leads to constitutive down-regulation of the kinases Src and Fyn. Curr. Biol. 9, 535–538 [DOI] [PubMed] [Google Scholar]
- 14. Su J., Muranjan M., Sap J. (1999) Receptor protein-tyrosine phosphatase α activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr. Biol. 9, 505–511 [DOI] [PubMed] [Google Scholar]
- 15. Bodrikov V., Leshchyns'ka I., Sytnyk V., Overvoorde J., den Hertog J., Schachner M. (2005) RPTPα is essential for NCAM-mediated p59fyn activation and neurite elongation. J. Cell Biol. 168, 127–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zeng L., D'Alessandri L., Kalousek M. B., Vaughan L., Pallen C. J. (1999) Protein-tyrosine phosphatase α (PTPα) and contactin form a novel neuronal receptor complex linked to the intracellular tyrosine kinase fyn. J. Cell Biol. 147, 707–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ye H., Tan Y. L., Ponniah S., Takeda Y., Wang S. Q., Schachner M., Watanabe K., Pallen C. J., Xiao Z. C. (2008) Neural recognition molecules CHL1 and NB-3 regulate apical dendrite orientation in the neocortex via PTPα. EMBO J. 27, 188–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Samayawardhena L. A., Pallen C. J. (2008) Protein-tyrosine phosphatase α regulates stem cell factor-dependent c-Kit activation and migration of mast cells. J. Biol. Chem. 283, 29175–29185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vacaresse N., Møller B., Danielsen E. M., Okada M., Sap J. (2008) Activation of c-Src and Fyn kinases by protein-tyrosine phosphatase RPTPα is substrate-specific and compatible with lipid raft localization. J. Biol. Chem. 283, 35815–35824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ardini E., Agresti R., Tagliabue E., Greco M., Aiello P., Yang L. T., Ménard S., Sap J. (2000) Expression of protein-tyrosine phosphatase α (RPTPα) in human breast cancer correlates with low tumor grade, and inhibits tumor cell growth in vitro and in vivo. Oncogene 19, 4979–4987 [DOI] [PubMed] [Google Scholar]
- 21. Wang P. S., Wang J., Xiao Z. C., Pallen C. J. (2009) Protein-tyrosine phosphatase alpha acts as an upstream regulator of Fyn signaling to promote oligodendrocyte differentiation and myelination. J. Biol. Chem. 284, 33692–33702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cabodi S., Calautti E., Talora C., Kuroki T., Stein P. L., Dotto G. P. (2000) A PKC-eta/Fyn-dependent pathway leading to keratinocyte growth arrest and differentiation. Mol. Cell 6, 1121–1129 [DOI] [PubMed] [Google Scholar]
- 23. Berwanger B., Hartmann O., Bergmann E., Bernard S., Nielsen D., Krause M., Kartal A., Flynn D., Wiedemeyer R., Schwab M., Schäfer H., Christiansen H., Eilers M. (2002) Loss of a FYN-regulated differentiation and growth arrest pathway in advanced stage neuroblastoma. Cancer Cell 2, 377–386 [DOI] [PubMed] [Google Scholar]
- 24. Colognato H., Ramachandrappa S., Olsen I. M., ffrench-Constant C. (2004) Integrins direct Src family kinases to regulate distinct phases of oligodendrocyte development. J. Cell Biol. 167, 365–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Osterhout D. J., Wolven A., Wolf R. M., Resh M. D., Chao M. V. (1999) Morphological differentiation of oligodendrocytes requires activation of Fyn tyrosine kinase. J. Cell Biol. 145, 1209–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perez M. J., Ortiz E. H., Roffé M., Soto E. F., Pasquini J. M. (2009) Fyn kinase is involved in oligodendroglial cell differentiation induced by apotransferrin. J. Neurosci. Res. 87, 3378–3389 [DOI] [PubMed] [Google Scholar]
- 27. Paez P. M., Garcia C. I., Soto E. F., Pasquini J. M. (2006) Apotransferrin decreases the response of oligodendrocyte progenitors to PDGF and inhibits the progression of the cell cycle. Neurochem. Int. 49, 359–371 [DOI] [PubMed] [Google Scholar]
- 28. Chen M., Chen S. C., Pallen C. J. (2006) Integrin-induced tyrosine phosphorylation of protein-tyrosine phosphatase-α is required for cytoskeletal reorganization and cell migration. J. Biol. Chem. 281, 11972–11980 [DOI] [PubMed] [Google Scholar]
- 29. Manser E., Leung T., Salihuddin H., Zhao Z. S., Lim L. (1994) A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367, 40–46 [DOI] [PubMed] [Google Scholar]
- 30. Singec I., Knoth R., Meyer R. P., Maciaczyk J., Volk B., Nikkhah G., Frotscher M., Snyder E. Y. (2006) Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nat. Methods 3, 801–806 [DOI] [PubMed] [Google Scholar]
- 31. Karnoub A. E., Weinberg R. A. (2008) Ras oncogenes: split personalities. Nat. Rev. Mol. Cell Biol. 9, 517–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bennett M. R., Rizvi T. A., Karyala S., McKinnon R. D., Ratner N. (2003) Aberrant growth and differentiation of oligodendrocyte progenitors in neurofibromatosis type 1 mutants. J. Neurosci. 23, 7207–7217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barnett S. C., Robertson L., Graham D., Allan D., Rampling R. (1998) Oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells transformed with c-myc and H-ras form high-grade glioma after stereotactic injection into the rat brain. Carcinogenesis 19, 1529–1537 [DOI] [PubMed] [Google Scholar]
- 34. Barnett S. C., Crouch D. H. (1995) The effect of oncogenes on the growth and differentiation of oligodendrocyte type 2 astrocyte progenitor cells. Cell Growth Differ. 6, 69–80 [PubMed] [Google Scholar]
- 35. Zhu Y., Parada L. F. (2002) The molecular and genetic basis of neurological tumours. Nat. Rev. Cancer 2, 616–626 [DOI] [PubMed] [Google Scholar]
- 36. Chew L. J., Coley W., Cheng Y., Gallo V. (2010) Mechanisms of regulation of oligodendrocyte development by p38 mitogen-activated protein kinase. J. Neurosci. 30, 11011–11027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Durand B., Fero M. L., Roberts J. M., Raff M. C. (1998) p27Kip1 alters the response of cells to mitogen and is part of a cell-intrinsic timer that arrests the cell cycle and initiates differentiation. Curr. Biol. 8, 431–440 [DOI] [PubMed] [Google Scholar]
- 38. Durand B., Gao F. B., Raff M. (1997) Accumulation of the cyclin-dependent kinase inhibitor p27/Kip1 and the timing of oligodendrocyte differentiation. EMBO J. 16, 306–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sa G., Stacey D. W. (2004) P27 expression is regulated by separate signaling pathways, downstream of Ras, in each cell cycle phase. Exp. Cell Res. 300, 427–439 [DOI] [PubMed] [Google Scholar]
- 40. Moeller S. J., Head E. D., Sheaff R. J. (2003) p27Kip1 inhibition of GRB2-SOS formation can regulate Ras activation. Mol. Cell Biol. 23, 3735–3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li X., Tang X., Jablonska B., Aguirre A., Gallo V., Luskin M. B. (2009) p27(KIP1) regulates neurogenesis in the rostral migratory stream and olfactory bulb of the postnatal mouse. J. Neurosci. 29, 2902–2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Casaccia-Bonnefil P., Tikoo R., Kiyokawa H., Friedrich V., Jr., Chao M. V., Koff A. (1997) Oligodendrocyte precursor differentiation is perturbed in the absence of the cyclin-dependent kinase inhibitor p27Kip1. Genes Dev. 11, 2335–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Friessen A. J., Miskimins W. K., Miskimins R. (1997) Cyclin-dependent kinase inhibitor p27kip1 is expressed at high levels in cells that express a myelinating phenotype. J. Neurosci. Res. 50, 373–382 [DOI] [PubMed] [Google Scholar]
- 44. Dyer M. A., Cepko C. L. (2001) p27Kip1 and p57Kip2 regulate proliferation in distinct retinal progenitor cell populations. J. Neurosci. 21, 4259–4271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Attali B., Wang N., Kolot A., Sobko A., Cherepanov V., Soliven B. (1997) Characterization of delayed rectifier Kv channels in oligodendrocytes and progenitor cells. J. Neurosci. 17, 8234–8245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Soliven B., Ma L., Bae H., Attali B., Sobko A., Iwase T. (2003) PDGF upregulates delayed rectifier via Src family kinases and sphingosine kinase in oligodendroglial progenitors. Am. J. Physiol. Cell Physiol. 284, C85–C93 [DOI] [PubMed] [Google Scholar]
- 47. Sperber B. R., McMorris F. A. (2001) Fyn tyrosine kinase regulates oligodendroglial cell development but is not required for morphological differentiation of oligodendrocytes. J. Neurosci. Res. 63, 303–312 [DOI] [PubMed] [Google Scholar]
- 48. Vidal A., Millard S. S., Miller J. P., Koff A. (2002) Rho activity can alter the translation of p27 mRNA and is important for RasV12-induced transformation in a manner dependent on p27 status. J. Biol. Chem. 277, 16433–16440 [DOI] [PubMed] [Google Scholar]
- 49. Zezula J., Casaccia-Bonnefil P., Ezhevsky S. A., Osterhout D. J., Levine J. M., Dowdy S. F., Chao M. V., Koff A. (2001) p21cip1 is required for the differentiation of oligodendrocytes independently of cell cycle withdrawal. EMBO Rep. 2, 27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Olson M. F., Paterson H. F., Marshall C. J. (1998) Signals from Ras and Rho GTPases interact to regulate expression of p21Waf1/Cip1. Nature 394, 295–299 [DOI] [PubMed] [Google Scholar]
- 51. Larocque D., Galarneau A., Liu H. N., Scott M., Almazan G., Richard S. (2005) Protection of p27(Kip1) mRNA by quaking RNA-binding proteins promotes oligodendrocyte differentiation. Nat. Neurosci. 8, 27–33 [DOI] [PubMed] [Google Scholar]
- 52. Hecker T. P., Ding Q., Rege T. A., Hanks S. K., Gladson C. L. (2004) Overexpression of FAK promotes Ras activity through the formation of a FAK/p120RasGAP complex in malignant astrocytoma cells. Oncogene 23, 3962–3971 [DOI] [PubMed] [Google Scholar]
- 53. Smida M., Posevitz-Fejfar A., Horejsi V., Schraven B., Lindquist J. A. (2007) A novel negative regulatory function of the phosphoprotein associated with glycosphingolipid-enriched microdomains: blocking Ras activation. Blood 110, 596–615 [DOI] [PubMed] [Google Scholar]
- 54. Lee J. S., Padmanabhan A., Shin J., Zhu S., Guo F., Kanki J. P., Epstein J. A., Look A. T. (2010) Oligodendrocyte progenitor cell numbers and migration are regulated by the zebra fish orthologs of the NF1 tumor suppressor gene. Hum. Mol. Genet. 19, 4643–4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thurnherr T., Benninger Y., Wu X., Chrostek A., Krause S. M., Nave K. A., Franklin R. J., Brakebusch C., Suter U., Relvas J. B. (2006) Cdc42 and Rac1 signaling are both required for and act synergistically in the correct formation of myelin sheaths in the CNS. J. Neurosci. 26, 10110–10119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wolf R. M., Draghi N., Liang X., Dai C., Uhrbom L., Eklöf C., Westermark B., Holland E. C., Resh M. D. (2003) p190RhoGAP can act to inhibit PDGF-induced gliomas in mice: a putative tumor suppressor encoded on human chromosome 19q13.3. Genes Dev. 17, 476–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Casaccia-Bonnefil P., Hardy R. J., Teng K. K., Levine J. M., Koff A., Chao M. V. (1999) Loss of p27Kip1 function results in increased proliferative capacity of oligodendrocyte progenitors but unaltered timing of differentiation. Development 126, 4027–4037 [DOI] [PubMed] [Google Scholar]
- 58. Tikoo R., Osterhout D. J., Casaccia-Bonnefil P., Seth P., Koff A., Chao M. V. (1998) Ectopic expression of p27Kip1 in oligodendrocyte progenitor cells results in cell cycle growth arrest. J. Neurobiol. 36, 431–440 [PubMed] [Google Scholar]
- 59. Tang X. M., Beesley J. S., Grinspan J. B., Seth P., Kamholz J., Cambi F. (1999) Cell cycle arrest induced by ectopic expression of p27 is not sufficient to promote oligodendrocyte differentiation. J. Cell Biochem. 76, 270–279 [DOI] [PubMed] [Google Scholar]
- 60. Tokumoto Y. M., Apperly J. A., Gao F. B., Raff M. C. (2002) Post-transcriptional regulation of p18 and p27 Cdk inhibitor proteins and the timing of oligodendrocyte differentiation. Dev. Biol. 245, 224–234 [DOI] [PubMed] [Google Scholar]
- 61. Bansal R., Marin-Husstege M., Bryant M., Casaccia-Bonnefil P. (2005) S-phase entry of oligodendrocyte lineage cells is associated with increased levels of p21Cip1. J. Neurosci. Res. 80, 360–368 [DOI] [PubMed] [Google Scholar]
- 62. Hu W., Bellone C. J., Baldassare J. J. (1999) RhoA stimulates p27(Kip) degradation through its regulation of cyclin E/CDK2 activity. J. Biol. Chem. 274, 3396–3401 [DOI] [PubMed] [Google Scholar]
- 63. Moon S. Y., Zang H., Zheng Y. (2003) Characterization of a brain-specific Rho GTPase-activating protein, p200RhoGAP. J. Biol. Chem. 278, 4151–4159 [DOI] [PubMed] [Google Scholar]
- 64. Taniguchi S., Liu H., Nakazawa T., Yokoyama K., Tezuka T., Yamamoto T. (2003) p250GAP, a neural RhoGAP protein, is associated with and phosphorylated by Fyn. Biochem. Biophys. Res. Commun. 306, 151–155 [DOI] [PubMed] [Google Scholar]
- 65. Lu Z., Ku L., Chen Y., Feng Y. (2005) Developmental abnormalities of myelin basic protein expression in fyn knock-out brain reveal a role of Fyn in post-transcriptional regulation. J. Biol. Chem. 280, 389–395 [DOI] [PubMed] [Google Scholar]