Abstract
Hypothyroidism is the most common thyroid disorder, affecting about 5% of the general population. Here we present the current largest genome-wide association study of hypothyroidism, in 3,736 cases and 35,546 controls. Hypothyroidism was assessed via web-based questionnaires. We identify five genome-wide significant associations, three of which are well known to be involved in a large spectrum of autoimmune diseases: rs6679677 near PTPN22, rs3184504 in SH2B3, and rs2517532 in the HLA class I region ( -values
-values  ,
,  , and
, and 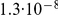 , respectively). We also report associations with rs4915077 near VAV3 (
, respectively). We also report associations with rs4915077 near VAV3 ( -value
-value 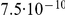 ) and rs925489 near FOXE1 (
) and rs925489 near FOXE1 (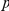 -value
-value 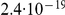 ). VAV3 is involved in immune function, and FOXE1 and PTPN22 have previously been associated with hypothyroidism. Although the HLA class I region and SH2B3 have previously been linked with a number of autoimmune diseases, this is the first report of their association with thyroid disease. The VAV3 association is also novel. We also show suggestive evidence of association for hypothyroidism with a SNP in the HLA class II region (independent of the other HLA association) as well as SNPs in CAPZB, PDE8B, and CTLA4. CAPZB and PDE8B have been linked to TSH levels and CTLA4 to a variety of autoimmune diseases. These results suggest heterogeneity in the genetic etiology of hypothyroidism, implicating genes involved in both autoimmune disorders and thyroid function. Using a genetic risk profile score based on the top association from each of the five genome-wide significant regions in our study, the relative risk between the highest and lowest deciles of genetic risk is 2.0.
). VAV3 is involved in immune function, and FOXE1 and PTPN22 have previously been associated with hypothyroidism. Although the HLA class I region and SH2B3 have previously been linked with a number of autoimmune diseases, this is the first report of their association with thyroid disease. The VAV3 association is also novel. We also show suggestive evidence of association for hypothyroidism with a SNP in the HLA class II region (independent of the other HLA association) as well as SNPs in CAPZB, PDE8B, and CTLA4. CAPZB and PDE8B have been linked to TSH levels and CTLA4 to a variety of autoimmune diseases. These results suggest heterogeneity in the genetic etiology of hypothyroidism, implicating genes involved in both autoimmune disorders and thyroid function. Using a genetic risk profile score based on the top association from each of the five genome-wide significant regions in our study, the relative risk between the highest and lowest deciles of genetic risk is 2.0.
Introduction
Hypothyroidism is characterized by deficiencies of thyroid hormones T3 (triiodothyronine) and T4 (thyroxine). Thyroid hormones are primarily responsible for the regulation of metabolism, but also play a major role in development. Hypothyroidism is typically marked by high thyroid-stimulating hormone (TSH) levels (usually indicative of impaired thyroid function). However, this is not always the case. For example, reduced T3/T4 levels may be caused by insufficient generation of TSH by the pituitary gland rather than thyroid dysfunction. While iodine deficiency is the most common cause of hypothyroidism worldwide, and there are rare forms of congenital hypothyroidism with a number of genetic causes [1], most cases in the developed world are due to autoimmune hypothyroidism (e.g., Hashimoto or Ord thyroiditis). Over the last five years, hundreds of genetic variants have been found that predispose to various autoimmune diseases [2], with many shared across multiple autoimmune diseases [3].
There has been only one published genome-wide association study (GWAS) of hypothyroidism, carried out in a sample of 1317 hypothyroidism cases and 5053 controls determined algorithmically from five electronic medical record databases. They found one association, near FOXE1 (forkhead box E1), also known as TTF-2 (thyroid transcription factor 2) [4]. Candidate gene studies have also suggested links between autoimmune hypothyroidism and PTPN22 (protein tyrosine phosphatase, non-receptor type 22 (lymphoid)), as well as the HLA (human leukocyte antigen) class II region, CTLA4 (cytotoxic T lymphocyte antigen 4), and 8q23-24 [5]–[13]. Graves' disease (another autoimmune thyroid disease, characterized by hyperthyroidism) has been studied in several GWAS, with many loci discovered [14]–[17].
In addition to hypothyroidism, FOXE1 has previously been associated with thyroid cancer and TSH levels [18]. A second SNP near this gene (rs755109) has also been associated with TSH levels in an isolated Pacific Island population [19]. FOXE1 is also involved in thyroid development and coding mutations cause congential hypothyroidism [20]. As untreated individuals with hypothyroidism typically have high TSH levels, it is possible that a GWAS of TSH levels could detect loci involved in hypothyroidism. These facts raise the question of whether other variants associated with thyroid function also influence hypothyroidism risk.
The genetic determinants of TSH levels are partially understood, with several established associations from GWAS, including PDE8B (phosphodiesterase 8B) [19], [21] CAPZB (capping protein (actin filament) muscle Z-line, beta) [22], and NKX2-1 (NK2 homeobox 1), also known as TTF-1 (thyroid transcription factor 1) [18]. CAPZB has also been associated with thyroid volume [23]. These genes have strong links to thyroid function: in addition to the thyroid transcription factors, PDE8B is primarily expressed in the thyroid [24] and encodes a phosphodiesterase with a high affinity for cAMP, which mediates TSH effects in the thyroid [25].
In this paper, we report on the largest GWAS to date of hypothyroidism. We find five variants significantly associated with hypothyroidism. Two are non-synonymous variants in genes associated with many autoimmune diseases (PTPN22 R620W and SH2B3 (SH2B adaptor protein 3) R262W) and a third is in the HLA class I region. A fourth is found in an intron of VAV3 (vav 3 guanine nucleotide exchange factor), a gene plausibly involved in immune function. The final variant is located upstream of the thyroid transcription factor FOXE1. In addition, among the SNPs marginally associated with hypothyroidism, we observe associations with two genes that have been linked to TSH levels: PDE8B and CAPZB. We also replicate a previously reported association of CTLA4 with hypothyroidism [13].
Results
We performed a GWAS in 3,736 cases and 35,546 controls from the customer base of 23andMe, Inc., a personal genetics company. All participants were of primarily European ancestry and were at most distantly related to each other. Figure S1 shows further details about the population structure. Hypothyroidism was assessed using online self-report. Briefly, participants responded to questions about their hypothyroidism diagnosis and related thyroid issues using web-based surveys. We classified as cases individuals who had been diagnosed with hypothyroidism, had elevated TSH levels, or were taking thyroid hormone replacement medication. Controls reported no to at least one of the above questions and yes to none of them. Participants reporting hyperthyroidism, thyroid cancer, thyroid removal, or treatment with radioactive iodine for hyperthyroidism were excluded from both the cases and controls. Details about the cohort can be found in Table 1 and the Methods. All analyses were controlled for age, sex, and five principal components. In addition, a conditional analysis was performed, adding the five genome-wide significant SNPs as covariates. Manhattan and quantile-quantile plots for both analyses are provided in Figures S2 and S3.
Table 1. Cohort statistics.
| Number | Male | Female |
|
46–55 | 56–65 |

|
V1 | V2 | V3 | |
| Control | 35546 | 22446 | 13100 | 18941 | 5528 | 6142 | 4935 | 271 | 15699 | 19572 |
| Case | 3736 | 953 | 2783 | 979 | 640 | 1120 | 997 | 29 | 1694 | 2013 |
Participants broken down by sex, age, and genotyping platform. V1, V2, and V3 refer to the three platforms used in this study, see Methods.
Table 2 shows lead SNPs from loci with 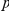 -values under
-values under 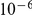 for hypothyroidism. Under our threshold for genome-wide significance of
for hypothyroidism. Under our threshold for genome-wide significance of  , five regions are significant (FOXE1, PTPN22, SH2B3, VAV3, and the HLA region. The strongest association is with rs925489, with a
, five regions are significant (FOXE1, PTPN22, SH2B3, VAV3, and the HLA region. The strongest association is with rs925489, with a  -value of
-value of  and odds ratio (OR) of
and odds ratio (OR) of 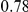 , near FOXE1 (Figure S4). This variant has been associated with hypothyroidism [4]. It is in linkage disequilibrium (LD) with rs965513, which has been associated with thyroid cancer and TSH levels [18] and is in weak LD (
, near FOXE1 (Figure S4). This variant has been associated with hypothyroidism [4]. It is in linkage disequilibrium (LD) with rs965513, which has been associated with thyroid cancer and TSH levels [18] and is in weak LD (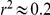 ) with rs1867277, which has been associated with thyroid cancer and shown to affect FOXE1 transcription [26]. Homozygous loss-of-function mutations in this gene cause congenital hypothyroidism due to thyroid dysgenesis and other developmental abnormalities [20].
) with rs1867277, which has been associated with thyroid cancer and shown to affect FOXE1 transcription [26]. Homozygous loss-of-function mutations in this gene cause congenital hypothyroidism due to thyroid dysgenesis and other developmental abnormalities [20].
Table 2. SNPs associated with hypothyroidism at 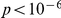 .
.
| SNP | Chr. | Pos. | Region | Alleles | MAF | HWE |

|
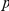 -value -value |
OR |
| rs925489 | 9 | 99586421 | FOXE1 | T/C | 0.332 | 0.69 | 38947 |
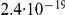
|
0.78 (0.74–0.82) |
| rs6679677 | 1 | 114105331 | PTPN22 | C/A | 0.091 | 0.34 | 38959 |

|
1.36 (1.26–1.48) |
| rs2476601 | 1 | 114179091 | PTPN22 | G/A | 0.092 | 0.26 | 39256 |

|
1.36 (1.25–1.47) |
| rs3184504 | 12 | 110368991 | SH2B3 | T/C | 0.497 | 0.49 | 39245 |
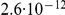
|
0.84 (0.79–0.88) |
| rs4915077 | 1 | 108167539 | VAV3 | T/C | 0.084 | 0.76 | 39248 |

|
1.30 (1.20–1.42) |
| rs2517532 | 6 | 31126386 | HLA | G/A | 0.403 | 0.094 | 39225 |
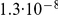
|
0.86 (0.82–0.91) |
| rs2516049 | 6 | 32678378 | HLA | T/C | 0.307 | 0.13 | 39241 |

|
1.15 (1.09–1.21) |
All genomic positions are given with respect to NCBI build 36.3. Alleles are listed as major/minor and are specified for the forward strand.  refers to the number of people successfully genotyped for each SNP. Odds ratios are per copy of the minor allele. One SNP is listed per region of the genome with the exception of HLA, which shows evidence of two independent signals, and PTPN22, for which we have included rs2476601, the non-synonymous change R620W.
refers to the number of people successfully genotyped for each SNP. Odds ratios are per copy of the minor allele. One SNP is listed per region of the genome with the exception of HLA, which shows evidence of two independent signals, and PTPN22, for which we have included rs2476601, the non-synonymous change R620W.
The second association is with rs6679677, with a 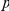 -value of
-value of  and OR of
and OR of  near PTPN22 (Figure S5). The SNP rs6679677 is in LD (
near PTPN22 (Figure S5). The SNP rs6679677 is in LD ( ) with rs2476601 (the missense mutation R620W in PTPN22). This mutation has previously been associated with Hashimoto thyroiditis in a relatively small candidate gene study [5]. PTPN22 also has well-established associations with multiple autoimmune conditions [27], including type 1 diabetes, rheumatoid arthritis, systemic lupus erythematosus, juvenile idiopathic arthritis [28], Graves' disease [29], [30], systemic sclerosis [31], and alopecia areata [32].
) with rs2476601 (the missense mutation R620W in PTPN22). This mutation has previously been associated with Hashimoto thyroiditis in a relatively small candidate gene study [5]. PTPN22 also has well-established associations with multiple autoimmune conditions [27], including type 1 diabetes, rheumatoid arthritis, systemic lupus erythematosus, juvenile idiopathic arthritis [28], Graves' disease [29], [30], systemic sclerosis [31], and alopecia areata [32].
Next, we see a novel association with rs3184504, a missense mutation (R262W) in SH2B3, with a 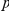 -value of
-value of  and an OR of
and an OR of 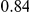 (Figure S6). This SNP has not previously been associated with thyroid disease; however, it has been associated with a number of autoimmune diseases, including type 1 diabetes [33], celiac disease [34], rheumatoid arthritis [35], and multiple sclerosis [36], as well as with hypertension and myocardial infarction [37]. The T allele of rs3184504 is the variant associated with increased risk in our data and corresponds to the W allele in the protein, which is also the risk variant for type 1 diabetes and rheumatoid arthritis and the protective variant for celiac and multiple sclerosis.
(Figure S6). This SNP has not previously been associated with thyroid disease; however, it has been associated with a number of autoimmune diseases, including type 1 diabetes [33], celiac disease [34], rheumatoid arthritis [35], and multiple sclerosis [36], as well as with hypertension and myocardial infarction [37]. The T allele of rs3184504 is the variant associated with increased risk in our data and corresponds to the W allele in the protein, which is also the risk variant for type 1 diabetes and rheumatoid arthritis and the protective variant for celiac and multiple sclerosis.
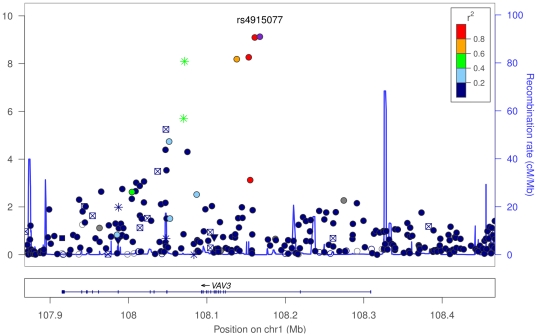
The next association, which is novel, is with rs4915077, in an intron of VAV3. This SNP has a 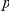 -value of
-value of  and OR of
and OR of 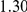 . See Figure 1 for SNPs in this region.
. See Figure 1 for SNPs in this region.
Figure 1. SNPs in the VAV3 region.
In the plot, circles represent unannotated SNPs, upside-down triangles represent non-synonymous variants, and boxes with an “x” are SNPs in regions that are highly conserved across 44 placental mammals. Colors depict the squared correlation (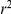 ) of each SNP with the most associated SNP (i.e., rs4915077, shown in purple). Gray indicates SNPs for which
) of each SNP with the most associated SNP (i.e., rs4915077, shown in purple). Gray indicates SNPs for which 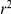 information was missing. Plots were produced using the LocusZoom program [50].
information was missing. Plots were produced using the LocusZoom program [50].
Finally, we found two associations between the HLA region and hypothyroidism (Table 2). The first, rs2517532, is genome-wide significant with a  -value of
-value of  and OR of 0.86. It lies in the HLA class I region, between HLA-E and HLA-C. The second association (rs2516049, about 2 mb away in the HLA class II region, near HLA-DRB1) shows only suggestive evidence of association (
and OR of 0.86. It lies in the HLA class I region, between HLA-E and HLA-C. The second association (rs2516049, about 2 mb away in the HLA class II region, near HLA-DRB1) shows only suggestive evidence of association ( ). However, a conditional genome-wide analysis (conditioning on the five significant associations) reveals that this second HLA association is independent of the first (see Figure 2).
). However, a conditional genome-wide analysis (conditioning on the five significant associations) reveals that this second HLA association is independent of the first (see Figure 2).
Figure 2. SNPs in the HLA region.
(A) shows statistics for the main GWAS, (B) shows statistics conditioned on 5 genome-wide significant SNPs (rs925489, rs6679677, rs3184504, rs4915077, rs2517532). For details, see Figure 1.
Using the five genome-wide significant SNPs, we constructed risk scores for all participants. In the top decile of risk, there were 555 cases and 3373 controls versus 277 cases and 3650 controls in the bottom decile, giving a relative risk of approximately 2.0.
Table 3 shows three associations for hypothyroidism with  -values under
-values under 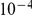 that are relevant for other conditions. Near CAPZB (Figure S7), rs1472565 is in moderate LD (
that are relevant for other conditions. Near CAPZB (Figure S7), rs1472565 is in moderate LD (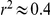 ) with rs12091047 (associated with thyroid volume [23]) but not in LD with rs10917469 (associated with TSH levels [22]). The SNP we observe near PDE8B, rs4704397 (Figure S8), has also been associated with TSH levels [21]. Finally, rs231779 near CTLA4 (Figure S9) has been associated with many other autoimmune diseases as well as with autoimmune hypothyroidism in candidate gene studies [3], [8]. All SNPs with
) with rs12091047 (associated with thyroid volume [23]) but not in LD with rs10917469 (associated with TSH levels [22]). The SNP we observe near PDE8B, rs4704397 (Figure S8), has also been associated with TSH levels [21]. Finally, rs231779 near CTLA4 (Figure S9) has been associated with many other autoimmune diseases as well as with autoimmune hypothyroidism in candidate gene studies [3], [8]. All SNPs with 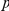 -values under
-values under 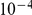 are shown in Table S1.
are shown in Table S1.
Table 3. Selected SNPs suggestively associated with hypothyroidism.
| SNP | Chr. | Pos. | Region | Alleles | MAF | HWE |
|
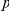 -value -value |
OR |
| rs1472565 | 1 | 19,627,617 | CAPZB | T/C | 0.478 | 0.39 | 39249 |
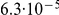
|
1.107 (1.05–1.16) |
| rs4704397 | 5 | 76,554,198 | PDE8B | G/A | 0.390 | 0.98 | 21622 |

|
1.179 (1.10–1.26) |
| rs231779 | 2 | 204,442,732 | CTLA4 | C/T | 0.366 | 0.81 | 39254 |

|
1.126 (1.07–1.19) |
Three SNPs with connections to other conditions that are suggestively associated with hypothyroidism. CAPZB and PDE8B have been associated with TSH levels and CTLA4 with a variety of autoimmune diseases, including autoimmune hypothyroidism. See Table 2 for nomenclature, Table S1 for all SNPs with  and Table S2 for 107 SNPs involved in autoimmune disease.
and Table S2 for 107 SNPs involved in autoimmune disease.
To search for further SNPs shared with other autoimmune diseases, we investigated a list of 107 SNPs that were studied across 7 autoimmune diseases [3]. Among this list, only the CTLA4, PTPN22, and SH2B3 loci show significant association with hypothyroidism after correction for 107 multiple tests. See Table S2 for details for all 107 SNPs.
Discussion
We have found five genome-wide significant associations for hypothryoidism (near FOXE1, PTPN22, SH2B3, VAV3, and the HLA class I region) as well as four others with suggestive evidence of biological interest (near CAPZB, PDE8B, and CTLA4 as well as in the HLA class II region). Of these, six seem to be involved in immune function (PTPN22, SH2B3, VAV3, CTLA4, and the two in HLA) and three in thyroid function (FOXE1, CAPZB, and PDE8B); see Figure 3. This suggests that while autoimmune loci may play a predominant role in genetic predisposition for hypothyroidism, there may be smaller effects due to genes more directly related to thyroid function and hormone levels.
Figure 3. Summary of results.
Regions associated with hypothyroidism classified by signficance level (genome-wide or suggestive), known function (thyroid versus autoimmune), and whether they had previously been associated with hypothyroidism.
Three of these associations are novel: the non-synonymous change R262W in SH2B3, the SNP rs4915077 near VAV3, and rs2517532 in the HLA class I region. The SH2B3 and HLA associations reinforce the connection between hypothyroidism and autoimmune genetics. VAV3 is a guanine nucleotide exchange factor for Rho guanosine triphosphatases. It has not yet been associated with autoimmune disease or thyroid function. However, it has been proposed as the candidate gene in the Idd18.1 region linked with type 1 diabetes in mouse [38], and the Vav1/Vav2/Vav3 family is necessary for adaptive immune function in mouse [39]. VAV3 is also expressed in the thyroid [40] and is down-regulated in a subset of thyroid tumors [41]. The second, suggestive association in the HLA class II region, rs2516049, lies near HLA-DRB1. Non-synonymous variants in HLA-DRB1 have been associated with autoimmune thyroiditis in humans and in mice [6].
We have also replicated several previously discovered associations. FOXE1 has recently been associated with hypothyroidism (as well as several other thyroid conditions) in a GWAS with 1,317 hypothyroidism cases determined from medical records [4]. Their strongest association was at rs7850258, with a reported OR of 0.74 (95% confidence interval of 0.67–0.82). For this SNP, we see a very similar OR of 0.78 (0.74–0.82). We have found one more association with a variant well known to be involved in autoimmune disease: R620W in PTPN22. This association was also seen in Denny et al. [4] ( -value of
-value of 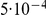 , OR of 1.29) as well as in a small candidate gene study of Hashimoto thyroiditis [5] (OR of 1.77 (1.31–2.40) in a sample of 194 cases and 2064 controls). We observe an OR for this SNP of 1.36 (1.25–1.47), which is similar to previous studies, despite slightly different phenotypes.
, OR of 1.29) as well as in a small candidate gene study of Hashimoto thyroiditis [5] (OR of 1.77 (1.31–2.40) in a sample of 194 cases and 2064 controls). We observe an OR for this SNP of 1.36 (1.25–1.47), which is similar to previous studies, despite slightly different phenotypes.
While many autoimmune diseases share genetic risk factors [42], there is evidence that these diseases form separate clusters based on genetics [3], [43]. For example, the 620W allele of PTPN22 has a protective effect in Crohn's disease [44] but is the risk allele for type 1 diabetes and others [45] (and hypothyroidism as reported here). SH2B3 also shows opposite directions of effect in multiple sclerosis and celiac compared to rheumatoid arthritis, psoriasis, type 1 diabetes, and hypothyroidism [3]. Thus our results begin to place hypothyroidism on the spectrum of autoimmune disease.
Our web-based design leads to a number of interesting trade-offs. On the one hand, the ease of online phenotype collection allows us to cheaply amass a very large study population (e.g., 3,736 cases in our study, compared to 1,317 cases in the only previous GWAS of hypothyroidism [4]). On the other hand, due to the nature of online self-reported data, we did not gather clinical measures such as TSH levels or hypothyroidism symptoms. The consequences of potential case misclassification arising from the unavailability of clinical data may manifest in two different ways. If some of our reported cases were misclassified at random (i.e., if a fraction 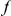 of the cases were actually controls who randomly reported having hypothyroidism), then the impact on our study would be a decrease in power as a consequence of a downward bias in the odds ratios observed in our study population, with no increase in false positive rate. In our study, however, the compensatory gain in power from having a larger sample more than makes up for the reduction in power due to random misclassification. For instance, conservatively assuming an
of the cases were actually controls who randomly reported having hypothyroidism), then the impact on our study would be a decrease in power as a consequence of a downward bias in the odds ratios observed in our study population, with no increase in false positive rate. In our study, however, the compensatory gain in power from having a larger sample more than makes up for the reduction in power due to random misclassification. For instance, conservatively assuming an 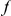 as high as 0.2, our power to detect the HLA class I association in Table 2 was 27%; reducing the sample size in half in order to obtain
as high as 0.2, our power to detect the HLA class I association in Table 2 was 27%; reducing the sample size in half in order to obtain  results in a power reduction to 15%. In practice, the close similarity of the odds ratios reported here with previous estimates suggest that random misclassification is unlikely to substantially affect our results.
results in a power reduction to 15%. In practice, the close similarity of the odds ratios reported here with previous estimates suggest that random misclassification is unlikely to substantially affect our results.
A separate concern is whether a substantial proportion of the cases in this study were not randomly misclassified controls but rather individuals who actually exhibited symptoms for a separate health condition (e.g., elevated TSH levels unrelated to hypothyroidism). Our study does not exclude this possibility. For example, about 7.5% of females and 2.8% of males are estimated to have elevated TSH levels and 2–3% of individuals are estimated to have overt symptoms of hypothyroidism [45]. These estimated rates of hypothyroidism are lower than the 9.5% prevalence of hypothyroidism (defined as either elevated TSH levels or hypothyroidism diagnosis) in our cohort, although the hypothyroidism is generally thought to be under-diagnosed (or possibly over-diagnosed in a health-conscious group of participants). As many of the SNPs discussed here show pleiotropic effects across autoimmune diseases or thyroid conditions, and as hypothyroidism is difficult to exactly define in a retrospective, web-based design, ultimately, the loci discovered here should be further replicated in a more deeply phenotyped hypothyroidism population, to determine the interplay between thyroid function genes and immune system genes that leads to this disorder.
Methods
Cohort
All participants in the study were customers of 23andMe, Inc., a personal genetics company, who had been genotyped as part of the 23andMe Personal Genome Service®. Individuals included in the cohort were selected for being of primarily European ancestry, as determined through an analysis of local ancestry via comparison to the three HapMap 2 populations, using an unpublished method substantially similar to Falush et al. [46]. All participants had over 97% of their genome estimated to be most similar to the HapMap CEU population. This subset was selected as it is the largest relatively unstructured set within the 23andMe customer base.
A maximal set of unrelated individuals was chosen for the analysis using a segmental identity-by-descent (IBD) estimation algorithm (as used in [47]). Individuals were defined as related if they shared more than 700 cM IBD, including both regions where the two individuals share either one or both genomic segments identical-by-descent. This level of relatedness (roughly 20% of the genome) corresponds approximately to the minimal expected sharing between first-cousins in an outbred population.
This study was conducted according to the principles expressed in the Declaration of Helsinki. The study protocol and informed consent form were approved by the external IRB, Ethical and Independent Review Services (E&I Review), which is accredited by the Association for the Accreditation of Human Research Protection Programs (AAHRPP). Informed consent was obtained from subjects online, in an IRB approved process. Our consent and privacy statement preclude the sharing of individual-level data without explicit consent. We have, however, shared summary statistics for all SNPs with  -values under
-values under 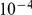 in Table S1.
in Table S1.
Genotyping
For the 23andMe cohort, DNA extraction and genotyping were performed on saliva samples by National Genetics Institute (NGI), a CLIA-certified clinical laboratory and subsidiary of Laboratory Corporation of America. Samples were genotyped on one of three different platforms. About half of the participants were genotyped on one of two platforms based on the Illumina HumanHap550+ BeadChip (called V1 and V2 in Table 1), which included SNPs from the standard HumanHap550 panel augmented with a custom set of approximately 25,000 SNPs selected by 23andMe. Two slightly different versions of this platform were used, as described in [47]. The remaining participants were genotyped on the Illumina OmniExpress+ Bead Chip. This platform has a base set of 730,000 SNPs. It was augmented by approximately 250,000 SNPs to make it approximately a superset of the HumanHap550+, as well as a custom set of about 30,000 SNPs. This platform was called V3 in Table 1. With the exception of the SNP rs4704397 near PDE8B (a V3-only SNP), all SNPs discussed here appeared at least on the V2 and V3 platforms that were used to genotype the vast majority of samples. Every sample that failed to reach a 98.5% call rate for SNPs on the standard platforms was re-analyzed. Individuals whose analyses failed repeatedly were re-contacted by 23andMe customer service to provide additional samples, as is done for all 23andMe customers. Our quality control procedures include genotyping the HapMap sample on our platforms and discarding discordant SNPs, manual examination of thousands of cluster plots, filtering based on Mendelian discordance in thousands of genotyped trios, filtering by Hardy-Weinberg, as well as independent validation of many probes. Quality control procedures for the genotyping are described in more detail in [47].
SNPs with a call rate under 95% or minor allele frequency under 0.01 were excluded from analysis. Call rates were calculated on a per-platform basis. Additionally, SNPs with Hardy-Weinberg  -values [48] less than than
-values [48] less than than 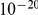 were excluded. Altogether, 870,065 SNPs (on the union of the two platforms) were retained with an average call rate of 99.78%.
were excluded. Altogether, 870,065 SNPs (on the union of the two platforms) were retained with an average call rate of 99.78%.
Phenotyping
Participants were able to fill out web-based questionnaires whenever they logged into their 23andMe accounts. Participants answered some of the following questions:
-
Have you ever been diagnosed by a doctor with any of the following thyroid conditions? (asked as part of a medical history questionnaire)
-
-
Hyperthyroidism
-
-
Hypothyroidism
-
-
-
Have you been diagnosed with any of the following? (asked as part of a questionnaire on baldness)
-
-
Hyperthyroidism (overactive thyroid)
-
-
Hypothyroidism (underactive thyroid)
-
-
Have you ever been diagnosed with hypothyroidism (underactive thyroid)?
Do you currently take medication for hypothyroidism (low thyroid hormone levels)?
Have you ever been told by a doctor that your thyroid stimulating hormone (TSH) levels were elevated, indicating hypothyroidism?
Have you ever been diagnosed with thyroid cancer?
Have you ever received radioactive iodine treatment for goiter or hyperthyroidism (overactive thyroid)?
Have you ever had all or part of your thyroid surgically removed?
-
Have you ever been diagnosed by a doctor with any of the following common cancers? (asked as part of a medical history questionnaire)
-
-
Thyroid cancer
-
-
Cases answered yes to hypothyroidism or to elevated TSH levels or to taking medication for hypothyroidism. Controls answered no to at least one of the qualifying questions and yes to none of them. Individuals reporting hyperthyroidism or thyroid cancer or treatment with radioactive iodine or thyroid removal were excluded, as all of these could cause hypothyroidism or could signal Graves' disease.
As customers of 23andMe, all participants had the opportunity to view reports based on their genetic information on over 100 traits and diseases. Among these diseases were reports on thyroid cancer (covering the FOXE1 and NKX2-1 SNPs from [18]) and Hashimoto thyroiditis (covering PTPN22). The context in which a question is asked can influence responses, as we have seen in a very different context in a previous paper [47]. While it is unlikely that a predicted high or low risk for thyroid cancer would lead to misreport of hypothyroidism, we sought to rule out any such bias in this case. Indeed, for PTPN22, there was no evidence of a difference in ORs for rs2476601 and hypothyroidism for people viewing their results before or after answering survey questions (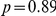 for interaction).
for interaction).
Statistical analysis
For the association analysis, all 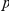 -values were calculated using a likelihood ratio test for the logistic regression model, adjusting for sex, age, and projections onto the first five principal components of the genotype data matrix. The principal components used were calculated within the subset of 23andMe customers with primarily European ancestry, as described previously [49]. Figure S1 shows the first two principal components with self-reported ancestry overlayed for a subset of participants. The inflation factor without any principal components included in the model was 1.032. After including five, this decreased slightly to 1.029. See Figure S3 for the quantile-quantile plot. The phenotypic status of each individual was coded as 0 for unaffected individuals and 1 for affected individuals. Genotypes were coded 0, 1, or 2, to indicate the number of minor alleles present for the tested SNP (corresponding to a log-additive model of association). For the conditional analysis, the 5 SNPs found to be genome-wide significant were added as predictors to the model. For SNPs appearing on a subset of genotyping platforms, analyses were restricted to individuals who were typed. Reported odds ratios for each SNP relative to the minor allele were defined as the exponential of the regression coefficients, and the alleles used throughout refer to the plus strand of NCBI build 36.3 of the human genome. We used a cutoff for genome-wide significance of
-values were calculated using a likelihood ratio test for the logistic regression model, adjusting for sex, age, and projections onto the first five principal components of the genotype data matrix. The principal components used were calculated within the subset of 23andMe customers with primarily European ancestry, as described previously [49]. Figure S1 shows the first two principal components with self-reported ancestry overlayed for a subset of participants. The inflation factor without any principal components included in the model was 1.032. After including five, this decreased slightly to 1.029. See Figure S3 for the quantile-quantile plot. The phenotypic status of each individual was coded as 0 for unaffected individuals and 1 for affected individuals. Genotypes were coded 0, 1, or 2, to indicate the number of minor alleles present for the tested SNP (corresponding to a log-additive model of association). For the conditional analysis, the 5 SNPs found to be genome-wide significant were added as predictors to the model. For SNPs appearing on a subset of genotyping platforms, analyses were restricted to individuals who were typed. Reported odds ratios for each SNP relative to the minor allele were defined as the exponential of the regression coefficients, and the alleles used throughout refer to the plus strand of NCBI build 36.3 of the human genome. We used a cutoff for genome-wide significance of  (corresponding to a Bonferroni correction assuming 1 million independent tests).
(corresponding to a Bonferroni correction assuming 1 million independent tests).
Supporting Information
Projections onto first two principal components for all participants in this study. The country codes are based on self-reported ancestry from a subset of 3363 participants. These participants reported four grandparents born in the given country (or in the case of “AJ”, four grandparents of Ashkenazi Jewish ancestry). The label for a country is placed at the median position of all participants reporting such ancestry, and the size is proportional to the number of such reports. Note that the label size is not proportional to the actual density of each subgroup in the study (the densities are approximately 85% northern European and 5% each Ashkenazi, eastern European, and southern European).
(TIFF)
Manhattan plots. (A) Negative log  -values for SNPs by genome position. Genome-wide significant SNPs are shown in red. (B) Same for conditional analysis adding 5 genome-wide signficiant SNPs (rs925489, rs6679677, rs3184504, rs4915077, rs2517532) as covariates.
-values for SNPs by genome position. Genome-wide significant SNPs are shown in red. (B) Same for conditional analysis adding 5 genome-wide signficiant SNPs (rs925489, rs6679677, rs3184504, rs4915077, rs2517532) as covariates.
(TIFF)
Quantile-quantile plot. Observed  -values versus theoretical
-values versus theoretical  -values under the null hypothesis of no association. The genomic control inflation factor for the study was
-values under the null hypothesis of no association. The genomic control inflation factor for the study was 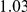 and is indicated by the red line. (A) Genome-wide analysis. (B) Conditional analysis with five SNPs included as covariates.
and is indicated by the red line. (A) Genome-wide analysis. (B) Conditional analysis with five SNPs included as covariates.
(TIFF)
SNPs in the
FOXE1
region. In the plot, circles represent unannotated SNPs, upside-down triangles represent non-synonymous variants, and boxes with an “x” are SNPs in regions that are highly conserved across 44 placental mammals. Colors depict the squared correlation (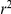 ) of each SNP with the most associated SNP (i.e., rs925489, shown in purple). Gray indicates SNPs for which
) of each SNP with the most associated SNP (i.e., rs925489, shown in purple). Gray indicates SNPs for which  information was missing. Plots were produced using the LocusZoom program.
information was missing. Plots were produced using the LocusZoom program.
(TIFF)
SNPs with
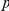 -values under
-values under
 . All genomic positions are given with respect to NCBI build 36.3. Alleles are listed as major/minor and are specified for the forward strand.
. All genomic positions are given with respect to NCBI build 36.3. Alleles are listed as major/minor and are specified for the forward strand.  refers to the number of people successfully genotyped for each SNP. Odds ratios are per copy of the minor allele.
refers to the number of people successfully genotyped for each SNP. Odds ratios are per copy of the minor allele.
(XLS)
SNPs identified in other autoimmune diseases. The 107 SNPs studied across 7 autoimmune diseases in Cotsapas et al., 2011. Under a threshold of 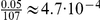 for significance, only PTPN22, SH2B3, and CTLA4 are significant. See Table S1 for details on columns.
for significance, only PTPN22, SH2B3, and CTLA4 are significant. See Table S1 for details on columns.
(XLS)
Acknowledgments
We thank the customers of 23andMe who answered surveys and participated in this research. Thanks to all the employees of 23andMe, who together have made this research possible, especially Kimberly Barnholt, Geoffrey Benton, Arnab Chowdry, Emily Drabant, Michael Macpherson, and Brian Naughton.
Footnotes
Competing Interests: The authors are employed by 23andMe and own stock options in the company. PLoS co-founder Michael B. Eisen is a member of the 23andMe Scientific Advisory Board. There are no patents related to this paper to declare. 23andMe may use the results of this paper to provide reports to customers regarding their genetic risk of hypothyroidism. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: There are no current external funding sources for this study.
References
- 1.Park SM, Chatterjee VK. Genetics of congenital hypothyroidism. J Med Genet. 2005;42:379–389. doi: 10.1136/jmg.2004.024158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho JH, Gregersen PK. Genomics and the multifactorial nature of human autoimmune disease. N Engl J Med. 2011;365:1612–1623. doi: 10.1056/NEJMra1100030. [DOI] [PubMed] [Google Scholar]
- 3.Cotsapas C, Voight BF, Rossin E, Lage K, Neale BM, et al. Pervasive sharing of genetic effects in autoimmune disease. PLoS Genet. 2011;7:e1002254. doi: 10.1371/journal.pgen.1002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denny JC, Crawford DC, Ritchie MD, Bielinski SJ, Basford MA, et al. Variants near FOXE1 are associated with hypothyroidism and other thyroid conditions: using electronic medical records for genome- and phenome-wide studies. Am J Hum Genet. 2011;89:529–542. doi: 10.1016/j.ajhg.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Criswell L, Pfeiffer K, Lum R, Gonzales B, Novitzke J, et al. Analysis of families in the multiple autoimmune disease genetics consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. Am J Hum Genet. 2005;76:561–71. doi: 10.1086/429096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menconi F, Monti MC, Greenberg DA, Oashi T, Osman R, et al. Molecular amino acid signatures in the MHC class II peptide-binding pocket predispose to autoimmune thyroiditis in humans and in mice. Proc Natl Acad Sci USA. 2008;105:14034–14039. doi: 10.1073/pnas.0806584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomer Y. Genetic susceptibility to autoimmune thyroid disease: past, present, and future. Thyroid. 2010;20:715–725. doi: 10.1089/thy.2010.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeitlin AA, Simmonds MJ, Gough SC. Genetic developments in autoimmune thyroid disease: an evolutionary process. Clin Endocrinol (Oxf) 2008;68:671–682. doi: 10.1111/j.1365-2265.2007.03075.x. [DOI] [PubMed] [Google Scholar]
- 9.Simmonds MJ, Gough SC. The search for the genetic contribution to autoimmune thyroid disease: the never ending story? Brief Funct Genomics. 2011;10:77–90. doi: 10.1093/bfgp/elq036. [DOI] [PubMed] [Google Scholar]
- 10.Donner H, Braun J, Seidl C, Rau H, Finke R, et al. Codon 17 polymorphism of the cytotoxic T lymphocyte antigen 4 gene in Hashimoto's thyroiditis and Addison's disease. J Clin Endocrinol Metab. 1997;82:4130–4132. doi: 10.1210/jcem.82.12.4406. [DOI] [PubMed] [Google Scholar]
- 11.Petrone A, Giorgi G, Mesturino CA, Capizzi M, Cascino I, et al. Association of DRB1*04-DQB1*0301 haplotype and lack of association of two polymorphic sites at CTLA-4 gene with Hashimoto's thyroiditis in an Italian population. Thyroid. 2001;11:171–175. doi: 10.1089/105072501300042901. [DOI] [PubMed] [Google Scholar]
- 12.Tandon N, Zhang L, Weetman AP. HLA associations with Hashimoto's thyroiditis. Clin Endocrinol (Oxf) 1991;34:383–386. doi: 10.1111/j.1365-2265.1991.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 13.Ikegami H, Awata T, Kawasaki E, Kobayashi T, Maruyama T, et al. The association of CTLA4 polymorphism with type 1 diabetes is concentrated in patients complicated with autoim- mune thyroid disease: a multicenter collaborative study in Japan. J Clin Endocrinol Metab. 2006;91:1087–1092. doi: 10.1210/jc.2005-1407. [DOI] [PubMed] [Google Scholar]
- 14.Chu X, Pan CM, Zhao SX, Liang J, Gao GQ, et al. A genome-wide association study identifies two new risk loci for Graves' disease. Nat Genet. 2011;43:897–901. doi: 10.1038/ng.898. [DOI] [PubMed] [Google Scholar]
- 15.Chen P, Fann C, Chu C, Chang C, Chang S, et al. Comprehensive genotyping in two homogeneous Graves' disease samples reveals major and novel HLA association alleles. PLoS One. 2011;6:e16635. doi: 10.1371/journal.pone.0016635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burton P, Clayton D, Cardon L, Craddock N, Deloukas P, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 2007;39:1329–37. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newby P, Pickles O, Mazumdar S, Brand O, Carr-Smith J, et al. Follow-up of potential novel Graves' disease susceptibility loci, identified in the UK WTCCC genome-wide nonsynonymous SNP study. Eur J Hum Genet. 2010;18:1021–6. doi: 10.1038/ejhg.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gudmundsson J, Sulem P, Gudbjartsson D, Jonasson J, Sigurdsson A, et al. Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. Nat Genet. 2009;41:460–4. doi: 10.1038/ng.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowe J, Maller J, Pe'er I, Neale B, Salit J, et al. Genome-wide association studies in an isolated founder population from the Pacific Island of Kosrae. PLoS Genet. 2009;5:e1000365. doi: 10.1371/journal.pgen.1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castanet M, Polak M. Spectrum of Human Foxe1/TTF2 Mutations. Horm Res Paediatr. 2010;73:423–429. doi: 10.1159/000281438. [DOI] [PubMed] [Google Scholar]
- 21.Arnaud-Lopez L, Usala G, Ceresini G, Mitchell B, Pilia M, et al. Phosphodiesterase 8B gene variants are associated with serum TSH levels and thyroid function. Am J Hum Genet. 2008;82:1270–80. doi: 10.1016/j.ajhg.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panicker V, Wilson SG, Walsh JP, Richards JB, Brown SJ, et al. A locus on chromosome 1p36 is associated with thyrotropin and thyroid function as identified by genome-wide association study. Am J Hum Genet. 2010;87:430–435. doi: 10.1016/j.ajhg.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teumer A, Rawal R, Homuth G, Ernst F, Heier M, et al. Genome-wide association study identifies four genetic loci associated with thyroid volume and goiter risk. Am J Hum Genet. 2011;88:664–673. doi: 10.1016/j.ajhg.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi M, Matsushima K, Ohashi H, Tsunoda H, Murase S, et al. Molecular cloning and characterization of human PDE8B, a novel thyroid-specific isozyme of 3′,5′-cyclic nucleotide phosphodiesterase. Biochem Biophys Res Commun. 1998;250:751–756. doi: 10.1006/bbrc.1998.9379. [DOI] [PubMed] [Google Scholar]
- 25.Bender A, Beavo J. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 26.Landa I, Ruiz-Llorente S, Montero-Conde C, Inglada-Prez L, Schiavi F, et al. The variant rs1867277 in FOXE1 gene confers thyroid cancer susceptibility through the recruitment of USF1/USF2 transcription factors. PLoS Genet. 2009;5:e1000637. doi: 10.1371/journal.pgen.1000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregersen P, Olsson L. Recent advances in the genetics of autoimmune disease. Annu Rev Immunol. 2009;27:363–91. doi: 10.1146/annurev.immunol.021908.132653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee YH, Rho YH, Choi SJ, Ji JD, Song GG, et al. The PTPN22 C1858T functional poly-morphism and autoimmune diseases–a meta-analysis. Rheumatology (Oxford) 2007;46:49–56. doi: 10.1093/rheumatology/kel170. [DOI] [PubMed] [Google Scholar]
- 29.Smyth D, Cooper JD, Collins JE, Heward JM, Franklyn JA, et al. Replication of an association between the lymphoid tyrosine phosphatase locus (LYP/PTPN22) with type 1 diabetes, and evidence for its role as a general autoimmunity locus. Diabetes. 2004;53:3020–3023. doi: 10.2337/diabetes.53.11.3020. [DOI] [PubMed] [Google Scholar]
- 30.Velaga M, Wilson V, Jennings C, Owen C, Herington S, et al. The codon 620 tryptophan allele of the lymphoid tyrosine phosphatase (LYP) gene is a major determinant of Graves' disease. J Clin Endocrinol Metab. 2004;89:5862–5. doi: 10.1210/jc.2004-1108. [DOI] [PubMed] [Google Scholar]
- 31.Gourh P, Tan F, Assassi S, Ahn C, McNearney T, et al. Association of the PTPN22 R620W polymorphism with anti-topoisomerase I- and anticentromere antibody-positive systemic sclerosis. Arthritis Rheum. 2006;54:3945–3953. doi: 10.1002/art.22196. [DOI] [PubMed] [Google Scholar]
- 32.Betz RC, Konig K, Flaquer A, Redler S, Eigelshoven S, et al. The R620W polymorphism in PTPN22 confers general susceptibility for the development of alopecia areata. Br J Dermatol. 2008;158:389–391. doi: 10.1111/j.1365-2133.2007.08312.x. [DOI] [PubMed] [Google Scholar]
- 33.Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39:857–864. doi: 10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhernakova A, Elbers C, Ferwerda B, Romanos J, Trynka G, et al. Evolutionary and functional analysis of celiac risk loci reveals SH2B3 as a protective factor against bacterial infection. Am J Hum Genet. 2010;86:970–7. doi: 10.1016/j.ajhg.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coenen MJ, Trynka G, Heskamp S, Franke B, van Diemen CC, et al. Common and different genetic background for rheumatoid arthritis and coeliac disease. Hum Mol Genet. 2009;18:4195–4203. doi: 10.1093/hmg/ddp365. [DOI] [PubMed] [Google Scholar]
- 36.Alcina A, Vandenbroeck K, Otaegui D, Saiz A, Gonzalez JR, et al. The autoimmune disease- associated KIF5A, CD226 and SH2B3 gene variants confer susceptibility for multiple sclerosis. Genes Immun. 2010;11:439–445. doi: 10.1038/gene.2010.30. [DOI] [PubMed] [Google Scholar]
- 37.Newton-Cheh C, Johnson T, Gateva V, Tobin M, Bochud M, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–676. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fraser H, Dendrou C, Healy B, Rainbow D, Howlett S, et al. Nonobese diabetic congenic strain analysis of autoimmune diabetes reveals genetic complexity of the Idd18 locus and identifies Vav3 as a candidate gene. J Immunol. 2010;184:5075–5084. doi: 10.4049/jimmunol.0903734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujikawa K, Miletic A, Alt F, Faccio R, Brown T, et al. Vav1/2/3-null mice define an essential role for Vav family proteins in lymphocyte development and activation but a differential requirement in MAPK signaling in T and B cells. J Exp Med. 2003;198:1595–1608. doi: 10.1084/jem.20030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Movilla N, Bustelo XR. Biological and regulatory properties of Vav-3, a new member of the Vav family of oncoproteins. Mol Cell Biol. 1999;19:7870–7885. doi: 10.1128/mcb.19.11.7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giordano TJ, Kuick R, Thomas DG, Misek DE, Vinco M, et al. Molecular classification of papillary thyroid carcinoma: distinct BRAF, RAS, and RET/PTC mutation-specific gene expression profiles discovered by DNA microarray analysis. Oncogene. 2005;24:6646–6656. doi: 10.1038/sj.onc.1208822. [DOI] [PubMed] [Google Scholar]
- 42.Lettre G, Rioux J. Autoimmune diseases: insights from genome-wide association studies. Hum Mol Genet. 2008;17:R116–121. doi: 10.1093/hmg/ddn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sirota M, Schaub M, Batzoglou S, Robinson W, Butte A. Autoimmune disease classification by inverse association with SNP alleles. PLoS Genet. 2009;5:e1000792. doi: 10.1371/journal.pgen.1000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet. 2008;40:955–962. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hakonarson H, Grant SF, Bradfield JP, Marchand L, Kim CE, et al. A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature. 2007;448:591–594. doi: 10.1038/nature06010. [DOI] [PubMed] [Google Scholar]
- 46.Falush D, Stephens M, Pritchard J. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–87. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eriksson N, Macpherson J, Tung J, Hon L, Naughton B, et al. Web-based, participant-driven studies yield novel genetic associations for common traits. PLoS Genet. 2010;6:e1000993. doi: 10.1371/journal.pgen.1000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet. 2005;76:887–893. doi: 10.1086/429864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Do CB, Tung JY, Dorfman E, Kiefer AK, Drabant EM, et al. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson's disease. PLoS Genet. 2011;7:e1002141. doi: 10.1371/journal.pgen.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Projections onto first two principal components for all participants in this study. The country codes are based on self-reported ancestry from a subset of 3363 participants. These participants reported four grandparents born in the given country (or in the case of “AJ”, four grandparents of Ashkenazi Jewish ancestry). The label for a country is placed at the median position of all participants reporting such ancestry, and the size is proportional to the number of such reports. Note that the label size is not proportional to the actual density of each subgroup in the study (the densities are approximately 85% northern European and 5% each Ashkenazi, eastern European, and southern European).
(TIFF)
Manhattan plots. (A) Negative log  -values for SNPs by genome position. Genome-wide significant SNPs are shown in red. (B) Same for conditional analysis adding 5 genome-wide signficiant SNPs (rs925489, rs6679677, rs3184504, rs4915077, rs2517532) as covariates.
-values for SNPs by genome position. Genome-wide significant SNPs are shown in red. (B) Same for conditional analysis adding 5 genome-wide signficiant SNPs (rs925489, rs6679677, rs3184504, rs4915077, rs2517532) as covariates.
(TIFF)
Quantile-quantile plot. Observed  -values versus theoretical
-values versus theoretical  -values under the null hypothesis of no association. The genomic control inflation factor for the study was
-values under the null hypothesis of no association. The genomic control inflation factor for the study was 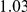 and is indicated by the red line. (A) Genome-wide analysis. (B) Conditional analysis with five SNPs included as covariates.
and is indicated by the red line. (A) Genome-wide analysis. (B) Conditional analysis with five SNPs included as covariates.
(TIFF)
SNPs in the
FOXE1
region. In the plot, circles represent unannotated SNPs, upside-down triangles represent non-synonymous variants, and boxes with an “x” are SNPs in regions that are highly conserved across 44 placental mammals. Colors depict the squared correlation (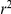 ) of each SNP with the most associated SNP (i.e., rs925489, shown in purple). Gray indicates SNPs for which
) of each SNP with the most associated SNP (i.e., rs925489, shown in purple). Gray indicates SNPs for which  information was missing. Plots were produced using the LocusZoom program.
information was missing. Plots were produced using the LocusZoom program.
(TIFF)
SNPs with
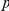 -values under
-values under
 . All genomic positions are given with respect to NCBI build 36.3. Alleles are listed as major/minor and are specified for the forward strand.
. All genomic positions are given with respect to NCBI build 36.3. Alleles are listed as major/minor and are specified for the forward strand.  refers to the number of people successfully genotyped for each SNP. Odds ratios are per copy of the minor allele.
refers to the number of people successfully genotyped for each SNP. Odds ratios are per copy of the minor allele.
(XLS)
SNPs identified in other autoimmune diseases. The 107 SNPs studied across 7 autoimmune diseases in Cotsapas et al., 2011. Under a threshold of 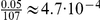 for significance, only PTPN22, SH2B3, and CTLA4 are significant. See Table S1 for details on columns.
for significance, only PTPN22, SH2B3, and CTLA4 are significant. See Table S1 for details on columns.
(XLS)