Abstract
Purpose
Benign epilepsy with centrotemporal spikes (BECTS), the most common childhood epilepsy syndrome, is a neurodevelopmental disorder with a genetic influence. In spite of its signature electroencephalogram pattern and distinct focal motor seizure semiology, little is known regarding the underlying brain anatomical alteration and its corresponding cognitive consequences. Given the motor manifestations of seizures in BECTS, we hypothesize that anatomical networks in BECTS involve a distributed corticostriatal circuit.
Methods
We investigated volumetric differences and shape deformities of caudate, putamen, pallidum and thalamus in a group of children with new and recent onset BECTS (N=13) compared to healthy controls (N=54). We correlated specific subcortical volumes in BECTS that were significantly different from healthy controls with performances in executive function.
Key findings
Children with BECTS demonstrated significantly hypertrophied putamen, which was selective among the subcortical regions examined. Shape analysis showed dorsal-ventral elongation of left caudate and bilateral putamen, with sub-nuclei expansion in ventral and dorsal striatum. Larger putamen volumes were linked to better cognitive performances on two complementary executive function tests.
Significance
Children with BECTS showed aberrant volume and shape in subcortical regions that are critical for both motor processing and executive function. Importantly, the hypertrophy appears to be cognitively adaptive, as enlargement was associated with improved cognitive performances. The anatomical abnormalities and their cognitive effects are evident in a group of children with new and recent onset epilepsy, suggesting that the structural brain anomalies occurred prior to the diagnosis of epilepsy.
Keywords: Benign epilepsy with centrotemporal spikes, striatum, cognition, executive function, shape analysis
Introduction
Benign epilepsy with centrotemporal spikes (BECTS) is the most common childhood epilepsy syndrome, affecting one out of every five children with epilepsy (Cavazzuti, 1980). Although the typical form is not associated with apparent brain abnormalities and seizures frequently remit during adolescence (Bouma et al., 1997), there is now compelling evidence to suggest that BECTS is a neurodevelopmental disorder with a genetic influence. First, this disorder is more common in boys than girls (3:2) and close relatives of children with BECTS have a higher prevalence of epilepsy when compared to controls (Bray & Wiser, 1964). Second, the centrotemporal spike wave electroencephalogram (EEG) trait has recently been mapped to a variant of the Elongator Complex 4 allele (Strug et al., 2009) - a gene that might be important for brain development, given its role in regulating cytoskeletal actin, cell motility and cell migration (Creppe et al., 2009; Nguyen et al., 2010). Third, children with BECTS have abnormal cognitive and behavior maturation, with impaired attention, executive function, and language, when compared to typically developing children (Kavros et al., 2008; Massa et al., 2001; Metz-Lutz et al., 1999; Monjauze et al., 2005). Despite the accumulating evidence of a neurodevelopmental etiology in BECTS, little is known about any underlying neuroanatomical alterations. Further, the cognitive consequences of potential structural anomalies have not been investigated.
The first goal of the current study was to investigate neuroanatomical alterations in BECTS. Children with BECTS have characteristic EEG patterns, with the negative component of the spike dipole orientated near the junction of the rolandic and sylvian fissures and the positive pole broadly distributed over the bilateral frontal regions (Gregory & Wong, 1984). The seizures also have a distinct focal motor semiology, manifesting as arm and oral facial tonic-clonic contractions (Engel, 2001). Based on these stereotyped electrographic and clinical phenomena, we hypothesize that neuroanatomical derangements involve a motor network – the frontostriatal network. Therefore, in the current study, we examined volumes of subcortical regions in the corticostriatal circuit including the caudate, putamen, pallidum, and thalamus. To minimize the effect that repetitive seizures might have on brain development, study participants only included children with BECTS at or near the time of seizure onset, with normal intelligence (attending regular schools), normal neurological examinations and normal clinical brain MRI scans.
The second goal of the study was to investigate potential variations in the shapes of subcortical structures in children with BECTS. In addition to volumetric changes, the developing brain also undergoes shape transformations (Hilgetag & Barbas, 2005). Brain shape and cortical convolution during maturation may, in part, reflect mechanical forces derived from the formation of cortical to cortical and cortical to subcortical connections (Rakic, 1988; Van Essen, 1997). The end result of these mechanical forces is that each subcortical region is topographically organized into distinct functional zones, based on its connections to the cortex (Behrens et al., 2003; Draganski et al., 2008). In light of the known topographical organization, uncovering anatomical deviations at sub-nuclei resolution within each subcortical structure would provide insights into aberrant cortical-subcortical networks in BECTS. Therefore, here we used three-dimensional shape analysis to localize specific sub-regions within each subcortical structure in which BECTS children might have relative hypertrophy or atrophy, compared to healthy controls.
The third goal of the study was to assess for functional consequences of structural alterations by determining the degree to which abnormal subcortical volumes in BECTS are related to cognitive performance. The subcortical regions evaluated in the current study were comprised of nodes in the corticostriatal circuit with a well-established pattern of connections – cortex to caudate/putamen (striatum) to pallidum to thalamus and back to cortex (Alexander et al., 1986; Parent & Hazrati, 1995; Postuma & Dagher, 2006). This network is critical for attention and executive function, cognitive domains that are significantly impaired in children with BECTS (Baglietto et al., 2001; Cerminara et al., 2010; Chevalier et al., 2000; D’Alessandro et al., 1990; Gunduz et al., 1999; Kavros et al., 2008; Lindgren et al., 2004; Massa et al., 2001; Metz-Lutz et al., 1999). Therefore, we correlated specific subcortical volumes in BECTS that were significantly different from healthy controls with performances in executive function.
Methods
Participants
Study participants included 13 children with new or recent onset BECTS and 54 healthy first-degree cousin controls, all attending regular schools. Entry criteria for the BECTS group included: 1) diagnosis of BECTS within the past 12 months, 2) chronological age between 8–18 years, 3) no other neurological disorders, and 4) normal routine brain MRI scans. The criteria for diagnosing BECTS were: 1) tonic-clonic convulsive nocturnal seizures or simple partial seizures during the waking hours, with seizure semiology compatible with this syndrome, 2) classic centrotemporal spikes on EEG, occurring independently in the right and left hemispheres, and 3) normal EEG background (Engel, 2001). Activation of spike wave charges with sleep was also considered a feature suggestive of this syndrome. Subjects with epileptiform abnormalities outside of the centrotemporal region were excluded from this study. In summary, children recruited in the current study have typical features of BECTS.
Control participants were first-degree cousins. Criteria for controls include no history of: 1) any initial precipitating event (e.g., simple or complex febrile seizures), 2) seizure or seizure-like event, 3) diagnosed neurological disorder, 4) loss of consciousness for longer than 5 minutes, or 5) other family history of a first-degree relative with epilepsy or febrile convulsions. We deliberately selected first-degree cousins rather than siblings to minimize shared genetic factors that might contribute to anomalies in brain structure and cognition, while at the same time controlling for potential socioeconomic confounds. Note that EEGs were not performed in controls participants.
This study was reviewed and approved by the Institutional Review Board of the University of Wisconsin School of Medicine and Public Health. On the day of study participation, families and children gave informed consent and assent.
Table 1 outlines the demographic characteristics of the study participants. The healthy control group was on average three-years older than the BECTS group (p=0.001; t-test). Gender and IQ scores were not significantly different between the two groups (gender: p>0.36; Fisher’s exact test; IQ: all p’s> 0.32; t-test). Eight of the 13 children with BECTS were on a single antiepileptic medication (3 on carbamazepine, 4 on oxcarbazepine and 1 on valproate). Of those children on an antiepileptic medication, the mean duration of exposure was 6.75 months.
Table 1.
Demographic features of study groups
| Healthy controls | BECTS | |
|---|---|---|
| N | 54 | 13 |
| Age (years) | 13.2 (3.0) | 10.2 (1.4) |
| Gender (F/M) | 30/24 | 5/8 |
| Verbal IQ | 107.1 (14.0) | 103.1 (9.4) |
| Performance IQ | 106.4 (12.5) | 105.0 (14.2) |
| Full-scale IQ | 107.7 (12.8) | 104.5 (12.2) |
| D-KEFS Correct Card Sort (scaled score) | 10.3 (2.2) | 9.6 (3.7) |
| BRIEF-BRI (T-score) | 43.4 (8.6) | 53.5 (5.8) |
| Age of seizure onset (years) | - | 9.5 (1.4) |
| Epilepsy duration (month) | - | 6.5 (3.8) |
Note: Data are shown as mean (standard deviation); F=female, M=male; IQ=Intelligence Quotient; D-KEFS=Delis–Kaplan Executive Function System; BRIEF=Behavior Rating Inventory of Executive Function; BRI=Behavior Regulation Index
Head circumference measurements
A nurse in the General Clinical Research Center (GCRC), University of Wisconsin, Madison, who was blinded to the clinical data, performed the head circumference measurements. The nurse was required to take a class on head circumference measurements, and was given specific guidelines. Each child’s head circumference was measured twice, at the beginning and end of the study day. The average of the two measures was used for the current study.
Image acquisition and processing
MRI data was obtained with a 1.5-Tesla GE Signa MR scanner. T1-weighted three-dimensional spoiled gradient recalled (SPGR) images were acquired using the following parameters: TR = 24 ms, TE = 5 ms, flip angle = 40°, NEX1, Slices = 124, plane = coronal, slice thickness = 1.5 mm, FOV = 200 mm, matrix = 256x256.
Image processing and analysis were performed using tools from the Oxford Center for Functional MRI of the Brain (FMRIB, FSL version 4.1.5, http://www.fmrib.ox.ac.uk/fsl). T1-weighted images were skull-stripped using the Brain Extraction Tool (BET) (Smith, 2002) and processed using FMRIB’s Automated Segmentation Tool (FAST) (Zhang et al., 2001) to correct for spatial intensity variations and segment images into gray matter, white matter, and CSF. Total intracranial volume (TIV) was calculated by summing the volumes of gray matter, white matter, and CSF. Subcortical gray matter structures, including caudate, putamen, pallidum and thalamus, were automatically segmented using FMRIB’s Integrated Registration and Segmentation Tool (FIRST). The subcortical segmentation procedures are described in detail in other reports and have been shown to be highly comparable to manual labeling techniques (Patenaude et al., 2011). The automated segmentation also generates a deformable mesh of vertices composed of a set of triangles. The relative position of each corresponding vertex is then compared between BECTS subjects and healthy controls in order to determine the specific regions of shape deviations. A multivariate F-test for each vertex using a Multivariate General Linear Model was carried out to compare statistical differences between BECTS and controls. Note that the F-statistics are sensitive to changes in any orientation of the coordinate system, with vector arrows denoting different directions (atrophy=inward vectors versus hypertrophy=outward vectors). Finally, multiple comparisons corrections were performed using False Discovery Rate (FDR). The resulting FDR-corrected p-values were displayed as color-coded surface maps, depicting regions within the subcortical structure that have significant shape differences.
Neuropsychological tests
Both control participants and children with BECTS completed a battery of comprehensive neuropsychological tests and their parents completed behavior questionnaires. Executive functioning was measured by the Sorting Test from the Delis–Kaplan Executive Function System (D-KEFS (Delis et al., 2004)) and the Behavior Regulation Index from the Behavior Rating Inventory of Executive Function (BRIEF-BRI (Gioia et al., 2000)). The Sorting Test is a measure of cognitive flexibility in which six cards are presented, displaying both stimulus words and perceptual features. Individuals are asked to sort the cards into two groups, according to as many concepts or rules as possible. The BRIEF is an 86-item parent questionnaire, focusing on inhibition, shifting, and emotional control. These two tests of executive function are complementary because the D-KEFS provides an objective score of the child’s performance, whereas the BRIEF offers an index of parental observations of the child’s behavior. Raw scores from the D-KEFS are scaled, with a mean of 10 and standard deviation of 3, while raw scores from the BRIEF-BRI are transformed to T-scores with a mean of 50 and standard deviation of 10. Thus both D-KEFS and BRIEF-BRI scores are standardized according test specific age and gender norms in both control and BECTS subjects.
Statistical Analysis
Volumetric analysis
Dependent measures included subcortical volumes for caudate, putamen, pallidum and thalamus. There were no significant differences between the BECTS and control subjects in the side-to-side differences (adjusted p>0.05) and therefore all volumetric analyses used total subcortical volumes (i.e. total caudate volume= right + left caudate volume) as dependent variables. There was a statistically significant difference in brain size between BECTS and controls (BECTS = 1634360 ± 156343 mm3, mean ± standard deviation, Controls 1498437 ± 127746 mm3, ANOVA with age as a covariate, p=0.01) which precluded its use as a covariate in anatomic comparisons as adjusting for this measure would lead to an underestimation of the effect of BECTS on brain structure. Therefore, occipital-frontal head circumference, which did not differ between groups (BECTS = 54.55 ± 2.76 cm, Controls 55.45 ± 2.19 cm, p=0.58) but was significantly correlated with total intracranial volume (spearman’s rho= 0.30, p=0.013), was used to correct for variations in brain size and more precisely evaluate the effects of BECTS on brain structures (Cheong et al., 2008; Tramo et al., 1998). Residual volumes were obtained after correcting for head circumference and gender using linear regression analyses. Comparisons between control and BECTS were performed using linear regression analyses, with Bonferroni correction for multiple comparisons (p<0.05/4=0.0125). Given that children with BECTS were on average younger than the controls, we performed a secondary analysis comparing children with BECTS to a subset of 26 age-matched controls (age: p=0.44). All analyses included age as a covariate. As will be described, there were no differences in findings when comparing BECTS to all controls or age-matched controls.
The effect sizes of the differences between groups for head circumference and gender corrected volumes were calculated for each subcortical region using Cohen’s d (Cohen, 1992). For consistency, a positive effect size indicates that the BECTS group has a larger brain volume when compared to controls. An effect size greater than 0.80 denotes a large magnitude of difference between the two groups.
Cognitive correlations
Right and left subcortical volumes were correlated with two cognitive endpoints: 1) D-KEFS correct card sort and 2) BRIEF-BRI. All scores were age-adjusted thereby rendering the scores directly comparable between the groups. Correlation analyses were performed using Spearman’s rho, with Bonferroni correction for multiple comparisons (significance level set at p<0.05/2).
Results
Subcortical volumes
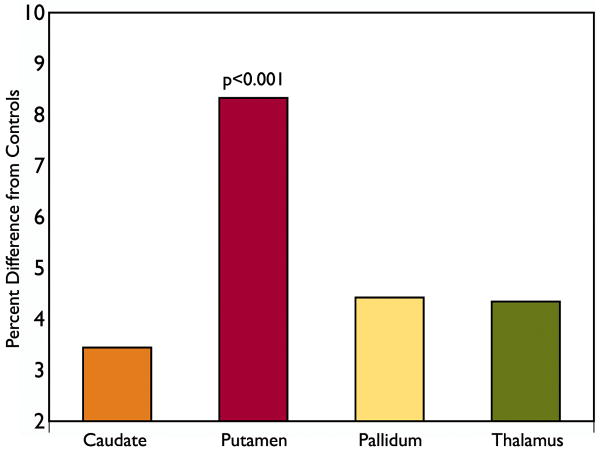
Head circumference and gender adjusted caudate, putamen, pallidum and thalamus volumes in children with BECTS were compared to controls, with age as a covariate. Significantly larger putamen volumes were present in children with BECTS, when compared to all control subjects (p=0.001, Figure 1; Supplemental Table 1). A secondary analysis comparing children with BECTS to a subset of age-matched controls yielded a similar result (p=0.001, Supplemental Table 1). On average, there was an 8.4% increase in putamen volumes with a large effect size (d=1.01). No significant volumetric differences were observed in caudate, pallidum or thalamus.
Figure 1.
BECTS subcortical volumes expressed as a percent difference from controls. Putamen volume was significantly increased in BECTS, as compared to controls (p<0.001). No significant volumetric differences were observed in caudate, pallidum or thalamus.
Subcortical Shape Analysis
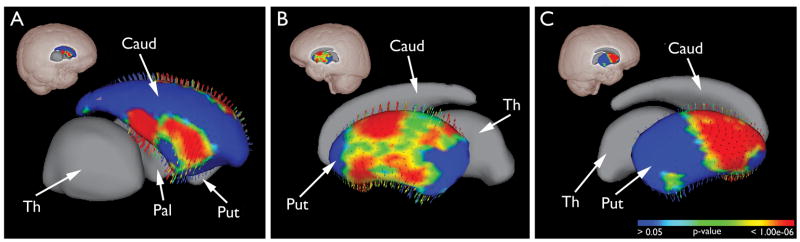
Shape analysis not only independently confirmed the significantly increased putaminal volumes but also revealed that the expansions were in a uniformly vertical direction (see vector arrows). Figure 2 shows the FDR-corrected statistical maps in the oblique sagittal view, revealing sub-regional tissue expansion within caudate and putamen (see Supplemental Figure 1 for additional views). Specifically, in the left caudate, there were two regions of enlargement in the dorsal and ventral head of the caudate (Figure 2A). Hypertrophic regions were more diffuse in the left putamen, involving both rostral and caudal putamen (Figure 2B). Small regions in the most rostral and caudal portions of the putamen were not involved (blue regions in Supplement Figure 1C, D). In contrast, more selective regions of hypertrophy were detected in the right putamen, located mainly in rostral half the nucleus (Figure 2C) and small islands of hypertrophic regions in the caudal putamen. No significant shape deviation was found in the right caudate and bilateral pallidum or thalamus.
Figure 2.
Subcortical shape analysis: regional striatal hypertrophy in BECTS group. The FDR corrected statistical maps of the subcortical structures are in the oblique sagittal view, with glass brains indicating their relative positions. In the left caudate, selective expansion was located in the ventral and dorsal head of the caudate (A). In the left putamen, the hypertrophic regions were more expansive, sparing the most rostral and caudal regions (B). In contrast, the enlarged areas in the right putamen were more selective, mainly located in the rostral half of the nucleus, and smaller regions of hypertrophy in the caudal putamen (C).
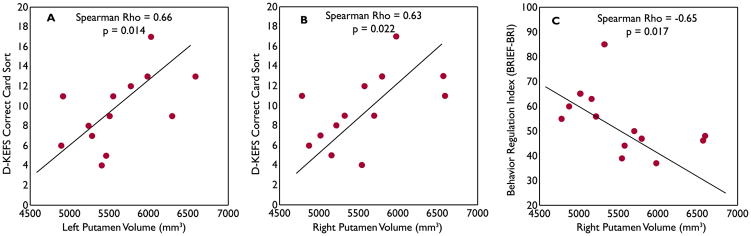
Putamen volume and executive function relationships
Whereas the left caudate and bilateral putamen in BECTS children showed significant hypertrophy, the behavior consequences of these anatomical changes have never been explored. Specifically, we tested whether such volume expansions have a positive or negative neurobehavioral or cognitive impact. Interestingly, larger left and right putamen volumes were significant correlated with better performances on D-KEF card sorting test (left: Spearman’s r=0.66, p=0.014; right: r=0.63, p=0.022, Figure 3A,B). Further, larger right putamen volumes were also linked to better executive function scores on parental questionnaires (lower scores on the BRIEF-BRI = less impairment; r=−0.65, p=0.017, Figure 3C). No correlation was found between left caudate volume and executive function performance. These associations were absent in the controls.
Figure 3.
Putamen volume and executive function relationship in BECTS group. Larger left (A) and right (B) putamen volumes were significantly correlated with better performances on D-KEFS card sorting test. Larger right putamen volumes (C) were linked to less impairment on BRIEF-BRI.
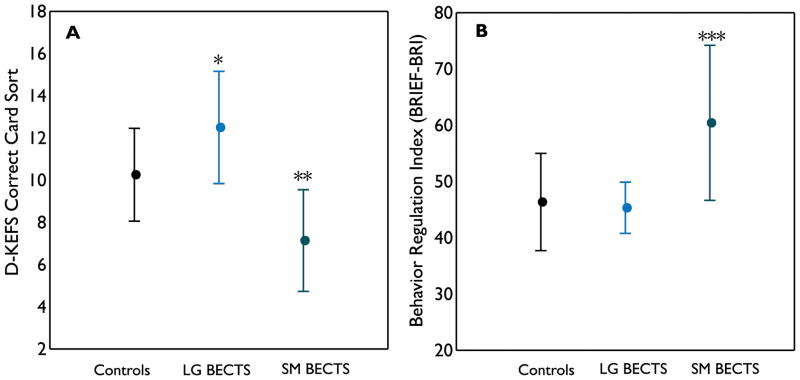
Although larger putamen volumes in BECTS were associated with better performances on executive function tests, variations in putamen volumes exist among children with BECTS. It is unknown whether BECTS children with larger putamen volumes performed markedly better than controls on executive function tests, or those with smaller putamen volumes performed significantly worse than controls. Therefore, we dichotomized the BECTS group based on total putamen volumes (median split, n=6, > 50th percentile; n=7, < or = 50th percentile) and compared their executive function scores to controls. A significant group difference in the age-adjusted D-KEF card sorting scores was found among the three groups (ANOVA, p=0.0003, Figure 4A). Pair-wise comparisons showed BECTS children with larger putamen volumes to perform on average significantly better than controls (p=0.024, t-test). In contrast, BECTS children with smaller putamen volumes performed on average significantly worse than controls (p=0.001, t-test). A significant group difference was also noted in the age-adjusted BRIEF-BRI scores (ANOVA, p=0.0013, Figure 4B). Pair-wise analyses showed BECTS children with larger putamen volumes had similar scores as controls, but BECTS children with smaller putamen had significantly more impairment than controls (p=0.0006).
Figure 4.
Executive function performances in controls, BECTS with larger (LG BECTS) and smaller (SM BECTS) putamen volumes. On both executive function tests, a significant group difference was found among the three groups (D-KEFS card sorting test, p=0.0003; BRIEF-BRI, p=0.0006, ANOVA). Pair-wise comparisons showed that BECTS children with larger putamen on average performed significantly better than controls on D-KEFS card sorting test (* p=0.024), while those with smaller putamen performed significantly worse than controls (** p=0.001). On BRIEF-BRI, BECTS children with larger putamen were not significantly different from controls but those with smaller putamen on average had significantly more impairment than controls (*** p=0.0006).
Discussion
The current study represents the first report to delineate subcortical brain morphometric profiles of children with new and recent onset BECTS and the cognitive consequences of these structural alterations. The five major finding are as follows: 1) Among the subcortical regions in corticostriatal network, the putamen is selectively hypertrophied in children with BECTS, compared to controls. 2) In addition to overall volume expansion, shape deformities were present in BECTS, with vertical expansion in the left caudate and bilateral putamen. 3) At the sub-nuclei level, the shape displacements were most evident in the rostral portion of the striatum, although smaller regions of expansion were also seen in the caudal putamen. 4) Larger putamen volumes were significantly linked to better performances on two complementary executive function tests. 5) BECTS children with larger putamen on average performed better than controls on D-KEF card sorting test, while those with smaller putamen performed worse than controls.
Larger striatal volumes and vertical shape expansion in BECTS children: significance for neurodevelopment abnormalities
An unanticipated and very interesting finding of the current study was the presence of bilateral putamen hypertrophy, documented by both overall volume and shape analysis, and left caudate hypertrophy found using shape analysis. Although the overall volume of the left caudate in BECTS was not different from controls, shape analysis showed significant hypertrophy in small subregions of the caudate. Shape analysis and other similar surface mapping techniques have been found to be more sensitive to disease specific differences, compared to analysis of overall volume (Apostolova et al., 2010a; Apostolova et al., 2010b). While striatum (putamen and caudate) volume abnormalities are often present in both chronic temporal lobe epilepsy (Bouilleret et al., 2008; Dreifuss et al., 2001; Geary et al., 2009; Riley et al., 2011) and idiopathic generalized epilepsy (Ciumas & Savic, 2006; Seeck et al., 2005), the volumes are reduced in those disorders, not increased. In fact, BECTS is the only epilepsy syndrome we are aware of in which hypertrophy is present in the striatum.
Whereas there is now accumulating evidence that the development of BECTS might be genetically influenced, the morphometric findings in the current study provide additional evidence of neurodevelopemental factors to the origin of this epilepsy syndrome. First, longitudinal quantitative MRI studies of healthy children have shown that striatal volumes linearly decline in late childhood and adolescence (Giedd et al., 1996; Ostby et al., 2009; Sowell et al., 2002). The enlargement observed in the current study is discordant with normal developmental trajectory of these brain regions. It is intriguing that enlarged striatum have been consistently demonstrated in autism, another neurodevelopmental disorder (Herbert et al., 2003; Langen et al., 2007). Second, the morphometric alteration in the current study is evident in a group of children with new and recent onset BECTS, suggesting anomalies in brain development occurred before the onset of seizures. Third, the ELP4 gene for the centrotemporal spike EEG trait is highly expressed in the striatum, among other brain regions (Allen Brain Atlas, www.brain-map.org). ELP4 belongs to the family of elongator genes that controls the trafficking of various proteins and organelles through acetylation of alpha-tubulin and as such, underlies the maturation of cortical projection neurons (Creppe et al., 2009; Nguyen et al., 2010). More recently, striatal and cortical neurons from patients with Huntington’s disease have shown a reduced level of alpha-tubulin and impaired axonal transport (Dompierre et al., 2007) but elongator’s role in BECTS remains to be elucidated.
Shape alterations identified in our study further support the notion that BECTS is coupled with abnormal brain development. The caudate and putamen are elongated in a vertical direction (dorsal-ventral). As brain structural development is tightly coupled with brain shape changes, such alterations are likely to have important neurodevelopmental implications. A recent craniometric study showed that brain maturation is accompanied with an anterior-posterior expansion of the cerebral hemisphere relative to the width of the brain (Vannucci et al., 2011). Because brain shape changes seen in that study were most dramatic during infancy, with minor reshaping in childhood and adolescence, the shape deviations found in the current study suggest that these changes might have occurred early in the course of brain development.
Putamen hypertrophy and executive function: the bigger the better?
Whereas the process that leads to putamen hypertrophy in BECTS is unclear, the functional consequences provide insights as to whether such anatomical anomaly is adaptive or maladaptive. The findings of the current study suggest that putamen hypertrophy in BECTS might be an adaptive response. First, larger putamen volumes are linked to better performance on two complementary executive function tests in children with BECTS. Second, a sub-group of BECTS children who have larger putamen volumes performed appreciably better than controls on D-KEF card sorting test, while those with smaller putamen volumes performed worse than controls. Third, parents did not perceive any behavior impairment in BECTS children with larger putamen volumes but observed that those with smaller putamen volumes had significantly more problems on executive function. However, it remains to be determined if such adaptive responses are due to nature or nurture. On the one hand, the human brain has considerable neuroplasticity and appears to be amendable to environmental influences. For example, active engagement in spatial navigation over time can significantly enlarge the posterior hippocampus (Maguire et al., 2000). On the other hand, human brain shape and size are largely determined prenatally. Hill and colleagues found that cortical shape and folder patterns in term infants are similar to adults in many respects, including pattern individual variability (Hill et al., 2010). Given that BECTS children with larger putamen performed even better than controls, it is intriguing to consider whether putamen hypertrophy might be a primary developmental process, which survived evolutionarily because it improved executive function (Bickart et al., 2011; Striedter, 2005).
Conclusions and future directions
In summary, the current study revealed the selective nature of striatal hypertrophy and links to executive functioning in children with recent and new-onset BECTS. Several limitations and opportunities for future research will now be addressed.
This study is a cross sectional study with a number of associated limitations. In the context of normal development, it is unknown if these structural alterations will normalize, as seizures usually remit in this epilepsy syndrome. A follow up longitudinal investigation may clarify whether changes seen in the striatum represent a temporary developmental anomaly and these children will “catch up” after the cessation of seizures and medication (Shaw et al., 2007). Further, the current study aimed to identify significant group differences and heterogeneity in striatal size among children with BECTS, and to determine whether these differences correlate with executive function. Longitudinal follow up of these children will afford us the opportunity to assess whether baseline striatal volume is a clinically useful biomarker for cognitive outcome. A prospective controlled study is underway to assess both brain and cognitive developmental trajectories in this cohort.
While specific striatal alterations are detected in children with BECTS, the exact frontostriatal network affected is unclear. With advances in diffusion tensor imaging analysis techniques, it is now possible to map specific frontostriatal connections and link their connection strength to cognitive performances (Cohen et al., 2009; Riley et al., 2011). Clearly, in future studies, characterizing specific frontostriatal tracts (i.e. frontal lobe to ventral vs. dorsal striatum) in relation to distinct aspects of executive function (working memory vs. reward-based decision) would further advance the understanding of how these neurodevelopmental changes impact cognition in BECTS.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke Grant No. K23 NS060993 (JJL) and R01 44351 (BPH, MS).
Footnotes
Disclosure
The authors have no conflicts of interests. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Beyer M, Green AE, Hwang KS, Morra JH, Chou YY, Avedissian C, Aarsland D, Janvin CC, Larsen JP, Cummings JL, Thompson PM. Hippocampal, caudate, and ventricular changes in Parkinson’s disease with and without dementia. Mov Disord. 2010a;25:687–688. doi: 10.1002/mds.22799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova LG, Mosconi L, Thompson PM, Green AE, Hwang KS, Ramirez A, Mistur R, Tsui WH, de Leon MJ. Subregional hippocampal atrophy predicts Alzheimer’s dementia in the cognitively normal. Neurobiol Aging. 2010b;31:1077–1088. doi: 10.1016/j.neurobiolaging.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglietto MG, Battaglia FM, Nobili L, Tortorelli S, De Negri E, Calevo MG, Veneselli E, De Negri M. Neuropsychological disorders related to interictal epileptic discharges during sleep in benign epilepsy of childhood with centrotemporal or Rolandic spikes. Dev Med Child Neurol. 2001;43:407–412. doi: 10.1017/s0012162201000755. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nat Neurosci. 2011;14:163–164. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouilleret V, Semah F, Chassoux F, Mantzaridez M, Biraben A, Trebossen R, Ribeiro MJ. Basal ganglia involvement in temporal lobe epilepsy: a functional and morphologic study. Neurology. 2008;70:177–184. doi: 10.1212/01.wnl.0000297514.47695.48. [DOI] [PubMed] [Google Scholar]
- Bouma PA, Bovenkerk AC, Westendorp RG, Brouwer OF. The course of benign partial epilepsy of childhood with centrotemporal spikes: a meta-analysis. Neurology. 1997;48:430–437. doi: 10.1212/wnl.48.2.430. [DOI] [PubMed] [Google Scholar]
- Bray PF, Wiser WC. Evidence for a Genetic Etiology of Temporal-Central Abnormalities in Focal Epilepsy. N Engl J Med. 1964;271:926–933. doi: 10.1056/NEJM196410292711803. [DOI] [PubMed] [Google Scholar]
- Cavazzuti GB. Epidemiology of different types of epilepsy in school age children of Modena, Italy. Epilepsia. 1980;21:57–62. doi: 10.1111/j.1528-1157.1980.tb04044.x. [DOI] [PubMed] [Google Scholar]
- Cerminara C, D’Agati E, Lange KW, Kaunzinger I, Tucha O, Parisi P, Spalice A, Curatolo P. Benign childhood epilepsy with centrotemporal spikes and the multicomponent model of attention: a matched control study. Epilepsy Behav. 2010;19:69–77. doi: 10.1016/j.yebeh.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Cheong JL, Hunt RW, Anderson PJ, Howard K, Thompson DK, Wang HX, Bear MJ, Inder TE, Doyle LW. Head growth in preterm infants: correlation with magnetic resonance imaging and neurodevelopmental outcome. Pediatrics. 2008;121:e1534–1540. doi: 10.1542/peds.2007-2671. [DOI] [PubMed] [Google Scholar]
- Chevalier H, Metz-Lutz MN, Segalowitz SJ. Impulsivity and control of inhibition in Benign Focal Childhood Epilepsy (BFCE) Brain Cogn. 2000;43:86–90. [PubMed] [Google Scholar]
- Ciumas C, Savic I. Structural changes in patients with primary generalized tonic and clonic seizures. Neurology. 2006;67:683–686. doi: 10.1212/01.wnl.0000230171.23913.cf. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Schoene-Bake JC, Elger CE, Weber B. Connectivity-based segregation of the human striatum predicts personality characteristics. Nat Neurosci. 2009;12:32–34. doi: 10.1038/nn.2228. [DOI] [PubMed] [Google Scholar]
- Creppe C, Malinouskaya L, Volvert ML, Gillard M, Close P, Malaise O, Laguesse S, Cornez I, Rahmouni S, Ormenese S, Belachew S, Malgrange B, Chapelle JP, Siebenlist U, Moonen G, Chariot A, Nguyen L. Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell. 2009;136:551–564. doi: 10.1016/j.cell.2008.11.043. [DOI] [PubMed] [Google Scholar]
- D’Alessandro P, Piccirilli M, Tiacci C, Ibba A, Maiotti M, Sciarma T, Testa A. Neuropsychological features of benign partial epilepsy in children. Ital J Neurol Sci. 1990;11:265–269. doi: 10.1007/BF02333856. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: an update. J Int Neuropsychol Soc. 2004;10:301–303. doi: 10.1017/S1355617704102191. [DOI] [PubMed] [Google Scholar]
- Dompierre JP, Godin JD, Charrin BC, Cordelieres FP, King SJ, Humbert S, Saudou F. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington’s disease by increasing tubulin acetylation. J Neurosci. 2007;27:3571–3583. doi: 10.1523/JNEUROSCI.0037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Kloppel S, Cook PA, Alexander DC, Parker GJ, Deichmann R, Ashburner J, Frackowiak RS. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J Neurosci. 2008;28:7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss S, Vingerhoets FJ, Lazeyras F, Andino SG, Spinelli L, Delavelle J, Seeck M. Volumetric measurements of subcortical nuclei in patients with temporal lobe epilepsy. Neurology. 2001;57:1636–1641. doi: 10.1212/wnl.57.9.1636. [DOI] [PubMed] [Google Scholar]
- Engel J., Jr A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- Geary EK, Seidenberg M, Hermann B. Atrophy of basal ganglia nuclei and negative symptoms in temporal lobe epilepsy. J Neuropsychiatry Clin Neurosci. 2009;21:152–159. doi: 10.1176/appi.neuropsych.21.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Child Neuropsychol. 2000;6:235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- Gregory DL, Wong PK. Topographical analysis of the centrotemporal discharges in benign rolandic epilepsy of childhood. Epilepsia. 1984;25:705–711. doi: 10.1111/j.1528-1157.1984.tb03481.x. [DOI] [PubMed] [Google Scholar]
- Gunduz E, Demirbilek V, Korkmaz B. Benign rolandic epilepsy: neuropsychological findings. Seizure. 1999;8:246–249. doi: 10.1053/seiz.1999.0293. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Lange N, Bakardjiev A, Hodgson J, Adrien KT, Steele S, Makris N, Kennedy D, Harris GJ, Caviness VS., Jr Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain. 2003;126:1182–1192. doi: 10.1093/brain/awg110. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Barbas H. Developmental mechanics of the primate cerebral cortex. Anat Embryol (Berl) 2005;210:411–417. doi: 10.1007/s00429-005-0041-5. [DOI] [PubMed] [Google Scholar]
- Hill J, Dierker D, Neil J, Inder T, Knutsen A, Harwell J, Coalson T, Van Essen D. A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. J Neurosci. 2010;30:2268–2276. doi: 10.1523/JNEUROSCI.4682-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavros PM, Clarke T, Strug LJ, Halperin JM, Dorta NJ, Pal DK. Attention impairment in rolandic epilepsy: systematic review. Epilepsia. 2008;49:1570–1580. doi: 10.1111/j.1528-1167.2008.01610.x. [DOI] [PubMed] [Google Scholar]
- Langen M, Durston S, Staal WG, Palmen SJ, van Engeland H. Caudate nucleus is enlarged in high-functioning medication-naive subjects with autism. Biol Psychiatry. 2007;62:262–266. doi: 10.1016/j.biopsych.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Lindgren S, Kihlgren M, Melin L, Croona C, Lundberg S, Eeg-Olofsson O. Development of cognitive functions in children with rolandic epilepsy. Epilepsy Behav. 2004;5:903–910. doi: 10.1016/j.yebeh.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa R, de Saint-Martin A, Carcangiu R, Rudolf G, Seegmuller C, Kleitz C, Metz-Lutz MN, Hirsch E, Marescaux C. EEG criteria predictive of complicated evolution in idiopathic rolandic epilepsy. Neurology. 2001;57:1071–1079. doi: 10.1212/wnl.57.6.1071. [DOI] [PubMed] [Google Scholar]
- Metz-Lutz MN, Kleitz C, de Saint Martin A, Massa R, Hirsch E, Marescaux C. Cognitive development in benign focal epilepsies of childhood. Dev Neurosci. 1999;21:182–190. doi: 10.1159/000017397. [DOI] [PubMed] [Google Scholar]
- Monjauze C, Tuller L, Hommet C, Barthez MA, Khomsi A. Language in benign childhood epilepsy with centro-temporal spikes abbreviated form: rolandic epilepsy and language. Brain Lang. 2005;92:300–308. doi: 10.1016/j.bandl.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Humbert S, Saudou F, Chariot A. Elongator - an emerging role in neurological disorders. Trends Mol Med. 2010;16:1–6. doi: 10.1016/j.molmed.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Riley JD, Moore S, Cramer SC, Lin JJ. Caudate atrophy and impaired frontostriatal connections are linked to executive dysfunction in temporal lobe epilepsy. Epilepsy Behav. 2011;21:80–87. doi: 10.1016/j.yebeh.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeck M, Dreifuss S, Lantz G, Jallon P, Foletti G, Despland PA, Delavelle J, Lazeyras F. Subcortical nuclei volumetry in idiopathic generalized epilepsy. Epilepsia. 2005;46:1642–1645. doi: 10.1111/j.1528-1167.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Striedter GF. Principles of brain evolution. Sinauer Associates; Sunderland, Mass: 2005. [Google Scholar]
- Strug LJ, Clarke T, Chiang T, Chien M, Baskurt Z, Li W, Dorfman R, Bali B, Wirrell E, Kugler SL, Mandelbaum DE, Wolf SM, McGoldrick P, Hardison H, Novotny EJ, Ju J, Greenberg DA, Russo JJ, Pal DK. Centrotemporal sharp wave EEG trait in rolandic epilepsy maps to Elongator Protein Complex 4 (ELP4) Eur J Hum Genet. 2009;17:1171–1181. doi: 10.1038/ejhg.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramo MJ, Loftus WC, Stukel TA, Green RL, Weaver JB, Gazzaniga MS. Brain size, head size, and intelligence quotient in monozygotic twins. Neurology. 1998;50:1246–1252. doi: 10.1212/wnl.50.5.1246. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Barron TF, Lerro D, Anton SC, Vannucci SJ. Craniometric measures during development using MRI. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.03.044. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.